Abstract
Objective:
To test whether children with epilepsy have impairments in myocardial mechanics compared to controls without epilepsy.
Methods:
Children with refractory epilepsy with epilepsy duration of at least 3 years underwent echocardiography including conventional measurements and speckle tracking to assess longitudinal and circumferential strain. Parent-completed surveys, capturing critical aspects of the children’s seizure history and cardiac risk factors, complemented retrospective chart reviews, which also included antiepileptic drug history. Normal echocardiograms from controls, matched for age and gender, were obtained from our institutional database and evaluated for strain.
Results:
Forty-one patients (median age = 10 years, interquartile range [IQR] = 5–15; 58.5% male) were enrolled. Epilepsy etiology included genetic (n = 26), structural (n = 6), genetic and structural (n = 5), infection (n = 3), and unknown (n = 1). No cardiac structural abnormalities were identified. Both longitudinal and circumferential strain were impaired (P < .03) in patients compared to controls (median [IQR] = 22.7% [21.2–24.2] vs 23.6% [22.2–26.1] and 22.0% [20.3–25.4] vs 24.5% [22.3–27.0], respectively), indicating decreased myocardial deformation/contraction. Shortening fraction was higher in patients (37.6% [35.7–39.7] vs 34.9% [32.5–38.7], P = .009); mitral valve E wave inflow velocity (84.8 cm/s [78.4–92.8] vs 97.2 cm/s [85.9–105.8], P = .005) and tissue Doppler lateral E’ wave (13.9 cm/s [12.3–16.1] vs 17.3 cm/s [15.4–18.5], P < .001) were decreased compared to controls. Findings were similar in the pairs with epilepsy patients distinguished by the ability to independently ambulate. There was no difference between patients and controls in ejection fraction. Among the epilepsy patients, there were no associations between cardiac measurements and epilepsy characteristics, including seizure type and frequency and cardiotoxic antiseizure medication exposure after correction for multiple comparisons.
Significance:
Children with refractory epilepsy had impaired systolic ventricular strain compared to controls, not correlated with epilepsy history. Further studies are needed to determine the significance of these changes.
Keywords: biomarker, cardiac, refractory epilepsy
1 |. INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) is a rare but devastating consequence of epilepsy. Proposed mechanisms implicate brainstem, cardiac, and respiratory pathways.1 Previous reports of prolonged or repeated convulsive seizure effects in adults demonstrate myocardial damage, including myocyte fibrosis, myocyte vacuolization, left ventricular apical ballooning, Takotsubo cardiomyopathy, and neurogenic stunning.2–4 Autopsies have linked histologic cardiac abnormalities with SUDEP compared to noncardiac deaths without epilepsy.5–7 A meta-analysis of postmortem findings from SUDEP studies reported cardiac abnormalities in approximately 25%, most commonly myocyte hypertrophy and myocardial fibrosis.7 In vivo evaluation of histological myocardial abnormalities is impractical, as myocardial biopsy is invasive, with an unfavorable risk/benefit profile for clinical care. However, advances in evaluation of myocardial function beyond typical parameters such as ejection fraction (EF) can provide insight into subclinical myocardial dysfunction. For example, tissue Doppler imaging, a technique to evaluate the velocity of myocardial movement, was seen to be abnormal in both untreated adults with epilepsy and children treated with antiepileptic drugs compared to newly diagnosed or healthy controls.8,9
Myocardial strain and strain rate measure the deformation (and rate of deformation) of the heart throughout the cardiac cycle and are easily measured noninvasively by speckle-tracking echocardiography.10–13 In this noninvasive technique, software automatically identifies and tracks individual “speckles” of the myocardial wall on a routine echocardiogram to directly quantify the extent of contraction. Strain has been evaluated in many disease states in adult and pediatric populations and may be more sensitive to early myocardial damage than traditional measures of systolic and diastolic function.14,15 Recently, Çelik et al evaluated pediatric patients with well-controlled epilepsy on monotherapy and found differences in strain as well as other secondary measures of cardiac function compared to controls.16 However, such an analysis has not been performed in patients with refractory epilepsy, and it is not known how the deformation parameters or other echocardiographic differences would compare in this group.
We aimed to determine whether speckle-tracking echocardiography would detect impaired myocardial strain in a pediatric epilepsy population with a history of refractory seizures compared to matched controls without epilepsy and whether these changes were related to details of the patients’ epilepsy histories. Due to high risk of SUDEP, we specifically sought patients with Dravet syndrome17 and those with a history of more than three generalized tonic-clonic seizures per year,18 as the myocardial abnormalities might be more pronounced.
2 |. MATERIALS AND METHODS
2.1 |. Study design and setting
This was a case-control study of pediatric epilepsy patients at higher risk for SUDEP compared to children without epilepsy and matched for gender and age. All children were registered patients at the Children’s National Medical Center (CNMC) based in Washington, DC.
2.2 |. Study population
Epilepsy patients eligible for the study were defined as children at least 3 years of age with a history of refractory generalized motor seizures for at least 3 years, >25 total generalized motor seizures, or a diagnosis of Dravet syndrome. Children were excluded if they had a history of cardiac disease. Eligible controls were defined as children who previously underwent only a single outpatient echocardiogram at CNMC and with normal findings based on traditional echocardiographic measures. Limiting the number of studies to a single echocardiogram per control substantiated the absence of symptoms worthy of follow-up. Furthermore, normal healthy children frequently have echocardiograms for symptoms that are only rarely due to cardiovascular causes, such as chest pain or murmur. In cases such as these, the echocardiogram is the gold standard for diagnosis, and if the test is normal, then the presenting symptoms are typically benign.
