Supplemental Digital Content is Available in the Text.
Key Words: HIV, COVID-19, key populations, Cameroon, Benin, mathematical model
Background:
The COVID-19 pandemic indirectly impacts HIV epidemiology in Central/West Africa. We estimated the potential impact of COVID–19-related disruptions to HIV prevention/treatment services and sexual partnerships on HIV incidence and HIV-related deaths among key populations including female sex workers (FSW), their clients, men who have sex with men, and overall.
Setting:
Yaoundé (Cameroon) and Cotonou (Benin).
Methods:
We used mathematical models of HIV calibrated to city population–specific and risk population–specific demographic/behavioral/epidemic data. We estimated the relative change in 1-year HIV incidence and HIV-related deaths for various disruption scenarios of HIV prevention/treatment services and decreased casual/commercial partnerships, compared with a scenario without COVID-19.
Results:
A 50% reduction in condom use in all partnerships over 6 months would increase 1-year HIV incidence by 39%, 42%, 31%, and 23% among men who have sex with men, FSW, clients, and overall in Yaoundé, respectively, and 69%, 49%, and 23% among FSW, clients, and overall, respectively, in Cotonou. Combining a 6-month interruption of ART initiation and 50% reduction in HIV prevention/treatment use would increase HIV incidence by 50% and HIV-related deaths by 20%. This increase in HIV infections would be halved by a simultaneous 50% reduction in casual and commercial partnerships.
Conclusions:
Reductions in condom use after COVID-19 would increase infections among key populations disproportionately, particularly FSW in Cotonou, who need uninterrupted condom provision. Disruptions in HIV prevention/treatment services have the biggest impacts on HIV infections and deaths overall, only partially mitigated by equal reductions in casual/commercial sexual partnerships. Maintaining ART provision must be prioritized to minimize short-term excess HIV-related deaths.
INTRODUCTION
Disruptions associated with the COVID-19 pandemic and response1 (hereafter simplified to “COVID-19”) may inadvertently impact the HIV epidemic in at least 2 ways.2–7 First, an increasing number of countries and organizations reported disruptions in traditional and safe in-person HIV prevention and treatment services due to COVID-19.8–21 Second, COVID-19 has been associated with disruptions in casual22,23 and commercial24,25 sexual partnerships due to increased physical distancing and closure of sex work venues.
Key populations (KPs), such as female sex workers (FSW),26 their clients, and men who have sex with men (MSM)27 may be particularly vulnerable to these disruptions.28,29 Community outreach and KP HIV prevention programs in sub-Saharan Africa have increased online services, but with decreased reported uptake.17,18,30–32 KPs experience higher levels of stigma,25,33 are more affected by the economic impact of COVID-19,29 and may have limited access to online health platforms, potentially reducing use of health services, as well as condoms and antiretroviral therapy (ART). For example, a recent global survey among MSM (n = 10,654) suggested that nearly half of MSM living with HIV (45%, N = 1254) were unable to refill their HIV medicine prescription remotely.34 However, limited demand for sex work from clients and the closure of venues where some commercial and same-sex sexual partnerships are formed could create fewer opportunities for HIV transmission.35,36
Early modeling studies assessed the potential overall population-level impact of different hypothetical HIV prevention/treatment disruption scenarios37–39 across sub-Saharan Africa. These studies suggested that a complete interruption of all ART services for 6 months among 50% of treated people living with HIV (PLHIV) may increase annual new HIV infections by 1.6 fold,38 but did investigate the heterogeneity in impact by the risk group, which is particularly important for HIV epidemics in Central and West Africa. Empirical programmatic or behavioral data measuring the effects of COVID-19 on intervention uptake or sexual behaviors are only starting to emerge, but translating these disruption estimates from such studies into population-level impact on HIV can only be done conducted using mathematical models.
Yaoundé and Cotonou are the largest cities of Cameroon and Benin, respectively, in Central and West Africa, with 3% and 1% HIV prevalence among people aged 15–49 years. As in most African settings, KPs have been shown to be disproportionately affected by HIV and are an important part in HIV transmission and control.40–42 Compared with the overall populations, FSW HIV prevalence in Yaoundé and Cotonou is approximately 5 and 9 times higher,43,44 respectively, and around 10 times higher among MSM in Yaoundé.43,45
The first COVID-19 cases in Cameroon and Benin were recorded on the 6th and 16th of March 2020, respectively, but the countries have since experienced different COVID-19 epidemics. In January 2021, Cameroon had recorded 455 COVID-19 deaths and 28,000 cases, whereas Benin had reported very few deaths (n = 46) and cases (n = 3400).1 On March 18th 2020, Cameroon implemented the first set of COVID-19 mitigation measures with the closure of international borders and schools46 and the closure of bars and restaurants in the evenings, which impacted commercial sex.47 Additional measures were implemented in April, including the creation of COVID-19 health centers in all regional capitals. The country started easing lockdown measures on May 1st, when bars/restaurants were allowed to stay open in the evenings,48 whereas schools and universities reopened in early June. Similar measures to those in Cameroon were implemented in Benin, including the closure of bars and nightclubs on March 23rd, but only within specific geographic hotspots, such as Cotonou.49 Although bar gatherings were still officially limited to 50 people in both countries in January 2021, it is not systematically enforced by Cameroon authorities.50,51 The COVID-19 pandemic and responses to it have substantially impacted countries' economies,52 which could subsequently affect commercial sex. KPs have been more severely affected by both countries' response and economic difficulties, and several mass arrests of KPs have been reported in Cameroon.53–55 Despite Cameroon having implemented universal free HIV testing and treatment in January 2020,56 a recent Ministry of Health report suggests a 10% reduction in new HIV diagnoses over January/June 2020 compared with January/June 2019.57 A survey conducted among FSW (n = 407) in Greater Cotonou in September and October 2020 assessed the impact of COVID-19-related disruption on sex work activity in the city.58 The large majority of respondents (88%) reported that clients had become “rare” in hotspots. More than a third (36%) of FSW reported a reduction in the price of sex work since the arrival of the COVID-19 pandemic in the country, and two-thirds of FSW (68%) reported a decrease in spending power due to COVID-19.
To inform countries on the likely impact of COVID-19 on HIV using currently available data,59 we conducted a comparative mathematical modeling study60 and estimated how various data driven and hypothetical scenarios of 3-month, 6-month, and 12-month disruptions in ART services and condom provision, and reductions in sexual activity, may influence the number of new HIV infections and HIV-related deaths over 1 and 5 years in Yaoundé and Cotonou, among KPs and the overall populations. We compared the independent and combined population-level impact of the different disruptions, overall, and by risk populations. Our analysis generates new results allowing to (1) determine and identify which type and levels of COVID-19 disruptions has the largest population-level impact on HIV, and therefore need to be prevented in priority impacts, and (2) to rapidly interpret and further our understanding of the likely impacts on HIV as more data emerge on different level of COVID-19 disruptions.
METHODS
Yaoundé and Cotonou HIV Models
For these analyses, we used 2 existing models specifically developed and calibrated to the population and HIV epidemiology of each setting.61,62 The models are very similar, and the slight differences reflect the specific epidemiology and data available in each city. Table 1 shows the main characteristics of the HIV epidemics and levels of interventions, including among KPs. After successful programs aiming to reduce FSW HIV acquisition and transmission, condoms are more readily used by FSW than the rest of the population, with an estimated 81% and 91% of commercial sex acts involving condoms in 2020 in Yaoundé and Cotonou, respectively.42,43,63–65 Similarly, condom use among MSM in Yaoundé is twice as frequent then among non-KPs.43,66 In early 2020, ∼43% of all PLHIV were estimated to be virally suppressed in both cities67,68 with higher suppression among FSW (70% and 57% in Yaoundé and Cotonou, respectively43,69).
TABLE 1.
