Abstract
BACKGROUND:
Specific clinical and demographic risk factors may be associated with improving or worsening neurologic outcomes within a population of acute ischemic stroke (AIS) patients with a history of obstructive sleep apnea (OSA). The objective of this study was to determine the changes in neurologic outcome during a 14-day recovery as it relates to initial stroke severity in AIS patients with OSA.
METHODS:
This retrospective study analyzed baseline clinical risk factors and demographic data collected in a regional stroke center from January 2010 to June 2016. Our primary endpoint measure was the National Institutes of Health Stroke Scale (NIHSS) score and our secondary endpoint measures included the clinical factors associated with improving (NIHSS score ≤7) or worsening (NIHSS score >7) neurological outcome. The relative contribution of each variable to stroke severity and related outcome was determined using a logistic regression. The regression models were checked for the overall correct classification percentage using a Hosmer–Lemeshow test, and the sensitivity of our models was determined by the area under the receiver operating characteristic curve.
RESULTS:
A total of 5469 AIS patients were identified. Of this, 96.89% did not present with OSA while 3.11% of AIS patients presented with OSA. Adjusted multivariate analysis demonstrated that in the AIS population with OSA, atrial fibrillation (AF) (odds ratio [OR] = 3.36, 95% confidence interval [CI], 1.289–8.762, P = 0.013) and changes in ambulatory status (OR = 2.813, 95% CI, 1.123–7.041, P = 0.027) showed an association with NIHSS score >7 while being Caucasian (OR = 0.214, 95% CI, 0.06–0.767, P = 0.018) was associated with NIHSS score ≤7.
CONCLUSION:
In AIS patients with OSA, AF and changes in ambulatory status were associated with worsening neurological outcome while Caucasian patients were associated with improving neurological outcome. Our findings may have significant implications for patient stratification when determining treatment protocols with respect to neurologic outcomes in AIS patients with OSA.
Keywords: Acute ischemic stroke, National Institutes of Health Stroke Scale score, obstructive sleep apnea, stroke severity
Introduction
Obstructive sleep apnea (OSA) is a treatable form of abnormal breathing in which the upper airway closes repeatedly during sleep.[1] This syndrome is known to be associated with vascular risk factors and represents an important risk factor for ischemic stroke.[2] While several studies[2,3,4] have shown the prevalence of OSA among acute ischemic stroke (AIS) patients, whether the observed severity in AIS patients with a history of OSA is linked to specific clinical risk factors is not fully understood. There are numerous potential clinical risk factors for stroke in the OSA population including obesity, hypertension (HTN), atherosclerosis, endothelial dysfunction, and cardiac arrhythmias.[5,6] These factors are also known to contribute to poor neurologic recovery in AIS patients.[7] Therefore, identification of specific risk factors associated with AIS patients with OSA could represent an important strategy for the management of AIS patients with OSA.[8]
The National Institutes of Health Stroke Scale (NIHSS) score is one of the most widely used tools in stroke neurology[9] to assess neurological deficits after an anterior stroke.[10] The threshold for NIHSS values less than or greater than 7 has been used in the stratification of stroke severity in AIS.[11,12] For example, a NIHSS score >7 within a 48-h period is a significant clinical indicator of neurologic worsening and poor prognosis at discharge,[12] while a score of ≤7 is linked to improved neurological outcome.[13] This finding reveals that the course of neurologic outcome following AIS depends on the initial stroke severity, and that a dichotomy in neurologic outcome exists based on the initial NIHSS score when stratified by ≤7 and >7.[12] This implies that in a standard statistical approach of modeling, baseline NIHSS score can help predict neurologic outcomes in AIS patients with OSA using their clinical risk factors.[12]
Despite presenting with more cardiovascular conditions, AIS patients with OSA have been shown to present with less neurological injury at the time of hospital admission when compared to patients without OSA.[14] A recent study[15] also indicates poor functional outcome in AIS patients with OSA. However, in these studies,[14,15] AIS-OSA patients were not stratified based on initial stroke severities using initial NIHSS scores. It is possible that AIS-OSA patients with varying severities present with a nonlinear profile of recovery after 14 days, and the early course of improvement may be greater at the lower end of the deficit NIHSS than at the higher end. If this is the case, then more AIS-OSA patients will show improvement if they present with lower NIHSS scores during admission. Another possibility is that there may also be a population of AIS-OSA patients with worsening presentation based on the initial severity of their stroke, resulting in poor neurologic outcome. In this context, specific baseline clinical factors may influence stroke severity differentially to affect neurologic outcome in AIS populations with and without OSA. Therefore, the objective of the current study is to identify clinical and demographic factors that contribute to worsening or improving neurologic outcome in AIS patients with OSA. Because neurological outcome following an acute stroke is dependent on the initial stroke severity, baseline NIHSS scores were analyzed to predict progression and improvement in neurologic outcomes. Understanding the potential effect of demographic and risk factors on the course of neurologic worsening and improvement may help in the development of management strategies for AIS patients with OSA.
Methods
Study population
This institutional review board (IRB)-approved, retrospective study analyzed data from 5,469 AIS patients who were admitted and treated at Prisma Health (previously Greenville Health System) located in Greenville, South Carolina, USA, from January 2010 to June 2016. Of the 5,469 AIS patients, 5,299 patients did not present with OSA while 170 patients presented with OSA. The IRB of PRISMA Health Institutional Committee for Ethics approved this study. Patients included in this study presented within 24 h of onset of AIS symptoms, based on relevant lesions demonstrating ischemia or injury on brain magnetic resonance imaging or computed tomography, such as decreased gray/white matter distinction, parenchymal hypodensity, sulcal swelling, or cerebral artery hyperdensity. The stroke registry, previously described in other studies,[16,17,18,19,20,21,22,23,24,25] provided data on patient demographics, laboratory values, clinical presentation, and medical history. The demographic variables studied include age, race, gender, ethnicity, body mass index (BMI), and medication history on admission. The clinical characteristics that were collected include atrial fibrillation (AF)/atrial flutter, coronary artery disease (CAD), carotid artery stenosis, depression, dyslipidemia, diabetes mellitus, drug or alcohol abuse, congestive heart failure, family history of stroke, hormonal replacement therapy, migraine, obesity, HTN, previous stroke or transient ischemic attack, peripheral vascular disease (PVD), sickle cell disease, chronic renal disease, OSA, and history of smoking. Ambulation data were obtained upon admission, during admission, and following discharge. Patients' ambulatory data were recorded from a range of 0–3: undocumented (0), patient not able to ambulate (1), patient able to ambulate with assistance (2), and patient able to ambulate independently (3). The ambulation score at discharge was compared to the ambulation score on admission which quantitatively showed if there was an improvement in ambulation. This method of scoring has been described in a previous study.[26]
Statistical analysis
A univariate statistical analysis of the risk factors and differences of AIS patients with or without OSA was performed. This analysis used the Pearson Chi-square test to analyze discrete variables and the Student's t-test to analyze all continuous variables. The data on demographic and clinical risk factors in patients with a NIHSS ≤7 or a NIHSS >7 dependent on their OSA diagnosis were analyzed through a univariate analysis done similarly to the first analysis. Next, a binary logistic multivariate analysis was constructed by including the established predictors with a probability value <0.3 from the univariate analysis building to identify independent predictors of higher NIHSS scores in the two groups based on sleep apnea status. A post hoc adjusted analysis (logistic regression) of baseline risk factors associated with lower or higher NIHSS was analyzed using the likelihood ratio backward selection method. The decision to use a backward selection model allowed all of the clinical and demographic risk factors that were approaching significance to be initially included in the model and then systematically removed if they did not add to the significance of the overall model. Another reason this was done is due to the retrospective nature of the data.
