Abstract
Hepatitis B virus (HBV) infection is a major worldwide health problem that requires the development of improved antiviral therapies. Here, a series of 4′-Azido-thymidine/4′-Azido-2′-deoxy-5-methylcytidine derivatives (6, 10-15) were synthesized, and their anti-HBV activities evaluated. Compounds 10–15 were synthesized via an SNAr reaction of 18, in which the 4-position of the thymine moiety was activated as the 2,4,6-triisopropylbenzenesulfonate. Compounds 11–15 showed no antiviral activity. However, 4′-Azido thymidine (6) and 4′-Azido-2′-deoxy-5-methylcytidine (10) displayed significant anti-HBV activity (EC50 = 0.63 and 5.99 μM, respectively) with no detectable cytotoxicity against MT-2 cells up to 100 μM.
Keywords: Anti-HBV nucleoside, 4′-azido nucleoside, modified pyrimidines
1. Introduction
Hepatitis B Virus (HBV) infection is a global public health concern, with more than 257 million people chronically infected with HBV worldwide.[1] Patients with chronic HBV-infection have a high risk of developing liver cirrhosis and hepatocellular carcinoma.[2,3] Interferons and nucleoside/nucleotide analogs (NAs) are currently used to treat HBV infection.[4,5] However, interferons are effective in only 30% of HBV antigen-positive and 40% of HBV antigen-negative cases.[6] In addition, the clinical application of interferons is limited due to multiple adverse effects and inconvenient methods of administration.[7] By contrast, NAs are both safe and highly effective in suppressing HBV replication.[5] HBV and Human immunodeficiency virus type 1 (HIV-1) both utilize a specific reverse transcription process for their proliferation. NAs target the reverse transcriptase (RT) enzyme involved in this process.[8,9] To date, six NAs (entecavir, lamivudine, adefovir dipivoxil, telbivudine, tenofovir disoproxil and tenofovir alafenamide) have been approved by the United States Federal Drug Administration for the treatment of HBV infection.[10] Among these, lamivudine and tenofovir are also effective against HIV-1.[11] However, prolonged treatment with NAs can lead to the emergence of drug-resistant mutations and severe adverse effects.[12] Thus, the development of novel anti-HBV agents that exhibit potent activity against both wild-type and drug-resistant HBV with fewer long-term adverse effects is crucial. To this end, various nucleoside analogs, such as anti-HBV agents, have been designed and synthesized.[13-16] Among these NAs, 4′-substituted NAs have attracted significant attention due to their potent antiviral activity. The antiviral activity of these 4′-substituted nucleosides might arise from a conformational change of the furanose ring.[17,18]
We previously reported that 4′-cyano-2′-deoxyguanosine (CdG, 1, Figure 1) and 4′-cyano-2-amino-2′-deoxyadenosine (CAdA, 2) block the replication of both wild-type and entecavir-resistant HBV.[19] In addition, these compounds also exhibit potent anti-HIV activity. Furthermore, we recently synthesized a series of purine NAs substituted with a fluoromethyl, methyl, vinyl, ethyl or azido group at the 4′-position.[20] Among them, 4′-azido-2′-deoxyguanosine (AdG, 3) and 4′-azido-2-amino-2′-deoxyadenosine (AAdA, 4) were found to exhibit potent anti-HBV activity (EC50 = 0.0097 and 0.0051 μM, respectively). Moreover, Chang and coworkers reported that 2′-deoxy-2′-P-fluoro-4′-azido cytidine (FNC, 5) exhibits strong anti-HBV activity (EC50 = 0.12 μM). [21] Taken together, these findings suggest 4′-azido modified NAs might be promising anti-HBV agents. Therefore, we aimed to synthesize and assess the anti-HBV activity of 4′-azido nucleosides bearing alterations on the nucleobase moiety.
Figure 1.
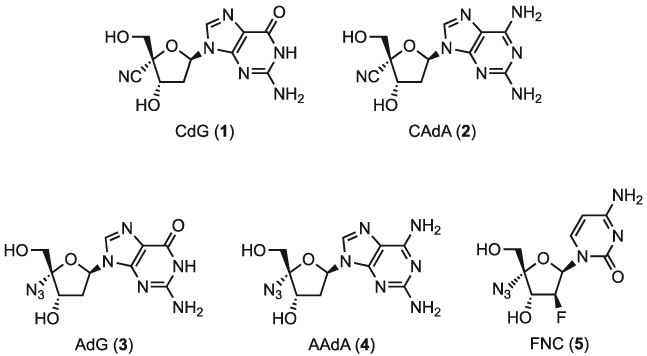
Structures of compounds 1–5.
