Abstract
Hydrogel microparticles (HMPs) are promising for biomedical applications, ranging from the therapeutic delivery of cells and drugs to the production of scaffolds for tissue repair and bioinks for 3D printing. Biologics (cells and drugs) can be encapsulated into HMPs of predefined shapes and sizes using a variety of fabrication techniques (batch emulsion, microfluidics, lithography, electrohydrodynamic (EHD) spraying and mechanical fragmentation). HMPs can be formulated in suspensions to deliver therapeutics, as aggregates of particles (granular hydrogels) to form microporous scaffolds that promote cell infiltration or embedded within a bulk hydrogel to obtain multiscale behaviours. HMP suspensions and granular hydrogels can be injected for minimally invasive delivery of biologics, and they exhibit modular properties when comprised of mixtures of distinct HMP populations. In this Review, we discuss the fabrication techniques that are available for fabricating HMPs, as well as the multiscale behaviours of HMP systems and their functional properties, highlighting their advantages over traditional bulk hydrogels. Furthermore, we discuss applications of HMPs in the fields of cell delivery, drug delivery, scaffold design and biofabrication.
Owing to their high water content, diverse properties and similarity to the native extracellular matrix (ECM), hydrogels are used as substrates for cell culture1, templates for tissue engineering2 and vehicles for drug and protein delivery3. Traditionally, hydrogels are cross-linked into continuous volumes (bulk hydrogels) with external dimensions at the millimetre scale or larger and a mesh size at the nanometre scale that permits molecule diffusion. A micrometre-scale porosity can be introduced into a bulk hydrogel using various processing techniques, such as porogen leaching4, cryogel formation5 or electrospinning6. However, bulk hydrogels are not always suited for their intended applications, particularly in cases in which injection is needed or smaller sizes are required.
As an alternative, various techniques have been developed to fabricate hydrogels as microscale particles (~1–1,000 μm), called hydrogel microparticles (HMPs) or microgels. HMPs can be made from both natural and synthetic polymers, and can be fabricated into a variety of shapes and sizes using techniques that are often compatible with the encapsulation of biologics (for example, cells and drugs). Whereas the dynamics of polymers in solution, such as prior to hydrogel formation, are driven by thermal fluctuations, the larger HMPs are dominated by gravity, which allows for particle settling. HMPs can be utilized as distinct units or in aggregation; thus, we classify HMP systems into three categories: HMP suspensions, granular hydrogels and HMP composites (FIG. 1). In HMP suspensions, the HMPs reside in a fluid (liquid or air), with minimal interactions between particles. When the particle-packing density is increased and the particle–particle interactions that govern bulk assembly properties arise, HMPs form granular hydrogels. Granular hydrogels primarily exist in a jammed state, where they can range from a loose-packing configuration with high porosity to an ultraclose-packing state in which HMPs deform and the interstitial space collapses, resulting in a loss of microporosity. Finally, HMP composites are obtained when HMPs are embedded within a bulk hydrogel.
Fig. 1 |. Categories of hydrogel microparticles.
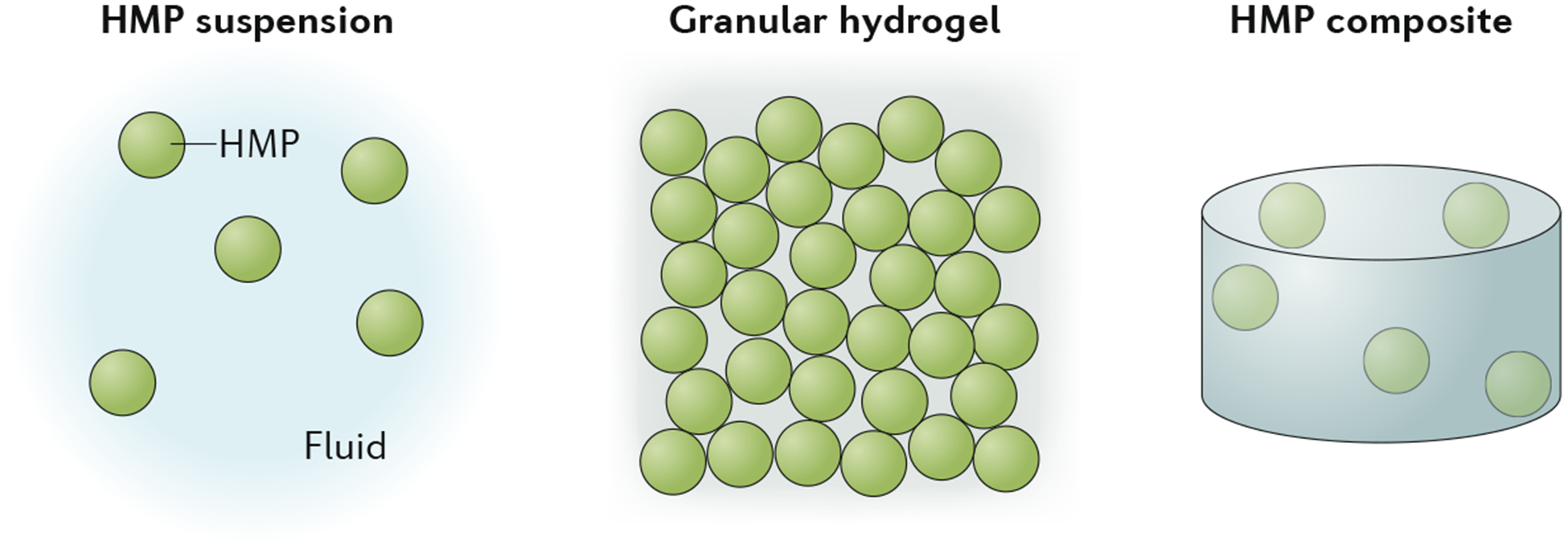
Hydrogel microparticles (HMPs) can be fabricated and used as distinct units or in aggregation. Their aggregates can be categorized as suspensions, granular hydrogels or composites if HMPs are embedded within a bulk hydrogel.
HMPs have a number of unique properties compared to bulk hydrogels that make them attractive for biomedical applications. First, their small size enables injection through small needles and catheters and inhalation of particles, which is advantageous for minimally invasive delivery of cells and biologics. The physical interactions between particles in granular systems often lead to a shear-thinning behaviour that permits injection and then a solid-like consistency after injection without the need for chemical modification7; however, interparticle cross-linking chemistries may also be incorporated to further alter the granular hydrogel properties8. Second, HMP systems are inherently modular, as multiple HMP populations with varying composition, size and contents can be mixed to create diverse materials9. Third, granular hydrogels can possess significant porosity (or void space) owing to the interstitial space between HMPs. The level of porosity scales with the size and packing density of HMPs and can be tuned to support cell proliferation and migration10.
In this Review, we discuss advances in the development of HMPs for biomedical applications. First, we introduce and compare methods used to fabricate HMPs, including batch and microfluidic emulsions, lithography, spraying and mechanical fragmentation. Then, we discuss the multiscale properties of HMP systems, including mechanical properties, injectability and porosity. Finally, we survey recent applications of HMP systems in cell delivery, drug delivery, scaffold building and biofabrication. The overall goal of this Review is to introduce the biomedical potential of these materials, as well as provide guidance for methods to fabricate and characterize them.
Fabrication of HMPs
A variety of fabrication techniques can be used to fabricate HMPs across various hydrogel types and cross-linking methods (FIG. 2). We can categorize them as batch emulsions, microfluidic emulsions, lithography, EHD spraying and mechanical fragmentation. In general, these approaches include the formation of droplets of hydrogel precursors that are then cured into HMPs, the spatial control of light to cure HMPs from hydrogel precursor solutions or the disruption of bulk hydrogels into HMPs.
Fig. 2 |. Fabrication of hydrogel microparticles.
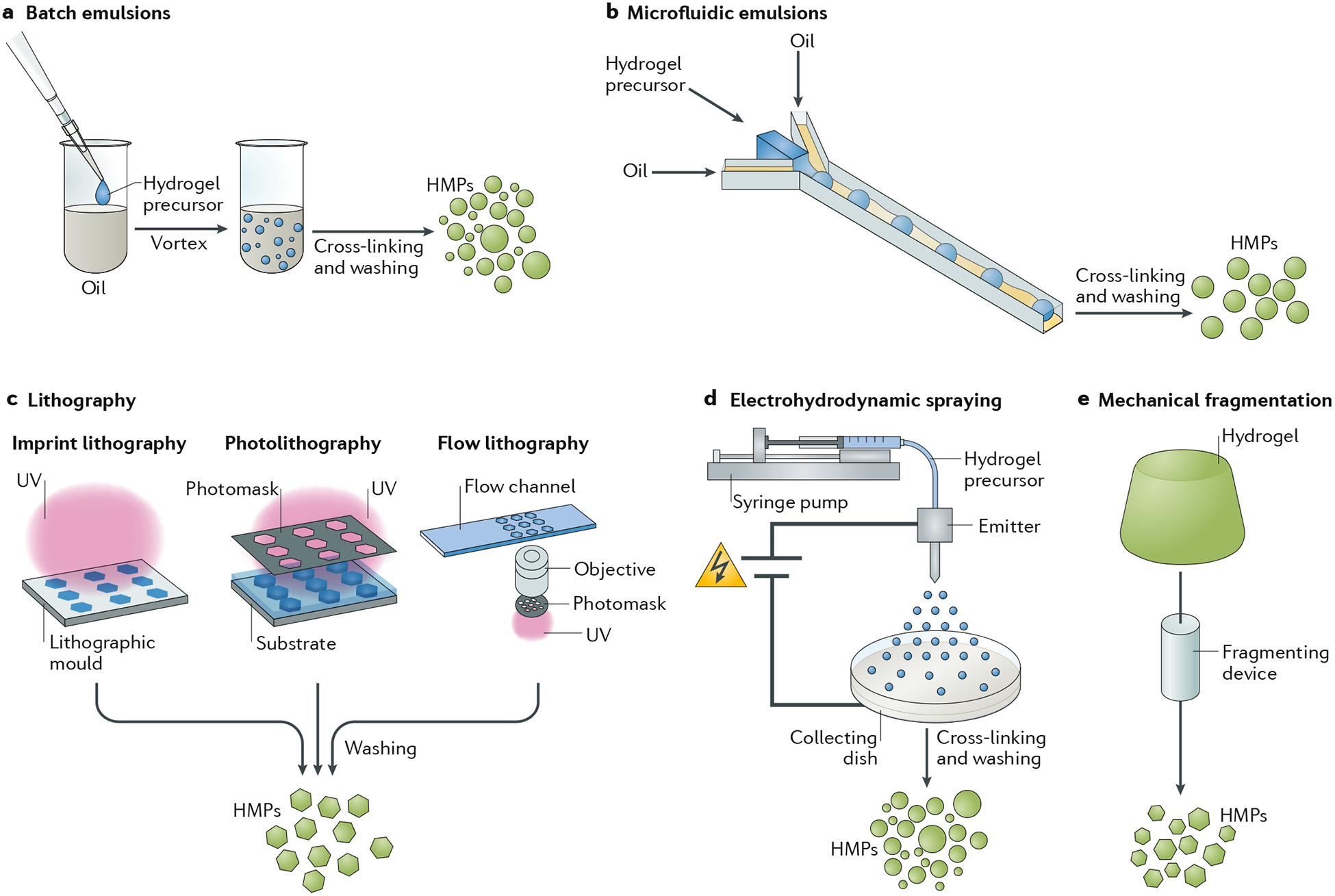
Examples of fabrication techniques include: a | Batch emulsions, in which immiscible liquids are mixed together (for example, water and oil) to generate droplets that can be cross-linked to form hydrogel microparticles (HMPs). b | Microfluidic emulsions, in which flow-focusing junctions are used to generate droplets that can be subsequently cross-linked to form HMPs. c | Lithography, in which masks or moulds are used as templates for hydrogels at the microscale. d | Electrohydrodynamic spraying, in which electrical forces are used to charge flowing solutions to form droplets that can then be cross-linked into hydrogels. e | Mechanical fragmentation techniques, in which mechanical energy is used to fragment preformed hydrogels into smaller particles. Blue shading refers to uncross-linked solution, green shading refers to cross-linked HMPs or hydrogel. UV, ultraviolet.
Approaches such as batch emulsions and mechanical fragmentation are popular because of their simplicity and the speed at which HMPs can be produced. However, more advanced strategies, such as microfluidic emulsions, lithography and EHD spraying, offer improved control over the formation of individual particles and have enabled the production of more monodisperse HMPs with variations in internal and external architectures. Key process parameters such as the rate of particle production and the particle size distribution can vary significantly across fabrication techniques: we outline these parameters in TABLE 1.
Table 1 |.
Key performance metrics for different techniques for the fabrication of hydrogel microparticles
| Production method | Particle size coefficient of variation* (CV%) | Minimum size range | Production rate | Particle geometry | Compatibility with cells |
|---|---|---|---|---|---|
| Batch emulsion | Large variation (>10%)11,230 | 1–10 μm17 | High (only limited by vessel size) | Spherical | Average (>80% viability)11,12 |
| Microfluidic emulsion | Low variation (1–2% possible)26 | 5–10 μm14,23,24,51 | Average (can be improved with parallel channels) | Spherical | Average (>80% viability)33,38,47 |
| Lithography | Low variation (1–3% possible)61,75,231 | <1 μm possible61,62 | Low (can be improved when integrated with microfluidics) | Arbitrary 2D and 3D geometries possible | High (>90%); no oil or surfactant required58,59 |
| Electrohydrodynamic spraying | Average variation >50%77,79,80 | 1–10 μm78 | Average | Spherical | Average (>80%)76,77 |
| Mechanical fragmentation | Average variation (2–8%)86 | 20–50 μm85,86 | High (only limited by vessel size) | Irregular | Unreported |
The coefficient of variation (CV%) is defined as the ratio of the standard deviation of particle size to the mean particle size.
Each fabrication technique imposes constraints on the hydrogels that can be processed. For example, the rheological behaviour and cross-linking mechanism of the hydrogel must be compatible with the chosen method. Thus, the fabrication technique should be selected based on its compatibility with the selected hydrogel, as well as on the desired HMP properties (such as size and dispersity) and access to specialized equipment (microfluidic emulsion, lithography and EHD spraying require more advanced equipment, whereas batch emulsions and fragmentation require relatively simple equipment). In this section, we discuss the strengths, limitations and compatibility with cell and drug encapsulation of each technique. We also highlight the hydrogel chemistry and cross-linking methods used in each fabrication approach, which are summarized in TABLE 2.
Table 2 |.
Hydrogel cross-linking chemistries used to generate hydrogel microparticles
| Base hydrogel | Chemical modification | Cross-linking method | Fabrication technique |
|---|---|---|---|
| Alginate | Unmodified77 | Ionic cross-linking with CaCl2 | EHD spraying |
| Unmodified47,112 | Ionic cross-linking in the presence of CaCl2 | Microfluidic emulsion | |
| Methacrylate232 | Radical polymerization (UV) | Microfluidic emulsion | |
| Gelatin | Unmodified86 | Temperature | Mechanical fragmentation |
| Methacrylate35,233 | Radical polymerization (UV) | Microfluidic emulsion | |
| Methacrylate194 | Temperature (cooling to 4 °C then radical polymerization (UV)) | Microfluidic emulsion | |
| Methacrylate58 | Radical polymerization (UV) | Lithography | |
| Hyaluronic acid | Methacrylate234 | Radical polymerization (UV) | Batch emulsion |
| Methacrylate67 | Radical polymerization (UV) | Lithography | |
| Acrylate103 | Radical polymerization (UV) | Microfluidic emulsion | |
| Norbornene7 | Thiol-ene reaction | Microfluidic emulsion | |
| Tyramine235 | Sequential enzymatic and radical polymerization (UV) | Lithography (two-photon) | |
| Tyramine + furan236 | Enzymatic cross-linking and/or Diels–Alder click chemistry | Microfluidic emulsion | |
| Vinyl ester69 | Thiol-ene reaction | Lithography (two-photon) | |
| Aldehyde + hydrazide237 | Hydrazone covalent cross-linking | Batch emulsion | |
| Methacrylate + tetrazole238 | Tetrazole–alkene photoclick chemistry (UV) | Microfluidic emulsion | |
| PEG | Acrylate59,239,240 | Radical polymerization (UV) | Microfluidic emulsion and lithography |
| Methacrylate34 | Radical polymerization (UV) | Lithography | |
| Norbornene195 | Thiol-ene reaction (UV) | EHD spraying | |
| Vinyl sulfone8 | Michael addition | Microfluidic emulsion | |
| Vinyl sulfone and thiol39,125 | Michael addition | Microfluidic emulsion | |
| Maleimide29,33,38 | Michael addition | Microfluidic emulsion | |
| PVA | Thiol and ene68 | Thiol-ene reaction (UV) | Lithography |
| Chitosan | Unmodified82 | Electrostatic interactions | EHD spraying |
| Silk/fibroin | Norbornene241 | Thiol-ene photoclick chemistry and β-sheet formation | Batch emulsion |
| Agarose | Unmodified7,37,242 | Temperature | Microfluidic emulsion |
EDH, electrohydrodynamic; PEG, poly(ethylene glycol); PVA, poly(vinyl alcohol); UV, ultraviolet.
Batch-emulsion techniques
Emulsion techniques use immiscible oil and aqueous hydrogel precursor solutions to generate droplets that can then be cross-linked into HMPs. In a batch process, an aqueous precursor solution containing the hydrogel prepolymer and an initiator and/or cross-linker is combined with the oil in a single container, potentially with a surfactant to stabilize the emulsion. Mechanical mixing is used to homogenize the solution and generate aqueous droplets surrounded by an oil phase (FIG. 2a). The extent and timing of mixing can influence the size and dispersity of the resulting droplets. The droplets are then cross-linked and the oil phase is removed using a series of washing, centrifugation and filtration steps. Several hydrogel cross-linking approaches are compatible with batch-emulsion techniques (TABLE 2). Photocross-linking is commonly used because external light sources induce cross-linking while the droplets of precursor solutions are suspended in the oil phase. For example, acrylated or methacrylated poly(ethylene glycol) (PEG) HMPs can be photocross-linked using radical polymerizations if a suitable initiator is included in the aqueous phase11. Alternatively, changes in temperature (cooling) can be used to cross-link thermosensitive hydrogels such as gelatin12.
