Abstract
This study aimed to investigate the effect of acute psychological stress on autonomic function and arterial stiffness, and to test a mediating role of changes in autonomic function between acute stress and arterial stiffness. Eighty-five healthy female adults were randomized into either an experimental or control group. The Trier Social Stress Test (TSST) was used to induce acute psychological stress. Autonomic function (measured by pre-ejection period [PEP] from cardiac impedance and high frequency [HF] of heart rate variability [HRV]) and arterial stiffness (measured by carotid and femoral pulse wave velocity [cfPWV] and augmentation index [AIx]) were assessed before and after the TSST. The mean age of the participants was 28.78 (±9.84) years old. Experimental group participants had a significant increase in cfPWV (p = .025) and AIx (p = .017) following the stressor, compared with those in the control group, after controlling for age, body mass index, and systolic blood pressure. However, no significant group differences were observed in changes in PEP (p = .181) and HF (p = .058). Changes in PEP and HF were neither associated with changes in cfPWV (p = .975 and p = .654, respectively), nor in AIx (p = .376 and p = .323, respectively). The results suggest that even a brief period of mild to moderate stress, which does not cause sustainable changes in autonomic function, may still exert significant adverse effects on arterial stiffness. The changes in arterial stiffness were not related to changes in autonomic function. Future experimental studies with several measurement points are recommended to identify distinct effects of stress on autonomic function and arterial stiffness.
Keywords: Psychological stress, Autonomic function, Arterial stiffness, The Trier Social Stress Test, Pulse wave velocity
1. Introduction
Hypertension is a well-known risk factor of cardiovascular morbidity and mortality that affects approximately 73 million adults (33.6%) in the United States. Despite the high prevalence of this disease, the causes of most cases of hypertension are unknown (Rosamond et al., 2008; Stergiou et al., 2004). Previous investigators have suggested that increases in blood pressure (BP) in response to chronic psychological stress contribute to the development of hypertension (DiBona and Jones, 1995; Lucini et al., 2005). The mechanisms by which chronic stress increases cardiovascular disease (CVD) risk include its effect on the sympathetic nervous system (SNS), rennin-angiotensin system, inflammation, cortisol levels, and unhealthy behaviors (Ghiadoni et al., 2000; Rozanski et al., 1999; Rozanski et al., 2005; Steptoe et al., 1982). Over time, elevated BP can lead to vascular remodeling, hypertrophy, and hyperplasia that induce intrinsic arterial stiffening, accelerating hypertension (Franklin, 2005; Risler et al., 2005).
Arterial stiffness is considered a hallmark of vascular aging (Laurent et al., 2016), which is an important predictor of CVD. While hypertension leads to arterial stiffness, population-based longitudinal studies have demonstrated that arterial stiffness can predict progression to hypertension among non-hypertensive persons (Dernellis and Panaretou, 2005; Liao et al., 1999). One explanation is that ventricular contraction generates a pulse pressure wave that propagates along the arterial tree (incident wave) and is reflected backward (reflected wave) at impedance mismatch sites. When the cushioning capacity of arteries is normal, the reflected wave appears during the diastolic period; it enhances the capacity for flow through the coronary system (O’Rourke, 1967). However, when the arterial wall loses its elastic properties and becomes stiff, the incident wave returns faster and the reflected wave appears as a secondary wave in the late systole period. This increases systolic BP and left ventricular load (Chung et al., 2012; Gosse et al., 2010). For this reason, it is important to identify risk factors contributing to arterial stiffness and to implement targeted interventions.
Previous studies have identified chronic psychological stress as a significant predictor of arterial stiffness in non-hypertensive people, independent of age and BP (Logan et al., 2012). Still, whether and how acute psychological stress might cause arterial stiffness remains unknown. By acute stress, we mean stress resulting from specific events or situations (not repeated, longstanding exposure to situations) that are usually unpredictable. The autonomic nervous system is one of the major pathways activated during stressful situations (Won and Kim, 2016). Autonomic function, defined as sympathetic and parasympathetic function (reflecting a dynamic state), has been extensively assessed to evaluate acute stress response (Pomeranz et al., 1985). Autonomic dysregulation, characterized by increased sympathetic activity and decreased parasympathetic tone (Licht et al., 2013), reflects a relatively stable trait-like individual difference that has been observed in people with hypertension (Erdem et al., 2013; Grassi, 2009; Palatini and Julius, 2009), particularly in those with high levels of psychological stress and/or newly diagnosed patients with hypertension (Pavithran et al., 2008; Shehab and Abdulle, 2011; Singh et al., 1998). Autonomic dysregulation may play a significant role in connecting stress with arterial stiffness. This study investigated the effect of acute psychological stress on autonomic function and arterial stiffness, and tested the role of changes in autonomic function as a mediator between acute stress and arterial stiffness. We hypothesized that changes in autonomic function would account for the effect of psychological stress on arterial stiffness.
2. Method
2.1. Participants
After Institutional Review Board (IRB) approval, 85 healthy female adults aged 18 years or above were recruited from Charlottesville, Virginia, using snowball sampling with the study flyers posted on public sites. Men and women tend to react to stress differently, possibly due to effects of gonadal steroids on hypothalamic-pituitary adrenocortical (HPA) axis arousal (Verma et al., 2011). Thus, to reduce heterogeneity of the sample, only women were included in this study. Given that arterial stiffness and autonomic dysregulation increase with age and CVD risk changes after menopause, women who were over 55 years old or had undergone menopause were excluded. Further, women who are taking any medications (e.g., anxiolytics and antipsychotics) were excluded given those medication may affect an individual’s experience of stress. Other exclusion criteria included having CVD that may increase arterial stiffness (e.g., hypertension, hyperlipidemia, or diabetes mellitus) and having an irregular heart rhythm that would prohibit measuring heart rate variability and arterial stiffness. Hypertension was screened by BP measure; hyperlipidemia and diabetes by fasting lipid profile and glucose level measured using Alere Cholestech LDX® system (Abbott, IL USA); and irregular heart rhythm by reviewing pulse wave forms for 10 s using the SphygmoCor system (AtCor medical, IL USA). These exclusion criteria were included to reduce the likelihood that any observed stress reactivity would be confounded by the participants’ cardiovascular condition.