Patients were identified in the neurology clinics held by one of the authors (J.M.S.) or through referrals from other neurologists at CNMC. The study procedures were explained to the parent and child, if possible, during a clinic visit, hospital admission, or a follow-up phone call by a member of the study team. All parents approached about the study agreed to participate. Two children were enrolled in the hospital after being admitted for status epilepticus (SE) and were re-evaluated 4–8 weeks after discharge in the outpatient cardiology clinic, where a second study echocardiogram was performed. In these two cases, we used this second echocardiogram for analysis, because it better represented the child’s baseline. The remaining children were enrolled during an outpatient study visit in the cardiology department, where the study procedures were conducted and received a single echocardiogram. All enrollment took place between October 2015 and June 2017. After completion of each patient study, an age- and gender-matched control was obtained by querying the CNMC institutional echocardiographic database for normal studies during the same time period, ensuring consistency of the echocardiographic hardware and software used.
2.3 |. Traditional echocardiography
Echocardiographic studies on the two children admitted for SE were completed at the bedside at the time of admission and then again 4–8 weeks after discharge in the outpatient cardiology department. All other echocardiographic studies on patients and controls were performed in the outpatient cardiology department at CNMC. Children underwent a focused echocardiography protocol on a standard ultrasound machine (Philips Healthcare iE33). Apical four-chamber and parasternal short-axis images were acquired for evaluation, and conventional echocardiographic measures including EF, shortening fraction (SF), mitral inflow E wave velocity, and lateral tissue Doppler E’ velocity were recorded. The traditional echocardiographic measurements (EF, SF, mitral E wave velocity, and tissue Doppler E’) were obtained prospectively on the patients and from the original echo report on the controls.
2.4 |. Strain measurements
Strain analysis for patients and controls was performed offline in the CNMC echocardiography laboratory using vendor-specific software (Philips Healthcare Qlab version 10.7). Strain and strain rate for controls were prospectively performed post hoc on the previous studies by the study staff. By convention, longitudinal and circumferential systolic strain are expressed as negative values as the heart contracts. Longitudinal strain and strain rate were measured in the four-chamber view; circumferential strain and strain rate were measured in the parasternal short-axis view at the level of the mitral valve papillary muscles. These measurements are semiautomated, with the investigator responsible for tracing the myocardium at standard landmarks. Representative myocardial tracings as well as strain and strain rate graphs in the four-chamber and short-axis view are shown in Figure 1. Each measurement was performed twice, and the mean was recorded. The investigators were not blinded to patient/control status, but the large degree of automation and duplicate measurements minimized the potential for bias.
FIGURE 1.
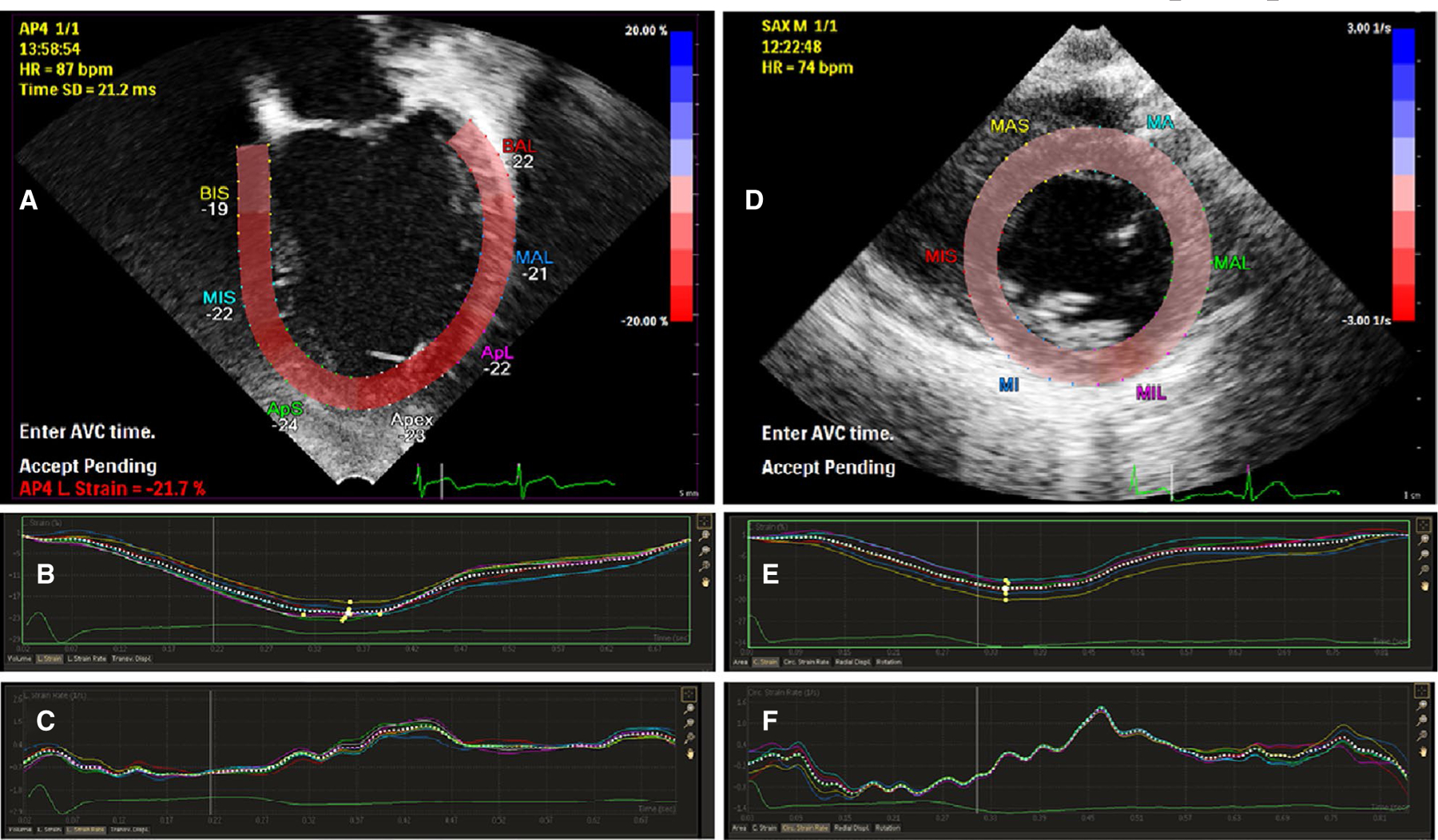