HIV Epidemic Profile of the 2 Cities: Population Structure, Sexual Behaviors, HIV Prevalence, and Intervention Levels in Yaoundé (Cameroon) and Cotonou (Benin)
| Yaoundé | Cotonou | |
| Population structure | ||
| % FSW among all females | 1.5% (0.5–3.0)43,120,121 | 0.30%* (0.27–0.38)72,91,122–125 |
| % Clients among all males | 16.0% (7.9–19.7)66,76,92,93,126 | 21.3% (12.6–25.9)64,90,122–125 |
| % MSM among all males | 1.8% (1.0–2.2)43,121 | NA |
| Sexual behaviors | ||
| FSW: | ||
| No. of commercial sex acts (per year) | 493 (304–932)43,76,78,121,127 | 648 (194–1546)44,65,80,82,84,88,90 |
| No. of noncommercial sex acts (per year) | 368 (231–303)43 | 27 (15–44)44,65,80,82–84,88 |
| Clients: | ||
| No. of sex acts (per year) | 233 (168–380)73,128 | 121 (89–162)44,82,84,90 |
| MSM: | ||
| No. of sex acts with male partners (per year) | 172 (109–212)43 | NA |
| No. of sex acts with female partners (per year) | 140 (120–157)43 | NA |
| Lower-risk populations: | ||
| No. of sex acts (per year) | 100 (71–120)128 | 38 (29–47)81,86 |
| Fraction of sex acts which are with casual partners. | 2093 | 20%† |
| HIV prevalence | ||
| FSW | 13.9% (11.9–16.4)43 | 8.5% (7.1–11.2)81,84,85,129 |
| Clients | 3.5% (2.5–4.6)73 | 1.5% (0.9–2.2)65 |
| MSM | 31.1% (26.9–34.8)43,130,131 | NA |
| Lower-risk females | 4.9% (3.9–5.9)66 | 1.0% (0.8–1.5)86 |
| Lower-risk males | 1.8% (1.3–2.4)66 | 0.6% (0.3–0.9)86 |
| % Condom use at last sex | ||
| FSW during paid sex | 81.2% (73.5–94.0)43 | 91.4% (86.0–98.2)44,81 |
| FSW during unpaid sex | 53.7% (47.5–65.0)43 | 39.9% (21.7–59.9)44,81,83 |
| MSM | 65.7% (57.7–77.5)43 | NA |
| Lower-risk populations | 29.1% (20.0–39.5)66,95 | 20.7% (16.2–25.5)81 |
| ART and viral suppression coverage in PLHIV | ||
| FSW: % on ART | 87.3% (81.3–91.9) | 72.0% (63.1–80.6) |
| % Virally suppressed | 69.9% (63.8–74.3)43 | 57.1% (49.7–66.2)68,69,99 |
| Clients: % on ART | 46.7% (40.5–52.5) | 58.8% (42.0–73.4) |
| % Virally suppressed | 37.4% (32.1–42.5)73 | 35.8% (20.2–55.8)68,69,99,101 |
| MSM: % on ART | 49.2% (43.4–54.8) | NA |
| % Virally suppressed | 39.6% (34.4–44.9)43 | |
| Lower-risk females: % on ART | 54.5% (50.4–59.9) | 74.9% (64.7–83.2) |
| % Virally suppressed | 43.8% (40.1–48.3)67,132 | 46.8% (31.4–65.4)68,69,99,101 |
| Lower-risk males: % on ART | 48.6% (42.7–53.4) | 68.3 (50.4–80.6) |
| % Virally suppressed | 39.2% (34.2–43.0)67,132 | 42.0% (23.3–63.0)68,69,99,101 |
Median and 95% UI (2.5th and 97.5th percentiles) of posterior distributions in January 2020 are shown.
The Cotonou model also represented women receiving less than half of their income from commercial sex [“part-time FSW,” 0.27% (0.23–0.33) of all women]. The combined posterior size of both groups was 0.70% (0.56–0.87).
FSW were defined as women reporting most of their income as originating from commercial sex.
Casual partnerships were not explicitly modeled in Cotonou, as no data were available. A fraction of 20% of noncommercial sex acts of all risk groups were assumed to be with casual partners, to match estimates from Yaoundé DHS.
NA, not available as not modeled in Cotonou.
Both models were deterministic compartmental models of HIV transmission which stratified the population by gender, sexual risk group, HIV infection stage, and ART status. They were parameterized to reflect city-specific empirical estimates of demographic (eg, growing population size) and sexual behavioral characteristics, as well as the HIV prevalence and HIV intervention levels (eg, condom use and male circumcision66,70) among relevant risk groups over time. Newly infected individuals progressed through an acute infection stage, then chronic stages, associated with HIV mortality (see Figures 1b, 8, Supplemental Digital Content, http://links.lww.com/QAI/B630). PLHIV could initiate and drop out from ART and a fraction of treated PLHIV were virally suppressed and experienced reduced HIV-related mortality and infectiousness.
The 2 models represented FSW (women reporting commercial sex as their major source of income), movement in and out of sex work [ending engagement in commercial sex and/or migration (Cotonou model only, information not available for FSW in Yaoundé)],71 and their clients. Each risk group could form heterosexual noncommercial (main and casual) partnerships. In the Yaoundé model, FSW could form sexual partnerships with all male groups,43,61 but commercial sex with clients only, whereas they could only form partnerships with clients in the Cotonou model, because of lower reported numbers of noncommercial sex acts by FSW in Cotonou, and the high proportion of seasonal migrants in this group (see Figures 1a, 7, Supplemental Digital Content, http://links.lww.com/QAI/B630). The Yaoundé model also included younger (aged 15–24 years) and older (aged 25–49 years) MSM because of their very high HIV prevalence.43 The Cotonou model did not represent MSM but represented part-time sex workers72 (ie, “bar girls”), who were excluded from the outcome calculations for the FSW group.
The per-capita rate at which HIV-uninfected people acquire HIV in each model depended on the frequency of commercial and noncommercial partnerships, number of sex acts per partnership with and without condoms, sexual mixing between groups, as well as HIV prevalence, viral suppression and disease stage among partners, and efficacies of different interventions (see equations in supplement, Supplemental Digital Content, http://links.lww.com/QAI/B630).
Parameterization and Calibration
Multiple data sources informed the parameters and fitted outcomes for the 2 models (Tables 1–3 and Figures 2–6 for Yaoundé; Tables 4–9 and Figures 9–18 for Cotonou, Supplemental Digital Content, http://links.lww.com/QAI/B630). Data included recent KP surveys in Yaoundé43,63,73–79 and Cotonou,44,72,80–91 as well as general population surveys.66–68,75,76,81,85–87,92–101 Both models were fitted within a Bayesian framework, accounting for uncertainties in parameters (eg, number of partners) and fitted data (eg, HIV prevalence).
Model Analysis: Base-Case Scenario
We defined a “base–case” scenario for each model, reflecting the expected course of the HIV epidemic in the absence of COVID–19-related disruptions over 2020–2024.
The base–case scenarios project that the UNAIDS 90-90-90 targets would not have been achieved by 2024. The fraction of PLHIV virally suppressed is expected to increase from 43% to 50% over 2020–2024 in Yaoundé, and from 44% to 45% over 2020–2024 in Cotonou (Table 1). The proportion of individuals on ART who are virally suppressed (80% and 70% in Yaoundé and Cotonou, respectively), and condom use within the different partnership types, was conservatively assumed to remain fixed at their last reported levels throughout the simulations (Figures 4 and 16, Supplemental Digital Content, http://links.lww.com/QAI/B630). Pre-exposure prophylaxis is not available in either country outside of KP-specific demonstration projects,102 and so is not modeled.
Model Analyses: Disruption Scenarios
We defined several disruption scenarios informed by the first empirical studies describing worldwide disruptions in HIV prevention/treatment and sexual partnerships due to COVID-19.16,20,22,23,29,35,57,58,103–105
These temporary disruptions were simulated independently and in different combinations when applied to the entire population or restricted to specific subgroups. The Table 10, Supplemental Digital Content, http://links.lww.com/QAI/B630 details the scenarios as well as their justification and plausibility with more details.