2.2. Experiment
The Trier Social Stress Test (TSST) is a standardized protocol used in laboratory settings to induce acute psychological stress (Birkett, 2011) and is considered a reliable tool to study biological responses to stress (Het et al., 2009). According to a meta-analysis of 208 laboratory studies, perceived uncontrollability and fears of negative social evaluation, which are key components to the TSST, are central to psychological stress and activate biological stress reactivity, such as activation of the HPA axis and sympathetic-adrenal medullary (SAM) axis (Dickerson and Kemeny, 2004). While many emotion inductions have failed to cause HPA activation, the TSST, which requires speech performance and verbal arithmetic performance in front of an audience, is reported to induce considerable changes in corticotropin (Kudielka et al., 2004), cortisol (Brody et al., 2002; Juster et al., 2012), BP (Brody et al., 2002), and heart rate (Polheber and Matchock, 2014).
2.3. Measures
2.3.1. Acute psychological stress
Acute psychological stress was operationalized as peak subjective distress during the stress induction and self-reported state anxiety.
Peak subjective distress during the stress induction was measured by the Subjective Units of Distress Scale (SUDS). The SUDS is a simple, widely-used one-item scale to measure the subjective intensity of distress experienced by an individual (Tanner, 2012). The question asked pre TSST was: “On a scale of 0 to 10, where 0 is ‘not distressed at all’ and 10 is ‘the most distressed, what is your distress level now?” The question asked post TSST was: “On a scale of 0 to 10, where 0 is ‘not distressed at all’ and 10 is ‘the most distressed’, what was your highest level of distress during the test?” The score ranges from 0 (no distress) to 10 (maximum distress).
State anxiety was measured by the state anxiety subscale of the Spielberger Sate-Trait Anxiety Inventory (STAI; Spielberger et al., 1970). This 20 item Likert-type scale contains response options ranging from 0 (“never”) to 4 (“very often”) to assess the intensity of an individual’s momentary feelings tied to “how one feels right now, that is, at this moment.” The total score is derived from the sum of the items with higher scores indicating greater anxiety. This scale has been used extensively in practice, and has strong construct and divergent validity, and internal consistency in large samples (Hammadah et al., 2017; Speilberger and Vagg, 1984). In the current study, the internal consistency of the baseline state anxiety scale was excellent (Cronbach’s α = 0.90).
2.3.2. Arterial stiffness
Arterial stiffness was measured by carotid and femoral pulse wave velocity and augmentation index.
Carotid and femoral pulse wave velocity (cfPWV) was obtained noninvasively using the commercially available SphygmoCor system (AtCor medical, IL USA). Each carotid and femoral waveform was acquired by applying a pressure sensitive transducer (tonometer) on carotid and femoral sites. The transit time of the pulse from the left ventricle to the carotid artery (t1) and the transit time of the pulse from the left ventricle to the femoral artery (t2) were calculated by the system software on the basis of ECG (electrocardiogram), using the foot-to-foot method. The distance from the suprasternal notch to the carotid artery site (d-carotid) and the distance from the suprasternal notch to the femoral artery site (d-femoral) were measured on the body using a tape measure. CfPWV(∆L/∆t) (m/s) was the difference in the distances from suprasternal notch to two arterial sites (∆L = d-femoral – d-carotid) divided by the mean time difference between t1 and t2 (∆t = t2−t1). A higher cfPWV denotes stiffer arteries. In a prior study, reference values of cfPWV for 1455 people aged 15 to 59 years with normal blood pressure ranged from 6.2 to 8.3 m/s (Mattace-Raso et al., 2010).
Augmentation index (AIx) was obtained from pulse wave analysis (PWA). PWA was performed by the SphygmoCor system, by applying a tonometer on the radial arterial site. AIx indicates the size of the increase in the pulse height as a result of the reflected wave. If the reflected peak is greater than the primary peak, AIx is positive; if the reflected peak is less than the primary peak, AIx is negative. Given AIx can be affected by heart rate (higher heart rate causes increased AIx), this parameter was adjusted to heart rate of 75 beats per minutes by the SphygmoCor system.
2.3.3. Autonomic function
Autonomic function was operationalized by sympathetic function and parasympathetic function.
Sympathetic function was assessed by measuring pre-ejection period (PEP) in impedance cardiography. PEP reflects cardiac contractility, which is primarily controlled by the beta-adrenergic system (Newlin and Levenson, 1979). While the ECG was being recorded (ECG data were used for heart rate variability), impedance cardiography wave form (dZ/dt) was derived from a 5-minute raw impedance cardiogram (Z) by the MP36 system (Biopac System, Inc. CA USA). PEP is the time in milliseconds between the ECG Q wave onset and the B point on the dZ/dt waveform (which corresponds to the interval from the onset of left ventricular depolarization to the opening of the aortic valve); shorter PEP indicates more sympathetic activity. PEP was analyzed by the AcqKnowledge 4.4.1. software (Biopac System, Inc. CA USA).
Parasympathetic function was assessed by measuring high frequency of heart rate variability (HRV). HRV is a noninvasive measure of the variation in beat-to-beat intervals. When investigating short-term HRV, a 5-minute segment is preferred to standardize different studies (Malliani, 2005). RR-intervals were generated, and high frequency (HF) power in normalized unit was calculated and averaged by the AcqKnowledge 4.4.1. software (Biopac System, Inc. CA USA).
