Abstract
Background:
Postpartum hemorrhage (PPH) is a threat to maternal mortality worldwide. Evidence supports active management of third stage labor (AMTSL) for preventing PPH. However, trials of AMTSL include women at varying risk levels, such as women undergoing physiologic labor and those with labor complications. Counseling women about their risk for PPH and AMTSL is difficult as many women who appear low-risk can still have PPH.
Methods:
This study uses outcomes of 2322 vaginal births from a hospital midwifery service in the United States to examine risks for PPH and effectiveness of AMTSL. Using a latent class analysis approach, physiologic birth practices and other risk factors for PPH were analyzed to understand if discrete classes of clinical characteristics would emerge. The effect of AMTSL on the PPH outcome was also considered by class.
Results:
A four-class solution best fit the data; each class was clinically distinct. The two largest Classes (A and B) represented women with term births and lower average parity, with higher rates of nulliparity in Class B. Class A women had more physiologic birth elements and less labor induction or labor dysfunction compared with Class B. PPH and AMTSL use was higher in Class B. In Class B, AMTSL lowered risk for PPH. However, in Class A, AMTSL was associated with higher risk for PPH and delayed placental delivery (>30 minutes).
Discussion:
AMTSL may not be as beneficial to women undergoing physiologic birth. Further study of the etiology of PPH in these women is indicated to inform preventive care.
Keywords: active management of third stage labor, oxytocin, physiologic childbirth, postpartum hemorrhage, third stage labor
1 ∣. INTRODUCTION
As all pregnant women are considered at-risk for postpartum hemorrhage (PPH), international health and professional childbirth organizations in the United States have called for the universal uterotonic medication use for PPH prophylaxis.1-3 These statements are based on evidence showing lower PPH (500 mL or higher) rates when uterotonic medication (an important component of active management of third stage labor [AMTSL]) has been given after vaginal birth.4 This recommendation (Level of Evidence: 1A)5 for PPH prophylaxis is important given rising PPH and uterine atony rates in developed nations.6-9 Reasons identified for increased uterine atony include rising labor induction rates, prolonged labor, epidural analgesia, instrument-assisted vaginal birth, maternal obesity, and higher intrapartum oxytocin dosage.6,8,10-13 Regardless of the call for universal AMTSL, both the statements from the Association of Women’s Health, Obstetric and Neonatal Nursing and the National Partnership for Maternal Safety Consensus Bundle on Obstetric Hemorrhage support that women at low risk for PPH can decline prophylactic oxytocin via informed consent. However, the definition of “low-risk women” is not provided by the statements. Given that 40% of PPH occurs in low-risk women,3 this lack of clarity about what constitutes low risk can cause difficulties in counseling women who desire a low-intervention approach to birth and that informed consent is limited by gaps in knowledge.
The PPH definition has been expanded by the American College of Obstetrics and Gynecology ReVITALize initiative to a (combined intrapartum/postpartum) blood loss totaling 1000 mL or greater, or hypovolemia symptoms accompanying any blood loss within 24 hours after any birth.14 By expanding the definition, this statement indicates that normal physiologic blood loss after vaginal birth is greater than previously thought. Most research conducted before the 2014 revised definition considers the 500 mL or greater threshold, and therefore, reports on data from some women who have blood loss volumes in the 500 mL to 999 mL range, that would now be considered physiologic. Some studies on PPH prevention, when defined at 1000 mL or higher, have also found AMTSL or prophylactic oxytocin beneficial when compared with expectant management or placebo.4,15 However, other studies have reported no difference between groups at the 1000 mL threshold,16-19 particularly among women who have had a physiologic labor20 or labors not stimulated by exogenous oxytocin.21
The third stage of labor relies intrinsically on the maternal oxytocin release after birth, as it is a dominant hormone for uterine contraction.22 Maternal positioning and putting the baby to breast to stimulate oxytocin release may assist the physiologic third stage.20,23,24 A physiologic childbirth model was promoted and defined by the 2012 joint consensus statement of midwifery organizations in the United States.25 The physiologic birth model emphasizes the importance of the innate biological forces of a woman’s body as the basis for an evidence-based approach to safe low-risk birth. Included in the definition of normal physiologic childbirth is an expectation of physiologic postpartum blood loss; it also promotes delayed cord clamping and early breastfeeding (Box 1). However, the definition or quantity of physiologic blood loss is not provided by the statement, nor does it address AMTSL/uterotonic medication use.
Box 1. Proposed Components of Physiologic Childbirth24.
Normal physiologic childbirth is one that is powered by the innate human capacity of the woman and fetus, and:
Is characterized by spontaneous onset and progression of labor;
Includes biologic and psychologic conditions that promote effective labor;
Results in the vaginal birth of the infant and placenta;
Results in physiologic blood loss;
Facilitates optimal newborn transition through skin-to-skin contact and keeping the mother and infant together during the postpartum period; and
Supports early initiation of breastfeeding.
The following factors disrupt normal physiologic childbirth:
-
7.
Induction or augmentation of labor;
-
8.
An unsupportive environment (ie, bright lights, cold room, lack of privacy, multiple providers, lack of supportive companions);
-
9.
Time constraints, including those driven by institutional policy and/or staffing;
-
10.
Nutritional deprivation (eg, food and drink);
-
11.
Opiates, regional analgesia, or general anesthesia;
-
12.
Episiotomy;
-
13.
Operative vaginal (vacuum, forceps) or abdominal (cesarean) birth;
-
14.
Immediate cord clamping;
-
15.
Separation of mother and infant and/or any situation in which the mother feels threatened or unsupported.
This study employs a latent class analysis procedure. Latent class analysis is a statistical technique, used to subdivide a study sample into naturally occurring but previously unknown classes or groups to help predict outcomes.26 It has been used in other scientific/clinical fields, such as heart failure27 and pelvic pain symptoms28 in an attempt to identify distinct patterns within populations, rather than testing averages. To our knowledge, it has not been used to study PPH or other obstetric risk types. As maternal risk for PPH consists of many contributing characteristics; this approach allows for the identification of classes of women based on naturally occurring patterns among contributing characteristics. This differs from multivariate regression models that allow for simultaneous analysis of independent variables but not latent patterns among independent variables.