In 1992, 4′-azido thymidine (6, Figure 2) was reported to exhibit potent anti-HIV activity (EC50 = 0.01 μM).[21] NAs, which display inhibitory activity against HIV-1, tend to exhibit anti-HBV activity. We recently showed that AdG (3) and AAdA (4) are highly potent against both HIV-1 and HBV.[20] However, to the best of our knowledge, the anti-HBV activity of 4′-azido thymidine (6) has not been reported. Moreover, telbivudine (7) is a clinically used thymidine nucleoside analog for the treatment of chronically HBV-infected patients. Therefore, we reasoned thymine might be a good nucleobase scaffold for 4′-azido nucleoside analogs as potential anti-HBV agents. Furthermore, Muller and coworkers reported 5-methyl cytosine nucleoside FddMeC (8) exhibits moderate anti-HBV activity (EC50 = 0.54 μM).[23] These findings suggest 5-methyl cytosine may be a good nucleobase scaffold. In addition, it was reported that an N4-alkylated 4′-azido cytidine derivative 9 significantly reduces the in vivo toxicity of the parent compound, FNC (5).[24] These findings prompted us to investigate the 4′-azido thymidine and 4′-azido methyl cytidine analogs with N4/O4 alkylation for activity against HBV.
Figure 2.
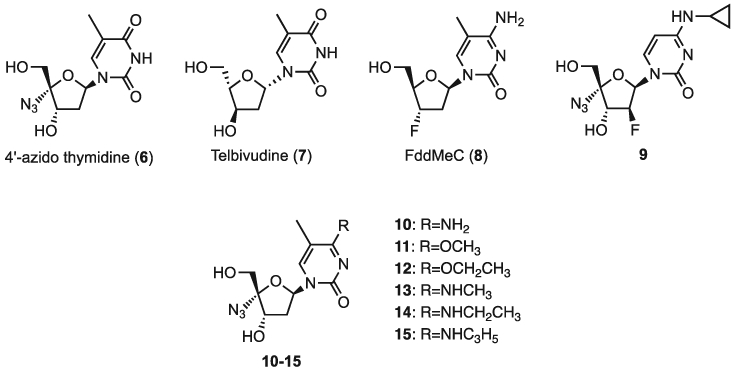
Structures of compounds 6–15.
Here, we describe the synthesis of a series of 4′-azido-4-substituted thymine/5-methylcytosine nucleosides 10–15. The anti-HBV activity of 6 and 10–15 was also assessed as a means of elucidating the structure-activity relationship of 4′-azido nucleosides.
2. Results and discussion
2.1. Chemistry
The synthetic strategy for 4′-azido-4-substituted thymine/5-methylcytosine nucleosides 10–15 is outlined in Scheme 1. Compound 16 was prepared from thymidine in 4 steps according to a previously described method.[22] Oxidative displacement of 5′-iodine with m-chloroperbenzoic acid and m-chlorobenzoic acid gave 17. Activation of the 4-position of the base moiety with 2,4,6-triisopropylbenzenesulfonyl chloride was followed by treatment with an alcohol or amine, and subsequent deprotection gave the corresponding 4′-azido-4-substituted thymine/5-methylcytosine nucleosides 10–15. Compound 6 was also synthesized according to the previously reported procedure.[22] The purities of all the tested compounds were at least 95% as determined by analytical RP-HPLC.
Scheme 1.
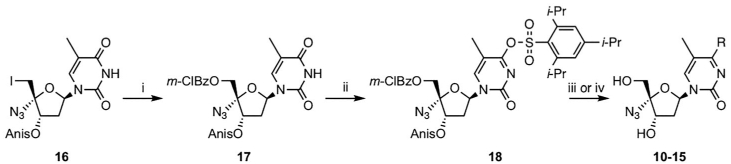
Synthesis of 4′-azido-4-substituted pyrimidine nucleosides 10–15.
Reagents and conditions: (i) m-CBA, m-CPBA, K2HPO4, nBu4NHSO4, DCM/H2O; (ii) 2,4,6-triisopro-pylbenzenesulfonyl chloride, TEA, DMAP, DCM; (iii) 1,4-diazabicyclo[2.2.2]octane, DBU, ROH, rt, overnight, then NH3/MeOH, 50 °C, overnight; (iv) RNH2, solvent, 50 °C, overnight, then NH3/MeOH, 50 °C, overnight.
