Abstract
Each year, over one hundred million people become ill and tens of thousands die from exposure to viruses and bacteria from sewage transported to the ocean by rivers, estuaries, stormwater, and other coastal discharges. Water activities and seafood consumption have been emphasized as the major exposure pathways to coastal water pollution. In contrast, relatively little is known about the potential for airborne exposure to pollutants and pathogens from contaminated seawater. The Cross Surfzone/Inner-shelf Dye Exchange (CSIDE) study was a large-scale experiment designed to investigate the transport pathways of water pollution along the coast by releasing dye into the surfzone in Imperial Beach, CA. Additionally, we leveraged this ocean-focused study to investigate potential airborne transmission of coastal water pollution by collecting complementary air samples along the coast and inland. Aerial measurements tracked sea surface dye concentrations along 5+ km of coast at 2 m × 2 m resolution. Dye was detected in the air over land for the first 2 days during two of the three dye releases, as far as 668 m inland and 720 m downwind of the ocean. These coordinated water/air measurements, comparing dye concentrations in the air and upwind source waters, provide insights into the factors that lead to the water-to-air transfer of pollutants. These findings show that coastal water pollution can reach people through an airborne pathway and this needs to be taken into account when assessing the full impact of coastal ocean pollution on public health. This study sets the stage for further studies to determine the details and importance of airborne exposure to sewage-based pathogens and toxins in order to fully assess the impact of coastal pollution on public health.
Keywords: Coastal pollution, Air pollution, Sea spray aerosol, Water pollution, Water quality, Tijuana River, Tracer dye, Imperial Beach, Surfzone, Aerosols
Introduction
Roughly half of the global population lives in coastal regions (U.S. Comission on Ocean Policy, 2004). The discharge of treated and untreated sewage, industrial effluents, agricultural drainage, and urban stormwater into coastal waters is a global public health concern (U.S. Comission on Ocean Policy, 2004; Shuval, 2003; Halpern et al., 2012). Chemical contaminants include metals, chlorinated pesticides, oil, fuel, soot, and butyltins, amongst others (National Oceanic and Atmospheric Administration, 2016). The United States Environmental Protection Agency lists 126 Priority Pollutants in industrial discharges, in addition to the many emerging contaminants of concern (United States Environmental Protection Agency, 2014; Hutchinson et al., 2013). But it is untreated sewage in coastal waters that is of primary concern because it often contains pathogens that can cause illness from a single exposure (Shuval, 2003; Gersberg et al., 2006; Griffin et al., 2003). Pathogenic viruses cause most cases of illness from contact with sewage contaminated waters (Shuval, 2003; Griffin et al., 2003). Serious illness can result from exposure to low numbers of viruses (i.e. 1’s–10’s) which have been shown to remain infectious in seawater longer than bacteria (Gersberg et al., 2006; Griffin et al., 2003; Schiff et al., 1983; Fong & Lipp, 2005; Munn, 2011). Over 100 enteric viruses—those affecting the gut—have been detected in contaminated waters, including hepatitis A virus (HAV) and norovirus, and SARS-CoV-2 virus is present in sewage (Gersberg et al., 2006; Munn, 2011; Lodder & de Roda Husman, 2020). Globally, over 120 million cases of gastrointestinal disease and more than 50 million cases of respiratory disease are estimated to be caused each year by people entering contaminated coastal waters (Shuval, 2003).
Coastal water quality is an increasing challenge for developed and developing countries alike. San Diego County, CA, USA, has 80 km of coastline, much of which is impacted by seasonal stormwater runoff that enters the ocean untreated, a common occurrence in coastal cities worldwide (Gersberg et al., 2006; Griffin et al., 2003; Dwight et al., 2011; Steele et al., 2018). Human fecal pollution associated with stormwater runoff has been documented at San Diego beaches (Gersberg et al., 2006; Steele et al., 2018). The problem is compounded at Imperial Beach, San Diego County’s most southwesterly city, which lies across the border from Tijuana, Mexico. Rainfall in Tijuana River watershed results in both stormwater and sewage being discharged untreated into the Tijuana River, which empties into the ocean 2 km north of the US/Mexico border and pollutes the coastal waters on both sides. Specific infrastructure failures have resulted in additional releases of untreated sewage in recent years (Dibble & Smith, 2017; Hernandez, 2017). Moreover, other point sources of untreated sewage further south broaden the problem. The health risks are significant, with HAV having been detected in Imperial Beach’s coastal waters following rainfall (Gersberg et al., 2006). Beach water quality is monitored in San Diego on a weekly basis; when poor water quality is detected, beaches are closed to direct water contact (i.e. swimming, surfing) (County of San Diego Department of Environmental Health and Quality, 2017). But other forms of exposure to polluted water are possible, including airborne exposure, as we aim to demonstrate in this study.
Sea spray aerosol (SSA) produced by breaking waves in the open ocean and surfzone transfers microscopic droplets of seawater into the atmosphere (Lewis & Schwartz, 2004; Lenain & Melville, 2017; Gantt & Meskhidze, 2013). Sea spray aerosol comprises a significant fraction of total aerosol at the coast and 20+ km downwind, especially during elevated winds, whitecaps, and surf (Clarke, Owens & Zhou, 2006; Van Eijk et al., 2011; de Leeuw et al., 2000). The majority of SSA particles have diameters ranging between 10’s of nm to 10 µm (Fröhlich-Nowoisky et al., 2016). SSA particles contain various chemical compounds and microorganisms, including bacteria and viruses (Quinn et al., 2015; Patterson et al., 2016). Airborne microorganisms have been found to be most abundant in SSA particles greater than 2 µm in diameter (Patterson et al., 2016; Shaffer & Lighthart, 1997; Montero, Dueker & O’Mullan, 2016). Genetic sequencing efforts have shown that coastal air contains a mixture of microorganisms from the ocean and land (Li et al., 2011; Urbano et al., 2011; Graham et al., 2018). Culturing approaches have demonstrated the presence of viable bacteria and viruses in coastal and marine aerosol (Shaffer & Lighthart, 1997; Li et al., 2011; Urbano et al., 2011; Baylor et al., 1977; Fahlgren et al., 2010; Smith et al., 2013; Ladino et al., 2019). Hazardous chemicals and microorganisms have been detected in SSA (Graham et al., 2018; Kirkpatrick et al., 2010; Fleming et al., 2011; Cheng et al., 2005; Pierce et al., 2003; Michaud et al., 2018). The detection of brevetoxins in coastal air downwind of harmful algal blooms confirmed reports of naturally occurring marine toxins reaching humans on land via airborne transport (Kirkpatrick et al., 2010; Fleming et al., 2011; Cheng et al., 2005; Pierce et al., 2003). Genetic sequencing of coastal aerosol has identified potentially pathogenic bacteria at the species and genera levels (Graham et al., 2018). Whole genome shotgun sequencing identified potentially pathogenic bacteria in isolated SSA (Michaud et al., 2018).
Coastal water pollution has the potential to transfer into the atmosphere in SSA and reach people inland, a growing public health concern (O’Mullan, Dueker & Juhl, 2017). Baylor and colleagues demonstrated sea-to-air virus transfer by releasing non-native viruses into coastal waters and recovering the same virus strains on the beach (Baylor et al., 1977). Exposure to aerosolized microorganisms has also been identified as a risk in other environments, including solid waste treatment facilities, wastewater treatment plants, and agricultural sites using wastewater for spray irrigation (Fannin et al., 1977; Teltsch & Katzenelson, 1978; Carducci, Arrighi & Ruschi, 1995; Li et al., 2021; Malakootian et al., 2013; Brisebois et al., 2018). This risk has been demonstrated for aerosols from sewage impacted rivers using aeration remediation (Dueker et al., 2012; Dueker & O’Mullan, 2014). Aerosols released into the air from rivers have been linked with illness (Pickup et al., 2005). The objectives of the present study were to: (a) use a nontoxic dye to simulate large-scale coastal water pollution events; (b) determine the conditions leading to the transfer of the dye to the atmosphere; and (c) relate dye concentrations between the ocean and atmosphere. This study is the first to examine the potential for inland transport of airborne water pollution in coastal regions. The detection of the dye in the air inland from the beach demonstrates that coastal water pollution transfers to the air in SSA and reaches communities via an airborne exposure pathway.
