Abstract
In 2018, some sartan medicinal products were reported to be contaminated with nitrosamine compounds, which are potent mutagenic carcinogens. Two nitrosamines received particular attention: N -nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA). These have since been confirmed in different types of medicinal products, including ranitidine and metformin. Consequently, the European Medicines Agency (EMA) started an investigation into the cause of contamination and an assessment of the risk to patients taking contaminated medicinal products. The main source of contamination were changes in production, which involves combinations of amines and nitrogen compounds and the use of specific catalysts and reagents. Withdrawals of medicinal products that took place in Croatia did not lead to a shortage of sartan- or metformin-containing medicines. Moreover, ranitidine had been preventively withdrawn all over the EU, including Croatia, creating shortages at the time, but was subsequently replaced with therapeutic alternatives.
Key Words: carcinogenicity, metformin, NDEA, NDMA, ranitidine, sartans
Abstract
Nitrozaminski su spojevi jaki kancerogeni i njihova je prisutnost potvrđena 2018. u nekim lijekovima s djelatnom tvari iz skupine sartana. Dva predstavnika nitrozamina, najčešće detektirana kao onečišćenja u lijekovima, jesu N-nitrozodimetilamin (NDMA) i N-nitrozodietilamin (NDEA). Od tada je njihova prisutnost potvrđena u različitim skupinama lijekova, što upućuje na njihovu učestalost. Slijedom toga, Europska agencija za lijekove (EMA) pokrenula je istragu uzroka onečišćenja te procjenu rizika kojima su pacijenti koji su uzimali lijekove onečišćenih serija bili izloženi. Glavni uzrok onečišćenja djelatne tvari uključuje promjene u proizvodnom postupku koji sadržava smjesu amina i dušikovih spojeva uz uporabu specifičnih katalizatora i reagensa. Nitrozamini su također pronađeni u gotovim lijekovima. Povlačenje lijekova u Hrvatskoj nije dovelo do nestašice lijekova koji sadržavaju sartan ili metformin, a lijekovi koji sadržavaju ranitidin povučeni su iz mjera opreza iz ljekarni i u Hrvatskoj i u Europskoj uniji.
Ključne riječi: kancerogenost, metformin, NDEA, NDMA, ranitidin, sartani
Nitrosamines are a group of organic compounds containing the nitroso functional group. They can be found in water, food, tobacco, pesticides, or plastics, but received public attention in mid-2018, when they were also found in medicinal products (1).
Among them, N-nitrosamines are so potent mutagenic carcinogens that they are referred to as the “cohort of concern” by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk (2). The two best-known nitrosamines, N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), have been classified by the International Agency for Research on Cancer (IARC) as possible human (class 2A) carcinogens, but they are also genotoxic (3, 4). Once N-nitrosamines are activated by microsomal liver enzymes, they can react with DNA base pairs to form unstable α-hydroxyalkylnitrosamines and produce alkyldiazonium ions, which alkylate DNA bases and induce carcinogenic response (5).
Discovery of nitrosamines in medicinal products
On 6 June 2018, active pharmaceutical ingredient (API) manufacturer Zhejiang Huahai Pharmaceutical (ZHP) from China detected NDMA contamination in the manufacturing process of valsartan. Investigation showed that nitrosamine contaminants in the ZHP plant appeared after July 2012, when parts of valsartan production changed to increase yields and reduce waste. More specifically, the manufacturer changed the synthetic process involving tetrazole ring formation by replacing tributyltin azide with a more toxic anhydrous sodium azide, while dimethylformamide (DMF) was used as the solvent. Sodium nitrite, which forms nitrous acid in an acidic medium, was used to quench the excess sodium azide, which resulted in the nitrosation of dimethylamine (DMA) impurity present in dimethylformamide to form NDMA (Figure 1) (6).
Figure 1.