In the binary regression model, the stroke severity based on NIHSS score stratification was the dependent variable. The demographic and clinical risk factors for the groups stratified by the presence or absence of OSA were the primary independent variables in this patient population. The odds for presenting with a more severe stroke (NIHSS >7) or a stroke of moderate severity (NIHSS ≤7) were analyzed separately for the group with sleep apnea and the group without sleep apnea upon presentation of symptoms. Odds ratios (ORs) and 95% confidence intervals (CIs) of outcome measures were obtained from this model with the significance set at a 0.05 probability level. The ORs were then used to predict which independent variables positively influenced a patient with or without sleep apnea to have a more severe stroke, as defined by a NIHSS >7. After the model was built, the specificity, sensitivity, and accuracy of the regression model was studied using the area under the receiver operating characteristic curve (ROC) and overall correct classification percentage. Multicollinearity and interactions between independent variables were identified by using Hosmer–Lemeshow test. All statistical analyses were done using the Statistical Package for Social Sciences (SPSS) version 26.0 for Windows (SPSS, Chicago, IL, USA).
Results
A total of 5,469 AIS patients were identified. In this population, 5,299 patients did not have OSA and 170 patients did have OSA [Table 1]. In comparison to patients without OSA, patients with OSA were more likely to be Caucasian, with a higher mean BMI, but were less likely to be female. The patients with OSA presented with higher rates of CAD, depression, diabetes, dyslipidemia, heart failure, HTN, migraines, obesity, PVD, and chronic renal disease. In addition, patients with OSA presented with a lower rate of history of smoking and were more likely to be taking a HTN medication, cholesterol reducer, diabetes, and antidepressant medications. They were also more likely to present with higher triglyceride levels, lipid levels, and blood glucose levels but lower high-density lipoprotein (HDL) levels. NIHSS scores on admission were not significantly different for patients with and without OSA.
Table 1.
Baseline characteristics of ischemic stroke patients divided by diagnosis of sleep apnea
| Characteristic | No sleep apnea, n (%) | Sleep apnea, n (%) | P |
|---|---|---|---|
| Number of patients | 5299 | 170 | |
| Age group | |||
| <50 | 644 (12.2) | 14 (8.2) | 0.003*,a |
| 50-59 | 956 (18.0) | 40 (23.5) | |
| 60-69 | 1256 (23.7) | 43 (25.3) | |
| 70-79 | 1181 (22.3) | 50 (29.4) | |
| ≥80 | 1262 (23.8) | 23 (13.5) | |
| Mean±SD | 67.28±14.81 | 66.33±12.3 | 0.326 |
| Race | |||
| White | 4143 (78.2) | 145 (85.3) | 0.018*,a |
| Black | 977 (18.4) | 25 (14.7) | |
| Other | 179 (3.4) | 0 | |
| Gender | |||
| Female | 2738 (51.7) | 69 (40.6) | 0.004*,a |
| Male | 2561 (48.3) | 101 (59.4) | |
| Hispanic ethnicity | 85 (1.6) | 0 | 0.096 |
| BMI (mean±SD) | 28.15±6.85 | 34.23±8.39 | <0.001*,b |
| Medical history | |||
| Atrial fib | 886 (16.7) | 38 (22.4) | 0.054 |
| CAD | 1590 (30.0) | 71 (41.8) | 0.001*,a |
| Carotid artery stenosis | 321 (6.1) | 13 (7.6) | 0.394 |
| Depression | 637 (12.0) | 84 (49.4) | <0.001*,a |
| Diabetes | 1836 (34.6) | 99 (58.2) | <0.001*,a |
| Drugs or alcohol | 327 (6.2) | 10 (5.9) | 0.878 |
| Dyslipidemia | 2634 (49.7) | 121 (71.2) | <0.001*,a |
| Stroke family history | 473 (8.9) | 21 (12.4) | 0.125 |
| Heart failure | 556 (10.5) | 34 (20.0) | <0.001*,a |
| Hormonal replacement therapy | 75 (1.4) | 4 (2.4) | 0.313 |
| HTN | 4152 (78.4) | 154 (90.6) | <0.001*,a |
| Migraine | 124 (2.3) | 10 (5.9) | 0.003*,a |
| Obesity | 2159 (40.7) | 152 (89.4) | <0.001*,a |
| Previous stroke | 1374 (25.9) | 50 (29.4) | 0.308 |
| Previous TIA (>24 h) | 462 (8.7) | 15 (8.8) | 0.962 |
| Prosthetic heart valve | 62 (1.2) | 0 | 0.156 |
| Peripheral vascular disease | 378 (7.1) | 22 (12.9) | 0.004*,a |
| Chronic renal disease | 418 (7.9) | 29 (17.1) | <0.001*,a |
| Sickle cell disease | 4 (0.1) | 0 | 0.720 |
| Smoker | 1461 (27.6) | 25 (14.7) | <0.001*,a |
| Medication history | |||
| HTN medication | 3656 (69.0) | 138 (81.2) | 0.001*,a |
| Cholesterol reducer | 2330 (44.0) | 98 (57.6) | <0.001*,a |
| Diabetes medication | 1413 (26.7) | 82 (48.2) | <0.001*,a |
| Antidepressant | 631 (11.9) | 80 (47.1) | <0.001*,a |
| Initial NIHSS score | |||
| 0-9 | 3177 (71.7) | 112 (72.7) | 0.952 |
| 10-14 | 489 (11.0) | 18 (11.7) | |
| 15-20 | 486 (11.0) | 15 (9.7) | |
| 21-25 | 281 (6.3) | 9 (5.8) | |
| Mean±SD | 8.32±8.27 | 7.3±7.54 | 0.127 |
| Laboratory values, mean±SD | |||
| Total cholesterol | 171.95±51.92 | 168.67±48.6 | 0.441 |
| Triglycerides | 139.09±105.7 | 156.11±86.33 | 0.049*,b |
| HDL | 41.92±13.88 | 37.63±11.85 | <0.001*,b |
| LDL | 104.68±41.33 | 102.75±39.99 | 0.568 |
| Lipids | 6.52±2.57 | 6.97±2.06 | 0.032*,b |
| Blood glucose | 146.78±80.59 | 163.48±92.82 | 0.022*,b |
| Serum creatinine | 1.29±1.18 | 1.34±0.91 | 0.601 |
| INR | 1.14±0.5 | 1.16±0.37 | 0.722 |
| Vital signs, mean±SD | |||
| Heart rate | 82.02±18.59 | 81.47±16.4 | 0.703 |
| BP systolic | 151.9±29.27 | 149.45±30.65 | 0.283 |
| BP diastolic | 82.48±19.04 | 81.35±21.53 | 0.451 |
| Ambulation status prior to event | |||
| Ambulate independently | 4735 (89.4) | 152 (89.4) | 0.852 |
| Ambulate with assistance | 199 (3.8) | 4 (2.4) | |
| Unable to ambulate | 205 (3.9) | 8 (4.7) | |
| Not documented | 159 (3.0) | 6 (3.5) | |
| Ambulation status on admission | |||
| Ambulate independently | 1279 (24.1) | 52 (30.6) | 0.013*,a |
| Ambulate with assistance | 1571 (29.6) | 55 (32.4) | |
| Unable to ambulate | 1676 (31.6) | 52 (30.6) | |
| Not documented | 773 (14.6) | 11 (6.5) | |
| Ambulation status on discharge | |||
| Ambulate independently | 2096 (39.6) | 78 (45.9) | 0.385 |
| Ambulate with assistance | 1771 (33.4) | 49 (28.8) | |
| Unable to ambulate | 1039 (19.6) | 30 (17.6) | |
| Not documented | 393 (7.4) | 13 (7.6) | |
| rtPA received | 1282 (24.2) | 45 (26.5) | 0.495 |
| First care received | |||
| Emergency department | 4158 (79.2) | 139 (82.7) | 0.259 |
| Direct admission | 1095 (20.8) | 29 (17.3) | |
| Improved ambulation | 1773 (36.0) | 52 (32.9) | 0.429 |
| NIHSS>7 | 1811 (38.7) | 56 (35.4) | 0.404 |
| Diastolic BP ≥80 mmHg | 2805 (53.0) | 83 (48.8) | 0.281 |
Results for continuous variables are presented as mean±SD, while discrete data are presented as percentage frequency. aPearson’s Chi-square test, bStudent’s t-test, *P<0.05. NIHSS: National Institutes of Health Stroke Scale, SD: Standard deviation, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, INR: International normalized ratio, HTN: Hypertension, TIA: Transient ischemic attack, CAD: Coronary artery disease, BP: Blood pressure, rtPA: recombinant tissue plasminogen activator
The clinical and demographic characteristics associated with stroke severity for patients separated by sleep apnea diagnosis are presented in Table 2. Ischemic stroke patients without OSA, with NIHSS >7 presented with higher rates of AF, heart failure. They were less likely to present with a family history of previous stroke, but presented with lower rates of diabetes, migraines, obesity, and a history of smoking. They were less likely to be taking a diabetes medication and presented with lower total cholesterol levels, triglyceride, and low-density lipoprotein levels. Ischemic stroke patients with OSA presented with higher levels of HDL, blood glucose levels, international normalized ratio levels, higher heart rates; however, they presented with lower systolic blood pressure (BP). They displayed improvement or changes in ambulation and were more likely to be treated with recombinant tissue plasminogen activator (rtPA) but were less likely to be directly admitted to the neurology unit. Patients with OSA and a NIHSS >7 were more likely to present with higher rates of AF and treated with HTN medications. These patients presented with higher heart rates, changes, or an improved ambulatory status and lower triglyceride levels.