Materials & methods
Tracer dye as a water pollution mimic
The CSIDE study simulated the impacts of coastal water pollution events on coastal air quality by releasing rhodamine WT (RWT) dye into the surfzone at Imperial Beach, CA, USA (Fig. 1) (Grimes et al., 2020). Rhodamine WT (molecular weight 566.99 g/mol) is a water soluble, non-toxic, fluorescent dye used as a semi-conservative water mass tracer, having no known oceanic sources and a minor sink in the form of photodegradation (Smart & Laidlaw, 1977; Wilson, Cobb & Kilpatrick, 1986; Suijlen & Buyse, 1994; Clark et al., 2009). It is a non-volatile, solid compound at room temperature with maximum fluorescence at excitation/emission wavelengths of 558/582 nm (Wilson, Cobb & Kilpatrick, 1986; Mackay & Shiu, 1981). We simulated three pollution events by releasing roughly 30 gallons of concentrated RWT solution into the surfzone on September 23 (early morning, 3.2 h release), October 8 (early morning, 3.8 h release), and October 12 (mid morning, 1.8 h release into estuary outflow on ebb tide), 2015 (Fig. 1). Sea surface concentrations of the fluorescent dye (Fig. 1, Table 1) were measured with a hyperspectral camera mounted on an airplane that flew over the study site multiple times each hour on the first 2 days of each dye release (DR) (Melville et al., 2016). The hyperspectral camera was calibrated with in-situ near-surface dye measurements to provide dye concentrations in surface waters with high spatial resolution (2 m × 2 m) (Grimes et al., 2020; Melville et al., 2016; Clark et al., 2014; Feddersen et al., 2016). This method is limited to daylight hours, and therefore no nighttime sea surface dye concentrations are reported.
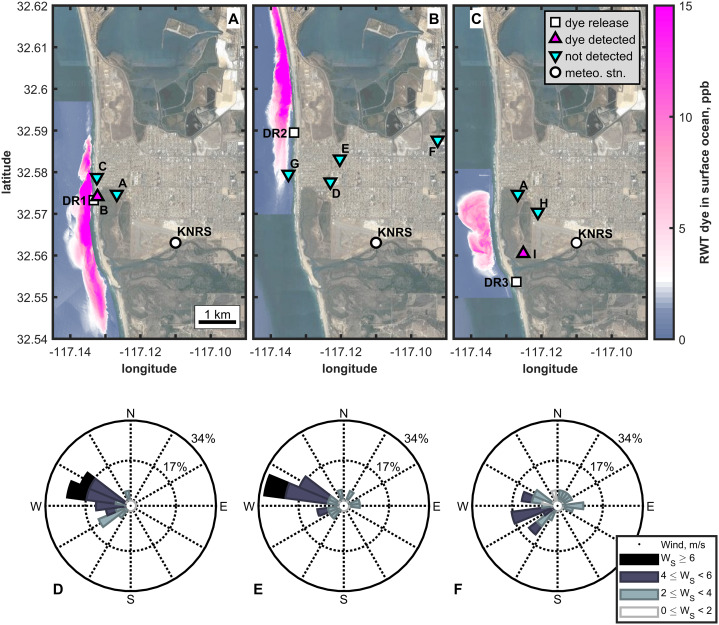
Figure 1. Overview of the three dye releases of the CSIDE study.
(A)–(C) present maximum RWT dye concentrations in coastal waters and aerosol sampling locations and results from each dye release (1–3). Magenta triangles pointing up indicate locations where dye was detected in the aerosol. Cyan triangles pointing down show the locations where aerosol was sampled and dye was not detected. (D)–(F) contain wind roses presenting daytime winds, responsible for onshore transport, for the first 2 days of each DR, when most aerosol samples were collected (4). Stacked bars in the wind roses indicate the percent of the time period the wind blew from the indicated direction and at the indicated speed (represented by color). (1) Zohar Bar-Yehuda (2017). zoharby/plot_google_map (https://github.com/zoharby/plot_google_map), GitHub. Retrieved June 1, 2017. (2) Map data: Imagery ©2020 Google. Data USGS, Data SIO, NOAA, U.S. Navy, NGA, GEBCO, Data LDEO-Columbia, NSF, NOAA, Imagery ©2020 TerraMetrics, Map data ©2020 INEGI. (3) Jonathan Sullivan (2017). Automatic Map ScaleGeneration (https://www.mathworks.com/matlabcentral/fileexchange/33545-automatic-map-scale-generation), MATLAB Central File Exchange. Retrieved June 1, 2017. (4) Daniel Pereira (2017). Wind Rose (https://www.mathworks.com/matlabcentral/fileexchange/47248-wind-rose), MATLAB Central File Exchange. Retrieved June 1, 2017.
Table 1. Data for the aerosol samples collected following the three dye releases.
Distance inland is the distance from the air sampling location to the nearest point along the shoreline. Distance downwind is the mean distance along the wind vector from the center of the 200 m × 100 m ocean source window to the aerosol sampling site. Distance inland is fixed, whereas distance downwind is subject to the relevant wind directions. [dye]air (pg/m3) is the concentration of dye in the air calculated from the fluorescence intensity and the volume of air sampled. The four samples which match our criteria for dye detection are highlighted in bold. The values for the samples that did not meet our criteria for dye detection are provided in parentheses. [dye]sea (ppb) indicates the sea surface dye concentration measured by the hyperspectral camera and are available for most daylight hours on the day of each dye release and the following day and are not available for aerosol samples collected at night.
| # | DR | date start | sample period | Site | inland (m) | downwind (m) | sampled air (m3) | dye pattern | above LoD | [dye]air (pg/m3) | [dye]sea, (ppb) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 9/23 | day | A | 615 | 678 | 198 | Yes | No | (14.3) | 4.4 |
| 2 | 1 | 9/23 | day | B | 101 | 154 | 135 | Yes | Yes | 759.0 | 3.6 |
| 3 | 1 | 9/23 | night | A | 615 | 653 | 398 | No | No | (4.8) | 0.6 |
| 4 | 1 | 9/24 | day | A | 615 | 664 | 226 | No | No | (5.2) | 0.7 |
| 5 | 1 | 9/24 | day | B | 101 | 148 | 162 | Yes | Yes | 43.3 | 0.8 |
| 6 | 1 | 9/24 | night | C | 122 | NA | 422 | No | No | (5.0) | NA |
| 7 | 1 | 9/24 | night | A | 615 | NA | 377 | No | No | (11.3) | NA |
| 8 | 1 | 9/25 | day | C | 122 | NA | 116 | No | No | (20.0) | NA |
| 9 | 1 | 9/25 | day | A | 615 | NA | 137 | No | No | (4.8) | NA |
| 10 | 2 | 10/8 | day | D | 976 | 1057 | 241 | No | No | (8.3) | 0.5 |
| 11 | 2 | 10/8 | day | E | 1256 | 1310 | 216 | No | No | (9.4) | 0.6 |
| 12 | 2 | 10/8 | day | F | 3867 | 3953 | 181 | No | No | (8.9) | 3.4 |
| 13 | 2 | 10/8 | night | E | 1256 | 1284 | 428 | No | No | (12.5) | 0.3 |
| 14 | 2 | 10/8 | day | D | 976 | 1037 | 54 | No | No | (20.4) | 0.3 |
| 15 | 2 | 10/9 | day | G | 108 | 57 | 140 | No | No | (9.2) | 0.4 |
| 16 | 2 | 10/9 | 24 h | E | 1256 | 1288 | 691 | No | No | (5.3) | 0.5 |
| 17 | 3 | 10/12 | day | A | 615 | 712 | 211 | No | Yes | (44.6) | 1.8 |
| 18 | 3 | 10/12 | day | H | 1193 | 1275 | 234 | No | No | (14.1) | 2.6 |
| 19 | 3 | 10/12 | day | I | 668 | 720 | 178 | Yes | Yes | 184.2 | 0.6 |
| 20 | 3 | 10/12 | night | H | 1193 | NA | 347 | No | No | (15.2) | NA |
| 21 | 3 | 10/12 | night | A | 615 | NA | 376 | No | No | (16.4) | 1.0 |
| 22 | 3 | 10/13 | day | I | 668 | 694 | 169 | Yes | Yes | 55.5 | 0.1 |
| 23 | 3 | 10/14 | day | I | 668 | NA | 135 | No | Yes | (60.9) | NA |
| 24 | 3 | 10/15 | day | I | 668 | NA | 152 | No | Yes | (34.0) | NA |
| 25 | blank | 10/7 | night | D | 976 | NA | 192 | No | NA | (2.0) | NA |
| 26 | blank | 10/7 | night | C | 122 | NA | 383 | No | NA | (7.3) | NA |
