Abstract
Purpose
Sarcopenia is an independent prognostic indicator for hepatocellular carcinoma (HCC). Our objective was to determine the effect of sarcopenia on response to systemic targeted therapy in patients with advanced HCC.
Materials and methods
This was a retrospective, Institutional Review Board approved study of 36 patients on systemic targeted therapy with immune checkpoint blockade (n = 25) or tyrosine kinase inhibitor (n = 11) for biopsy-proven advanced HCC. Skeletal muscle index (SMI) was calculated from erector spinae muscle area (SMA) at the level of T12 on pretreatment CT: [SMI = SMA (cm2)/height (m2)]. SMI was compared to treatment response defined as overall survival ≥ 1 year (nonsurgical patients) or > 50% HCC necrosis (surgical patients). Receiver operating characteristic curve and area under the curve was used for analysis with p < 0.05 for statistical significance.
Results
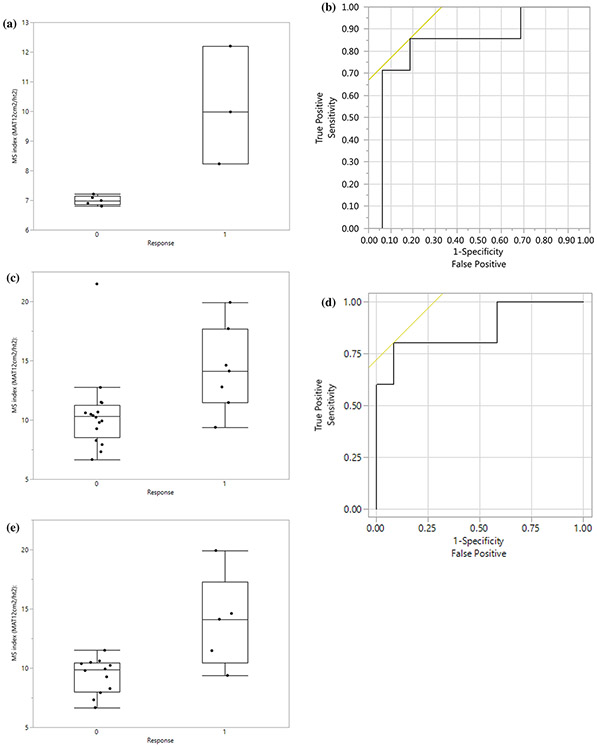
Median age of men and women was 66.5 years (range 32–83) and 70 years (range 54–78), respectively. Liver disease etiology was nonalcoholic steatohepatitis (n = 9), hepatitis C (n = 10), hepatitis B (n = 5), alcohol (n = 3) and unknown (n = 9). Mean (± SD) height and SMI for men were 1.7 m (± 0.1) and 11.4 (± 3.6); values for women were 1.7 m (± 0.1) and 8.2 (± 1.9). Treatment was withdrawn in five patients due to treatment intolerance. Response occurred in 10/31 (32.3%) patients (23 men, 8 women). T12SMI correlated with treatment response using a threshold of 7.21-8.23 for women (AUC = 1; p = 0.037), and 11.47 for men (AUC = 0.83; p = 0.015); correlation was increased for men ≥ 60 years, (AUC = 0.87; p = 0.023).
Conclusion
Sarcopenia was associated with reduced survival and HCC necrosis in patients treated with systemic targeted therapy.
Clinical relevance
Sarcopenia may help in predicting outcomes to targeted therapy in advanced HCC.