Table 2.
Baseline characteristics of an National Institutes of Health Stroke Scale score >7 in ischemic stroke patients stratified by absence or presence of sleep apnea
| Characteristic | No sleep apnea | P | Sleep apnea | P | ||
|---|---|---|---|---|---|---|
| NIHSS ≤7, n (%) | NIHSS >7, n (%) | NIHSS ≤7, n (%) | NIHSS >7, n (%) | |||
| Number of patients | 2865 | 1811 | 102 | 56 | ||
| Age group (years) | ||||||
| <50 | 391 (13.6) | 182 (10.0) | <0.001*,a | 8 (7.8) | 5 (8.9) | 0.662 |
| 50-59 | 552 (19.3) | 298 (16.5) | 28 (27.5) | 10 (17.9) | ||
| 60-69 | 738 (25.8) | 358 (19.8) | 26 (25.5) | 14 (25.0) | ||
| 70-79 | 650 (22.7) | 404 (22.3) | 29 (28.4) | 18 (32.1) | ||
| ≥80 | 534 (18.6) | 569 (31.4) | 11 (10.8) | 9 (16.1) | ||
| Age, mean±SD | 65.62±14.37 | 69.8±15.08 | <0.001*,b | 65.69±12.11 | 67.23±12.49 | 0.449 |
| Race | ||||||
| White | 2262 (79.0) | 1401 (77.4) | 0.318 | 92 (90.2) | 45 (80.4) | 0.081 |
| Black | 514 (17.9) | 342 (18.9) | 10 (9.8) | 11 (19.6) | ||
| Other | 89 (3.1) | 68 (3.8) | 0 | 0 | ||
| Gender | ||||||
| Female | 1398 (48.8) | 1018 (56.2) | <0.001*,a | 37 (36.3) | 25 (44.6) | 0.303 |
| Male | 1467 (51.2) | 793 (43.8) | 65 (63.7) | 31 (55.4) | ||
| Hispanic ethnicity | 51 (1.8) | 28 (1.5) | 0.545 | 0 | 0 | |
| BMI, mean±SD | 28.72±6.9 | 27.55±6.73 | <0.001*,b | 33.86±7.27 | 34.73±9.02 | 0.515 |
| Medical history | ||||||
| Atrial fib | 361 (13.6) | 423 (23.4) | <0.001*,a | 15 (14.7) | 21 (37.5) | 0.001*,a |
| CAD | 852 (29.7) | 548 (30.3) | 0.705 | 40 (39.2) | 27 (48.2) | 0.274 |
| Carotid artery stenosis | 167 (5.8) | 105 (5.8) | 0.965 | 9 (8.8) | 4 (7.1) | 0.713 |
| Depression | 355 (12.4) | 236 (13.0) | 0.521 | 52 (51.0) | 28 (50.0) | 0.906 |
| Diabetes | 1029 (35.9) | 573 (31.6) | 0.003*,a | 63 (61.8) | 31 (55.4) | 0.433 |
| Drugs or alcohol | 163 (5.7) | 121 (6.7) | 0.166 | 5 (4.9) | 4 (7.1) | 0.561 |
| Dyslipidemia | 1464 (51.1) | 877 (48.4) | 0.075 | 68 (66.7) | 43 (76.8) | 0.183 |
| Stroke family history | 322 (11.2) | 114 (6.3) | <0.001*,a | 15 (14.7) | 5 (8.9) | 0.296 |
| Heart failure | 231 (8.1) | 240 (13.3) | <0.001*,a | 16 (15.7) | 15 (26.8) | 0.093 |
| Hormonal replacement therapy | 41 (1.4) | 27 (1.5) | 0.868 | 2 (2.0) | 2 (3.6) | 0.538 |
| HTN | 2224 (77.6) | 1423 (78.6) | 0.446 | 92 (90.2) | 53 (94.6) | 0.331 |
| Migraine | 86 (3.0) | 29 (1.6) | 0.003*,a | 6 (5.9) | 4 (7.1) | 0.756 |
| Obesity | 1291 (45.1) | 701 (38.7) | <0.001*,a | 90 (88.2) | 52 (92.9) | 0.357 |
| Previous stroke | 710 (24.8) | 507 (28.0) | 0.015*,a | 32 (31.4) | 15 (26.8) | 0.546 |
| Previous TIA (>24 h) | 260 (9.1) | 156 (8.6) | 0.590 | 9 (8.8) | 6 (10.7) | 0.698 |
| Prosthetic heart valve | 35 (1.2) | 25 (1.4) | 0.638 | 0 | 0 | |
| Peripheral vascular disease | 192 (6.7) | 117 (6.5) | 0.747 | 12 (11.8) | 9 (16.1) | 0.446 |
| Chronic renal disease | 227 (7.9) | 130 (7.2) | 0.350 | 16 (15.7) | 10 (17.9) | 0.725 |
| Sickle cell disease | 3 (0.1) | 1 (0.1) | 0.573 | 0 | 0 | |
| Smoker | 846 (29.5) | 454 (25.1) | 0.001*,a | 15 (14.7) | 7 (12.5) | 0.702 |
| Medication history | ||||||
| HTN medication | 1951 (68.1) | 1270 (70.1) | 0.144 | 80 (78.4) | 51 (91.1) | 0.043*,a |
| Cholesterol reducer | 1302 (45.4) | 770 (42.5) | 0.050 | 57 (55.9) | 39 (69.6) | 0.090 |
| Diabetes medication | 802 (28.0) | 431 (23.8) | 0.002*,a | 50 (49.0) | 28 (50.0) | 0.906 |
| Antidepressant | 352 (12.3) | 242 (13.4) | 0.282 | 50 (49.0) | 27 (48.2) | 0.923 |
| Laboratory values, mean±SD | ||||||
| Total cholesterol | 174.73±54.01 | 168.41±47.29 | <0.001*,b | 174.31±47.45 | 158.98±50.36 | 0.070 |
| Triglycerides | 147.58±114.88 | 126.39±91.33 | <0.001*,b | 168.29±88.99 | 136.39±78.77 | 0.033*,b |
| HDL | 41.33±13.4 | 43.13±14.33 | <0.001*,b | 38.15±11.77 | 36.92±11.51 | 0.546 |
| LDL | 106.58±41.14 | 102.42±40.37 | 0.001*,b | 105.42±39.87 | 97.9±41 | 0.279 |
| Lipids | 6.55±2.74 | 6.4±2.3 | 0.073 | 7.19±2.22 | 6.65±1.77 | 0.132 |
| Blood glucose | 143.23±74.84 | 149.52±82.46 | 0.007*,b | 170.58±105.52 | 157.91±69.36 | 0.421 |
| Serum creatinine | 1.23±1.09 | 1.25±1.07 | 0.405 | 1.26±0.61 | 1.36±1.05 | 0.470 |
| INR | 1.11±0.43 | 1.16±0.54 | 0.001*,b | 1.1±0.19 | 1.18±0.32 | 0.130 |
| Vital signs, mean±SD | ||||||
| Heart rate | 79.02±16.39 | 85±19.84 | <0.001*,b | 79.14±15.01 | 84.71±17.75 | 0.038*,b |
| BP systolic | 153.46±28.04 | 151.71±29.4 | 0.043*,b | 153.88±29.87 | 149.63±25.86 | 0.371 |
| BP diastolic | 82.31±17.79 | 83.44±20.07 | 0.052 | 82.14±20.83 | 83.89±21.01 | 0.614 |
| Ambulation status prior to event | ||||||
| Ambulate independently | 2721 (95.0) | 1534 (84.7) | <0.001*,a | 97 (95.1) | 49 (87.5) | 0.117 |
| Ambulate with assistance | 81 (2.8) | 79 (4.4) | 3 (2.9) | 1 (1.8) | ||
| Unable to ambulate | 43 (1.5) | 109 (6.0) | 1 (1.0) | 3 (5.4) | ||