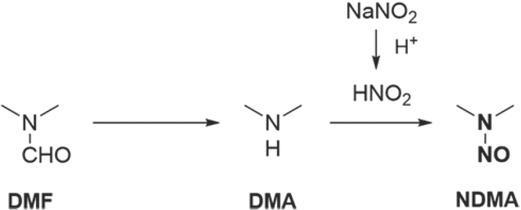
Production changes that caused formation of NDMA in the Zhejiang Huahai Pharmaceutical plant (6)
Regulatory response
In response to that discovery, medicines agencies of the member states of the European Union (EU) withdrew a series of medicines containing valsartan API manufactured by ZHP. On 5 July 2018, the European Commission (EC) put the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency in charge of assessing valsartan medicines in the EU that contained the ZHP-manufactured API, pursuant to Article 31 of the Directive 2001/83/EC on the Community code relating to medicinal products for human use (7). In the following weeks, the presence of another nitrosamine, NDEA, was also confirmed in certain products. Furthermore, investigations showed that N-nitrosamine contamination was not limited to the ZHP plant and valsartan API production but also affected other sartans containing tetrazole rings (Figure 2). That discovery widened the assessment to all sartans with a tetrazole ring (6, 8).
Figure 2.
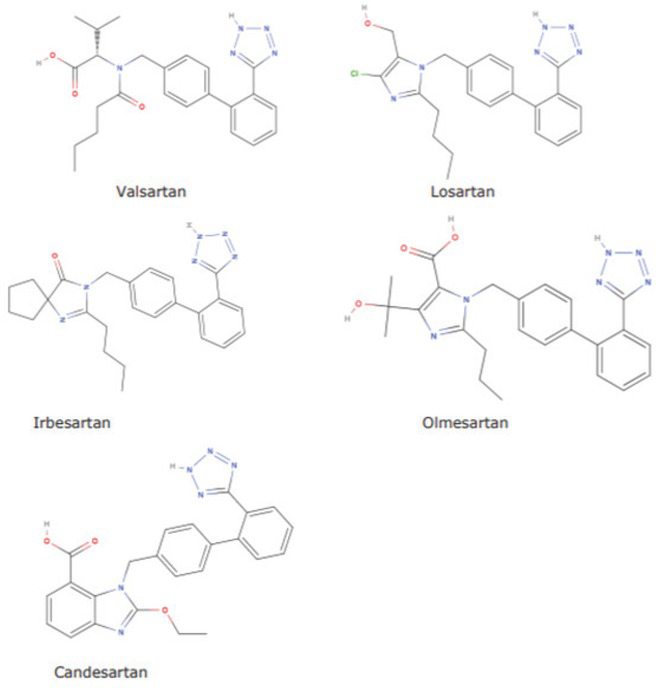
Sartans with a tetrazole ring contaminated with impurities (4)
On 31 January 2019, the CHMP concluded its review and published a final retrospective risk assessment (9) taking into account that every second person in the EU is at risk of developing cancer during their lifetime. Preliminary assessments included nitrosamine exposure from other sources and extrapolated risk results from animal studies. The worst-case scenario of the final risk assessment was that if 100,000 patients took the highest dose (320 mg) of valsartan manufactured by ZHP (which had the highest levels of contamination) every day for six years (that is, since 2012, when the production process was changed), that could lead to 22 additional cases of cancer due to NDMA over their lifetime. NDEA impurities, in turn, could lead to eight additional cancer cases per 100,000 patients if they took valsartan in the highest dose every day for four years. As for sartans containing the tetrazole ring, the review reported that a vast majority had no nitrosamine impurities or these were detected in very small amounts (9).
Having identified the sources of N-nitrosamine contamination (Table 1), the CHMP considered short- and long-term measures to address the issue. In the short term, it set temporary limits for NDMA and NDEA impurities in APIs based on maximum daily intake of 96.0 ng and 26.5 ng, respectively, derived from animal testing and calculated according to ICH M7 (R1) methodology (linear extrapolation of cancer potency metrics). These limits should apply over a two-year transition period following the Commission’s decision adopted on 2 April 2019. During this period, marketing authorisation holders (MAHs) are to assess the risk of contamination and ensure that control strategies are in place for the batches of sartan APIs used in their finished products. In the long term, all MAHs concerned must make the necessary changes in API production to minimise nitrosamine contamination. In the meantime, the temporary limits will be used to recall sartans that exceed them from national markets (4, 6, 8, 9).