The purpose of this paper is to examine risk for total postpartum blood loss, PPH, and blood transfusion, and evaluate outcomes associated with AMTSL for women undergoing physiologic birth. We hypothesize that in accordance with the normal physiologic childbirth model: (a) Women with higher rates of physiologic childbirth will have lower PPH rates and (b) AMTSL in the third stage will not reduce postpartum blood loss outcomes for women with physiologic births.
2 ∣. METHODS
De-identified data from a prospectively gathered birth repository attended by a nurse-midwifery faculty practice in the northwestern United States was analyzed. A waiver for Institutional Review Board approval was granted for this study. This repository contains detailed clinical outcomes for clients in 2012-2017. A data collection instrument was initiated at the first prenatal appointment, updated during intrapartum admission, at discharge, and finally at 6 weeks postpartum. Faculty and student nurse-midwives completed the data collection. Each variable was defined on a datasheet, aiding accuracy. Data were entered into REDCap software by paid staff. Data verification took place before analysis, examining discrepant blood loss data.
Outcome variables include total blood loss, PPH defined as 500 mL or higher (to reflect the historic definition), and PPH 1000 mL or higher. Blood loss was reported by the attending certified nurse-midwife (CNM) as either estimated or measured (if weighed). We used the higher of the two when both were listed. The time frame for totaling blood loss varied depending on how long the CNM was present in the room before recording the total. General practice among attending CNMs was to remain present in the first 30-60 minutes after birth. Later PPH within the first 2 hours postpartum (before transfer to the postpartum unit) would be reflected in the total blood loss as well. Maternal hypovolemic symptoms were not recorded in the repository, though women with severe symptoms would have been offered blood transfusion. Blood transfusion was recorded on the instrument before patient discharge.
AMTSL was defined by the instrument as prophylactic oxytocin, controlled cord traction, and uterine massage after placental delivery. Another variable, “modified AMTSL,” was defined in the instrument as oxytocin being given after the placenta was delivered, or no umbilical cord traction for expediting third stage. This was included due to individual practice variations in third stage management. Expectant management was considered the absence of either AMTSL or modified AMTSL. No data were collected in this data set specific to further uterotonic treatment for heavier bleeding beyond initial third stage management.
The sample was limited to live singleton, vaginal, and instrument-assisted vaginal births, 34 or more weeks of gestation. Variables relevant to physiologic childbirth were identified in the data set as presence, or absence, of: induction/augmentation methods, instrument-assisted delivery, estimated or measured blood loss, immediate skin-to-skin contact, early breastfeeding (within 30 minutes of birth), epidural or intravenous(IV) analgesia, episiotomy, cord-clamping timing, mother/infant separation, general diet during active labor, and IV fluids use (Table 1).
TABLE 1.
Generalized linear model regression on factors predicting total blood loss in 2322 hospital vaginal births attended by nurse-midwives in the United States, 2012-2017
| Physiologic birth factors | n (%) | Full GLM coefficient (95% CI) |
Stepwise GLM coefficient (95% CI) |
|---|---|---|---|
| Induction of labor | 516 (22.3) | ||
| Oxytocina | 409 (17.6) | 24.96 (−19.81 to 69.73) | 43.35 (8.95-77.76) |
| Prostaglandin | 263 (11.3) | −29.64 (−79.94 to 20.65) | |
| Mechanical | 145 (6.2) | −35.89 (−99.11 to 27.35) | |
| Alternative methods | 22 (0.9) | n/a | |
| Multiple methods | 253 (10.9) | 65.16 (−16.12 to 146.44) | |
| Augmentation of labor | 600 (25.8) | ||
| Oxytocin | 388 (16.7) | −15.41 (−61.01 to 30.19) | |
| Artificial rupture of membranes | 284 (12.2) | 1.75 (−34.53 to 38.03) | |
| Nipple stimulation/ambulation | 97 (4.2) | n/a | |
| Immediate skin-to-skin contact with infant | 2194 (94.9) | 17.39 (−39.55 to 74.35) | |
| Breastfeeding in 30 min after birtha | 1745 (75.2) | −36.72 (−69.04 to −4.41) | −34.08 (−64.71 to −3.44) |
| Umbilical cord clamping: <1 min | 315 (13.6) | n/a | |
| 1–2 min | 339 (14.6) | ||
| >2 min | 748 (32.2) | ||
| Epidural | 884 (38.1) | −12.67 (−42.59 to 17.25) | |
| IM/IV medication for sedation or pain | 760 (32.7) | 11.83 (−13.94 to 37.61) | |
| NICU admission or mother-infant separation | 217 (9.3) | −18.24 (−59.64 to 23.15) | |
| Regular diet during active labora | 796 (34.2) | −26.22 (−49.86 to −2.57) | −26.13 (−49.29 to −2.98) |
| IV fluids during labora | 1222 (52.6) | 20.31 (−8.71 to 49.32) | 17.04 (−8.55 to 42.64) |
| Covariate factors | Mean ± SD/n(%) | Full GLM Coefficient (95% CI) |
Stepwise GLM Coefficient (95% CI) |
| Maternal age (y) | 30.6 ± 5.1 | 5.31 (−21.24 to −31.86) | |
| BMI (prepregnancy) | 24.7 ± 5.2 | 1.62 (−11.63 to 14.81) | |
| Total pregnancy weight gain(lbs)a | 30.0 ± 12 | −0.51 (−1.42 to 0.40) | −0.62 (−1.49 to 0.25) |
| Weeks of gestationa | 39.9 ± 1.3 | 7.38 (−1.55 to −16.33) | 7.68 (−1.20 to 16.56) |
| First stage labor <12 ha | 1287 (55.4) | Reference | |
| 12–24 h | 590 (25.4) | 15.47 (−12.80 to 43.74) | 18.02 (−7.84 to 43.88) |
| >24 h | 445 (19.2) | −0.15 (−34.92 to 34.62) | |
| Second stage labor 0–30 mina | 1029 (44.3) | Reference | |
| 31–60 min | 395 (17.0) | 41.34 (7.49 - 75.19) | 41.09 (10.09-72.09) |
| 61–120 min | 393 (16.9) | −3.36 (−37.06 to 30.33) | |
| 121–180 min | 232 (9.9) | 54.91 (4.58-105.24) | 52.70 (6.73-98.67) |
| >180 min | 273 (11.8) | 10.49 (−35.76 to 56.76) | |