| Not documented | 20 (0.7) | 88 (4.9) | 1 (1.0) | 3 (5.4) | ||
| Ambulation status on admission | ||||||
| Ambulate independently | 1050 (36.6) | 94 (5.2) | <0.001*,a | 48 (47.1) | 2 (3.6) | <0.001*,a |
| Ambulate with assistance | 1133 (39.5) | 283 (15.6) | 41 (40.2) | 12 (21.4) | ||
| Unable to ambulate | 222 (7.7) | 1196 (66.0) | 6 (5.9) | 40 (71.4) | ||
| Not documented | 460 (16.1) | 238 (13.1) | 7 (6.9) | 2 (3.6) | ||
| Ambulation status on discharge | ||||||
| Ambulate independently | 1618 (56.5) | 339 (18.7) | <0.001*,a | 65 (63.7) | 10 (17.9) | <0.001*,a |
| Ambulate with assistance | 1009 (35.2) | 546 (30.1) | 27 (26.5) | 21 (37.5) | ||
| Unable to ambulate | 178 (6.2) | 694 (38.3) | 6 (5.9) | 18 (32.1) | ||
| Not documented | 60 (2.1) | 232 (12.8) | 4 (3.9) | 7 (12.5) | ||
| rtPA administration | 602 (21.0) | 670 (37.0) | <0.001*,a | 25 (24.5) | 20 (35.7) | 0.136 |
| First care received | ||||||
| Emergency department | 2226 (78.4) | 1489 (82.9) | <0.001*,a | 82 (81.2) | 49 (87.5) | 0.308 |
| Direct admission | 612 (21.6) | 307 (17.1) | 19 (18.8) | 7 (12.5) | ||
| Improved ambulation | 940 (33.4) | 684 (43.0) | <0.001*,a | 26 (26.3) | 24 (49.0) | 0.006*,a |
| Diastolic BP ≥80 mmHg | 1535 (53.6) | 977 (54.1) | 0.772 | 51 (50.0) | 31 (55.4) | 0.519 |
Results for continuous variables are presented as mean±SD, while discrete data are presented as percentage frequency. aPearson’s Chi-square test, bStudent’s t-test, *P<0.05. NIHSS: National Institutes of Health Stroke Scale, SD: Standard deviation, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, INR: International normalized ratio, HTN: Hypertension, TIA: Transient ischemic attack, CAD: Coronary artery disease, BP: Blood pressure, rtPA: recombinant tissue plasminogen activator
In the adjusted analysis for the patient cohort without OSA, increasing age, being female, AF, heart failure, history of previous stroke, antidepressant use, higher blood glucose level, higher heart rate, rtPA administration, and changes in ambulatory status were associated with worsening neurologic outcomes, while family history of stroke, obesity, diabetic medication, higher triglyceride levels, higher systolic BP readings, and direct admission were associated with an improvement in neurologic outcomes [Table 3 and Figure 1]. The model demonstrated moderately strong discriminating capability see Figure 2, and the area under the curve (AUROC) was 0.704 (95% CI, 0.687–0.721, P < 0.001).
Table 3.
Factors associated with a National Institutes of Health Stroke Scale score >7 for ischemic stroke patients without sleep apnea
| Variables | B | Wald | OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Increasing age | 0.022 | 40.877 | 1.022 | 1.015 | 1.029 | <0.001* |
| Female | 0.316 | 14.147 | 1.372 | 1.163 | 1.617 | <0.001* |
| Atrial fibrillation | 0.323 | 8.281 | 1.381 | 1.108 | 1.72 | 0.004* |
| History of drugs or alcohol abuse | 0.34 | 3.705 | 1.405 | 0.994 | 1.987 | 0.054 |
| Family history of stroke | −0.481 | 10.463 | 0.618 | 0.462 | 0.827 | 0.001* |
| Heart failure | 0.277 | 4.271 | 1.319 | 1.014 | 1.716 | 0.039* |
| Migraines | −0.536 | 3.739 | 0.585 | 0.34 | 1.007 | 0.053 |
| Obesity | −0.209 | 5.882 | 0.811 | 0.685 | 0.961 | 0.015* |
| History of previous stroke | 0.315 | 11.572 | 1.371 | 1.143 | 1.644 | 0.001* |
| History of smoking | 0.186 | 3.4 | 1.204 | 0.988 | 1.466 | 0.065 |
| Cholesterol reducer | −0.151 | 3.043 | 0.86 | 0.726 | 1.019 | 0.081 |
| Diabetic medication | −0.233 | 4.789 | 0.792 | 0.643 | 0.976 | 0.029* |
| Antidepressant | 0.267 | 4.694 | 1.306 | 1.026 | 1.662 | 0.03* |
| Triglycerides | −0.002 | 11.774 | 0.998 | 0.998 | 0.999 | 0.001* |
| Blood glucose | 0.002 | 11.206 | 1.002 | 1.001 | 1.003 | 0.001* |
| Heart rate | 0.017 | 43.7 | 1.017 | 1.012 | 1.022 | <0.001* |
| Systolic BP | −0.004 | 5.301 | 0.996 | 0.993 | 0.999 | 0.021* |
| Diastolic BP | 0.005 | 3.508 | 1.005 | 1 | 1.011 | 0.061 |
| rtPA | 0.918 | 109.91 | 2.505 | 2.11 | 2.975 | <0.001* |
| Direct admission | −0.387 | 10.55 | 0.679 | 0.538 | 0.858 | 0.001* |
| Changes in ambulation | 0.394 | 22.175 | 1.482 | 1.258 | 1.746 | <0.001* |
Adjusted OR <1 denotes factors that are associated with not having a NIHSS score >7 while OR >1 denotes factors that are associated with having a NIHSS score >7. *Statistical significance (P <0.05) with a 95% CI. Backward stepwise model based on likelihood ratio was applied. Model assumptions were fulfilled. Multicollinearity and interactions among independent variables were checked, and no significant interactions were found. Hosmer-Lemeshow test (P=0.069), Cox and Snell (R2=0.123). OR: Odds ratio, CI: Confidence interval, NIHSS: National Institutes of Health Stroke Scale, BP: Blood pressure, rtPA: Recombinant tissue plasminogen activator
Figure 1.