Representative echocardiogram views and corresponding strain curves. A, Apical four-chamber view with representative myocardial tracing. B, Representative strain curve showing longitudinal strain throughout the cardiac cycle. C, Representative strain rate curve showing longitudinal strain rate throughout the cardiac cycle. D, Parasternal short-axis view with representative myocardial tracing. E, Representative strain curve showing circumferential strain throughout the cardiac cycle. F, Representative strain rate curve showing circumferential strain rate throughout the cardiac cycle. Colored text denotes labels of regional myocardium, not individually analyzed in this study. Panel A: ApL, apical lateral wall; ApS, apical septum; BAL, basal anterolateral wall; BIS, basal interventricular septum; MAL, mid anterolateral wall; MIS, mid interventricular septum. Panel D: MA, mid anterior wall; MAL, mid anterolateral wall; MAS, mid anterior septum; MI, mid inferior wall; MIL, mid inferolateral wall; MIS, mid inferior septum
2.5 |. Other data collection and coding
Parents completed a short survey that included current and historical seizure frequency, seizure severity, treatment history, cardiac risk factors, and family history of heart disease and sudden death. Information abstracted from the child’s medical record included age at epilepsy onset, current and past seizure type(s) and frequency, history of SE, epilepsy syndrome and etiology, current and past use of antiseizure medications (ASMs), ambulatory status, presence of scoliosis, and any abnormal cardiac phenotype. Seizures categorized as tonic-clonic included generalized tonic-clonic and focal to bilateral tonic-clonic, and together are referred to as GTCs. ASMs considered cardiotoxic were those that are arrhythmogenic or negative inotropic (weaken myocardial contractility). ASMs coded as arrhythmogenic included clobazam, lamotrigine, phenytoin, carbamazepine, topiramate, gabapentin, pregabalin,19 and lacosamide (https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022255lbl.pdf). Those coded as negative inotropic included phenytoin, carbamazepine, valproate, and pregabalin.19 We did not include discontinued ASMs.
2.6 |. Statistical analysis
Data analyses were conducted in SAS version 9.4. For children with two echocardiographic studies, results from only the second study were included in the analysis, because they should more closely represent the child’s baseline. Categorical variables were summarized as frequency counts and percentages. Continuous variables were summarized using the median and interquartile range (IQR). All measurements of cardiac function were compared between patients and controls using one-sample t tests of the difference between observations in the two groups. The null hypothesis was that the average difference was zero. Tissue Doppler E’ velocity was missing in one patient, and the value for the matched control was excluded from the mean calculation. There were no other missing data. Although we hypothesized a priori that cardiac measurements from the patients would indicate greater impairment, all t tests were two-sided, and significance was determined at the alpha level of .05. To evaluate for associations between measures of cardiac function and epilepsy characteristics in the population of cases only, logistic regression, adjusted for age at enrollment, was performed. Significance was determined at the alpha level of .05 after Bonferroni correction for multiple comparisons.
2.7 |. Standard protocol approvals, registrations, and patient consents
The protocol was approved by the CNMC Institutional Review Board, which included selection of retrospective controls from the CNMC echocardiology database. Written informed consent, and assent when applicable, were obtained by a member of the study team for the children with epilepsy.
2.8 |. Data availability
The study protocol and template data collection forms are available upon request. The raw data are currently being combined with an ongoing study of strain measurements in the Dravet syndrome population. Upon publication of the larger study, the raw data will be shared with interested researchers upon request.