| Third stage 0–15 mina | 1984 (85.4) | Reference | |
| 16–29 min | 245 (10.5) | 6.18 (−29.41 to 41.78) | |
| ≥30 min | 93 (4.0) | 217.36 (124.66-310.05) | 221.89 (129.90-313.87) |
| Apgar score 5 min | 8.8 ± 0.6 | −4.00 (−22.31 to 14.31) | |
| Newborn weight (grams)a | 3488 ± 455.7 | 0.07 (0.04-0.10) | 0.07 (0.04-0.10) |
| Not European descenta (reference European) | 241 (10.7) | 38.23 (1.17-75.28) | 37.65 (1.09-74.21) |
| Hispanic/Latina (reference not)a | 428 (19.1) | 41.10 (9.04-73.14) | 40.40 (9.48-71.32) |
| Parity (before delivery)a | 0.92 ± 1.1 | −10.97 (−21.61 to −0.32) | −10.03 (−19.77 to −0.28) |
| Antepartum anemia | 192 (8.3) | 2.25 (−36.34 to 40.85) | |
| Gestational diabetes | 163 (7.0) | 14.22 (−30.37 to 58.82) | |
| VBAC | 226 (9.7) | 13.89 (−27.72 to 55.49) | |
| Dysfunctional labora | 589 (25.4) | −21.16 (−64.52 to 22.19) | −27.18 (−55.75 to 1.39) |
| Laceration score = 0b | 866 (37.3) | Reference | |
| 1 | 836 (36.0) | 53.09 (25.95-80.23) | 55.22 (28.97-81.45) |
| 2 or more | 601 (26.1) | 114.62 (80.73-148.51) | 118.18 (85.18-151.18) |
Variables left out of generalized linear model were variables < 5% of sample (fetal intolerance to labor, intrauterine growth restriction, chorioamnionitis, preeclampsia, gestational hypertension, instrument assisted delivery, retained placenta, episiotomy). BMI, body mass index; IM, intramuscular; NICU, neonatal intensive care unit; VBAC, vaginal birth after cesarean.
Indicates selected for latent class analysis model.
Laceration score: cumulative number of genital tract lacerations or episiotomy requiring suturing.
Covariates included maternal age, body mass index, pregnancy weight gain, race, ethnicity, parity, gestational age, antepartum anemia, vaginal birth after cesarean, labor duration, genital tract lacerations, obstetric complications (fetal intolerance to labor, intrauterine growth restriction, dysfunctional labor, chorioamnionitis, gestational hypertension, preeclampsia/eclampsia, diabetes), and newborn Apgar scores.
After descriptive and bivariate analysis, step-wise, forwards-backwards, generalized linear modeling (GLM) using a gamma distribution, determined physiologic childbirth variables and covariates significantly associated with total blood loss using Stata SE 15.1 (College Station, TX, USA). Gamma distribution was selected due to the significantly nonnormal total blood loss distribution (right skew). Variables were included in the latent class analysis if the P-value was <0.2 in the stepwise GLM. Variables occurring at <5% of the sample were not included in the GLM due to difficulties in fitting latent class analysis with low frequency data. Dropped from further analysis was the cord-clamping variable because these data were not collected before 2014. Latent class analysis was determined by best fitting structure comparing model fit parameters with MPlus 1.5(1) (Los Angeles, CA, USA), using procedures described by Ram and Grimm (2009).29 Model fit was tested for 2-7 possible classes. Vuong-Lo-Mendell-Rubin likelihood ratio test (LRT), adjusted Lo-Mendell-Rubin LRT, and bootstrapped LRT test were considered along with model convergence (entropy close to 1), class size (more than 5% in each group), and the correct class assignment probability (posterior probabilities over 0.8). With a latent model identified, regression using class assignment as the predictor was performed for total blood loss, risk for PPH, and blood transfusion outcomes in Stata. Interactions of the latent class with AMTSL on total blood loss and risk for PPH were analyzed. Post hoc tests addressed variations in AMTSL procedure and risk for delayed placental delivery.
3 ∣. RESULTS
After exclusions, 2322 vaginal births were included in the GLM and 15 physiologic birth and covariate factors met criteria to be considered in latent class analysis (Table 1). Sample mean postpartum blood loss was 390 mL (± 295 mL) with a median of 300 mL. Rate of postpartum blood loss between 500-999 mL was n = 345 (14.9%), 93 women (4%) had 1000-2000 mL blood loss, and 13 (0.6%) had higher than 2000 mL. There were 42 blood transfusions in the sample (1.8%). Management of third stage of labor was 24% (n = 558) “expectant management,” 55.3% (n = 1283) “AMTSL,” while 20.7% (n = 481) were labeled “modified AMTSL.”
3.1 ∣. Latent class model
Latent class analysis best fit the data with four classes. The classes represent 44.3%, 35.3%, 6.3%, and 14.2% of the sample. Fit statistics are as follows: final classes all higher than 5% of the sample, Entropy = 0.823, Posterior Probabilities = 0.90, 0.89, 0.86, 0.92, Vuong-Lo-Mendell-Rubin LRT P < 0.00001, Lo-Mendell-Rubin adjusted LRT P < 0.00001, bootstrapped LRT P < 0.00001.
Table 2 describes the relative characteristics of each class and third stage management, blood loss, and PPH outcomes. Overall, the four groups are clinically distinct. Class A (44%) was termed the “physiologic” class, identified by term births, lower average parity (30% nulliparous), and low proportions of labor dysfunction or multiple lacerations needing suturing. Class A women also had more physiologic birth elements including low IV fluid administration, general diet in active labor, early breastfeeding, and less oxytocin labor induction. Class B (35%), labeled the “dysfunctional” class, also included term births with low parity (76% nulliparous), but had higher induction rates, longer labors, more labor dysfunction, and more need for genital tract suturing, and lower proportions of the physiologic birth elements. Class C (6.3%), the “preterm” class, represented data from preterm births and had a higher percentage of women from non-European racial background, while Class D (14.2%), the “high multiparous” class, had more multiparous with greater than three prior births and Hispanic women (Table 2).