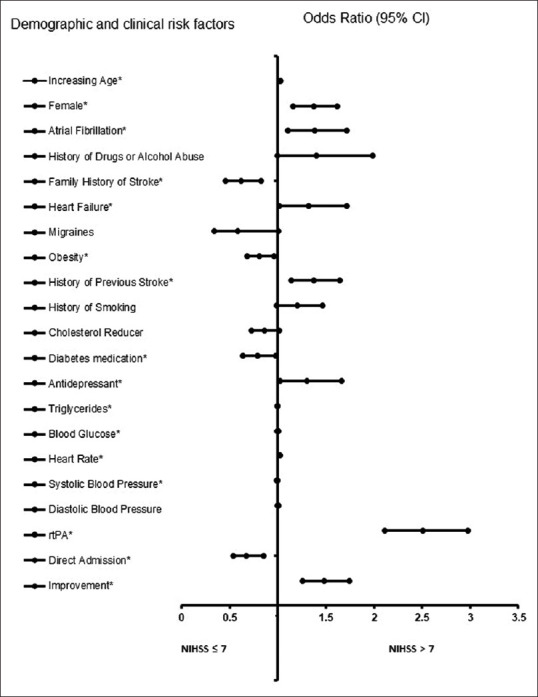
Forest plot representation of Table 3. Confidence interval band below 1 denotes factors that are associated with a National Institutes of Health Stroke Scale score ≤7 while confidence interval band above 1 denotes factors that are associated with a National Institutes of Health Stroke Scale score >7. *Statistical significance (P < 0.05) with a 95% confidence interval.
Figure 2.
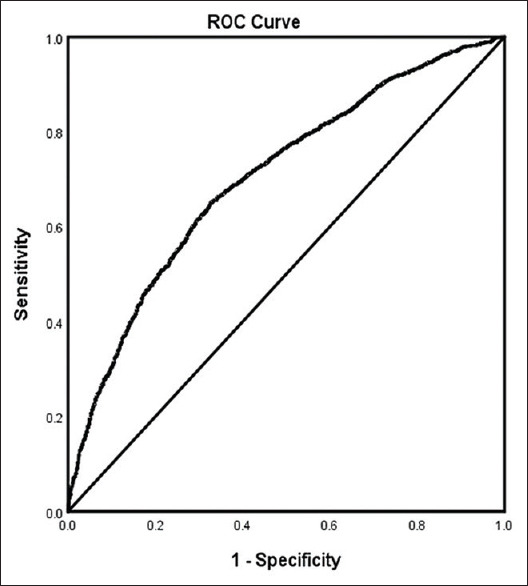
ROC curve associated with prediction of a National Institutes of Health Stroke Scale score >7 for acute ischemic stroke patients without sleep apnea. Classification table (overall correctly classified percentage = 68.8%) and area under the ROC curve (Area under the ROC Curve = 0.704, 0.687–0.721) were applied to check model fitness
In the adjusted analysis for AIS with OSA [Table 4 and Figure 3], AF and changes of an improvement in ambulation were associated with worsening neurologic outcomes, while Caucasians were more likely to be associated with improving neurologic outcomes. As presented in Figure 4, the logistic regression demonstrated strong predictive power. The AUROC is 0.761 (95% CI, 0.679–0.843, P < 0.001).
Table 4.
Factors associated with a National Institutes of Health Stroke Scale score >7 for ischemic stroke patients with sleep apnea
| Variables | B | Wald | OR | 95% CI (lower-upper) | P | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Caucasian | −1.542 | 5.609 | 0.214 | 0.06 | 0.767 | 0.018* |
| Atrial fibrillation | 1.212 | 6.145 | 3.36 | 1.289 | 8.762 | 0.013* |
| Dyslipidemia | 0.822 | 2.707 | 2.274 | 0.855 | 6.053 | 0.1 |
| Heart failure | 0.897 | 2.753 | 2.451 | 0.85 | 7.067 | 0.097 |
| Lipids | −0.21 | 2.91 | 0.81 | 0.636 | 1.032 | 0.088 |
| Changes in ambulation | 1.034 | 4.878 | 2.813 | 1.123 | 7.041 | 0.027* |
Adjusted OR <1 denotes factors that are associated with not having a NIHSS score >7 while OR >1 denotes factors that are associated with having a NIHSS score >7. *Statistical significance (P <0.05) with a 95% CI. Backward stepwise model based on likelihood ratio was applied. Model assumptions were fulfilled. Multicollinearity and interactions among independent variables were checked, and no significant interactions were found. Hosmer-Lemeshow test (P=0.988), Cox and Snell (R2=0.191). OR: Odds ratio, CI: Confidence interval, NIHSS: National Institutes of Health Stroke Scale, rtPA: Recombinant tissue plasminogen activator
Figure 3.
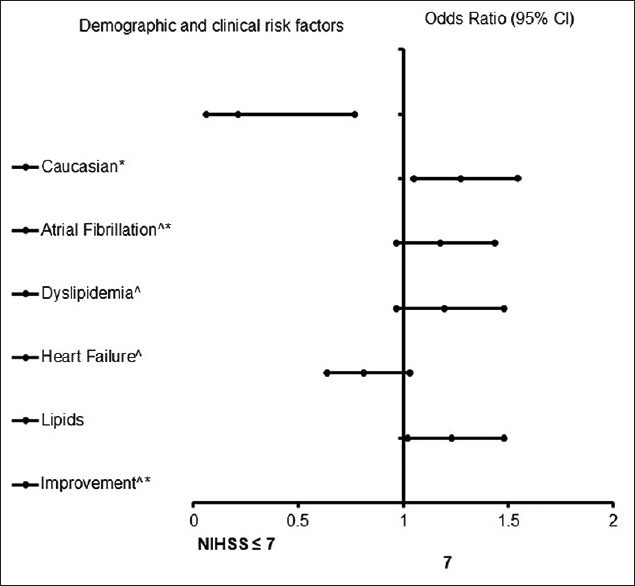
Forest plot representation of Table 4. Confidence interval band below 1 denotes factors that are associated with a National Institutes of Health Stroke Scale score ≤7 while confidence interval band above 1 denotes factors that are associated with National Institutes of Health Stroke Scale score >7. *Indicates statistical significance (P < 0.05) with a 95% confidence interval. ^Indicates that data were modified by taking the 5th square root
Figure 4.