Table 1.
| Sources of nitrosamine contamination/formation in the API | |||||
|---|---|---|---|---|---|
| Critical combinations of compounds | Nitrosamine | NOx source | Amine source | Amine nitrosated by NOx | Critical combination |
| NDMA | NaNO2 | Dimethylformamide (DMF) | Dimethylamine (DMA) | reagent / solvent | |
| NDEA | NaNO2 | Triethylamine (TEA) | Diethylamine (DEA) | reagent / reagent | |
| Triethylamine hydrochloride (TEA HCl) | Triethylamine (TEA) | reagent / catalyst | |||
| Reagents, solvents, and catalysts | The use of azide reagents in the synthesis of tetrazole, under the acidic conditions required for the isolation and extraction of tetrazole, releases the dangerous hydrazoic acid which can give e.g. amines. The remaining amounts of azide can be decomposed into gaseous by-products such as nitrogen and nitrous oxide (N2O) by the addition of sodium nitrite (NaNO2). | ||||
| Faults in the production process | Inappropriate or poorly controlled reaction conditions concerning temperature, pH, or the order of addition of reagents, intermediates, or solvents. | ||||
| Recovered and recycled materials | If the solvents used in the reaction step involving azide are collected and reused, there is a possibility that nitrosamines may be inadvertently introduced into the production process if the waste stream is quenched with nitrite in the presence of secondary or tertiary amines and subsequently treated with nitric acid, e.g. to destroy residual azides without adequate process control. There is also a possibility that the process water used in the production of the API may contain low levels of nitrosamines due to environmental pollution. | ||||
| Third-party | External recovery companies do not receive sufficiently specific information on the composition of the materials they process and they use routine processing procedures and generally accepted equipment. This can lead to cross-contamination of solvents, reagents, and catalysts (originating from different sources and processes) if the equipment is not properly cleaned between customer orders or if precautions are not taken to prevent the formation of nitrosamines. | ||||
| Contaminated raw materials and intermediates | Distributors using recovered materials or raw materials that contain nitrites or amines and whose processing can lead to the formation of nitrosamines. Problematic if an API manufacturer who exclusively uses nitrosamine-free processes is not aware that there is a risk of nitrosamine formation. | ||||
| Sources of nitrosamine contamination of finished products | |||||
|---|---|---|---|---|---|
| Use of certain packaging materials | Nitrosamine | NOsource x | Amine source | Amine nitrosated by NOx | Critical combination |
| NDMA | Nitrocellulose (on lidding foil) | Printing ink | Dimethylamine (DMA) | Lidding foil / printing ink | |
| NDEA | Nitrocellulose (on lidding foil) | Printing ink | Diethylamine (DEA) | Lidding foil / printing ink | |
| Storage conditions | Some stored medicinal products may undergo degradation pathways that form nitrosamine impurities. The chemical structure of metformin also indicates the possibility of subsequent nitrosamine formation during storage. The formation of metformin hydroperoxide can lead to the oxidation of other metformin molecules or the formation of nitrosamines by intramolecular oxidation, which leads to rearrangements and fragmentation reactions. Due to the high nitrogen content in metformin, numerous positions for N-oxidations are possible. Besides, numerous fragmentation reactions enable decomposition of the molecular structure, which can lead to the formation of thermodynamically stable nitrosamine derivatives. | ||||
| Endogenous production from drugs | In vitro studies of different pH solutions of ranitidine with and without nitrite have shown the possibility of NDMA formation from ranitidine in acidic gastric pH in the presence of high levels of nitrites, which nitrosate the diamine part of the ranitidine molecule. | ||||
In 2019, NDMA and NDEA were also found in ranitidine and metformin. When quality testing showed that some of ranitidine medicines contained NDMA in higher concentrations than the acceptable daily intake limit, EMA in September 2019 started another review of medicinal products containing ranitidine, which led to a conclusion that MAHs should extend their risk evaluation to all medicinal products containing a chemically synthesised active substance (3). As for metformin, EMA review is still ongoing as it awaits further test results from Official Medicines Control Laboratories (OMCLs) (10).
Carcinogenicity
The carcinogenicity of NDMA and NDEA stems from their biotransformation via microsomal liver enzymes, primarily CYP2E1, to their respective alkyl diazonium ions. These ions react with the DNA and form DNA adducts, which cause endogenous DNA damage (6, 8, 9, 10, 11).
