Abstract
Impaired gait in Parkinson’s disease is marked by slow, arrhythmic stepping, and often includes freezing of gait episodes where alternating stepping halts completely. Wearable inertial sensors offer a way to detect these gait changes and novel deep brain stimulation (DBS) systems can respond with clinical therapy in a real-time, closed-loop fashion. In this paper, we present two novel closed-loop DBS algorithms, one using gait arrhythmicity and one using a logistic-regression model of freezing of gait detection as control signals. Benchtop validation results demonstrate the feasibility of running these algorithms in conjunction with a closed-loop DBS system by responding to real-time human subject kinematic data and pre-recorded data from leg-worn inertial sensors from a participant with Parkinson’s disease. We also present a novel control policy algorithm that changes neurostimulator frequency in response to the kinematic inputs. These results provide a foundation for further development, iteration, and testing in a clinical trial for the first closed-loop DBS algorithms using kinematic signals to therapeutically improve and understand the pathophysiological mechanisms of gait impairment in Parkinson’s disease.
I. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, and over 80% of people with moderate to advanced PD develop freezing of gait (FOG) [1]. FOG is characterized as an intermittent, involuntary and often sudden inability to perform alternating stepping and usually occurs when patients attempt to initiate walking, turn, or navigate obstacles. Dopaminergic medication and chronic, open-loop deep brain stimulation have variable effects on FOG [2], [3]. Subthalamic deep brain stimulation (DBS) at either 60 Hz or 140 Hz can improve FOG and markers of FOG such as gait arrhythmicity in forward walking and stepping in place tasks [4], as well as during walking in a turning and barrier course [5]. 60 Hz was superior to 140 Hz DBS in improving regularity of ongoing movement in both a stepping in place task [4] and an upper limb task [6]. Other studies suggest variable benefits of lower or higher frequencies of DBS [8], and differential effects across people with Parkinson’s disease, imploring for more personalized therapy.
The feasibility and efficacy of closed-loop DBS for tremor in Parkinson’s disease using a kinematic marker of tremor sensed by a smart watch [7], [8] has been demonstrated. However, it is unknown whether kinematic markers of gait impairment and FOG can be used as control variables for closed-loop DBS systems, and there are currently no control policy algorithms available to respond to such inputs by changing the frequency of DBS. Based on the wide availability of gait detecting kinematic sensors in the consumer space, an opportunity exists to directly monitor FOG and adjust neurostimulation based on gait parameters in real time.
This paper demonstrates the first step toward such a system and leverages the next generation abilities of the Summit™ RC+S (Medtronic Inc., FDA IDE approved), an investigational human-use rechargeable sensing neurostimulator. We developed a preclinical benchtop system using the accompanying Summit API (Medtronic Inc.) programming interface that allows for external control of the RC+S neurostimulator using a PC-in-the-loop, Figure 1. We developed and successfully implemented novel classifier and control policy algorithms on the benchtop closed-loop system that detected impaired gait or freezing of gait and adjusted either stimulation frequency or current intensity. This was done using real-time human subject kinematic data and kinematic data previously recorded from a person with Parkinson’s disease, who demonstrated typical gait impairment and FOG. This allowed for real-time testing and iteration of these novel control policies using a closed-loop stimulation system that will be eventually implemented in human subjects.
FIGURE 1. Closed-loop kinematic adaptive deep brain stimulation system in freely moving human.