2.3.4. Contextual factors
The contextual factors included age, race, per capita income, education, body mass index (BMI), blood pressure (BP), and heart rate (HR).
Age, race, per capita income, education, body mass index, were self-reported by the participants.
To calculate BMI, height (m) was measured by a wall stadimeter (Accu-Hite, USA), and weight (kg) was measured with an electronic scale (Penn Scale, USA). These two measures were used to determine BMI in kilogram per square meter (kg/m2).
BP and HR were measured using the WelchAllyn Vital Signs Monitor 300 Series. The mid-section circumference of the dominant upper arm was measured with a measuring tape, and the proper sized cuff was selected according to the upper arm circumference. The artery marker on the cuff was placed over the brachial artery and the cuff was applied snuggly, allowing no more than two fingers underneath (Maxwell et al., 1982). While the arm was kept at the level of the heart without movement, BP was measured twice on the nondominant arm.
2.4. Procedures
The study procedures complied with the European Society of Cardiology’s recommendations to standardize participant conditions for cardiovascular measures (De Backer et al., 2003; S. Laurent et al., 2006; Malik et al., 1996). All participants were asked to refrain from vigorous exercise and consuming coffee, tea, bananas, chocolate, cocoa, citrus fruits, and vanilla for one day before data collection, because vigorous exercise and consumption of those food items may change cardiovascular hemodynamics. The participants had the same breakfast containing cereal (35 g), milk (8 oz), and orange juice (8 oz) at 8 am followed by fasting until data collection was completed in the afternoon. The study assessments took place in a quiet room between 1 pm and 3 pm.
2.4.1. Pre-test data collection
Participants were asked to complete the SUDS and the state anxiety subscale to obtain the baseline measures of stress. Participants were then asked to change into a hospital gown and have their height and weight measured. This was followed by resting for 10 min in the supine position and assessments of BP and HR. To measure pre-ejection period (PEP) and heart rate variability (HRV), 7 electrodes were placed on participants’ chest and back, followed by 5-min recording of ECG and impedance cardiography. CfPWV were obtained from carotid and femoral arterial sites. Next, pressure wave was obtained from radial arterial site, and AIx was derived from pulse wave analysis. The assessments of cfPWV and AIx were performed by a researcher who had extensive training in using the SphygmoCor device. During physiological data measurements, participants were instructed not to speak. Next, the participants were randomized to either the intervention group or the control group.
2.4.2. Intervention
Individuals who were assigned to the intervention group were led to an intervention room and were given instructions on how to complete the upcoming TSST tasks. Each individual had 10 min to prepare for a 5-minute job interview speech in which they were asked to convince two interviewers that they were a strong candidate for a position. The participants were informed that their performances would be video-taped and that their body language would be evaluated by the interviewers. The participants stood in front of a camera and the two interviewers, who were research members dressed in lab coats, representing the selection committee. The timer was set for 5 min. During the performance, the interviewers remained as impassive as possible and maintained eye contact with participants, periodically taking notes. If participants stopped early, they were asked to continue. If the participants repeatedly looked at the interviewers or stopped talking for more than 10 s, they were told that “you have X minutes left but you can let me know if you wish to stop”. Immediately after the speech, each participant was asked to complete the math portion of the test, which involved sequentially subtracting the number 13 from 1022. A digital timer was set for 5 min. They needed to start over from the beginning every time they made a mistake. Those who were in the control group spent about 20 min quietly sitting in the same intervention room, instead of undergoing the TSST.
2.4.3. Post-test data collection
Immediately after the intervention, participants in both groups were asked to complete the SUDS. After taking 5 min of rest in a supine position, BP, HR, PEP, HRV, cfPWV, and AIx were measured in the same manner as in the pretest. Finally, participants were asked to complete the state anxiety subscale before being fully debriefed and compensated for their participation.
2.5. Data analysis
All analyses were performed with SPSS (version 24 for Windows). Differences by condition in characteristics of the study participants were assessed by independent samples t-tests. The effects of acute stress on the outcome variables after controlling for age, BMI, and systolic BP were tested by using a repeated measures analysis of covariance (RM-ANCOVA) with one between-subjects factor (intervention and control) and one within-subject factor (pre-test and post-test). The mediation analysis was no longer appropriate given the lack of changes on the proposed mediator indicators, PEP and HRV. Finally, relationships among the changes in PEP, HF, cfPWV, and AIx were examined with Pearson’s correlations. Statistical significance was inferred at a 2-sided probability value less than 0.05. The sample of 85 subjects provided 80% power to observe group differences based on a medium effect size (Partial η2 = 0.06 for the effect of TSST on cfPWV).
3. Results
3.1. Characteristics of participants
Table 1 displays the characteristics of the study participants. Of the 85 female adults participating in this study, 44 individuals were randomly assigned to the experimental group and 41 to the control group. The sample reported their race as: White (n = 23), Asian (n = 39), and Black (n = 23). The participants’ age ranged between 22 and 57 years, with a mean of 28.78 (±9.84) years. Most of the sample was not obese, had low income, and had completed at least an associate degree. There were no significant differences in these characteristics between the two groups (all p > .05).
Table 1.