TABLE 2.
Physiologic and covariate characteristics of latent class assignment among 2322 hospital vaginal births attended by nurse-midwives in the United States, 2012-2017
| Sample mean ± SD/n(%) |
Physiologic Class A mean ± SD/n(%) |
Dysfunctional Class B mean ± SD/n(%) |
Preterm Class C mean ± SD/n(%) |
High multiparous Class D mean ± SD/n(%) |
A vs B t-test/χ2 P-value |
|
|---|---|---|---|---|---|---|
| Class membership | n = 2322 | 1028 (44.3) | 819 (35.3) | 146 (6.3) | 329 (14.2) | |
| Mean parity | 0.9 ± 1.1 | 0.8 ± 0.7 | 0.3 ± 0.5 | 0.5 ± 0.7 | 3.0 ± 1.1 | <0.001 |
| Para 0 | 1033 (44.5) | 327 (31.8) | 624 (76.2) | 82 (56.2) | 0 (0) | |
| Para 1 | 777 (33.5) | 549 (53.4) | 176 (21.5) | 51 (34.9) | 1 (0.30) | |
| Para 2-3 | 431 (18.6) | 150 (14.6) | 18 (2.2) | 13 (8.9) | 250 (75.9) | |
| Para >3 | 81 (3.5) | 2 (0.2) | 1 (0.1) | 0(0) | 78 (23.7) | |
| Gestational age | 39.9 ± 1.3 | 40.1 ± 1.0 | 40.4 ± 1.0 | 37.3 ± 1.3 | 39.6 ± 1.2 | <0.001 |
| Total pounds gained during Pregnancy | 30 ± 12 | 31.5 ± 11.2 | 32.4 ± 12.1 | 23.32 ± 11.7 | 22.9 ± 11.4 | 0.12 |
| Newborn weight (grams) | 3488 ± 455.7 | 3536.6 ± 417.8 | 3564.4 ± 419.0 | 2801.3 ± 349.9 | 3480.2 ± 418.7 | 0.09 |
| Race (European/Caucasian) | 2003 (89.3) | 909 (88.4) | 698 (85.2) | 117 (80.1) | 281 (85.4) | 0.04 |
| Ethnicity (Hispanic/Latina) | 428 (19.1) | 108 (10.5) | 89 (10.8) | 36 (24.6) | 195 (59.3) | 0.83 |
| Laceration/episiotomy score index | ||||||
| No suturing needed | 866 (37.3) | 425 (41.3) | 102 (12.5) | 63 (41.2) | 276 (83.9) | <0.001 |
| One repaired | 836 (36.0) | 367 (35.7) | 378 (46.2) | 46 (31.5) | 45 (13.7) | |
| Two or more repaired | 536 (23.0) | 229 (22.3) | 238 (29.1) | 36 (24.6) | 8 (2.4) | |
| First stage | ||||||
| <12 h | 1287 (55.4) | 816 (79.4) | 188 (22.9) | 81 (55.5) | 202 (61.4) | <0.001 |
| 12-24 h | 590 (25.4) | 182 (17.7) | 286 (34.9) | 40 (27.4) | 82 (24.9) | |
| >24 h | 445 (19.2) | 30 (2.9) | 345 (42.1) | 25 (17.1) | 45 (13.7) | |
| Second stage | ||||||
| <30 min | 1029 (44.3) | 625 (60.8) | 58 (7.1) | 77 (52.7) | 269 (81.7) | <0.001 |
| 31-60 min | 395 (17.0) | 216 (21.0) | 118 (14.4) | 24 (16.4) | 27 (8.2) | |
| 61-120 min | 393 (16.9) | 148 (14.1) | 209 (25.5) | 25 (17.1) | 11 (3.3) | |
| 121-180 min | 232 (9.9) | 37 (3.5) | 180 (21.9) | 10 (6.8) | 5 (1.5) | |
| >180 min | 273 (11.7) | 2 (0.1) | 254 (31.0) | 10 (6.8) | 7 (2.1) | |
| Third stage | ||||||
| <15 min | 1984 (85.4) | 885 (86.1) | 687 (83.8) | 118 (80.8) | 294 (89.3) | 0.14 |
| 15-30 min | 245 (10.6) | 112 (10.9) | 93 (11.3) | 18 (12.3) | 22 (6.6) | |
| >30 min | 93 (4.0) | 31 (3.0) | 39 (4.7) | 10 (6.8) | 13 (3.9) | |
| Dysfunctional labor Diagnosis | 589 (25.4) | 31 (3.1) | 469 (57.3) | 24 (16.4) | 65 (19.7) | <0.001 |
| IV fluids during labor | 1222 (52.6) | 224 (21.8) | 715 (87.3) | 93 (63.7) | 190 (57.7) | <0.001 |
| Regular diet in active labor | 796 (34.2) | 508 (49.4) | 161 (19.6) | 36 (24.6) | 91 (27.6) | <0.001 |
| Pitocin for induction of labor | 409 (17.6) | 97 (9.4) | 198 (24.1) | 41 (28.1) | 73 (22.2) | <0.001 |
| Breast feeding in first 30 min | 1745 (78.6) | 858 (83.5) | 529 (64.6) | 87 (59.6) | 271 (82.4) | <0.001 |
| Management of third stagea | ||||||
| Expectant | 558 (24.0) | 351 (34.1) | 116 (14.2) | 58 (17.6) | 33 (22.6) | <0.001 |
| Active management | 1283 (55.3) | 453 (44.1) | 546 (66.7) | 201 (61.1) | 83 (56.9) | |
| Modified active Management | 481 (20.7) | 224 (21.8) | 157 (19.2) | 70 (21.3) | 30 (20.6) | |
Not included in latent model.
3.2 ∣. Blood loss and PPH outcomes
GLM and logistic regression using the latent class determination on blood loss and PPH outcomes is shown in Table 3. Women in the “dysfunctional” class had higher rates of, and risk for, blood loss, PPH, and blood transfusion compared with women in the “physiologic” class (Figure 1), whereas outcomes for “preterm” and “high multiparous” classes did not differ from the “physiologic” class. Interactions with performance of AMTSL were examined for the four outcomes as well (Table 4).