Keywords: HCC, Sarcopenia, Immunotherapy
Introduction
Hepatocellular carcinoma (HCC) usually presents with advanced stage disease not amenable to curative surgical treatment and has a poor prognosis. Molecular targeted therapy has become standard-of-care for the treatment of advanced HCC. Oral tyrosine kinase inhibitors (sorafenib and lenvatinib) have shown some improvement in survival and have been used for frontline systemic treatment of advanced HCC in patients with preserved liver function. The median overall survival is increased from 7.9 to 10.7 months in patients treated with sorafenib [1]. Patients with failure of response or intolerance to these agents are treated with immune checkpoint blockade (ICB) using anti-programmed cell death protein 1 (anti-PD-1) monoclonal antibodies (e.g. pembrolizumab and nivolumab) [2]. More recently anti-PD-1 therapy (atezolizumab) in combination with anti-VEGF (bevacizumab) has been approved as frontline standard-of-care therapy [3]. Identification of patients suitable for treatment with molecular targeted therapy depends not only on stage of disease but also assessment of patient’s ability to tolerate treatment. Important considerations include degree of preservation of liver function as indicated by a Child–Pugh class A liver disease score, and a good patient performance status (functional capacity) as indicated by a Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1. A more objective determination of patient performance status or frailty can be obtained through assessment of sarcopenia (low muscle mass) [4-6]. Sarcopenia refers to skeletal muscle loss which occurs in 20-70% of cancer patients and is associated with chemotherapy toxicity (e.g. capecitabine and epirubicin) and reduced survival patients [7-9]. Sarcopenia has also been associated with poor outcomes or toxicity to tyrosine kinase inhibition (sorafenib) in patients with HCC and to immune checkpoint blockade in patients with lung and breast cancer [9, 10]. However, the relationship of sarcopenia to immunotherapy outcome in HCC is unknown. The purpose of our study was to determine the effect of sarcopenia on response to systemic targeted therapy, including immunotherapy, in patients with advanced HCC.
Materials and methods
We retrospectively reviewed pretreatment clinical chest CT images of patients identified from 2016 to 2019 through our institution’s liver cancer clinic. All patients had biopsy-proven advanced HCC and were enrolled in a targeted therapy clinical trial. The thoracic CT scans were obtained as part of patient care at the time of entry into the clinical trial. The study was approved by our Institutional Review Board with waiver of informed consent. Some of the patients had liver MRI data used in a previously published study with different objectives [11]. All patients were over the age of 18 years and had an ECOG performance score of 0 or 1 (i.e. high level of patient function with respect to daily activities), and Child–Pugh class A liver disease (i.e. preservation of liver function). Although all patients had a thoracic CT and liver MRI within 1 week of starting treatment in accordance to the parent clinical trial, we chose the thoracic CT to obtain muscle area measurements to ensure consistency of vertebral level selection for all subjects. Measurements of the erector spinae muscle area was obtained at the level of T12 (approximate level of the celiac axis). The population consisted of 36 patients (16 surgical and 20 non-surgical patients) including 28 men and 8 women treated with systemic targeted therapy. Twenty-five of the patients were treated with immune checkpoint blockade (pembrolizumab or nivolumab ± ipilimumab) and 11 patients were treated with a tyrosine kinase inhibitor (sorafenib). All surgical patients had neoadjuvant immune checkpoint blockade therapy for 6 weeks prior to HCC resection. Nonsurgical patients had clinical follow-up for up to 2 years. In the nonsurgical group, 11 of 20 were treated with a tyrosine kinase inhibitor while the remainder were also treated with immune checkpoint blockade. The dates of documented disease progression and death were obtained from the patients’ electronic medical records. Since both surgical and nonsurgical patients were included we used different endpoint indicators of response to systemic targeted therapy. Treatment response for the study was defined as overall survival (OS) ≥ 1 year in nonsurgical patients and > 50% necrosis in resected HCC (after 6 weeks of neoadjuvant treatment) by pathology review for surgical patients.
Imaging
CT scans through the chest were obtained on a 64-slice multi-detector CT (GE, Milwaukee, Wisconsin). Intravenous iodinated contrast (2.0 mL/kg iohexol 300) was administered at a rate of 3–5 mL/s. Images were acquired in the arterial phase (20 s after start of contrast injection). CT images reconstructed at 2.5 mm slice thickness were used for interpretation.
Image analysis
Bilateral erector spinae muscle area measurements were obtained at the level of the lower third of the T12 vertebral body. Image analysis was performed in a blinded manner by a single radiologist. Although outcome data with liver MRI findings for some of the subjects have been previously published [11], the muscle measurements were performed on images from the pretreatment clinical chest CT without data on treatment response. The erector spinae muscles were manually segmented on our picture archiving and communication system (Philips Medical, Andover MA) using a single axial CT image, Fig. 1. The single axial image was selected from the lower third of the T12 vertebral body. The segmented area of the erector spinae muscle area measurement (cm2) was converted to a skeletal muscle index (SMI) by dividing by the square of the patient’s height (m2) [8, 9]. The pretreatment skeletal muscle index for men and women was compared to treatment response defined as overall survival ≥ 1 year or > 50% necrosis of the resected HCC.