2.2. Biological evaluation
Anti-HBV activity was evaluated using the HBV-producing HepG2 2.2.15 human hepatoblastoma cell line, and cytotoxicity was assessed in MT-2 cells. The anti-HBV activity and cytotoxicity of each 4′-azido-4-substituted thymine/5-methylcytosine nucleoside is summarized in Table 1. The EC50 value is the concentration of the compound that inhibits 50% of virus replication. Entecavir was included as a control. In the present study, 4′ azido thymidine (6) showed significant anti-HBV activity (EC50 = 0.63 μM), with no cytotoxicity up to 100 μM. 4′-Azido-2′-deoxy-5-methylcytidine (10) also displayed anti-HBV activity with an EC50 value of 5.99 μM without any cytotoxicity at concentrations up to 100 μM. By contrast, compounds 11-15 displayed no detectable anti-HBV activity and were not cytotoxic. These results clearly indicate that O4-alkylation of 6 and N4-alkylation of 10 completely abolishes the anti-HBV activity. Further structural optimization of 6 and 10, including sugar modification, might lead to the identification of novel compounds that are active against HBV with acceptable levels of cytotoxicity.
Table 1.
Anti-HBV activity and cytotoxicity of 6 and 10–15.
| Compund | EC50 (μM) | CC50 (μM) |
|---|---|---|
| 6 | 0.63 ± 0.29 | >100 |
| 10 | 5.99 ± 5.54 | >100 |
| 11 | >100 | >100 |
| 12 | >100 | >100 |
| 13 | >100 | >100 |
| 14 | >100 | >100 |
| 15 | >100 | >100 |
| Entecavir | 0.00675 ± 0.000353 | 57.2 ± 5.20 |
Abbreviations: EC50, 50% effective concentration; CC50, 50% cytotoxic concentration.
3. Conclusions
In conclusion, a synthetic route for the novel 4′-azido-4-substituted thymine/5-methylcytosine nucleosides (10–15) has been developed. Additionally, we have studied the structure-activity relationships of 6 and 10–15 as potential anti-HBV agents. Evaluation of compounds 6 and 10 revealed they displayed moderate anti-HBV activity with an EC50 of 0.63 μM and 5.99 μM, respectively. Conversely, 11–15 displayed no activity as anti-HBV agents. However, compounds 6 and 10–15 showed no cytotoxicity up to 100 μM. Interestingly, it was reported that 4′-azido-2′-deoxycytidine shows high levels of cytotoxicity (CC25 = 0.21 μM).[22] These results suggest that the 5-methyl group of pyrimidine reduces the toxicity of the compound. Taking these considerations into account, thymine and 5-methylcytosine might be a suitable scaffold for further structural optimization of 4′-substituted nucleosides to develop anti-HBV drug with no associated cytotoxicity. We believe the structural information presented here will prove useful in the development of potent but safe anti-HBV agents.
4. Experimental
4.1. General methods
Reagents and anhydrous solvents were purchased and used without further purification. TLC was performed using Merck precoated TLC plates (Silica gel 60 F254, 0.25 mm). Flash column chromatography was performed using pre-packed cartridges (Yamazen Hi-flash or Biotage ZIP sphere) on a Yamazen AI-580S automated flash chromatography system. 1H and 13C NMR spectra were recorded on a JEOL ECA 500 spectrometer operating at room temperature. Chemical shifts were reported in parts per million (δ) relative to the residual solvent peak. Multiplicities are described as singlet (s), doublet (d), doublet of doublets (dd), triplet (t), or multiplet (m). Coupling constants (J) are reported in hertz (Hz). ESI-Mass spectral analysis was performed on a JEOL JMS-T100LP system. Analytical HPLC was performed on a JASCO instrument equipped with a YMC Hydrosphere C18 column (6.0 × 150 mm) with UV detection at 254 nm.
