New progressive tobacco control legislation in Israel restricted advertising of all tobacco and related products, including heated tobacco products and electronic cigarettes. The three main elements comprised: (1) advertising bans in all media, including broadcast, outdoor, internet and print media, but excluding newspapers (effective March 2019); (2) bans on point-of-sale displays except in specialised tobacco and alcohol outlets (effective January 2020); and (3) plain packaging (effective January 2020).1–3 During the initial implementation phase, an active tobacco control coalition communicated via social media (‘WhatsApp’) regarding tobacco industry tactics to bypass legislative efforts, test the limits of the legislation and/or test the government’s ability to enforce the legislation. The coalition included civil society and professional organisations from a wide range of disciplines, including public health, medicine, public policy, public administration and communications.3 Table 1 provides a detailed account of the policies, industry reactions and lessons learnt (also see figures 1 and 2).
Table 1.
Policies implemented, tobacco industry responses and lessons learnt
| Tobacco control policies | Provisions content | Tobacco industry response | Lessons learnt and suggested actions |
|---|---|---|---|
| Tobacco advertising, promotion and sponsorship | Restrictions on advertising, promotion and sponsorship, except:
|
|
|
Point-of-sales display ban, except:
|
|
|
|
| Tobacco packaging and labelling | Plain packaging using the colour Pantone 448 C, for all tobacco products, including heated tobacco and electronic cigarettes. Only specific wording is allowed on the packaging (including the name of the product and the manufacturer). Textual health warnings will be included on both the cover and back of the package, covering 65% of the package for tobacco products and 30% for electronic cigarette products. All packaged products will also include an insert with health warnings and smoking cessation support options.‡ |
|
|
Internet sales of products are only allowed to include the name of the product, country of manufacturer, price, parts and components of the product and their amount, and regarding electronic cigarettes—also the nicotine concentration in the product.
It is not completely clear whether these new signs are considered a violation of the new law. The wording in the legislation does not permit any advertisement at the point-of-sales. However, it is not clear whether that refers to advertisements that include a specific brand or whether that includes also general advertisements that are not specific to a certain brand. Differing legal advice has been issued on this topic, but due to lack of enforcement, this was never challenged publicly.
The inserts require the Ministry of Health, after authorisation from the Economic Committee, to publish specific regulations regarding the inserts size, design, form, language used, messaging turnover and the inclusion of graphic health warnings. This has not been done yet by the Ministry of Health and therefore the use of inserts is not currently implemented.