Characteristics of the study participants.
| All | Experimental group | Control group | p-Value | |
|---|---|---|---|---|
| N | 85 | 44 | 41 | |
| Age (year) | 28.78 ± 9.84 | 27.16 ± 8.38 | 30.51 ± 11.04 | 0.121 |
| Race | 0.366 | |||
| White | 23 (27.1%) | 15 (65.2%) | 8 (34.8%) | |
| Asian | 39 (45.9%) | 20 (51.3%) | 19 (48.7%) | |
| Black | 23 (27.1%) | 9 (39.1%) | 14 (60.9%) | |
| Body mass index | 24.54 ± 6.59 | 23.48 ± 4.10 | 25.68 ± 8.41 | 0.134 |
| Per Capita Income | 21,483 ± 15,197.00 | 22,370.56 ± 17,469.47 | 20,494.02 ± 12,363.47 | 0.599 |
| Education | 0.127 | |||
| High school graduate | 12 (14.1%) | 4 (33.3%) | 8 (66.7%) | |
| Associate degree | 31 (36.5%) | 21 (67.7%) | 10 (32.3%) | |
| College degree | 28 (32.9%) | 12 (42.9%) | 16 (57.1%) | |
| Graduate degree | 14 (16.5%) | 7 (50%) | 7 (50%) |
3.2. Effects of the TSST on psychological stress and physiological measures
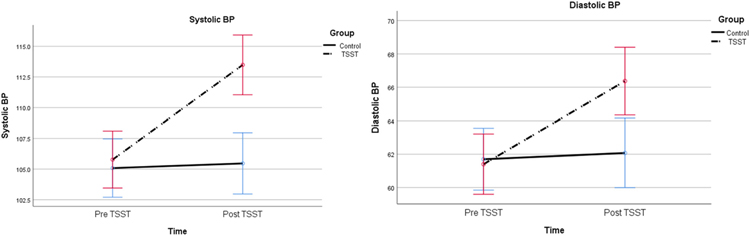
Table 2 presents the results of the RM-ANCOVA. There were significant increases in the mean scores of SUDS (F1, 67 = 70.35, p = .000) and STAI (F1, 79 = 17.54, p = .000), systolic & diastolic BP (F1, 80 = 34.09, p = .000 & F1, 80 = 17.41, p = .000), HR (F1, 79 = 6.01, p = .016), cfPWV (F1, 78 = 5.23, p = .025), and AIx (F1, 74 = 5.95, p = .017) among the participants who completed the TSST, compared with those who did not, after controlling for age, BMI, and systolic BP. However, no significant changes were observed for PEP (F1, 79 = 1.82, p = .181) and HF (F1, 79 = 0.31, p = .579) between the two groups. Changes in PEP and HF were not reliably associated with changes in cfPWV (p = .975 and p = .654, respectively) or AIx (p = .376 and p = .323, respectively). The changes in BP, HR, PEP, HF, cfPWV, and AIx are displayed in Figs. 1–4.
Table 2.
Effects of the TSST on psychological stress and physiological measures after controlling for Age, BMI, and Systolic BPa.
| Mean (SD) |
p-Value for repeated ANCOVA | ||||
|---|---|---|---|---|---|
| Pre-test |
Post-test |
||||
| Exp. | Control | Exp. | Control | ||
| Acute psychological stress | |||||
| SUDS | 1.66 ± 1.77 | 2.56 ± 2.12 | 4.87 ± 2.08 | 1.67 ± 1.63 | 0.000* |
| STAI | 31.00 ± 7.59 | 32.59 ± 8.91 | 35.81 ± 10.78 | 30.02 ± 8.92 | 0.000* |
| Blood pressurea | |||||
| Systolic BP | 104.91 ± 7.63 | 106.00 ± 9.48 | 112.49 ± 8.78 | 106.51 ± 9.12 | 0.000* |
| Diastolic BP | 60.65 ± 5.36 | 62.49 ± 7.80 | 65.72 ± 6.28 | 62.76 ± 7.84 | 0.000* |
| Heart rate | |||||
| Heart Rate | 60.33 ± 8.65 | 60.93 ± 8.89 | 63.12 ± 9.33 | 60.51 ± 7.22 | 0.016* |
| Autonomic function | |||||
| PEP | 0.092 ± 0.017 | 0.089 ± 0.018 | 0.0930 ± 0.032 | 0.083 ± 0.023 | 0.181* |
| HF | 66.95 ± 16.30 | 65.70 ± 16.86 | 67.11 ± 15.81 | 64.08 ± 18.82 | 0.579* |
| Arterial stiffness | |||||
| cfPWV | 5.68 ± 0.81 | 6.18 ± 1.29 | 6.13 ± 0.90 | 6.32 ± 1.19 | 0.025* |
| AIx | −0.03 ± 13.89 | 6.68 ± 13.56 | 2.11 ± 15.45 | 4.47 ± 14.22 | 0.017* |
TSST = Trier social stress test.
BMI = body mass index.
BP = blood pressure.
Exp. = experimental group.
SUDS = Subjective units of distress scale.
STAI = Spielberger Sate-Trait Anxiety Inventory – state anxiety subscale.
PEP = pre-ejection period.
HF = high frequency power in normalized unit.
cfPWV = carotid and femoral pulse wave velocity.
AIx = augmentation index.
Controlled for age and BMI.
Significant at α level < .05 of repeated measures analysis of covariance.
Fig. 1.
The effect of the acute psychological stress on systolic and diastolic BP after controlling for age and BMI (p = .000 and p = .000, respectively).
Fig. 4.
The effect of the acute psychological stress on arterial stiffness after controlling for age, BMI, and systolic BP (p = .025 and p = .017, respectively). TSST = Trier social stress test; BMI = body max index; BP = blood pressure; * = estimated marginal means; Error bars = +/− standard error.
4. Discussion
In this study, acute stress was strongly associated with a significant increase in arterial stiffness, independent of common cardiovascular risk factors including age, BMI, and systolic BP. The authors hypothesized that stress would cause arterial stiffness through its effect on increasing sympathetic function and decreasing parasympathetic function. Unexpectedly, no changes in autonomic function were recorded during the time of assessment after the stress induction, and the changes in arterial stiffness were not related to the changes in autonomic function.