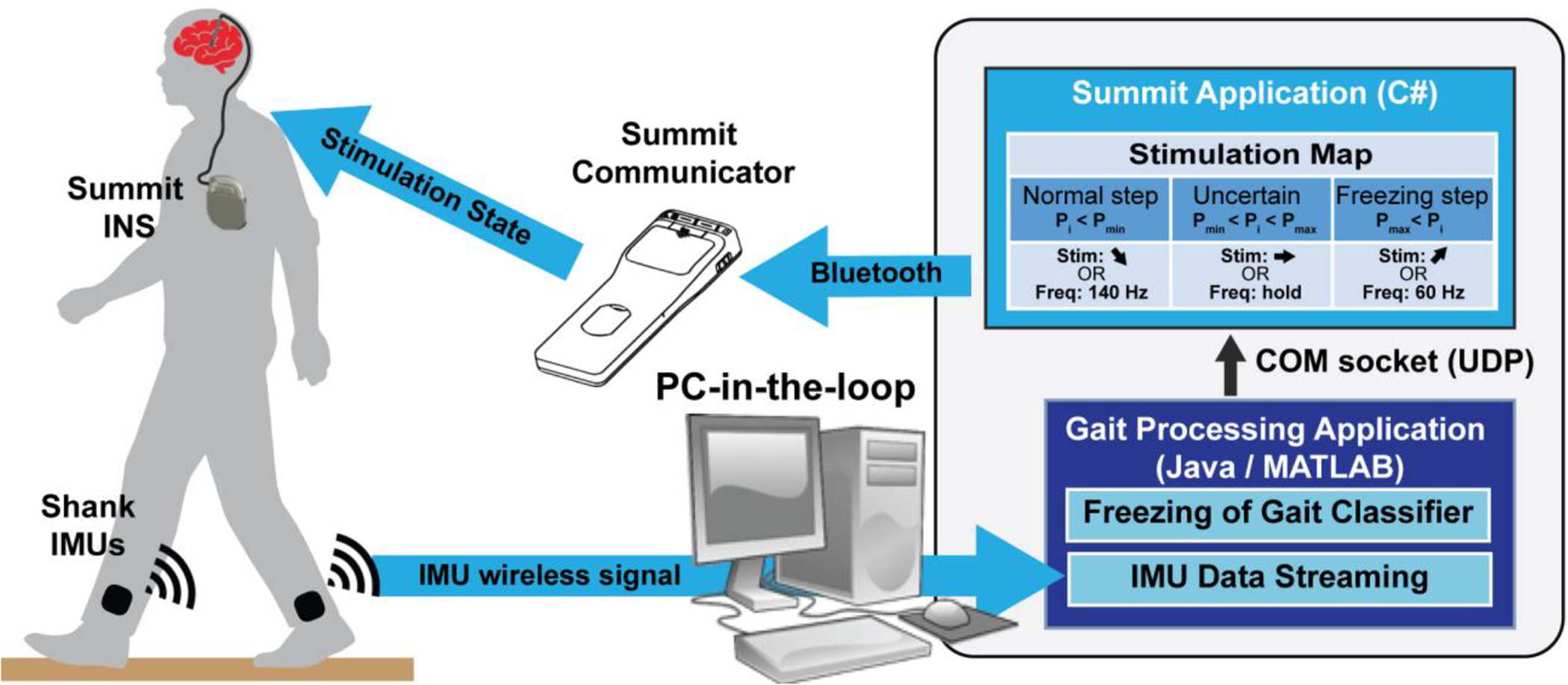
Inertial measurement unit (IMU) sensor data stream to PC-in-the-loop where Gait Processing Application classifies freezing in the closed-loop algorithm. The Gait Processing Application communicates through a COM socket (UDP) with the Summit Application to change the stimulation state on the Summit INS according to stimulation map. For the freezing of gait classifier, this maps probabilities (Pi) of freezing for one timepoint (i) to a stimulation change in intensity or frequency based on whether Pi < normal step threshold Pmin, Pi is uncertain: Pmin ≤ Pi ≤ Pmax, or Pi > freezing step threshold Pmax. Summit communicator and INS images are both credited to Medtronic.
II. Methods
A. System Architecture
We developed a system that included wearable inertial measurement unit sensors (IMUs) that streamed kinematic data to a PC-in-the-loop, which wirelessly communicated with the Summit™ RC+S System, Figure 1. The Summit™ System consists of the Summit™ RC+S, a rechargeable implanted neurostimulator (INS) with sensing and closed-loop stimulation capabilities, a bidirectional Summit Communicator, and a Summit Application or C# API [9]. The C# API allows for development of customized Summit Applications to enable closed-loop therapy investigations through real-time data streaming and stimulation adjustment from a PC [10]. We built custom Gait Processing Applications in Java and MATLAB to wirelessly stream real-time (Java) and pre-recorded (MATLAB) IMU data, through which we implemented our novel freezing of gait algorithms. The Gait Processing Applications communicated state changes (i.e. freezing or normal stepping) to the Summit Application through a User Datagram Protocol (UDP) socket. The Summit Application adapted either stimulation frequency or intensity according to a custom Stimulation Map.