Fig. 1.
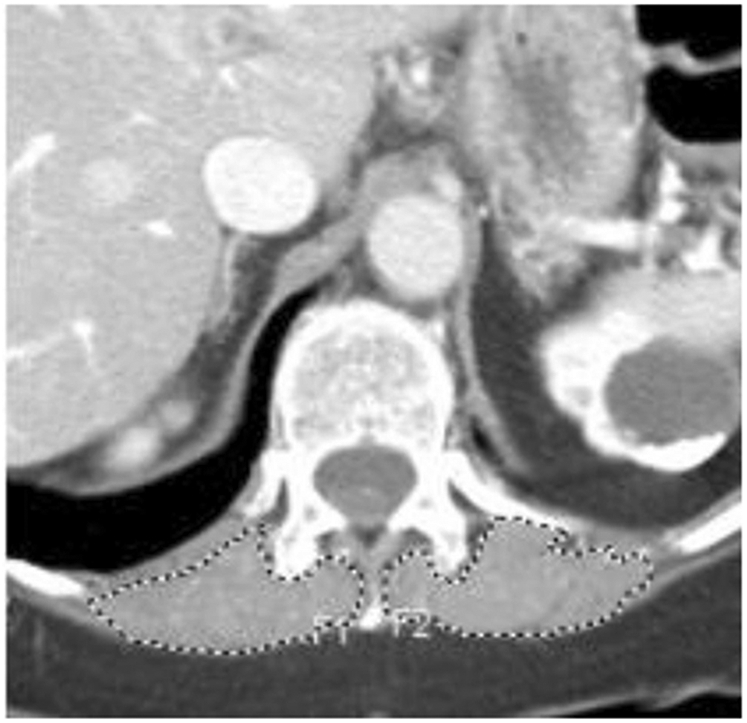
Axial CT scan at the level of T12 showing segmented erector spinae muscle (broken line)
Pathologic analysis
The pathologic review was performed as part of the parent clinical trial by a liver pathologist (A.R.) with more than 20 years of experience. Study-specific identifiers were used to ensure blinded interpretation of pathology. Entire tumors and representative sections of larger tumors were embedded for histology. Hematoxylin- and eosin-stained slides were prepared with formalin-fixed, paraffin-embedded tissue sections. Resected HCC specimens were analyzed for treatment effect, which was recorded as percent viable tumor versus necrosis. Accurate assessment of the percent HCC necrosis was not possible from the liver core biopsies obtained in nonsurgical patients. Study-specific identifiers were used to ensure blinded interpretation of pathology.
Statistical analysis
Response to targeted therapy was defined as an overall survival duration of at least 1 year for nonsurgical patients and greater than 50% tumor necrosis according to pathology for surgical patients. The area under the receiver operating characteristic curve (AUROC) was used to assess the diagnostic performance of the pretreatment T12 skeletal muscle index as a predictor of response in men and in women. An optimal cutoff threshold was selected using Youden’s index, and sensitivity and specificity of imaging measurements were reported. All tests were two-sided, and p values of 0.05 or less were considered significant. Box plots were used to display and compare the distributions of imaging measurements between response groups. Statistical analyses were performed using JMP Pro 14 (SAS Institute Inc., Cary, NC).
Results
The median age of men and women was 66.5 years (range 32 to 83) and 70 years (range 54 to 78), respectively. Liver disease etiology was NASH (n = 9), HCV (n = 10), HBV (n = 5), alcohol (n = 3) and unknown (n = 9). Patient characteristics for men and women are shown in Table 1. For men, mean ± standard deviation (± SD) value for height was 1.7 m (± 0.1) and for erector spinae skeletal muscle area (SMA) was 33.6 cm2 (± 10.3). For women, mean (± SD) value for height was 1.7 m (± 0.1) and for SMA was 22.5 cm2 (± 5.5). The mean (± SD) T12 skeletal muscle index (T12SMI) for men and women were 11.4 (± 3.6) and 8.2 (± 1.9), respectively. The mean (± SD) HCC size for men and women was 5.1 cm (± 3.8) and 4.3 cm (± 2.8).