| 27 | blank | 10/7 | day | D | 976 | NA | 119 | No | NA | (18.9) | NA |
| 28 | blank | 10/7 | day | C | 122 | NA | 224 | No | NA | (10.0) | NA |
| 29 | rinse | 9/23 | NA | rinse | NA | NA | NA | No | NA | NA | NA |
| 30 | rinse | 10/9 | NA | rinse | NA | NA | NA | No | NA | NA | NA |
| blanks mean | 9.5 | ||||||||||
| blanks SD | 7.1 | ||||||||||
| LoD | 32.8 | ||||||||||
Aerosol sampling
Atmospheric aerosols were sampled at up to 3 sites over 2–4 days following each DR, at a total of 9 locations (Fig. 1 and Table 1). Aerosol sampling began the morning of each DR and continued into the afternoon, when samples were recovered and new sampling periods began, yielding ~6 h daytime and ~16 h overnight collection periods. Aerosol sampling locations were selected each morning based on the current dye observations (aerial and in-situ) in an effort to sample downwind of the ocean dye. The oceanic dye plume continued to evolve over time such that the aerosol sampling sites did not always remain downwind, and the downwind distance varied. Aerosols were collected using a PAS450-10 SpinCon I (heretofore “SpinCon I”; see Supplemental Materials for more information), which utilizes a swirling technique to transfer aerosols and water soluble compounds from the air into a fixed volume of sterile water (Michaud et al., 2018; Yooseph et al., 2013). The SpinCon I samples air at 450 lpm and can sample continuously for hours, greatly concentrating airborne constituents into a 10 ml liquid sample. Internal surfaces of the SpinCon I were cleaned with 70% ethanol and tubing was flushed with 10% bleach followed by sodium thiosulfate to neutralize the bleach. In between samples, rinse cycles, in which the instrument is run for 30 s and then the sample is discarded, were used to flush surfaces. Field blanks were collected on October 7–8, between the first and second DRs, after the dye had dissipated in the coastal waters for 2 weeks.
RWT measurement in aerosol samples
Samples were analyzed for RWT using fluorescence spectroscopy, normalizing to the water Raman peak (Lawaetz & Stedmon, 2009; Murphy, 2011). The fluorescence measurements were made the day the samples were recovered, using the Horiba Aqualog fluorescence spectrometer (Aqualog UV 800C, Horiba Ltd., Kyoto, Japan), at 425–625 nm excitation wavelengths, at 5 nm increments, and reading at emission wavelengths of 248–827 nm, at ~4.5 nm increments. A calibration curve was generated to quantitatively relate fluorescence to RWT concentration, which also provided the RWT fluorescence pattern in the excitation-emission matrix, which we used to identify RWT in aerosol samples (Fig. S1). Positive detection of RWT in the aerosol samples are those that showed the RWT fluorescence pattern and a RWT fluorescence intensity above that of our limit of detection derived from the field blanks (Eq. 1) (Armbruster & Pry, 2008):
| (1) |
where α is the mean of the field blanks and β is the standard deviation of the field blanks. The RWT fluorescence pattern was not detected in any aerosol field blanks nor any sampler rinses.
Atmospheric dye concentrations
The average concentration of RWT in the air during each sampling period was determined by dividing the amount of RWT in the SpinCon I liquid sample by the total volume of air sampled. Figure S2 shows fluorescence spectra from a RWT standard (Fig. S2A), from aerosol samples with strong and weak RWT signatures (Figs. S2B & S2C), and from a field blank (Fig. S2D), as well as the peaks used for quantitation and the region for background correction. See Supplemental Information. RWT concentrations are reported for all air samples and those which met both criteria for RWT detection are noted (Table 1).
Dye concentrations in upwind waters
To determine sea surface dye concentrations upwind of the aerosol samplers ([dye]sea (ppb); Table 1; x-axis in Fig. 3), wind vectors were used from a local meteorological station (KNRS, Fig. 1) and interpolated to the time intervals of aerial sea surface dye measurements. No lag between source and wind times was imposed because the observed ~5 m/s winds advected material from the surfzone to the furthest sampler (~1.5 km) in ~5 min, much shorter than the multi-hour sampling intervals. The intersection of the interpolated upwind vector with the tidally varying shoreline location, estimated using the 2012 NOAA Tsunami DEM and local water-level (including tides, but neglecting wave set-up), provided the center location for the upwind dye source in the ocean. A 200 m alongshore by 100 m offshore sea surface dye concentration window was extracted for each sea surface dye measurement taken during each aerosol sampling period. This box was limited to 100 m in the offshore direction because SSA was mainly generated in the surfzone region of depth-limited breaking waves (Van Eijk et al., 2011; de Leeuw et al., 2000). The spatial and temporal average of the sea surface dye concentration in those windows was taken for each aerosol sampling period ([dye]sea (ppb)). This allowed us to precisely define the RWT aerosol source waters and quantitatively relate RWT concentrations in the ocean and atmosphere.
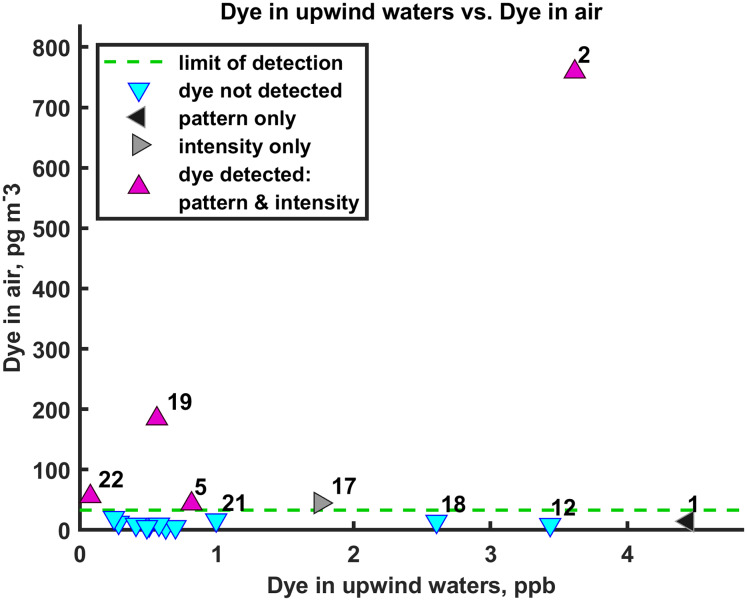
Figure 3. Dye concentrations in air and upwind waters.
For aerosol samples that were collected during periods of hyperspectral measurements of ocean dye concentrations (primarily daytime), the RWT concentration in the air (vertical axis) is compared against the dye concentration in the upwind source waters (horizontal axis). Symbol labels are sample numbers. A dye concentration in the air can be calculated whether dye was detected (magenta) or not (cyan) as dye detection relies both on values being above the blank and containing the RWT signature. The green dashed line indicates the limit of detection determined from the air field blanks and Eq. (1) (32.8 pg/m3). False positives would show up along a vertical line near 0 on the horizontal axis. Here, two air samples met one of two criteria for dye detection and two other air samples did as well, but do not appear here because sea surface RWT concentrations are not available for them.
