Abstract
Objective:
To examine the association between body mass indexes (BMI) and clinical outcomes among patients with COVID-19 infection.
Methods:
We included 10,861 patients with COVID-19 infection admitted to the Northwell Health system hospitals during the period of March 1 to April 27, 2020. BMI was classified as: underweight, normal, overweight, obesity class I, II, and III. Primary outcomes are invasive mechanical ventilation (IMV) and death.
Results:
There were 243 (2.2%) underweight, 2,507 (23.1%) normal weight, 4,021 (37.0%) overweight, 2,345 (21.6%) obesity class I, 990 (9.1%) obesity class II, and 755 (7.0%) obesity class III patients. Patients who are overweight (OR=1.27[95% CI, 1.11-1.46]), obesity class I (OR=1.48 [95% CI, 1.27-1.72]), obesity class II (OR=1.89[95% CI, 1.56-2.28]), and obesity class III (OR=2.31 [95% CI, 1.88-2.85]) had increased risk of requiring IMV. Underweight and obesity classes II and III were statistically associated with death (OR=1.44 [95% CI, 1.08-1.92]; OR=1.25 [95% CI 1.03-1.52]; OR=1.61 [95% CI 1.30-2.00], respectively). Among patients who were on IMV, BMI was not associated with inpatient deaths.
Conclusion:
Patients who are underweight or with obesity are at a risk for mechanical ventilation and death, suggesting pulmonary complications (indicated by IMV) is a significant contributor for poor outcomes in COVID-19 infection.
Keywords: obesity; body-mass index, BMI; mortality; prevalence; respiratory
Introduction
The health impact of coronavirus disease 2019 (COVID-19) in the United States—with New York City at the epicenter—is staggering. As of August 9, 2020, there are over 4.9 million confirmed cases and over 161,000 deaths from COVID-19 in the United States.1 Preliminary data reveals that obesity may be a significant contributing factor for poor health outcomes among patients with COVID-19 infection.2,3 Additionally, studies show that patients with obesity are also more likely to require mechanical ventilation.4,5
Metabolic inflammation, unfavorable hormonal milieu, and a predisposition to the enhanced release of cytokines-pathophysiology accompanying severe obesity have been implicated in multi-organ failure seen in patients with COVID-19.6,7 Common clinical complications of obesity include pulmonary embolism, increased atelectasis in acute respiratory distress syndrome (ARDS), and acute kidney injury in critical illness.8,9 These obesity-related complications are also common among patients with severe COVID-19 infection.10-12 Therefore, it is not surprising that obesity is being suggested as a risk factor for poor outcomes in individuals affected by COVID-19.
Although obesity has been associated with poor clinical outcomes in patients with COVID-19, the association between full spectrums of body habitus based on body mass index (BMI)—including underweight, overweight, and obesity classes I, II, and III—and COVID-19 outcomes is yet to be elucidated. We used data from a large integrated network to examine whether BMI, stratified by category, is associated with poorer clinical outcomes for COVID-19 including invasive mechanical ventilation (IMV) or death.
Methods
We included all adult patients admitted to 12 Northwell Health system acute care hospitals in New York between March 1, 2020, and April 27, 2020, with a confirmed diagnosis of COVID-19 by polymerase chain reaction of nasopharyngeal swabs. The health system serves patients throughout Long Island, New York City, and Westchester areas. Data were collected from inpatient electronic health records (Sunrise Clinical Manager, Allscripts, Chicago, IL). BMI was documented on admission either as self-report or obtainment by nursing staff and classified as follows: underweight (less than 18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obesity class I (30-34.9 kg/m2), obesity class II (35-39.9 kg/m2), and obesity class III (greater than or equal to 40 kg/m2). Patients were identified to have missing BMI if they did not have BMI or both weight and height information during their hospitalizations (Figure 1).
Figure 1.