We considered potential disruptions in levels of ART initiation and adherence due to reduced outreach and access to ART clinics, as well as ART supply issues. Early data from Cameroon suggest a 32% decrease in ART initiations during January–June 2020 compared with January–June 2019106 (despite Cameroon having implemented free ART testing and treatment in January 2020), as well as a 10% decrease in the proportion of PLHIV on ART who are virally suppressed in June 2020 compared with December 2019 (80% vs 88%).57 Several African countries (n = 7) reported being at medium or high risk of ART stockout in a World Health Organisation (WHO) survey conducted between April and June 2020.9 However, this survey also showed that African national health systems prescribed ART for 3–6 months, which could increase the resilience of ART access channels. We considered reductions in overall condom use resulting from reduced outreach and potential disruption in condom provision.29,104,105 Larger reductions were anticipated to occur among KP because of their higher dependency on HIV programs, as well as the larger impact of the COVID-19 response on their income.25,59 In Cotonou, 21% of FSW have reported a decrease in the distribution of free condoms due to the COVID-19 response.58 In the 2016 IBBS in Cameroon, FSW reported being offered more money for sex without a condom an average of 5.2 times in the past month, and accepted more money for sex without a condom an average of 2.3 times.43 There are no data on the price of sex by condom use in Yaoundé, whereas the reported median price received by FSW in Cotonou for a condomless sex acts was 4000 franc de la Communauté financière africaine, vs 2250 franc de la Communauté financière africaine for sex act with a condom,107 suggesting that the loss of income due to economic circumstances could lead to a decrease in condom use by FSW. Finally, we considered reductions in number of casual and commercial partners because of increased physical distancing. Although no data are currently available on changes in partner numbers in Africa, it has been reported by many studies from high-income countries, and suggested by African press articles.22,23,103,104 Significant reductions in the number of commercial partners are suggested by a recent survey in Cotonou where 88% of surveyed FSW reported that clients were “rare” in hotspots.58
We defined as single disruptions reductions in (1) ART initiations, (2) proportion of PLHIV on ART who are virally suppressed, (3) condom use, (4) number of casual partnerships among all risk groups, and (5) commercial partnerships between FSW and their clients (details in Table 2). We explored 2 specific condom use scenarios: reductions within commercial (3a) and noncommercial partnerships (3b) separately. In addition, 3 setting-specific scenarios (reflecting specificities in HIV epidemics and models) were defined as follows: reductions in (6) HIV testing in Cotonou, (7) number of noncommercial partnerships between MSM in Yaoundé, and (8) condom use to the same levels as among the lower-risk population among MSM, FSW, and clients in Yaoundé (corresponding to >50% decrease in their condom use), which reflects higher HIV prevention efforts and intervention use among KP compared with lower-risk groups. Two Cotonou-specific scenarios explored the closure of bars and cessation of sex work by bar girls assuming either that (9a) there is no concomitant increase in commercial sex of clients with full-time FSW to compensate for the contacts “lost with bar girls”, or (9b) the client demand for sex work with bar girls is maintained and instead met by full-time FSW.
TABLE 2.
Overall Impact of Risk group–specific Disruptions: Relative Increase in New HIV Infections and HIV-Related Deaths overall Over 1 year Due to: A) Cessation of New ART Initiations and 50% Reduction in the Proportion of Virally Suppressed Among Those on ART in all and Specific Risk Groups and B) 50% Reduction in all Condom Use in all and Specific Partnerships (Impact on HIV Incidence Only)
| % HIV-Related Deaths | % New HIV Infections | |||
| Yaoundé | Cotonou | Yaoundé | Cotonou | |
| A) | ||||
| Affected risk group | ||||
| All | 15.7% (11.2–19.0) | 22.4% (11.8–46.9) | 21.4% (18.3–26.0) | 19.0% (10.5–32.7) |
| FSW and clients | 1.7% (0.9–2.7) | 3.3% (1.5–7.9) | 5.5% (3.0–8.5) | 10.3% (5.3–17.6) |
| Non-FSW and non-clients | 13.7% (10.0–17.5) | 19.5% (9.4–39.2) | 16.2% (12.5–20.1) | 9.0% (4.9–15.9) |
| FSW | 0.7% (0.3–1.6) | 0.3% (0.2–0.5) | 2.3% (0.8–4.4) | 3.8% (1.9–6.3) |
| Clients | 1.0% (0.4–1.6) | 3.1% (1.3–7.6) | 3.2% (1.5–5.0) | 6.0% (2.7–12.5) |
| MSM | 1.1% (0.4–1.6) | NA | 5.5% (2.1–8.5) | NA |
| FSW, clients, and MSM | 2.9% (1.7–3.9) | NA | 11.1% (6.5–14.6) | NA |
| Non-FSW, non-clients, and non-MSM | 12.6% (8.8–16.6) | NA | 11.1% (6.7–15.3) | NA |
| B) | ||||
| Affected partnerships | ||||
| All | 22.6% (14.6–31.1) | 23.1% (12.3–48.6) | ||
| FSW and clients with all partners | 8.5% (4.8–13.7) | 21.4% (11.4–46.8) | ||
| Between FSW and clients | 4.5% (2.0–9.3) | 18.5% (8.9–42.0) | ||
| Non-FSW and non-clients with all partners | 17.3% (11.2–25.2) | 4.2% (2.6–6.3) | ||
| Between non-FSW and nonclients | 14.2% (7.7–21.1) | 1.5% (0.8–2.6) | ||
| MSM with all partners | 10.6% (4.7–17.9) | NA | ||
| FSW with all partners | 5.9% (2.6–10.8) | 18.5% (8.9–42.0) | ||
| Clients with all partners | 7.0% (4.0–12.2) | 21.4% (11.4–46.8) | ||
| MSM, FSW, and clients with all partners | 18.9% (13.1–28.4) | NA | ||
| Between MSM, FSW, and clients | 10.9% (6.3–17.0) | NA | ||
| Non-MSM, non-FSW, and non-clients with all partners | 10.3% (6.4–16.0) | NA | ||
| Between non-MSM, non-FSW, and non-clients | 3.5% (1.4–6.9) | NA | ||
NA, not available as not modeled in Cotonou.
The second set of scenarios combined the 5 main single disruptions additively (eg, 1, 1 + 2, …, 1 + 2 + 3 + 4 + 5) to assess the impact of simultaneous disruptions. We explored reductions of 10/25/50% in each scenario. In scenario 1, we also examined no ART initiations. The “most realistic” scenario, exploring reductions of 10% in levels of HIV viral suppression, large reductions in ART initiations, as well as decreases in condom use and risky sex, best reflected early empirical estimates from Cameroon's National AIDS Control Committee and a recent survey among FSW in Cotonou.57,58
Finally, the third set explored disruptions to ART initiations and viral suppression (1 + 2) and condom use (3) among KPs alone, to estimate what proportion of the overall impact was attributable to these groups.
The indirect impacts of COVID-19 on HIV outcomes were calculated as the relative change (expressed as a percentage of the base-case) in new HIV infections and HIV-related deaths over 1 and 5 years from the start of disruptions [assumed to last 6 months (primary analysis) and 3 and 12 months (sensitivity analyses)] starting in January 2020 in Cotonou, and April 2020 in Yaoundé). These impacts were evaluated for the overall population and within each risk group. We report the median and 95% uncertainty interval of the predicted disruption impact.
RESULTS
Single HIV Prevention/Treatment and Sexual Behavior Disruptions—Overall Impact
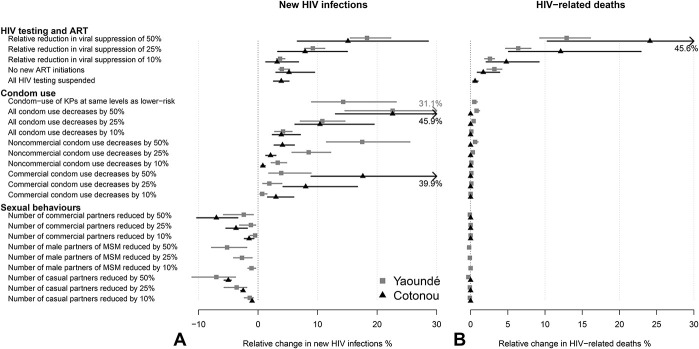
Our model-predicted impacts of COVID-19 on HIV outcomes were of similar magnitude in Cotonou and Yaoundé. We found that COVID–19-related changes in condom use may impact HIV incidence slightly more than disruptions to ART and viral suppression but have a negligible impact on HIV-related mortality compared with any of the ART disruptions (Fig. 1).
FIGURE 1.
First set of scenarios: relative change in the cumulative number of HIV infections (A) and HIV-related deaths (B) over 1 year under individual scenarios assuming 6-month disruptions, compared with base–case in Yaoundé (gray) and Cotonou (black). Dots represent median estimates across model predictions, whereas lines represent 95% UI (2.5th and 97.5th percentile of the estimates). UI, uncertainty interval.
Our worst-case single scenario (temporary 50% reduction in viral suppression over 6 months) increased new overall HIV infections by 18% (95% uncertainty interval 16–22) and 14% (7–27) and increased HIV-related deaths by 13% (9–16) and 21% (11–44) over 1 year in Yaoundé and Cotonou, respectively, compared with the base–case (Figs. 1A, B). When assessed over 5 years, the impact of the 6-month disruptions was reduced to 6% (5–7) and 4% (2–7) relative increases in HIV infections, and 4% (3–5) and 4.0% (2–9) increases in HIV-related deaths in Yaoundé and Cotonou, respectively (see Figure 19, Supplemental Digital Content, http://links.lww.com/QAI/B630). Temporarily ceasing ART initiations over 6 months, suspending HIV testing, and 10% reduction in viral load suppression are expected to have small impacts on HIV incidence and mortality (<5%).
A temporary 50% reduction in all condom use over 6 months was predicted to increase new HIV infections by 23% (15–31) and 23% (12–49) over 1 year in Yaoundé and Cotonou, respectively [8% (5–10) and 8% (4–18) over 5 years, see Figure 19, Supplemental Digital Content, http://links.lww.com/QAI/B630]. This will also result in a small increase in HIV-related deaths even over 5 years (≤2%). In Cotonou, the impact of a 6-month 50% decrease in condom use during commercial sex on overall new HIV infections [19% (9–42)] was substantially larger than during noncommercial sex (4% (3–6)), whereas it was the opposite for Yaoundé [4% (2–9) during commercial sex vs 18% (12–26) during noncommercial sex, Figure 1].