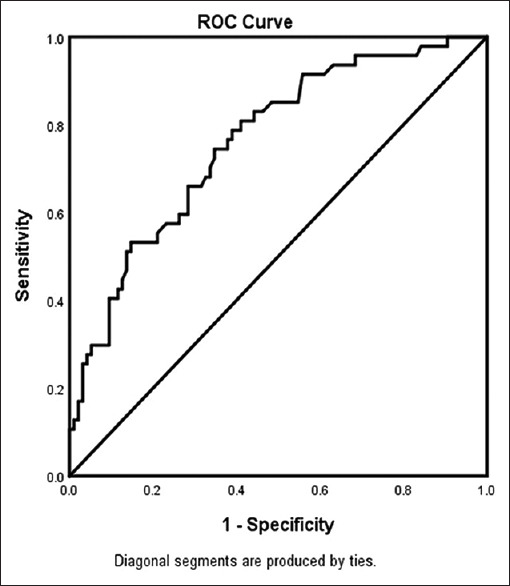
ROC curve associated with prediction of having a National Institutes of Health Stroke Scale score >7 for acute ischemic stroke patients with sleep apnea. Classification table (overall correctly classified percentage = 71.9%) and area under the ROC curve (Area under the ROC Curve = 0.761, 0.679–0.843) were applied to check model fitness
Discussion
OSA has been linked with vascular diseases, including stroke, but it is unclear whether specific demographic or clinical factors may differentially influence neurologic outcomes in AIS populations with OSA. There are conflicting studies pointing to OSA having both a protective effect and an increased risk for AIS indicating the need to understand the baseline clinical risk factors in AIS patients with a history of OSA. Baseline NIHSS score is a strong predictor of outcomes and represents a major evaluation tool to determine stroke severity and neurological deficits after an anterior stroke.[27] In this study, stroke severity was between 7.3 and 8.32 for AIS with or without OSA. This finding indicates that NIHSS is a predictor of neurological deficits in AIS with or without OSA and does account for stroke severity and neurologic outcome among our AIS population with OSA.
In the adjusted analysis, AIS patients without OSA that present with a worsening neurologic outcome were more likely to be older female patients with a history of heart failure, AF, previous stroke, antidepressant use, higher blood glucose, increased heart rate, and rtPA administration. Because females have longer life expectancies, the majority of stroke deaths are known to occur in women compared with men.[28] While stroke incidence rates are higher for men than women in most age groups,[29] as age increases, the incidence of stroke in females increases drastically such that after the age of 85 years, more women suffer from strokes than men.[30] In addition, several studies have reported risk factors associated with poor neurologic outcome in elderly stroke populations.[31,32,33] Findings indicate that women were more likely to have a poor outcome than men. While hormonal factors and comorbid function are known to contribute to the poor functional recovery in older women, our study reveals that well-established factors including previous stroke,[34,35] AF,[36] heart failure,[37] antidepressant use,[38] higher blood glucose level,[39] higher heart rate,[40] and rtPA administration[41] are all linked to worsening outcome in the elderly patient population. After adjusting for the effect of confounding variables, our findings reveal that several clinical risk factors contribute to the continuous decline in neurologic outcome in older female AIS patients, which suggests that long-term management strategies are needed for older stroke populations.
We observed that AIS patients without OSA, and with a family history of stroke, obesity, diabetic medication use, higher triglyceride levels, higher systolic BP, and direct admission, were associated with an improvement in neurologic outcomes. A similar finding demonstrating an increased risk in AIS patients with a family or parental history of stroke has been reported.[42] In addition, high triglyceride levels have been reported to be associated with low NIHSS scores and improving neurologic outcome,[43] while a systolic BP ≥185 mmHg was considered to be a contraindication for thrombolytic therapy and associated with poor outcomes.[44] However, in our current study, we observed a systolic BP of 153.46 mmHg to be associated with poor outcomes which is significantly lower than previously reported values.[44] There is a J- and U-shape relationship between BP variables and outcomes,[44] indicating that higher BP levels linked with worse or improved clinical outcomes may not produce direct evidence for causality of neurologic outcome with certainty.[45] Therefore, future studies will be necessary to definitively determine the potential association of BP variables and improved neurologic outcomes in AIS.
We found that a direct admission of patients was associated with improving neurologic outcomes. In general, direct admission is known to contribute to shorter onset-to-need times and therefore better outcomes in AIS patients undergoing thrombolysis.[46,47,48] This is because direct admission has been shown to reduce the door-to-needle time to <60 min in >70% of AIS patients and to <50 min in >45% of AIS patients.[49,50,51] Such a time improvement could be helpful when implementing the protocol of mixing and administering rtPA thrombolytic therapy to stroke patients, resulting in timely intervention that could lead to improved neurologic outcome.
Our finding that AF was associated with the odds of a worse neurologic outcome in AIS patients with OSA is not surprising. This is because AF is a common cardiac arrhythmia and is known to increase stroke severity while OSA represents an independent risk factor for stroke.[52] The pathophysiology of AF and OSA is complex and bidirectional, such that while OSA may contribute to the development of AF, AF may also promote OSA.[53] Although the mechanism for the pathological effects of AF and OSA on stroke severity and neurologic outcome is beyond the scope of the current study, there is extensive evidence[24,54] that these comorbidities are linked, and can independently worsen neurologic outcomes in AIS patients. Our finding that AIS patients with worsening neurologic outcomes are associated with AF indicates that the pathophysiology linking AF, OSA, and AIS is complex and likely multifactorial. We know that the pathophysiologic changes associated with sleep apnea, such as changes in intrathoracic pressure and blood gas levels, may cause structural and electrical changes that predispose patients to arrhythmias like AF.[53] While OSA is considered to be an independent risk for AIS, it is possible that its effect could also contribute to cardiac conduction abnormalities in the rate of AF. The abnormality in AF not only leads to stasis and abnormal cardiac circulation but also leads to increased endothelial activation, and coagulation factor release,[55] which may contribute to worsening neurologic outcome in AIS populations with OSA. This possibility demonstrates the multidirectional interplay between OSA and AF in AIS which may lead to the observed result of worsening neurologic outcomes in our AIS population with OSA. This possibility also suggests the need to develop care and management plans to address OSA and AF in AIS patients.
Our finding that Caucasian AIS patients with OSA were more likely to present with improving neurologic outcomes is not surprising. It is well documented that African Americans are twice as likely to have a stroke, with worsening neurologic outcome, and the mortality rate is 35% greater than that of Caucasian AIS patients.[56,57,58] Other studies[59,60] note that this disparity also affects Hispanics, Native Americans, and Asian Americans.[61] A retrospective study found that when insurance, access to health care, and treatment plans were controlled for, African Americans still had worsening neurologic outcome when compared to Caucasians[59] indicating the possibility that race contributes to worsening neurologic outcome in AIS patients with OSA. This possibility suggests that race needs to be considered in developing treatment plans for OSA to reduce the likelihood of worsening neurological outcomes in AIS patients with OSA.
Changes in ambulatory outcome were predictive of worsening neurologic outcomes in AIS patients with OSA. NIHSS scores are traditionally used to measure the severity of stroke, and several studies have shown its efficacy in being used for the prediction of worsening neurologic outcomes.[9,10] Other models including modified Rankin score and Barthel's index try to quantify functional outcomes of stroke recovery,[11,48,49] but none of these tests specifically measure motor recovery.[18,50] Our finding that changes in ambulatory outcome were associated with higher NIHSS scores and worsening neurologic outcome reveals that ambulatory function may serve as a significant quantitative measure and predictor of future outcomes following an anterior stroke in AIS patients with OSA.[27]
There are several limitations that must be taken into consideration. In this study, only patients with a diagnosis of OSA prior to stroke were classified as having OSA. Experts estimate that approximately 80% of OSA cases go undiagnosed, and many patients are not diagnosed with sleep apnea until after hospitalization for AIS. This could have led to the misclassification of some patients with undiagnosed OSA as patients without OSA leading to decreased differences between the two groups. This data set also lacked information about severity of OSA, treatment of OSA, and compliance with treatment of OSA limiting our ability for further study. Because this was a retrospective study, there is always the possibility of selection bias. One strength of this study is the use of a large data set from a primary stroke center. Data from the primary stroke center contributed to the get with the guidelines national stroke registry. Another strength is the use of logistic regression which allows us to make predictions about future patients.