MOH, Ministry of Health; PMI, Philip Morris International.
Figure 1.
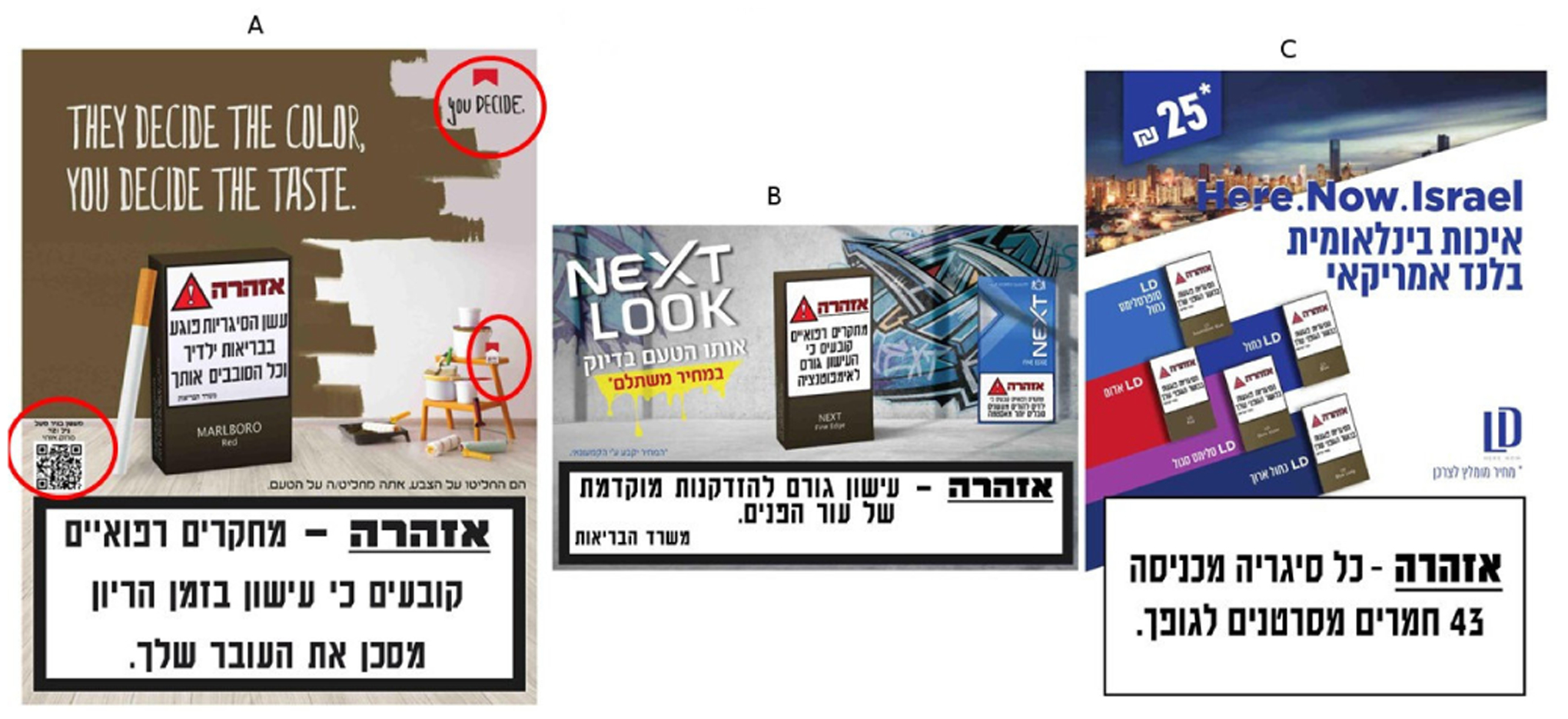
(A) Branded elements appeared in the background of the print media advertisements (red circles); January 2020. (B) When the inclusion of the small branded elements was not met with a response, the industry included the full size previous packaging in the background; March 2020. (C) Colours and fonts not allowed to be used in plain packaging, were used in print media advertisement to differentiate and overcome the plain packaging.
Figure 2.

(A) Before the legislation. (B) In transition—plain packaging appearing in the market, but products are visible at point-of-sale. (C) After the legislation went into effect. Note - (1) case is not hermetic and some products are still visible and (2) large sign stating that cigarettes and tobacco products are sold here (highlighted in red).
Major weaknesses of the new legislation, which may be relevant to other countries as well, are the lack of: (1) specificity in the language of the legislation; (2) strong implementation guidelines; and (3) comprehensive enforcement plans. With regard to specificity, tobacco control advocates and legislators can leverage the robust global tobacco control network, which has expertise and experience in crafting legislation and anticipating industry response to policy. Implementation guidelines are crucial prior to the implementation phase and should include a campaign for the public and for stakeholders (such as the point-of-sale owners) and practical assistance and guidance for field implementation (such as distributing specific guidelines through local municipalities, and conducting planned and pre-announced enforcement operations without fines for instructional purpose). The Israel legislation requires enforcement at both the national (to enforce media outlet advertising bans) and local levels (to enforce point-of-sale advertisement restrictions and display bans). The proposed bill was a private bill, not initiated by the government. Therefore, including a comprehensive enforcement plan, which would have required allocating significant funding and personnel, may have resulted in lack of governmental support for the bill, and further delay in the approval of the bill.
These limitations to the legislation resulted in tobacco industry exploitation (table 1) and ultimately the undermining of the legislation’s impact. Some of the industry responses to the new legislation were not necessarily considered policy violations. Rather, they expose the ways in which the industry takes advantage of ambiguities in the legislation to undermine its intent and impact. These experiences highlight that passing legislation is insufficient without specificity of provisions and plans for implementation and enforcement.