TABLE 3.
Blood loss outcomes by latent class assignment among 2322 hospital vaginal births attended by nurse-midwives in the United States, 2012-2017
| Physiologic Class A |
Dysfunctional Class B |
Preterm Class C |
High multiparous Class D |
||
|---|---|---|---|---|---|
| Total blood loss (mL) | Mean ± SD | 367.3 ± 258.7 | 440.5 ± 333.8 | 350.5 ± 275.8 | 356.5 ± 293.5 |
| Coefficient (95% CI) | Reference | 73.12 (45.05-101.18)*** | −16.82 (−62.45 to 28.81) | −10.85 (−44.13 to 22.43) | |
| ≥500 mL PPH | n (%) | 178 (17.32) | 198 (24.2) | 21 (14.4) | 54 (16.4) |
| OR (95% CI) | Reference | 1.52 (1.21-1.91)*** | 0.80 (0.49-1.31) | 0.94 (0.67-1.31) | |
| ≥1000 mL PPH | n (%) | 37 (3.6) | 47 (5.7) | 5 (3.4) | 17 (5.2) |
| OR (95% CI) | Reference | 1.63 (1.05-2.53)* | 0.95 (0.37-2.46) | 1.46 (0.81-2.63) | |
| Blood transfusion | n (%) | 13 (1.3) | 22 (2.7) | 2 (1.4) | 5 (1.5) |
| OR (95% CI) | Reference | 2.16 (1.08, 4.30)* | 1.08 (0.24, 4.85) | 1.20 (0.43-3.41) |
P < 0.05,
P < 0.01,
P < 0.001.
FIGURE 1.
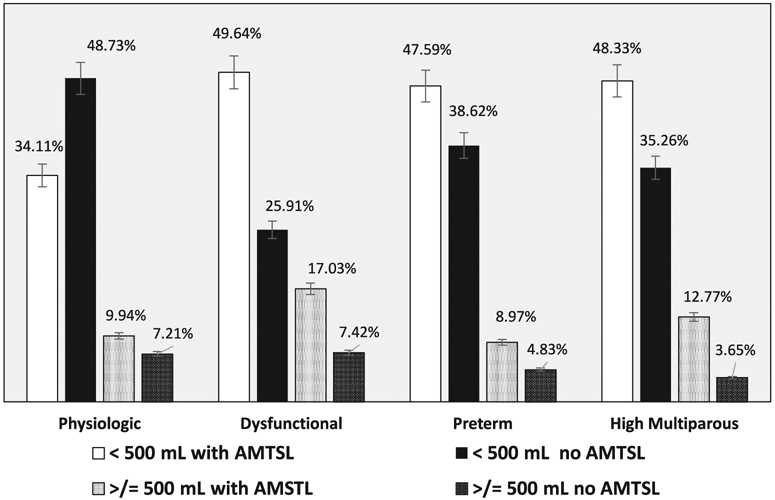
Blood loss after birth by latent class assignment and role of active management of third stage in 2322 vaginal births
TABLE 4.
Moderation of blood loss outcomes by latent class with AMTSL among 2322 hospital vaginal births attended by nurse-midwives in the United States, 2012-2017
| Class | Total blood loss coefficient (95% CI) |
≥500 mL PPH OR (95% CI) |
≥1000 mL PPH OR (95% CI) |
Blood transfusion OR (95% CI) |
|
|---|---|---|---|---|---|
| Main effects | Physiologic: A | Reference | Reference | Reference | Reference |
| Class | Dysfunctional: B | 70.38 (29.41-111.16)** | 1.88 (1.29-2.73)* | 2.16 (0.95-4.87) | 1.27 (0.30-5.34) |
| Preterm: C | −22.86 (−83.44 to 37.72) | 0.83 (0.36-1.89) | 0.76 (0.09-5.92) | 1.37 (0.29-6.59) | |
| Multiparous: D | −23.94 (−68.70 to 20.82) | 0.69 (0.36-1.31) | 1.51 (0.48-4.77) | 0.89 (0.10-7.75) | |
| AMTSL | (if Class A) | 64.02 (30.16-97.89)*** | 1.96 (1.41-2.72)* | 2.74 (1.36-5.52)* | 2.05 (0.67-6.31) |
| Interaction | Physiologic: A | Reference | Reference | Reference | Reference |
| Dysfunctional: B | −17.45 (−73.92 to 39.02) | 0.61 (0.38-0.98)* | 0.54 (0.21-1.43) | 1.58 (0.29-8.36) | |
| Preterm: C | −3.78 (−92.72 to 85.17) | 0.83 (0.29-2.31) | 1.15 (0.11-11.71) | Dropped collinearity | |
| Multiparous: D | 3.59 (−61.49 to 68.77) | 1.31 (0.61-2.78) | 0.78 (0.20-2.99) | 1.26 (0.11-14.91) |
P < 0.05,
P < 0.01,
P < 0.001.
OR, odds ratio.
According to interaction analyses, total blood loss and risk for PPH was differentially affected by class when considering AMTSL. In the main effects, not using AMTSL for women in the “dysfunctional” class was associated with 70.38 mL more bleeding (95% CI 29.41, 111.16) compared with the “physiologic” class. Risk for PPH (500 mL or higher) was 88% higher (OR = 1.88, 95% CI 1.29, 2.73) when not using AMTSL, in the main effects and the interaction term showed a 39% decrease in PPH for women in the “dysfunctional” class with AMTSL. In contrast, for women in the “physiologic” class, AMTSL may have had an adverse effect on blood loss outcomes. AMTSL used in the “physiologic” class was associated with higher total bleeding (64.02 mL) (95% CI 30.16-97.89) and increased risk for both PPH at ≥500 mL (OR = 1.96, 95% CI 1.41-2.72) or ≥1000 mL (OR = 2.74, 95% CI 1.36-5.52). AMTSL did not influence risk for blood transfusion in any class.
Another driver of total blood loss was tissue trauma, and need for suturing. Notably, “dysfunctional” class had the highest number of women needing two or more lacerations sutured (40%), and low numbers having no suturing needed (13%), which likely contributed to elevated blood loss in this class.