B. Kinematic Algorithm Design and Implementation
Two kinematic classifier algorithms, which detected freezing of gait or arrhythmic gait were employed in the Gait Processing Applications. For the Gait Processing Application written in MATLAB, previously collected IMU data was streamed in real time through the system to validate algorithm performance with real Parkinsonian data. This data was collected from a subject (60 years old) with moderate Parkinson’s disease and FOG, who walked around a turning and barrier course that we have designed to elicit freezing events [11], [12]. For the Java-based Gait Processing Application, IMU data was streamed from a healthy subject freely walking around a laboratory in real time to validate experimental system performance. For both subjects, IMU sensors (APDM Inc.) were positioned in a standardized manner, laterally on both shanks to measure sagittal angular velocity, which captures the kinematics of leg swinging. IMU data was sampled at 128 Hz. Participants provided informed written consent and all study procedures were approved by the Stanford University Institutional Review Board.
Individual steps were identified as positive peaks in the left and right sagittal shank angular velocity plot over time, Figure 2, top panel. Three gait parameters were calculated for each leg separately. Stride time was defined as the time period between two successive positive peaks on the angular velocity plot. Swing angular range was defined as the area under a peak on the sagittal angular velocity plot. Swing time (SWT) was computed as the time between the initiation of the swing phase and the end of the swing