Abstract
Background
Cytokine storm is a marker of severity and severe mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia. Immunomodulatory treatments may reduce morbidity and mortality.
Objectives
To determine whether a 7-day course of methylprednisolone (MP) administered with and without tocilizumab improves outcomes in patients with severe COVID-19 (SARS-CoV-2) pneumonia requiring oxygen therapy, relative to historical controls.
Study design and method
In this randomized controlled study, patients hospitalized with severe COVID-19 at Rashid Hospital, Dubai, in June 2020 were randomized 1:1 to receive intravenous MP (40 mg twice daily for 7 days) with or without a single dose of intravenous tocilizumab (400 mg). While data from the control arm, consisting of patients administered usual care, were obtained through retrospective review of their electronic medical records. The patients in the three arms were matched by disease severity and inclusion and exclusion criteria. The primary outcomes were day 45 all-cause mortality after randomization, rate of admission to the intensive care unit (ICU), length of ICU stay, days on ventilators, and length of hospital stay.
Results
In total, 76 patients were recruited, including 23 treated with MP, 26 with MP plus tocilizumab, and 27 historical controls. The rates of admission to the ICU and invasive mechanical ventilation were lowest in patients treated with MP alone, with the rates in this group being significantly lower than the rates in the control group (p = 0.04). Time on a ventilator was lowest in the MP group (1.09 ± 3.68 days) and highest in the control group (7.93 ± 14.86 days). The number of days in the ICU was significantly lower in the MP group than in the control and MP plus tocilizumab groups (p = 0.043). One patient (4.3%) in the MP group and five (18.5%) in the control arm died within 45 days. Survival was highest in patients treated with MP alone, with the addition of tocilizumab not improving survival or any of the other outcomes significantly.
Interpretation/conclusion
In patients with severe COVID-19 pneumonia on oxygen support, administration of MP daily for 7 days had reduced mortality at 45 days and was associated with significantly lower ICU admission and ventilation rates compared with usual. Adding tocilizumab to MP did not improve any of the studied outcomes significantly.
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; MP, methylprednisolone; WHO, World Health Organization; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein (CRP); LDH, lactate dehydrogenase; IL-6, interleukin-6; CCS, COVID-19 cytokine storm; RT-PCR, real-time PCR; HIV, human immunodeficiency virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase
Keywords: Methylprednisolone therapy, Severe COVID-19 pneumonia, Tocilizumab
Background
Since first reported, coronavirus disease 2019 (COVID-19) infection has spread worldwide, prompting the World Health Organization (WHO) to characterize it as a pandemic on 11 March 2020 [1]. Although most patients with COVID-19 develop mild to moderate disease, approximately 15% develop severe disease that requires oxygen support, and around 5% have critical disease with complications such as respiratory failure, acute respiratory distress syndrome (ARDS), sepsis and septic shock, thromboembolism, and/or multiorgan failure, including acute kidney injury and cardiac injury [2,3]. Early retrospective data showed increased mortality rates in patients with elevated inflammatory markers, such as ferritin, C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6 (IL-6), and D-dimer [4,5]. Uncontrolled and persistent cytokine release and a hyperinflammatory response, termed the COVID-19 cytokine storm (CCS), have been reported to be major determinants of poor survival [5,6], suggesting that immunomodulatory treatments may reduce patient morbidity and mortality.
Corticosteroids are commonly prescribed as adjuvant therapy in acute respiratory distress syndrome because of their perceived anti-inflammatory effects. However, for patients with severe viral pneumonia, corticosteroid therapy remained highly controversial [[7], [8], [9], [10], [11]]. The benefits of corticosteroids in improving outcomes of COVID-19 pneumonia were realized when evidence started to evolve in the form of retrospective data. In a systematic review and meta-analysis of 73 comparative studies describing the experience of corticosteroids in COVID-19 in early 2020, severely ill patients showed a statistically significant mortality benefit from corticosteroids [12]. Randomized trials soon emerged to reveal survival benefits of corticosteroids compared with usual care in severe COVID-19 pneumonia [[13], [14], [15], [16], [17]]. For example, the UK RECOVERY trial showed that treatment with 6 mg dexamethasone once daily reduced mortality rates by one-third in ventilated patients with severe COVID-19 and by one-fifth in patients receiving oxygen, when compared with usual care [13]. Several trials examining use of corticosteroids for COVID-19 were halted after publication of the RECOVERY trial results; however, a prospective meta-analysis from the WHO rapid evidence appraisal for COVID-19 therapies (REACT) pooled data from 7 randomized clinical trials that totaled 1703 critically ill patients with COVID19 revealed that the administration of corticosteroids was associated with lower all-cause mortality at 28 days after randomization. The ORs for the association between corticosteroids and mortality were similar for dexamethasone and hydrocortisone. Optimal dose and duration of treatment could not be assessed in this analysis [14]. This led the WHO to include corticosteroids in treatment guidelines for patients with severe COVID 19 [15].