The positive impact of 50% reductions in the number of casual or commercial partners on reducing HIV infections was smaller [Yaoundé: 7% (4–11), Cotonou: 5% (4–6) over 1 year] than the negative impact for the corresponding 50% reductions in condom use.
Finally, our model results suggest that closing bars in Cotonou is likely to have a negligible impact on HIV if clients demand for sex work with bar girls was not compensated by an increased demand for full-time FSW. If instead client demand for sex with bar girls was met by full-time FSW over 6 months, annual new HIV infections could increase by 1% (-1-3) and 5% (0%–15%) overall and among FSW, respectively. This modest impact was due to the small number of partnerships of part-time FSW with clients compared with full-time FSW.
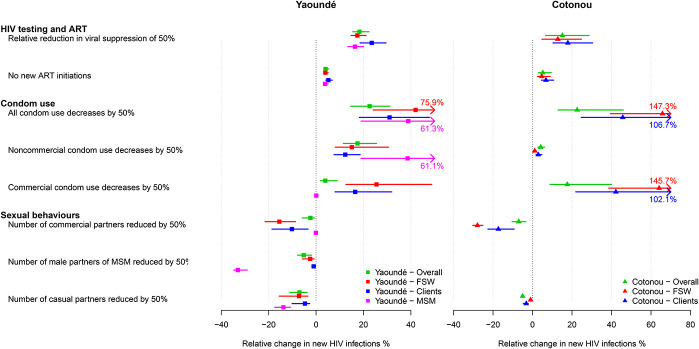
Single HIV Prevention/Treatment and Sexual Behavior Disruptions—Impact Among Different Risk Groups
We estimated that KPs would be more severely impacted by the effects of COVID-19 disruptions compared with the rest of the population. For example, the impact of a 6-month 50% reduction in all condom use on new HIV infections would be 1.7-fold, 1.9-fold, and 1.4-fold larger over 1 year among MSM, FSW, and their clients than among the overall population in Yaoundé, respectively (Fig. 2). Differences were even more pronounced for Cotonou where it would be 3.0-fold and 2.1-fold larger among FSW and their clients than overall, respectively, reflecting higher consistency in condom use during commercial sex in the Cotonou model compared with the Yaoundé model. Estimated impacts on HIV-related mortality and infections over 5 years are shown in Figures 20–22, Supplemental Digital Content, http://links.lww.com/QAI/B630.
FIGURE 2.
Impact of disruptions among risk groups: relative change in the cumulative number of new HIV infections in Yaoundé (squares) and Cotonou (triangles) over 1 year under individual scenarios assuming 6-months disruptions, calculated overall (green dots and lines), among MSM (pink dots and lines), FSW (red dots and lines), and their clients (blue dots and lines). Dots represent median point estimates across model predictions, whereas lines represent 95% UI (2.5th and 97.5th percentile of the estimates).
Combined Disruptions—Impact Among Specific Risk Groups and Overall
Our models predicted qualitatively similar impacts of combined disruptions on HIV outcomes across the 2 cities (Fig. 3). Although the negative impact of combined ART and condom provision disruptions could be substantial, the effect on HIV infections, but not mortality, could be partially offset (and largely among FSW and their clients) by a reduction in numbers of partners.
FIGURE 3.
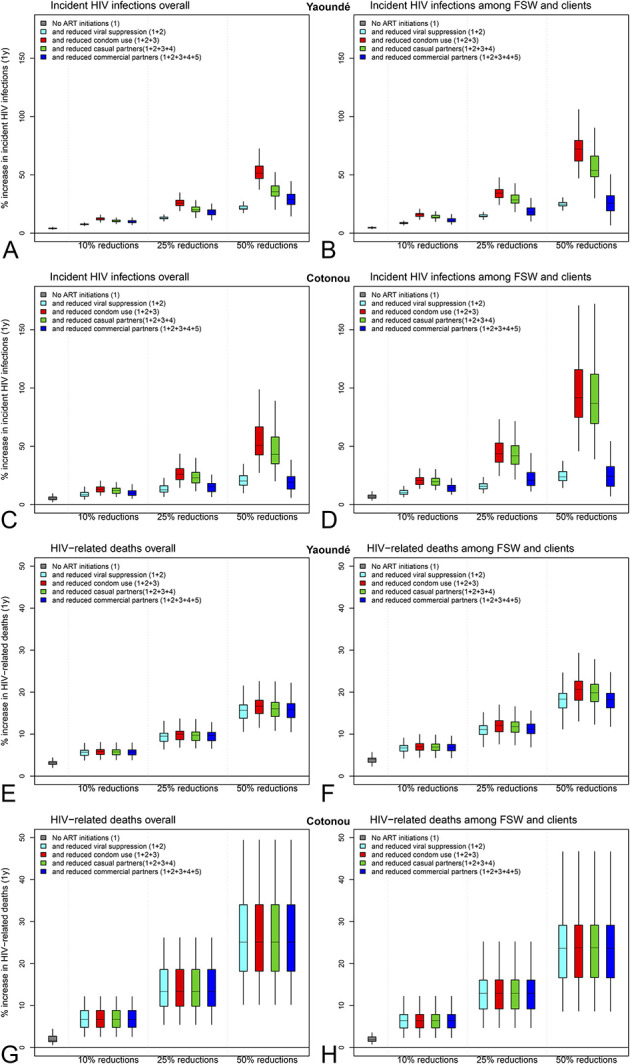
Impact of combined disruption scenarios: relative change in the cumulative number of new HIV infections among (A) overall population and (B) FSW and their clients in Yaoundé, (E) overall population and (F) FSW and their clients in Cotonou, and HIV-related deaths over 1 year among (C) overall population and (D) FSW and their clients in Yaoundé, (G) overall population and (H) FSW and their clients in Cotonou under additive combined scenarios assuming no ART initiations and 10/25/50% reductions. Boxplots represent median, interquartile range and 95% UI across model predictions.
The most realistic scenario, best reflecting early data from the cities (assuming a 3-month universal cessation of new ART initiations, and 10% reduction in viral suppression coverage, condom use, and number of casual and commercial partnerships) led to an overall increase in annual new HIV infections of less than 10% in both cities (Figure 23, Supplemental Digital Content, http://links.lww.com/QAI/B630).
In our worst-case combined scenario [6-month concomitant universal cessation of new ART initiations, 50% reduction in viral suppression coverage and in condom use (scenarios 1 + 2 + 3)] overall 51% (39–67) and 51% (29–90) more new HIV infections over 1 year were predicted in Yaoundé and Cotonou (Figs. 3A, C), respectively. This impact is double what was achieved for any of the 5 single disruptions alone, and 1.4-fold and 1.9-fold higher among FSW and clients combined compared with the overall in Yaoundé and Cotonou, respectively (Figs. 3B–D).
However, if these disruptions coincide with a 50% reduction in casual and commercial partnerships (scenarios 1 + 2 + 3 + 4 + 5), the impact on new HIV infections is attenuated to 29% (19–42, corresponding to 990 (710–1420) excess infections) and 19% (8–36, corresponding to 60 (30–130) excess infections) in Yaoundé and Cotonou over 1 year, with little heterogeneity in impact across risk groups, apart from among MSM in Yaoundé for which it was 1.2-fold higher than overall.
The estimated increases in HIV-related mortality in combined scenarios (1 + 2 + 3 + 4 + 5) were around 20% in both cities [corresponding to 390 (264–484) and 150 (80–270) excess HIV-related deaths over 1 year], among FSW and their clients combined and overall (Figs. 3E–H). However, the specific estimates among FSW alone were 2.1-fold and 1.6-fold higher than overall in Yaoundé and Cotonou, respectively (not shown). The estimated impacts of combined disruptions over 5 years and impacts among MSM, FSW, and clients combined were more modest (see supplement, Supplemental Digital Content, http://links.lww.com/QAI/B630).
ART and Condom Use Disruptions Among Specific Risk Groups—Overall Impact
In both settings, KPs contributed disproportionately to the overall impact of disruptions on new HIV infections. We estimated that a 50% reduction in viral load suppression among MSM, FSW, and their clients increased new HIV infections by over half the amount incurred by 50% reduction in all groups. (Table 2). Similarly, the overall impact of disruptions in condom use on new HIV infections in Cotonou could be mainly due to disruptions among FSW and their clients (21% vs 23% overall) and mainly due to disruptions among KP in Yaoundé (19% vs 23% overall). However, most of the impact on HIV-related mortality was due to reductions in lower-risk populations as they comprise most PLHIV on ART in both cities (Table 2).