Conclusion
This study reveals that baseline clinical and demographic factors influence stroke severity differently in AIS patients with and without incidence of OSA. Our findings suggest that in patients with a history of OSA, AF and changes in ambulation are associated with worsening neurological outcomes while Caucasian AIS patients with OSA were associated with improving neurological outcome. These findings may have significant implications for patient stratification when determining treatment protocols with respect to neurologic outcomes in AIS patients with OSA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roque DG, Valenciano JM, Aquines AG, Carriles RG, Resendez NV, Luevanos BE, et al. Prevalence of nocturnal tachyarrhythmias and long-term functional outcome in patients with obstructive sleep apnea syndrome and acute ischemic stroke. J Sleep Res. 2020;29:102–3. [Google Scholar]
- 2.Ifergane G, Ovanyan A, Toledano R, Goldbart A, Abu-Salame I, Tal A, et al. Obstructive sleep apnea in acute stroke: A role for systemic inflammation. Stroke. 2016;47:1207–12. doi: 10.1161/STROKEAHA.115.011749. [DOI] [PubMed] [Google Scholar]
- 3.Mattaliano P, Lombardi C, Sangalli D, Faini A, Corrà B, Adobbati L, et al. Impact of obstructive sleep apnea on cardiac organ damage in patients with acute ischemic stroke. J Hypertens. 2018;36:1351–9. doi: 10.1097/HJH.0000000000001697. [DOI] [PubMed] [Google Scholar]
- 4.Wu ZS, Chen FH, Yu F, Wang Y, Guo ZD. A meta-analysis of obstructive sleep apnea in patients with cerebrovascular disease. Sleep Breath. 2018;22:729–42. doi: 10.1007/s11325-017-1604-4. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Liu Y, Zhao Y, Wu X, Tong J, Hua Y. Cerebrovascular reactivity in young and old patients with obstructive sleep apnea. Sleep Med. 2020;73:125–9. doi: 10.1016/j.sleep.2020.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Rana D, Torrilus C, Ahmad W, Okam NA, Fatima T, Jahan N. Obstructive sleep apnea and cardiovascular morbidities: A review article. Cureus. 2020;12:e10424. doi: 10.7759/cureus.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens D, Martins RT, Mukherjee S, Vakulin A. Post-stroke sleep-disordered breathing-pathophysiology and therapy options. Front Surg. 2018;5:9. doi: 10.3389/fsurg.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: Diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–72. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 9.Lyden P. Using the National Institutes of Health Stroke Scale: A cautionary tale. Stroke. 2017;48:513–9. doi: 10.1161/STROKEAHA.116.015434. [DOI] [PubMed] [Google Scholar]
- 10.Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono ML, et al. National Institutes of Health Stroke Scale Score and Vessel Occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–7. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 11.Phuong V, Huy TV. Prediction of acute stroke progression by the National Institutes of Health Stroke Scale. J Geriatr Cardiol. 2007;4:225–8. [Google Scholar]
- 12.DeGraba TJ, Hallenbeck JM, Pettigrew KD, Dutka AJ, Kelly BJ. Progression in acute stroke: Value of the initial NIH Stroke Scale score on patient stratification in future trials. Stroke. 1999;30:1208–12. doi: 10.1161/01.str.30.6.1208. [DOI] [PubMed] [Google Scholar]
- 13.Wouters A, Nysten C, Thijs V, Lemmens R. Prediction of outcome in patients with acute ischemic stroke based on initial severity and improvement in the first 24 h. Front Neurol. 2018;9:308. doi: 10.3389/fneur.2018.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Festic N, Alejos D, Bansal V, Mooney L, Fredrickson PA, Castillo PR, et al. Sleep apnea in patients hospitalized with acute ischemic stroke: Underrecognition and associated clinical outcomes. J Clin Sleep Med. 2018;14:75–80. doi: 10.5664/jcsm.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair R, Radhakrishnan K, Chatterjee A, Gorthi SP, Prabhu VA. Sleep apnea-predictor of functional outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:807–14. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Nathaniel TI, Williams JA, Fazzone B, Yi SR, Morris G, Black LA, et al. Contraindications and Exclusion Criteria in Guidelines for Rt-pa in Acute Ischemic Stroke: Can the new AHA/ASA Guideline Expand the Use of Rt-pa? Hypertension. 2016;68:245. [Google Scholar]
- 17.Gainey J, Blum B, Bowie B, Cooley K, Madeline L, Ervin EL, et al. Stroke and dyslipidemia: Clinical risk factors in the telestroke versus non-telestroke. Lipids Health Dis. 2018;17:226. doi: 10.1186/s12944-018-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathaniel TI, Ubah C, Wormack L, Gainey J. The telestroke and thrombolysis therapy in diabetic stroke patients. Diabetol Metab Syndr. 2019;11:36. doi: 10.1186/s13098-019-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gainey J, Wormack L, Brechtel L, Nathaniel IT. A functional outcome model for a telestroke-guided tissue plasminogen activator treatment of stroke patients. Stroke. 2018;49(Suppl 1):89. [Google Scholar]
- 20.Colello MJ, Ivey LE, Gainey J, Faulkner RV, Johnson A, Brechtel L, et al. Pharmacological thrombolysis for acute ischemic stroke treatment: Gender differences in clinical risk factors. Adv Med Sci. 2018;63:100–6. doi: 10.1016/j.advms.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Nathaniel TI, Cochran T, Chaves J, Fulmer E, Sosa C, Yi S, et al. Co-morbid conditions in use of recombinant tissue plasminogen activator (rt-PA) for the treatment of acute ischaemic stroke. Brain Inj. 2016;30:1261–5. doi: 10.1080/02699052.2016.1186840. [DOI] [PubMed] [Google Scholar]
- 22.Wapshott T, Blum B, Kelsey W, Nathaniel TI. Investigation of gender differences and exclusive criteria in a diabetic acute ischemic stroke population treated with recombinant tissue-type plasminogen activator (rtPA) J Vasc Interv Neurol. 2017;9:26–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Shuler RP, Stafford C, Adkins A, Efird J, Colello M, Nathaniel IT. Contraindications with recombinant tissue plasminogen activator (rt-PA) in acute ischemic stroke population. Neurol Psychiatry Brain Res. 2018;27:6–11. [Google Scholar]
- 24.Shugart RM, Poupore N, Moraney RA, Tate M, George K, Brown K, et al. Improvements and deficits progression among ischemic stroke patients with pre-stroke depression and thrombolytic therapy. Neurol Psychiatry Brain Res. 2020;37:43–51. [Google Scholar]
- 25.Reynolds M, Blum B, Brechtel L, Gainey G, Nathaniel IT. Clinical risk factors associated with functional outcomes of thrombolytic therapy in stroke and non-stroke units. J Exp Stroke Transl Med. 2017;1:22–8. [Google Scholar]
- 26.Lawson TR, Brown IE, Westerkam DL, Blackhurst DW, Sternberg S, Leacock R, et al. Tissue plasminogen activator (rt-PA) in acute ischemic stroke: Outcomes associated with ambulation. Restor Neurol Neurosci. 2015;33:301–8. doi: 10.3233/RNN-140480. [DOI] [PubMed] [Google Scholar]
- 27.Heldner MR, Mattle HP, Jung S, Fischer U, Gralla J, Zubler C, et al. Thrombolysis in patients with prior stroke within the last 3 months. Eur J Neurol. 2014;21:1493–9. doi: 10.1111/ene.12519. [DOI] [PubMed] [Google Scholar]