The main advantages of batch-emulsion methods are their simplicity and the high particle-production rates, which are only limited by the container volume and by the ability to mix the emulsion. These methods are also compatible with biologics such as small molecules and growth factors, which can be simply dissolved in the hydrogel precursor solutions before the formation of the emulsion13. Batch-emulsion techniques have also been successfully used to encapsulate cells within HMPs through suspension in the aqueous solution prior to emulsification11·12.
One of the main limitations of batch emulsions is the polydispersity of the resulting HMPs, as there is limited control over the formation of individual particles14. This may also lead to batch-to-batch variations if the mixing procedures are not performed identically each time. The importance of polydispersity depends on the application: for example, when HMPs are used to release growth factors, diffusion may depend on their polydispersity and on particle sizes. It has been demonstrated that HMP populations with comparable mean diameters but prepared with either batch or microfluidic emulsions possess distinct release profiles, with greater variability in the population prepared by batch emulsion, which displays an earlier burst release of the encapsulated drug14. Further, HMP polydispersity makes it challenging to control the number of cells contained within each HMP; however, polydisperse HMP suspensions can be serially filtered with increasingly smaller filters to achieve a more monodisperse suspension15. If the batch-emulsion process is performed in a repeatable manner and its influence on HMP properties is understood, the drawbacks related to polydispersity can be minimal.
Although this method is used less frequently, HMPs can also be produced using all-aqueous, two-phase separation techniques16. Early work in this area leveraged phase-separation phenomena that occur through simple mixing of aqueous solutions of PEG and methacrylated dextran and subsequent cross-linking with light after separation17. PEG can also be used to form HMPs, exploiting phase separation above a lower critical solution temperature, including across a range of sizes with control over the thermodynamics and kinetics of the phase-separation process18. For example, HMPs were formed from reactive PEG derivatives that underwent thermally induced phase separation, and their size was dependent on the kinetics of gelation, influenced by temperature and pH19. Gelatin-based HMPs have also been fabricated with two-phase separation techniques exploiting the formation of simple coacervates in the presence of polyanions such as alginate under low-pH conditions20. For example, spontaneous HMP formation was observed following mixing of oxidized and methacrylated alginate and gelatin methacrylamide (GelMA) in aqueous solutions at low pH, resulting in a composite hydrogel system containing growth-factor-loaded GelMA compartments surrounded by an alginate bulk phase20.
Microfluidic-emulsion techniques
The limitations of batch-emulsion protocols motivated seminal studies in the early 2000s that demonstrated the potential of more controlled emulsions in HMP fabrication, such as in flow-focusing microfluidic devices21,22. Controlled droplet formation can be achieved by directing the flow of oils and aqueous solutions at intersection points, where shear forces and hydrophobic interactions induce the formation of aqueous droplets within an oil phase (FIG. 2b). Importantly, by varying the geometry of the intersection and the relative flow rates between the two phases, it is possible to control the droplet diameter, which is in the micrometre range (5–500 μm)23·24. In addition, if the flow rates are maintained, monodisperse particle populations with dispersity indexes as low as 1–2% can be produced25,26. As a result, microfluidic technology has been widely adopted to produce HMPs across a wide variety of hydrogels8,23,27 (TABLE 2). Microfluidic emulsions are also compatible with small-molecule or protein encapsulation; the improved size distribution offers greater control over subsequent release profiles13,14,28–30. In addition, by incorporating cells in the aqueous phase at a defined concentration, it is possible to predict and control the number of cells encapsulated in single HMPs31–33.
Microfluidic methods impose two main requirements on the hydrogel precursor solution. First, the solution must have a relatively low viscosity, so that it can be pumped through narrow microchannels at low pressures. Second, the droplets must cross-link rapidly during collection to prevent droplet coalescence. Many cross-linking strategies have been introduced in which droplets are cross-linked either ‘on-chip’ or ‘off-chip’; on-chip approaches are preferred because they limit droplet coalescence. Photocross-linking has been used with macromers such as polyethylene (glycol) diacrylate (PEGDA) and GelMA because they can be rapidly cross-linked using externally applied light34,35. Photoinitiated thiol-ene cross-linking has also been used to process HMPs, for example with norbornene-modified hyaluronic-acid and PEG macromers, which were cross-linked in the presence of dithiol cross-linkers and radical initiators7,36. Thermoresponsive hydrogels such as agarose can also be processed into HMPs by cooling the droplets during collection7,37.
Gelation of the hydrogel precursor can also be achieved through mixing or introduction of reactive components on-chip. Cross-linkers or initiators can be added to the oil phase, which subsequently diffuses into the aqueous phase to initiate cross-linking. For example, PEG maleimide HMPs have been fabricated by incorporating dithiol cross-linkers into the oil phase, where they can diffuse into the droplets to induce a Michael-addition reaction38. Advanced microfluidic devices can also be used to mix reactive components, for example using multiple flow-focusing junctions. The mixed aqueous phase can then be rapidly focused at a second emulsion junction for droplet formation and cross-linking over time. Using this approach, HMPs have been fabricated through a Michael-addition reaction between PEG thiol and PEG vinyl sulfone39. In a similar approach, degradable HMPs were formed through a reaction between cysteine-terminated, degradable matrix metalloproteinase (MMP)-sensitive cross-linkers and PEG vinyl sulfone8.
The main advantage of microfluidic approaches is the good control over the droplet-formation process, which can be precisely engineered by both designing microfluidic channels with specified geometries and controlling inputs such as flow rates. Typically, microfluidic junctions are made using microfabricated polydimethylsiloxane (PDMS) moulds that can be engineered to user-defined dimensions using soft lithography or 3D printing. Capillary-based microfluidic devices, in which glass capillaries are coaxially aligned in either co-flow or flow-focusing configurations, can also be used to generate microfluidic emulsions. A range of microfluidic techniques for generating compartmentalized HMPs have been developed40–42. For example, Janus HMPs were obtained by directing two aqueous solutions at a primary junction with laminar flow and then breaking them into droplets by further flow focusing at a secondary emulsion junction43 (FIG. 3a). Glass microcapillary devices can be used to form double emulsions, where particles of various internal complexities can be made, dependent upon the relative flow rates between the outer, middle and inner fluids25 (FIG. 3b). This high level of control over the droplet-formation process has enabled the formation of HMPs with either multiple drug-loaded compartments, where the drug-release rate from a particular compartment can be independently tuned by varying the hydrogel properties of that compartment and the overall release is based on the total release over time from all compartments44,45, or with structured co-cultures of cells46,47. A promising alternative is the design of centrifuge-based microfluidic devices to produce compartmental microparticles without oil, with the particle size controlled by the capillary diameter and centrifugal force48.
Fig. 3 |. Microfluidic and lithographic templating of compartmentalized hydrogel microparticles.
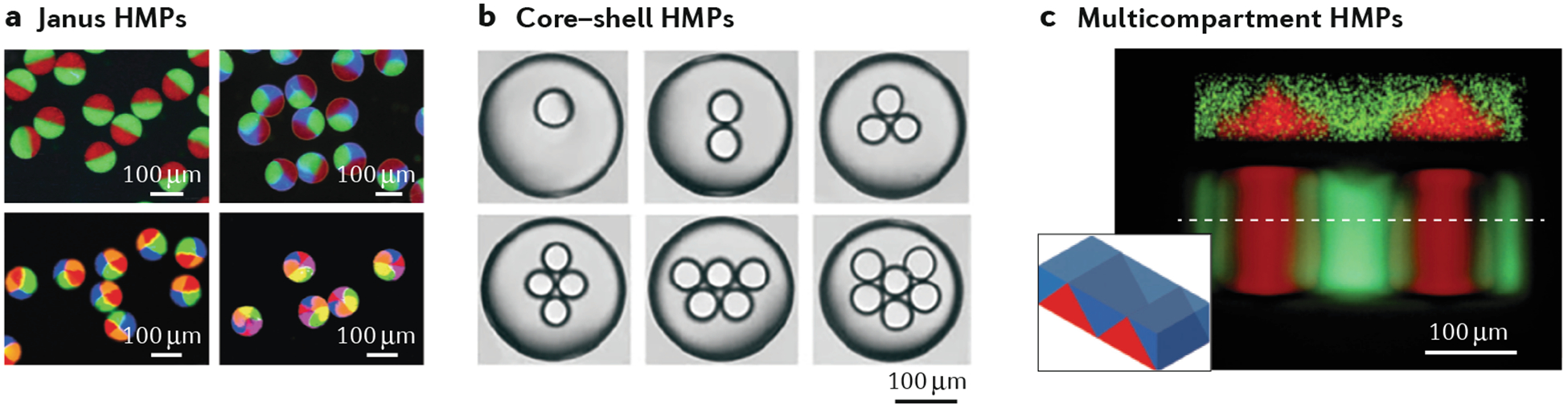
a | Microfluidic formation of Janus (multiple-sided) hydrogel microparticles (HMPs) containing up to six distinct compartments, which is achieved by using multibarrel microcapillaries123. b | Formation of structured core–shell HMPs containing multiple compartments using co-axial, flow-focusing, microcapillary needle arrangements42. c | Complex 3D structures fabricated using membrane-assisted photolithography, which facilitates sequential, layered polymerizations. The confocal microscopy image shows the cross section of the particle, the inset its 3D morphology56. Panel a is adapted from reF.123, panel b from reF.42, panel c from reF.56.
As an alternative to chip-based microfluidics, in-air microfluidic methods have been developed. In these methods, two microscale liquid streams are jetted together and forced to collide, leading to droplet formation49,50. Compared to microchannel-based on-chip techniques, higher liquid-flow rates can be achieved, which leads to faster particle-production rates (10–100 times faster). In addition, when reactive liquids are jetted towards each other (for example, alginate and calcium chloride), the HMPs can be directly deposited into 3D constructs. There is great potential in techniques such as these, which need to be further explored to exploit their advantages over other emulsion-based methods.
The major limitation of microfluidic approaches is that they are relatively low throughput compared to batch emulsions. This issue becomes exacerbated when attempting to produce smaller-diameter HMPs because the volumetric throughput decreases with the cube of the droplet diameter. However, higher throughput rates can be achieved using parallelized microfluidic devices containing multiple junctions on a single device51–54. To ensure that monodisperse particle populations are produced when using parallelized devices, the channel geometry must be designed to accommodate pressure drops and flow fluctuations throughout the device. For example, the successful encapsulation of viable cells within monodisperse PEG HMPs was demonstrated using a parallelized, double-layer PDMS device with a throughput six times higher than that of single-channel devices33. Microfluidic devices also classically produce spherical particles as a product of the surface-tension-driven droplet formation and alternative techniques may be needed if non-spherical particle geometries are desired.
Lithography
HMPs can also be produced using lithographic methods, in which photopolymerization is used to template hydrogels at the microscale. There are three main classes of lithography technologies: imprint lithography, photolithography and flow lithography55 (FIG. 2c). In imprint lithography, a hydrogel precursor is loaded into a templated mould with the negative features of the desired HMPs for cross-linking and then cured. Photolithography uses templated photomasks to selectively cure regions of a hydrogel precursor to form HMPs. Lastly, flow lithography uses a photomask to cure regions of a flowing hydrogel precursor solution at regular intervals to form HMPs. The main advantage of lithography technologies is that the geometrical features of the mask or mould can be tightly controlled, resulting in great control over the geometry and monodispersity of the HMPs56–58. In addition, lithographic approaches are widely compatible with cell encapsulation and no oil or surfactants are required to induce particle formation34,59.
Advances in microfabrication techniques have enabled the production of moulds and masks with features defined at the nanometre scale, making it possible to generate designer HMPs with tailored internal and external architectures that could not be achieved with other fabrication methods56–58,60–62. However, there are several constraints on the HMP geometries that can be obtained. Imprint-lithography approaches present the practical constraint of needing to remove the cross-linked HMPs from the mould, which limits the complexity of the achievable internal or external features. With photolithography, only relatively simple geometries such as cubes, cuboids, discs, bars and stars can be obtained55,61. To enable the production of HMPs with complex 3D geometries, multiphoton light sources have to be used63,64. Advanced lithographic assemblies that facilitate sequentially layered polymerization to produce complex 3D HMPs have also been developed56 (FIG. 3c).
A range of photocross-linking chemistries have been explored to produce HMPs using lithography (TABLE 2). The most commonly used hydrogels are acrylated or methacrylated PEGs, which are simple, tunable and biocompatible59,65,66. For example, PEGDA and poly(ethylene glycol) dimethacrylate (PEGMA) hydrogels can be cross-linked in seconds through photoinduced radical polymerization in the presence of a photoinitiator. Naturally derived hydrogels such as hyaluronic acid and gelatin can also be functionalized with reactive groups that make them processable by lithography58,67. Thiol-ene-based, step-growth photocross-linking approaches have been used to generate HMPs using modified poly(vinyl alcohol) (PVA)68 or hyaluronic acid vinyl ester with two-photon photolithography69.
A major challenge with lithography approaches is that they are relatively low throughput compared to other methods. Specifically, the particle-production rate is limited by the size of the moulds or masks that can be prepared using available microfabrication techniques and by the field of view of the light source or objective. Fast hydrogel curing times are generally preferred because they increase particle-production rates. For example, by increasing the number of functional groups on the macromer (for example, acrylate or norbornene) or the macromer concentration, the rate of polymerization and the final mechanical properties increased70–72. Further, increasing the light-source intensity or the initiator concentration (for example, I2959 for ultraviolet light and lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) for visible light) reduces the curing time required to reach a desired cross-linking density73,74. Flow-lithography techniques offer the greatest throughput because they can be integrated with microfluidic techniques61,65 and parallelized75. A method that is not widely adopted but has good performance is the particle replication in non-wetting templates (PRINT) system, which can produce HMPs with controlled sizes and shapes in a high-throughput manner through integration with a roll-to-roll manufacturing process that facilitates scalable particle-production rates62,66.
Electrohydrodynamic-spraying methods
EHD-spraying methods are less commonly used than the techniques discussed so far. During EHD spraying, a hydrogel precursor solution is extruded through a syringe while a voltage is applied at the needle tip. Beyond a critical threshold, the applied voltage overcomes the surface tension at the needle tip, leading to the formation of a charged jet of droplets that are attracted towards a collecting substrate76 (FIG. 2d). The droplets are then cross-linked in the collecting bath, and their size depends on parameters such as the applied voltage, needle diameter and polymer flow rate77, and can be as small as 1 μm78. However, achieving monodisperse particle populations is challenging, and dispersity indexes higher than 5% are often reported79,80. Alginate is widely used for EHD spraying because it can be directly sprayed into a calcium-chloride solution to induce immediate cross-linking of the droplets76,77,81. Chitosan HMPs have been processed by spraying into a tripolyphosphate solution to cross-link via electrostatic interactions82. Photocross-linkable hydrogels have been processed by spraying into a bath that is simultaneously exposed to light79,80. Finally, this technique is compatible with cell encapsulation76,77,83,84. Similar to emulsion approaches, the polydisperse particles can be passed through filters to achieve a more monodisperse population.
Mechanical-fragmentation methods
In mechanical-fragmentation methods, HMPs are produced by mechanically breaking a preformed bulk hydrogel into microscale particles (FIG. 2e). For example, a cross-linked hydrogel can be mechanically forced through a fine steel mesh to form smaller particles. The size of the HMPs can then be controlled through the pore size ofthe mesh. HMPs with diameters of 15–30 μm have been obtained this way85. Another simple fragmentation method involves breaking a cross-linked hydrogel into particles using a simple rotational blender. This method has been used to generate blended gelatin slurries composed of microparticles with diameters of120–300 μm86. The main advantages of microparticulation approaches are their speed and simplicity, which make it possible to rapidly generate large volumes of microparticles in a single process. However, a major limitation is that there is little control over the formation of individual particles, which results in polydisperse size distributions. It is also not clear whether these approaches are compatible with cell encapsulation, and, to the best of our knowledge, no studies on this aspect are available in the literature.
Properties of HMP systems
The physical properties of HMP systems are multiscale and depend on the properties of the individual HMPs, on their packing density and on the properties of the continuous phase. HMP suspensions, granular hydrogels and HMP composites each possess unique functional properties not present in bulk hydrogels. For example, HMP suspensions can be easily delivered through small needles or inhalers for minimally invasive delivery of cells and biologics. Granular hydrogels (FIG. 4) form particle scaffolds that open up new building approaches, including microporosity (owing to the interstitial space among HMPs) and scaffold modularity (owing to the mixing of multiple HMP populations)87. Granular hydrogels also display a shear-thinning behaviour that can be exploited for injectability and 3D printing applications9. In this section, we describe the different classes of HMP systems, with particular focus on their macroscale and microscale mechanics, porosity and diffusivity. We then describe some of the unique functional features of HMPs (injectability, heterogeneity and porosity) relevant for biomedical applications.
Fig. 4 |. Structure and properties of granular hydrogels.