In the meanwhile, observational studies have found that treatment with other immunomodulator like IL-6 inhibitors was associated with reduced risks of intubation and/or death in patients with severe COVID-19 infection [[18], [19], [20], [21]]. By contrast, the results of randomized trials found that these agents did not have survival benefits or other clear clinical benefits in these patients [[22], [23], [24]].
At the time we conducted our study, prospective data on steroid use in patients with severe COVID19 on oxygen support but not yet requiring mechanical respiratory support remained limited. The RECOVERY trial results showed a modest benefit of corticosteroids in less severely ill patients receiving oxygen without invasive mechanical ventilation [13]. Furthermore, the WHO rapid evidence appraisal for COVID-19 therapies (REACT) could not asses steroid optimal dose and duration of treatment [14]. Therefore, reporting the effect of steroids other than dexamethasone, identifying their therapeutic doses and duration of treatment in severely ill COVID-19 patients on oxygen support, and examining their effectiveness when combined with other immunomodulators have become important. This prospective randomised controlled study compared clinical outcomes in three groups of patients with severe COVID-19 pneumonia requiring oxygen support: those treated with methylprednisolone (MP) alone, those administered MP plus tocilizumab, and patients receiving usual care alone.
Objectives
The primary objective of this prospective randomized controlled study is to evaluate whether early intravenous MP at dose of 40 mg twice daily for 7 days is effective in improving clinical outcomes in hospitalized patients with severe COVID-19 (SARS-CoV-2) pneumonia on oxygen support, relative to historical controls. The secondary objective is to explore whether adding a single dose of intravenous tocilizumab (400 mg) will lead to better clinical outcomes compared to control in the same group of patients.
Patients and methods
Study design
This prospective study with a retrospective control arm was performed June 2020 at Rashid Hospital, the main designated hospital for management of moderate, severe, and critical COVID-19 patients in Dubai. Patients were randomized 1:1 to receive intravenous MP, with or without tocilizumab. Data from the control arm, consisting of patients administered usual care, were obtained through retrospective review of their electronic medical records. Patients in the three groups were matched by disease severity and inclusion and exclusion criteria. The study was approved by the Dubai Health Authority ethics committee. Written informed consent was obtained from each patient or from a legal representative if patients in the prospective groups were unable to provide consent.
Eligibility criteria
Patients aged ≥18 years were deemed eligible if they were hospitalized at Rashid Hospital, Dubai, with clinically and laboratory confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as shown by positive results on real-time PCR (RT-PCR) of nasopharyngeal swab samples. Other eligibility criteria included the development lung infiltrates involving >50% of the lung fields, as shown on chest X-rays, within 48 h of admission; and O2 saturation <93% at rest on room air. Patients could receive concomitant antiviral and other usual care.
Exclusion criteria
Women of child-bearing age, patients with known hypersensitivity to tocilizumab, and organ transplant recipients were excluded. Patients were also excluded if they had other active infections, bowel diverticulitis or perforation, heart failure, decompensated liver cirrhosis, cancer, human immunodeficiency virus (HIV) positive, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >5 times the upper limit of normal, neutrophil counts <500/μl, or platelet counts <50.000/μl.
Intervention
Patients were prospectively randomized 1:1 to receive intravenous MP (40 mg twice daily for 7 days), alone or with a single dose of intravenous tocilizumab (400 mg). By review of their electronic medical records, patients in the historical control group were retrospectively matched with patients in the prospective groups by disease severity and the same inclusion and exclusion criteria, except that they did not receive MP or the combination of MP and tocilizumab. However, 11 patients in the retrospective control group received tocilizumab as part of their usual care. All eligible patients in the three groups received usual care, which included antiviral agents (chloroquine or hydroxychloroquine; lopinavir/ritonavir and/or favipiravir) and anticoagulants, in addition to supportive care with oxygen and intravenous fluids. Baseline data were obtained from the electronic medical records maintained by the Dubai Health Authority.
Outcome measures
All patients were followed-up until day 45 after randomization. The primary outcomes were all-cause mortality, rate of admission to the intensive care unit (ICU), length of ICU stay, days on ventilators, and length of hospital stay.