3.3 ∣. Post hoc analysis 1: retained placenta
While third stage length was an important driver of overall blood loss, in the sample, third stage over 30 minutes was infrequent (n = 93/4%). In the “physiologic” class, third stage over 30 minutes was higher among women with AMTSL (n = 20, 4.4%) than without (n = 11, 1.9%, P = 0.02). Post hoc interaction analyses (Table 5) examined the role of AMTSL on a third stage lasting over 30 minutes within each class. Main effects showed that women in the “physiologic” class were at higher risk for retained placenta with AMTSL. The interaction terms (data not shown) showed a nonsignificant reduced risk of retained placenta for the “dysfunctional” class and the “high multiparous” classes when AMTSL was used. Figure 2 displays the statistically distinct relationship between length of third stage and blood loss in the “physiologic” class, trend lines represent AMTSL and expectant management. The relationship between third stage length and bleeding quantity was stronger for women undergoing AMTSL in the “physiologic” class.
TABLE 5.
Post hoc tests: role of modified AMTSL by latent class assignment and effect of active management on prolonged third stage length among 2322 hospital vaginal births attended by nurse-midwives in the United States, 2012-2017
| Class | ≥500 mL PPH OR (95% CI) |
≥1000 mL PPH OR (95% CI) |
Blood transfusion OR (95% CI) |
Third stage labor ≥30 min |
|
|---|---|---|---|---|---|
| Main effects | Physiologic: A | Reference | Reference | Reference | Reference |
| Class | Dysfunctional: B | 1.48 (0.99-2.19) | 1.70 (0.75-3.89) | 1.08 (0.25-4.66) | 1.55 (0.61-3.89) |
| Preterm: C | 0.72 (0.31-1.67) | 0.67 (0.08-5.28) | 1.38 (0.29-6.65) | 2.40 (1.02-5.65)* | |
| Multiparous: D | 0.53 (0.28-1.03) | 1.23 (0.39-3.92) | 0.78 (0.09-6.86) | 1.23 (0.34-4.47) | |
| AMTSL | (if Class A) | 4.53 (2.95-6.93)*** | 6.20 (2.36-16.27)*** | 3.12 (0.76-12.71) | 2.37 (1.12-4.99)* |
| Modified AMSTL | (overall effect) | 4.84 (3.23-7.26)*** | 4.29 (1.72-10.74)** | 2.34 (0.57-9.61) | Not included |
P < 0.05,
P < 0.01,
P < 0.0001.
OR, odds ratio.
FIGURE 2.

Length of third stage labor and blood loss
3.4 ∣. Role of modified AMTSL practice
As mentioned, midwives in the practice recorded if a modified version of AMTSL was performed at the birth. The effect of this alternative practice was controlled for in the model (Table 4). PPH risk (≥500 mL) for women in the “physiologic” class with AMTSL was 3.53 times higher and 5.20 times higher for ≥1000 mL while controlling for modified AMTSL. Modified AMTSL was also independently associated with increased risk for PPH (≥500 mL). Interaction terms were nonsignificant (data not shown) but followed the trend for AMTSL in the “dysfunctional” class conferring lowering risk (OR 0.78, 95% CI 0.47-1.27).
4 ∣. DISCUSSION
The purpose of this study was to examine risk for postpartum blood loss, PPH, and blood transfusion using a latent class analysis, and to test the role of AMTSL on the outcomes. Both hypotheses were supported by the results. (a) Women with higher rates of physiologic childbirth did have lower rates of PPH/blood transfusion, and (b) prophylactic oxytocin in the third stage did not improve postpartum blood loss outcomes (total blood loss, rates of PPH) for women with physiologic births. Unexpectedly, use of AMTSL for women undergoing a more physiologic and less complicated birth (“physiologic” class), was associated with higher risk for PPH and higher odds for prolonged third stage labor.
There are several sources of postpartum bleeding and more than one of them may occur, resulting in a total blood loss that meets criteria for PPH. Assuming normal coagulation pathways, a retained placenta, uterine atony, and/or genital trauma all could potentiate blood loss. Partially mitigating some blood loss from uterine atony or expediting the third stage with AMTSL benefits women who are most at risk for cumulative PPH from complicated, prolonged labors.9,30 However, according to these data, for women undergoing a nonpathologic labor, who have been mostly orally nourished in labor and experienced lower rates of genital tract trauma, the role of AMTSL may be less beneficial, raise PPH risk, or may increase the length of third stage labor.
One possible reason for this finding is that the midwife attending the birth identified some other indication or risk factor and recommended AMTSL. For example, a history of PPH was not part of the data collection tool, and could have been an important clinical consideration at the time of birth. Alternatively, heavy bleeding at birth from genital trauma may have been interpreted as uterine bleeding and AMTSL recommended—thus the link between AMTSL and higher PPH outcomes.
Current clinical recommendations, based on compelling meta-analyses of randomized controlled trial data show reduced PPH with oxytocin administration or AMTSL.4,20 However, the generalizability of these meta-analyses outcomes to women undergoing a physiologic birth, or one not stimulated by exogenous oxytocin has been questioned in the literature.21 Data from the current study contribute to knowledge about PPH prevention in lower-risk populations, but alone cannot recommend for practice changes until further randomized trials specifically address this question. Conceptually, a physiologic blood loss should follow a physiologic birth, as introduced by the midwifery consensus statement. Yet, despite lower complicated birth rates and overall less genital tract trauma in the “physiologic” class, some women still experienced either a delayed placental delivery (3%, n = 31) or PPH ≥1000 mL (3.6%, n = 37). The possibility that prophylactic oxytocin or AMTSL can increase rates of retained placenta has been addressed in two Cochrane meta-analysis papers.4,20 Both reports found nonsignificant increases in manual removal of placenta with prophylactic oxytocin/AMTSL compared with no uterotonic or expectant management. Notably, participants in these studies were of varied risk and intrapartum care (induction, augmentation). The possible mechanisms by which AMTSL could increase the possibility of retained placenta are not clear. One hypothesis is that in the setting of a physiologically stimulated process, a supraphysiologic dose of synthetic oxytocin could lead to partial detachment or trapping the placenta with early cervical closure.