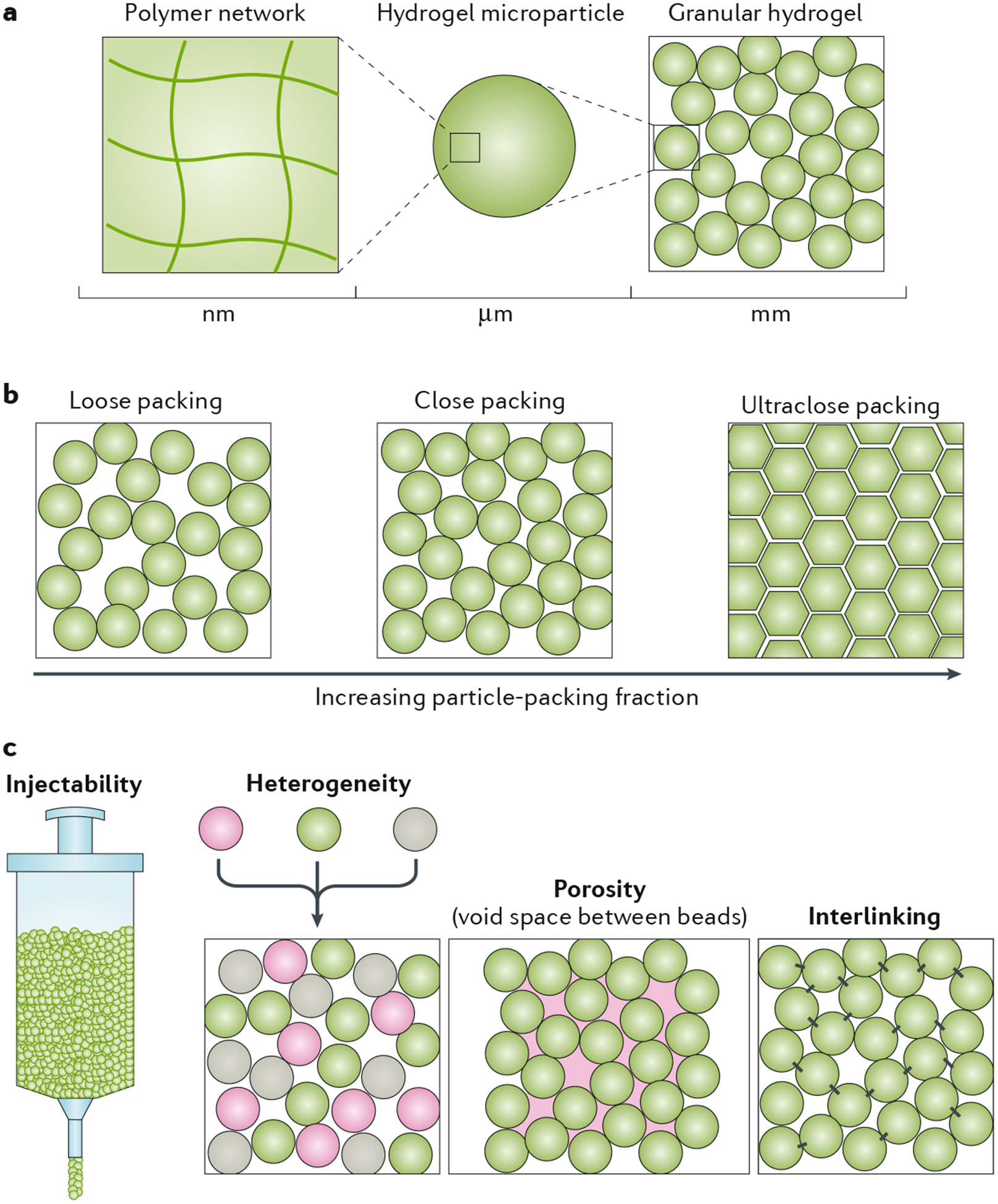
a | Granular hydrogels have multiscale features, with the polymer network at the nanoscale, individual hydrogel microparticles (HMPs) at the microscale and the granular structure at the millimetre scale. b | When the particle-packing fraction of HMPs in a granular hydrogel increases, the system evolves from loose packing to close packing to, eventually, an ultraclose-packing state, in which the particles deform and void spaces collapse. The packing density affects physical properties such as porosity, transport and mechanical properties. c | Granular hydrogels have unique features, including injectability, heterogeneity (if different types of HMPs are mixed together) and porosity, which allows for passage through the structure. Interlinking between particles further stabilizes the structure.
Multiscale properties of HMP systems
HMP suspensions.
HMP suspensions comprise dilute HMPs in a fluid, such as liquid or air (FIG. 1). The macroscale mechanical and rheological behaviours of HMP suspensions are largely governed by the properties of the continuous phase because interparticle interactions are negligible. The individual mechanical properties of the HMPs within the system depend on the polymer type and density, as well as the extent of cross-linking between polymer chains. Due to the relatively low packing density of HMPs in suspensions, the overall system diffusivity is high; however, diffusivity within an individual HMP is governed by the polymer mesh size. The concept of microporosity does not apply to HMP suspensions because microporosity is defined in the context of a solid-like material, but the particle-packing density is too low in these systems. Larger and denser microparticles (usually larger than 10 μm in diameter) are less sensitive to thermal forces that lead to Brownian motion and more affected by gravitational forces. This, in conjugation with interparticle friction, promotes HMP settling and packing88–90. Therefore, under static, non-mixing, non-flow conditions, suspensions of HMPs greater than 10 μm readily settle into a jammed state and transition to a granular hydrogel. HMP suspensions are generally used for cell or drug delivery, when interactions between particles are not needed to achieve effective therapeutic outcomes.
Granular hydrogels.
Granular hydrogels (FIG. 4) comprise an agglomeration of HMPs in the jammed state, where the term ‘jammed’ implies that an inside particle can only move if its neighbouring particles also move91. Generally, granular materials are composed of solid particles that readily sediment and experience frictional forces between touching particles88,90. Interparticle friction among HMPs in granular hydrogels is attributed to polymer interactions on the surface of HMPs; the amount of friction is influenced by the polymer type, microparticle chemistry and properties of the continuous phase of the material (such as viscosity and polymer-solvent interactions)92. As the HMP-packing density reaches that of the jammed state, interparticle friction aids in the transition from a liquid-like to a solid-like state88. Looking at the properties of granular hydrogels across various length scales, the polymer network is important at the nanoscale and it can influence the properties of individual HMPs at the microscale and those of the granular hydrogel at the millimetre scale (FIG. 4a).
The jamming transition occurs when the particle-packing density (or particle-volume fraction, ϕ) is sufficiently high and under appropriate conditions of stress and temperature87,90. Reducing the continuous phase of the material can promote HMP packing towards the jammed state7. The resulting jammed system behaves as a solid-like mass of touching particles; the precise particle-packing density largely influences hydrogel dynamics. Classically, hard-sphere packing is categorized into three general ranges: random loose packing (ϕ > 0.58), random close packing (0.58 < ϕ < 0.64) and maximally jammed, perfect packing (ϕ ≈ 0.74). At ϕ ≈ 0.58, particles are minimally jammed but configurationally stable. At ϕ ≈ 0.64, particles are maximally jammed in a random configuration in which additional jamming would require particle deformation90,93. Thus, granular hydrogels of spherical HMPs theoretically lie within a range of ϕ ≈ [0.58–0.64]. In reality, however, HMP systems are much more complex, owing to HMP deformability, interparticle friction and non-spherical, non-uniform HMP shapes, all of which may allow for states with ϕ > 0.74 (REF94). External forces such as centripetal forces or compression can be used to reach a state of ultraclose packing (ϕ 1), in which the interstitial space begins to collapse and flat-faced facets form between touching, deformed HMPs (FIG. 4b). To understand these behaviours, numerical simulations have been used to study the packing of particles of heterogeneous shapes, sizes and deformability93,95,96.
Granular hydrogels can swell and compress more than bulk hydrogels, owing to their two-scale matrix structure: intraparticle polymer cross-linking makes up individual HMPs, whereas an interparticle microporous matrix is formed when particles are packed together. The intraparticle matrix is nearly identical to that of a bulk hydrogel, but the microporous scaffold is specific to the jammed state, in which touching particles form the interconnected secondary matrix. This two-scale structure results in a two-phase response to swelling and osmotic compression. Bulk hydrogels can only swell up to the capacity of their polymer matrix, whereas granular hydrogels comprising fully swollen HMPs may continue to swell into the interstitial space of the packed particles until particles begin to pull apart (transitioning towards an HMP suspension)92. By contrast, osmotic forces can make HMP suspensions transition to a granular hydrogel phase. Once the jammed state is reached, further compression past a critical force will expel solvent from the decreasing interstitial space until the granular hydrogel begins to behave as a solid bulk gel. Such phenomena are sensitive to HMP stiffness but not to HMP size92.
The particulate nature of granular hydrogels makes them respond differently than bulk hydrogels to uniaxial compression, indentation and shear tests. Viscoelastic materials such as bulk hydrogels fracture under an applied force surpassing the yield stress. Granular hydrogels behave in a similar way under exceedingly high compression, when particles are densely packed and deformed. However, below this critical threshold, local slipping and sliding of HMPs helps to accommodate the imposed stress89,92. Unlike homogeneous bulk hydrogels, granular hydrogels contain load-bearing force chains, which are paths of touching particles through which stress is transmitted; these chains govern the stability and flowability of the system. Over the range of strains at which granular hydrogels exhibit elastic behaviour, force-chain networks remain constant. However, in the plastic regime, force chains become dynamic and respond to changes in the external load to maintain stable stress states89,97. The surface area of the applied load dramatically influences the mechanical response of a granular system: a large surface-area compression may result in minimal HMP displacement, whereas a focused indentation can penetrate the scaffold without fracture by causing local particle displacement. Shear loading of granular hydrogels results in a higher loss modulus (viscous response) under low shear rates than in bulk hydrogels.
Many materials properties influence the mechanical response of granular hydrogels, including polymer type, HMP stiffness, HMP density, HMP charge and interparticle friction. Incorporating a heterogeneous population of HMPs can introduce other interparticle forces that contribute to the dynamics of granular gels, including electrostatic interactions and hydrophobic-hydrophilic interactions. Granular hydrogels that comprise heterogeneous particle sizes may contain ‘rattlers’ or ‘floaters’ (smaller particles that freely traverse the interstitial space of the scaffold)91,95; however, these should not significantly impact the jamming properties of the system. When an external force is applied to a granular hydrogel, interparticle friction reduces HMP rearrangement92. To fully immobilize particles and fix the packing configuration of a scaffold, HMPs may be cross-linked together to create what is referred to as a microporous annealed particle (MAP) scaffold8. Annealing between HMPs can be achieved using a number of covalent or non-covalent techniques. The mechanical properties of MAP scaffolds are different from those of non-annealed HMP systems10, and, although both systems behave as solids under low shear stress, non-annealed HMP systems flow under high shear stress. By contrast, MAP scaffolds reach a higher modulus and do not behave as a liquid at high shear stress unless the annealing chemistry is reversible or non-covalent. Note that MAP scaffolds do not match the mechanical properties of bulk hydrogels with a matching formulation, owing to the lower number of HMP contact points relative to polymer entanglement in a bulk network15,98. Material degradation kinetics can also be modulated by HMP annealing, which introduces a tunable degradation parameter that does not exactly mirror the properties of the non-porous matrix composing the HMPs. Additionally, by limiting HMP rearrangement, the void space microarchitecture of the scaffold becomes fixed, offering a more controllable infrastructure.
HMP composites.
In HMP composites, the particles are incorporated in a secondary material (such as a hydrogel) (FiG. 1). The mechanical properties of the secondary material typically dominate the behaviour of composite systems, unless the concentration of HMPs is substantial and depending on their size and density. Embedding HMPs in a hydrogel helps to stabilize individual HMPs: the hydrogel acts as a cement to hold HMPs together and can potentially introduce desirable chemical or molecular interactions. HMP composites can also be used to fabricate microporous bulk hydrogels by using the HMPs as a sacrificial porogen that is then washed out (for example, gelatin through heating and cooling steps) after cross-linking the bulk hydrogel phase99,100. The dual material inherent to HMP composites can also be exploited for a more complex mechanical behaviour. For example, HMP composites have been used to engineer mechanically tough hydrogels in which the dispersed microgel phase blunts crack propagation under load101. HMP composites can also be used to decouple the mechanical and biological properties of a hydrogel. For example, rather than simply increasing the cross-linking density of a hydrogel to make it tougher, which may influence cell behaviour, adding HMPs (such as stiff gellan-gum microgels) to a soft hydrogel was shown to increase its toughness102.
Functional properties of HMP systems
Injectability.
A noteworthy feature of HMP systems is that they can be injected using a syringe or catheter (FiG. 4). Preformed bulk hydrogels are challenging to deliver using minimally invasive techniques and must, instead, be shaped and implanted, often involving surgical incisions. Bulk hydrogels are normally only injectable as a homogeneous precursor solution that exhibits liquid-like (viscous) rheological properties until gelation is induced using in situ cross-linking approaches or through the incorporation of shear-thinning cross-links. By contrast, unannealed HMP systems are easily injectable due to their particulate nature and small particle size. Frictional, non-covalent and electrostatic forces among neighbouring HMPs affect their injectability and become increasingly influential as particle packing increases. For example, jammed granular hydrogels exhibit shear-thinning behaviour; thus, large injection forces allow HMPs to collectively flow as a fluid and, once the applied force is attenuated, the HMPs return to a viscoelastic solid state with gel-like rheological properties7. Gel stiffness may be further enhanced by annealing HMPs post injection8. The injectability of HMPs has been exploited for several applications, including wound healing and bioprinting, using a wide range of hydrogels. For example, modified hyaluronic-acid HMPs (made using microfluidics) were injected into ischaemic sites within cardiac9 and brain103 tissue in rats and mice, respectively, to promote wound healing in a minimally invasive way.
Particle jamming, which regularly occurs under syringe pressure, minimizes turbulent flow and allows HMPs to move as a plug during injection54,104 (FIG. 4c). When injected into a cavity, HMPs at appropriate packing fractions readily fill the space and take the shape of the cavity, similar to a precursor hydrogel solution; however, the viscoelasticity of particulate hydrogels minimizes their dispersion upon injection8,103. Note that unannealed, jammed HMPs behave as a solid unit when injected into tissues that experience low pressure, such as the skin; however, injection into pressurized, confined spaces, such as fluid-filled stroke cavities, may require post-injection HMP annealing to avoid backflow after the syringe is removed103. Annealing after injection into highly motile tissues, such as cardiac tissues, may also help to minimize particle dislodging9 but, depending on the formulation, is not always needed105.
Heterogeneity.
Incorporating intraparticle and/or interparticle heterogeneity introduces additional complexity to HMP systems that may elicit multifactorial effects at the particle or system level (FIG. 4c). Intraparticle heterogeneity may involve compartmentalizing individual HMPs, altering surface chemistries post production, layering individual HMPs or varying porosity within individual HMPs. Regarding interparticle heterogeneity, HMP species can differ from one another in a multitude of ways, including hydrogel formulation or cargo type. A heterogeneous material can be produced by simply mixing multiple HMP populations until particles are uniformly distributed. Macroscale anisotropy can be achieved by exploiting HMP jamming, which minimizes HMP mixing and allows for physical separation of different HMP agglomerations104. To demonstrate the potential complexity of HMP systems, consider a collection of spherical (100-μm) HMPs in an aqueous medium. In the jammed state, there are roughly 1,000 HMPs per μL of material. Scaling up, 1 mL of material would contain 1 million distinct HMPs. Thus, there is substantial room for incorporating HMP heterogeneity across various HMP systems.
Void space and porosity.
The interstitial space (or void space) among packed HMPs is referred to as the pores of the granular scaffold, and the size of the pores is pro-portional to the size of the HMPs. Thus, packed HMPs produce micrometre-sized pockets of interstitial space87. Cells with diameters on this length scale can, therefore, easily infiltrate and traverse a granular scaffold without needing to degrade the hydrogel, whereas degradation is often needed for cells to infiltrate a bulk hydrogel. Relative to non-porous hydrogels, the microporosity of granular hydrogels increases fluid-flow, mass-transport, permeability and cell-infiltration rates. The system can also accommodate other large entities, such as ECM proteins (which often exceed 200 kDa in size). A reduction in scaffold porosity generally correlates with a reduction in mass-transport rates. The average pore size of a scaffold is affected by HMP shapes and sizes, packing density and stiffness8,106. Depending on the degree of HMP jamming and softness, the application of a large external force may result in the collapse of the interstitial space, so that flat-faced facets form between touching, deformed particles94 (ultraclose packing in FIG. 4c).
Applications of HMPs
The properties of HMPs make them promising for numerous biomedical applications, several of which are covered in this section, including cell delivery, drug delivery, scaffold building and biofabrication. The ability to extrude HMP-based biomaterials from syringes or catheters is particularly important for their delivery to tissues. HMPs can be delivered, for example, by intraarticular injection or direct injection into tissues: the modes of delivery of HMPs to various tissues are summarized in FIG. 5.
Fig. 5 |. Hydrogel microparticles delivery to various tissues in the body.