Results
RWT transport by ocean currents
The three DRs demonstrated different ocean transport pathways of the RWT, driven by the coastal ocean currents. Figures 1A–1C show the maximum sea surface dye concentration at each point in the ocean on the first day of each dye release as measured from the air with the hyperspectral camera (Melville et al., 2016; Clark et al., 2014). Although temporal information such as persistence/transience is obscured in these figures, they provide a broad visual comparison of the major dye distribution for each DR. At the broadest level, the three DRs showed that the pollution mimic did not immediately disperse offshore, but instead remained within 1 km of the shoreline during the first day of each release, consistent with multiple studies showing surfzone trapping (Boehm, 2003; Grant et al., 2005; Clark, Feddersen & Guza, 2010; Rodriguez, Giddings & Kumar, 2018). For DRs 1 and 3 (Figs. 1A & 1C), the dye remained within ~2 km alongshore of the release location. For DR2 (Fig. 1B) a strong, wave-driven, alongshore current rapidly advected the dye ~10 km northward along the coast (Grimes et al., 2020; Wu et al., 2020). Thus for DR2, although high sea surface dye concentrations are seen along the coast, they only persisted at any given location for a short time due to the rapid northward advection. Sea surface dye concentrations are overall much lower for DR3, likely a result of localized mixing and, to a lesser extent, missing aerial measurements due to flight access limitations in the first few hours following the DR. Combined, the three DRs show the variability in water and pollution transport expected for the coastal ocean and the motivation for the CSIDE study. The ocean transport aspects of the CSIDE study are discussed in greater depth in other publications (Grimes et al., 2020; Wu et al., 2020).
RWT detected in coastal aerosols
Dye was detected in coastal aerosols on days 1 and 2 of DRs 1 and 3 in four total aerosol samples (Figs. 1A & 1C, Table 1). Dye was detected as far as 668 m inland and 720 m downwind from source waters. Wind rose plots (Figs. 1D–1F) for daytime winds show that onshore winds, typical of coastal meteorology patterns, were common. Wind data link the oceanic source waters with locations of atmospheric dye detection over land. Figure 1 broadly shows that atmospheric dye was detected downwind of the dye plume. The well characterized distribution of RWT in coastal waters allows quantitative comparison between dye concentrations in the air and in the source waters.
Comparing ocean and atmospheric observations
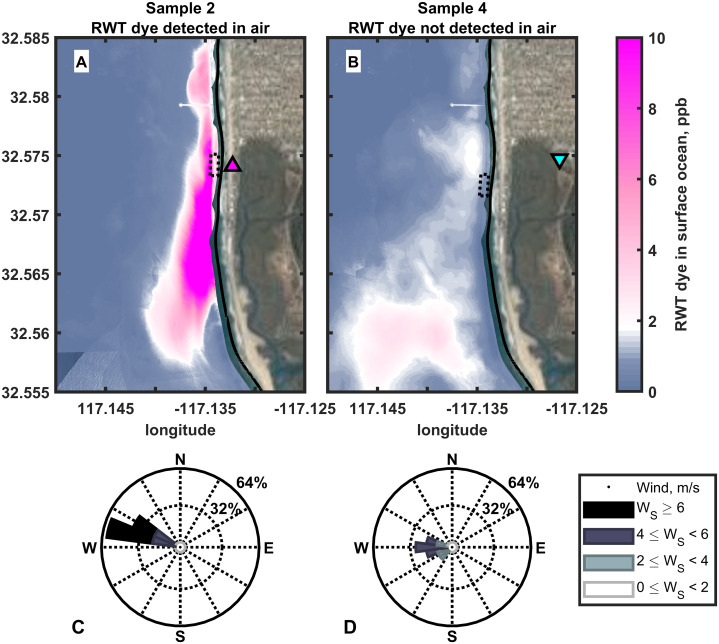
Repeated measurements of dye in the coastal waters allow us to examine dye concentrations in the source waters during the atmospheric sampling periods. Figure 2 shows the comparison of ocean dye data from two exemplary samples for when dye was and was not detected in the air. The mean sea surface dye concentration in the upwind waters during the aerosol sampling period is presented relative to the aerosol sampling location on land. The inset dotted box shows a 200 m × 100 m region centered where the mean wind direction during the aerosol sampling period intersected the coastal waters. Sample 2 (left panel) was collected on the first day of DR1 and sample 4 (right panel) was collected the day after, i.e. day 2 of DR1. Dye was detected in sample 2, which was collected downwind of waters with high dye concentrations. Dye was not detected in sample 4, which was collected downwind of waters with little to no detectable dye. Analogous plots for all air samples with coupled ocean measurements are provided in the Supplemental Materials (Fig. S3).
Figure 2. Mean sea surface dye concentrations upwind of aerosol sampling locations.
(A) and (B) show average sea surface dye concentrations during the period each aerosol sample was collected at the location on land indicated by the triangle (5–7). The 200 m × 100 m dotted black box indicates where the average wind direction during each sampling interval intersected the coastal waters from the sampling site. Figure 3 finds the location of that box and its mean dye value for each hourly sea surface dye measurement. Here we show the mean dye field during each air sampling period and the mean upwind location (box). (C) and (D) are wind roses for the winds observed at the KNRS meteorological station during the aerosol sampling periods (8). Aerosol sample 2 (A & C) contained dye and was collected downwind of high dye concentrations in coastal water. Aerosol sample 4 (B & D) did not contain detectable dye and was collected downwind of seawater containing very low dye concentrations near the detection limit. (5) Zohar Bar-Yehuda (2017). zoharby/plot_google_map (https://github.com/zoharby/plot_google_map), GitHub. Retrieved June 1, 2017. (6) Map data: Imagery ©2020 Google. Data USGS, Data SIO, NOAA, U.S. Navy, NGA, GEBCO, Data LDEO-Columbia, NSF, NOAA, Imagery ©2020 TerraMetrics, Map data ©2020 INEGI. (7) Jonathan Sullivan (2017). Automatic Map ScaleGeneration (https://www.mathworks.com/matlabcentral/fileexchange/33545-automatic-map-scale-generation), MATLAB Central File Exchange. Retrieved June 1, 2017. (8) Daniel Pereira (2017). Wind Rose (https://www.mathworks.com/matlabcentral/fileexchange/47248-wind-rose), MATLAB Central File Exchange. Retrieved June 1, 2017.
Discussion
Atmospheric transfer of RWT in SSA
Surfzone production of SSA transferred RWT from the ocean to the atmosphere. Rhodamine WT’s transfer in SSA is consistent with the detection of other chemical species in SSA (Fleming et al., 2011; Cheng et al., 2005; Hawkins & Russell, 2010; Cochran et al., 2017; Kuznetsova, Lee & Aller, 2005) and larger entities including viruses and bacteria (Patterson et al., 2016; Baylor et al., 1977; Michaud et al., 2018; Aller et al., 2005). Dissolved RWT has a low surface activity, therefore it acts as a tracer of bulk seawater, which transfers to SSA primarily in jet drops. Sea spray aerosol production, specifically from depth-limited waves breaking in the surfzone, must have been the dominant RWT transfer mechanism because surfzone production of SSA has been shown to be dominant for low to moderate wind speeds (Van Eijk et al., 2011; de Leeuw et al., 2000). Dye transfer outside the surfzone was negligible because whitecap conditions were not common during the study period, due to low wind speeds often below 6 m/s.
Atmospheric transport of RWT SSA
We understand that the major factors influencing atmospheric concentrations of RWT SSA include: dye concentrations in aerosol source waters, which depends on advection and dispersion of the dye in the ocean, driven by waves, regional pressure gradients, buoyant plumes, winds, tides, and internal tides; ocean-to-atmosphere RWT SSA flux, which occurred primarily through SSA production by surfzone depth-limited wave breaking; downwind advection and horizontal and vertical dispersion in the atmosphere, driven by coastal winds and atmospheric boundary layer dynamics; and dry deposition (gravitational settling) of RWT SSA. We understand that dye was not detected in most aerosol samples due to high variability in ocean and air transport patterns. Photobleaching of RWT is negligible on our <4 d timescales (Suijlen & Buyse, 1994). We compare dye concentrations in the ocean and atmosphere to test whether source water dye concentrations influenced airborne dye concentrations (Fig. 3). Dye was not always detected when upwind waters contained elevated dye concentrations, due to downwind distance, 3D atmospheric dispersion, and the lack of a vertical sampling array. When RWT dye was detected in the aerosol (Fig. 3, magenta points), the data suggest dye concentrations in the air are proportional to dye concentrations in source waters. Importantly, there were no false positive atmospheric dye values because higher RWT concentrations in the air correspond to higher RWT concentrations in the ocean; there are no data points high in air RWT and low in ocean RWT (Fig. 3). More experiments with sampling at higher temporal and vertical/horizontal spatial resolution are needed to confirm a robust, quantitative relationship between SSA and upwind source waters, and to better understand the role of atmospheric dispersion.