phase for each step, determined from the zero-crossings on the angular velocity plot. Gait arrhythmicity was defined as the average coefficient of variation (CV) for the previous three stride times of the left and right leg. Gait asymmetry was defined as 100 × |ln(SSWT/LSWT)|, where SSWT and LSWT correspond to the leg with the shortest and longest mean swing time over the trials, respectively [13].
FIGURE 2. Freezing detection comparisons.
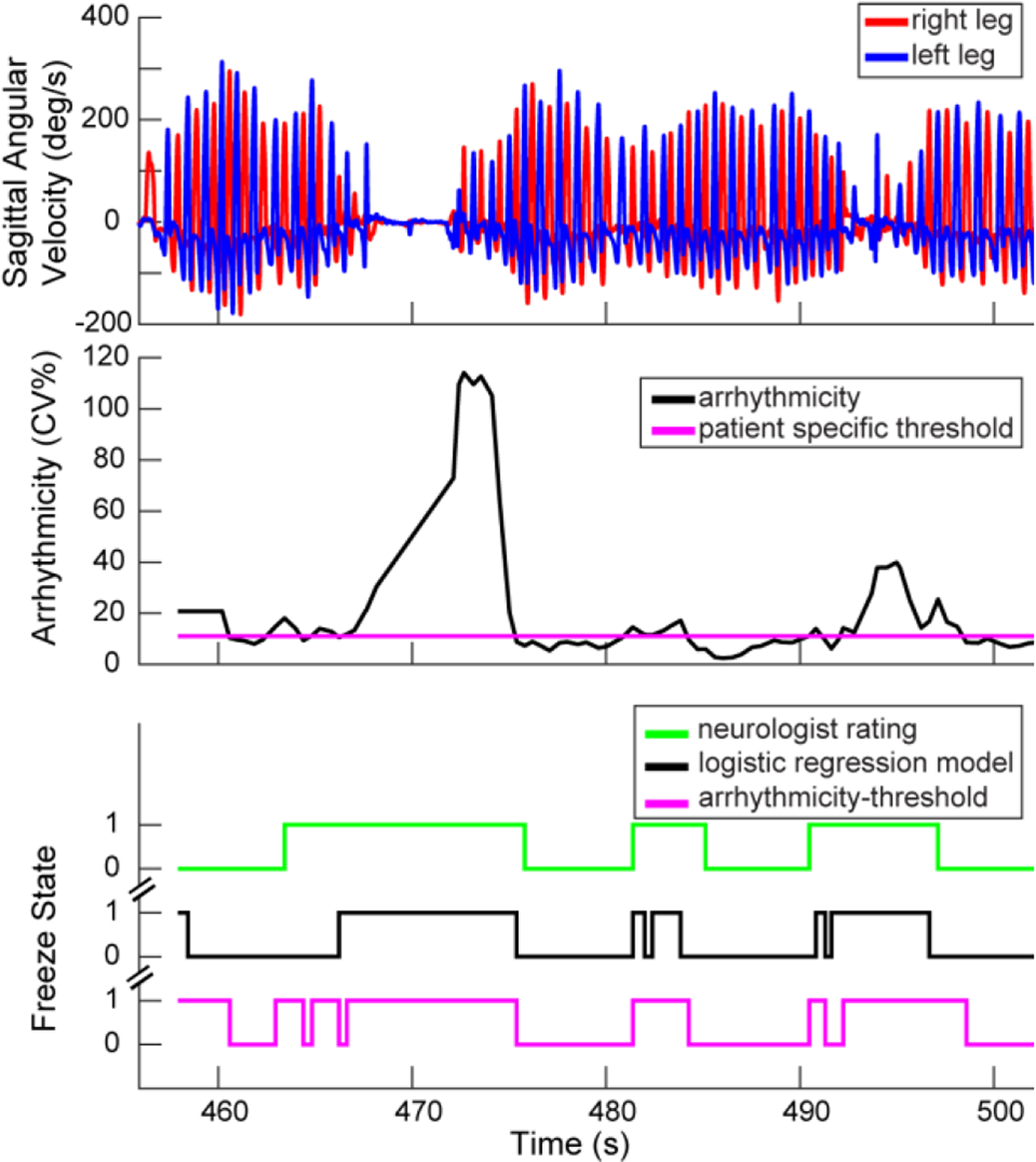
Excerpt of sagittal angular velocities from Parkinson’s gait data (top), from which arrhythmicity was calculated over time (middle) and where freezing behavior was identified by: a neurologist, a validated logistic regression model, or by an arrhythmicity-threshold model (bottom).
The kinematic algorithm that detected freezing was a previously validated logistic regression model that utilized four calculated gait parameters, arrhythmicity over the last six steps (AR), stride time (ST), swing angular range (SA), and asymmetry over the last six steps (AS), to predict a subject’s probability of freezing [12], Figure 2 bottom panel. Specifically, probability was calculated using the aforementioned gait parameters denoted as Xar, Xst, Xsa, Xas, respectively:
We also employed a “guardian angel” arrhythmicity single threshold model as a surrogate for FOG. This model identified arrhythmic steps above a patient-specific threshold and classified these steps as freezing behavior, Figure 2, middle. The patient-specific threshold (11 CV%) was determined from previously recorded walking data for this patient. Both algorithms signaled state changes to the Summit™ RC+S system.