3 |. RESULTS
Gender and age distribution of the 41 patients and 41 controls included 24 males and 17 females with median age = 10 years and IQR = 5–15 years. Patients had a median duration of epilepsy of 7.8 years (IQR = 4.6–11.5). Table 1 presents a summary of the other epilepsy characteristics of the patient population. Thirteen patients (31.7%) were nonambulatory compared to none of the controls. Four patients (all nonambulatory) had scoliosis. Specific pathogenic variants (classified as such according to guidelines established by the American College of Medical Genetics20), when identified, are listed in Table 2. Etiology was genetic in 26 (63.4%), including 11 with Dravet syndrome (all 11 with a heterozygous pathogenic variant or deletion of SCN1A). Six had presumed genetic epileptic encephalopathies (three Lennox-Gastaut syndrome, one epilepsy of infancy with migrating focal seizures, and two epileptic encephalopathies of unknown cause). Six (14.6%) had only structural causes, five (12.2%) combined structural and genetic, three (7.3%) infectious, and one (2.4%) unknown—a patient with nonlesional focal epilepsy. One child with Dravet syndrome had a history of ventricular ectopy. The patient with a mosaic DCX pathogenic variant had a family history of sudden death.
TABLE 1.
Epilepsy characteristics of pediatric patient population
| Characteristic | All patients, n (%) |
|---|---|
| Epilepsy etiology | |
| Genetic | 26 (63.4) |
| Dravet syndrome, SCN1A pathogenic variant | 11 (26.8) |
| SCN2A-related epilepsy | 2 (4.9) |
| SCN8A-related epilepsy | 2 (4.9) |
| Aicardi-Goutières syndrome | 1 (2.4) |
| CDKL5 deficiency disorder | 1 (2.4) |
| SHANK3 pathogenic variant | 1 (2.4) |
| SCL35A2 pathogenic variant | 1 (2.4) |
| Unknown epileptic encephalopathy | 6 (14.6) |
| Structural | 6 (14.6) |
| Focal cortical dysplasia, one with hippocampal sclerosis | 3 (7.3) |
| Hypoxic-ischemic injury | 3 (7.3) |
| Genetic and structural | 5 (12.2) |
| Lissencephaly/pachygyria | 2 (4.9) |
| Polymicrogyria, one GPR56 | 2 (4.9) |
| Subcortical band heterotopia, DCX | 1 (2.4) |
| Infection | 3 (7.3) |
| Cerebral malaria | 1 (2.4) |
| Group B streptococcus meningitis | 1 (2.4) |
| Congenital CMV | 1 (2.4) |
| Unknown | 1 (2.4) |
| Status epilepticus history | |
| Yes | 16 (39.0) |
| No | 25 (61.0) |
| Number of seizures in past month | |
| <13 | 11 (26.8) |
| 13–100 | 12 (28.6) |
| >100 | 18 (42.9) |
| Number of lifetime seizures | |
| <25 | 4 (9.8) |
| 25–100 | 9 (22.0) |
| 101–500 | 8 (19.5) |
| >500 | 20 (48.8) |
| History of GTCs | |
| Yes | 31 (73.8) |
| No | 10 (26.2) |
| Number of current ASMs | |
| 1 | 4 (9.8) |
| 2 | 14 (34.1) |
| 3 or more | 23 (56.1) |
| Number of current cardiotoxic ASMs | |
| 0 | 5 (12.2) |
| 1 | 21 (51.2) |
| 2 or more | 15 (36.6) |
| Number of current negative inotropic ASMs | |
| 0 | 20 (48.8) |
| 1 | 21 (51.2) |
| Ambulatory status | |
| Independent mobility | 28 (68.3) |
| Wheelchair bound | 13 (31.7) |
Abbreviations: ASM, antiseizure medication; CMV, cytomegalovirus; GTCs, generalized tonic-clonic seizure (or focal to bilateral tonic-clonic seizure).
TABLE 2.
Specific pathogenic variants identified in patients
| Gene | Pathogenic variant |
|---|---|
| SCN1A | c.1128del (p.Phe376fs) |
| SCN1A | c.2134C>T (p.Arg712Ter) |
| SCN1A | IVS14+3A> T |
| SCN1A | c.5010_5013del (p.Leu1670fs) |
| SCN1A | c.4879_4880dup (p.Ile1627fs) |
| SCN1A | c.3976_3977dup (p.Val1326fs) |
| SCN1A | c.602+1G>A (+duplication of 8p23.1p21.3) |
| SCN1A | c.1034_1038delGTCCAinsGTCCATCCA (p.Glu347SerfsTer11) |
| SCN1A | c.829T>C (p.Cys277Arg) |
| SCN1A | c.4762delT (p.Tyr1588fs) |
| SCN1A | arr[hg19]2q24.3 (166,838,380– 167,285,059) × 1 (involves SCN1A, SCN9A, and SCN7A) |
| SCN2A | c.5339G>T (p.Ser1780Ile) |
| SCN2A | c.823C>T (p.Arg275Ter) |
| SCN8A | c.4850G>A (p.Arg1617Gln) |
| SCN8A | c.2549G>A (p.Arg850Gln) |
| CDKL5 | c.2828_2829delGA (p.Arg943fs) |
| SHANK3 | c.3424_3425delCT (p.L1143VfsX153) |
| SLC35A2 | mosaic c.164G>C (p.Arg55Pro) |
| TREX1 | compound heterozygous c.341G>A (p.Arg114His) and c.667G>A (p.Ala223Thr) |
| GPR56 | homozygous c.739_745del (p.Gln247fs) |
| DCX | mosaic c.683T>C (p.Leu228Pro) |
Of the 16 (39.0%) patients with a history of SE, nine of 16 (56%) had Dravet syndrome. A history of GTCs occurred in 31 (73.8%), and 26 (63.4%) had at least one GTC in the 6 months prior to study. Total seizure frequency in the previous 6 months included four patients (9.8%) with zero seizures and 18 (42.9%) with >100 seizures. Four patients (9.8%) were currently taking one ASM, 23 (56.1%) were taking three or more ASMs, and >50% of patients were currently being treated with a negative inotropic ASM. Table 3 lists indications for echocardiography in the controls.