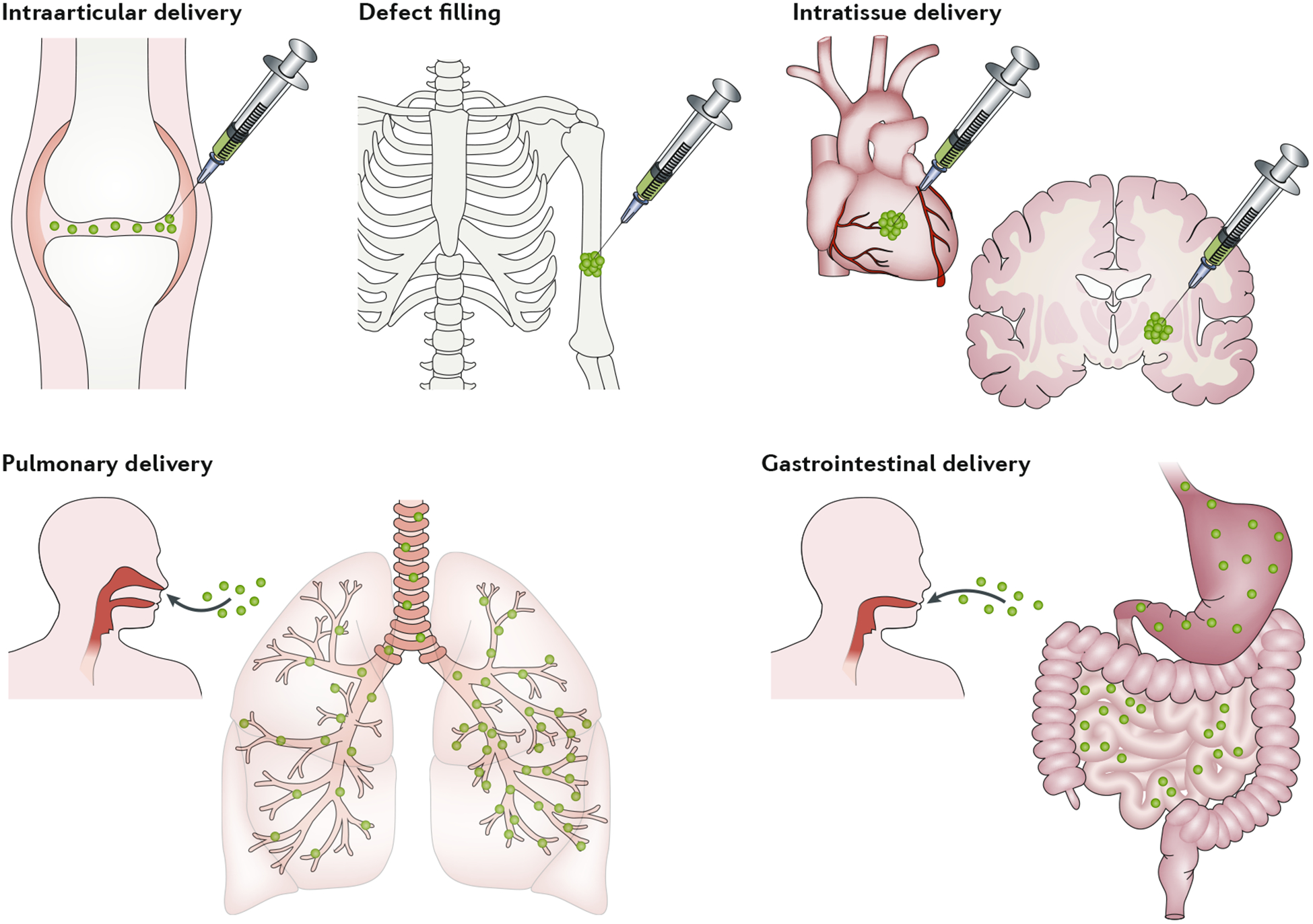
Examples of hydrogel microparticle delivery include: delivery to the intraarticular space; delivery to bone defects; intratissue delivery (for example, in the heart or brain); delivery to the lungs via aerosols; and delivery through the gastrointestinal tract to the intestine. Hydrogel microparticles can be delivered as suspensions, granular hydrogels or composites, and they may contain biologics, such as cells or drugs.
HMPs for cell delivery
The delivery of cells to damaged and diseased tissues holds tremendous potential for numerous applications; however, challenges — including limited cell survival and difficult engraftment of transplanted cells following delivery — has hampered progress in this area. This has led to increased interest in the use of delivery vehicles to enhance the integration, viability and function of injected cells107. Hydrogels emerged as a platform to increase the localization of cells at the target site following injection and to provide appropriate biophysical and biochemical cues to promote cellular integration and desired function108–111. The high water content and tunable properties of hydrogels further motivated their use in this area. Although significant progress has been made, challenges still remain; for example, the large diffusion lengths in bulk hydrogels may limit the oxygen and nutrients that reach transplanted cells. Further, the limited porosity and lack of injectability of many bulk hydrogels impede their use in several applications. These limitations have stimulated interest in the use of HMP systems for cell delivery. In this section, we describe concepts and advances in the use of HMPs for cell delivery and highlight some successful applications.
Cell encapsulation within HMPs.
As discussed, a range of HMP fabrication techniques are compatible with cell encapsulation (batch emulsion, microfluidic emulsion, lithography and EHD spraying). Microfluidic emulsions and lithography are often used due to the improved control over particle size and shape that they offer. By controlling process parameters such as the particle size and density of cells within the precursor solution, it is possible to precisely control the number of cells per HMP, down to the single-cell level33,112. In addition, by parallelizing channels, it is possible to generate cell-laden HMPs in a high-throughput manner33,75.
Cell encapsulation within HMPs offers several advantages compared to encapsulation within bulk hydrogels. A significant challenge with hydrogel-based cell encapsulation is the limited nutrient diffusion within core regions, which is exacerbated as the hydrogel size is increased to clinically relevant dimensions. A common approach to address this problem is the incorporation of nutrient microchannels into the hydrogel113–116; however, this can be technically challenging when an injectable material is desired. Packed HMP systems provide an alternative because the microporous interstitial space surrounding the particles enables diffusion. Additionally, granular scaffolds enhance vascularization when compared to bulk hydrogels in a variety of in vivo environments, which supports the engineering of thicker tissues8,103.
When considering the design of HMP cell-encapsulation techniques, it is important to consider both the process that is used to encapsulate the cells (in terms of achieving high viability) and the environment in which the cells are embedded (in terms of enabling function). The importance of the cellular microenvironment on cell function has emerged over the past decades: features such as the mechanics of the environment may change cellular mechanosensing, and the incorporation of ligands may enable cell adhesion1,117; thus, there is now a focus on these features when designing HMP environments. Further, properties such as HMP degradation may be important to control the timing of cell release or their integration with the surrounding tissue environment. This may happen through the incorporation of hydrolytic or enzymatic cross-links or even through the use of phototriggered degradation processes118. In some cases, cells are made to adhere to the outside of HMPs or between populations of HMPs for delivery to tissues instead of encapsulated.
The cytocompatibility of the HMP encapsulation process is regulated by the method of gelation and the presence of shear forces, radicals and chemicals that may be harmful to cells. This is mostly similar to encapsulation of cells within bulk hydrogels, although there are some differences due to the fabrication processes to scale down the HMPs. For example, the use of lithography for HMP cell encapsulation does not impose significant added constraints compared to cell encapsulation within bulk hydrogels, and cell viability within such systems is generally high, at >90%58,59. However, achieving high cell viability within microfluidic emulsions is not trivial and depends on several factors, including the total batch-processing time, the pH of the precursor solution, the medium used to achieve effective droplet formation and the level of shear experienced by the precursor solution while travelling through the microfluidic channels.
Several approaches can be employed to improve cell viability when designing chips for microfluidic emulsions. First, the width of the channels should be carefully considered, as narrower channels (35 μm) resulted in significantly decreased cell viability compared to wider channels (100 μm), with viabilities of 71% and 91%, respectively33. The length of exposure to potentially harsh oil and surfactants can also decrease cell viability. The traditional approach to separate HMPs from oil is to apply cycles of centrifugation and washing steps; however, these processes can impact cell viability and reduce the yield of collected particles. Recently, a range of on-chip filtration processes to rapidly separate cross-linked HMPs from the continuous oil phase have been developed, and significant improvements in cell viability (up to 20%) have been reported119,120. Double-microfluidic-emulsion water-oil-water approaches have been used to generate cell-laden GelMA HMPs containing an ultra-thin oil shell on the particle that quickly dewets upon transfer into an aqueous solution121. This reduces cell exposure to the oil phase, resulting in improved viability compared to conventional, single-emulsion approaches. Oil-free, all-aqueous microfluidic approaches can also be used to generate HMPs based on the immiscibility between specific aqueous phases (such as PEG (30% w/v), sodium alginate (1% w/v) and dextran (15% w/v)122). An oil-free, centrifuge-based microfluidic device was also developed that can produce alginate-based HMPs loaded with cells with viability as high as 70% (REFS48,123).
The distribution of cells within HMPs is another important consideration. Within emulsions, cell distributions may be influenced by HMP polydispersity (in batch emulsions) or long processing times (in microfluidics); however, increased control over the particle-production process and hydrogel cross-linking33,38,124 and techniques that prevent cell settling in the hydrogel precursor solution can improve control over cell distributions125. Achieving consistent cell distributions with EHD-spraying techniques can also be challenging because excessive voltages may lead to cell aggregation towards the periphery of the HMPs79. Lithography approaches generally result in homogeneous cell distributions across particles, although particle cross-linking must occur sufficiently fast to prevent cell settling within the uncross-linked hydrogel precursor58.
Applications of HMPs in cell delivery.
HMPs have been used in a variety of cell applications, typically either as HMP suspensions or as granular hydrogels (often with low particle-packing density). The cell-laden HMPs may be cultured in vitro prior to delivery or delivered soon after cell encapsulation, and approaches often include co-cultures of cells. One major advantage of using HMPs for cell delivery is that cells are protected during the delivery process. Although bulk hydrogels may be injectable by exploiting shear thinning, shear forces acting on cells during injection may impact their viability126,127.
The shear-thinning behaviour of bulk hydrogels is based on the relative motion of adjacent polymer chains, which results in the transfer of shear forces to cells encapsulated within the hydrogel. In contrast, when cells are encapsulated in HMPs, the relative motion between particles during injection, especially in granular hydrogels, results in limited force transfer, which could enhance cell viability during extrusion through small needles. Although not specifically explored in a HMP system, hydrogel cross-linking has been shown to enhance cell protection during injection: lightly cross-linked alginate (~30 Pa) enhanced cell survival compared to non-cross-linked solutions128. In many HMPs, encapsulated cells are surrounded by a stable hydrogel, which likely improves cell viability during syringe delivery.
One area that has garnered attention is the use of HMPs to deliver bone-marrow-derived stromal cells (BMSCs) for the repair of bone defects. For example, injectable osteogenic microtissues were generated by encapsulating BMSCs and bone morphogenetic protein-2 (BMP-2) within GelMA HMPs fabricated using a microfluidic emulsion device129. Cells proliferated within the HMPs and, following delivery into a bone defect, induced robust bone regeneration129. In a similar approach, BMSCs were encapsulated in chitosan-collagen HMPs using a batch emulsion and differentiated along an osteogenic lineage in vitro before being delivered into mouse calvarial defects130. Predifferentiation of cells within the HMPs enhanced bone defect repair compared to undifferentiated controls, which demonstrates how HMP technologies can be used to deliver preformed, engineered microtissues that are otherwise challenging to deliver using minimally invasive techniques. A synergistic enhancement of bone formation was also observed when BMSCs and bone marrow mononuclear cells were co-delivered by distinct chitosan-collagen HMP populations (fabricated by batch emulsion)131.
HMPs are also being explored as platforms for cell delivery in the repair of cartilage tissue. Early studies demonstrated that, following microencapsulation within HMPs, BMSCs differentiate along a chondrogenic lineage in the presence of the transforming growth factor β3 (TGF-β3)132,133. Promising in vitro studies also reported enhanced synthesis of cartilage matrix components in gelatin-norbornene HMPs (produced by microfluidic emulsion) compared to cartilage synthesis promoted by bulk hydrogels, which was attributed to the microporosity among particles134. HMP systems have also been explored as templates for directing the repair of cartilage defects. For example, cartilage ECM-derived particles were fabricated by performing a series of fragmentation pulverization, sieving and decellularization steps on fresh cartilage tissues135. Following seeding with BMSCs, the ECM-derived particles promoted the repair of osteochondral defects in a rabbit model. In a similar study, chitosan-based and cellulose-based ECM mimetic nanofibrous microparticles were seeded with BMSCs to promote cartilage regeneration following delivery into chondral defects136.
HMPs have also been explored as platforms for delivering cells to promote cardiac repair. In one approach, cardiac side population cells (a progenitor cell in the heart) were seeded onto the surface of GelMA HMPs made using microfluidic emulsions35. The cells adhered and proliferated on the surface of the HMPs, and the inclusion of a silica hydrogel coating on the HMPs provided protection against oxidative stress to promote survival following delivery in vivo35. In another study, it was demonstrated that the delivery of cardiac progenitor cells (CPCs) to the ischaemic myocardium using gelatin HMPs enhanced cell engraftment compared to CPC-only controls137. Interestingly, despite improvements in cell engraftment, only marginal improvements in cardiac function were observed compared to the results obtained delivering CPC controls.
HMPs can also be used for applications in which physical isolation of transplanted cells from the immune system is required to promote a therapeutic effect following delivery in vivo. For example, delivery of insulin-producing islets has been widely explored for the treatment of diabetes; however, overcoming premature immune rejection following transplantation has been a major challenge. HMPs are an ideal platform for islet transplantation because they can be used to provide an immunoprotective barrier; the shorter diffusion distances (compared to those of bulk hydrogels) ensure the efficient exchange of nutrients and oxygen with the surrounding tissue to maintain islet survival and enhance the transfer of insulin from the islets to the surrounding vasculature138. Islets encapsulated in alginate HMPs (fabricated using EHD spraying) can be used as a platform to achieve immunoisolation and enhance the therapeutic window following delivery139. Interestingly, a size-dependent immune response was observed, with larger particles (up to 1.9 mm in diameter) promoting a reduced foreign-body response and fibrosis compared to smaller particles (500 μm in diameter)139. An enhanced therapeutic window was achieved by co-delivering islets and PEG HMPs containing Fas ligand fabricated using microfluidic emulsions140. The Fas-ligand-containing HMPs localized immune suppression through selective apoptosis of adaptive immune cells, which, in turn, promoted survival of the transplanted islets.
Although not developed specifically for delivery to tissues, the ability to structurally organize cells with predefined arrangements using microfluidic emulsions and lithography has made it possible to engineer in vitro models that mimic the complexity of in vivo microenvironments. Core-shell assemblies are promising for models in which homotypic and heterotypic interactions need to be balanced. For example, HMPs containing hepatocytes in the core and fibroblasts in the shell enhance the production of liver-specific functions compared to isolated or unstructured co-cultures46. In another experiment, pneumatic-aided imprint lithography was used to fabricate HMPs that mimicked the structural and cellular organization of a liver lobule with radially aligned hepatocytes and endothelial cells encapsulated in collagen124. It was demonstrated that the hepatocytes cultured within the structured 3D model were more sensitive to acetaminophen than those in unstructured 2D and 3D cultures, highlighting the influence of cell-cell and cell-ECM interactions in regulating in vitro drug-toxicity screening.
HMPs for drug delivery
Hydrogels are very promising for the delivery of drugs (for example, small-molecule pharmaceuticals or growth factors), owing to their ability to protect, deliver and locally release bioactive factors in a controllable manner141, overcoming many of the limitations of traditional drug-administration methods (such as oral and intravenous) that often require high doses and repeated administration, and can lead to off-target effects. Traditionally, hydrogels for drug delivery have been macroscale bulk hydrogels, potentially delivered using minimally invasive techniques142,143 and with controlled-release profiles3; however, the use of HMP systems is growing, owing, as discussed, to their distinct advantages over macroscale hydrogels. The small size of HMPs enables minimally invasive delivery through small needles and catheters8 (FIG. 5), without the need for the specific in situ or shear-thinning cross-linking chemistries needed for the delivery of bulk hydrogels. Additionally, HMPs are highly versatile because multiple HMP populations can be easily combined to include multiple release profiles and degradation behaviours into a single injection9, which may be advantageous for many tissue-repair strategies to match biological signalling cascades. In this section, we discuss advances in the use of HMPs for drug delivery and highlight their increased versatility compared to traditional delivery strategies (FIG. 6).
Fig. 6 |. Drug release from hydrogel microparticles.
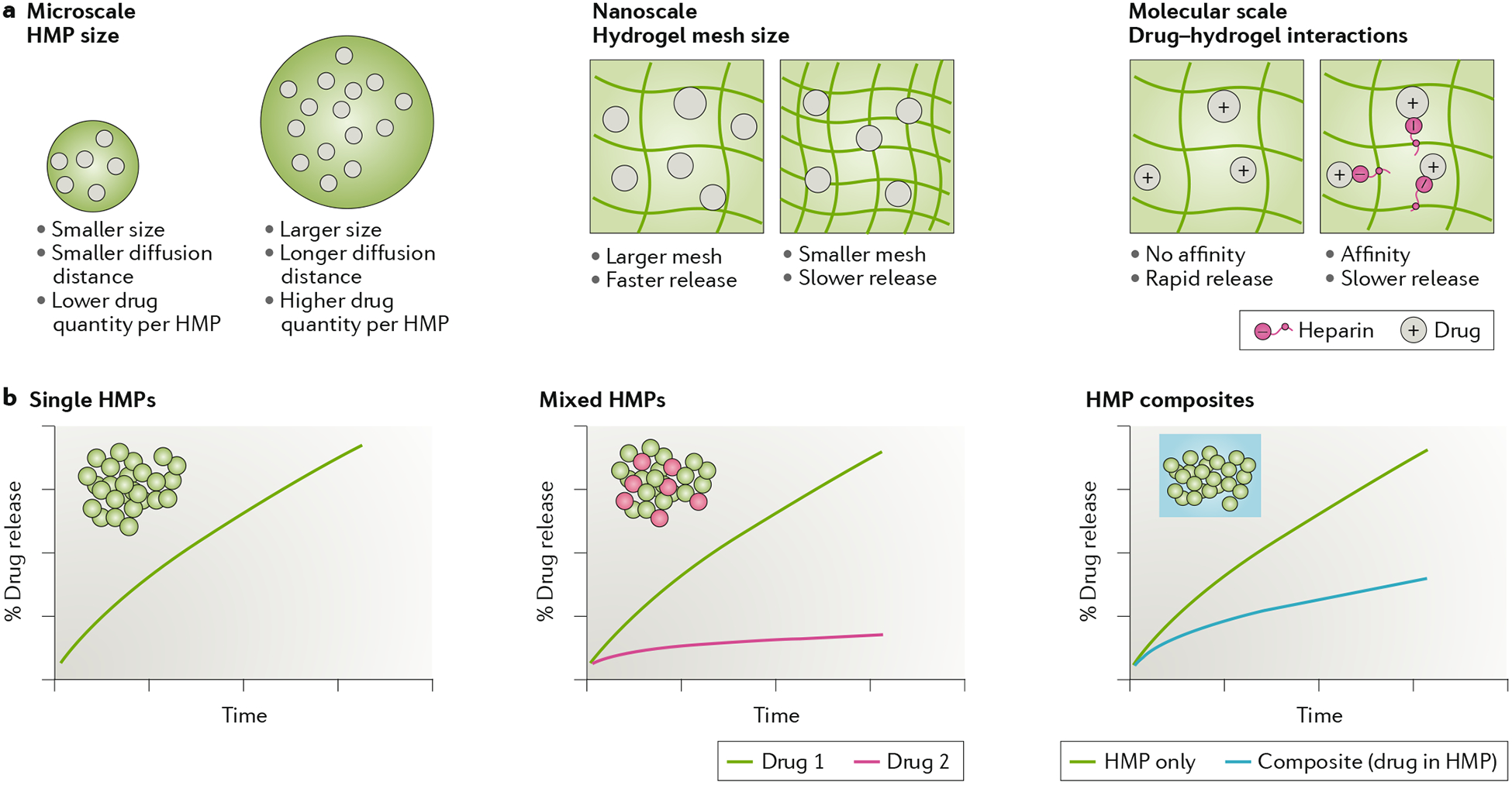
a | General parameters that influence drug release from hydrogel microparticles (HMPs) are the particle size, network mesh size and molecular interactions between drug and hydrogel. b | Potential release profiles of drugs from HMPs for single HMP formulations, mixed HMP formulations to deliver multiple drugs and composites in which HMPs are embedded within a bulk hydrogel.