Statistical analysis
Continuous variables are reported as mean ± standard deviation, and categorical variables as numbers and percentages. Continuous variables in the three groups were compared by one-way ANOVA (for normally distributed data) or the Kruskal–Wallis nonparametric test (for non-normally distributed data). Categorical variables in the three groups were compared by Chi-square tests or Fisher’s exact tests, as warranted. Survival outcomes were determined by the Kaplan–Meier method and compared by log-rank tests. p-Values <0.05 were considered statistically significant.
Results
A total of 76 patients were recruited, including 23 treated with MP alone, 26 treated with MP and tocilizumab, and 27 in the historical control group; of the latter, 11 were treated with tocilizumab, whereas 16 were not. The most common stratification factors at baseline were comparable in the three groups (Table 1 ). The 76 patients included 65 (85.5%) men and 11 (14.5%) women, of mean age 48 years (range, 23–77 years). Of these patients, 55% had at least one major comorbidity, including 47% who had diabetes and 30% who had hypertension. At enrolment, all the participants had clinically and laboratory confirmed SARS-CoV-2 infection, with chest X-rays showing lung infiltrates involving >50% of the lung fields developing within 48 h, with O2 saturation <93% at rest on room air.
Table 1.
Baseline demographic and clinical characteristics of the patients with severe COVID-19 pneumonia. MP: methylprednisolone, CRP: C-reactive protein; SOB: shortness of breath. Results are presented as mean ± SD, unless otherwise indicated.
| Variables | Total (n = 76) | Control group (n = 27) | MP group (n = 23) | MP plus tocilizumab group (n = 26) | p-Value | |
|---|---|---|---|---|---|---|
| Age, year | 48 (23–77) | 47.26 ± 12.5 | 45.04 ± 11.07 | 51.50 ± 10.75 | 0.07 | |
| Gender no. (%) | Male | 65 (85.5) | 25 (92.6) | 18 (78.3) | 22 (84.6) | |
| Females | 11 (14.5) | 2 (7.4) | 5 (21.7) | 4 (15.3) | ||
| Duration of symptoms, days | 5.364 ± 2.73 | 5.478 ± 2.428 | 6.307 ± 2.936 | 0.4 | ||
| Comorbidities (diabetes and/or hypertension) | All | 42, 55.2% | 10, 37.03% | 12,52.17% | 20, 76.92% | |
| 0 | 34, 44.7% | 17, 62.96% | 11, 47.83% | 6, 23.08% | 0.02 | |
| 1 | 28, 36.8% | 7, 25.93% | 8, 34.78% | 13, 50% | ||
| 2 | 14, 18.4% | 3, 11.11% | 4, 17.39% | 7, 26.92% | ||
| CRP (mg/dl) on arrival | 127 ± 88.17 | 148.91 ± 99.81 | 155.23 ± 100.13 | 0.06 | ||
| Symptoms at presentation | Fever | 69 (90%) | 24 (89%) | 23 (100%) | 22 (85%) | |
| Cough | 59 (77.6%) | 22 (81.4%) | 17 (74%) | 20 (77%) | ||
| SOB | 50 (65.8%) | 19 (70%) | 15 (65%) | 16 (61.5%) | ||
| Chest X-ray infiltrates involving >50% | 75 (98.7%) | 26 (96%) | 23 (100%) | 26 (100%) | ||
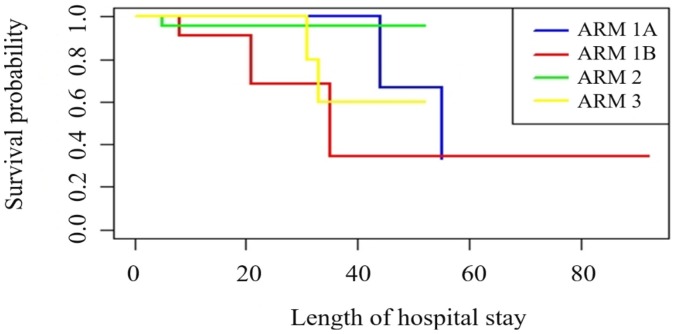
Table 2 shows the main study outcomes in the three groups. The rates of admission to the ICU and of progression to invasive mechanical ventilation were lowest in patients treated with MP alone, and were significantly lower in the MP group than in the control group (p = 0.04 each). Mean time on a ventilator was lowest in the MP group (1.09 ± 3.68 days) and highest in the control group (7.93 ± 14.86 days). Mean length of ICU stay was significantly lower in the MP group than in the control and MP plus tocilizumab group (p = 0.04), whereas mean length of hospital stay was similar in the three groups. Overall, one patient in the MP group (4.3%) and five (18.5%) in the control group died within 45 days of study enrolment. Kaplan–Meier analysis showed that the overall survival rate was highest in the MP group, and that the addition of tocilizumab to MP did not significantly improve survival (Fig. 1 ). Kaplan–Meier analysis also showed that patients in the control group who were not treated with tocilizumab showed the most precipitous early reduction, indicating a higher early mortality rate, although the difference between these patients and those in the control group who received tocilizumab was not statistically significant, likely because the sample sizes were very small.