Our results on the effect of acute psychological stress on arterial stiffness are consistent with the results of a previous study, in which 19 healthy individuals between 23 and 32 years of age demonstrated a sustained increase of cfPWV and AIx up to 1 h after a mental arithmetic test (Vlachopoulos et al., 2006). That study was unique in terms of measuring arterial stiffness using cfPWV, a gold standard measure of arterial stiffness. Although two studies reported a nonsignificant association between acute stress and arterial stiffness (Liu et al., 2009; Tabara et al., 2008), several other studies using different measures of arterial stiffness, such as AIx, carotid stiffness, and brachial-ankle PWV (baPWV) did find that mental stress and cold pain stress increased arterial stiffness (Johnson et al., 2013; Kalfon et al., 2015; Lipman et al., 2002). While previous studies were conducted with much smaller sample sizes (fewer than 30), Hammadah and colleagues reported increased cfPWV after mental stress in 660 patients with stable coronary arterial disease (Hammadah et al., 2017). Despite the great interest in vascular response to psychological stress, none of these studies has evaluated the simultaneous changes in autonomic function and arterial stiffness.
It is well-established that psychological stress stimulates SNS and causes catecholamine release. The activity of SNS constricts the vascular smooth muscle of the arteries by activating α1-adrenergic receptors, resulting in increased BP (William Tank and Lee Wong, 2014). There is evidence that norepinephrine increases AIx and pulse pressure, which are surrogate measures of arterial stiffness (Vallée et al., 2017; Wilkinson et al., 2001). However, it is not clear whether SNS activity and norepinephrine release directly cause stiffness of central arteries. To accommodate high pressure from ventricular ejection, central arteries have an abundance of elastic tissue and less quantity of smooth muscle compared with peripheral arteries (Tucker and Mahajan, 2019). The clinical importance of elastic central arteries in buffering high ejection pressure is well documented in epidemiological studies, showing that cfPWV (rather than other measures of arterial stiffness in peripheral arteries) has the most significant prognostic value of cardiovascular morbidity and mortality. Although a few studies have reported the association between SNS activity and central arterial stiffness in healthy humans (Nakao et al., 2004; Swierblewska et al., 2010), more studies have demonstrated nonsignificant associations (Ahn and Kong, 2011; Yeragani et al., 2006). For example, one study reported that plasma levels of catecholamines had no significant correlation with aortic compliance measured by PWV (Potocka-Plazak et al., 1998). Another recent study that examined the effect of autonomic function on arterial stiffness reported that in young healthy participants, the autonomic nervous system does not have a pressure-independent role in the regulation of aortic stiffness (Mäki-Petäjä et al., 2016). Consistent with these negative results, our study demonstrated no relationship between the changes in autonomic function and arterial stiffness.
Interestingly, after stress induction, sympathetic function slightly decreased (i.e., PEP increased) and parasympathetic function increased (i.e., HF of HRV increased). On the other hand, PEP and HF of HRV decreased in the control group. Although these changes were not statistically significant, these apparent discrepancies from the norm in autonomic responses to psychological stress may be related to the time point of measuring autonomic function. Before remeasuring the physiological factors after stress induction, all participants were asked to rest for 5 min in a supine position. The decreased sympathetic function and increased parasympathetic function observed in the experimental group may reflect physiological compensation of autonomic function in healthy individuals during this rest period. In addition, the mild intensity of induced stress and the relatively young participants (mean age less than 30 years) might also explain the unexpected findings of autonomic function in this study. The SUDS, our overall stress indicator, has a score range from 1 to 10. Given the mean score among our intervention group was 4.9, the stress induced by the intervention was, perhaps, not extremely intense. The mild to moderate levels of stress induced in this study are also indicated by the slightly, but significantly, increased BP and HR observed in the experimental group. Young adults may be more resilient to stress and less likely to present a sustainable change in autonomic function when undergoing mild to moderate stress. While the participants in the experimental group went through the stress and rest periods, the participants in the control group spent the entire time not being engaged in any activity. The researcher observed that they became somewhat bored and irritable about the monotonous study process. This may have contributed to the unexpected results for the control group.
The average increase of cfPWV was 0.45 m/s in the experimental group. Given that the level of cfPWV increases 0.1 m/s/year (Cecelja and Chowienczyk, 2012), our study suggests that a brief period of mild to moderate stress may exert significant adverse changes in arterial stiffness. Plausible mechanisms by which psychological stress increases arterial stiffness could be via endothelin-1. Endothelin-1 induces endothelial dysfunction and directly regulates cfPWV (Iglarz and Clozel, 2007; McEniery et al., 2003). Stress evokes the HPA axis as well as SNS activity. In line with previous studies, corticotropin-releasing hormone (CRH), which is the major hormone of the HPA axis, significantly released endothelin-1, and the release of endothelin-1 was abolished by the CRH-receptor antagonist astressin (Wilbert-Lampen et al., 2006). Cortisol is also known to induce endotheline-1 (Kanse et al., 1991). In support of the role of cortisol in endothelial dysfunction and endethelin-1, there is evidence that endothelial dysfunction induced by stress was prevented by blocking cortisol production with metyrapone (Broadley et al., 2005). Another study has demonstrated that endothelial dysfunction induced by stress was prevented by selective endothelin-A receptor (Endothelin 1 selective) antagonism (Spieker et al., 2002). Interestingly, their study showed that intraarterial infusion of norepinephrine did not inhibit endothelial function. Future experimental studies to compare the relative contributions of HPA axis and SNS activity to stress-induced arterial stiffness may provide insights to developing a therapeutic strategy to mitigate the adverse effect of stress on increased arterial stiffness.