4.2. 4′-Azido-5′-(3-chlorobenzoyl)-3′-O-(4-methoxybenzoyl)thymidine (17)
A mixture of 16 (3.24 g, 6.14 mmol), K2HPO4 (6.80 g, 39.0 mmol), n-Bu4NHSO4 (6.63 g, 19.5 mmol), m-CBA (3.06 g, 19.6 mmol), and m-CPBA (70%, 6.40 g, 26.0 mmol) in CH2Cl2/H2O (2:1, v/v, 120 mL) was stirred at room temperature overnight. Then, 10% Na2S2O3 aq. was added, and the mixture was extracted with CH2Cl2. The combined organic layer was washed with satd. NaHCO3 and brine, and then dried over MgSO4. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/EtOAc, 100:0 to 82:18, v/v) to give compound 17 (1.60 g, 2.87 mmol, 47% yield) as a pale yellow foam. 1H NMR (CDCl3, 500 MHz) δ 8.11 (s, 1 H), 8.04-8.01 (m, 2 H), 7.93-7.91 (m, 1 H), 7.58-7.55 (m, 1 H), 7.39-7.36 (m, 1 H), 7.11 (d, J = 1.2 Hz, 1 H), 6.96-6.93 (m, 2 H), 6.47 (dd, J = 7.5, 5.2 Hz, 1 H), 5.80 (dd, J = 8.0, 6.3 Hz, 1 H), 4.78 (d, J = 12.0 Hz, 1 H), 4.72 (d, J = 12.0 Hz, 1 H), 3.89 (s, 3 H), 2.79-2.66 (m, 2 H), 1.82 (d, J = 1.2 Hz, 3 H); 13C NMR (CDCl3, 126 MHz) δ 165.1, 164.4, 164.1, 163.1, 149.8, 135.1, 134.9, 133.7, 132.2, 130.6, 130.0, 129.8, 127.9, 120.7, 113.9, 112.2, 96.9, 85.3, 72.9, 65.3, 55.6, 35.8, 12.4; MS (ESI) m/z (M + Na)+ calcd. 578.1055; found 578.1024.
4.3. 4′-Azido-51-(3-chlorobenzoyl)-3′-O-(4-methoxybenzoyl)-O4-(2,4,6-triisopropylbenzenesulfonyl) thymidine (18)
A mixture of 17 (144 mg, 0.259 mmol), 2,4,6-triisopropylbenzenesulfonyl chloride (165 mg, 0.545 mmol), triethylamine (152 μL, 1.09 mmol), and DMAP (3.3 mg, 0.027 mmol) in dry CH2Cl2 (4 mL) was stirred at room temperature overnight. Then, the mixture was diluted with CH2Cl2, washed with satd. NaHCO3 and brine, and then dried over MgSO4. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (Hexane/EtOAc, 85:15 to 65:35, v/v) to give compound 18 (101 mg, 0.123 mmol, 47% yield) as a white foam. 1H NMR (CDCl3, 500 MHz) δ 8.02-7.97 (m, 3 H), 7.91-7.88 (m, 1 H), 7.67 (s, 1 H), 7.59-7.57 (m, 1 H), 7.39 (t, J = 8.0 Hz, 1 H), 7.20 (s, 2 H), 6.94 (d, J = 8.6 Hz, 2 H), 6.38 (dd, J = 6.3, 5.7 Hz, 1 H), 5.67 (dd, J = 7.5, 6.9 Hz, 1 H), 4.76 (s, 2 H), 4.29 (sep, 6.9 Hz, 2 H), 3.88 (s, 3 H), 2.97-2.86 (m, 2H), 2.51 (ddd, J = 13.2, 7.5, 5.2 Hz, 1 H), 1.91 (s, 3 H), 1.32-1.24 (m, 18 H); 13C NMR (CDCl3, 126 MHz) δ 166.7, 165.0, 164.5, 164.2, 154.5, 153.2, 151.2, 141.6, 135.0, 133.9, 132.2, 130.52, 130.47, 130.1, 129.8, 127.8, 124.1, 120.6, 113.9, 104.6, 97.6, 86.7, 72.4, 65.5, 55.5, 37.1, 34.3, 29.6, 24.5, 24.4, 23.5, 12.2; MS (ESI) m/z (M + Na)+ calcd. 844.2395; found 844.2392.
4.4. 4′-Azido-2′-deoxy-5-methylcytidine (10)
Compound 18 (272 mg, 0.331 mmol) was dissolved in 5M ammmonia in methanol (10 mL) and stirred at 70 °C overnight. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 97:3 to 97:13, v/v) to give compound 10 (28 mg, 0.100 mmol, 30% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz) δ 7.78 (s, 1 H), 6.37 (dd, J = 7.5, 4.0 Hz, 1 H), 4.53 (dd, J = 8.0, 7.5 Hz, 1 H), 3.83 (d, J = 12.0 Hz, 1 H), 3.76 (d, J = 12.0 Hz, 1 H), 2.47 (ddd, J = 13.8, 8.0, 7.5 Hz, 1 H), 2.34 (ddd, J = 13.8, 7.5, 4.0 Hz, 1 H), 1.96 (s, 3 H); 13C NMR (methanol-d4, 126 MHz) δ 167.4, 158.1, 139.9, 104.6, 101.3, 86.7, 72.0, 63.7, 39.4, 13.3; MS (ESI) m/z (M + Na)+ calcd. 305.0974; found 305.0930.