Other observational studies of low-risk women receiving institutional midwifery care have noted increased PPH with AMTSL.31,32 These studies report a physiologic approach to third stage labor management (delayed cord clamping, draining the placenta after cutting the cord, not administering routine uterotonic, and using gravity or gentle cord traction for placental expulsion). Rates of PPH ≥1000 mL were 2-fold higher in women having AMTSL in one study,31 and PPH rates and manual removal of the placenta were increased with AMTSL in the other.32 This points to the idea that not all interventions benefit all laboring women equally, even if randomized controlled trials show an average difference. Using the latent class approach examines subgroups of women (rather than averages) and could help clinicians counsel women on their collective risk profile.
Most studies on AMTSL have not considered the role of early breastfeeding on third stage labor outcomes. Early breastfeeding promotes uterine involution through endogenous oxytocin release.33,34 In our study, women in the “dysfunctional” class had lower rates of early breastfeeding and higher rates of PPH. This is consistent with a study that found a reduction of PPH with early contact and breastfeeding among women.24 Interestingly, this paper also noted increased PPH when AMTSL was used. A similar study examined “psychophysiologic care” and a hands-off approach to the third stage while ensuring skin-to-skin contact and breastfeeding when possible35 also reported low PPH rates. For our data, however, an alternative explanation is that those already experiencing early heavy postpartum blood loss or undergoing significant laceration repair may have been unable to latch the infant within 30 minutes after birth; therefore the effect of early latching on reducing blood loss should continue to be studied.
An assumption that birth care providers make in supporting a physiologic third stage is that the maternal pituitary will secrete oxytocin after birth. One study from 1988 challenged this premise, using serial blood samples (obtained every 30 seconds from crowning of the fetal head until 15 minutes after birth) of 25 spontaneously laboring women.36 Third stage management included immediate cord clamping and modified Brandt-Andrews technique. Ten women received an intramuscular synthetic oxytocin and ergotamine injection immediately after birth and 15 women did not have the injection. Only 6 of the 15 expectantly managed women had a surge in their oxytocin levels that was “similar” to those who had injections. In all, the authors concluded that prophylaxis should be administered to all women. However, perineal trauma, epidural, skin-to-skin contact, early breastfeeding, maternal positioning, or gravitational forces were not specified. Further examination of third stage oxytocin secretion in the setting of early breastfeeding and delayed cord clamping is needed.
PPH prevention should be considered throughout labor and postpartum, not focused on the minutes between newborn and placental delivery or the first PP hour. Attention to maternal nutrition and hydration will help support effective uterine muscle contraction.37 Minimizing synthetic oxytocin use and considering decreasing or discontinuing use when active effective labor is established may help maximize oxytocin receptor availability such that women’s uteri can respond to endogenous or exogenous postpartum oxytocin.38-40 Strategies for prevention or quick repair of genital tract trauma should be a priority;41 as it may be an overlooked driver of blood loss.42 Finally, encouraging endogenous oxytocin release by assisting with early breastfeeding deserves further study.
This study has several strengths including a large sample size and use of latent class analysis for identifying risk using clinically relevant phenotypes of laboring women. Data were collected prospectively and included details of intrapartum care relevant to physiologic birth. Limitations of this study are that it describes women seeking midwifery care in the northwestern United States, limiting generalizability. The PPH rate was higher than other midwifery cohort studies,32,35 which may indicate higher-risk parturient women. Other limitations include not having data about women’s symptoms of hypovolemia in the data collection nor a total 24-hour blood loss. In addition, there was incomplete data on cord clamping and the exact timing of uterotonic administration was not recorded. Finally, low rates of certain pregnancy/labor complications precluded them from inclusion in the latent model. Replication of this study in more diverse data sets would help evaluate pregnancy-related complications and PPH outcomes.
In conclusion, risk for PPH is a multifaceted concept that deserves more attention through analyses that consider the distinctions between laboring women and intrapartum and physiologic birth characteristics. More research is needed on the effect of AMTSL and/or prophylactic oxytocin, particularly within the context of physiologic childbirth.
ACKNOWLEDGEMENTS
This work was supported by the Jonas Veteran Healthcare Scholar Award and Jonas Foundation.
REFERENCES
- 1.World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. 2012. Geneva: WHO Google Scholar; 2014. [PubMed] [Google Scholar]
- 2.Association of Women's Health, Obstetric and Neonatal Nurses. Guidelines for oxytocin administration after birth: AWHONN practice brief number 2. J Obstet Gynecol Neonatal Nurs. 2015;44(1):161–163. [DOI] [PubMed] [Google Scholar]
- 3.Main EK, Goffman D, Scavone BM, et al. National partnership for maternal safety consensus bundle on obstetric hemorrhage. J Midwifery Womens Health. 2015;60(4):458–464. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013;10:CD001808. [DOI] [PubMed] [Google Scholar]
- 5.Hull A, Lagrew D. Active Management of Third Stage Labor. California Maternal Quality Care Collaborative. Obstetric Hemorrhage Toolkit; 2009:1–2. [Google Scholar]
- 6.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(4):353. e1–e6. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449. e1–e7. [DOI] [PubMed] [Google Scholar]
- 8.Grotegut CA, Paglia MJ, Johnson LNC, Thames B, James AH. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204(1):56. e1–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita ATN. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol. 2013;209(1):51. e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin Agten A, Passweg D, von Orelli S, Ringel N, Tschudi R, Tutschek B. Temporal trends of postpartum haemorrhage in Switzerland: a 22-year retrospective population-based cohort study. Swiss Med Wkly. 2017;147(4546):w14551. [DOI] [PubMed] [Google Scholar]