Several major limitations should be noted in this study. First, stress responses were not measured during the TSST and were measured only one time after the participations lay supine for 5 min after the stress induction; thus, instantaneous effects of acute stress on autonomic function and arterial stiffness were not examined. A study design that measures stress responses during the TSST and has multiple points of measurements afterward will help increase understanding of stress responses. Second, the TSST conducted in this study induced only mild to moderate levels of stress based on the subjective measures of SUDS and state anxiety. People may exhibit different trajectories of autonomic function and arterial stiffness in response to higher levels of acute psychological stress, Third, the study included relatively young and healthy female adults recruited from one city, limiting the generalizability of the study results. People with clinical conditions as well as men and older adults may have different stress responses. Replicating the findings in larger and more representative samples will be important. Fourth, the participants in the control group may have been bored or annoyed about not being engaged in any activity over the entire study period, potentially affecting the results. Future studies that include an alternative activity for the control group may help prevent unexpected adverse responses of autonomic function in the control group. Last, we did not obtain information about history of stress among the participants. Acute stress reactivity might be confounded by individuals’ exposure to chronic stress in the past. The study also has several strengths, including evaluation of the causal effect of acute psychological stress by using a randomized controlled design, standardizing the physiological condition of participants through the control of diet and physical activity before the study participation, assessing stiffness of central arteries using cfPWV, and recruiting participants with no known CVD to reduce the likelihood of confounding effects of pre-existing conditions.
In conclusion, to our knowledge, the literature does not include other randomized controlled studies that examine the effects of acute stress on autonomic function and arterial stiffness simultaneously in healthy female adults. The results suggest that after an acute stress induction, no significant changes in autonomic function were observed, but arterial stiffness measured by cfPWV and AIx significantly increased, independent of age, BMI and systolic BP. Our study demonstrates that acute stress, which was brief and did not cause measurable changes in autonomic function, still had a deteriorating effect on arterial stiffness. The results also show no association between changes in autonomic function and arterial stiffness. This finding suggests that SNS activity may not directly regulate stiffness of central arteries independent of BP. Despite the strong emphasis on the role of arterial stiffness in the development of CVD, underlying mechanisms by which an independent but modifiable factor (such as psychological stress) contributes to arterial stiffness are not fully understood. Experimental studies with concurrent measures of plausible mechanisms at multiple points may elucidate the complex link between psychological stress and arterial stiffness, giving rise to more precise prevention recommendations.
Fig. 2.
The effect of the acute psychological stress on heart rate after controlling for age, BMI, and systolic BP (p = .016).
Fig. 3.
The effect of the acute psychological stress on autonomic function after controlling for age, BMI, and systolic BP (p = .181 and p = .579, respectively). TSST = Trier social stress test; BMI = body max index; BP = blood pressure; * = estimated marginal means; Error bars = +/− standard error.
Acknowledgements
This study was supported by the K23NR016215 grant from the National Institutes of Health/National Institute of Nursing Research.
Footnotes
The authors do not have any credit or conflicts of interest to disclose.
References
- Ahn J-H, Kong M, 2011. The relationship among pulse wave velocity, ankle-brachial pressure index and heart rate variability in adult males. Korean Journal of Family Medicine 32 (7), 406. 10.4082/kjfm.2011.32.7.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett MA, 2011. The Trier Social Stress Test protocol for inducing psychological stress. Journal of Visualized Experiments: JoVE(56). 10.3791/3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley AJM, Korszun A, Abdelaal E, Moskvina V, Jones CJH, Nash GB, Frenneaux MP, 2005. Inhibition of cortisol production with metyrapone prevents mental stress-induced endothelial dysfunction and baroreflex impairment. J. Am. Coll. Cardiol 46 (2), 344–350. 10.1016/j.jacc.2005.03.068. [DOI] [PubMed] [Google Scholar]
- Brody S, Preut R, Schommer K, Schürmeyer TH, 2002. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 159 (3), 319–324. 10.1007/s00213-001-0929-6. [DOI] [PubMed] [Google Scholar]
- Cecelja M, Chowienczyk P, 2012. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis 1 (4), 1–10. 10.1258/cvd.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C-M, Lin Y-S, Chang S-T, Cheng H-W, Yang T-Y, Hsiao J-F, Chu C-M, 2012. Arterial stiffness is the independent factor of left ventricular hypertrophy determined by electrocardiogram. Am J Med Sci 344 (3), 190–193. 10.1097/MAJ.0b013e318242a354. [DOI] [PubMed] [Google Scholar]
- De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, … European Society of Cardiology Committee for Practice Guidelines, 2003. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). European Journal of Cardiovascular Prevention and Rehabilitation: Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology 10 (4), S1–S10. 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- Dernellis J, Panaretou M, 2005. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension 45 (3), 426–431. 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY, 1995. Analysis of renal sympathetic nerve responses to stress. Hypertension (Dallas, Tex.: 1979) 25 (4 Pt 1), 531–538. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/7721394. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull 130 (3), 355–391. 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Erdem A, Uenishi M, Küçükdurmaz Z, Matsumoto K, Kato R, Hara M, Yazıcı M, 2013. Cardiac autonomic function measured by heart rate variability and turbulence in pre-hypertensive subjects. Clin. Exp. Hypertens 35 (2), 102–107. 10.3109/10641963.2012.690475. [DOI] [PubMed] [Google Scholar]