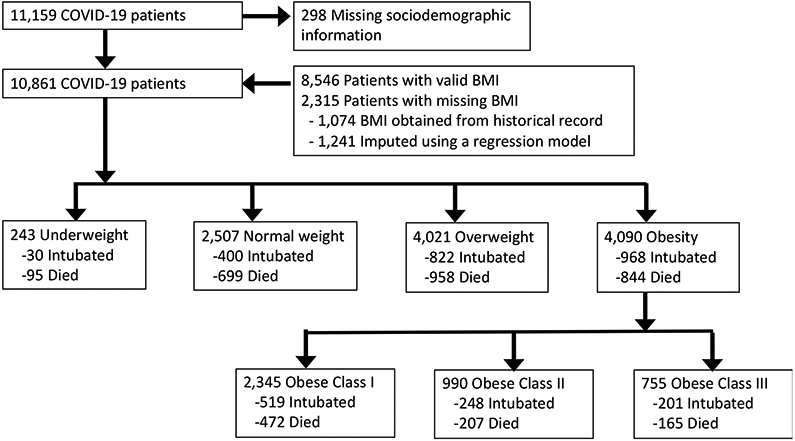
Flowchart of patients admitted with COVID-19 infection by their BMI groups
For patients with missing BMI (n=2,315), we used historical BMI data and linear regression models to impute missing BMI. This was necessary because there were differences in sociodemographic and clinical characteristics, including use of IMV and death, between patients with and without BMI information (online Supporting Information Table S1). For patients with system historical records (any clinical visits with BMI or a combination of weight and height information), we first used the most recent BMI values (n=1,074) from 2018 to 2020. For the remaining 1,241 patients, we imputed missing BMI using regression models based upon predictors of this variable: age, sex, race, and if possible, weight or height (not both).13 The linear predictive models were validated using patients with BMI information.
We obtained demographic information (sex, age, race/ethnicity), comorbidities, smoking status, and hospital type (tertiary or not). Primary outcomes included mechanical ventilation and death. All patients were followed until discharge, death, or until May 12, 2020.
Descriptive statistical analyses were performed using Chi-square for categorical variables and analysis of variance (ANOVA) for continuous variables. Next, we performed multivariable regression models adjusting for patient characteristics (age, sex, race/ethnicity, presence of comorbidities, smoking status, hospital type, and BMI groups) and present odds ratio (OR) and adjusted odds ratio (AOR) with 95% confidence intervals (CI) to determine characteristics associated with the outcome variables of IMV and death. We also conducted a secondary analysis using a Cox proportional-hazards model to examine death as a clinical outcome because a proportion of patients remained hospitalized. Statistical significance was considered for p-value less than 0.05. All statistical analyses were done in SAS v9.4 (SAS Institute). This study protocol was approved by the Feinstein Institutes for Medical Research Institutional Review Board.
Results
There were 10,861 patients with COVID-19 infection with valid BMI information (Figure 1); 243 (2.2%) patients were underweight, 2,507 (23.1%) patients were normal weight, 4,021 (37.0%) patients were overweight, and 4,090 (37.7%) patients were obese. For patient characteristics, 6,468(59.6%) were male, the median age was 65 years [IQR: 54-77], and the median BMI was 28.3 [IQR: 24.9-32.3] (Table 1). There were 3,675 (33.8%) White; 2,270 (20.9%) Black; 2,475 (22.8%) Hispanic; 932 (8.6%) Asian; and 1,509 (13.9%) Other/Unknown. A total of 2,220 (20.4%) patients required IMV and 2,596 (23.9%) died. There were significant differences in IMV and death among different BMI categories: 12.4% of underweight, 16.0% of normal weight, 20.4% of overweight, 22.1% of obesity class I, 25.1% of obesity class II, and 26.6% of obesity class III required IMV (p-value less than 0.001). Additionally, 39.2% of underweight, 27.9% of normal weight, 23.8% of overweight, 20.1% of obesity class I, 20.9% of obesity class II, and 21.9% of obesity class III died.