Controlling drug release from HMPs.
The design of HMPs for drug delivery resembles that of bulk hydrogels, and the different strategies for controlling drug loading and release from bulk gels can be adapted to HMPs3. However, there are unique features that apply only to HMPs or affect them more than bulk systems. For example, microscale features such as the particle size and shape influence the total volume of cargo that can be encapsulated (FIG. 6a). The particle diameter is an important consideration; larger particles display more sustained release due to larger diffusion distances and smaller surface per hydrogel volume compared to smaller particles144,145. At the nanoscale, the particle mesh size influences drug diffusion through the network, and the release profile is slower for smaller mesh sizes146,147. Smaller mesh sizes can be achieved by increasing the polymer concentration and cross-linking density during HMP formation. Finally, at the molecular scale, chemical interactions between the drug and hydrogel can be used to delay the release of cargo from the particle.
A range of interactions such as covalent conjugation and electrostatic and hydrophobic associations can be used to increase the drug-hydrogel affinity to control release3,148. For example, heparin, which is an important molecule in drug delivery because of its ability to reversibly bind proteins owing to its highly sulfated nature, has been incorporated into HMPs (using a batch-emulsion process) to sustain the delivery of BMP-2, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF-2)149. Similar approaches can be adopted through sulfation of uronic acids that are present in commonly used hydrogels, such as hyaluronic acid and alginate150,151. For example, enhanced binding of growth factors within alginate HMPs has been achieved through sulfation152.
One advantage of using HMPs in drug delivery is that multiple particle populations can be mixed together to develop injectables with multifunctional behaviours. For example, two distinct drugs can be encapsulated in separate particle populations engineered with independent release profiles (FIG. 6b). Early work in this area demonstrated how the sequential release of distinct growth factors using HMP suspensions enhances tissue formation and repair153–155. Mixed particle populations containing different degradation behaviours can also be used to selectively deliver therapeutics in response to the local biological environment. For example, mixed hyaluronic-acid particle populations cross-linked with either stable or degradable cross-linkers (fabricated using a microfluidic emulsion) selectively degrade and release their cargo in response to the upregulated MMP activity present after myocardial infarction9. Further, spatial control over drug presentation following delivery is challenging to achieve using traditional injectable hydrogels. Recently, distinct populations of hyaluronic-acid HMPs formed using microfluidics were delivered in a spatially controlled manner by sequentially loading different particle populations into the syringe before injection104.
HMPs are often delivered as HMP composites, in which they are mixed into a secondary bulk hydrogel (FIG. 6b). Composite systems are appealing because they can address some of the limitations of both HMPs and macroscale hydrogels. For example, the secondary hydrogel can prevent particle migration from target sites when drug localization is required. As in heterogeneous particle systems, different drugs can be encapsulated into the HMPs and bulk hydrogels, with the drug release controlled by the independent properties of the two phases. Purely particle-based systems present short diffusion distances that can result in early ‘burst release’ of encapsulated drugs, whereas composite systems reduce burst effects by providing a secondary diffusional barrier around the particle phase156,157. HMP composites and bulk hydrogels are often exploited for their ability to trap cells in the hydrogel phase and drugs in the HMPs; HMPs loaded with growth factors have been used to direct the differentiation of stem cells encapsulated in the bulk hydrogel158,159. This makes it possible to create HMP composites containing distinct microscale and macroscale functionalities, with the growth-factor release controlled through HMP design, and the bulk hydrogel independently tunable to direct cell behaviour.
Applications of HMPs in drug delivery.
Due to their ease of injection and versatility, HMPs have been used in a wide variety of drug-delivery applications (FIG. 5). Many of the earliest applications were in the orthopaedic field, where HMP suspensions were used to provide sustained release of growth factors to promote bone and cartilage repair160,161. For example, genipin-cross-linked, gelatin-based microparticles, often made using batch emulsions, have been widely used to sustain the release of BMP-2 to promote bone repair162. Mixed particle populations have also shown promise for bone-repair applications. For example, the dual delivery of VEGF and BMP-2 using gelatin HMPs enhanced bone repair compared to the delivery of either factor alone163. In another example, oppositely charged chitosan particles formed using a batch emulsion were mixed to separately deliver osteoinductive and antibacterial factors in a sustained manner to promote bone regeneration164. The controlled delivery of chondroinductive factors, such as TGF-β1/3 and FGF-2 from gelatin particles, has been widely used for both cartilage-tissue engineering and cartilage-defect-repair applications165–167. HMP suspensions have also been explored for intraarticular delivery strategies: in a rat osteoarthritis model, degeneration of the articular surface was attenuated by delivering chitosan microparticles containing kartogenin (which can promote chondrogenesis) to the joint space168.
HMPs have also found utility in cardiac-repair applications, as they can be successfully delivered to the myocardium using minimally invasive, catheter-based strategies169. Following delivery, HMP suspensions provide sustained delivery of therapeutics to promote repair of the myocardium. For example, the sustained delivery of FGF from gelatin microspheres promoted angiogenesis and improved ventricular function in a model of myocardial infarction170,171 and polyethylene glycol/polybutylene tere-phthalate (PEG-PBT) HMPs loaded with VEGF demonstrated a therapeutic effect when delivered after myocardial infarction172. More recently, it was demonstrated that hydrolytically degradable, hyaluronic-acid HMPs (engineered using a microfluidic emulsion) loaded with interleukin-10 promoted cardiac repair following delivery through a shear-thinning supramolecular hydrogel173.
HMPs are commonly used for pulmonary-drug-delivery applications because they can be fabricated with sizes suitable for bronchial-airway delivery (~5 μm)174. Bulk hydrogels are not suitable for pulmonary delivery because of the risk of airway blockage. In an early experiment, alginate particles containing antitubercular drugs caused a nine-fold enhancement in drug bioavailability in the respiratory tract compared to bolus injection175. To facilitate sustained delivery to the lungs, therapeutics are often delivered via aerosols; aerodynamic particles with diameters of 0.5–5 μm are required for deep lung penetration176. Upon penetration into the airways, the dried drug-loaded particles swell and increase in size once they are deposited on the moist surfaces of the lung177. Final sizes of >5 μm are required to avoid premature uptake and clearance by alveolar macrophages174. Reduced clearance rates can also be achieved by using particles that are mucoadhesive to provide sustained therapeutic delivery within the respiratory tract178. PEG HMPs (made by batch emulsion) have been developed that degrade in response to proteases (such as MMP-2) that are overexpressed in a range of pulmonary diseases, such as lung cancer, tuberculosis and chronic obstructive pulmonary diseases179,180.
For many years, effective and reliable oral insulin-delivery strategies have been sought for the treatment of diabetes. Hydrogels have been widely explored because they can protect insulin from degradation during the passage through the stomach, and release it in the intestinal system for transfer into the blood-stream181. HMP suspensions are ideal for insulin delivery because they can be administered orally and their small size enables easy passage to the intestine, where they offer a substantial surface-area coverage following delivery. A large body of work has focused on developing pH-responsive particles that can protect insulin from degradation while passing through the low-pH environment of the stomach, and then release their cargo to the small intestine. where the pH is higher181. One example is poly(methacrylic acid) (PMAA), which contains free carboxylic-acid groups that undergo pH-dependent protonation and deprotonation that leads to swelling and deswelling182–184. To delay particle clearance, mucoadhesive polymers such as chitosan can be incorporated into the HMPs185–187. Glucose-responsive particles that can release insulin in response to hyperglycaemic conditions have also been developed82: chitosan HMPs containing insulin and nanocapsules loaded with glucose-specific enzymes were prepared using EHD spraying. Their swelling changed under hyperglycaemic conditions as a result of the enzymatic conversion of glucose to gluconic acid, which led to protonation.
HMPs are also used as platforms for delivering growth factors within cell aggregates and microtissues. For example, gelatin HMPs loaded with TGF-β3 were embedded within mesenchymal stem/stromal cell (MSC) microtissues. HMPs were mixed with the cell suspension and then centrifuged; following aggregation, the particles were evenly distributed throughout the microtissues and supported levels of chondrogenic differentiation comparable to those obtained by exogenous delivery of the growth factor in the culture media147,188,189. Similar approaches have been used to control morphogen presentation (BMP4/noggin) within pluripotent stem-cell aggregates, including cases in which spatial control of the HMPs within the microtissue influenced the spatial differentiation of cells190.
HMPs for building scaffolds
For nearly three decades, hydrogel scaffolds have been explored for numerous applications, including the repair and regeneration of tissues191,192. In many of these platforms for both in vitro and in vivo studies, cells are either embedded within the material during fabrication or seeded onto the scaffold after fabrication. Scaffolds must provide an environment that supports cell viability, enables desired cellular interactions (such as adhesion and remodelling), contains encapsulated soluble factors (such as growth factors, chemokines and cytokines) that control cellular outcomes and matches timescales relevant for tissue development and repair. Granular scaffolds are fabricated by packing HMPs until they reach a jammed state; as discussed, these systems are becoming popular because of their microporosity, injectability and tunability (FIG. 4). Granular scaffolds are inherently microporous, owing to the interstitial space among packed HMPs, which supports cellular invasion. By contrast, to allow for cell infiltration, bulk hydrogels must be engineered with features that permit material remodelling by the cells, which can lead to impaired mechanical properties over time. Granular scaffolds offer good potential for mechanical matching between tissue and scaffold, as well as long-lasting mechanical support for cells. This section explores the design of granular scaffolds and provides examples of their use.
Designing granular scaffolds.
As with drug-delivery systems, the design of granular scaffolds borrows many concepts originally developed for bulk-hydrogel systems3,193. Of particular interest for granular scaffolds are HMP annealing methods, the resulting HMP and bulk mechanical properties and the incorporation of adhesion and other bioactive signals (FIG. 7). Many granular scaffolds are made of HMPs annealed to each other to provide stabilization in the presence of excessively aqueous environments or mechanical agitation. Annealing granular scaffolds results in MAP scaffolds8. In wet, cutaneous wounds, annealing is often essential to limit inflammation and promote wound closure8. The annealing chemistry offers further control over the engineering of HMPs, and various covalent, reversible and electrostatic bonds have been explored (FIG. 7a). There are numerous cross-links per HMP, and, thus, increased cross-linking results in a more stable and rigid scaffold. Covalent bonds result in the most stable scaffolds, with more robust mechanical properties (such as storage and Young’s modulus) compared to scaffolds comprising non-annealed HMPs or HMPs annealed with non-covalent bonds. Covalent cross-linking has been achieved by a variety of chemistries, including enzymatic (FXIIIa) chemistry8,10,54, carboxylic/amine chemistry10,134, light-mediated radical reactions10,194, light-mediated thiol-ene reactions195, azide/alkyne click chemistry106 and norbornene-tetrazine cycloadditions15,98. An example of reversible bonds are host-guest interactions based on cyclodextrin-adamantine; for example, hyaluronic-acid HMPs (made using a microfluidic emulsion) were modified with adamantane and then bonded together with cyclodextrin-modified polymers9. The reversibility of these host-guest bonds causes the material to flow upon shear stress, allowing for its injectability, followed by self-healing, during which gel properties are recovered after the shear is removed. Similar shear-thinning and self-healing behaviours are observed when HMPs are annealed using electrostatic interactions between HMPs, which are particularly appealing due to their simplicity196.
Fig. 7 |. Design considerations for building scaffolds from hydrogel microparticles.
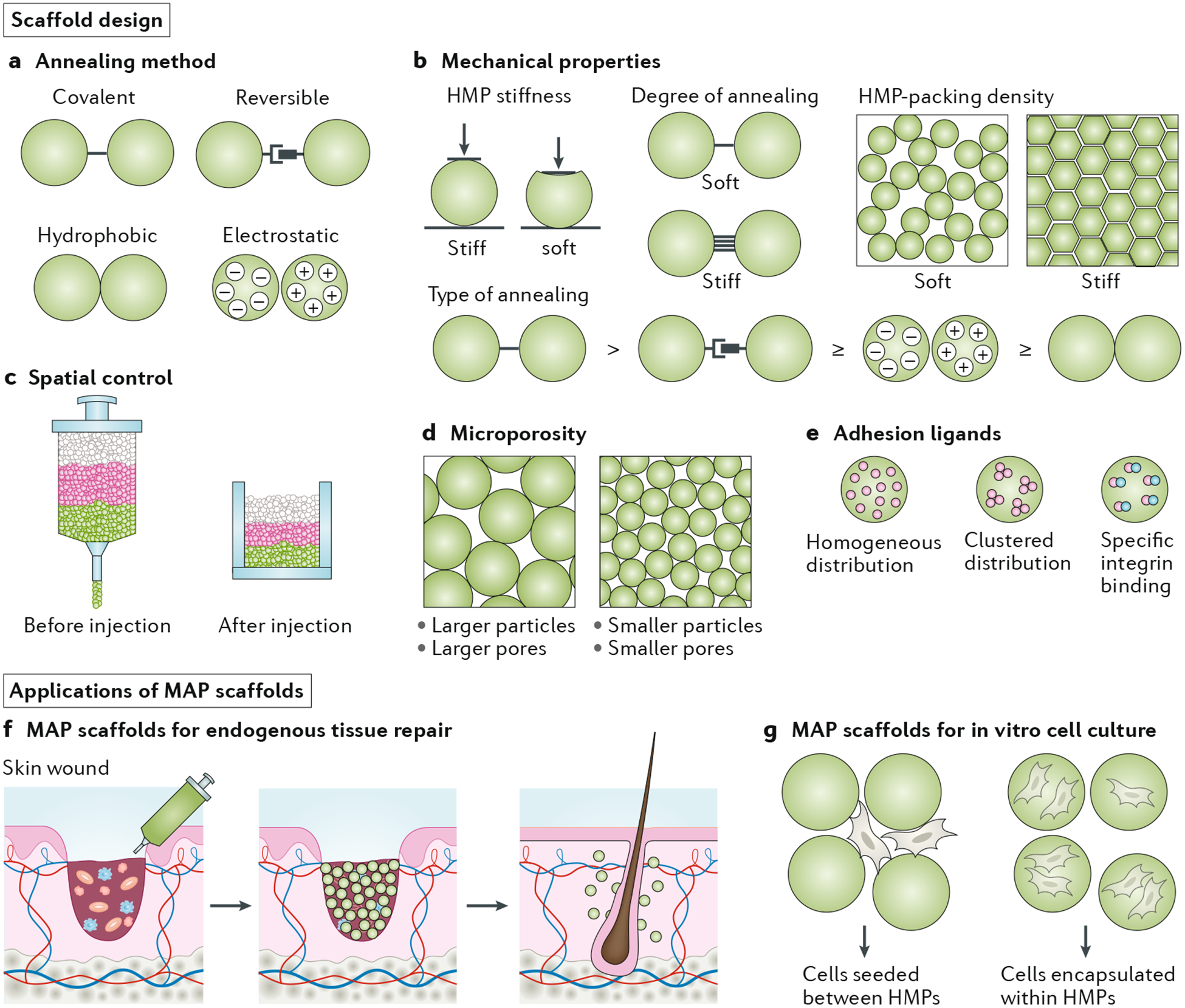
Scaffold design includes the engineering of: a | The annealing chemistry (which can be covalent, reversible, electrostatic or hydrophobic, resulting in various strengths of interactions) used to form microporous annealed particle (MAP) scaffolds. b | The mechanical properties, which are modulated by the stiffness of the individual hydrogel microparticles (HMPs), the degree of annealing (number of bonds between HMPs), the HMP-packing density and the chemistry of annealing (for example, covalent bonding is stronger than non-covalent bonding). c | Spatial control during injection. d | HMP size, which influences microporosity. e | Ligand modification for adhesion (distribution and type of ligand presentation). Applications of HMPs include: f | Cutaneous endogenous repair. g | Cell culture (intraparticle and interparticle culture platforms).