Table 2.
Primary and secondary outcomes in patients with severe COVID-19 pneumonia receiving methylprednisolone (MP) alone, MP combined with tocilizumab, or usual care alone. One-way ANOVA test for all, Kruskal–Wallis test, and Fisher’s exact test/Chi-square test of proportionality were used. Results are presented as mean ± SD, unless otherwise indicated. ICU: intensive care unit.
| Variables | Control group (n = 27) | MP group (n = 23) | MP plus tocilizumab group (n = 26) | p-Values |
||
|---|---|---|---|---|---|---|
| Total (n = 76) | MP group versus control | MP plus tocilizumab versus control | ||||
| Length of hospital stay, days | 21 ± 19.85 | 20.83 ± 12.22 | 23.27 ± 8.96 | 0.800 | 0.9 | 0.5 |
| Admission to ICU | 9, 33.33% | 2, 8.69% | 6, 23.08% | 0.116 | 0.04 | 0.4 |
| Invasive mechanical ventilation | 9, 33.33% | 2, 8.69% | 6, 23.08% | 0.116 | 0.04 | 0.4 |
| Days on ventilators | 7.93 ± 14.86 | 1.09 ± 3.68 | 3.38 ± 7.11 | 0.052 | .03 | 0.1 |
| Length of ICU stay, days | 9.96 ± 16.73 | 2 ± 6.79 | 3.92 ± 7.99 | 0.043 | 0.03 | 0.1 |
| Day 45 mortality | 5, 18.5% | 1, 4.35% | 2, 7.69% | 0.231 | 0.129 | 0.2 |
Fig. 1.
Kaplan–Meier probability of survival according to treatment assignment. Kaplan–Meier survival curve showing the probabilities of survival in each treatment group, including sub analysis of the usual care group. Arm 1A, patients administered usual care without tocilizumab; Arm 1B, patients administered usual care plus tocilizumab; Arm 2, patients treated with methylprednisolone alone; Arm 3, patients treated with methylprednisolone plus tocilizumab.
Discussion
The present study investigated the use of MP in a homogeneous group of patients diagnosed with severe COVID-19 pneumonia who received supplemental oxygen only at the time of randomization. The results showed that the 45-day mortality rate was significantly lower in patients treated with 40 mg intravenous MP twice daily for 7 days than in a matched group of patients treated with usual care alone. These findings were comparable to those observed in patients in the RECOVERY trial receiving oxygen and treated with dexamethasone [12]. MP reduced the requirement for invasive mechanical ventilation to 8.7%, compared with 33.3% in the control group. Moreover, patients on MP spent less time on a ventilator and less time in the ICU than control patients. The addition of tocilizumab to MP did not improve patient outcomes significantly, including patient survival rates. This result is consistent with most prospective randomized trials of tocilizumab and other IL6 blockers [[9], [10], [11]], which found that these agents did not enhance survival or have any other clear clinical benefits. However, comorbidity rates at the time of enrolment were higher in the MP plus tocilizumab group than in the control or MP groups, which may have masked any additive impact of tocilizumab on patient outcomes.
The results of the present study indicate that early use of MP at a dose of 40 mg twice daily for 7 days may benefit patients with Covid-19 with severe pneumonia in need of supplemental oxygen and prior to receiving respiratory support. Because this clinical stage is dominated by immunopathological elements, timely and appropriate application of glucocorticoid may play a crucial role in the treatment of these patients. Adding tocilizumab to prednisolone did not improve any of the studied outcomes in this group of patients.
Our study had several limitations. First, the control group was selected retrospectively. Although the control group had similar disease severity and received the same usual care as patients treated with MP, with or without tocilizumab, the latter agent was administered to 11 of the 26 patients in the control group as part of their usual care. Furthermore, adverse events related to treatment were not evaluated and the encountered comorbidities were limited to diabetes and hypertensin. Unfortunately, the number of diabetic patients in each arm were not large enough to be analyzed separately. Moreover, although patients in the MP and MP plus tocilizumab groups were randomized, treatment assignment was open labelled. In addition, this was a modest-sized case series of patients admitted to a single hospital. Therefore, the results of statistical testing and the resultant p-values should be interpreted with caution. Specifically, non-significant p-values may not necessarily rule out differences between treatment modalities.