Implications
Our results demonstrate the ability to trace SSA back to its source waters with high spatial and temporal resolution, and to quantitatively relate dye concentrations in source waters and air masses. More importantly, this study confirms coastal water pollution can be transferred from the surf into the atmosphere by releasing a tracer dye into the ocean and detecting it in the air inland from the beach. These observations highlight an under-appreciated airborne exposure pathway to coastal water pollution. We expand on the pioneering work of Baylor et al. (1977) by simulating large-scale artificial water pollution events, measuring sea surface dye concentrations at high spatio-temporal resolution (2 m × 2 m, hourly), tightly constraining RWT SSA source waters, and making quantitative ocean-atmosphere comparisons that extend inland beyond the beach. Whereas many atmospheric aerosol studies detect particles from distant sources, and aerosol dispersion models provide insight into particle origin at regional level resolution (~100 km), we were able to pinpoint SSA source waters on the <1 km scale. Although we were unable to detect RWT SSA beyond 668 m inland (Fig. 1, Table 1), it is well documented that SSA can travel much further (Smith et al., 2013; Bondy et al., 2017; Prospero et al., 2005; Smith et al., 2012). A 2 µm SSA particle has a predicted dry deposition residence time of ~1.5 weeks with an estimated travel distance of 10,000 km (Lewis & Schwartz, 2004). SSA particles this size have been detected hundreds to thousands of km from their oceanic source region (Bondy et al., 2017). Bacteria can remain viable after travelling thousands of km in the atmosphere (Prospero et al., 2005; Smith et al., 2012). Our findings emphasize that species transfer from the ocean to the atmosphere includes not just natural seawater components but also pollutants. This study also demonstrates that coastal water pollution reaches people beyond the shoreline through atmospheric transport. These results detail a promising approach for linking pollution in source waters, including the surfzone, estuaries, and other outflows, to atmospheric pollutants and health impacts downwind.
Conclusions
In summary, this study confirms that coastal water pollution can be transferred from the surf to the atmosphere by measuring tracer dye concentrations in the ocean and air. Airborne transport of coastal water pollution can expose people beyond the water and beach, including entire coastal communities, to biological and chemical pollutants. The magnitude of this risk is a function of various parameters. Dispersion in the atmosphere dilutes airborne pollution, and pathogens can die or deactivate in the atmosphere, but exposure to a relatively small number of viruses have been shown to cause illness (Schiff et al., 1983; Fong & Lipp, 2005; Munn, 2011). This study draws attention to an underappreciated airborne exposure pathway and sets the stage for future studies to better quantify the magnitude of the airborne exposure. Additional work is needed to assess public health threats from known coastal pathogens and emerging pathogens, like SARS-CoV-2, which is present in wastewater (Lodder & de Roda Husman, 2020; Colwell, 1996). Coastal water pollution is an increasing global problem that will only worsen as the human population grows and climate change leads to more extreme precipitation events. Ultimately, a thorough understanding of how pathogens and toxins are transported by coastal water currents and wind patterns will allow timely predictions of potential health risks to coastal communities and beyond.
Supplemental Information
Calibration curve produced by measuring the fluorescence of RWT solutions at known concentrations. The data were background corrected using the same technique used for the collected samples.
(A) a 2 PPB RWT standard, (B) sample #19 - dye detected, (C) sample #20 - dye not detected, and (D) sample #27 - aerosol field blank; dye not detected Red dots indicate the 3 excitation/emission pairs determined from the calibration to be used for RWT dye quantification. The mean fluorescence intensity from the black rectangle was subtracted from the entire spectrum as an internal background correction.
Like Figure 2, for all aerosol samples with data available (day samples). Right: Average sea surface dye concentrations during the period each aerosol sample was collected at the location on land indicated by the triangle. The 200 m × 100 m dotted box indicates where the average wind direction during each sampling interval intersected the coastal waters from the sampling site. Figure 3 finds the location of that box and its mean dye value for each hourly sea surface dye measurement. Here we show the mean dye field during each air sampling period and the mean upwind location (box). Left: wind rose for the winds observed at the KNRS meteorological station during the aerosol sampling periods.
More details on methods used
Acknowledgments
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We thank Ken Melville, Luc Lenain, Nick Statom, and Stephen Holleman for acquisition and preliminary processing of MASS data and SIO CDIP for wave data. We thank YMCA: Camp Surf for providing a base of operations; the cities of Imperial Beach and Coronado, the Imperial Beach lifeguards, the Tijuana River National Estuary Research Reserve, Mar Vista High School, and CA State Parks for accommodating our research activities. We thank the US Navy and Naval Base Coronado for their support during the CSIDE experiment. Extra thanks to CA Border Patrol, Adriana Corrales, Josh Cox, Jeff Crooks, Chris Peregrin, Julio Lorda, Capt. Robert Stabenow, Sgts. Ayala & Lindquist, Kevin Willard, Randy Rosenheim, the Blake/Giardina household, and the Pendergraft household for sampling assistance and access.
Funding Statement
This work was supported by the National Science Foundation (grant # OCE1459389; GRFP to Derek J. Grimes) and the Dankberg Family Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Matthew A. Pendergraft, Email: mpenderg@ucsd.edu.