Mechanical properties are important bioactive cues for cells both in vitro and in vivo; thus, their modulation is of particular interest. The mechanical properties of MAP scaffolds can be modified by modulating the stiffness of individual HMPs, the chemistry of annealing, the amount of annealing between HMPs and the particle-packing density (FIG. 7b). The method of annealing can modify the bulk mechanics without influencing the local mechanics; thus, MAP scaffolds can present different mechanical cues at the local and bulk levels. Individual HMP stiffness can be tuned during fabrication by increasing the material concentration or degree of polymer cross-linking98,195; MAP scaffolds produced from HMPs with different stiffnesses influence cell behaviour differently: stiffer particles generally lead to increased cell spreading and proliferation54,98. The incorporation of soft-to-stiff gradients in PEG HMPs (produced by microfluidic emulsion) led to fibroblast migration towards the stiff end of the material54. By layering HMP species of varying stiffnesses in a syringe or catheter, material gradients may be produced upon injection, owing to the unique jamming properties of granular hydrogels (FIG. 7c). System-wide stiffness is further affected by the particle-packing density. Increasing particle-packing density generally leads to increased stiffness, but if HMPs are packed ‘beyond jamming’ so that they deform and lose interstitial space, they no longer function as a scaffold and, instead, exhibit characteristics similar to those of bulk hydrogels92. The HMP-packing density can be modulated by changing the particle-settling time before annealing10 or by removing liquid from the particle suspension via vacuum or centrifugation.
The local geometry of a cell’s microenvironment also plays a role in modulating cell behaviour197–199. In granular scaffolds, HMP surfaces and the void space among particles comprise the microarchitecture of the cellular environment. Therefore, the size and shape of HMPs, which instructs the porosity of the system, may influence cell behaviour (FIG. 7d). Seeding fibroblast cells on different MAP scaffolds comprising spherical HMPs (hyaluronic acid-PEG produced using a batch emulsion followed by sieving) that ranged in diameter from small (20–60 μm), medium (60–100 μm) and large (100–200 μm) highlighted differences in cell spreading and proliferation depending on HMP size. In the large-particle group, cells visibly wrapped around large particles and adopted a more flattened shape98. These results were attributed to the differences in pore sizes and HMP surface area available to cells. Similarly, seeding stromal cells in MAP scaffolds comprising HMPs with diameters of 10 μm or 100 μm led to increased spreading of cells in the scaffolds with larger HMPs106.
The engineering of integrin-binding cues within materials remains essential for many cellular responses in vitro and in vivo, such as angiogenesis200. Functionalizing HMP polymers with integrin-binding peptides, such as arginine-glycine-aspartic acid (RGD), has proven crucial to improve cell affinity for HMP surfaces. As in bulk hydrogel scaffolds, the concentration and spatial distribution of integrin-binding proteins within HMPs affects cellular responses (FIG. 7e). For example, increasing the concentration of RGD per HMP resulted in increased cell spreading and proliferation98.
As in 2D cultures201, the presentation of RGD is important; RGD islands spaced too far apart or excessively homogeneously distributed resulted in ineffective binding98. In addition to adhesion, the degradation of HMPs is also an important material feature to control, for example through the inclusion of cross-links that respond to local enzymes9. The mixing of particles with variable modes of degradation results in selective HMP degradation from a scaffold, which again highlights the importance of heterogeneity in the use of HMPs in biomedical applications.
Applications of granular scaffolds.
The use of granular HMP scaffolds in tissue repair has grown extensively in recent years. Most of the earliest works utilizing granular scaffolds were focused on bone and cartilage tissue engineering202–204. In one study, large chitosan hydrogel particles with diameters in the millimetre range were annealed to form a granular scaffold for osteoblast seeding204. Since this work, the applications of granular scaffolds have expanded; here, we present a number of applications in the context of in vivo wound healing, as well as ex vivo cell studies.
Granular scaffolds are promising for the promotion of wound healing in numerous tissues (FIG. 7f). The first use of MAP scaffolds was in a seminal study comparing them to a bulk hydrogel in a murine, cutaneous-wound-healing model8. PEG-based HMPs made using a microfluidic emulsion were injected into cutaneous-tissue defects and then covalently annealed in vivo using factor XIII-mediated K and Q peptide amide linkages. The presence of the MAP scaffold not only accelerated re-epithelialization but, five days post injection, the wound bed displayed hair follicles and sebaceous glands — structures that are characteristically not seen in scar tissue — which indicated a truly regenerative process. Such results could not be obtained with non-annealed HMP gels. These surprising findings led to several subsequent applications of MAP scaffolds. Hyaluronic-acid-based HMPs were designed to covalently anneal in vivo after being applied to stroke cavities induced in mice103. Within ten days from injection, the MAP scaffolds showed vascularization and neural progenitor cell migration towards the wound site, suggesting a tissue remodelling response. MAP scaffolds have also been engineered into modular implants to accommodate the long, narrow geometry of axon defects found in spinal-cord injuries205. PEG-based HMPs made using a batch emulsion were moulded around narrow cylinders and covalently annealed, after which the cylinder was removed to create a tubular scaffold containing a hollow lumen that provided directional guidance for growing axons. Stacked tubes implanted into spinal-cord defects in mice showed a reduction of glial scarring compared to a bulk hydrogel control, suggesting regenerative potential due to the scaffold microstructure.
Non-covalent, reversible annealing techniques have also been used for MAP materials in vivo. A self-healing cross-linking method involving host-guest interactions was developed to assemble hyaluronic-acid-based HMPs9. Heterogeneity was explored by experimenting with HMP species that differed in degradability and payload. Delivery to infarcted cardiac tissue in rats demonstrated a two-component response that resulted in successful interaction with inflammatory cells. Granular scaffolds have also been used in bone repair in more recent studies, in which packed heparin-modified gelatin HMPs produced using a batch emulsion were applied to rat mandibular-bone defects in a type 2 diabetes mellitus model206. The HMPs were loaded with IL-4 to polarize macrophages towards an anti-inflammatory phenotype, which successfully reduced the inflammation, enhancing osteogenesis and promoting a regenerative state. There is consensus among these studies that the microporosity of granular scaffolds plays a significant role in the improved cellular responses.
The microporosity of granular hydrogels, the ability of cells to spread without scaffold degradation and the tunability of HMPs makes granular scaffolds ideally suited as 3D culturing devices for studying cell behaviour in vitro. Cells can be seeded on top of granular hydrogels to capture information about cell infiltration or they can be mixed with the HMPs to observe their responses, such as spreading, proliferation and multicellular structure formation (FIG. 7g). Mesenchymal stem cells were loaded inside the HMPs of a MAP scaffold to study maturation and long-term maintenance of the cells within the scaffold. The results showed an upregulation of chondrogenic gene expression and the presence of hyaline-like cartilage tissue surrounding the gelatin-norbornene HMPs (made by microfluidic emulsion)134. MAP scaffolds formed using hyaluronic acid-PEG HMPs (made by batch emulsion) have also been studied as a framework for gene transfer because tunable changes in the MAP microenvironment influence cell behaviour, which, in turn, impacts transfection dynamics15.
The modular properties of granular systems have been leveraged to engineer scaffolds containing property gradients for improved tissue repair. For example, three forms of PEG HMPs with different functionalities were used to form scaffolds: the first provided mechanical support, the second provided controlled delivery of the angiogenesis-promoting molecule (sphingosine 1-phosphate) and the third acted as a porogen to impose pockets of macroporosity in the scaffold16. Gradients in scaffold porosity were produced by increasing the porogen fraction through the depth of the scaffold, which promoted endothelial cell migration into the construct. Using a similar approach, gradients of glial-cell-derived human neurotrophic factor were produced using heparinated PEG HMPs for nerve-regeneration applications207. Different cross-linking densities were used to tune the rate of growth-factor release within each region of the scaffold, which led to the formation of a growth-factor gradient. The graded scaffolds promoted axonal regeneration following implantation into severed sciatic nerves207,208.
Lastly, as a prelude to microtissue-like cell-transplant platforms, MAP scaffolds that utilize cells themselves to anneal HMPs have been developed209,210. In these applications, HMP surfaces are modified to accommodate cell adhesion. One approach also adopted a method called thermoswitching to influence the cell-release dynamics210.
HMPs for biofabrication
HMPs have also been used in biofabrication, where additive manufacturing is used to develop in vitro models or implantable constructs that mimic the complexity of native tissues211. Biofabrication processes can be categorized as either bioassembly, in which preformed building blocks are assembled into 3D constructs using an automated-assembly technology, or bioprinting, in which extrusion-based and lithography-based printing techniques are used to build 3D structures212. Some of the earliest examples of HMPs in biofabrication involved bioassembly, in which cell-encapsulating HMPs were used as building blocks in automated-assembly approaches (FIG. 8). In an early example, a fluidic-based bioassembly process was developed in which railed microfluidic channels were used to guide the movement of PEGDA HMPs into 3D constructs57 (FIG. 8a). External magnetic fields have also been leveraged to guide magnetic-nanoparticle-loaded GelMA and PEG HMPs into multilayered 3D structures213, and untethered, magnetically responsive microrobots were used to manipulate HMPs into 3D assemblies214 (FIG. 8b). Acoustic-wave technologies can also be used to manipulate HMPs to assemble 3D constructs215 (FIG. 8c).
Fig. 8 |. Hydrogel microparticles in biofabrication.
Examples of hydrogel microparticle (HMP) bioassembly approaches, which include: a | Railed microfluidic bioassembly, in which cell-containing poly(ethylene glycol) diacrylate HMPs are fluidically guided along complimentarily grooved microchannels57. b | Magnetically guided bioassembly of poly(ethylene glycol) diacrylate HMPs into 2D structures using untethered microrobots214. c | Acoustically guided bioassembly of complimentary shaped HMPs215. d | Extrusion printing of jammed-particle ink filaments prepared using hyaluronic-acid HMPs7. e | Ear-shaped and nose-shaped structures printed using jammed poly(ethylene glycol) HMPs217. f | Schematic illustrating the printing of a hydrogel ink into a HMP-based support medium, accompanied by an example of this approach, in which gelatin HMPs were used as a thermoreversible support bath in which the hydrogel precursor ink was deposited to form the letters CMU86. Panel a is adapted from reF.57, panel b from reF.214, panel c from reF.215, panel d from reF.7, panel e from reF.217, panel f from reF.86.
HMPs have also been used in the production of bioinks for extrusion-based bioprinting. Traditionally, extrudable bioinks are prepared using single-network or double-network hydrogels, and their biophysical and biochemical properties are kept homogeneous; however, heterogeneity may be desirable for printing complex tissues. To address this limitation, PEG HMPs (made using microfluidics) were combined with an extrudable bulk hydrogel precursor to engineer a composite bioink platform containing distinct microscale and macroscale environments216. The modular nature of the system was demonstrated by encapsulating MSCs in the PEG HMPs, which were then surrounded by a proangiogenic bulk hydrogel composed of fibrin loaded with endothelial cells. Further advances in this area should enable the development of a diverse set of multifunctional bioinks in which the local microenvironment can be tailored for distinct encapsulated cell types and/or bioactive factors.
More recently, it has been demonstrated that extrudable bioinks can be made from granular hydrogels7,217. When HMPs are packed closely together in a jammed state, they behave as a solid, but when external forces are applied, they display fluidic collective movement. This shear-thinning behaviour has been exploited for extrusion bioprinting because it enables extrusion through a needle and immediate post-printing stabilization7,217 (FIG. 8d,e). Inks for extrusion bioprinting have generally been engineered at the molecular scale, often leveraging reversible cross-linking chemistries. However, the shear-thinning behaviour of jammed HMPs is based predominately on the physical interactions between particles, which expands the range of materials that can be extruded7,217. Another advantage of HMP-based inks is their inherently modularity: multiple particle populations can be fabricated and then jammed together. Further, the HMPs themselves can be cross-linked to stabilize the final printed structure.
Traditional extrusion-bioprinting approaches involve layer-by-layer printing onto a 2D surface. However, because the printed structure is under the influence of gravity, it is challenging to print complex structures with high aspect ratios in the vertical direction. Gel-in-gel printing approaches have been developed in which inks are directly extruded into a secondary support hydrogel that serves to minimize the effect of gravity218 (FIG. 8f). This enables the printing of taller, more complex 3D structures. Jammed HMP systems are an ideal support for gel-in-gel printing because, during extrusion, the particles around the translating nozzle locally displace to support the ink and printed object219. The most commonly used support hydrogel for 3D printing is Carbopol, which is a commercial, particle-based hydrogel composed of poly(acrylic acid) HMPs (average diameter 0.2 μm). Carbopol-support hydrogels are compatible with a wide range of bioinks and can be used to print diverse, multicellular structures220. Support hydrogels for 3D printing have also been made using a granular gelatin HMP system prepared by fragmentation86.
Other applications of HMPs
HMP suspensions and granular hydrogels have been utilized in applications that extend beyond those previously discussed. For decades, hydrogels (in particular, hyaluronic acid) have been used in the cosmetic industry as dermal fillers for soft-tissue augmentation owing to their injectability, swelling and consistency that is similar to that of human cutaneous tissue221,222. Hydrogel fillers are commonly manufactured by forcing a bulk gel through a fine screen (mechanical fragmentation). The resulting HMPs are then either mixed with additional polymer solution to reduce interparticle friction for smoother injection or left as packed particles. Smaller particles make for better superficial fillers that target wrinkles or scar divots, whereas larger particles are used for deep-tissue volumizers221. Products on the market vary in terms of formulation, manufacturing techniques, consistency, degradation times, intended usage and cost.
Owing to their micrometre size scale and tunable units, HMPs have also proved useful as microtopo-graphical cues for cells. HMPs delivered to damaged tissue serve as local anchor points for cell adhesion and contact guidance that help to restore the mechanical properties of the ECM. Microrod suspensions (100 μm) made from hyaluronic acid were fabricated for this purpose using a lithographical process and were delivered to fibrotic cardiac tissue in rats105. Previous work showed that a minimal elastic modulus of 20 kPa was necessary for microrod HMPs to elicit fibroblast cell responses, indicating the importance of material stiffness for topographical applications. The hyaluronic-acid HMPs stimulated attachment by local cells, discouraged the pro-scarring myofibroblast phenotype and resulted in improved organ function105. In an attempt to target the linear, directional aspect of nerve cells, an HMP composite material was developed containing PEG acrylate microrod HMPs (made by lithography) that were laced with small amounts of superparamagnetic nanoparticles, which allowed for HMP alignment in an external magnetic field223. The microrod HMPs could align fibroblasts and unidirectionally guide nerve cells in vitro, demonstrating the efficacy of targeting contact guidance to influence cell behaviour. Much work has also been done to study how HMPs can be used as microstructures that offer environmental heterogeneity in terms of stiffness and topography both in vitro and in vivo224.
Inspired by synthetic-cell biology, HMPs have recently been engineered to contain multiple compartments that mimic distinct organelles within a cell. For example, microfluidics was used to fabricate PEG-based HMPs containing two compartments225. Each compartment was designated for a single chemical reaction and the product from the first compartment was used as reactant for the second. Reactions in each compartment were pH-specific to demonstrate effective compartmen-talization. The elegantly designed synthetic cells were highly successful at performing their task and showcased the potential for new, ‘smart’ HMP technologies.
Future perspectives
We have covered the main classes of HMP systems, the fabrication techniques to manufacture them, their properties across length scales and a number of their biomedical applications. Overall, their injectability, modular design and porosity constitute clear advantages over traditional bulk hydrogels in many applications. Advances are expected across all areas discussed, particularly as new fabrication methods make diverse HMPs accessible to a broad user base and as new reports generate further enthusiasm for their use.
Regarding HMPs fabrication, microfluidic technologies and other techniques will continue to improve, allowing for the scaling up of HMP fabrication with uniform particle populations and batch-to-batch consistency at high production rates. Recent years have already seen a scaling up of microfluidic platforms with these goals in mind33,226, and additional advances will likely allow the fabrication of heterogeneous HMP systems on a single chip by controlling hydrogel precursor flows and mixing. With these advances plus improved material consistency, the characterization of HMP-based biomaterials (based on numerous microscopy and rheological methods) will also improve, including better visualization of individual HMPs and their interactions under mechanical load. Broadening the classes of materials used to make HMPs will expand the material properties of HMP systems, for example introducing stimuli-responsive materials that change under various endogenous and exogenous signals.
To better design and understand HMP systems, computational models and numerical approaches will be useful. For example, multiscale models have been developed that use the discrete element method to capture behaviour that follows Newtonian mechanics at the microscopic level (for granular and composite systems) and then either the finite difference method or elasto-plasticity theory to model behaviour that follows continuum mechanics at the macroscopic level227,228, where, ultimately, first-order homogenization and kinematic averaging are used to couple the two. Such computational approaches offer a deeper understanding for how granular systems respond to mechanical loading, and many more approaches have and can be used to aid in predicting bulk material properties and behaviours, as well as local internal, featural structures. Computational models and numerical simulations will be invaluable in both expediting experimental design and analysing fabricated materials.
Beyond the fabrication and understanding of HMP systems, the applications of these materials will likely broaden as more investigators recognize their advantages. Already, HMP systems have been inhaled, ingested, injected into intraarticular space and injected into tissues (such as dermal, neural and muscle tissues). Additionally, with rapid advances in stem-cell and drug-design technologies (such as the discovery of induced pluripotent stem cells and CRISPR), the options for HMP cargo will continue to grow. A major concern with the use of the CRISPR technology is off-target effects, so approaches that enable precise activation within targeted tissues are desirable229. It is possible that the versatility and modularity of HMP systems will provide a platform for controlling and localizing the activity of CRISPR-Cas9 following delivery in vivo. As discussed, the potential complexity of mixing many particle populations has only been briefly investigated, despite their immense potential for delivering sequential signals to tissues and cells.
HMP systems are also likely to be developed with new and interesting properties. The properties of native tissues (such as bone and cartilage) are often the result of interactions between multiple tissue components across multiple length scales. Granular systems offer a modular platform for recreating physical and chemical properties that can be independently controlled across these length scales, which include the nanoscale (hydrogel mesh size), microscale (interparticle interactions) and macroscale (particle-packing fraction). It has already been demonstrated that interparticle motion can induce shear-thinning behaviour, and that static8 and dynamic85 interparticle cross-links can be used to alter particle rearrangements under load. Further advances in this area will likely lead to the creation of a novel class of materials that better mimic the multiscale mechanical behaviour of native tissues.