4.5. 4′-Azido-O4-methylthymidine (11)
A mixture of 18 (300 mg, 0.359 mmol), 1,4-diazabicyclo[2.2.2]octane (81 mg, 0.720 mmol), and dry 3Å molecular sieves (600 mg) in dry 1,4-dioxane (7.0 mL) was stirred at room temperature for 30 min. Then, methanol (165 μL, 4.07 mmol) and DBU (161 μL, 1.08 mmol) were added to the solution. After being stirred at room temperature overnight, the mixture was filtered and evaporated. The residue was re-dissolved in 7M methanolic ammonia (10 mL) and stirred at 55 °C for 16 h. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 99:1 to 94:6, v/v) to give compound 11 (24 mg, 0.081 mmol, 22% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz), δ 7.99 (d, J = 1.2 Hz, 1 H), 6.25 (dd, J = 7.5, 4.0 Hz, 1 H), 4.43 (dd, J = 8.0, 7.5 Hz, 1 H), 3.87 (s, 3 H), 3.76 (d, J = 12.0 Hz, 1 H), 3.68 (d, J = 12.0 Hz, 1 H), 2.43 (ddd, J = 13.8, 8.0, 7.5 Hz, 1 H), 2.26 (ddd, J = 13.8, 7.5, 4.0 Hz, 1 H), 1.88 (d, J = 1.2 Hz, 3 H); 13C NMR (methanol-d4, 126 MHz) δ 172.6, 158.0, 141.9, 106.8, 101.5, 87.2, 71.7, 63.4, 55.2, 39.5, 12.2; MS (ESI) m/z (M + Na)+ calcd. 320.0971; found 320.0974.
4.6. 4′-Azido-O4-ethylthymidine (12)
A mixture of 18 (218 mg, 0.265 mmol), 1,4-diazabicyclo[2.2.2]octane (59 mg, 0.530 mmol), and dry 3Å molecular sieves (200 mg) in dry 1,4-dioxane (5.3 mL) was stirred at room temperature for 30 min. Then, ethanol (154 μL, 2.65 mmol) and DBU (99 μL, 0.663 mmol) were added to the solution. After being stirred at room temperature overnight, the mixture was filtered and evaporated. The residue was re-dissolved in 7M methanolic ammonia (10 mL) and stirred at 55 °C for 16 h. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 97:2 to 96:4, v/v) to give compound 12 (27 mg, 0.086 mmol, 27% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz) δ 7.98 (d, J = 1.2 Hz, 1 H), 6.25 (dd, J = 7.5, 4.0 Hz, 1 H), 4.43 (dd, J = 8.0, 7.5 Hz, 1 H), 4.32 (q, J = 6.9 Hz, 2 H), 3.76 (d, J = 12.0 Hz, 1 H), 3.68 (d, J = 12.0 Hz, 1 H), 2.43 (ddd, J = 13.8, 8.0, 7.5 Hz, 1 H), 2.30 (ddd, J = 13.8, 7.5, 4.0 Hz, 1 H), 1.87 (d, J = 1.2 Hz, 3 H), 1.28 (t, J = 6.9 Hz, 3 H). 13C NMR (126 MHz, methanol-d4) δ 170.7, 156.5, 140.3, 105.4, 100.0, 85.6, 70.1, 63.1, 61.9, 38.0, 13.0, 10.7; MS (ESI) m/z (M + Na)+ calcd. 334.1127; found 334.1157.
4.7. 4′-Azido-2′-deoxy-N4, 5-dimethylcytidine (13)
Compound 18 (101 mg, 0.123 mmol) was dissolved in 30% methylamine in ethanol (10 mL) and stirred at 70 °C overnight. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 92:8 to 85:15, v/v) to give compound 13 (30 mg, 0.101 mmol, 82% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz) δ 7.67 (s, 1 H), 6.39 (dd, J = 7.5, 4.0 Hz, 1 H), 4.53 (dd, J = 7.7, 7.2 Hz, 1 H), 3.82 (d, J = 12.0 Hz, 1 H), 3.75 (d, J = 12.0 Hz, 1 H), 2.93 (s, 3 H), 2.27-2.17 (m, 2 H), 1.92 (s, 3 H); 13C NMR (methanol-d4, 126 MHz) δ 165.4, 158.4, 138.1, 105.3, 101.2, 86.5, 72.1, 63.7, 39.3, 28.3, 13.2; MS (ESI) m/z (M + Na)+ calcd. 319.1131; found 319.1109.