Kimberly A. Prather, Email: kprather@ucsd.edu.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Matthew A. Pendergraft conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Derek J. Grimes conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Sarah N. Giddings conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Falk Feddersen conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Charlotte M. Beall conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Christopher Lee conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Mitchell V. Santander conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Kimberly A. Prather conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Pendergraft, Matthew A.; Grimes, Derek J.; Giddings, Sarah N.; Feddersen, Falk; Beall, Charlotte M.; Lee, Christopher; Santander, Mitchell V.; Prather, Kimberly A. (2021). Data from: Airborne transmission pathway for coastal water pollution. In Center for Aerosol Impacts on Chemistry of the Environment (CAICE). UC San Diego Library Digital Collections. https://doi.org/10.6075/J0V40SSD
References
- Aller et al. (2005).Aller JY, Kuznetsova MR, Jahns CJ, Kemp PF. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. Journal of Aerosol Science. 2005;36(5–6):801–ss12. doi: 10.1016/j.jaerosci.2004.10.012. [DOI] [Google Scholar]
- Armbruster & Pry (2008).Armbruster DA, Pry T. Limit of Blank, Limit of Detection and Limit of Quantitation. 2008;29(August):49–52. [PMC free article] [PubMed] [Google Scholar]
- Baylor et al. (1977).Baylor AER, Baylor MB, Blanchard DC, Syzdek LD. Virus transfer from surf to wind. Science. 1977;198(4317):575–580. doi: 10.1126/science.918656. [DOI] [PubMed] [Google Scholar]
- Boehm (2003).Boehm AB. Model of microbial transport and inactivation in the surf zone and application to field measurements of total coliform in Northern Orange County, California. Environmental Science and Technology. 2003;37(24):5511–5517. doi: 10.1021/es034321x. [DOI] [PubMed] [Google Scholar]
- Bondy et al. (2017).Bondy AL, Wang B, Laskin A, Craig RL, Nhliziyo MV, Bertman SB, Pratt KA, Shepson PB, Ault AP. Inland Sea spray aerosol transport and incomplete chloride depletion: varying degrees of reactive processing observed during SOAS. Environmental Science and Technology. 2017;51(17):9533–9542. doi: 10.1021/acs.est.7b02085. [DOI] [PubMed] [Google Scholar]
- Brisebois et al. (2018).Brisebois E, Veillette M, Dion-Dupont V, Lavoie J, Corbeil J, Culley A, Duchaine C. Human viral pathogens are pervasive in wastewater treatment center aerosols. Journal of Environmental Sciences (China) 2018;67(10):45–53. doi: 10.1016/j.jes.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci, Arrighi & Ruschi (1995).Carducci A, Arrighi S, Ruschi A. Detection of coliphages and enteroviruses in sewage and aerosol from an activated sludge wastewater treatment plant. Letters in Applied Microbiology. 1995;21(3):207–209. doi: 10.1111/j.1472-765X.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2005).Cheng YS, Zhou Y, Irvin CM, Pierce RH, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden DG. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environmental Health Perspectives. 2005;113(5):638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, Feddersen & Guza (2010).Clark DB, Feddersen F, Guza RT. Cross-shore surfzone tracer dispersion in an alongshore current. Journal of Geophysical Research: Oceans. 2010;115(10):1–18. doi: 10.1029/2009JC005683. [DOI] [Google Scholar]
- Clark et al. (2009).Clark DB, Feddersen F, Omand MM, Guza RT. Measuring fluorescent dye in the bubbly and sediment-laden surfzone. Water, Air, and Soil Pollution. 2009;204(1–4):103–115. doi: 10.1007/s11270-009-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark et al. (2014).Clark DB, Lenain L, Feddersen F, Boss E, Guza RT. Aerial imaging of fluorescent dye in the near shore. Journal of Atmospheric and Oceanic Technology. 2014;31(6):1410–1421. doi: 10.1175/JTECH-D-13-00230.1. [DOI] [Google Scholar]
- Clarke, Owens & Zhou (2006).Clarke AD, Owens SR, Zhou J. An ultrafine sea-salt flux from breaking waves: implications for cloud condensation nuclei in the remote marine atmosphere. Journal of Geophysical Research Atmospheres. 2006;111(6):1–14. doi: 10.1029/2005JD006565. [DOI] [Google Scholar]
- Cochran et al. (2017).Cochran RE, Jonathan V, Elizabeth A, Prather KA, Grassian H, Cochran RE, Laskina O, Trueblood JV, Estillore AD, Morris HS, Jayarathne T, Sultana CM, Lee C, Lin P, Laskin J, Laskin A, Dowling JA, Qin Z, Cappa CD, Bertram TH, Tivanski AV, Stone EA, Prather KA, Grassian VH. Molecular diversity of sea spray aerosol particles: impact of ocean biology on particle composition and hygroscopicity. Chem. 2017;2(5):655–667. doi: 10.1016/j.chempr.2017.03.007. [DOI] [Google Scholar]
- Colwell (1996).Colwell RR. Global climate and infectious disease: The cholera paradigm. Science. 1996;274(5295):2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- County of San Diego Department of Environmental Health and Quality (2017).County of San Diego Department of Environmental Health and Quality Beach and Bay Water Quality Program. 2017. http://www.sdbeachinfo.com. http://www.sdbeachinfo.com (accessed 1 January 2017)
- de Leeuw et al. (2000).de Leeuw G, Neele FP, Hill M, Smith MH, Vignati E. Production of sea spray aerosol in the surf zone. Journal of Geophysical Research. 2000;105(D24):29397–29409. doi: 10.1029/2000JD900549. [DOI] [Google Scholar]
- Dibble & Smith (2017).Dibble S, Smith JE. Massive Tijuana Sewage Spill That Polluted San Diego Beaches Part of Larger Problem. https://www.sandiegouniontribune.com/news/california/la-me-sewage-mexico-20170313-story.html San Diego Union Tribune. 2017 [Google Scholar]
- Dueker & O’Mullan (2014).Dueker ME, O’Mullan GD. Aeration remediation of a polluted waterway increases near-surface coarse and culturable microbial aerosols. Science of the Total Environment. 2014;478:184–189. doi: 10.1016/j.scitotenv.2014.01.092. [DOI] [PubMed] [Google Scholar]
- Dueker et al. (2012).Dueker ME, O’Mullan GD, Juhl AR, Weathers KC, Uriarte M. Local environmental pollution strongly influences culturable bacterial aerosols at an urban aquatic superfund site. Environmental Science & Technology. 2012;46(20):10926–10933. doi: 10.1021/es301870t. [DOI] [PubMed] [Google Scholar]
- Dwight et al. (2011).Dwight RH, Caplan JS, Brinks MV, Catlin SN, Buescher G, Semenza JC. Influence of variable precipitation on coastal water quality in Southern California. Water Environment Research. 2011;83(12):2121–2130. doi: 10.2175/106143011X12928814444574. [DOI] [PubMed] [Google Scholar]
- Fahlgren et al. (2010).Fahlgren C, Hagström Å, Nilsson D, Zweifel UL. Annual variations in the diversity, viability, and origin of airborne bacteria. Applied and Environmental Microbiology. 2010;76(9):3015–3025. doi: 10.1128/AEM.02092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin et al. (1977).Fannin KF, Gannon JJ, Cochran KW, Spendlove JC. Field studies on coliphages and coliforms as indicators of airborne animal viral contamination from wastewater treatment facilities. Water Research. 1977;11(2):181–188. doi: 10.1016/0043-1354(77)90124-5. [DOI] [Google Scholar]
- Feddersen et al. (2016).Feddersen F, Olabarrieta M, Guza RT, Winters D, Raubenheimer B, Elgar S. Observations and modeling of a tidal inlet dye tracer plume. Journal of Geophysical Research: Oceans. 2016;121(10):7819–7844. doi: 10.1002/2016JC011922. [DOI] [Google Scholar]
- Fleming et al. (2011).Fleming LE, Kirkpatrick B, Backer LC, Walsh CJ, Nierenberg K, Clark J, Reich A, Hollenbeck J, Benson J, Cheng YS, Naar J, Pierce R, Bourdelais AJ, Abraham WM, Kirkpatrick G, Zaias J, Wanner A, Mendes E, Shalat S, Hoagland P, Stephan W, Bean J, Watkins S, Clarke T, Byrne M, Baden DG. Review of Florida red tide and human health effects. Harmful Algae. 2011;10(2):224–233. doi: 10.1016/j.hal.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong & Lipp (2005).Fong T-T, Lipp EK. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews. 2005;69(2):357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich-Nowoisky et al. (2016).Fröhlich-Nowoisky J, Kampf CJ, Weber B, Huffman JA, Pöhlker C, Andreae MO, Lang-Yona N, Burrows SM, Gunthe SS, Elbert W, Su H, Hoor P, Thines E, Hoffmann T, Després VR, Pöschl U. Bioaerosols in the earth system: climate, health, and ecosystem interactions. Atmospheric Research. 2016;182:346–376. doi: 10.1016/j.atmosres.2016.07.018. [DOI] [Google Scholar]