Nitrosamine exposure can be either endogenous or exogenous. Exogenous exposure can result from consuming food or beverages (e.g., beer), inhaling tobacco smoke, or using rubber items and cosmetics. Endogenous exposure is the result of nitrosation of nitrosamine precursors in the stomach. Quantification of endogenous N-alkylnitrosamine formation and its contribution to total N-nitrosamine exposure was extensively studied years ago (3, 12), and there is no recent research on intragastric formation of NDMA/NDEA from complex mixtures of precursors and inhibitors in humans.
It is important to note that the target organs of NDMA/ NDEA toxicity and tumours may differ between species. Although nitrosamine metabolism takes place in the liver, it is not concentrated in the liver, and according to studies performed in rats (6, 13), only about 1 %, is excreted unchanged in the urine. The target organs of NDMA and NDEA in humans are still unknown, but several human studies have shown a risk of developing nitrosamine-related cancer, stomach in particular.
Causes of NDMA and NDEA impurities in medicinal products
N-Nitrosamines can form in the presence of certain raw materials (including starting materials and intermediates) under suitable reaction conditions and may end up in the final product if purification in the various stages of production is incomplete (3, 4).
According to the CHMP review of sartans (4), the formation of N-nitrosamine in the API was caused by the reaction of NaNO2 – a common source of NOx – with different sources of secondary and tertiary amines. In addition to these causes for sartan impurities, Table 1 lists other possible causes of nitrosamine contamination/ formation in metformin and ranitidine.
The situation on the Croatian market
Medicinal products containing sartans with tetrazole rings (valsartan, losartan, olmesartan, irbesartan), candesartan, or metformin are still present on the Croatian market. Withdrawal of certain product batches did not jeopardise regular supply, because medicines manufactured by other manufacturers were not affected by withdrawals (14).
However, ranitidine, which had been available as over-the-counter (OTC, non-prescription) or prescription medicine, has been withdrawn from the Croatian market since September 2019 (15, 16), when EMA’s assessment of ranitidine started. All ranitidine products in Croatia were recalled as a precautionary measure. Other EU countries have done the same for as long as the EC decision is pending. Ranitidine withdrawal has created a shortage, i.e. a permanent interruption in the supply of the Croatian market, and the patients have been provided replacement therapy.
Before withdrawal, ranitidine was among the 50 most used medicinal products in Croatia and reached its peak in 2018, when ranked 31st.
Although valsartan is still available on the market, it has seen a major decline in its consumption since 2018, when it ranked 25th. Namely, in 2019 it dropped to the 37th place (17, 18).
References
- 1.World Health Organization (WHO) Information Note Nitrosamine impurities [displayed 24 September 2020] https://www.who.int/medicines/publications/drugalerts/InformationNote_Nitrosamine-impurities/en/ Available at. [Google Scholar]
- 2.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) ICH guideline M7(R1) on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk [displayed 7 March 2021] https://www.ema.europa.eu/en/documents/scientific-guideline/ch-guideline-m7r1-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit_en.pdf p. 6. Available at. [Google Scholar]
- 3.European Medicines Agency (EMA) Procedure under Article 5(3) of Regulation EC (No) 726/2004. Nitrosamine impurities in human medicinal products [displayed 24 September 2020] https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf Available at. [Google Scholar]
- 4.European Medicines Agency (EMA) Lessons learnt from presence of N-nitrosamine impurities in sartan medicines [displayed 24 September 2020] https://www.ema.europa.eu/en/documents/report/essons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf Available at. [Google Scholar]