- 28.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gainey J, Brechtel L, Konklin S, Madeline E, Lowther E, Blum B, et al. In a stroke cohort with incident hypertension; are more women than men likely to be excluded from recombinant tissue-type plasminogen activator (rtPA)? J Neurol Sci. 2018;387:139–46. doi: 10.1016/j.jns.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Gu HQ, Yang X, Rao ZZ, Wang CJ, Zhao XQ, Wang YL, et al. Disparities in outcomes associated with rural-urban insurance status in China among inpatient women with stroke: A registry-based cohort study. Ann Transl Med. 2019;7:426. doi: 10.21037/atm.2019.08.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SJ, Kim BK, Kim BS, Kim JM, Kim SK, Moon HS, et al. Associations of elderly onset headache with occurrence of poor functional outcome, cardiovascular disease, and cognitive dysfunction during long-term follow-up. Ann Geriatr Med Res. 2018;22:176–83. doi: 10.4235/agmr.18.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blum B, Brechtel L, Nathaniel T. Thrombolysis therapy in specialized and non-specialized stroke units. Arch Med Res. 2018;49:588–97. doi: 10.1016/j.arcmed.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Blum B, Penwell A, Wormack L, Walker B, Lari S, Nathaniel TI. Gender and thrombolysis therapy in acute ischemic stroke patients with incidence of obesity. Neurol Sci. 2019;40:1829–39. doi: 10.1007/s10072-019-03902-7. [DOI] [PubMed] [Google Scholar]
- 34.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in Ischemic stroke pathology. Endocrinology. 2018;159:3120–31. doi: 10.1210/en.2018-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brechtel L, Poupore N, Stoikov T, Roley LT, Emerson JF, Nathaniel T. Comorbidities associated with different levels of total cholesterol in male and female acute ischemic stroke patients. Medicine (Baltimore) 2020;99:e23870. doi: 10.1097/MD.0000000000023870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Zou C, Wang C, Zhang Y, Wang S. Long-term outcomes after stroke in elderly patients with atrial fibrillation: A hospital-based follow-up study in China. Front Aging Neurosci. 2016;8:56. doi: 10.3389/fnagi.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: A focus on risk factors. Trends Cardiovasc Med. 2015;25:140–51. doi: 10.1016/j.tcm.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao M, Ni J, Zhou LX, Peng B, Zhu YC, Cui LY, et al. Elevated fasting blood glucose is predictive of poor outcome in non-diabetic stroke patients: A sub-group analysis of SMART. PLoS One. 2016;11:1–10. doi: 10.1371/journal.pone.0160674. [doi: 10.1371/journal.pone.0160674] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Böhm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, et al. Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J. 2012;33:2804–12. doi: 10.1093/eurheartj/ehs250. [DOI] [PubMed] [Google Scholar]
- 41.Sagnier S, Galli P, Poli M, Debruxelles S, Renou P, Olindo S, et al. The impact of intravenous thrombolysis on outcome of patients with acute ischemic stroke after 90 years old. BMC Geriatr. 2016;16:156. doi: 10.1186/s12877-016-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wannamethee SG, Shaper AG, Ebrahim S. History of parental death from stroke or heart trouble and the risk of stroke in middle-aged men. Stroke. 1996;27:1492–8. doi: 10.1161/01.str.27.9.1492. [DOI] [PubMed] [Google Scholar]
- 43.Cheng KH, Lin JR, Anderson CS, Lai WT, Lee TH SRICHS Group. Lipid paradox in statin-naïve acute ischemic stroke but not hemorrhagic stroke. Front Neurol. 2018;9:541. doi: 10.3389/fneur.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangalore S, Schwamm L, Smith EE, Hellkamp AS, Suter RE, Xian Y, et al. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J. 2017;38:2827–35. doi: 10.1093/eurheartj/ehx330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhotra K, Ahmed N, Filippatou A, Katsanos AH, Goyal N, Tsioufis K, et al. Association of elevated blood pressure levels with outcomes in acute ischemic stroke patients treated with intravenous thrombolysis: A systematic review and meta-analysis. J Stroke. 2019;21:78–90. doi: 10.5853/jos.2018.02369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fredwall M, Sternberg S, Blackhurst D, Lee A, Leacock R, Nathaniel TI. Gender differences in exclusion criteria for recombinant tissue-type plasminogen activator. J Stroke Cerebrovasc Dis. 2016;25:2569–74. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Scalise M, Brechtel L, Conn Z, Bailes B, Gainey J, Nathaniel IT. Predicting ambulatory recovery in acute ischemic stroke patients with thrombolytic therapy. Future Neurol. 2020;4:34–9. [Google Scholar]
- 48.Rotimi OR, Ajani IF, Penwell A, Lari S, Walker B, Nathaniel TI. In acute ischemic stroke patients with smoking incidence, are more women than men more likely to be included or excluded from thrombolysis therapy? Womens Health (Lond) 2020;16:1–10. doi: 10.1177/1745506520922760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moraney R, Poupore N, Shugart R, Tate M, Snell A, Brown K, et al. Thrombolytic therapy in ischemic stroke patients with pre-stroke depression in the telestroke vs non-telestroke. J Stroke Cerebrovasc Dis. 2020;29:1–12. doi: 10.1016/j.jstrokecerebrovasdis.2020.104890. [DOI] [PubMed] [Google Scholar]
- 50.Poupore N, Strat D, Mackey T, Brown K, Snell A, Nathaniel IT. Thrombolytic therapy in Ischemic Stroke Patients with a preceding transient ischemic attack (TIA) in a telestroke and non-telestroke setting. Neurol Clin Neurosci. 2020;5:34–9. [Google Scholar]
- 51.Fleming T, Blum B, Averkamp B, Sullivan J, Nathaniel T. Effect of antihypertensive medications on thrombolysis therapy and outcomes in acute ischemic stroke patients. J Clin Hypertens (Greenwich) 2019;21:271–9. doi: 10.1111/jch.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipford MC, Flemming KD, Calvin AD, Mandrekar J, Brown RD, Jr, Somers VK, et al. Associations between cardioembolic stroke and obstructive sleep apnea. Sleep. 2015;38:1699–705. doi: 10.5665/sleep.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marulanda-Londoño E, Chaturvedi S. The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol. 2017;8:668. doi: 10.3389/fneur.2017.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poupore N, Strat D, Mackey T, Nathaniel TI. The association between an antecedent of transient ischemic attack prior to onset of stroke and functional ambulatory outcome. Clin Appl Thromb Hemost. 2020;26:1–11. doi: 10.1177/1076029620906867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YH, Roh SY. The mechanism of and preventive therapy for stroke in patients with atrial fibrillation. J Stroke. 2016;18:129–37. doi: 10.5853/jos.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, et al. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:E255–61. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:154–60. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Wing JJ, Baek J, Sanchez BN, Lisabeth LD, Smith MA, Morgenstern LB, et al. Differences in initial stroke severity between Mexican Americans and non-Hispanic whites vary by age: The brain attack surveillance in Corpus Christi (BASIC) project. Cerebrovasc Dis. 2014;38:362–9. doi: 10.1159/000366468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores LE, Verduzco-Gutierrez M, Molinares D, Silver JK. Disparities in health care for Hispanic patients in physical medicine and rehabilitation in the United States: A narrative review. Am J Phys Med Rehabil. 2020;99:338–47. doi: 10.1097/PHM.0000000000001342. [DOI] [PubMed] [Google Scholar]
- 60.Ellis C, Boan AD, Turan TN, Ozark S, Bachman D, Lackland DT. Racial differences in poststroke rehabilitation utilization and functional outcomes. Arch Phys Med Rehabil. 2015;96:84–90. doi: 10.1016/j.apmr.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Jones EM, Okpala M, Zhang X, Parsha K, Keser Z, Kim CY, et al. Racial disparities in post-stroke functional outcomes in young patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:1–9. doi: 10.1016/j.jstrokecerebrovasdis.2020.104987. [doi: 10.1016/j.jstrokecerebrovasdis.2020.104987] [DOI] [PubMed] [Google Scholar]