- Gantt & Meskhidze (2013).Gantt B, Meskhidze N. The physical and chemical characteristics of marine primary organic aerosol: a review. Atmospheric Chemistry and Physics. 2013;13(8):3979–3996. doi: 10.5194/acp-13-3979-2013. [DOI] [Google Scholar]
- Gersberg et al. (2006).Gersberg RM, Rose MA, Robles-Sikisaka R, Dhar AK. Quantitative detection of hepatitis a virus and enteroviruses near the United States-Mexico border and correlation with levels of fecal indicator bacteria. Applied and Environmental Microbiology. 2006;72(12):7438–7444. doi: 10.1128/AEM.01024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham et al. (2018).Graham KE, Prussin AJ, II, Marr LC, Sassoubre LM, Boehm AB. Microbial community structure of sea spray aerosols at three california beaches. FEMS Microbiology Ecology. 2018;94(3):1–10. doi: 10.1093/femsec/fiy005. [DOI] [PubMed] [Google Scholar]
- Grant et al. (2005).Grant SB, Kim JH, Jones BH, Jenkins SA, Wasyl J, Cudaback C. Surf zone entrainment, along-shore transport, and human health implications of pollution from tidal outlets. Journal of Geophysical Research C: Oceans. 2005;110(10):1–20. doi: 10.1029/2004JC002401. [DOI] [Google Scholar]
- Griffin et al. (2003).Griffin DW, Donaldson KA, Paul JH, Rose B, Griffin DW, Donaldson KA, Paul JH, Rose JB. Pathogenic human viruses in coastal waters. American Society for Microbiology. 2003;16(1):129–143. doi: 10.1128/CMR.16.1.129-143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes et al. (2020).Grimes DJ, Feddersen F, Giddings SN, Pawlak G. Cross-shore deformation of a surfzone released dye plume by an internal tide on the inner-shelf. Journal of Physical Oceanography. 2020;50(1):35–54. doi: 10.1175/JPO-D-19-0046.1. [DOI] [Google Scholar]
- Halpern et al. (2012).Halpern BS, Longo C, Hardy D, Mcleod KL, Samhouri JF, Katona SK, Kleisner K, Lester SE, Oleary J, Ranelletti M, Rosenberg AA, Scarborough C, Selig ER, Best BD, Brumbaugh DR, Chapin FS, Crowder LB, Daly KL, Doney SC, Elfes C, Fogarty MJ, Gaines SD, Jacobsen KI, Karrer LB, Leslie HM, Neeley CE, Pauly D, Polasky S, Ris B, Martin KS, Stone GS, Sumaila UR, Zeller D. An index to assess the health and benefits of the global ocean. Nature. 2012;488(7413):615–620. doi: 10.1038/nature11397. [DOI] [PubMed] [Google Scholar]
- Hawkins & Russell (2010).Hawkins LN, Russell LM. Polysaccharides, proteins, and phytoplankton fragments: four chemically distinct types of marine primary organic aerosol classified by single particle spectromicroscopy. Advances in Meteorology. 2010;2010(3642):1–14. doi: 10.1155/2010/612132. [DOI] [Google Scholar]
- Hernandez (2017).Hernandez D. 143 Million Gallons of Sewage Spill into Tijuana River. https://www.sandiegouniontribune.com/news/environment/sd-me-sewage-spill-20170224-story.html San Diego Union Tribune. 2017 [Google Scholar]
- Hutchinson et al. (2013).Hutchinson TH, Lyons BP, Thain JE, Law RJ. Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Marine Pollution Bulletin. 2013;74(2):517–525. doi: 10.1016/j.marpolbul.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick et al. (2010).Kirkpatrick B, Pierce R, Cheng YS, Henry MS, Blum P, Osborn S, Nierenberg K, Pederson BA, Fleming LE, Reich A, Naar J, Kirkpatrick G, Backer LC, Baden D. Inland transport of aerosolized Florida red tide toxins. Harmful Algae. 2010;9(2):186–189. doi: 10.1016/j.hal.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, Lee & Aller (2005).Kuznetsova M, Lee C, Aller J. Characterization of the proteinaceous matter in marine aerosols. Marine Chemistry. 2005;96(3–4):359–377. doi: 10.1016/j.marchem.2005.03.007. [DOI] [Google Scholar]
- Ladino et al. (2019).Ladino LA, Raga GB, Alvarez-Ospina H, Andino-Enríquez MA, Rosas I, Martínez L, Salinas E, Miranda J, Ramírez-Díaz Z, Figueroa B, Chou C, Bertram AK, Quintana ET, Maldonado LA, García-Reynoso A, Si M, Irish VE. Ice-nucleating particles in a coastal tropical site. Atmospheric Chemistry and Physics. 2019;19(9):6147–6165. doi: 10.5194/acp-19-6147-2019. [DOI] [Google Scholar]
- Lawaetz & Stedmon (2009).Lawaetz AJ, Stedmon CA. Fluorescence intensity calibration using the Raman Scatter Peak of Water. Applied Spectroscopy. 2009;63(8):936–940. doi: 10.1366/000370209788964548. [DOI] [PubMed] [Google Scholar]
- Lenain & Melville (2017).Lenain L, Melville WK. Evidence of sea-state dependence of aerosol concentration in the marine atmospheric boundary layer. Journal of Physical Oceanography. 2017;47(1):69–84. doi: 10.1175/JPO-D-16-0058.1. [DOI] [Google Scholar]
- Lewis & Schwartz (2004).Lewis ER, Schwartz SE. Sea salt aerosol production: mechanisms, methods, measurements and models - a critical review. Washington D.C.: American Geophysical Union; 2004. [Google Scholar]
- Li et al. (2021).Li P, Li L, Yang K, Zheng T, Liu J, Wang Y. Characteristics of microbial aerosol particles dispersed downwind from rural sanitation facilities: size distribution. Source Tracking and Exposure Risk. Environmental Research. 2021;195:110798. doi: 10.1016/j.envres.2021.110798. [DOI] [PubMed] [Google Scholar]
- Li et al. (2011).Li M, Qi J, Zhang H, Huang S, Li L, Gao D. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao Coastal Region. Science of the Total Environment. 2011;409(19):3812–3819. doi: 10.1016/j.scitotenv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Lodder & de Roda Husman (2020).Lodder W, de Roda Husman AM. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet Gastroenterology and Hepatology. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay & Shiu (1981).Mackay D, Shiu WY. A critical review of Henry’s Law constants for chemicals of environmental interest. Journal of Physical Chemistry. 1981;10(4):1175. [Google Scholar]
- Malakootian et al. (2013).Malakootian M, Radhakrishna N, Mazandarany MP, Hossaini H. Bacterial-aerosol emission from wastewater treatment plant. Desalination and Water Treatment. 2013;51(22–24):4478–4488. doi: 10.1080/19443994.2013.769668. [DOI] [Google Scholar]
- Melville et al. (2016).Melville WK, Lenain L, Cayan DR, Kahru M, Kleissl JP, Linden PF, Statom NM. The modular aerial sensing system. Journal of Atmospheric and Oceanic Technology. 2016;33(6):1169–1184. doi: 10.1175/JTECH-D-15-0067.1. [DOI] [Google Scholar]
- Michaud et al. (2018).Michaud JM, Thompson LR, Kaul D, Espinoza JL, Richter RA, Xu ZZ, Lee C, Pham KM, Beall CM, Malfatti F, Azam F, Knight R, Burkart MD, Dupont CL, Prather KA. Taxon-specific aerosolization of bacteria and viruses in an experimental ocean-atmosphere mesocosm. Nature Communications. 2018;9(1):1. doi: 10.1038/s41467-018-04409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero, Dueker & O’Mullan (2016).Montero A, Dueker ME, O’Mullan GD. Culturable Bioaerosols along an urban waterfront are primarily associated with coarse particles. PeerJ. 2016;2016(12):1–18. doi: 10.7717/peerj.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn (2011).Munn C. Marine microbiology: ecology and applications. New York: Taylor & Francis; 2011. [Google Scholar]
- Murphy (2011).Murphy KR. A note on determining the extent of the water Raman Peak in Fluorescence Spectroscopy. Applied Spectroscopy. 2011;65(2):233–236. doi: 10.1366/10-06136. [DOI] [Google Scholar]
- National Oceanic and Atmospheric Administration (2016).National Oceanic and Atmospheric Administration Bioeffects Program. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/bioeffects-program/ National Centers for Coastal Ocean Science. 2016 (accessed 1 January 2017) [Google Scholar]
- O’Mullan, Dueker & Juhl (2017).O’Mullan GD, Dueker EM, Juhl AR. Challenges to managing microbial fecal pollution in coastal environments: extra-enteric ecology and microbial exchange among water, sediment, and air. Current Pollution Reports. 2017;3(1):1–16. doi: 10.1007/s40726-016-0047-z. [DOI] [Google Scholar]