C. Kinematic System Software Development
The Gait Processing Applications (Java/MATLAB) communicated through a UDP socket communication layer with a customized Summit Application which was developed using the Summit API. The Gait Processing Application outputs a datagram packet corresponding to the probability of FOG at the current step as determined by our algorithm. The datagram signaled a stimulation frequency- or intensity-based state change. We used the Summit™ INS on-board state table, which can be used to ramp stimulation parameters to desired setpoints based on an embedded stimulation table [14]. We used test functionality built into the Summit API to force the Summit™ INS to manually change between state table entries based on our external analysis. An example of the state table implemented for the freezing detection algorithm is shown in the Stimulation Map, Figure 1 inset. This mapped probabilities (Pi) of freezing for one timepoint (i) to a stimulation state change in intensity or frequency based on the following: Pi < Pmin (a normal step was detected): ramp stimulation up to 140 Hz, Pmin ≤ Pi ≤ Pmax (uncertain if step is normal or freeze): hold at the current frequency of stimulation, or Pi > Pmax (a freeze was detected): ramp stimulation down to 60 Hz. Probabilities were set as follows: Pmin = 30%, Pmax = 70%. In addition, the Stimulation Map enabled changing stimulation current intensities according to the following control policy: the INS was configured to ramp up the intensity (at a safe rate and within safe ranges) when there was a high probability of freezing, hold at the current intensity when the probability of freezing was uncertain, and ramp down slowly and safely when the probability of freezing was unlikely. For the arrhythmicity threshold control policy, the states were configured to ramp stimulation frequency to 140 Hz when the patient’s gait was detected as “rhythmic” or below the arrhythmicity threshold, and ramp down to 60 Hz when their gait was detected as “arrhythmic” or above the arrhythmicity threshold. Similarly, when changing stimulation current intensities, the INS was configured to ramp up intensity (at a safe rate and within safe ranges) when arrhythmic gait was detected, and ramp down slowly and safely when the gait was rhythmic.
D. Benchtop Validation
To test the system, we used an un-implanted Summit™ INS connected to an oscilloscope with wires representing contacts 0–2 in the DBS leads as well as a voltage divider network soldered onto a breadboard. This benchtop setup allowed monitoring of stimulation response through resistors simulating resistances found in the biological brain. We tested the real-time kinematic streaming and stimulation responses with the Java Gait Processing Application and the custom Summit Application from a healthy control subject wearing the IMU sensors on both shanks and walking around the lab. The subject walked both regularly to represent rhythmic kinematic behavior as well as with slow and voluntary stops to represent potential freezing behavior. We also streamed previously recorded PD gait data interspersed with freezing events through the MATLAB Gait Processing Application which communicated with the custom Summit Application to also make stimulation changes in real time. For both the Java and MATLAB implementations, the stimulation intensity was bounded between 2 mA (Imin) and 5 mA (Imax) and the ramp up and down rates were set to 1 mA/s. The termination delay, or the duration of time over which the subject had to exhibit normal walking before stimulation ramped down to 140 Hz, was set to 1 s. In both cases, the Summit logs were used to validate stimulation parameter changes. These Summit logs have been previously validated as an accurate representation of output stimulation, as would be expected with a validated medical device. This allowed us to demonstrate the full closed-loop system in a fully functional manner before use in human trials.
III. Results
Benchtop testing demonstrated the ability of the system to correctly process and respond to kinematic data in real time, Figure 3. The arrhythmicity-based control policy, Figure 3A, evoked six periods of freeze-related stimulation changes while the logistic regression model control policy only evoked two periods of freeze-related stimulation change over the walking trial, Figure 3B. The arrhythmicity-based control policy classified intermittent periods of rhythmic and arrhythmic gait, while the logistic regression model-based FOG algorithm classified nearly the first half of the walking trial as below the freezing threshold (either as normal gait or uncertain to be normal or freezing gait), during which the INS output 140 Hz or decreased stimulation intensity, Figure 3B.
FIGURE 3. Real-time demonstration of system.