Table 1.
Baseline Characteristics of Patients Hospitalized with COVID-19 by Body Mass Index
| BMIa (kg/m2) | All | Underweight (< 18.5) |
Normal (18.5-24.9) |
Overweight (25-29.9) |
Obesity | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Class I (30-34.9) |
Class II (35-39.9) |
Class III (≥40) |
||||||
| N (%) | 10861(100.0) | 243(2.2) | 2507(23.1) | 4021(37.0) | 2345(21.6) | 990(9.1) | 755(7.0) | |
| Age, n (%) | <0.001 | |||||||
| 18 -39 | 779(7.2) | 6(2.5) | 119(4.8) | 218(5.4) | 182(7.8) | 112(11.3) | 142(18.8) | |
| 40-59 | 3136(28.9) | 23(9.5) | 425(16.9) | 1190(29.6) | 791(33.7) | 397(40.1) | 310(41.1) | |
| 60-79 | 4788(44.1) | 85(35.0) | 1095(43.7) | 1832(45.6) | 1105(47.1) | 408(41.2) | 263(34.8) | |
| >=80 | 2158(19.9) | 129(53.1) | 868(34.6) | 781(19.4) | 267(11.4) | 73(7.4) | 40(5.3) | |
| Sex, n (%) | <0.001 | |||||||
| Male | 6468(59.6) | 112(46.1) | 1451(57.9) | 2648(65.8) | 1382(58.9) | 522(52.7) | 353(46.8) | |
| Female | 4393(40.5) | 131(53.9) | 1056(42.1) | 1373(34.2) | 963(41.1) | 468(47.3) | 402(53.3) | |
| Race/Ethnicityb, n (%) | <0.001 | |||||||
| White | 3675(33.8) | 105(43.2) | 948(37.8) | 1304(32.4) | 729(31.1) | 336(34.0) | 253(33.5) | |
| Black | 2270(20.9) | 50(20.6) | 436(17.4) | 699(17.4) | 573(24.4) | 257(26.0) | 255(33.8) | |
| Hispanic | 2475(22.8) | 33(13.6) | 442(17.6) | 1056(26.3) | 592(25.3) | 221(22.3) | 131(17.4) | |
| Asian | 932(8.6) | 29(11.9) | 314(12.5) | 373(9.3) | 142(6.1) | 44(4.4) | 30(4.0) | |
| Other/Unknown | 1509(13.9) | 26(10.7) | 367(14.6) | 589(14.7) | 309(13.2) | 132(13.3) | 86(11.4) | |
| Comorbiditiesc, n (%) | ||||||||
| Hypertensiond | 6555(60.4) | 160(65.8) | 1508(60.2) | 2362(58.7) | 1398(59.6) | 639(64.6) | 488(64.6) | <0.001 |
| Coronary Artery Disease | 1447(13.3) | 44(18.1) | 392(15.6) | 529(13.2) | 297(12.7) | 119(12.0) | 66(8.7) | <0.001 |
| Diabetes Mellituse | 3995(36.8) | 66(27.2) | 789(31.5) | 1415(35.2) | 954(40.7) | 423(42.7) | 348(46.1) | <0.001 |
| Heart Failure | 932(8.6) | 21(8.6) | 249(9.9) | 317(7.9) | 175(7.5) | 90(9.1) | 80(10.6) | 0.006 |
| Chronic Kidney Diseasef | 515(4.7) | 10(4.1) | 127(5.1) | 204(5.1) | 94(4.0) | 40(4.0) | 40(5.3) | 0.29 |
| End Stage Renal Diseaseg | 430(4.0) | 22(9.1) | 144(5.7) | 134(3.3) | 68(2.9) | 34(3.4) | 28(3.7) | <0.001 |
| Cancer | 937(7.7) | 33(13.6) | 236(9.4) | 313(7.8) | 162(6.9) | 64(6.5) | 29(3.8) | <0.001 |