4.8. 4′-Azido-2′-deoxy-N4-ethyl-5-methylcytidine (14)
Compound 18 (151 mg, 0.184 mmol) was dissolved in 2M ethylamine in ethanol (10 mL) and stirred at 70 °C overnight. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 93:7 to 9:1, v/v) to give compound 14 (40 mg, 0.129 mmol, 70% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz) δ 7.67 (s, 1 H), 6.39 (dd, J = 7.5, 4.6 Hz, 1 H), 4.53 (dd, J = 8.0, 7.5 Hz, 1 H), 3.82 (d, J = 12.0 Hz, 1 H), 3.75 (d, J = 12.0 Hz, 1 H), 3.47 (q, J = 7.5 Hz, 2 H), 2.48-2.42 (m, 1 H), 2.33 (ddd, J = 13.8, 7.5, 4.6 Hz, 1 H), 1.93 (s, 3 H), 1.19 (t, J = 7.5 Hz, 3 H); 13C NMR (methanol-d4, 126 MHz) δ 164.7, 158.5, 138.4, 105.3, 101.2, 86.6, 72.1, 63.7, 39.4, 36.8, 14.7, 13.3; MS (ESI) m/z (M + Na)+ calcd. 333.1287; found 333.1322.
4.9. 4′-Azido-N′-cyclopropyl-2′-deoxy-5-methylcytidine (15)
A mixture of 18 (210 mg, 0.255 mmol), 1,4-diazabicyclo[2.2.2]octane (57 mg, 0.510 mmol), and dry 3Å molecular sieves (200 mg) in dry 1,4-dioxane (5.7 mL) was stirred at room temperature for 30 min. Then, cyclopropylamine (179 μL, 2.55 mmol) and DBU (95 μL, 0.637 mmol) were added to the solution. After being stirred at room temperature overnight, the mixture was filtered and evaporated. The residue was re-dissolved in 7M methanolic ammonia (10 mL) and stirred at 55 °C for 10 h. After removal of the solvent, the residue was purified by flash column chromatography on silica gel (CH2Cl2/MeOH, 96:4 to 9:1, v/v) to give compound 15 (17 mg, 0.0536 mmol, 21% yield) as a white solid. 1H NMR (methanol-d4, 500 MHz) δ 7.70 (d, J = 1.2 Hz, 1 H), 6.39 (dd, J = 7.5, 4.0 Hz, 1 H), 4.53 (dd, J = 8.0, 7.5 Hz, 1 H), 3.83 (d, J = 12.0 Hz, 1 H), 3.76 (d, J = 12.0 Hz, 1 H), 2.91-2.87 (m, 1 H), 2.47 (ddd, J = 13.8, 8.0, 7.5 Hz, 1 H), 2.34 (ddd, J = 13.8, 7.5, 4.0 Hz, 1 H), 1.91 (d, J = 1.2 Hz, 3 H), 0.82-0.78 (m, 2 H), 0.630.59 (m, 2 H); 13C NMR (methanol-d4, 126 MHz) δ 166.4, 158.2, 138.5, 105.2, 101.2, 86.6, 72.0, 63.7, 39.4, 25.2, 13.2, 7.2; MS (ESI) m/z (M + Na)+ calcd. 345.1287; found 345.1311.
4.10. Anti-HBV and cytotoxicity assay
Anti-HBV assays were conducted as previously described.[25] Briefly, HepG2 2.2.15 cells were seeded in 96-well cell culture plates at a density of 4 × 103 cells in 200 μL per well together with various concentrations of a compound. On days 3 and 7 after plating, culture medium was removed and fresh medium and a drug were replenished. On day 14, the cells were harvested for DNA collection and the DNA samples were extracted from the cells using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and was re-suspended in 100 μ1 Tris-EDTA buffer. Total cellular DNA samples were amplified using the genesig Advanced Kit for quantification of Hepatitis B Virus genomes (PrimerDesign™Ltd., Southampton, UK) and Applied Biosystems®7500 Fast Real-time PCR System (Life Technologies Japan Ltd., Tokyo Japan). PCR preparations were incubated at 95 °C for 10 min, followed by 50 cycles at 95 °C for 10 s and 60 °C for 1 min. The data were analyzed using 7500 Fast Software Version 2.0.6 (Life Technologies Japan Ltd., Tokyo Japan) and threshold cycle (CT) values were obtained. To derive the HBV copy numbers from the CT values, a standard curve was generated with 10-fold serial dilutions of an HBV plasmid (pHBVWT; 20 to 2 × 108 copies per reaction). The amount of HBV DNA in each assay sample was compared to that in compound-free control samples.