- 5.Šulc M, Hodek P, Stiborová M. The binding affinity of c a r c in o gen i c N- nit r o so dim et h y l a mine a n d N-nitrosomethylaniline to cytochromes P450 2B4, 2E1 and 3A6 does not dictate the rate of their enzymatic N-demethylation. Gen Physiol Biophys. 2010;29:175–85. doi: 10.4149/gpb_2010_02_175. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency (EMA) Assessment report Referral under Article 31 of Directive 2001/83/EC [displayed 24 September 2020] https://www.ema.europa.eu/en/documents/variation-report/sartans-article-31-referral-chmp-assessment-report_en.pdf Available at. [Google Scholar]
- 7.Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use [displayed 7 March 2021] https://eur-lex.europa.eu/eli/dir/2001/83/ Available at. [Google Scholar]
- 8.European Medicines Agency (EMA) EU inspection finds Zhejiang Huahai site non-compliant for manufacture of valsartan: EMA and national authorities considering impact on other active substances produced at the site [displayed 24 September 2020] https://www.ema.europa.eu/en/news/eu-inspection-finds-zhejiang-huahai-site-non-compliant-manufacture-valsartan-ema-national Available at. [Google Scholar]
- 9.European Medicines Agency (EMA) Sartan medicines: companies to review manufacturing processes to avoid presence of nitrosamine impurities [displayed 24 September 2020] https://www.ema.europa.eu/en/news/sartan-medicines-companies-review-manufacturing-processes-avoid-presence-nitrosamine-impurities Available at. [Google Scholar]
- 10.European Medicines Agency (EMA) Nitrosamine impurities [displayed 24 September 2020] https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/nitrosamine-impurities#metformin-containing-medicines-section Available at. [Google Scholar]
- 11.World Health Organization (WHO) Guidelines for Drinking-water Quality. 3rd ed. Vol 1, Incorporating first and second addenda. Geneva: WHO; 2008. [PubMed] [Google Scholar]
- 12.Krul CAM, Zeilmaker MJ, Schothorst RC, Havenaar R. Intragastric formation and modulation of N-nitrosodimethylamine in a dynamic in vitro gastrointestinal model under human physiological conditions. Food Chem Toxicol. 2004;42:51–63. doi: 10.1016/j.fct.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients. 2015;7:9872–95. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agency for Medicinal Products and Medical Devices of Croatia (HALMED). Medicinal products database [displayed 24 September 2020] https://www.halmed.hr/Lijekovi/Baza-lijekova/ Available at. [Google Scholar]
- 15.Agency for Medicinal Products and Medical Devices of Croatia (HALMED) Obavijest o nestašici lijekova s djelatnom tvari ranitidin [Notice of shortage of drugs with the active substance ranitidine, in Croatian] [displayed 24 September 2020] https://www.halmed.hr/Novosti-i-edukacije/Novosti/2019/Obavijest-o-nestasici-lijekova-s-djelatnom-tvari-ranitidin-/2239 Available at. [Google Scholar]
- 16.Agency for Medicinal Products and Medical Devices of Croatia (HALMED) Obustava izvršenja odobrenja za stavljanje u promet lijekova koji sadrže djelatnu tvar ranitidin u Europskoj uniji [Suspension of the marketing authorization for medicinal products containing the active substance ranitidine in the European Union, in Croatian] [displayed 24 September 2020] https://www.halmed.hr/Novosti-i-edukacije/Novosti/2020/Obustava-izvrsenja-odobrenja-za-stavljanje-u-promet-lijekova-koji-sadrze-djelatnu-tvar-ranitidin-u-Europskoj-uniji/2360/ Available at. [Google Scholar]
- 17.Agency for Medicinal Products and Medical Devices of Croatia (HALMED) Izvješće o potrošnji lijekova u Republici Hrvatskoj u 2018. [Report on medicinal product utilization in the Republic of Croatia in 2018, in Croatian] [displayed 24 September 2020] https://halmed.hr/Novosti-i-edukacije/Publikacije-i-izvjesca/Izvjesca-o-potrosnji-lijekova/Izvjesce-o-potrosnji-lijekova-u-Republici-Hrvatskoj-u-2018/ Available at. [Google Scholar]
- 18.Agency for Medicinal Products and Medical Devices of Croatia (HALMED) Izvješće o potrošnji lijekova u Republici Hrvatskoj u 2019. [Report on medicinal product utilization in the Republic of Croatia in 2019, in Croatian] [displayed 24 September 2020] https://halmed.hr/Novosti-i-edukacije/Publikacije-i-izvjesca/Izvjesca-o-potrosnji-lijekova/Izvjesce-o-potrosnji-lijekova-u-Republici-Hrvatskoj-u-2019/ Available at. [Google Scholar]