| Asthma | 903(8.3) | 14(5.8) | 151(6.0) | 275(6.8) | 222(9.5) | 114(11.5) | 127(16.8) | <0.001 |
| COPD | 677(6.2) | 18(7.4) | 175(7.0) | 234(5.8) | 114(4.9) | 78(7.9) | 58(7.7) | 0.002 |
| Smoking statuse, n (%) | <0.001 | |||||||
| Active | 260(2.4) | 6(2.5) | 54(2.2) | 90(2.2) | 55(2.4) | 32(3.2) | 23(3.1) | |
| Former | 1537(14.2) | 27(11.1) | 326(13.0) | 594(14.8) | 334(14.2) | 137(13.8) | 119(15.8) | |
| Never | 7996(73.6) | 161(66.3) | 1809(72.2) | 2945(73.2) | 1787(76.2) | 737(74.4) | 557(73.8) | |
| Unknown | 708(6.5) | 35(14.4) | 208(8.3) | 271(6.7) | 106(4.5) | 52(5.3) | 36(4.8) | |
| Clinical Outcomes, n (%) | ||||||||
| Ventilator | 2220(20.4) | 30(12.4) | 400(16.0) | 822(20.4) | 519(22.1) | 248(25.1) | 201(26.6) | <0.001 |
| Death | 2596(23.9) | 95(39.2) | 699(27.9) | 958(23.8) | 472(20.1) | 207(20.9) | 165(21.9) | |
| Intensive care unit (ICU) entry | 2625(24.2) | 39(16.2) | 500(19.9) | 968(24.1) | 611(26.1) | 277(28.0) | 230(30.5) | <0.001 |
| Pressor | 2256(20.8) | 34(14.0) | 423(16.9) | 837(20.8) | 520(22.2) | 243(24.6) | 199(26.4) | <0.001 |
| Inotrope | 141(1.3) | 1(0.4) | 28(1.1) | 55(1.4) | 32(1.4) | 20(2.0) | 5(0.7) | 0.12 |
| Hospital Type, n (%) | <0.001 | |||||||
| Community | 3630(33.4) | 89(36.6) | 938(37.4) | 1317(32.8) | 734(31.3) | 313(31.6) | 239(31.7) | |
| Tertiary | 7231(66.6) | 154(63.4) | 1569(62.6) | 2704(67.3) | 1611(68.7) | 677(68.4) | 516(68.3) | |
| Length of stay, n (%) | 0.05 | |||||||
| <7 | 5741 (52.9) | 139 (57.2) | 1348 (53.8) | 2138 (53.2) | 1250 (53.3) | 501 (50.6) | 365 (48.3) | |
| >=7 | 5120 (47.1) | 104 (42.8) | 1159 (46.2) | 1883 (46.8) | 1095 (46.7) | 489 (49.4) | 390 (51.7) | |
| Median (IQR) | 6.7 (3.5-12.1) | 5.9 (3.3-11.6) | 6.5 (3.7-11.8) | 6.6 (3.4-12.1) | 6.6 (3.3-11.9) | 6.9 (3.7-12.8) | 7.2 (3.9-13.1) | 0.005 |
| Discharge Disposition | <0.001 | |||||||
| Currently Admitted | 449(4.1) | 5(2.1) | 109(4.4) | 162(4.0) | 99(4.2) | 40(4.0) | 34(4.5) | |
| Discharged | 7435(68.5) | 138(56.8) | 1630(65.0) | 2747(68.3) | 1679(71.6) | 712(71.9) | 529(70.1) | |
| Transfer | 381(3.5) | 5(2.1) | 69(2.8) | 154(3.8) | 95(4.1) | 31(3.1) | 27(3.6) | |
Body Mass Index (BMI) is calculated as weight (kilograms)/height (meters) squared. BMI classified as underweight at less than 18.5kg/m2, normal at 18.5-24.9 kg/m2, overweight at 25-29.9 kg/m2, class I at 30-34.9 kg/m2, class II at 35-39.9 kg/m2, and class III at greater than 40 kg/m2.