- Franklin SS, 2005. Arterial stiffness and hypertension. Hypertension 45 (3), 349–351. 10.1161/01.HYP.0000157819.31611.87. [DOI] [PubMed] [Google Scholar]
- Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, Deanfield JE, 2000. Mental stress induces transient endothelial dysfunction in humans. Circulation 102 (20), 2473–2478. 10.1161/01.CIR.102.20.2473. [DOI] [PubMed] [Google Scholar]
- Gosse P, Pichot V, Guilhot M, Dauphinot V, Da Costa A, Barthelemy J-C, Roche F, 2010. Relationship of cardiac involvement with arterial stiffness in a general population of 65-year-olds in the PROOF study. J. Hypertens 28 (2), 389–394. 10.1097/HJH.0b013e328333d1a4. [DOI] [PubMed] [Google Scholar]
- Grassi G, 2009. Assessment of sympathetic cardiovascular drive in human hypertension. Hypertension 54 (4), 690–697. 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, … Quyyumi AA, 2017. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT, 2009. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test.’ Psychoneuroendocrinology 34 (7), 1075–1086. 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Clozel M, 2007. Mechanisms of ET-1-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 50 (6), 621–628. 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- Johnson J, Håkansson F, Shahgaldi K, Manouras A, Norman M, Sahlén A, 2013. Impact of tachycardia and sympathetic stimulation by cold pressor test on cardiac diastology and arterial function in elderly females. Am. J. Physiol. Heart Circ. Physiol 304 (7), H1002–H1009. 10.1152/ajpheart.00837.2012. [DOI] [PubMed] [Google Scholar]
- Juster R-P, Perna A, Marin M-F, Sindi S, Lupien SJ, 2012. Timing is everything: anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress 15 (6), 569–577. 10.3109/10253890.2012.661494. [DOI] [PubMed] [Google Scholar]
- Kalfon R, Campbell J, Alvarez-Alvarado S, Figueroa A, 2015. Aortic hemodynamics and arterial stiffness responses to muscle Metaboreflex activation with concurrent Cold Pressor Test. Am. J. Hypertens 28 (11), 1332–1338. 10.1093/ajh/hpv043. [DOI] [PubMed] [Google Scholar]
- Kanse SM, Takahashi K, Warren JB, Ghatei M, Bloom SR, 1991. Glucocorticoids induce endothelin release from vascular smooth muscle cells but not endothelial cells. Eur. J. Pharmacol 199 (1), 99–101. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/1893930. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C, 2004. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 29 (1), 83–98. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/14575731. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, … European Network for Non-invasive Investigation of Large Arteries, 2006. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal 27 (21), 2588–2605. 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Laurent Stéphane, Marais L, Boutouyrie P, 2016. The noninvasive assessment of vascular aging. Can. J. Cardiol 32 (5), 669–679. 10.1016/j.cjca.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G, 1999. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension (Dallas, Tex.: 1979) 34 (2), 201–206. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/10454441. [DOI] [PubMed] [Google Scholar]
- Licht CMM, de Geus EJC, Penninx BWJH, 2013. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism 98 (6), 2484–2493. 10.1210/jc.2012-3104. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Grossman P, Bridges SE, Hamner JW, Taylor JA, 2002. Mental stress response, arterial stiffness, and baroreflex sensitivity in healthy aging. J. Gerontol. A Biol. Sci. Med. Sci 57 (7), B279–B284. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/12084798. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hesse C, Curry TB, Pike TL, Issa A, Bernal M, Eisenach JH, 2009. Ambulatory arterial stiffness index is not correlated with the pressor response to laboratory stressors in normotensive humans. J. Hypertens 27 (4), 763–768. 10.1097/HJH.0b013e328324eb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JG, Barksdale DJ, Carlson J, Carlson BW, Rowsey PJ, 2012. Psychological stress and arterial stiffness in Korean Americans. J. Psychosom. Res 73 (1), 53–58. 10.1016/j.jpsychores.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M, 2005. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension 46 (5), 1201–1206. 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Mäki-Petäjä KM, Barrett SML, Evans SV, Cheriyan J, McEniery CM, Wilkinson IB, 2016. The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension (Dallas, Tex.: 1979) 68 (5), 1290–1297. 10.1161/HYPERTENSIONAHA.116.08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Camm AJ, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, … Singer DH, 1996. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal 17 (3), 354–381. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/8737210. [PubMed] [Google Scholar]
- Malliani A, 2005. Heart rate variability: from bench to bedside. European Journal of Internal Medicine 16, 12–20. 10.1016/j.ejim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FUS, Hofman A, Verwoert GC, Wittemana JCM, Wilkinson I, Cockcroft J, … Dolejsova M, 2010. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values.’ European Heart Journal 31 (19), 2338–2350. 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M, Schroth P, Waks A, Karam M, Dornfeld L, 1982. Error in blood-pressure measurement due to incorrect cuff size in obese patients. Lancet 320 (8288), 33–36. 10.1016/S0140-6736(82)91163-1. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB, 2003. Endothelin-1 regulates arterial pulse wave velocity in vivo. J. Am. Coll. Cardiol 42 (11), 1975–1981. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/14662262. [DOI] [PubMed] [Google Scholar]
- Nakao M, Nomura K, Karita K, Nishikitani M, Yano E, 2004. Relationship between brachialankle pulse wave velocity and heart rate variability in young Japanese men. Hypertension Research: Official Journal of the Japanese Society of Hypertension 27 (12), 925–931. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15894832. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Levenson RW, 1979. Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology 16 (6), 546–553. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/229507. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, 1967. Steady and pulsatile energy losses in the systemic circulation under normal conditions and in simulated arterial disease. Cardiovasc. Res 1 (4), 313–326. 10.1093/cvr/1.4.313. [DOI] [PubMed] [Google Scholar]