TABLE 3.
Indications for echocardiogram in controls
| Indication | n (%) |
|---|---|
| Murmur | 16 (39.0) |
| Chest pain | 11 (26.8) |
| Syncope | 4 (9.8) |
| Abnormal ECG | 3 (7.3) |
| Dizziness | 2 (4.9) |
| Palpitations | 2 (4.9) |
| Tachycardia | 1 (2.4) |
| Fever | 1 (2.4) |
| Prechemotherapy for Hodgkin lymphoma | 1 (2.4) |
Abbreviation: ECG, electrocardiogram.
Standard echocardiography and strain measurements for the 41 patients and 41 controls are shown in Table 4. There were no structural cardiac abnormalities identified in either group, and all electrocardiograms showed sinus rhythm. Although all conventional measurements were within normal ranges, there was a higher SF in patients compared to controls (median = 37.6% [IQR = 35.7–39.7] vs 34.9% [IQR 32.5–38.7], P = .009). Patients had a lower mitral valve E wave inflow velocity (median = 84.8 cm/s [IQR = 78.4–92.8] vs 97.2 cm/s [IQR = 85.9–105.8], P = .005) and tissue Doppler lateral E’ wave (median = 13.9 cm/s [IQR = 12.3–16.1] vs 17.3 cm/s [IQR = 15.4–18.5], P < .001) compared to controls. With respect to the measurements of cardiac deformation, both longitudinal (median = 22.7% [IQR = 21.2–24.2] vs 23.6% [IQR = 22.2–26.1], P = .002) and circumferential strain (median = 22.0% [IQR = 20.3–25.4] vs 24.5% [IQR = 22.3–27.0], P = .025) were decreased in the patients compared to the controls. Longitudinal and circumferential strain rates were similar between groups. There was no significant effect on any of the findings based on the ambulatory status of the patients.
TABLE 4.
Traditional and strain echocardiography measurements in patients and controls
| Patients, median (IQR) | Controls, median (IQR) | P | |
|---|---|---|---|
| n | 41 | 41 | |
| Ejection fraction, % | 65.2 (63.8–66.6) | 64.6 (61.7–66.6) | .948 |
| Shortening fraction, % | 37.6 (35.7–39.7) | 34.9 (32.5–38.7) | .009 |
| Mitral valve E wave inflow velocity, cm/s | 84.8 (78.4–92.8) | 97.2 (85.9–105.8) | .005 |
| Tissue Doppler lateral E’ wave, cm/sa | 13.9 (12.3–16.1) | 17.3 (15.4–18.5) | <.001 |
| Longitudinal strain, % | 22.7 (21.2–24.2) | 23.6 (22.2–26.1) | .002 |
| Longitudinal strain rate, 1/s | 1.3 (1.1–1.4) | 1.2 (1.1–1.4) | .958 |
| Circumferential strain, % | 22.0 (20.3–25.4) | 24.5 (22.3–27.0) | .026 |
| Circumferential strain rate, 1/s | 1.5 (1.4–1.6) | 1.5 (1.4–1.6) | 1.000 |
Abbreviation: IQR, interquartile range.
Missing in one patient/control pair.
Among the 41 patients with epilepsy, there were no associations between age at the time of the study and any echocardiogram measurement. In age-adjusted analysis, there were no associations between any of the echocardiogram measurements and the epilepsy characteristics, including age at epilepsy onset, duration of epilepsy, epilepsy etiology, history of SE, lifetime or recent frequency of any seizure type or of GTCs, or current use of any ASM considered cardiotoxic or negative inotropic.
4 |. DISCUSSION
We demonstrated altered systolic ventricular strain in children with refractory epilepsy compared to age- and gender-matched controls in the absence of standard systolic functional echocardiographic abnormalities, suggesting impaired myocardial fiber shortening. Additionally, mitral valve E wave inflow and left ventricular lateral tissue Doppler E’ velocities were also reduced compared to controls, suggesting impaired diastolic function as well. Whereas normal ranges for the conventional measures are widely accepted, for example, EF > 55% and SF > 28%, deformation measurements are less widely accepted and vary based on software vendor.21 For our Philips analysis package, strain values of less than −16% to 18% are considered the threshold for normal. Although the conventional and novel deformation measures are consistent with these established normal values and ranges, taken together, the systolic and diastolic changes suggest a statistically significant subclinical alteration in myocardial function. Although these values may not be considered clinically significant, the differences seen may suggest potential cardiac alterations in these patients that may provide insight into mechanisms of cardiovascular complications such as arrhythmias and SUDEP.