The cytotoxicities of the compounds in MT-2 cells were also determined. Cells were plated in a 96-well plate at a density of 2 × 103 cells in 200 μL per well and were continuously exposed to the compound at a certain concentration throughout the entire culturing period. The number of viable cells in each well was determined using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).
Acknowledgments
The present work was supported in part by the Japan Agency for Medical Research and Development (AMED) for research on innovative development and the practical application of new drugs for hepatitis B under Grant Numbers JP16fk0310501 and JP19fk0310113 (SI and HM); a grant from Japan Society for the Promotion of Sciences (HM); a grant from National Center for Global Health and Medicine Research Institute (HM); and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (HM). We are grateful to Mrs. Misato Yamakawa for assistance on NMR measurements.
References
- [1].WHO fact sheet. http://www.who.int/mediacentre/factsheets/fs204/en/
- [2].Shepard CW; Simard EP; Finelli L; Fiore AE; Bell BP Hepatitis B Virus Infection: Epidemiology and Vaccination. Epidemiol. Rev 2006, 28, 112–125. DOI: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- [3].Trépo C; Chan HLY; Lok A Hepatitis B Virus Infection. Lancet 2014, 384, 2053–2063. DOI: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- [4].Terrault NA; Lok ASF; McMahon BJ; Chang K-M; Hwang JP; Jonas MM; Brown RS; Bzowej NH Jr; Wong JB Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. DOI: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang LSY; Covert E; Wilson E; Kottilil S Chronic Hepatitis B Infection a Review. J. Am. Med. Assoc 2018, 319, 1802–1813. DOI: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- [6].Perrillo R Benefits and Risks of Interferon Therapy for Hepatitis B. Hepatology 2009, 49, S103–S111. DOI: 10.1002/hep.22956. [DOI] [PubMed] [Google Scholar]
- [7].Konerman MA; Lok AS Interferon Treatment for Hepatitis B. Clin Liver Dis. 2016, 20, 645–665. DOI: 10.1016/j.cld.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [8].Menéndez-Arias L; Álvarez M; Pacheco B Nucleoside/Nucleotide Analog Inhibitors of Hepatitis B Virus Polymerase: mechanism of Action and Resistance. Curr. Opin. Virol 2014, 8, 1–9. DOI: 10.1016/j.coviro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- [9].Cihlar T; Ray AS Nucleoside and Nucleotide HIV Reverse Transcriptase Inhibitors: 25 Years after Zidovudine. Antiviral Res. 2010, 85, 39–58. DOI: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- [10].Hepatitis B and C treatments. https://www.fda.gov/patients/hepatitis-b-c/hepatitis-b-and-c-treatments
- [11].Soriano V; Puoti M; Peters M; Benhamou Y; Sulkowski M; Zoulim F; Mauss S; Rockstroh J Care of HIV Patients with Chronic Hepatitis B: Updated Recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008, 22, 1399–1410. [DOI] [PubMed] [Google Scholar]
- [12].Zoulim F; Locarnini S Hepatitis B Virus Resistance to Nucleos(t)Ide Analogues. Gastroenterol. 2009, 137, 1593–1608. DOI: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- [13].Iyer RP; Padmanabhan S; Zhang G; Morrey JD; Korba BE Nucleotide Analogs as Novel anti-Hepatitis B Virus Agents. Curr. Opin. Pharmacol 2005, 5, 520–528. DOI: 10.1016/j.coph.2005.04.019. [DOI] [PubMed] [Google Scholar]
- [14].Liu C; Dumbre SG; Pannecouque C; Huang C; Ptak RG; Murray MG; Jonghe SD; Herdewijn P Amidate Prodrugs of Deoxythreosyl Nucleoside Phosphonates as Dual Inhibitors of HIV and HBV Replication. J. Med. Chem 2016, 59, 9513–9531. [DOI] [PubMed] [Google Scholar]