Table 1.
Distribution of patient age, height, erector spinae muscle area at the level of the T12 vertebral body (MAT12), skeletal muscle index (SMI) and HCC size for male (M) and female (F) patients
| Gender | N | Mean | Std dev | Min | Median | Max | |
|---|---|---|---|---|---|---|---|
| Age | F | 8 | 68.3 | 8.4 | 54.00 | 70 | 78.00 |
| M | 28 | 64.4 | 11.0 | 32.00 | 66.5 | 83.00 | |
| Height (m) | F | 8 | 1.7 | 0.1 | 1.53 | 1.68 | 1.72 |
| M | 28 | 1.7 | 0.1 | 1.54 | 1.74 | 1.96 | |
| T12 muscle area: MAT12 (cm2) | F | 8 | 22.5 | 5.5 | 18.78 | 21.16 | 35.62 |
| M | 28 | 33.6 | 10.3 | 18.10 | 31.87 | 68.09 | |
| SMI (MAT12 cm2/ht2): | F | 8 | 8.2 | 1.9 | 6.80 | 7.15 | 12.20 |
| M | 28 | 11.4 | 3.6 | 6.05 | 10.64 | 21.48 | |
| HCC size (cm) | F | 8 | 4.3 | 2.8 | 1.5 | 4.45 | 10.1 |
| M | 28 | 5.1 | 3.8 | 1.5 | 4.6 | 14 |
Treatment with sorafenib, was discontinued in five patients due to drug-related complications: anemia (n = 1), low platelets (n = 1), hand and foot syndrome (n = 1), colon perforation (n = 1), unspecified gastro-intestinal complication (n = 1). Treatment response was determined in the remaining 31 patients (surgical n = 16; nonsurgical n = 15). Treatment response occurred in 10 of the 31 (32.3%) remaining patients, Table 2. The mean (± SD) HCC size was 5.8 cm (± 3.7) and 3.8 cm (± 3.2) for surgical and nonsurgical patients, respectively. Four of the 16 surgical patients showed treatment response with greater than 50% HCC necrosis, i.e. less than 50% viable tumor, Fig. 2. Six of the 15 nonsurgical patients showed treatment response with an overall survival of 1 year or more. The T12SMI was significantly correlated with treatment response for women and men, Fig. 3. A T12SMI threshold of 7.21 to 8.23 for women was associated with an AUC of 1, p = 0.037, and a threshold of 11.47 for men was associated with an AUC of 0.83, p = 0.015. The correlation of T12SMI with treatment response was increased (AUC = 0.87, p = 0.023) for men aged 60 years or more. All 5 nonsurgical patients who had discontinuation of treatment with sorafenib due to complications were men with a mean (± SD) T12SMI of 10.3 (± 2.4).
Table 2.
Mean, and median values of male (M) and female (F) skeletal muscle index (SMI) in patients with and without treatment response (n = 31)
| Gender | Responsea 1 = Y; N = 0 |
N | Mean SMI (MAT12 cm2/ht2) |
Std dev SMI (MAT12 cm2/ht2) |
Min SMI (MAT12 cm2/ht2) |
Median SMI (MAT12 cm2/ht2) |
Max SMI (MAT12 cm2/ht2) |
|---|---|---|---|---|---|---|---|
| F | 0 | 5 | 6.998 | 0.159906 | 6.8 | 6.99 | 7.21 |
| 1 | 3 | 10.13667 | 1.989631 | 8.23 | 9.98 | 12.2 | |
| M | 0 | 16 | 10.54188 | 3.331585 | 6.66 | 10.3 | 21.48 |
| 1 | 7 | 14.28429 | 3.600152 | 9.38 | 14.12 | 19.92 |
Response to treatment was defined as an overall survival duration of at least 1 year for nonsurgical patients and greater than 50% tumor necrosis according to pathology for surgical patients
Fig. 2.