Sensitivity Analysis: Impact of Shorter/Longer Combined Disruptions
Both models estimated that the overall impact of universal disruptions to prevention and treatment interventions on new HIV infections and HIV-related deaths would vary relatively proportionately with their duration (Figure 24, Supplemental Digital Content, http://links.lww.com/QAI/B630). The exception was disruptions related to HIV testing in Cotonou and ART initiation in Yaoundé, for which longer disruptions were associated with proportionately lower relative impact on HIV outcomes.
DISCUSSION
The results presented here suggest that HIV prevention and treatment disruptions due to COVID-19 are likely to disproportionately impact KP and that severe disruptions (50% reduction in HIV prevention and treatment levels over 6 months) may increase overall HIV infections and HIV-related mortality by 1.5-fold in Yaoundé and Cotonou. Although additional HIV infections due to COVID-19 would impact the quality of life of populations, they would not necessarily translate into additional future HIV-related deaths if HIV treatment coverage quickly increases above pre-COVID-19 levels, after a resumption of full services. Most HIV-related deaths would be due to declines in the proportion of PLHIV that were virally suppressed or initiated on ART.
Our most realistic scenario assuming modest reductions (10%) in levels of viral suppression, condom use, and nonsteady partnerships, as well as a substantial decrease in the number of new ART initiations over a period of 3 months was consistent with early data collected in both countries during the first wave of the COVID-19 pandemic. The impact of these disruptions on HIV outcomes in both cities was relatively modest, with less than a 10% increase in annual new HIV infections. Early information for sub-Saharan Africa, such as the WHO Global AIDS Monitoring data9 suggest that ART delivery and countries responses, for example through multimonth dispensing of ART, has reduced ART disruptions during the first COVID-19 pandemic wave in Africa, indicating that our scenarios reflecting severe disruptions should be considered as relatively unlikely. That said, both models suggest that even small disruptions to ART could lead to substantial increases in number of HIV-related deaths. More data could become available and show more severe disruptions; in that case, our scenarios would be useful to determine whether these disruptions are likely to have a large impact on HIV.
Our analysis highlights the disproportionate impact of COVID-19 disruptions on KPs. These populations are already more vulnerable to HIV, often living in precarious financial situations,28 and our findings suggest that high levels of HIV prevention and treatment must be maintained to minimize the overall impact of disruptions on new infections. Sex work and sex between men are legal in Benin, but still criminalized in Cameroon where KPs are subjected to increased arbitrary arrests during the COVID-19 era,108 which means that they hide even more, making it difficult to reach them, especially in a context where street outreach activities are reduced for legitimate safety concerns.17,18 In both models, a modest reduction in condom use, especially during commercial sex,55 translated into a substantial increase in absolute numbers of condomless sex acts. For example, a 50% decrease in condom use during commercial sex resulted in a 6.8-fold increase in number of condomless acts for FSW in Cotonou, reflecting the achievements and importance of programs leading to extremely frequent condom use during commercial sex.
Our estimates of the impact of 6-month universal interruptions of ART initiation on new HIV infections and HIV-related deaths (∼4% and 2% increase) were similar to published impact estimates across sub-Saharan Africa.38,109 For example, a 5-model comparison exercise by Jewell et al38 suggested ART interruptions could increase new HIV infections and HIV-related deaths by 0%–4% and 0%–6%, respectively. Our estimated impact from a 50% decrease in viral load suppression for 6 months among treated PLHIV was also similar to estimates from the 5-model exercise.38 However, our models predicted a larger impact from a 50% reduction in condom use on new HIV infections (23% increase in both cities) compared with the 5-model study [range: 3% (0–13) to 19% (7–30) across models38] due to higher levels of baseline condom use among PLHIV in our study settings.
Our models predicted that the negative impact of HIV-related service disruptions could only be partially offset by changes in sexual partnerships. Temporary reductions in sexual partnerships had less impact than temporary reductions in condom use due to high levels of condom use among KP before the COVID-19 outbreak in these cities. We found that changes in sexual partnerships, and especially commercial sex in Cotonou, could partly offset the impact of service disruptions on new HIV infections, but would not compensate for the impact of an equal reduction in viral suppression alone (Fig. 3). Thus, taken together with the disproportionate impact of HIV-related service disruptions on KPs, our findings underscore the need for maintaining successful condom promotion and use among KPs through safe access of services. Early program data on intervention levels among KPs in West and Central Africa suggest increasing and decreasing trends of the number of FSW reached by programs in Cote d'Ivoire and Togo, respectively, over January-July 2020, a decrease in the number of MSM reached by interventions in Togo over 2020, and substantial decreases in the number of condoms distributed to MSM in Togo over 2020.110
Although the overall impact of the different disruptions was qualitatively similar across the 2 cities, our analysis highlighted some differences reflecting variation in underlying epidemic dynamics and intervention coverage. Commercial sex partnerships and condom use are more important determinants of HIV transmission in Cotonou compared with Yaoundé, because the HIV epidemic is more concentrated among FSW and their clients in Cotonou (despite the relatively smaller size of the FSW and part-time sex worker populations in Cotonou). The level of overall viral suppression is similar across the 2 cities; however, 70% of FSW living in HIV in Yaoundé are virally suppressed, compared with less than 60% in recent surveys among FSW in Cotonou, ART adherence being substantially affected by high turnover and migration in this population.111 Nevertheless, the similarities suggest that our results may be broadly applicable to places where KPs such as FSW are already disproportionately affected by HIV.
Strengths of our analysis include comprehensive reviews of city-specific and risk group–specific data over time to characterize the dynamics of the modeled populations and the HIV epidemic in 2020. To the best of our knowledge, this is the first analysis of the impact of COVID-19 disruptions on HIV outcomes among KPs, allowing us to better identify how to minimize disruption impacts. Limitations include simulating disruption scenarios derived from early published studies and reports of HIV prevention/treatment services and of sexual behaviors affected by COVID-19, most of which were conducted outside Africa. The use of hypothetical magnitudes of disruptions was due to the absence of quantitative data from Cotonou and scarcity of data for Yaoundé106 (which only describes reductions in ART initiations) at the time of this analysis. Thus, an important next phase of the study includes empiric data characterizing service disruptions and changes in the practice of sex work, sex between men, and condomless sex. Combining hypothetical magnitudes of disruptions with mathematical models allowed the understanding of which specific magnitudes of disruptions would induce “tolerable” increases in new HIV infections or deaths (eg, <5% over 1 year), and conversely, which disruption levels must be prevented. Our approach will allow the assessment of the potential impact of COVID–19-related disruptions on HIV epidemics in Central and West Africa when more data become available. However, our study highlights the fact that “tolerable” increases at the overall level might obscure much more significant impacts among KPs. Our models only accounted for the secondary and indirect effects of the COVID-19 pandemic and did not account for direct and specific COVID-19 mortality among PLHIV.112–116 We did not explicitly model other potential consequences of the economic fallout from COVID-19 on commercial sex, such as an increase in riskier (eg, condomless) commercial sex,117,118 which could amplify the negative impact of the COVID-19 outbreak on the local HIV epidemics.
To conclude, the COVID-19 pandemic might have a substantive negative impact on new HIV infections and deaths, especially among key populations, undermining recent efforts in controlling the HIV epidemic across Central and West Africa.119 Innovative approaches addressing and maintaining ART services for all PLHIV, and in particular, the HIV prevention and treatment needs of KPs—including their safe access—would constitute an efficient way to minimize negative impacts on new HIV infections in subsequent waves of the COVID-19 pandemic.
Footnotes
This work was partly supported by the HPTN Modelling Centre, which is funded by the NIH (NIH UM1 AI068617) through the HPTN Statistical and Data Management Center. R.S., L.G., K.M.M., and M.C.B. acknowledge funding from the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 program supported by the European Union. L.G. is funded by an MRC-DTP scholarship. P.V. also acknowledges support from the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at the University of Bristol. A.B. is supported by an Australian National Health and Medical Research Council Early Career Fellowship. M.M.R. is supported by Charles A. King Trust Postdoctoral Research Fellowship. M.A. acknowledges support from the Canadian Institutes of Health Research (grant # FDN-143218) and the Bill & Melinda Gates Foundation (grant # OPP-1098973), outside the submitted work. M.M.G. and S.M. are supported Tier 2 Canada Research Chairs. The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated, NIHR, World Health Organization, MRC.
K.M.M. has received an honorarium from Gilead for speaking, outside the submitted work. The remaining authors have conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Lily Geidelberg, Email: l.geidelberg@ic.ac.uk.