Although many abnormal cardiac findings in association with epilepsy or individual seizures describe conduction abnormalities including impaired heart rate variability,22 repolarization abnormalities,23,24 and ictal/postictal arrhythmias or asystole,25 functional abnormalities have been reported. These include diastolic dysfunction as assessed by tissue Doppler in untreated patients with epilepsy8 and in children on antiepileptic drugs.9 These studies showed a reduction in tissue Doppler E’ velocity consistent with our results. More significantly, our results are consistent with the results of the only other comparable study of advanced echocardiography and deformation in pediatric epilepsy patients. 16 These include normal EF and SF, impaired measures of systolic deformation, and impaired diastolic parameters as measured by mitral inflow and tissue Doppler velocities. Notably, the details of our subjects’ epilepsy histories were not correlated with echocardiographic data. Furthermore, although it is difficult to compare deformation measurements from different software packages, both studies had subtle changes in the echo parameters listed, suggesting that our more profoundly clinically affected epilepsy population may not have had worse echo evaluations. In a proposed schema involving inherited and acquired cardiac channelopathies in epilepsy (where cardiac ion channel dysfunction may be due to inherited ion channel mutations and/or secondary acquired changes in cardiac ion channel expression due to epilepsy),26 these similar findings despite different epilepsy severity may suggest that the inherited channelopathy plays a larger role in these echo differences.
The underlying mechanism for these functional changes is unclear. Similar changes in strain have been described in adolescent cancer survivors previously treated with anthracyclines,27 which may represent early fibrosis. Cardiac magnetic resonance imaging feature tracking, a technique analogous to speckle-tracking echocardiography, has been shown to correlate with late gadolinium enhancement (LGE) and areas of fibrosis in Duchenne muscular dystrophy,28 and a similar relationship has been found between speckle-tracking echocardiography and LGE in patients with rheumatic mitral stenosis.29 Although these disease processes are heterogeneous, the association of LGE with altered strain and the previously described autopsy series suggest that early fibrosis may be the cause of the impaired strain observed in our patients. Fibrotic areas of myocardium would not be expected to have the same degree of shortening (systolic function) or relaxation (diastolic function) as normal myocardium would.
Fibrosis may be a mechanism for the functional changes seen, but the biological mechanism of this potential structural/histologic change with seizures and epilepsy is poorly understood. Proposed pathophysiologic mechanisms for the autopsy reports demonstrating myocardial fibrosis and myocyte hypertrophy or vacuolization in SUDEP patients5–7 include seizure-related excessive catecholamine release, hypoxia, circulatory changes, and cardiac ischemia,6,30 which are more often found with convulsive seizures. A history of GTCs, childhood onset epilepsy, and longer duration of epilepsy are known risk factors for SUDEP in adults,18,31 although we were unable to find an association between any epilepsy-related factors and impaired strain in our patients. It is not clear why a seizure suddenly becomes fatal after years of previous similar seizures. Progressive myocardial injury may explain some of these findings.
There is increasing evidence that variants in genes affecting cardiac function are more common in SUDEP cases.32 Additionally, mouse myocytes expressing a human Dravet syndrome SCN1A pathogenic variant exhibit conduction abnormalities that translate to QT prolongation, ventricular ectopy, ventricular fibrillation, and bradycardia.33 This is particularly important in our cohort, given the large number with genetic etiologies. Specific variants in genes such as SCN1A, KCNQ1, KCNQ3, CACNA1C, KCNE1, and others segregate in families with SUDEP/sudden death.34 SCN8A epileptic encephalopathy is another genetically mediated epilepsy where there is mounting evidence of direct deleterious effects on the myocardium35 and a higher risk of SUDEP.36,37 Although no data exist regarding heart function in these patients, ventricular myocytes have been shown to be reduced in size in mice with a brain-type sodium channel pathogenic variant.38 Furthermore, patients with clinical long QT syndrome have been shown to have abnormal ventricular wall thickening during systolic contraction as detected by M-mode echocardiography,39,40 and these changes disappear with calcium channel blockage.41 Importantly, patients with long QT syndrome (including documented KCNQ1 and KCNH2 pathogenic variants) have been shown to have mechanical alterations including similar changes in both tissue Doppler E’ velocities and longitudinal strain to the patients in our study.42,43 This suggests a direct link between channelopathies and alterations in mechanical ventricular function and may point to impaired intracellular ion handling as an explanation for our findings.
There were several limitations to our study. We used prospective patients and retrospective healthy controls. Although the speckle-tracking analysis was performed in a prospective manner for all subjects, conventional echo measurements on the control group were obtained from the original report. A reason for the lack of differences or associations in cardiac measures among subgroups with epilepsy may be that this study included only patients with refractory epilepsy, who are more alike than different in their SUDEP risk factors compared to the typical pediatric epilepsy population. Pediatric patients with “complicated” epilepsy (with associated neuro-disability or underlying brain condition) are known to have a higher risk of mortality, including SUDEP.44 More patients across the spectrum of epilepsy and of SUDEP risk, as well as serial measurements on patients, are needed to adequately examine the significance of myocardial strain among the various patient subgroups. Similarly, we cannot exclude potential ASM effects on strain measures. A small number of our patients were nonambulatory, and the effects of this on strain are not known. Finally, although this study was open to all epilepsy patients at our institution meeting inclusion and exclusion criteria, the patients included are a convenience sample, not an exhaustive sample of all eligible patients. This may explain the high proportion with a known genetic etiology and limits generalizability.