Race and ethnicity data were collected by self-report in pre-specified fixed categories.
Comorbidities defined as medical diagnosis included in medical history by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coding. These include, but are not limited to, those presented in the table.
Hypertension defined as provider-entered diagnosis with ICD-10 I10-I16.
Assessed based on a diagnosis of diabetes mellitus, includes diet-controlled and non-insulin dependent diabetes; provider-entered diagnosis, with ICD10 E08-E13.
Based on a diagnosis of chronic kidney disease in medical history by ICD-10 coding.
Diagnosis of end-stage kidney disease in medical history by ICD-10 coding.
After adjusting for covariates (Table 2), overweight (adjusted odds ratio (AOR), 1.27 [1.11-1.46]), obesity class I (AOR 1.48 [1.27-1.72]), obesity class II (AOR, 1.89 [1.56-2.28]), and obesity class III (AOR, 2.31 [1.88-2.85]) were associated with IMV. Other characteristics associated with increased IMV include males (AOR, 1.57 [1.41-1.74]), an age of 60-79 years (AOR, 1.59 [1.41-1.79]), Hispanic ethnicity (AOR, 1.40 [1.23-1.60]), and Asian race (AOR, 1.36 [1.14-1.64]). Having diabetes (AOR, 1.24 [1.12-1.38]) and heart failure (AOR 1.49 [1.25-1.77]) had increased odds of IMV. Patients who were underweight (AOR, 1.44 [1.08-1.92]), obesity class II (AOR, 1.25 [1.03-1.52]), and obesity class III (AOR, 1.61 [1.30-2.00]) had increased odds of death. Among patients requiring IMV (n=2,220), neither being underweight (AOR, 1.03 [0.43-2.50] nor being obesity class III (AOR, 1.21 [0.82-1.79]) was associated with death. Patients who were 80 years and older (AOR, 4.16 [2.89-6.01]), of Asian race (AOR 1.49 [1.05-2.11]), and/or had end stage renal disease (AOR, 1.80 [1.09-3.00]) had increased odds of death. In a Cox proportional-hazards regression model, patients who were underweight (hazard ratio=1.46 [1.17-1.81]) or with obesity class 3 (hazard ratio=1.23 [1.03-1.48]) had increased risk of death.
Table 2.
Model-Adjusted Odds Ratios of Invasive Mechanical Ventilation (IMV) and Death
| Odds Ratio [95% Confidence Interval] | |||
|---|---|---|---|
| All patients | IMV | ||
| IMV | Death | Death | |
| Males (reference: female) | 1.57[1.41-1.74] | 1.55[1.40-1.71] | 1.47[1.21-1.79] |
| Age group, year old (reference: 40-59 years old) | |||
| Less than 40 | 0.61[0.48-0.76] | 0.51[0.37-0.70] | 0.61[0.39-0.94] |
| 60-79 | 1.59[1.41-1.79] | 2.63[2.29-3.01] | 2.06[1.66-2.56] |
| 80 or greater | 0.89[0.74-1.06] | 5.91[5.03-6.95] | 4.16[2.89-6.01] |
| Race/Ethnicity (reference: non-Hispanic White) | |||
| Black | 0.83[0.72-0.96] | 0.82[0.72-0.94] | 1.19[0.90-1.58] |
| Hispanic | 1.40[1.23-1.60] | 1.02[0.90-1.17] | 1.26[0.98-1.62] |
| Asian | 1.36[1.14-1.64] | 1.17[0.98-1.40] | 1.49[1.05-2.11] |
| Other/unknown | 1.24[1.06-1.44] | 1.00[0.86-1.17] | 1.22[0.91-1.63] |
| BMI (reference: normal BMI) | |||
| Underweight | 0.85[0.57-1.27] | 1.44[1.08-1.92] | 1.03[0.43-2.50] |
| Overweight | 1.27[1.11-1.46] | 1.04[0.92-1.17] | 0.94[0.72-1.23] |
| Obesity Class I | 1.48[1.27-1.72] | 1.00[0.87-1.16] | 0.93[0.70-1.25] |
| Obesity Class II | 1.89[1.56-2.28] | 1.25[1.03-1.52] | 1.00[0.70-1.43] |
| Obesity Class III | 2.31[1.88-2.85] | 1.61[1.30-2.00] | 1.21[0.82-1.79] |
| Comorbidities (reference: no comorbidities) | |||
| Hypertension | 1.02[0.91-1.14] | 0.95[0.853-1.07] | 0.94[0.76-1.15] |