Kate M. Mitchell, Email: kate.mitchell@imperial.ac.uk.
Sharmistha Mishra, Email: sharmisthak@gmail.com.
Dobromir Dimitrov, Email: ddimitro@scharp.org.
Anna Bowring, Email: anna.bowring@burnet.edu.au.
Luc Béhanzin, Email: bphilus2013@gmail.com.
Fernand Guédou, Email: guedaf@yahoo.fr.
Souleymane Diabaté, Email: dsouleym@hotmail.com.
Sheree Schwartz, Email: sschwartz@jhu.edu.
Serge C. Billong, Email: sergebillong@yahoo.fr.
Iliassou Mfochive Njindam, Email: imfochi1@jhu.edu.
Daniel Levitt, Email: daniel_levitt@me.com.
Christinah Mukandavire, Email: cmukandavire@gmail.com.
Mathieu Maheu-Giroux, Email: m.maheu.giroux@gmail.com.
Shona Dalal, Email: dalals@who.int.
Peter Vickerman, Email: Peter.Vickerman@bristol.ac.uk.
Stefan Baral, Email: sbaral@jhu.edu.
Michel Alary, Email: michel.alary@crchudequebec.ulaval.ca.
Marie-Claude Boily, Email: marieclaude.boily@gmail.com.
REFERENCES
- 1.World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed May 8, 2020. [Google Scholar]
- 2.El-Sadr WM, Justman J. Africa in the path of covid-19. N Engl J Med. 2020;383:e11. [DOI] [PubMed] [Google Scholar]
- 3.Drain PK, Garrett N. SARS-CoV-2 pandemic expanding in sub-Saharan Africa: considerations for COVID-19 in people living with HIV. EClinicalMedicine. 2020;22:100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adepoju P. Tuberculosis and HIV responses threatened by COVID-19. Lancet Hiv. 2020;7:e319–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amimo F, Lambert B, Magit A. What does the COVID-19 pandemic mean for HIV, tuberculosis, and malaria control? Trop Med Health. 2020;48:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiau S, Krause KD, Valera P, et al. The burden of COVID-19 in people living with HIV: a syndemic perspective. Aids Behav. 2020;24:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sands P. HIV, tuberculosis, and malaria: how can the impact of COVID-19 be minimised? Lancet Glob Health. 2020;8:e1102–e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Global Fund. Majority of HIV, TB and Malaria Programs Face Disruptions as a Result of COVID-19. Available at: https://www.theglobalfund.org/en/covid-19/news/2020-06-17-global-fund-survey-majority-of-hiv-tb-and-malaria-programs-face-disruptions-as-a-result-of-covid-19. Accessed April 7, 2020. [Google Scholar]
- 9.World Health Organisation. Disruption in HIV, Hepatitis and STI Services Due to COVID-19. Paper presented at: AIDS2020; July 9, 2020; San Francisco and Oakland.
- 10.United Nations Population Fund. UNFPA Supplies, COVID-19 UPDATE. New York, NY: United Nations Population Fund; 2020. [Google Scholar]
- 11.Luis H, Fridayantara WD, Mahariski P, et al. Evolving ART crisis for people living with HIV in Indonesia. Lancet Hiv. 2020;7:e384–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet Hiv. 2020;7:e308–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyoni T, Okumu M. COVID-19-Compliant strategies for supporting treatment adherence among people living with HIV in sub-saharan Africa. Aids Behav. 2020;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odhiambo F, Mulwa E, Ayieko J, et al. Implementation of HIV care in Western Kenya during corona virus disease 2019 response. AIDS. 2020;34:F1–F2. [DOI] [PubMed] [Google Scholar]
- 15.Quilantang MIN, Bermudez ANC, Operario D. Reimagining the future of HIV service implementation in the Philippines based on lessons from COVID-19. Aids Behav. 2020;24:3003–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagendra G, Carnevale C, Neu N, et al. The potential impact and availability of sexual health services during the COVID-19 pandemic. Sex Transm Dis. 2020;47:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponticiello M, Mwanga-Amumpaire J, Tushemereirwe P, et al. “Everything is a mess”: how COVID-19 is impacting engagement with HIV testing services in rural southwestern Uganda. Aids Behav. 2020;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mhango M, Chitungo I, Dzinamarira T. COVID-19 lockdowns: impact on facility-based HIV testing and the case for the scaling up of home-based testing services in sub-saharan Africa. Aids Behav. 2020;24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Times Live. Almost 11,000 HIV-Positive Patients in Gauteng Have Skipped ARV Collection during Lockdown. 2020. Available at: https://www.timeslive.co.za/news/south-africa/2020-05-19-almost-11000-hiv-positive-patients-in-gauteng-have-skipped-arv-collection-during-lockdown/. Accessed May 21, 2020 [Google Scholar]
- 20.Guo W, Weng HL, Bai H, et al. Quick community survey on the impact of COVID-19 outbreak for the healthcare of people living with HIV [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:662–666. [DOI] [PubMed] [Google Scholar]
- 21.Mukwenha S, Dzinamarira T, Mugurungi O, et al. Maintaining robust HIV and TB services in the COVID-19 era: a public health dilemma in Zimbabwe. Int J Infect Dis. 2020;100:394–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow EPF, Hocking JS, Ong JJ, et al. Changing the use of HIV pre-exposure prophylaxis Among men who have sex with men during the COVID-19 pandemic in melbourne, Australia. Open Forum Infect Dis. 2020;7:ofaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammoud MA, Maher L, Holt M, et al. Physical distancing due to COVID-19 disrupts sexual behaviours among gay and bisexual men in Australia: implications for trends in HIV and other sexually transmissible infections. J Acquir Immune Defic Syndr. 2020;85:309–315. [DOI] [PubMed] [Google Scholar]
- 24.Tan RKJ, Lim JM, Lo JJ, et al. Conducting rapid qualitative research to support sex workers' health and social needs in the face of COVID-19: capitalising on stakeholder networks from the HIV response in Singapore to drive policymaking. Sex Transm Infect. 2021;97:84. [DOI] [PubMed] [Google Scholar]
- 25.Kimani J, Adhiambo J, Kasiba R, et al. The effects of COVID-19 on the health and socio-economic security of sex workers in Nairobi, Kenya: emerging intersections with HIV. Glob Public Health. 2020;15:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:538–549. [DOI] [PubMed] [Google Scholar]
- 27.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNAIDS. Sex Workers Must Not Be Left behind in the Response to COVID-19 [press Release]. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]
- 29.Lamontagne E. COVID-19 Pandemic Increases Socioeconomic Vulnerability of LGBTI+ Communities and Their Susceptibility to HIV. Paper presented at: IAS 2020; July 6, 2020; San Francisco, CA.
- 30.Newman PA, Guta A. How to have sex in an epidemic redux: reinforcing HIV prevention in the COVID-19 pandemic. Aids Behav. 2020;24:2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Operario D, King EJ, Gamarel KE. Prioritizing community partners and community HIV workers in the COVID-19 pandemic. Aids Behav. 2020;24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CARE Cameroon. Impact of COVID-19 on CHAMP. Atlanta, GA: Care; 2020. [Google Scholar]
- 33.Logie CH, Turan JM. How do we balance tensions between COVID-19 public health responses and stigma mitigation? Learning from HIV research. Aids Behav. 2020;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao A, Rucinski K, Jarrett B, et al. Potential interruptions in HIV prevention and treatment services for gay, bisexual, and other men who have sex with men associated with COVID-19. medRxiv. 2020. DOI: 10.1097/QAI.0000000000002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callander D, Meunier E, DeVeau R, et al. Investigating the effects of COVID-19 on global male sex work populations: a longitudinal study of digital data. Sex Transm Infect. 2021;97:93–98. [DOI] [PubMed] [Google Scholar]
- 36.Campbell R, Sanders T, Hassan R, et al. Global Effects of COVID-19, government restrictions and implications for sex workers: a focus on Africa. LIAS Working Paper Ser. 2020;3. [Google Scholar]
- 37.Jewell BL, Smith JA, Hallett TB. The potential impact of interruptions to HIV services: a modelling case study for South Africa. medRxiv. 2020. DOI: 10.1016/j.eclinm.2020.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet Hiv. 2020;7:e629–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukandavire C, Walker J, Schwartz S, et al. Estimating the contribution of key populations towards the spread of HIV in Dakar, Senegal. J Int AIDS Soc. 2018;21(suppl 5):e25126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maheu-Giroux M, Vesga JF, Diabate S, et al. Changing dynamics of HIV transmission in Cote d'Ivoire: modeling who acquired and transmitted infections and estimating the impact of past HIV interventions (1976-2015). Jaids-J Acq Imm Def. 2017;75:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams JR, Alary M, Lowndes CM, et al. Positive impact of increases in condom use among female sex workers and clients in a medium HIV prevalence epidemic: modelling results from project SIDA1/2/3 in Cotonou, Benin. PLoS One. 2014;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns Hopkins School of Public Health. 2016 Integrated Biological and Behavioral Surveillance (IBBS) survey among female sex workers and men who have sex with men in Cameroon, Final Report, Yaoundé, 2018. Available at: http://onsp.minsante.cm/sites/default/files/publications/245/IBBS2016_preliminaryreport_final_Mar2017_linked.pdf.