Biologic pathways leading to SUDEP are likely heterogeneous. We have identified alterations in ventricular function that may serve as one potential biomarker for SUDEP risk that can be evaluated serially. The exact mechanism by which myocardial fibrosis may contribute to SUDEP is unclear, but could relate to hypoxia, circulatory changes, cardiac ischemia, and increased risk for arrhythmia. Speckle-tracking echocardiography and tissue Doppler imaging are relatively easy to perform and noninvasive. Future longitudinal studies should be performed to assess for any change in strain over time in larger epilepsy cohorts, including adult patients with epilepsy, those newly diagnosed, and those with well-controlled seizures. We do not suggest that anyone change their practice based on this study alone. Additional study is also important to determine whether there is a subpopulation at higher risk for impaired strain and whether there is any change in cardiac strain over time. Identification of children or adults with markedly impaired ventricular strain or diastolic function may provide the opportunity to implement a targeted treatment or monitoring strategy for SUDEP prevention.
Key Points.
Speckle-tracking echocardiography is a sensitive measure of early myocardial injury
Children with refractory epilepsy demonstrate impaired systolic ventricular strain compared to controls
Echocardiographic changes are not correlated with clinical epilepsy history
There was no difference in ejection fraction between cases and controls; all electrocardiograms showed normal sinus rhythm
Acknowledgments
Funding information
Children’s National Board of Visitors 2016 Grant #17 and CTSI-CN 2017 Pilot Grant. This project was supported by Award Number UL1TR001876 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL APPROVAL
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 2016;15:1075–88. [DOI] [PubMed] [Google Scholar]
- 2.Legriel S, Bruneel F, Dalle L, Appere-de-Vecchi C, Georges JL, Abbosh N, et al. Recurrent takotsubo cardiomyopathy triggered by convulsive status epilepticus. Neurocrit Care 2008;9:118–21. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu M, Kagawa A, Takano T, Masai H, Miwa Y. Neurogenic stunned myocardium associated with status epilepticus and postictal catecholamine surge. Intern Med 2008;47:269–73. [DOI] [PubMed] [Google Scholar]
- 4.Stöllberger C, Wegner C, Finsterer J. Seizure-associated takotsubo cardiomyopathy. Epilepsia 2011;52:e160–7. [DOI] [PubMed] [Google Scholar]
- 5.Natelson BH, Suarez RV, Terrence CF, Turizo R. Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol 1998;55:857–60. [DOI] [PubMed] [Google Scholar]
- 6.P-Codrea Tigaran S, Dalager-Pedersen S, Baandrup U, Dam M, Vesterby-Charles A. Sudden unexpected death in epilepsy: is death by seizures a cardiac disease? Am J Forensic Med Pathol 2005;25:99–105. [PubMed] [Google Scholar]
- 7.Nascimento FA, Tseng ZH, Palmiere C, Maleszewski JJ, Shiomi T, McCrillis A, et al. Pulmonary and cardiac pathology in sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav 2017;73:119–25. [DOI] [PubMed] [Google Scholar]
- 8.Bilgi M, Yerdelen D, Cölkesen Y, Müderrisoğlu H. Evaluation of left ventricular diastolic function by tissue Doppler imaging in patients with newly diagnosed and untreated primary generalized epilepsy. Seizure 2013;22:537–41. [DOI] [PubMed] [Google Scholar]
- 9.Kibar AE, Unver O, Oflaz MB, Güven AS, Balli S, Ece İ, et al. Effect of antiepilepsy drug therapy on ventricular function in children with epilepsy: a tissue Doppler imaging study. Pediatr Cardiol 2014;35:280–8. [DOI] [PubMed] [Google Scholar]
- 10.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351–69. [DOI] [PubMed] [Google Scholar]
- 11.Mondillo S, Galderisi M, Mele D, Lomoriello VS, Zacà V, Ballo P, et al. Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 2011;30:71–83. [DOI] [PubMed] [Google Scholar]
- 12.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/ EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 14.Sims A, Frank L, Cross R, Dimock D, Purdy J, Mikhail I, et al. Abnormal cardiac strain in children and young adults with HIV acquired early in life. J Am Soc Echocardiogr 2012;25:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu S, Frank LH, Fenton KE, Sable CA, Levy RJ, Berger JT. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med 2012;13:259–64. [DOI] [PubMed] [Google Scholar]
- 16.Çelik SF, Baratalı E, Güven AS, Torun YA. Left ventricular myocardial deformation abnormalities in seizure-free children with epilepsy. Seizure 2018;61:153–7. [DOI] [PubMed] [Google Scholar]
- 17.Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav 2016;64:69–74. [DOI] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–9. [DOI] [PubMed] [Google Scholar]
- 19.Finsterer J, Stöllberger C. Cardiac and pulmonary risk factors and pathomechanisms of sudden unexplained death in epilepsy patients. In: Lathers CM, Schraeder PL, Bungo MW, Leestma JE, editors Sudden death in epilepsy Boca Raton, FL: CRC Press, 2011; p. 679–92. [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using digital imaging and communications in medicine (DICOM) data and vendor independent speckle tracking software. Echocardiography 2011;24:37–44. [DOI] [PubMed] [Google Scholar]