| Coronary artery disease | 0.91[0.78-1.05] | 1.02[0.90-1.17] | 1.11[0.82-1.50] |
| Diabetes mellitus | 1.24[1.12-1.38] | 1.20[1.08-1.32] | 1.04[0.86-1.26] |
| Heart failure | 1.49[1.25-1.77] | 1.59[1.37-1.86] | 1.27[0.91-1.78] |
| Chronic kidney disease | 0.93[0.74-1.17] | 1.19[0.97-1.45] | 1.53[0.93-2.51] |
| End stage renal disease | 0.95[0.74-1.22] | 1.40[1.11-1.76] | 1.80[1.09-3.00] |
| Cancer | 0.85[0.70-1.03] | 1.15[0.97-1.35] | 1.31[0.88-1.95] |
| Asthma | 0.89[0.75-1.07] | 0.81[0.67-0.98] | 0.84[0.60-1.18] |
| COPD | 0.97[0.78-1.19] | 1.33[1.11-1.60] | 1.20[0.79-1.81] |
| Smoking status (reference: never/unknown smoker) | |||
| Active/former smoker | 0.81[0.71-0.93] | 0.73[0.64-0.83] | 0.87[0.67-1.12] |
| Hospital type (reference: non- tertiary hospital) | |||
| Tertiary | 1.13[1.02-1.25] | 0.81[0.73-0.89] | 0.61[0.50-0.75] |
COPD=Chronic obstructive pulmonary disease
Discussion
Consistent with other studies,14,15 our study showed that overweight and all classes of obesity were associated with increased odds of IMV. Additionally, underweight, obesity class II, and obesity class III were associated with higher odds of death. The presence of obesity incurs multiple risks for IMV and death among patients with COVID-19 infection. This is likely due to associations between obesity and pulmonary complications as well as common medical conditions including diabetes, hypertension, sleep apnea, hepatic steatosis, and kidney diseases.16
Interestingly, within the cohort of intubated patients, BMI was not statistically associated with greater odds of death – persons with obesity did not have increased odds of death compared to patients with normal weight. This suggests that while patients who suffer from obesity are more likely to experience a severe COVID-19 course (reflected by increased odds of IMV), once mechanically ventilated, all patients regardless of BMI have similar odds of death. Greater need for mechanical ventilation in patients with severe obesity indicates a trend towards more severe pulmonary complications, which are significant contributors of death. People with obesity are already known to have increased pulmonary complications from upper respiratory illnesses from reduced lung volume, hypoventilation, congestive heart failure, and acute respiratory distress syndrome. Furthermore, emerging studies have revealed that COVID-19 infection results in hypercoagulable states, with increased risks of developing pulmonary embolism, stroke, and arterial thrombosis.17 Obesity being associated with both hypercoagulable and hyperinflammatory states, may augment risks of developing complications from COVID-19 infection. As more details on the pathophysiology of severe COVID-19 emerge, the trend towards higher rates of mechanical ventilation—and thus, mortality—in populations with obesity might be explained.
Our study included underweight patients and being underweight was not associated with IMV but had higher odds of death, which is consistent with data showing that underweight patients have higher all-cause mortality.18 This group may reflect a fragile population such as elderly patients with multiple chronic diseases, as the majority of underweight patients (53.1%) were older than 80 years of age. Due to poor prognoses, it is possible that this underweight cohort deferred IMV based on goals of care discussions, thus reflecting our findings that showed no increased odds of IMV.