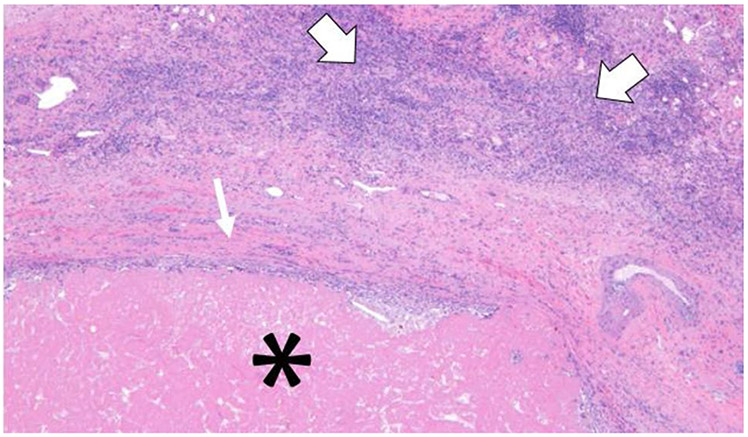
A slide showing part of a right hepatectomy specimen containing a 5.0 cm necrotic mass with no viable tumor. The Hematoxylin & Eosin stain at × 10 magnification shows the necrotic mass (*) rimmed by fibrous scar (small arrow) with peri-tumoral inflammatory cells (large arrows)
Fig. 3.
a Box and whisker plots of T12 skeletal muscle index (SMI) for women with (1) and without (0) treatment response. A T12SMI threshold value of 7.21 to 8.23 for women was associated with an AUC of 1, p = 0.037. b ROC curve for T12SMI as a predictor of treatment response for all men; the AUC was 0.83. A threshold T12SMI value of ≥ 11.47 to predict treatment response had a sensitivity of 86% and specificity of 81%. c Box and whisker plots for T12SMI for all men with (1) and without (0) treatment response. d ROC curve for T12SMI as a predictor of treatment response for men aged ≥ 60 years; the AUC was 0.87. A threshold SMI value of ≥ 11.47 to predict treatment response had a sensitivity of 80% and specificity of 92%. e Box and whisker plots for T12SMI for men aged ≥ 60 years with (1) and without (0) treatment response. a Box and whisker plots of T12 skeletal muscle index (SMI) for women with (1) and without (0) treatment response. A preoperative T12SMI yielded an AUC of 1, p = 0.037. A T12SMI threshold value of 7.21 to 8.23 for women was associated with an AUC of 1, p = 0.037. b ROC curve for T12SMI as a predictor of treatment response for all men; the AUC was 0.83. A threshold T12SMI value of ≥ 11.47 to predict treatment response had a sensitivity of 86% and specificity of 81%. c Box and whisker plots for T12SMI for all men with (1) and without (0) treatment response. d ROC curve for T12SMI as a predictor of treatment response for men aged ≥ 60 years; the AUC was 0.87. A threshold SMI value of ≥ 11.47 to predict treatment response had a sensitivity of 80% and specificity of 92%. e Box and whisker plots for T12SMI for men aged ≥ 60 years with (1) and without (0) treatment response
Discussion
In our study we found that a lower SMI was associated with shorter survival (< 1 year) and less HCC necrosis (< 50% necrosis or > 50% viable tumor) with targeted therapy. The five patients requiring discontinuation of treatment due drug intolerance also had a low T12SMI. Our findings are consistent with prior studies that have shown a negative association of sarcopenia to chemotherapy and targeted therapy in other solid tumor types [12-15].
Various techniques at different vertebral levels in the chest and abdomen have been employed to determine skeletal muscle loss. The axial level selected for calculation of SMI is often dependent on the anatomical coverage of CT scans that are acquired for clinical care purposes [16]. Thus the L3 level has been commonly used for CT scans of the abdomen and pelvis, while the T12 level has been used for CT scans of the chest. However, a CT of the abdomen only or a liver MRI may not include the L3 vertebral body, and in this situation T12 can be a helpful surrogate. In our retrospective study, the L3 vertebral body was not consistently included on liver MRI. We therefore selected the T12 level on the pretreatment chest CT to avoid additional imaging studies. Derstine et al. [16] reported an excellent correlation between muscle area measurements performed at multiple levels from T10 to L5 supporting the validity of calculating SMI at different axial levels. In a large study of healthy adults in the U.S. the mean (± SD) T12SMI values for men and women were reported as 44.1 (± 7.7) and 34 (± 6.6), respectively [16]. The T12SMI values were much lower in our study since we only included the erector spinae muscle, Table 1. However, our measurements were closer to those of a study of 180 patients with idiopathic pulmonary fibrosis, which also only included erector spinae muscles and reported mean (± SD) T12SMI values for men and women of 10.7 (± 3.3) and 9.9 (± 3.1) [17]. The slight difference between the T12SMI values in patients with idiopathic pulmonary fibrosis and those in our study could in part be attributed to differences in slice selection for the T12 vertebral level. The major contributing factor for SMI differences in comparison to healthy adults is that cancer and chronic disease are associated muscle loss [6, 18].