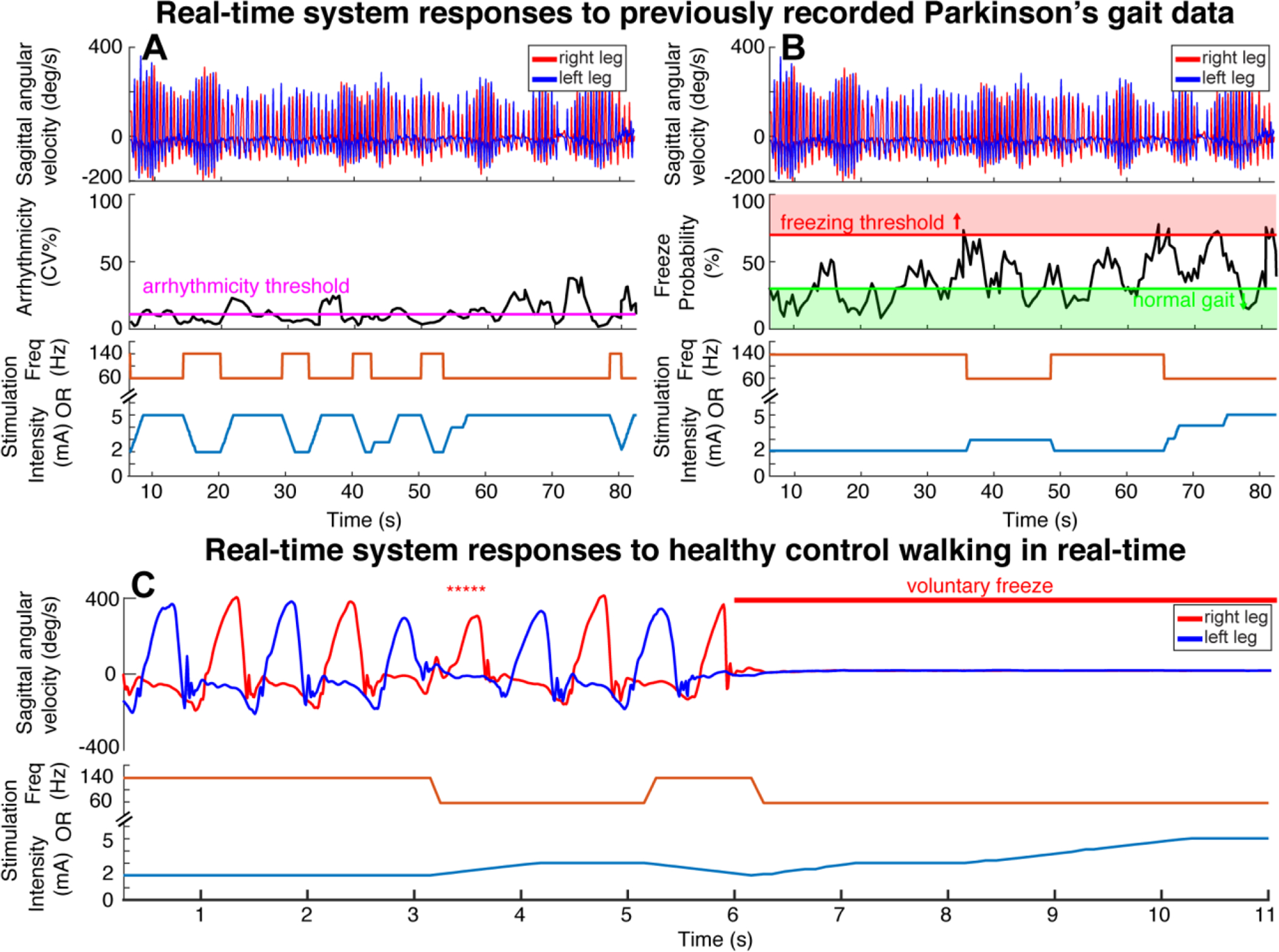
PC-in-the-loop architecture utilizing the Summit communicator to provide arrhythmicity-based (A) and freeze detection-based (B, C) adaptive stimulation. Arrhythmicity single threshold (B) and freezing, normal gait thresholds shown for freeze probabilities (B). Stimulation rate (frequency) changes shown independent of stimulation intensity changes (A/B/C bottom plots, orange and blue lines respectively). System responds to previously recorded IMU data from Parkinson’s patient with FOG and gait impairment (A, B) or IMU data from healthy control walking in real time with voluntary freezing episodes and voluntary freezing behavior (denoted by *****) (C).