- 22.Baysal-Kirac L, Serbest NG, Sahin E, Dede HÖ, Gürses C, Gökyiğit A, et al. Analysis of heart rate variability and risk factors for SUDEP in patients with drug-resistant epilepsy. Epilepsy Behav 2017;71:60–4. [DOI] [PubMed] [Google Scholar]
- 23.Strzelcyzk A, Cenusa M, Bauer S, Hamer HM, Mothersill IW, Grunwald T, et al. Management and long-term outcome in patients presenting with ictal asystole or bradycardia. Epilepsia 2011;52:1160–7. [DOI] [PubMed] [Google Scholar]
- 24.Surges R, Adjei P, Kallis C, Erhuero J, Scott CA, Bell GS, et al. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia 2010;51:233–42. [DOI] [PubMed] [Google Scholar]
- 25.van der Lende M, Surges R, Sander JW, Thijs RD. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry 2016;87:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MCH, O’Brien TJ, Todaro M, Powell KL. Acquired cardiac channelopathies in epilepsy: evidence, mechanisms, and clinical significance. Epilepsia 2019;60:1753–67. [DOI] [PubMed] [Google Scholar]
- 27.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr 2012;25:733–40. [DOI] [PubMed] [Google Scholar]
- 28.Siegel B, Olivieri L, Gordish-Dressman H, Spurney CF. Myocardial strain using cardiac MR feature tracking and speckle tracking echocardiography in Duchenne muscular dystrophy patients. Pediatr Cardiol 2018;39:478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soesanto AM, Desandri DR, Haykal TM, Kasim M. Association between late gadolinium enhancement and global longitudinal strain in patients with rheumatic mitral stenosis. Int J Cardiovasc Imaging 2019;35:781–9. [DOI] [PubMed] [Google Scholar]
- 30.Schuele SU. Effects of seizures on cardiac function. J Clin Neurophysiol 2009;26:302–8. [DOI] [PubMed] [Google Scholar]
- 31.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia 2012;53:249–52. [DOI] [PubMed] [Google Scholar]
- 32.Bagnall RD, Crompton DE, Petrovski S, Lam L, Cutmore C, Garry SI. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol 2016;79:522–34. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, et al. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One 2013;14:e77843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coll M, Striano P, Ferrer-Costa C, Campuzano O, Matés J, del Olmo B, et al. Targeted next-generation sequencing provides novel clues for associated epilepsy and cardiac conduction disorder/ SUDEP. PLoS One 2017;12:e0189618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frasier CR, Wagnon JL, Bao YO, McVeigh LG, Lopez-Santiago LF, Meisler MH, et al. Cardiac arrhythmia in a mouse model of sodium channel SCN8A epileptic encephalopathy. Proc Natl Acad Sci USA 2016;113:12838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagnon JL, Korn MJ, Parent R, Tarpey TA, Jones JM, Hammer MF, et al. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum Mol Genet 2014;24: 506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammer MF, Wagnon JL, Mefford HC, Meisler MH. SCN8A-related epilepsy with encephalopathy. 2016. August 25. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, editors GeneReviews Seattle, WA: University of Washington, 1993–2020. https://www.ncbi.nlm.nih.gov/books/NBK379665/. Accessed June 18, 2020. [Google Scholar]
- 38.Noujaim SF, Kaur K, Milstein M, Jones JM, Furspan P, Jiang D, et al. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J 2012;26:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nador F, Beria G, De Ferrari GM, Stramba-Badiale M, Locati EH, Lotto A, et al. Unsuspected echocardiographic abnormality in the long QT syndrome. Diagnostic, prognostic, and pathogenetic implications. Circulation 1991;84:1530–42. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama K, Yamanari H, Otsuka F, Fukushima K, Saito H, Fujimoto Y, et al. Dispersion of regional wall motion abnormality in patients with long QT syndrome. Heart 1998;80: 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Ferrari GM, Nador F, Beria G, Sala S, Lotto A, Schwartz PJ. Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation 1994;89: 2126–32. [DOI] [PubMed] [Google Scholar]
- 42.Leren IS, Hasselberg NE, Saberniak J, Håland TF, Kongsgård E, Smiseth OA, et al. Cardiac mechanical alterations and genotype specific differences in subjects with long QT syndrome. JACC Cardiovasc Imaging 2015;8:501–10. [DOI] [PubMed] [Google Scholar]
- 43.Haugaa KH, Amlie JP, Berge KE, Leren TP, Smiseth OA, Edvardsen T. Transmural differences in myocardial contraction in long-QT syndrome: mechanical consequences of ion channel dysfunction. Circulation 2010;122:1355–63. [DOI] [PubMed] [Google Scholar]
- 44.Berg AT, Nickels K, Wirrell EC, Geerts AT, Callenbach PMC, Arts WF, et al. Mortality risks in new-onset childhood epilepsy. Pediatrics 2013;132:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol and template data collection forms are available upon request. The raw data are currently being combined with an ongoing study of strain measurements in the Dravet syndrome population. Upon publication of the larger study, the raw data will be shared with interested researchers upon request.