Sarcopenia occurs with aging, in chronic diseases and in patients with cancer. The process of ageing is variable among individuals and is influenced by multiple factors such as genetics, co-morbidities and environment. Similarly, immune changes seen with aging are also variable. Importantly, the degree of frailty (functional limitations of performance and physical disabilities) in a patient affects immune-competence [19]. Sarcopenia is a key feature of frailty. The clinical assessment of a patient’s ability to perform daily activities is used to determine the degree of frailty, and is performed using the ECOG performance status but may be better quantified using skeletal muscle index. The development of immune senescence (decline in adaptive and innate immunity) with age is likely a result of many interacting cytokine and hormonal alterations. Nonetheless, increased age, muscle loss and immune senescence are thought to be interlinked [19-22]. Skeletal muscle is known to produce cytokines (myokines) [21] and it has been suggested that sarcopenia causes a change in cytokine expression which alters the immune cell population. For example, skeletal muscle expresses high levels of interleukin-15 (IL-15) which is important for the development and survival of natural killer (NK) lymphocytes. The NK lymphocytes are important for controlling intracellular infectious agents and cancer [19-22]. Alterations in other immune cell populations, such increased myeloid-derived suppressor cells (MDSCs), that have been reported with increasing age may also be linked to skeletal muscle loss through changes in production of myokines [23, 24]. Our observation of the association of reduced targeted therapy response with sarcopenia in patients with HCC may be related to immune function impairment. Larger studies would be required to demonstrate the incremental effect of sarcopenia on increasing patient age.
Our study had some limitations. First, our patient number is small since this was a retrospective review of CT scans obtained for patients enrolled in clinical trials. Second, patient therapy with targeted agents was not uniform. The majority of patients (25 of 36 or 69%) in our study were treated with immune checkpoint blockade, while 11 patients were treated with a tyrosine kinase inhibitor (sorafenib). However, our observation of poor outcomes linked to lower SMI is consistent with that of studies using sorafenib for advanced HCC. Similarly, a poor response to immune checkpoint blockade in association with low SMI has been described in lung cancer. Third, we included both surgical and nonsurgical patients because of the small number of patients enrolled in early clinical trials. Therefore, it was necessary to include different criteria as indicators of treatment response. However, irrespective of whether patients had nonsurgical or surgical treatment, all clinical trial patients had preserved liver function and advanced HCC, and overall larger HCCs occurred in the surgical patients.
In summary, our preliminary study suggests that quantification of skeletal muscle index in patients with advanced HCC may be a predictive factor of treatment tolerance and response to targeted therapy including immune checkpoint blockade.
Conclusion
Sarcopenia was associated with reduced survival and HCC necrosis in patients treated with targeted therapy for HCC.
Acknowledgments
Funding Supported by the MD Anderson Cancer Center SPORE in Hepatocellular Carcinoma Grant P50 CA217674 and by the NIH/NCI under Award Number P30CA016672.