- Patterson et al. (2016).Patterson JP, Collins DB, Michaud JM, Axson JL, Sultana CM, Moser T, Dommer AC, Conner J, Grassian VH, Stokes MD, Deane GB, Evans JE, Burkart MD, Prather KA, Gianneschi NC. Sea spray aerosol structure and composition using cryogenic transmission electron microscopy. ACS Central Science. 2016;2(1):40–47. doi: 10.1021/acscentsci.5b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup et al. (2005).Pickup RW, Rhodes G, Arnott S, Sidi-Boumedine K, Bull TJ, Weightman A, Hurley M, Hermon-Taylor J. Mycobacterium Avium Subsp. Paratuberculosis in the Catchment Area and Water of the River Taff in South Wales, United Kingdom, and Its Potential Relationship to Clustering of Crohn’s Disease Cases in the City of Cardiff. Applied and Environmental Microbiology. 2005;71(4):2130–2139. doi: 10.1128/AEM.71.4.2130-2139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce et al. (2003).Pierce RH, Henry MS, Patricia Blum JL, Yung Sung Cheng DY, Zhou Y. Brevetoxin concentrations in marine aerosol. Human Exposure Levels During a Karenia Brevis Harmful Algal Bloom. 2003;70(1):161–165. doi: 10.1007/s00128-002-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospero et al. (2005).Prospero JM, Blades E, Mathison G, Naidu R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia. 2005;21(1):1–19. doi: 10.1007/s10453-004-5872-7. [DOI] [Google Scholar]
- Quinn et al. (2015).Quinn PK, Collins DB, Grassian VH, Prather KA, Bates TS. Chemistry and related properties of freshly emitted sea spray aerosol. Chemical Reviews. 2015;115(10):4383–4399. doi: 10.1021/cr500713g. [DOI] [PubMed] [Google Scholar]
- Rodriguez, Giddings & Kumar (2018).Rodriguez AR, Giddings SN, Kumar N. Impacts of nearshore wave-current interaction on transport and mixing of small-scale buoyant plumes. Geophysical Research Letters. 2018;45(16):8379–8389. doi: 10.1029/2018GL078328. [DOI] [Google Scholar]
- Schiff et al. (1983).Schiff G, Stefanovic G, Young E, Pennekamp JK. Determination of Minimal Infectious Dose of an Enterovirus in Drinking Water. U.S. Environmental Protection Agency, Washington, D.C., EPA/600/1-83/004. 1983 [Google Scholar]
- Shaffer & Lighthart (1997).Shaffer BT, Lighthart B. Survey of culturable airborne bacteria at four diverse locations in Oregon: urban, rural, forest, and coastal. Microbial Ecology. 1997;34(3):167–177. doi: 10.1007/s002489900046. [DOI] [PubMed] [Google Scholar]
- Shuval (2003).Shuval H. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. Journal of Water and Health. 2003;1(2):53–64. doi: 10.2166/wh.2003.0007. [DOI] [PubMed] [Google Scholar]
- Smart & Laidlaw (1977).Smart PL, Laidlaw IMSS. An evaluation of some fluorescent dyes for water tracing. Water Resources Research. 1977;13(1):15–33. doi: 10.1029/WR013i001p00015. [DOI] [Google Scholar]
- Smith et al. (2012).Smith DJ, Jaffe DA, Birmele MN, Griffin DW, Schuerger AC, Hee J, Roberts MS. Free tropospheric transport of microorganisms from Asia to North America. Microbial Ecology. 2012;64(4):973–985. doi: 10.1007/s00248-012-0088-9. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2013).Smith DJ, Timonen HJ, Jaffe DA, Griffin DW, Birmele MN, Perry KD, Ward PD, Roberts MS. Intercontinental dispersal of bacteria and archaea by transpacific winds. Applied and Environmental Microbiology. 2013;79(4):1134–1139. doi: 10.1128/AEM.03029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele et al. (2018).Steele JA, Blackwood AD, Griffith JF, Noble RT, Schiff KC. Quantification of pathogens and markers of fecal contamination during storm events along popular surfing beaches in San Diego. California Water Research. 2018;136(2):137–149. doi: 10.1016/j.watres.2018.01.056. [DOI] [PubMed] [Google Scholar]
- Suijlen & Buyse (1994).Suijlen JM, Buyse JJ. Potentials of photolytic rhodamine WT as a large-scale water tracer assessed in a long-term experiment in the Loosdrecht Lakes. Limnology and Oceanography. 1994;39(6):1411–1423. doi: 10.4319/lo.1994.39.6.1411. [DOI] [Google Scholar]
- Teltsch & Katzenelson (1978).Teltsch B, Katzenelson E. Airborne enteric bacteria and viruses from spray irrigation with wastewater. Applied and Environmental Microbiology. 1978;35(2):290–296. doi: 10.1128/AEM.35.2.290-296.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano et al. (2011).Urbano R, Palenik B, Gaston CJ, Prather KA. Detection and phylogenetic analysis of coastal bioaerosols using culture dependent and independent techniques. Biogeosciences. 2011;8(2):301–309. doi: 10.5194/bg-8-301-2011. [DOI] [Google Scholar]
- U.S. Comission on Ocean Policy (2004).U.S. Comission on Ocean Policy An Ocean Blueprint for the 21st Century. Final Report. 2004. https://govinfo.library.unt.edu/oceancommission/documents/full_color_rpt/000_ocean_full_report.pdf https://govinfo.library.unt.edu/oceancommission/documents/full_color_rpt/000_ocean_full_report.pdf
- United States Environmental Protection Agency (2014).United States Environmental Protection Agency Toxic and Priority Pollutants Under the Clean Water Act. 2014. https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act. https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed 1 January 2017)
- Van Eijk et al. (2011).Van Eijk AMJ, Kusmierczyk-Michulec JT, Francius MJ, Tedeschi G, Piazzola J, Merritt DL, Fontana JD. Sea-spray aerosol particles generated in the surf zone. Journal of Geophysical Research Atmospheres. 2011;116(19):1–20. doi: 10.1029/2011JD015602. [DOI] [Google Scholar]
- Wilson, Cobb & Kilpatrick (1986).Wilson JF, Cobb ED, Kilpatrick FA. U.S. Geological Survey, Techniques of Water-Resources Investigations, 03-A12. Washington D.C.: US Government Printing Office; 1986. Fluorometric Procedures for Dye Tracing. [DOI] [Google Scholar]
- Wu et al. (2020).Wu X, Feddersen F, Giddings SN, Kumar N, Gopalakrishnan G. Mechanisms of mid-to outer-shelf transport of shoreline-released tracers. Journal of Physical Oceanography. 2020;50(7):1813–1837. doi: 10.1175/JPO-D-19-0225.1. [DOI] [Google Scholar]
- Yooseph et al. (2013).Yooseph S, Andrews-Pfannkoch C, Tenney A, McQuaid J, Williamson S, Thiagarajan M, Brami D, Zeigler-Allen L, Hoffman J, Goll JB, Fadrosh D, Glass J, Adams MD, Friedman R, Venter JC. A metagenomic framework for the study of airborne microbial communities. PLOS ONE. 2013;8(12):e81862. doi: 10.1371/journal.pone.0081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calibration curve produced by measuring the fluorescence of RWT solutions at known concentrations. The data were background corrected using the same technique used for the collected samples.
(A) a 2 PPB RWT standard, (B) sample #19 - dye detected, (C) sample #20 - dye not detected, and (D) sample #27 - aerosol field blank; dye not detected Red dots indicate the 3 excitation/emission pairs determined from the calibration to be used for RWT dye quantification. The mean fluorescence intensity from the black rectangle was subtracted from the entire spectrum as an internal background correction.
Like Figure 2, for all aerosol samples with data available (day samples). Right: Average sea surface dye concentrations during the period each aerosol sample was collected at the location on land indicated by the triangle. The 200 m × 100 m dotted box indicates where the average wind direction during each sampling interval intersected the coastal waters from the sampling site. Figure 3 finds the location of that box and its mean dye value for each hourly sea surface dye measurement. Here we show the mean dye field during each air sampling period and the mean upwind location (box). Left: wind rose for the winds observed at the KNRS meteorological station during the aerosol sampling periods.
More details on methods used
Data Availability Statement
The following information was supplied regarding data availability:
Pendergraft, Matthew A.; Grimes, Derek J.; Giddings, Sarah N.; Feddersen, Falk; Beall, Charlotte M.; Lee, Christopher; Santander, Mitchell V.; Prather, Kimberly A. (2021). Data from: Airborne transmission pathway for coastal water pollution. In Center for Aerosol Impacts on Chemistry of the Environment (CAICE). UC San Diego Library Digital Collections. https://doi.org/10.6075/J0V40SSD