Recent technological advances have enabled the analysis of complex biological systems at the single-cell level. Microfluidic technologies are being used for cell separation during single-cell sequencing, and it was demonstrated that single cells can be encapsulated in thin and tunable HMPs using microfluidics112. This area is likely to continue advancing, and this technology opens up numerous avenues for biological exploration. For example, the screening of biological cues (stiffness, viscoelasticity or drug concentration) is typically only explored across a small range of parameters and is usually based on conventional bulk assays that mask cell-to-cell variability. The integration of HMPs and sequencing technologies could enable high-throughput screening at the single-cell level to understand responses to multiple biophysical environments or drugs, as well as the interplay between cells in these environments.
We have only scratched the surface in terms of discussing the use of HMPs for scaffold building in vitro and in vivo. Besides performing more experiments to understand how the fine-tuning of physical and biochemical properties (such as mixing different HMP formulations) affects cells within MAP scaffolds, there are opportunities to explore features of MAP scaffolds that are not present in bulk hydrogels. For example, the local-void-space geometry within MAP systems has been minimally studied and almost exclusively in the context of spherical HMPs of different sizes. It would be interesting to more rigorously analyse local geometry formed from non-spherical or non-convex HMP shapes to gain more general knowledge about the role of void-space geometry on cell behaviour, multicellular arrangements and endogenous repair. Compared to bulk systems, the versatility of HMP systems makes them appealing as platforms to target endogenous repair processes that are spatially and temporally complex. The ability to form multiple microenvironments within a MAP scaffold by combining fractions of HMPs with different properties (such as stiffness or loading with bioactive cues) can give rise to superior organoid culture systems and endogenous repair through the generation of spatiotemporal microgradients.
Lastly, the use of HMPs for biofabrication is still in its infancy, with jammed HMP systems leveraged for extrusion bioprinting7 and individual HMPs having been patterned using a range of bioassembly technologies213,215. The shear-thinning behaviour of jammed HMPs is based on the physical interactions between the particles; thus, the range of materials that can be processed with extrusion printing will likely expand. In addition, the modularity of jammed systems will probably enable the preparation of more diverse inks with multifunctional behaviours. Acoustic, robotic and magnetic guidance of HMPs have only been explored in relatively 2D systems within the bioassembly field; further advances in this area will enable high-resolution positioning within 3D systems. Overall, there are likely important developments ahead in the field of HMPs for biomedical applications.
Acknowledgements
J.A.B. acknowledges funding through the National Science Foundation through the PENN MRSEC (DMR-1720530) and STC Program (CMMI: 15-48571). T.S. acknowledges funding from the National Institutes of Health (R01NS094599) and Duke Biomedical Engineering. The authors would like to thank their laboratories for the helpful input and suggestions on the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caliari SR & Burdick JA A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Vlierberghe S, Dubruel P & Schacht E Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12, 1387–1408 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Li J & Mooney DJ Designing hydrogels for controlled drug delivery. Nat. Rev. Mater 1, 16071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annabi N et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev 16, 371–383 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson TMA, Ladewig K, Haylock DN, McLean KM & O’Connor AJ Cryogels for biomedical applications. J. Mater. Chem. B 1, 2682–2695 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Wade RJ, Bassin EJ, Rodell CB & Burdick JA Protease-degradable electrospun fibrous hydrogels. Nat. Commun 6, 6639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Highley CB, Song KH, Daly AC & Burdick JA Jammed microgel inks for 3D printing applications. Adv. Sci 6, 1801076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D & Segura T Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater 14, 737–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mealy JE et al. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater 30, 1705912 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Sideris E et al. Particle hydrogels based on hyaluronic acid building blocks. ACS Biomater. Sci. Eng 2, 2034–2041 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Franco CL, Price J & West JL Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomater 7, 3267–3276 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Leong W, Lau TT & Wang DA A temperature-cured dissolvable gelatin microsphere-based cell carrier for chondrocyte delivery in a hydrogel scaffolding system. Acta Biomater 9, 6459–6467 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Liu AL & Garcia AJ Methods for generating hydrogel particles for protein delivery. Ann. Biomed. Eng 44, 1946–1958 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q et al. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small 5, 1575–1581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truong NF, Lesher-Pérez SC, Kurt E & Segura T Pathways governing polyethylenimine polyplex transfection in microporous annealed particle scaffolds. Bioconjug. Chem 30, 476–486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott EA, Nichols MD, Kuntz-Willits R & Elbert DL Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater 6, 29–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenekes RJH, Franssen O, van Bommel EMG, Crommelin DJA & Hennink WE The preparation of dextran microspheres in an all-aqueous system: effect of the formulation parameters on particle characteristics. Pharm. Res 15, 557–561 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Elbert DL Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: a tutorial review. Acta Biomater 7, 31–56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols MD, Scott EA & Elbert DL Factors affecting size and swelling of poly(ethylene glycol) microspheres formed in aqueous sodium sulfate solutions without surfactants. Biomaterials 30, 5283–5291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon O, Wolfson DW & Alsberg E In-situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv. Mater 27, 2216–2223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorsen T, Roberts RW, Arnold FH & Quake SR Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett 86, 4163–4166 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Anna SL, Bontoux N & Stone HA Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett 82, 364–366 (2003). [Google Scholar]
- 23.De Geest BG, Urbanski JP, Thorsen T, Demeester J & De Smedt SC Synthesis of monodisperse biodegradable microgels in microfluidic devices. Langmuir 21, 10275–10279 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Pittermannová A et al. Microfluidic fabrication of composite hydrogel microparticles in the size range of blood cells. RSC Adv 6, 103532–103540 (2016). [Google Scholar]
- 25.Utada AS et al. Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Nisisako T & Torii T Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 8, 287–293 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Kim J-W, Utada AS, Fernández-Nieves A, Hu Z & Weitz DA Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem 119, 1851–1854 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Greenwood-Goodwin M, Teasley ES & Heilshorn SC Dual-stage growth factor release within 3D protein-engineered hydrogel niches promotes adipogenesis. Biomater. Sci 2, 1627–1639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster GA et al. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 113, 170–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deveza L et al. Microfluidic synthesis of biodegradable polyethylene-glycol microspheres for controlled delivery of proteins and DNA nanoparticles. ACS Biomater. Sci. Eng 1, 157–165 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Li M, Chen Z & Leong KW Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 16, 4482–4506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selimović Š, Oh J, Bae H, Dokmeci M & Khademhosseini A Microscale strategies for generating cell-encapsulating hydrogels. Polymers 4, 1554–1579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Headen DM, García JR & García AJ Parallel droplet microfluidics for high throughput cell encapsulation and synthetic microgel generation. Microsyst. Nanoeng 4, 17076 (2018). [Google Scholar]
- 34.Koster S et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 8, 1110–1115 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Cha C et al. Microfluidics-assisted fabrication of gelatin-silica core–shell microgels for injectable tissue constructs. Biomacromolecules 15, 283–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Z, Xia B, McBride R & Oakey J A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres. J. Mater. Chem . B 5, 173–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumachev A, Tumarkin E, Walker GC & Kumacheva E Characterization of the mechanical properties of microgels acting as cellular microenvironments. Soft Matter 9, 2959–2965 (2013). [Google Scholar]
- 38.Headen DM, Aubry G, Lu H & Garcia AJ Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater 26, 3003–3008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allazetta S, Hausherr TC & Lutolf MP Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules 14, 1122–1131 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Seiffert S, Thiele J, Abate AR & Weitz DA Smart microgel capsules from macromolecular precursors. J. Am. Chem. Soc 132, 6606–6609 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Seiffert S & Weitz DA Microfluidic fabrication of smart microgels from macromolecular precursors. Polymer 51, 5883–5889 (2010). [Google Scholar]
- 42.Chu LY, Utada AS, Shah RK, Kim JW & Weitz DA Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed 46, 8970–8974 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Seiffert S, Romanowsky MB & Weitz DA Janus microgels produced from functional precursor polymers. Langmuir 26, 14842–14847 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Zhao CX Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliv. Rev 65, 1420–1446 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Duncanson WJ et al. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip 12, 2135–2145 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Chen Q et al. Controlled assembly of heterotypic cells in a core–shell scaffold: organ in a droplet. Lab Chip 16, 1346–1349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L et al. Microfluidic templated multicompartment microgels for 3D encapsulation and pairing of single cells. Small 14, 1702955 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Yoshida S, Takinoue M & Onoe H Compartmentalized spherical collagen microparticles for anisotropic cell culture microenvironments. Adv. Healthc. Mater 6, 1601463 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Kamperman T, Trikalitis VD, Karperien M, Visser CW & Leijten J Ultrahigh-throughput production of monodisperse and multifunctional Janus microparticles using in-air microfluidics. ACS Appl. Mater. Interfaces 10, 23433–23438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser CW, Kamperman T, Karbaat LP, Lohse D & Karperien M In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Science Adv 4, eaao1175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardin D, Kendall MR, Dayton PA & Lee AP Parallel generation of uniform fine droplets at hundreds of kilohertz in a flow-focusing module. Biomicrofluidics 7, 034112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muluneh M & Issadore D Hybrid soft-lithography/laser machined microchips for the parallel generation of droplets. Lab Chip 13, 4750–4754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Greener J, Voicu D & Kumacheva E Multiple modular microfluidic (M3) reactors for the synthesis of polymer particles. Lab Chip 9, 2715–2721 (2009). [DOI] [PubMed] [Google Scholar]
- 54.de Rutte JM, Koh J & Di Carlo D Scalable high-throughput production of modular microgels for in situ assembly of microporous tissue scaffolds. Adv. Funct. Mater 29, 1900071. [Google Scholar]
- 55.Helgeson ME, Chapin SC & Doyle PS Hydrogel microparticles from lithographic processes: novel materials for fundamental and applied colloid science. Curr. Opin. Colloid Interface Sci 16, 106–117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SA, Chung SE, Park W, Lee SH & Kwon S Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography. Lab Chip 9, 1670–1675 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Chung SE, Park W, Shin S, Lee SA & Kwon S Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat. Mater 7, 581–587 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Nichol JW et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panda P et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 8, 1056–1061 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang J-H, Dendukuri D, Hatton TA, Thomas EL & Doyle PS A route to three-dimensional structures in a microfluidic device: stop-flow interference lithography. Angew. Chem. Int. Ed 119, 9185–9189 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Dendukuri D, Pregibon DC, Collins J, Hatton TA & Doyle PS Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater 5, 365–369 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Rolland JP et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc 127, 10096–10100 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Nielson R, Kaehr B & Shear JB Microreplication and design of biological architectures using dynamic-mask multiphoton lithography. Small 5, 120–125 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Laza SC et al. Two-photon continuous flow lithography. Adv. Mater 24, 1304–1308 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Dendukuri D, Gu SS, Pregibon DC, Hatton TA & Doyle PS Stop-flow lithography in a microfluidic device. Lab Chip 7, 818–828 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Merkel TJ et al. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release 162, 37–44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khademhosseini A et al. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J. Biomed. Mater. Res. A 79, 522–532 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Baudis S et al. Modular material system for the microfabrication of biocompatible hydrogels based on thiol–ene-modified poly(vinyl alcohol). J. Polym. Sci. Part A Polym. Chem 54, 2060–2070 (2016). [Google Scholar]
- 69.Qin X-H et al. Enzymatic synthesis of hyaluronic acid vinyl esters for two-photon microfabrication of biocompatible and biodegradable hydrogel constructs. Polym. Chem 5, 6523–6533 (2014). [Google Scholar]
- 70.Gramlich WM, Kim IL & Burdick JA Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 34, 9803–9811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ifkovits JL & Burdick JA Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng 13, 2369–2385 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Nguyen KT & West JL Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 23, 4307–4314 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Bahney CS et al. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cell Mater 22, 43–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bryant SJ, Nuttelman CR & Anseth KS Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed 11, 439–457 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Le Goff GC, Lee J, Gupta A, Hill WA & Doyle PS High-throughput contact flow lithography. Adv. Sci 2, 1500149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naqvi SM et al. Living cell factories - electrosprayed microcapsules and microcarriers for minimally invasive delivery. Adv. Mater 28, 5662–5671 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Gansau J, Kelly L & Buckley CT Influence of key processing parameters and seeding density effects of microencapsulated chondrocytes fabricated using electrohydrodynamic spraying. Biofabrication 10, 035011 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Pancholi K, Ahras N, Stride E & Edirisinghe M Novel electrohydrodynamic preparation of porous chitosan particles for drug delivery. J. Mater. Sci. Mater. Med 20, 917–923 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Qayyum AS et al. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 9, 025019 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Young CJ, Poole-Warren LA & Martens PJ Combining submerged electrospray and UV photopolymerization for production of synthetic hydrogel microspheres for cell encapsulation. Biotechnol. Bioeng 109, 1561–1570 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Kim PH et al. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 187, 1–13 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Gu Z et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 7, 6758–6766 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Jayasinghe SN & Townsend-Nicholson A Stable electric-field driven cone-jetting of concentrated biosuspensions. Lab Chip 6, 1086–1090 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Jayasinghe SN, Qureshi AN & Eagles PA Electrohydrodynamic jet processing: an advanced electric-field-driven jetting phenomenon for processing living cells. Small 2, 216–219 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Sinclair A et al. Self-healing zwitterionic microgels as a versatile platform for malleable cell constructs and injectable therapies. Adv. Mater 30, 1803087 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hinton TJ et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Adv 1, e1500758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riley L, Schirmer L & Segura T Granular hydrogels: emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol 60, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Behringer RP & Chakraborty B The physics of jamming for granular materials: a review. Rep. Prog. Phys 82, 012601 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Hurley RC, Hall SA, Andrade JE & Wright J Quantifying interparticle forces and heterogeneity in 3D granular materials. Phys. Rev. Lett 117, 098005 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Weeks ER in Statistical physics of complex fluids (eds Maruyama S & Tokuyama M) 1–53 (Tohoku University Press, 2007). [Google Scholar]
- 91.Torquato S & Stillinger FH Jammed hard-particle packings: From Kepler to Bernal and beyond. Rev. Mod. Phys 82, 2633–2672 (2010). [Google Scholar]
- 92.Menut P, Seiffert S, Sprakel J & Weitz DA Does size matter? Elasticity of compressed suspensions of colloidal- and granular-scale microgels. Soft Matter 8, 156–164 (2012). [Google Scholar]
- 93.Liu AJ & Nagel SR The jamming transition and the marginally jammed solid. Annu. Rev. Condens. Matter Phys 1, 347–369 (2010). [Google Scholar]
- 94.van Hecke M Jamming of soft particles: geometry, mechanics, scaling and isostaticity. J. Phys. Condens. Matter 22, 033101 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Yuan Y, Liu L, Zhuang Y, Jin W & Li S Coupling effects of particle size and shape on improving the density of disordered polydisperse packings. Phys. Rev. E 98, 042903 (2018). [Google Scholar]
- 96.Haustein M, Gladkyy A & Schwarze R Discrete element modeling of deformable particles in YADE. SoftwareX 6, 118–123 (2017). [Google Scholar]
- 97.Sun Q, Jin F, Liu J & Zhang G Understanding force chains in dense granular materials. Int. J. Mod. Phys. B 24, 5743–5759 (2010). [Google Scholar]
- 98.Truong NF et al. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater 94, 160–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J, Yaszemski MJ & Lu L Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens. Tissue Eng. Part C Methods 15, 583–594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, Lu S, Lam J, Kasper FK & Mikos AG Fabrication of cell-laden macroporous biodegradable hydrogels with tunable porosities and pore sizes. Tissue Eng. Part C Methods 21, 263–273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J et al. Microgel-reinforced hydrogel films with high mechanical strength and their visible mesoscale fracture structure. Macromolecules 44, 7775–7781 (2011). [Google Scholar]
- 102.Shin H, Olsen BD & Khademhosseini A Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J. Mater. Chem. B 2, 2508–2516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nih LR, Sideris E, Carmichael ST & Segura T Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater 29, 1606471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Darling NJ, Sideris E, Hamada N, Carmichael ST & Segura T Injectable and spatially patterned microporous annealed particle (MAP) hydrogels for tissue repair applications. Adv. Sci 5, 1801046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le LV et al. Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials 169, 11–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caldwell AS, Campbell GT, Shekiro KMT & Anseth KS Clickable microgel scaffolds as platforms for 3D cell encapsulation. Adv. Healthc. Mater 6, 1700254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madl CM, Heilshorn SC & Blau HM Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seliktar D Designing cell-compatible hydrogels for biomedical applications. Science 336, 1124–1128 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Wang C, Varshney RR & Wang D-A Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Deliv. Rev 62, 699–710 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Rosales AM & Anseth KS The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater 1, 15012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prince E & Kumacheva E Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater 4, 99–115 (2019). [Google Scholar]