Abbreviations
- HCC
Hepatocellular carcinoma
- ICB
Immune checkpoint blockade
- MDSCs
Myeloid-derived suppressor cells
- NK
Natural killer
- PD-1
Programmed cell death protein 1
- RECIST
Response evaluation criteria in solid tumors
- SMA
Skeletal muscle area
- SMI
Skeletal Muscle Index
- TKI
Tyrosine kinase inhibitor
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gore GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol. 2019. October;16(10):589–604. 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, Kudo M, Breder V, Merle P, Kaesb AO, Li D, Verret W, Xu D-Z, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng A-L, IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382(20):1894–1905. 10.1056/nejmoa1915745 [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495 [DOI] [PubMed] [Google Scholar]
- 6.Ooi PH, Hager A, Mazurak VC, Dajani K, Bhargava R, Gilmour SM, Mager DR. Sarcopenia in chronic liver disease: Impact on outcomes. Liver Transpl 2019. September;25(9):1422–1438. 10.1002/lt.25591 [DOI] [PubMed] [Google Scholar]
- 7.Ryan AM, Power DG2, Daly L, Cushen SJ, Bhuachalla EN, Prado CM. Cancer-associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc Nutr Soc. 2016. May;75(2):199–211. 10.1017/s002966511500419x. [DOI] [PubMed] [Google Scholar]
- 8.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9: 629–635 [DOI] [PubMed] [Google Scholar]
- 9.Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, Ropert S, Videl M, Pol S, Chaussade S, Goldwasser F. Sarcopenia Predicts Early Dose-Limiting Toxicities and Pharmacokinetics of Sorafenib in Patients with Hepatocellular Carcinoma. PLoS One. 2012;7(5):e37563. 10.1371/journal.pone.0037563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, Tanimura K, Harita S, Imabayashi T, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J Clin Med. 2019. April; 8(4): 450. 10.3390/jcm8040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qayyum A, Hwang KP, Stafford J, Verma A, Maru D, Sandesh S, Sun J, Pestana RC, Avritscher R, Hassan M, Amin HM, Rashid A, Wistuba II, Ehman RL, Ma J, Kaseb A. Immunotherapy response evaluation with Magnetic Resonance Elastography (MRE) in advanced HCC. J Immunother Cancer 2019;7:329. 10.1186/s40425-019-0766-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonelli G, Gigante E, Iavarone M, Begini P, Sangiovanni A, Iannicelli E, et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing Sorafenib treatment. United European Gastroenterol. Journal 2018; 6: 1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharagozlian S, Mala T, Brekke HK, Kolbjørnsen LC, Ullerud AA, Johnson E. Nutritional status Sarcopenia, Gastrointestinal Symptoms and Quality of Life After Gastrectomy for Cancer - A Cross-Sectional Pilot study. Clin Nutr ESPEN. 2020. June;37:195–201. 10.1016/j.clnesp.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, Nishimura T, Kita R, Kimura T, Iijima H, Nishiguchi S, Osaki Y. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett. 2017. August; 14(2): 1637–1647. 10.3892/ol.2017.6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch L, Bellesoeur A, Boudou-Rouquette P, Arrondeau J, Thomas-Schoemann A, Kirchgesner J, Gervais C, Jouinot A, Chapron J, Giraud F, Wislez M, Alexandre J, Blanchet B, Goldwasser F. The Impact of Body Composition Parameters on Severe Toxicity of Nivolumab. Eur J Cancer. 2020. January;124:170–177. 10.1016/j.ejca.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8, 11369 (2018). 10.1038/s41598-018-29825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon SW, Choi JS, Lee SH, Jung KS, Jung JY, Kang YA, Park MS, Kim YS, Chang J, Kim SY. Thoracic skeletal muscle quantification: Low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. 2019. February 15;20(1):35. 10.1186/s12931-019-1001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanimura K, Sato S, Fuseya Y, Hasegawa K, Uemasu K, Sato A, Oguma T, Hirai T, Mishima M, Muro S,. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived Index for prognosis. Ann Am Thorac Soc. 2016. March;13(3):334–41. 10.1513/annalsats.201507-446oc [DOI] [PubMed] [Google Scholar]
- 19.Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging, immune senescence, and immunotherapy: A comprehensive review. Semin Oncol. 2018. August;45(4):187–200. 10.1053/j.seminoncol.2018.08.006. epub 2018 Oct 24. [DOI] [PubMed] [Google Scholar]
- 20.Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. Crit Rev Oncol/Hematol 2010:75(2): 165–72. 10.1016/j.critrevonc.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011:214:337–346. 10.1242/jeb.048074 [DOI] [PubMed] [Google Scholar]
- 22.Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: Altered cytokine levels as a common mechanism. Aging (Albany NY). 2012. August;4(8):535–46. 10.18632/aging.100482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J 2010:16(4):348–53. 10.1097/ppo.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 24.Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, Bowdish DME. Blood CD33( + )HLA-DR(−) myeloid- derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol. 2013. April 93(4):633–7 10.1189/jlb.0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]