- [15].Kumamoto H; Fukano M; Nakano T; Iwagami K; Takeyama C; Kohgo S; Imoto S; Amano M; Kuwata-Higashi N; Aoki M; et al. Diastereoselective Synthesis of 6″-(Z)- and 6″-(E)-Fluoro Analogues of anti-Hepatitis B Virus Agent Entecavir and Its Evaluation of the Activity and Toxicity Profile of the Diastereomers. J. Org. Chem 2016, 81, 2827–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Imoto S; Kohgo S; Tokuda R; Kumamoto H; Aoki M; Amano M; Kuwata-Higashi N; Mitsuya H; Haraguchi K Design, Synthesis, and Evaluation of anti-HBV Activity of Hybrid Molecules of Entecavir and Adefovir: Exomethylene Acycloguanine Nucleosides and Their Monophosphate Derivatives. Nucleos. Nucleot. Nucl. Acids 2015, 34, 590–602. DOI: 10.1080/15257770.2015.1037456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maag H; Nelson JT; Steiner JL; Prisbe EJ Solid-State and Solution Conformations of the Potent HIV Inhibitor, 4′-Azidothymidine. J. Med. Chem 1994, 37, 431–438. DOI: 10.1021/jm00030a001. [DOI] [PubMed] [Google Scholar]
- [18].Kirby KA; Singh K; Michailidis E; Marchand B; Kodama EN; Ashida N; Mitsuya H; Parniak MA; Sarafianos SG The Sugar Ring Conformation of 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine and Its Recognition by the Polymerase Active Site of HIV Reverse Transcriptase. Cell. Mol. Biol 2011, 57, 40–46. [PMC free article] [PubMed] [Google Scholar]
- [19].Takamatsu Y; Tanaka Y; Kohgo S; Murakami S; Singh K; Das D; Venzon DJ; Amano M; Kuwata N; Aoki M; et al. 4′-modified Nucleoside Analogs: Potent Inhibitors Active against Entecavir-Resistant Hepatitis B Virus. Hepatol. 2015, 62, 1024–1036. DOI: 10.1002/hep.27962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kohgo S; Imoto S; Tokuda R; Takamatsu Y; Higashi-Kuwata N; Aoki M; Amano M; Kansui H; Onitsuka K; Maeda K; Mitsuya H Synthesis of 4′-Substituted Purine 2′-Deoxyucleosides and Their Activity against Human Immunodeficiency Virus Type 1 and Hepatitis B Virus. Chem. Select 2018, 3, 3313–3317. [Google Scholar]
- [21].Zhou Y; Zhang Y; Yang X; Zhao J; Zheng L; Sun C; Jiang J; Yang Q; Wang Q; Chang J Novel Nucleoside Analogue FNC Is Effective against Both Wild-Type and Lamivudine-Resistant HBV Clinical Isolates. Antiviral Therapy 2012, 17, 1593–1599. [DOI] [PubMed] [Google Scholar]
- [22].Maag H; Rydzewski RM; McRoberts MJ; Crawford-Ruth D; Verheyden JPH; Prisbe EJ Synthesis and anti-HIV Activity of 4′-Azido- and 4′-Methoxynucleosides. J. Med. Chem 1992, 35, 1440–1451. DOI: 10.1021/jm00086a013. [DOI] [PubMed] [Google Scholar]
- [23].Matthes E; Langen P; Von Janta-Lipinski M; Will H; Schroder HC; Merz H; Weiler BE; Muller WEG Potent Inhibition of Hepatitis B Virus Production in Vitro by Modified Pyrimidine Nucleosides. Antimicrob. Agents Chemother 1990, 34, 1986–1990. DOI: 10.1128/AAC.34.10.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang Q; Kang J; Zheng L; Wang X-J; Wan N; Wu J; Qiao Y; Niu P; Wang S-Q; Peng Y; et al. Synthesis and Biological Evaluation of 4-Substituted Fluoronucleoside Analogs for the Treatment of Hepatitis B Virus Infection. J. Med. Chem 2015, 58, 3693–3703. DOI: 10.1021/jm5012963. [DOI] [PubMed] [Google Scholar]
- [25].Higashi-Kuwata N; Hayashi S; Das D; Kohgo S; Murakami S; Hattori S; Imoto S; Venzon DJ; Singh K; Sarafianos SG; et al. CMCdG, a Novel Nucleoside Analog, Exerts Potent Activity against Wild-Type and Entecavir-Resistant HBV with Favorable Safety Feature. Antimicrob. Agents Chemother 2019, 63. DOI: 10.1128/AAC.e02143-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