- 11.Nyfløt LT, Stray-Pedersen B, Forsén L, Vangen S. Duration of labor and the risk of severe postpartum hemorrhage: a case-control study. Hawkins SM, ed. PLoS ONE. 2017;12(4):e0175306–e0175310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looft E, Simic M, Ahlberg M, Snowden JM, Cheng YW, Stephansson O. Duration of second stage of labour at term and pushing time: risk factors for postpartum haemorrhage. Paediatr Perinat Epidemiol. 2017;31(2):126–133. [DOI] [PubMed] [Google Scholar]
- 13.Butwick AJ, Abreo A, Bateman BT, et al. Effect of maternal body mass index on postpartum hemorrhage. Anesthesiology. 2018;128(4):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Obstetrics and Gynecology. ReVITALize. 1st ed.; 2014. https://www.acog.org/-/media/Departments/Patient-Safety-and-Quality-Improvement/2014reVITALizeObstetricDataDefinitionsV10.pdf. Accessed July 23, 2018. [Google Scholar]
- 15.Jangsten E, Mattsson L-Å, Lyckestam I, Hellström A-L, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG. 2011;118(3):362–369. [DOI] [PubMed] [Google Scholar]
- 16.Sosa CG, Althabe F, Belizán JM, Buekens P. Use of oxytocin during early stages of labor and its effect on active management of third stage of labor. Am J Obstet Gynecol. 2011;204(3):238. e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. Int J Gynaecol Obstet. 2010;111(1):32–36. [DOI] [PubMed] [Google Scholar]
- 18.Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. Br J Obstet Gynaecol. 1991;98(6):528–530. [DOI] [PubMed] [Google Scholar]
- 19.de Groot AN, van Roosmalen J, van Dongen PW, Borm GF. A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstet Gynecol Scand. 1996;75(5):464–468. [DOI] [PubMed] [Google Scholar]
- 20.Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412. [DOI] [PubMed] [Google Scholar]
- 21.Erickson EN, Lee CS, Emeis CL. Role of prophylactic oxytocin in the third stage of labor: physiologic versus pharmacologically influenced labor and birth. J Midwifery Womens Health. 2017;62(4):418–424. [DOI] [PubMed] [Google Scholar]
- 22.Vannuccini S, Bocchi C, Severi FM, Challis JR, Petraglia F. Endocrinology of human parturition. Ann Endocrinol. 2016;77(2):105–113. [DOI] [PubMed] [Google Scholar]
- 23.Saxton A, Fahy K, Hastie C. Effects of skin-to-skin contact and breastfeeding at birth on the incidence of PPH: a physiologically based theory. Women Birth. 2014;27(4):250–253. [DOI] [PubMed] [Google Scholar]
- 24.Saxton A, Fahy K, Rolfe M, Skinner V, Hastie C. Does skin-to-skin contact and breast feeding at birth affect the rate of primary postpartum haemorrhage: results of a cohort study. Midwifery. 2015;31(11):1110–1117. [DOI] [PubMed] [Google Scholar]
- 25.American College of Nurse-Midwives, Midwives Alliance of North America, National Association of Certified Professional Midwives. Supporting healthy and normal physiologic childbirth: a consensus statement by the American College of Nurse-Midwives, Midwives Alliance of North America, and the National Association of Certified Professional Midwives. J Midwifery Womens Health. 2012;57(5):529–532. [DOI] [PubMed] [Google Scholar]
- 26.Lanza ST, Rhoades BL. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci. 2011;14(2):157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CS, Gelow JM, Denfeld QE, et al. Physical and psychological symptom profiling and event-free survival in adults with moderate to advanced heart failure. J Cardiovasc Nurs. 2014;29(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenton BW, Grey SF, Tossone K, McCarroll M, Von Gruenigen VE. Classifying patients with chronic pelvic pain into levels of biopsychosocial dysfunction using latent class modeling of patient reported outcome measures. Pain Res Treat. 2015;2015(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram N, Grimm KJ. Methods and measures: growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev. 2009;33(6):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khireddine I, Le RC, Dupont C, Rudigoz R-C, Bouvier-Colle M-H, Deneux-Tharaux C. Induction of labor and risk of postpartum hemorrhage in low risk parturients. Hawkins SM, ed. PLoS ONE. 2013;8(1):e54858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis D, Baddock S, Pairman S, et al. Risk of severe postpartum hemorrhage in low-risk childbearing women in New Zealand: exploring the effect of place of birth and comparing third stage management of labor. Birth. 2012;39(2):98–105. [DOI] [PubMed] [Google Scholar]
- 32.Dixon L, Tracy SK, Guilliland K, Fletcher L, Hendry C, Pairman S. Outcomes of physiological and active third stage labour care amongst women in New Zealand. Midwifery. 2013;29(1):67–74. [DOI] [PubMed] [Google Scholar]
- 33.Yuko M, Kataoka Y. Uterine activity during the two hours after placental delivery among low-risk pregnancies: an observational study. J Matern Fetal Neonatal Med. 2017;1–7. [DOI] [PubMed] [Google Scholar]
- 34.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. [DOI] [PubMed] [Google Scholar]
- 35.Fahy K, Hastie C, Bisits A, Marsh C, Smith L, Saxton A. Holistic physiological care compared with active management of the third stage of labour for women at low risk of postpartum haemorrhage: a cohort study. Women Birth. 2010;23(4):146–152. [DOI] [PubMed] [Google Scholar]
- 36.Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ. 1988;297(6642):167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Providing oral nutrition to women in labor: American College of Nurse-Midwives. J Midwifery Womens Health. 2016;61(4):528–534. [DOI] [PubMed] [Google Scholar]
- 38.Balki M, Ramachandran N, Lee S, Talati C. The recovery time of myometrial responsiveness after oxytocin-induced desensitization in human myometrium in vitro. Anesth Analg. 2016;122(5):1508–1515. [DOI] [PubMed] [Google Scholar]
- 39.Bor P, Ledertoug S, Boie S, Knoblauch NO, Stornes I. Continuation versus discontinuation of oxytocin infusion during the active phase of labour: a randomised controlled trial. BJOG. 2016;123(1):129–135. [DOI] [PubMed] [Google Scholar]
- 40.Tran G, Kanczuk M, Balki M. The association between the time from oxytocin cessation during labour to Cesarean delivery and postpartum blood loss: a retrospective cohort study. Can J Anaesth. 2017;64(8):820–827. [DOI] [PubMed] [Google Scholar]
- 41.Aasheim V, Nilsen ABV, Reinar LM, Lukasse M. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6:CD006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girault A, Deneux-Tharaux C, Sentilhes L, Maillard F, Goffinet F. Undiagnosed abnormal postpartum blood loss: incidence and risk factors. PLoS ONE. 2018;13(1):e0190845. [DOI] [PMC free article] [PubMed] [Google Scholar]