A major limitation of our study is the use of administrative databases that may limit clinical information including dyspnea severity and temporal hospital trends that manual chart review could otherwise address. Also, we do not have information about resuscitation and/or intubation statuses nor processes behind clinical decision making to explain which patients are intubated. Due to hospital data, information about whether discharged patients expired in the community are lacking. Importantly, the utilization of IMV in patients who are overweight and with obesity may be affected by clinical bias toward earlier intervention based on established pulmonary complications seen in patients with obesity. The study finding may be limited in generalizability due to differences in patient population, which is also reflected by the finding that Blacks having decreased adverse outcomes.
Conclusion
Our study showed that underweight, overweight, and obesity are associated with poor outcomes among patients hospitalized with COVID-19 infection. This study further emphasizes obesity as a significant adverse health condition, including COVID-19 infection, and reinforces an urgency to address obesity as a public health problem.
Supplementary Material
Study Importance:
Clinical observations suggest that obesity is a risk factor for more severe disease in patients infected with COVID-19, but no study has examined the statistical association between different BMI groups (underweight, overweight, obesity class I, obesity class II, and obesity class III) and COVID-19 clinical outcomes.
Our findings show that overweight and people with obesity hospitalized with COVID-19 infection have increased pulmonary complications, suggesting that it is important to properly allocate resources necessary for the care of patients with COVID-19 infection based on their BMI.
Acknowledgements
The Northwell Health COVID-19 Research Consortium: Sara Abrahams, BS; Stuart L. Cohen, MD; Bruce Hirsch, MD; Zachery Kozel, MD; Charlotte Kvasnovsky, MD, PhD, MPH; Douglas Lambert, MD; Lyndonna Marrast, MD, MPH; Johanna Martinez, MD, MS; Ernesto P. Molmenti, MD, PhD, MBA, FACS; Safiya Richardson, MD, MPH; Daniel Zarif, MD.
Funding:
This work was supported by grants R24AG064191 from the National Institute on Aging of the National Institutes of Health and R01LM012836 from the National Library of Medicine of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this paper are those of the authors and do not represent the views of the National Institutes of Health, the United States Department of Health and Human Services, or any other government entity.
Footnotes
Data Sharing Statement
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.
Disclosure:
The authors declared no conflicts of interest.
References
- 1.Prevention CfDCa. Cases in the U.S. . Cases, Data and 2020; https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 10, 2020, 2020. [Google Scholar]
- 2.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid-19 in New York City. New England Journal of Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassir R Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020:2020.2004.2008.20057794. [Google Scholar]
- 6.Petrakis D, Margină D, Tsarouhas K, et al. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol Med Rep. 2020;22(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond). 2020;44(8):1790–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res. 2011;128(6):518–523. [DOI] [PubMed] [Google Scholar]
- 9.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geier MR, Geier DA. Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Medical Hypotheses. 2020;140:109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International. 2020;97(5):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert PL, Taylor LT, Wang JJ, Bergman MA. Methods for using data abstracted from medical charts to impute longitudinal missing data in a clinical trial. Value Health. 2011;14(8):1085–1091. [DOI] [PubMed] [Google Scholar]
- 14.Hajifathalian K, Kumar S, Newberry C, et al. Obesity is associated with worse outcomes in COVID-19: Analysis of Early Data From New York City. Obesity. 2020;n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon JB. The effect of obesity on health outcomes. Molecular and Cellular Endocrinology. 2010;316(2):104–108. [DOI] [PubMed] [Google Scholar]
- 17.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringbäck Weitoft G, Eliasson M, Rosén M. Underweight, overweight and obesity as risk factors for mortality and hospitalization. Scand J Public Health. 2008;36(2):169–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