Benchtop testing of the real-time Java Gait Processing Application using the logistic regression model control policy was also demonstrated, Figure 3C. The initial steps taken by the healthy control subject represented walking “normally”, or rhythmically, and as a result the INS delivered 140 Hz or presented a low stimulation intensity. The INS delivered 60 Hz or increased stimulation intensity when the subject voluntarily froze, as delineated at the end of the walking trial. The healthy control subject also exhibited simulated freezing behavior of slowed shank angular velocity and increased stride time during the trial, which triggered the INS to deliver 60 Hz or increased stimulation intensity, asterisks, Figure 3C. The termination delay for these runs was set to 1 s, which delayed stimulation to closely adapt to each step, occurring every second. These results confirmed that both the Java Gait Processing Application streaming real-time IMU data, and the MATLAB Gait Processing Application streaming previously recorded PD IMU data, can detect freezes and, together with the Summit™ RC+S system respond with therapeutic stimulation.
IV. Discussion
We have successfully developed novel kinematic classifier algorithms that identified FOG from IMU gait data from a person with Parkinson’s disease and in real time from a healthy control, and novel control policy algorithms that instructed changes in frequency or current intensity of stimulation based on the kinematic classifier algorithms. We demonstrated the feasibility of these novel algorithms for closed-loop kinematic neurostimulation for PD FOG using a customized test bench closed-loop stimulation system, which successfully adapted the frequency or intensity of stimulation in real time based on the kinematic inputs. The gait data was classified using two different kinematic algorithms: (1) a validated logistic regression model of FOG based on four gait parameters and (2) a single arrhythmicity threshold. This is the first time a kinematically controlled closed-loop system focused on FOG has been developed and the first time that such a system has been shown to successfully change stimulation frequency based on real human subject kinematic data using gait parameters.
This closed-loop laboratory test bench system is important because it allows for non-invasive, rapid design, testing, and iterating of algorithms and system improvements in a safe environment before use in patients. We have made this even more translatable to human testing by using kinematic data recorded from a person with PD and implanted with a DBS system, who performed a gait task that we developed that mimicked real-life situations where patients are liable to experience FOG [11], [12]. Different classifier and control policy algorithms can target different treatment needs. If a patient’s treatment goal is focused on restoring rhythmic gait overall and not focused on treating interspersed freezing episodes, then the arrhythmicity single threshold algorithm may be more efficacious as stimulation was triggered more often (Figure 3A) than with the logistic regression model dual threshold algorithm, Figure 3B. However, if the goal is to treat intermittent freezing episodes, then the logistic regression model dual threshold algorithm may be superior, as the thresholds for normal (Pmin) and freezing gait (Pmax) can be adjusted to create more conservative versus more liberal FOG detection. Our test bench is especially important with regards to safety, as no control policy algorithms have been developed that change frequency instantaneously, although this is possible for the first time in the implantable Summit™ RC+S system. There are a number of system parameters that can be adjusted in a patient-specific manner to optimize for safety, including the Imin, Imax which correspond to current intensity limits. The termination delay corresponds to the delay that occurs after a therapeutic stimulation state (e.g. 60 Hz, higher current intensity) is entered, and stimulation is maintained at this state until the delay has passed. This could be lengthened to encourage the stimulation to stay in these therapeutic states for longer durations. These initial studies on the bench demonstrate functionality required for future human subjects which will be performed using the developed software and systems. The success of bench and clinical testing of kinematic closed-loop DBS for gait impairment and FOG in PD will enable future fully embedded closed-loop neurostimulators capable of sensing behavioral signals and adapting optimized therapy automatically, while a person is maneuvering around a home environment.
In summary we have developed and implemented novel kinematic classifiers and control policy algorithms for use in an upcoming clinical trial of closed-loop DBS therapy for FOG and gait impairment in Parkinson’s disease. We developed a customized test bench kinematic closed-loop stimulation system and for the first time demonstrated that the system was capable of changing DBS frequency and current intensity in response to kinematic signals representative of FOG, both in real time and from a person with PD and FOG. The successful translation of these algorithms to the clinical trial will pave the way for novel, personalized closed-loop DBS systems for people with Parkinson’s disease and will improve knowledge of the underlying mechanisms of gait impairment and FOG in PD.