- 44.Ministry of Health, Benin. Enquête de surveillance de deuxième génération relative aux IST, VIH et SIDA au Bénin (ESDG-2015): Professionnelles de Sexe & Serveuses de Bar et Restaurants. Cotonou, Bénin: Benin Ministry of Health; 2015. [Google Scholar]
- 45.Hessou PHS, Glele-Ahanhanzo Y, Adekpedjou R, et al. Comparison of the prevalence rates of HIV infection between men who have sex with men (MSM) and men in the general population in sub-Saharan Africa: a systematic review and meta-analysis. Bmc Public Health. 2019;19:1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Republique du Cameroun. Coronavirus: mesures instruites par le Président Paul BIYA [press release]. Yaoundé, Cameroon: République du Cameroun; 2020. [Google Scholar]
- 47.Le business du sexe en confinement à Yaoundé. 2020. Available at: https://www.camer.be/79743/11:1/cameroun-le-business-de-sexe-en-confinement-a-yaounde-cameroon.html. Accessed July 30, 2020. [Google Scholar]
- 48.BBC. Le Cameroun assouplit ses mesures de lutte contre le Covid-19, 2020. Available at: https://www.bbc.com/afrique/52496808. Accessed May 13, 2020. [Google Scholar]
- 49.Gouvernement de la R. é. publique du B. é. nin. CORONAVIRUS - Communiqué du Ministre de l'intérieur au sujet du Cordon Sanitaire, 2020. Available at: https://www.gouv.bj/actualite/571/coronavirus---communique-ministre-interieur-sujet-cordon-sanitaire/. Accessed July 10, 2020. [Google Scholar]
- 50.lanationbenin.info. Fermeture des bars Vip, discothèques et boîtes de nuit: Les promoteurs implorent l’indulgence des autorités, 2020. Available at: https://lanationbenin.info/fermeture-des-bars-vip-discotheques-et-boites-de-nuit-les-promoteurs-implorent-lindulgence-des-autorites/. Accessed January 4, 2021. [Google Scholar]
- 51.Guardian.ng. CAF Approves Cameroon 2021 CHAN to Hold with Fans, 2021. Available at: https://guardian.ng/sport/caf-approves-cameroon-2021-chan-to-hold-with-fans/. Accessed January 20, 2021 [Google Scholar]
- 52.Republique du Cameroun. Evaluation des effets socioeconomiques du Coronavirus (COVID-19) Au Caneroun—Phase 1. Yaoundé, Cameroon: République du Cameroun; 2020. [Google Scholar]
- 53.76crimesfr.com. Cameroun : Arrestation de 50 personnes présumées LGBTQI à Bafoussam. Available at: https://76crimesfr.com/2020/05/25/cameroun-arrestation-de-50-personnes-presumees-lgbtqi-a-bafoussam/. Accessed July 10, 2020. [Google Scholar]
- 54.cameroun24.net. Une cinquantaine de prostituées arrêtée pour avoir eu des contacts avec des personnes mise en quarantaine. Available at: https://www.cameroun24.net/actualite-cameroun-info-UnU_iinqiiniiinU_dU_prosiiii_C3_A9Us_irr_C3_AAi_C3_A9U_poir_iv-54078.html. Accessed June 8, 2020. [Google Scholar]
- 55.UNAIDS. COVID-19 Responses Must Uphold and Protect the Human Rights of Sex Workers [press Release]. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]
- 56.Comité National de Lutte Contre le Sida. Le ministere de la sante publique officialise la gratuite de la prise en charge des patients, 2019. Available at: http://www.cnls.cm/actualit%C3%A9s/cameroun-%E2%80%93-vih-sida-le-ministere-de-la-sante-publique-officialise-la-gratuite-de-la-prise. Accessed September 6, 2019.
- 57.Republique du Cameroun. Rapport Semestriel 2020 Des Activites De Lutte Contre Le VIH, Le SIDA et Les IST. Yaounde, Cameroon: République du Cameroun; 2020. [Google Scholar]
- 58.Guedou FA. Rapport sur le volet impact de COVID-19 sur la prévention de VIH: extrait de l’enquête évaluative de l’utilisation de l'autotest VIH salivaire chez les TS à Cotonou et environs. Québec, Canada: Université Laval; 2020. [Google Scholar]
- 59.FHI 360. Strategic Considerations for Mitigating the Impact of COVID-19 on Key-Population-Focused HIV Programs. Durham, NC: FHI360; 2020. [Google Scholar]
- 60.Lesosky M, Myer L. Modelling the impact of COVID-19 on HIV. Lancet Hiv. 2020;7:e596–e598. [DOI] [PubMed] [Google Scholar]
- 61.Silhol R, Baral S, Bowring AL, et al. Quantifying the evolving contribution of HIV interventions and key populations to the HIV epidemic in Yaounde, Cameroon. J Acquir Immune Defic Syndr. 2021;86:396–405. [DOI] [PubMed] [Google Scholar]
- 62.Geidelberg L, Mitchell KM, Alary M, et al. A mathematical model impact analysis of a real-life pre-exposure prophylaxis and treatment-as-prevention study among female sex workers in Cotonou, Benin. J Acquir Immune Defic Syndr. 2021;86:e28–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Calleja JM, Ngoumou P, Boupda A. HIV Seroprevalence Study Among Commercial Sex Workers in Yaounde and Douala. Yaounde, Cameroon; 1992. [Google Scholar]
- 64.The Global HIV/AIDS Program World Bank. West Africa HIV/AIDS Epidemiology and Response Synthesis: Implications for Prevention. Washington, DC: The world bank; 2008. [Google Scholar]
- 65.Ministry of Health Benin. Enquête de surveillance de deuxième génération relative aux IST, VIH et SIDA au Bénin (ESDG‐2015): Clients des TS, Camionneurs et Personnes Privées de Liberté. Cotonou, Benin: Benin Ministry of Health; 2015. [Google Scholar]
- 66.République du Cameroun. Enquête démographique et de santé et à indicateurs multiples (EDS-MICS) 2011. Yaoundé, Cameroon: République du Cameroun; 2012. [Google Scholar]
- 67.PEPFAR. Cameroon population-based HIV impact assessment, CAMPHIA 2017. In: Summary Sheet: Preliminary Findings. Washington, DC: PEPFAR; 2018. [Google Scholar]
- 68.Ministry of Health Benin. Rapport de l'audit de la file active des personnes vivant avec le VIH au Bénin (Audit report). Cotonou, Benin: Ministry of Health Bénin; 2017. [Google Scholar]
- 69.Mboup A, Béhanzin L, Guédou FA, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc. 2018;21:e25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.République du Bénin. Enquête Démographique et de Santé (EDSB-IV) 2011-2012, Benin. Cotonou, Bénin: République du Bénin; 2013. [Google Scholar]
- 71.Geidelberg L, Mitchell KM, Aza-Gnandji M, et al. A Model Impact Analysis of PrEP and TasP in FSW Demonstration Project in Benin. Paper presented at: CROI2019; March 5, 2019, Seattle, WA.
- 72.Projet Équité en Santé Sexuelle et Santé. Cartographie des Travailleuses de Sexe des communes de Cotonou, Abomey-Calavi, Sèmè-Podji et Parakou: Rapport Synthèse du Mapping, Benin, 2013-2014. Cotonou, Bénin: Équité en Santé Sexuelle et Santé.