- 112.Mao AS et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat. Mater 16, 236–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daly AC, Sathy BN & Kelly DJ Engineering large cartilage tissues using dynamic bioreactor culture at defined oxygen conditions. J. Tissue Eng 9, 2041731417753718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheehy EJ, Buckley CT & Kelly DJ Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J. Tissue Eng. Regen. Med 5, 747–758 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Daly AC & Kelly DJ Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 197, 194–206 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Kolesky DB, Homan KA, Skylar-Scott MA & Lewis JA Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 113, 3179–3184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Madl CM & Heilshorn SC Engineering hydrogel microenvironments to recapitulate the stem cell niche. Annu. Rev. Biomed. Eng 20, 21–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rapp TL, Highley CB, Manor BC, Burdick JA & Dmochowski IJ Ruthenium-crosslinked hydrogels with rapid, visible-light degradation. Chem 24, 2328–2333 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohamed MGA et al. An integrated microfluidic flow-focusing platform for on-chip fabrication and filtration of cell-laden microgels. Lab Chip 19, 1621–1632 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Deng Y et al. Rapid purification of cell encapsulated hydrogel beads from oil phase to aqueous phase in a microfluidic device. Lab Chip 11, 4117–4121 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Choi CH et al. One-step generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell. Lab Chip 16, 1549–1555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu K et al. All-aqueous-phase microfluidics for cell encapsulation. ACS Appl. Mater. Interfaces 11, 4826–4832 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Maeda K, Onoe H, Takinoue M & Takeuchi S Controlled synthesis of 3D multi-compartmental particles with centrifuge-based microdroplet formation from a multi-barrelled capillary. Adv. Mater 24, 1340–1346 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Ma C, Tian C, Zhao L & Wang J Pneumatic-aided micro-molding for flexible fabrication of homogeneous and heterogeneous cell-laden microgels. Lab Chip 16, 2609–2617 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Allazetta S, Kolb L, Zerbib S, Bardy J & Lutolf MP Cell-instructive microgels with tailor-made physicochemical properties. Small 11, 5647–5656 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Blaeser A et al. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater 5, 326–333 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Chen MH et al. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng 3, 3146–3160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aguado BA, Mulyasasmita W, Su J, Lampe KJ & Heilshorn SC Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao X et al. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater 26, 2809–2819 (2016). [Google Scholar]
- 130.Annamalai RT et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials 208, 32–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wise JK, Alford AI, Goldstein SA & Stegemann JP Synergistic enhancement of ectopic bone formation by supplementation of freshly isolated marrow cells with purified MSC in collagen–chitosan hydrogel microbeads. Connect. Tissue Res 57, 516–525 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang L, Rao RR & Stegemann JP Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs 197, 333–343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daley ELH, Coleman RM & Stegemann JP Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy. J. Mater. Chem. B 3, 7920–7929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li F et al. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater 77, 48–62 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Yin H et al. Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration. Acta Biomater 77, 127–141 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Wang Y et al. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 171, 118–132 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Feyen DAM et al. Gelatin microspheres as vehicle for cardiac progenitor cells delivery to the myocardium. Adv. Healthc. Mater 5, 1071–1079 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Shrestha P, Regmi S & Jeong J-H Injectable hydrogels for islet transplantation: a concise review. Int. J. Pharm. Investig 1–17 (2019). [Google Scholar]
- 139.Veiseh O et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater 14, 643–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Headen DM et al. Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat. Mater 17, 732–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoare TR & Kohane DS Hydrogels in drug delivery: Progress and challenges. Polymer 49, 1993–2007 (2008). [Google Scholar]
- 142.Guvendiren M, Lu HD & Burdick JA Shear-thinning hydrogels for biomedical applications. Soft Matter 8, 260–272 (2012). [Google Scholar]
- 143.Dimatteo R, Darling NJ & Segura T In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev 127, 167–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen W, Palazzo A, Hennink WE & Kok RJ Effect of particle size on drug loading and release kinetics of gefitinib-loaded PLGA microspheres. Mol. Pharm 14, 459–467 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Freiberg S & Zhu XX Polymer microspheres for controlled drug release. Int. J. Pharm 282, 1–18 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Nguyen AH, McKinney J, Miller T, Bongiorno T & McDevitt TC Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater 13, 101–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Solorio LD, Dhami CD, Dang PN, Vieregge EL & Alsberg E Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-β1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Transl. Med 1, 632–639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Censi R, Di Martino P, Vermonden T & Hennink WE Hydrogels for protein delivery in tissue engineering. J. Control. Release 161, 680–692 (2012). [DOI] [PubMed] [Google Scholar]
- 149.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE & McDevitt TC Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials 35, 7228–7238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng Q et al. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater 53, 329–342 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Öztürk E et al. Sulfated hydrogel matrices direct mitogenicity and maintenance of chondrocyte phenotype through activation of FGF signaling. Adv. Funct. Mater 26, 3649–3662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Freeman I, Kedem A & Cohen S The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 29, 3260–3268 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Buket Basmanav F, Kose GT & Hasirci V Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials 29, 4195–4204 (2008). [DOI] [PubMed] [Google Scholar]
- 154.Jaklenec A et al. Sequential release of bioactive IGF-I and TGF-β1 from PLGA microsphere-based scaffolds. Biomaterials 29, 1518–1525 (2008). [DOI] [PubMed] [Google Scholar]
- 155.Wang Y, Cooke MJ, Sachewsky N, Morshead CM & Shoichet MS Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J. Control. Release 172, 1–11 (2013). [DOI] [PubMed] [Google Scholar]
- 156.McGillicuddy FC et al. Novel “plum pudding” gels as potential drug-eluting stent coatings: controlled release of fluvastatin. J. Biomed. Mater. Res. A 79, 923–933 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Sivakumaran D, Maitland D & Hoare T Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 12, 4112–4120 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Almeida HV et al. Controlled release of transforming growth factor-β3 from cartilage-extra-cellular-matrix-derived scaffolds to promote chondrogenesis of human-joint-tissue-derived stem cells. Acta Biomater 10, 4400–4409 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Bian L et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425–6434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patel ZS, Yamamoto M, Ueda H, Tabata Y & Mikos AG Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater 4, 1126–1138 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kavanaugh TE, Werfel TA, Cho H, Hasty KA & Duvall CL Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv. Transl. Res 6, 132–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li M, Liu X, Liu X & Ge B Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin. Orthop. Relat. Res 468, 1978–1985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patel ZS et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai B et al. Injectable gel constructs with regenerative and anti-infective dual effects based on assembled chitosan microspheres. ACS Appl. Mater. Interfaces 10, 25099–25112 (2018). [DOI] [PubMed] [Google Scholar]
- 165.DeFail AJ, Chu CR, Izzo N & Marra KG Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials 27, 1579–1585 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Holland TA, Tabata Y & Mikos AG Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 101, 111–125 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Park H, Temenoff JS, Holland TA, Tabata Y & Mikos AG Delivery of TGF-β1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 26, 7095–7103 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Kang ML, Ko J-Y, Kim JE & Im G-I Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35, 9984–9994 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Hoshino K et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther 13, 1320–1327 (2006). [DOI] [PubMed] [Google Scholar]
- 170.Iwakura A et al. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels 18, 93–99 (2003). [DOI] [PubMed] [Google Scholar]
- 171.Liu Y, Sun L, Huan Y, Zhao H & Deng J Effects of basic fibroblast growth factor microspheres on angiogenesis in ischemic myocardium and cardiac function: analysis with dobutamine cardiovascular magnetic resonance tagging. Eur. J. Cardiothorac. Surg 30, 103–107 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Uitterdijk A et al. VEGF165A microsphere therapy for myocardial infarction suppresses acute cytokine release and increases microvascular density but does not improve cardiac function. Am. J. Physiol. Heart Circ. Physiol 309, H396–H406 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Chen MH et al. Injectable supramolecular hydrogel/microgel composites for therapeutic delivery. Macromol. Biosci 19, e1800248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Du J, Du P & Smyth HD Hydrogels for controlled pulmonary delivery. Ther. Deliv 4, 1293–1305 (2013). [DOI] [PubMed] [Google Scholar]
- 175.ul-Ain Qurrat, Sharma S, Khuller GK & Garg SK Alginate-based oral drug delivery system for tuberculosis: pharmacokinetics and therapeutic effects. J. Antimicrob. Chemother 51, 931–938 (2003). [DOI] [PubMed] [Google Scholar]
- 176.Selvam P, El-Sherbiny IM & Smyth HD Swellable hydrogel particles for controlled release pulmonary administration using propellant-driven metered dose inhalers. J. Aerosol. Med. Pulm. Drug Deliv 24, 25–34 (2011). [DOI] [PubMed] [Google Scholar]
- 177.El-Sherbiny IM, McGill S & Smyth HD Swellable microparticles as carriers for sustained pulmonary drug delivery. J. Pharm. Sci 99, 2343–2356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hwang SM, Kim DD, Chung SJ & Shim CK Delivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosis. J. Control. Release 129, 100–106 (2008). [DOI] [PubMed] [Google Scholar]
- 179.Secret E, Crannell KE, Kelly SJ, Villancio-Wolter M & Andrew JS Matrix metalloproteinase-sensitive hydrogel microparticles for pulmonary drug delivery of small molecule drugs or proteins. J. Mater. Chem. B 3, 5629–5634 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Secret E, Kelly SJ, Crannell KE & Andrew JS Enzyme-responsive hydrogel microparticles for pulmonary drug delivery. ACS Appl. Mater. Interfaces 6, 10313–10321 (2014). [DOI] [PubMed] [Google Scholar]
- 181.Chaturvedi K, Ganguly K, Nadagouda MN & Aminabhavi TM Polymeric hydrogels for oral insulin delivery. J. Control. Release 165, 129–138 (2013). [DOI] [PubMed] [Google Scholar]
- 182.Bell CL & Peppas NA Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-g-ethylene glycol). Biomaterials 17, 1203–1218 (1996). [DOI] [PubMed] [Google Scholar]
- 183.Mundargi RC, Rangaswamy V & Aminabhavi TM Poly(N-vinylcaprolactam-co-methacrylic acid) hydrogel microparticles for oral insulin delivery. J. Microencapsul 28, 384–394 (2011). [DOI] [PubMed] [Google Scholar]
- 184.Sajeesh S, Bouchemal K, Marsaud V, Vauthier C & Sharma CP Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J. Control. Release 147, 377–384 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF & Ponchel G Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials 28, 2233–2243 (2007). [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y, Wei W, Lv P, Wang L & Ma G Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm 77, 11–19 (2011). [DOI] [PubMed] [Google Scholar]
- 187.He P, Davis SS & Illum L In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm 166, 75–88 (1998). [Google Scholar]
- 188.Solorio LD, Fu AS, Hernández-Irizarry R & Alsberg E Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-β1 from incorporated polymer microspheres. J. Biomed. Mater. Res. A 92A, 1139–1144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Solorio LD, Vieregge EL, Dhami CD, Dang PN & Alsberg E Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-β1. J. Control. Release 158, 224–232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bratt-Leal AM, Nguyen AH, Hammersmith KA, Singh A & McDevitt TC A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 34, 7227–7235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Collins MN & Birkinshaw C Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym 92, 1262–1279 (2013). [DOI] [PubMed] [Google Scholar]
- 192.Chan BP & Leong KW Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J 17, 467–479 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ahmed EM Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res 6, 105–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sheikhi A et al. Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads. Biomaterials 192, 560–568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xin S, Wyman OM & Alge DL Assembly of PEG microgels into porous cell-instructive 3D scaffolds via thiol-ene click chemistry. Adv. Healthc. Mater 7, e1800160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hsu RS et al. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Adv. Sci 6, 1900520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McWhorter FY, Wang T, Nguyen P, Chung T & Liu WF Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 110, 17253–17258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Werner M et al. Surface curvature differentially regulates stem cell migration and differentiation via altered attachment morphology and nuclear deformation. Adv. Sci 4, 1600347 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mitra A et al. Cell geometry dictates TNFα-induced genome response. Proc. Natl. Acad. Sci.USA 114, E3882–E3891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li S et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater 16, 953–961 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bae M-S, Lee KY, Park YJ & Mooney DJ RGD island spacing controls phenotype of primary human fibroblasts adhered to ligand-organized hydrogels. Macromol. Res 15, 469–472 (2007). [Google Scholar]
- 202.Cruz DM et al. Chitosan microparticles as injectable scaffolds for tissue engineering. J. Tissue Eng. Regen. Med 2, 378–380 (2008). [DOI] [PubMed] [Google Scholar]
- 203.Malafaya PB, Santos TC, van Griensven M & Reis RL Morphology, mechanical characterization and in vivo neo-vascularization of chitosan particle aggregated scaffolds architectures. Biomaterials 29, 3914–3926 (2008). [DOI] [PubMed] [Google Scholar]
- 204.Kucharska M et al. Fabrication and characterization of chitosan microspheres agglomerated scaffolds for bone tissue engineering. Mater. Lett 64, 1059–1062 (2010). [Google Scholar]
- 205.Dumont CM et al. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater 86, 312–322 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hu Z, Ma C, Rong X, Zou S & Liu X Immunomodulatory ECM-like microspheres for accelerated bone regeneration in diabetes mellitus. ACS Appl. Mater. Interfaces 10, 2377–2390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Roam JL, Nguyen PK & Elbert DL Controlled release and gradient formation of human glial-cell derived neurotrophic factor from heparinated poly(ethylene glycol) microsphere-based scaffolds. Biomaterials 35, 6473–6481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Roam JL et al. A modular, plasmin-sensitive, clickable poly(ethylene glycol)-heparin-laminin microsphere system for establishing growth factor gradients in nerve guidance conduits. Biomaterials 72, 112–124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Custódio CA et al. Functionalized microparticles producing scaffolds in combination with cells. Adv. Funct. Mater 24, 1391–1400 (2014). [Google Scholar]
- 210.Jgamadze D, Liu L, Vogler S, Chu LY & Pautot S Thermoswitching microgel carriers improve neuronal cell growth and cell release for cell transplantation. Tissue Eng. C Methods 21, 65–76 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Moroni L et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater 3, 21–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Groll J et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 013001 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Xu F et al. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater 23, 4254–4260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tasoglu S, Diller E, Guven S, Sitti M & Demirci U Untethered micro-robotic coding of three-dimensional material composition. Nat. Commun 5, 3124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu F et al. The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials 32, 7847–7855 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kamperman T et al. Single cell microgel based modular bioinks for uncoupled cellular micro- and macroenvironments. Adv. Healthc. Mater 6, 1600913 (2017). [DOI] [PubMed] [Google Scholar]
- 217.Xin S, Chimene D, Garza JE, Gaharwar AK & Alge DL Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci 7, 1179–1187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Highley CB, Rodell CB & Burdick JA Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater 27, 5075–5079 (2015). [DOI] [PubMed] [Google Scholar]
- 219.Bhattacharjee T et al. Writing in the granular gel medium. Sci. Adv 1, e1500655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bhattacharjee T et al. Liquid-like solids support cells in 3D. ACS Biomater. Sci. Eng 2, 1787–1795 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Gilbert E, Hui A & Waldorf HA The basic science of dermal fillers: past and present part I: background and mechanisms of action. J. Drugs Dermatol 11, 1059–1068 (2012). [PubMed] [Google Scholar]
- 222.Tezel A & Fredrickson GH The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther 10, 35–42 (2008). [DOI] [PubMed] [Google Scholar]
- 223.Rose JC et al. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett 17, 3782–3791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le LV, Mkrtschjan MA, Russell B & Desai TA Hang on tight: reprogramming the cell with microstructural cues. Biomed. Microdevices 21, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tan H et al. Heterogeneous multi-compartmental hydrogel particles as synthetic cells for incompatible tandem reactions. Nat. Commun 8, 663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yadavali S, Jeong HH, Lee D & Issadore D Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun 9, 1222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Andrade JE, Avila CF, Hall SA, Lenoir N & Viggiani G Multiscale modeling and characterization of granular matter: from grain kinematics to continuum mechanics. J. Mech. Phys. Solids 59, 237–250 (2011). [Google Scholar]
- 228.Liu J, Bosco E & Suiker ASJ Multi-scale modelling of granular materials: numerical framework and study on micro-structural features. Comput. Mech 63, 409–427 (2019). [Google Scholar]
- 229.Zhu H et al. Spatial control of in vivo CRISPR–Cas9 genome editing via nanomagnets. Nat. Biomed. Eng 3, 126–136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lu L, Stamatas GN & Mikos AG Controlled release of transforming growth factor β1 from biodegradable polymer microparticles. J. Biomed. Mater. Res 50, 440–451 (2000). [DOI] [PubMed] [Google Scholar]
- 231.Pregibon DC, Toner M & Doyle PS Multifunctional encoded particles for high-throughput biomolecule analysis. Science 315, 1393–1396 (2007). [DOI] [PubMed] [Google Scholar]
- 232.Wang S et al. An in-situ photocrosslinking microfluidic technique to generate non-spherical, cytocompatible, degradable, monodisperse alginate microgels for chondrocyte encapsulation. Biomicrofluidics 12, 014106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang H et al. One-step generation of core–shell gelatin methacrylate (GelMA) microgels using a droplet microfluidic system. Adv. Mater. Technol 4, 1800632 (2019). [Google Scholar]
- 234.Jha AK, Malik MS, Farach-Carson MC, Duncan RL & Jia X Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter 6, 5045–5055 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Loebel C, Broguiere N, Alini M, Zenobi-Wong M & Eglin D Microfabrication of photo-cross-linked hyaluronan hydrogels by single- and two-photon tyramine oxidation. Biomacromolecules 16, 2624–2630 (2015). [DOI] [PubMed] [Google Scholar]
- 236.Ma T, Gao X, Dong H, He H & Cao X High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or Diels–Alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today 9, 49–59 (2017). [Google Scholar]
- 237.Jia X et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules 7, 3336–3344 (2006). [DOI] [PubMed] [Google Scholar]
- 238.Chen J et al. Tailor-making fluorescent hyaluronic acid microgels via combining microfluidics and photoclick chemistry for sustained and localized delivery of herceptin in tumors. ACS Appl. Mater. Interfaces 10, 3929–3937 (2018). [DOI] [PubMed] [Google Scholar]
- 239.Sonnet C et al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J. Orthop. Res 31, 1597–1604 (2013). [DOI] [PubMed] [Google Scholar]
- 240.Chung SE et al. Optofluidic maskless lithography system for real-time synthesis of photopolymerized microstructures in microfluidic channels. Appl. Phys. Lett 91, 041106 (2007). [Google Scholar]
- 241.Ryu S et al. Dual mode gelation behavior of silk fibroin microgel embedded poly(ethylene glycol) hydrogels. J. Mater. Chem. B 4, 4574–4584 (2016). [DOI] [PubMed] [Google Scholar]
- 242.Kumachev A et al. High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation. Biomaterials 32, 1477–1483 (2011). [DOI] [PubMed] [Google Scholar]