There is a clear need to instigate an amendment to the law to include quick and efficient mechanisms for responding to the industry that do not require going through the entire legislative process, for example, by establishing a national authority for tobacco control, similar to the US FDA (Food and Drug Administration) authority granted by the Family Smoking Prevention and Tobacco Control Act.4 A government decision to establish such an authority was passed in 2011, but never implemented.5
Other attempts to undermine the new legislation were identified. Two months after the legislation was passed in parliament, Juul Labs, joined after-wards by The Vaping Products Manufacturers and Importers Forum, appealed to the Supreme Court to exempt vaping products from the law on the basis that the legislative process was unjust. This appeal underscores the need to anticipate and prepare for such legal challenges and ensure proper legislative processes occur while developing and implementing legislation. The appeal was denied by the court, which ruled that the legislative process was just and that the industry had ample opportunity to voice their concern. This was possible due to actions taken in advance by the Chair of the Parliament Economics Committee (which prepared the bill for the Parliament vote): following recommendations in Article 5.3 of the Framework Convention of Tobacco Control, the Israeli Ministry of Health refused meeting the tobacco or vaping industry representatives to discuss the bill. However, the Chair of the Parliament Economics Committee demanded that the Ministry of Health meet with the opposing bodies. This was resolved through formal meetings with other stakeholders such as the Chambers of Commerce Union (representing the commercial interests of the trade and service sector), which included tobacco industry representatives.6 The Economics Committee chair allowed industry representatives to appear in the committee (despite opposition from the tobacco control coalition) and requested that a Ministry of Health representative respond to each industry argument. These actions ultimately led to: (1) the Ministry of Health consulting relevant regulatory agencies to ensure legislation endorsement of all necessary commerce requirements; (2) all responses to industry arguments being publicly transparent and fully-documented (a legal requirement for all Israeli parliament committee meetings). This provided a solid legal basis for the positive Supreme Court ruling. This emphasises that in setting and implementing tobacco control policies, and in accordance with article 5.3, the tobacco industry might need to be part of the process, but this should be conducted in a regulated, controlled and transparent manner; and preferably initiated by the regulator.
In summary, there are several lessons learnt from this case study. First, the industry uses ‘loopholes’ in legislation; that is, anything not specified and verbiage open to interpretation provides openings for the industry to circumvent the intentions of the legislation. Second, if such efforts are not met with quick responses, the industry will continue and expand their non-compliance and circumvention. Therefore, it is crucial that regulations be as specific and detailed as possible, anticipate industry efforts to identify such loopholes, compel industry compliance through planned, efficient and quick implementation guidelines and enforcement and provide mechanisms to quickly address new industry tactics that defy the spirit of legislation.
Funding
This research was supported by the National Cancer Institute (R01CA239178-01A1; MPIs: CJB, HL). Dr Berg is also supported by the National Cancer Institute (R01CA215155-01A1; PI: CJB; R01CA179422-01; PI: CJB), the US Fogarty International Center/National Cancer Institute (1R01TW010664-01; MPIs: CJB, Kegler), and Fogarty/NIEHS (D43ES030927-01; MPIs: CJB, Marsit, Sturua).
Footnotes
Competing interests YB-Z has received fees for lectures from Pfizer Israel Ltd, Novartis NCH and GSK Consumer Health (distributors of smoking cessation pharmacotherapy in Israel) in the past (2012-07/2019). HL had received fees for lectures from Pfizer Israel Ltd (distributor of a smoking cessation pharmacotherapy in Israel) in 2017. LA receives royalties for the sale of Text2Quit and is a shareholder in Welltok, Inc.
Data availability statement All data relevant to the study are included in the article
REFERENCES
- 1.Baumer L Israel to paint cigarette, e-cigarette packages with world’s ugliest color, 2019. Available: https://www.calcalistech.com/ctech/articles/0,7340,L-3753353,00.html [Accessed 3 Jan 2019].
- 2.Wootliff R Knesset stubs out ads for cigarettes with ‘historic, life-saving’ law. Times of Israel, 2019. [Google Scholar]
- 3.Rosen L, Kislev S, Bar-Zeev Y, et al. Historic tobacco legislation in Israel: a moment to celebrate. Isr J Health Policy Res 2020;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GPO. Public law 111–31—June 22, 2009. family smoking prevention and tobacco control, 2009. Available: https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.Pdf [Google Scholar]
- 5.Prime Minister Office. Government decision number 3247, 29/05/2011. National plan for reducing tobacco use and it’s harm.. Available: https://www.gov.il/he/Departments/policies/2011_des3247 [Google Scholar]
- 6.Ministry of Health. Meeting summary from 28/11/2018 with representatives of the chambers of Commerce Union in regard to the proposed bill on advertisiment and marketing of tobacco products, 2018. Available: https://www.health.gov.il/Subjects/KHealth/smoking/Pages/open-records-tobacco.aspx [Google Scholar]