- 73.Tamoufe U, Mfochive I, Bowring A, et al. Review of Preliminary Data from a Study of HIV and Risk Among Clients of Female Sex Workers in Yaounde, Cameroon. Yaoundé, Cameroon: Johns Hopkins Bloomberg School of Public Health; 2018. [Google Scholar]
- 74.Garcia-Calleja JM. HIV Prevalence in Yaoundé within Surveys Conducted in the Late 80's-Early 90's. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 75.Buve A, Carael M, Hayes RJ, et al. Multicentre study on factors determining differences in rate of spread of HIV in sub-Saharan Africa: methods and prevalence of HIV infection. Aids. 2001;15:S5–S14. [DOI] [PubMed] [Google Scholar]
- 76.Morison L, Weiss HA, Buve A, et al. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. AIDS. 2001;15(suppl 4):S61–S69. [DOI] [PubMed] [Google Scholar]
- 77.Mosoko JJ, Macauley IB, Zoungkanyi ACB, et al. Human immunodeficiency virus infection and associated factors among specific population subgroups in Cameroon. Aids Behav. 2009;13:277–287. [DOI] [PubMed] [Google Scholar]
- 78.Tamoufe U, Medang R. Enquete séroépidemiologique et compartmentale sur le VIH/SIDA et à la Syphilis chez les travailleurs du sexe au Cameroun. Yaoundé, Cameroon: Johns Hopkins Bloomberg School of Public Health; 2010. [Google Scholar]
- 79.Lai Y. Regional Differences in HIV Epidemic and HIV Testing Services Usage Among Cameroonian Female Sex Workers: Comparing Grand North and Grassland Regions. Baltimore, Maryland: Johns Hopkins Bloomberg School of Public Health; 2015. [Google Scholar]
- 80.Alary M, Mukenge-Tshibaka L, Bernier F, et al. Decline in the prevalence of HIV and sexually transmitted diseases among female sex workers in Cotonou, Benin, 1993-1999. AIDS. 2002;16:463–470. [DOI] [PubMed] [Google Scholar]
- 81.Behanzin L, Diabate S, Minani I, et al. Decline in the prevalence of HIV and sexually transmitted infections among female sex workers in Benin over 15 years of targeted interventions. J Acquir Immune Defic Syndr. 2013;63:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowndes CM, Alary M, Gnintoungbe CA, et al. Management of sexually transmitted diseases and HIV prevention in men at high risk: targeting clients and non-paying sexual partners of female sex workers in Benin. AIDS. 2000;14:2523–2534. [DOI] [PubMed] [Google Scholar]
- 83.Ministry of Health Benin. Enquête de surveillance de deuxième génération des IST/VIH/SIDA au Bénin (ESDG-2012): Travailleuses du sexe et Serveuses de bars/restaurants. Cotonou, Bénin: Benin Ministry of Health; 2012. [Google Scholar]
- 84.Ministry of Health Benin. Enquête de surveillance de deuxième génération des IST/VIH/SIDA au Bénin (ESDG-2012): Camionneurs et Clients des Travailleuses du sexe. Cotonou, Bénin: Benin Ministry of Health; 2012. [Google Scholar]
- 85.Ministry of Health Benin. Serology Report by Department. Benin: Ministry of Health Bénin; 2015. [Google Scholar]
- 86.Institut National de la Statistique et de l. Analyse. É. conomique (INSAE). Institut National de la Statistique et de l'Analyse Économique - INSAE/Bénin and ICF International. 2013. In: Enquête Démographique et de Santé du Bénin 2011-2012. Calverton, MA: INSAE and ICF International; 2011. [Google Scholar]
- 87.Behanzin L, Diabate S, Minani I, et al. Decline in HIV prevalence among young men in the general population of Cotonou, Benin, 1998-2008. PLoS One. 2012;7:e43818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Behanzin L, Diabate S, Minani I, et al. Assessment of HIV-related risky behaviour: a comparative study of face-to-face interviews and polling booth surveys in the general population of Cotonou, Benin. Sex Transm Infect. 2013;89:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.West Africa HIV/AIDS Epidemiology and Response Synthesis Report. Washington, DC: The world bank; 2008. [Google Scholar]
- 90.Lowndes CM, Alary M, Meda H, et al. Role of core and bridging groups in the transmission dynamics of HIV and STIs in Cotonou, Benin, West Africa. Sex Transm Infect. 2002;78(suppl 1):i69–i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vandepitte J, Lyerla R, Dallabetta G, et al. Estimates of the number of female sex workers in different regions of the world. Sex Transm Infect. 2006;82(suppl 3):iii18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.République du Cameroun. Enquête Démographique et de Santé, Cameroun 2004. Yaoundé, Cameroon: Republique du Cameroun; 2005. [Google Scholar]
- 93.République du Cameroun. Enquête Démographique et de Santé, Cameroun 1998. Yaoundé, Cameroon: Republique du Cameroun; 1999. [Google Scholar]
- 94.Direction Nationale du Deuxième Recensement Général de la Population et de l'Habitat Y, Cameroun. In: Enquête Démographique et de Santé Cameroun 1991. Yaoundé, Cameroon: Republique du Cameroun; 1992. [Google Scholar]
- 95.R. é. publique du Cameroun. Enquête Démographique et de Santé du Cameroun 2018. Yaoundé, Cameroon: Republique du Cameroun; 2019. [Google Scholar]
- 96.Lagarde E, Auvert B, Chege J, et al. Condom use and its associations with HIV/sexually transmitted diseases in four urban communities of sub-Saharan Africa. Aids. 2001;15:S71–S78. [DOI] [PubMed] [Google Scholar]
- 97.Ferry B, Carael M, Buve A, et al. Comparison of key parameters of sexual behaviour in four African urban populations with different levels of HIV infection. AIDS. 2001;15(suppl 4):S41–S50. [DOI] [PubMed] [Google Scholar]
- 98.Lagarde E, Auvert B, Carael M, et al. Concurrent sexual partnerships and HIV prevalence in five urban communities of sub-Saharan Africa. Aids. 2001;15:877–884. [DOI] [PubMed] [Google Scholar]
- 99.Ministry of Health Benin. “File Active”: anonymised database of current female sex workers on ART in Benin. Benin: Ministry of Health; 2017. [Google Scholar]
- 100.UNAIDS; PNLS. Rapport de suivi de la déclaration de politique sue le VIH/SIDA au Bénin 2010 (Country progress report, Benin). Geneva, Switzerland: UNAIDS; 2010. [Google Scholar]
- 101.UNAIDS; Ministry of Health Benin; CNLS. Rapport de suivi de la déclaration de politique sur le VIH/SIDA au Bénin 2016 (Country progress report, Benin). Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 102.AVAC. PrEPWatch Country Updates, 2020. Available at: https://www.prepwatch.org/in-practice/country-updates/. Accessed May 8, 2020. [Google Scholar]
- 103.Torres TS, Hoagland B, Bezerra DRB, et al. Impact of COVID-19 pandemic on sexual minority populations in Brazil: an analysis of social/racial disparities in maintaining social distancing and a description of sexual behavior. Aids Behav. 2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchez TH, Zlotorzynska M, Rai M, et al. Characterizing the impact of COVID-19 on men who have sex with men across the United States in April, 2020. Aids Behav. 2020;24:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santos GM, Ackerman B, Rao A, et al. Economic, mental health, HIV prevention and HIV treatment impacts of COVID-19 and the COVID-19 response on a global sample of cisgender gay men and other men who have sex with men. Aids Behav. 2020;25:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ze Meka A. HIV Response in the Context of the Covid-19 Epidemic in the City of Yaounde, Cameroon. Paper presented at: Fast-Track Cities 2020; Virtual. Available at: https://www.iapac.org/files/2020/09/Albert-Zeh-Meka.pdf. Accessed Sepetmber 12, 2020. [Google Scholar]
- 107.Guedou FA. Comparaison coût de la passe Utilisation condom Vs non utilisation condom. Quebec, Canada: Université Laval; 2020. [Google Scholar]
- 108.UNAIDS. Targeting Sex Workers Is Not the Answer [press Release]. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]
- 109.Stover J, Chagoma N, Taramusi I, et al. Estimation of the potential impact of COVID-19 responses on the HIV epidemic: analysis using the goals model. Cold Spring Harbor Laboratory; 2020. [Google Scholar]
- 110.UNAIDS. HIV Service Disruptions in 2020: January—July 2020. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]
- 111.Diabate S, Chamberland A, Geraldo N, et al. Gonorrhea, Chlamydia and HIV incidence among female sex workers in Cotonou, Benin: a longitudinal study. PLoS One. 2018;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davies M-A. Western Cape: COVID-19 and HIV/Tuberculosis. Cape Town, South Africa: Western Cape Government; 2020. [Google Scholar]
- 113.Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:2276–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sigel K, Swartz T, Golden E, et al. Covid-19 and people with HIV infection: outcomes for hospitalized patients in New York city. Clin Infect Dis. 2020;71:2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with HIV and coronavirus disease-19. Clin Infect Dis. 2020;9:ciaa1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robinson J, Yeh E. Transactional sex as a response to risk in western Kenya. Am Econ J Appl Econ. 2011;3:35–64. [Google Scholar]
- 118.Elmes J, Nhongo K, Ward H, et al. The price of sex: condom use and the determinants of the price of sex among female sex workers in eastern Zimbabwe. J Infect Dis. 2014;210(suppl 2):S569–S578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.UNAIDS. Global AIDS Update 2020: Seizing the Moment. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]