- Palatini P, Julius S, 2009. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr. Hypertens. Rep 11 (3), 199–205. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/19442329. [DOI] [PubMed] [Google Scholar]
- Pavithran P, Madanmohan R, Mithun R, Jomal M, Nandeesha H, 2008. Heart rate variability in middle-aged men with new-onset hypertension. Ann. Noninvasive Electrocardiol 13 (3), 242–248. 10.1111/j.1542-474X.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polheber JP, Matchock RL, 2014. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J. Behav. Med 37 (5), 860–867. 10.1007/s10865-013-9546-1. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay JB, Caudill MA, 1985. Assessment of autonomic functions in humans by heart rate spectral analysis. Am. J. Physiol. Heart Circ. Physiol 17 (1). 10.1152/ajpheart.1985.248.1.h151. [DOI] [PubMed] [Google Scholar]
- Potocka-Plazak K, Kolasa R, Poplawski T, Kulczycka J, Plazak W, 1998. Correlation between aortic pulse wave velocity and norepinephrine, epinephrine, aldosterone and plasma renin activity in very elderly subjects and in patients with congestive heart failure. Aging (Milan, Italy) 10 (1), 48–52. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/9589751. [DOI] [PubMed] [Google Scholar]
- Risler NR, Cruzado MC, Miatello RM, 2005. Vascular remodeling in experimental hypertension. Review Article TheScientificWorldJOURNAL 5, 959–971. 10.1100/tsw.2005.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, … Hong Y, 2008. Heart Disease and Stroke Statistics—2008 Update. Circulation 117 (4). 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J, 1999. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99 (16), 2192–2217. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/10217662. [DOI] [PubMed] [Google Scholar]
- Rozanski Alan, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L, 2005. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice. J. Am. Coll. Cardiol 45 (5), 637–651. 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Shehab A, Abdulle A, 2011. Cognitive and autonomic dysfunction measures in normal controls, white coat and borderline hypertension. BMC Cardiovasc. Disord 11, 3. 10.1186/1471-2261-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D, 1998. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension (Dallas, Tex.: 1979) 32 (2), 293–297. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/9719057. [DOI] [PubMed] [Google Scholar]
- Speilberger CD, Vagg PR, 1984. Psychometric properties of the STAI: a reply to Ramanaiah, Franzen, and Schill. J. Pers. Assess 48 (1), 95–97. 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, … Noll G, 2002. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 105 (24), 2817–2820. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/12070106. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, 1970. Manual for the state-trait anxiety inventory Retrieved from. https://ubir.buffalo.edu/xmlui/handle/10477/2895.
- Steptoe A, Melville D, Ross A, 1982. Essential hypertension and psychological functioning: a study of factory workers. Br. J. Clin. Psychol 21 (4), 303–311. 10.1111/j.2044-8260.1982.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Stergiou GS, Salgami EV, World Health Organization-International Society of Hypertension (WHO-ISH), USA Joint National Committee on Prevention, Detection, Evalutiaon, and Treatment of High Blood Pressure (JNC-7), European Soceity of Hypertension-European Society of Cardiology (ESH-ESC), 2004. New European, American and International guidelines for hypertension management: agreement and disagreement. Expert. Rev. Cardiovasc. Ther 2 (3), 359–368. 10.1586/14779072.2.3.359. [DOI] [PubMed] [Google Scholar]
- Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Narkiewicz K, 2010. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J. Hypertens 28 (5), 979–984. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/20408258. [DOI] [PubMed] [Google Scholar]
- Tabara Y, Kohara K, Nakagawa S, Handa J, Hayashi M, Hamada C, Konishi M, 2008. Effects of obesity and smoking on mental stress-induced blood pressure and augmentation index responses in normotensive young males: the J-SHIPP study. Hypertens. Res 31. 10.1291/hypres.31.1219. [DOI] [PubMed] [Google Scholar]
- Tanner BA, 2012. Validity of global physical and emotional SUDS. Applied Psychophysiology and Biofeedback 37 (1), 31–34. 10.1007/s10484-011-9174-x. [DOI] [PubMed] [Google Scholar]
- Tucker WD, Mahajan K, 2019. Anatomy, blood vessels. In: StatPearls, Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/29262226. [PubMed]
- Vallée F, Passouant O, Le Gall A, Joachim J, Mateo J, Mebazaa A, Gayat E, 2017. Norepinephrine reduces arterial compliance less than phenylephrine when treating general anesthesia-induced arterial hypotension. Acta Anaesthesiol. Scand 61 (6), 590–600. 10.1111/aas.12905. [DOI] [PubMed] [Google Scholar]
- Verma R, Balhara YPS, Gupta CS, 2011. Gender differences in stress response: role of developmental and biological determinants. Ind. Psychiatry J 20 (1), 4–10. 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C, 2006. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom. Med 10.1097/01.psy.0000203171.33348.72. [DOI] [PubMed]
- Wilbert-Lampen U, Trapp A, Modrzik M, Fiedler B, Straube F, Plasse A, 2006. Effects of corticotropin-releasing hormone (CRH) on endothelin-1 and NO release, mediated by CRH receptor subtype R2: a potential link between stress and endothelial dysfunction? J. Psychosom. Res 61 (4), 453–460. 10.1016/j.jpsychores.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ, 2001. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J. Physiol 530 (Pt 3), 541–550. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/11158283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William Tank A, Lee Wong D, 2014. Peripheral and central effects of circulating catecholamines. In: Comprehensive Physiology 5. pp. 1–15. 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- Won E, Kim Y-K, 2016. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol 14 (7), 665–673. 10.2174/1570159x14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Tancer M, Seema KP, Josyula K, Desai N, 2006. Increased pulse-wave velocity in patients with anxiety: implications for autonomic dysfunction. J. Psychosom. Res 61 (1), 25–31. 10.1016/j.jpsychores.2005.10.011. [DOI] [PubMed] [Google Scholar]