Our study findings show promising results and recommend the use of methylprednisolone in the severe form of the disease; despite these promising results, further confirmation is still needed due to the current study’s limitations.
Conclusion
In patients with severe COVID-19 who required oxygen support, treatment with 40 mg MP twice daily for up to 7 days reduced mortality rates significantly at 45 days compared to a control group receiving usual care alone. Moreover, MP treatment was associated with significantly lower rates of ICU admission and ventilation and a shorter length of stay in the ICU. Adding tocilizumab to MP did not improve any of the studied outcomes. Our results suggest that methylprednisolone therapy could be an efficient therapeutic agent for hospitalized severe COVID-19 patients.
Authors’ contributions
DMH and KMB designed the study; NAA, FG, MM, MAD, MN, and MM collected patients’ data. KMB analyzed the data and interpreted it; KMB and MA drafted the manuscript.
Ethics approval and consent to participate consent for publication
The study was approved by the Dubai Health Authority ethics committee. Written informed consent was obtained from each patient or from a legal representative if patients in the prospective groups were unable to provide consent.
Availability of data and materials
Data are available up on request.
Funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Competing interests
The authors declare that they have no conflict of interest.
Acknowledgements
We extend our appreciations to all healthcare providers who serviced during the critical phase of Corona outbreak.
References
- 1.World Health Organization. Health topics. Health emergency. Coronavirus disease (COVID-19) pandemic https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov.
- 2.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(February (8)):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(March (11)):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (Br Ed) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(December (12)):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Corticosteroids in Severe Influenza A/H1N1 Pneumonia and Acute Respiratory Distress Syndrome. Christian Brun-Buisson 1,2,3, Jean-Christophe M. Richard 4, Alain Mercat 5, Anne C. M. Thiébaut 3,6, Laurent Brochard 1,2,7, and https://www.atsjournals.org/doi/10.1164/rccm.201101-0135OC?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed&. [DOI] [PubMed]
- 8.Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. I. Martin-Loeches,1 T. Lisboa,1,2 A. Rhodes,3 R. P. Moreno,4 E. Silva,5 C. Sprung,6 J. D. Chiche,7 D. Barahona,8 M. Villabon,9 C. Balasini,10 R. M. Pearse,11 R. Matos,4 J. Rello, 12 and The ESICM H1N1 Registry Contributors https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7079858/. [DOI] [PMC free article] [PubMed]
- 9.Corticosteroid Treatment in Critically Ill Patients with Pandemic Influenza A/H1N1 2009 Infection Analytic Strategy Using Propensity Scores Sung-Han Kim1, Sang-Bum Hong2, Sung-Choel Yun3, Won-Il Choi4, Jong-Joon Ahn5, Young Joo Lee6, Heung-Bum Lee7, Chae-Man Lim2, and Younsuck Koh2; for the Korean Society of Critical Care Medicine H1N1 Collaborative*https://www.atsjournals.org/doi/pdf/10.1164/rccm.201101-0110OC. [DOI] [PubMed]
- 10.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2020;(October) doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. 3Medical Letter on the CDC & FDA 2020 July 12:216.
- 14.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol. 2020;146(August (2)):325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(October (13)):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dequin P.F., Heming N., Meziani F., Plantefève G., Voiriot G., Badié J. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg K.P., Hudson L.D., Goodman R.B., Hough C.L., Lanken P.N., Hyzy R. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 18.Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19 A Meta-analysis The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. file:///C:/Users/hp/Downloads/jama_sterne_2020_oi_200104_1601660282.95474%20(3).pdf. [DOI] [PMC free article] [PubMed]
- 19.Lamontagne F., Agoritsas T., Macdonald H., Leo Y., Diaz J., Agarwal A. A living WHO guideline on drugs for covid-19. BMJ. 2020;370(September):m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 20.Kaye Avi Gurion, Siegel Robert. The efficacy of IL-6 inhibitor tocilizumab in reducing severe COVID-19 mortality: a systematic review. PeerJ (San Francisco, CA) 2020;8(November) doi: 10.7717/peerj.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(November (6)):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;(October) doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(December (24)):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermine O., Mariette X., Tharaux P., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;(October) doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available up on request.