Acknowledgment
The authors would like to thank the members of the Bronte-Stewart Lab and the participant in the study. This work was supported by the Parkinson’s Foundation, the Robert and Ruth Halperin Foundation, John A. Blume Foundation, Helen M. Cahill Award for Research in Parkinson’s Disease, the Stanford Bio-X Graduate Fellowship. Medtronic Inc., provided the devices at no charge to the patients and provided components of the bench system used in this study but no additional financial support.
Contributor Information
Jeffrey A. Herron, University of Washington, Seattle, WA 98195 USA.
Helen M. Bronte-Stewart, Stanford University, Stanford, CA 94305 USA
References
- [1].Amboni M et al. , “Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study,” Park. Relat. Disord, vol. 21, no. 6, pp. 644–649, 2015, doi: 10.1016/j.parkreldis.2015.03.028. [DOI] [PubMed] [Google Scholar]
- [2].Merola A et al. , “Parkinson’s disease progression at 30 years: a study of subthalamic deep brain-stimulated patients,” Brain, vol. 134, no. 7, pp. 2074–2084, July. 2011, doi: 10.1093/brain/awr121. [DOI] [PubMed] [Google Scholar]
- [3].Schlenstedt C, Shalash A, Muthuraman M, Falk D, Witt K, and Deuschl G, “Effect of high-frequency subthalamic neurostimulation on gait and freezing of gait in Parkinson’s disease: a systematic review and meta-analysis,” Eur. J. Neurol, vol. 24, no. 1, pp. 18–26, January. 2017, doi: 10.1111/ene.13167. [DOI] [PubMed] [Google Scholar]
- [4].Anidi C et al. , “Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease,” Neurobiol. Dis, vol. 120, 2018, doi: 10.1016/j.nbd.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Day J, Syrkin-Nikolau J, Anidi C, Kidzinski L, Delp S, and Bronte-Stewart H, “The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation,” PLoS One, vol. 15, no. 4, p. e0231984, April. 2020, doi: 10.1371/journal.pone.0231984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blumenfeld Z et al. , “Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations,” Mov. Disord, vol. 32, no. 1, pp. 80–88, 2017, doi: 10.1002/mds.26837. [DOI] [PubMed] [Google Scholar]
- [7].Malekmohammadi M et al. , “Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease,” Mov. Disord, vol. 31, no. 3, pp. 426–428, 2016, doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]
- [8].Velisar A et al. , “Dual Threshold Neural Closed Loop Deep Brain Stimulation in Parkinson disease Patients,” Brain Stimul, vol. 12, February. 2019, doi: 10.1016/j.brs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- [9].Herron J, Stanslaski S, Chouinard T, Corey R, Denison T, and Orser H, “Bi-directional brain interfacing instrumentation,” I2MTC 2018 – 2018 IEEE Int. Instrum. Meas. Technol. Conf. Discov. New Horizons Instrum. Meas. Proc, pp. 1–6, 2018, doi: 10.1109/I2MTC.2018.8409795. [DOI] [Google Scholar]
- [10].Stanslaski S et al. , “A Chronically Implantable Neural Coprocessor for Investigating the Treatment of Neurological Disorders,” IEEE Trans. Biomed. Circuits Syst, vol. 12, no. 6, pp. 1230–1245, 2018, doi: 10.1109/TBCAS.2018.2880148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Syrkin-Nikolau J et al. , “Subthalamic neural entropy is a feature of freezing of gait in freely moving people with Parkinson’s disease,” Neurobiol. Dis, vol. 108, no. June, pp. 288–297, 2017, doi: 10.1016/j.nbd.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Day JJ, Syrkin-Nikolau J, Anidi CM, Kidzinski L, Delp SL, and Bronte-Stewart HM, “The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation,” bioRxiv, p. 671479, January. 2019, doi: 10.1101/671479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Plotnik M and Hausdorff JM, “The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease.,” Mov. Disord, vol. 23 Suppl 2, pp. S444–50, 2008, doi: 10.1002/mds.21984. [DOI] [PubMed] [Google Scholar]
- [14].Herron J et al. , Embedding adaptive stimulation algorithms for a new implantable deep-brain stimulation research tool. 2018.


