Graphical abstract
Keywords: Breast cancer, Metastasis, Pre-clinical models, Nanomedicines, Organoids, Patient-derived xenografts, Animal models, Biomarkers
Abbreviations: 2D, Two-dimensional; 3D, Three-dimensional; AGM, Aminoglutethimide; ALOX5, Arachidonate 5-lipoxygenase; ATM, Ataxia-telangiectasia mutated; AuNR, Gold nanorod; BRCA1, Breast cancer type 1 susceptibility protein; BRCA2, Breast cancer type 2 susceptibility protein; CAF, Cancer-associated fibroblasts; CCPM, Core-crosslinked block copolymer micelle; CDX, Cell-derived xenograft; CHEK2, Checkpoint kinase 2; CSC, Cancer stem cells; DC, Dendritic cells; DMBA, 7,12-dimethylbenzantracene; DOX, Doxorubicin; ECM, Extracellular matrix; EGFR, Epithelial growth factor receptor; EPR, Enhanced permeability and retention; ER, Estrogen receptor; ERS1, Estrogen receptor gene; FA, Folic acid; FDA, Food and Drug Administration; FITC, Fluorescein isothiocyanate; GEMM, Genetically modified mouse model; GSH, Glutathione; HER2, Epidermal growth factor receptor 2; HIF1α, Hypoxia-inducible factor 1 alpha; HPMA, Hydroxypropyl methacrylamide; ICAM1, Intercellular adhesion molecule–1; IHC, Immunohistochemistry; IO, Iron oxide; IONP, Iron oxide nanoparticle; iPSC, induced pluripotent stem cells; LTR, Long terminal repeat; MDR1, Multidrug resistance protein 1; MEF, Mouse embryonic fibroblasts; MMDOX, Doxorubicin-loaded mixed micelles; MMP, Metallopeptidases; MMTV, Mouse mammary tumor virus; MUC1, Mucin 1; NF1, Neurofibromin 1; NIR, Near-infrared; NK, Natural killer; NMU, N-methyl-n-nitrosourea; NOD-SCID, non-obese diabetic-severe combined immunodeficient; NOG, NOD/Shi-scid/γc−/− null; NP, Nanoparticle; NSG, NOD scid gamma; Nude, Athymic nude; PALB2, Partner and localizer of BRCA2; pDNA, Plasmid desoxyribonucleic acid; PDNA, Plasmid DNA; PDOX, Patient-derived organoid-derived xenograft; PDX, Patient-derived xenograft; PEG, Polyethylene glycol; PGA, Poly-L-glutamic acid; PI3KCA, Phosphatidylinositol 3-kinase; PIMs, Porcine pulmonary intravascular macrophages; PLA, Poly(lactide); PLGA, poly(lactide-co-glycolide); PPTT, Plasmonic photothermal therapy; PR, Progesterone receptor; PTBPC, Poly(2‐((tert‐butoxycarbonyl)amino)‐3‐propyl carbonate; PTEN, Phosphatase and tensin homolog; PyMT, Polyoma middle tumor-antigen; QbD, Quality by design; RAG, Rag-deficient; ROS, Reactive oxygen species; SCID, Severe combined immunodeficient; SPIO, Superparamagnetic iron oxide nanoparticle; SSMM, Sterically-stabilized mixed phospholipid nanomicelle; STK11, Serine/Threonine Kinase 11; TAM, Tumor-associated macrophages; Th1, Type 1 T helper; Th2, Type 2 T helper; TME, Tumor microenvironment; TNBC, Triple negative breast cancer; TP53, tumor protein p53; TPGS, D-α-tocopheryl polyethylene glycol 1000 succinate; uPAR, Urokinase plasminogen activator receptor; VIP, Vasoactive intestinal peptide; WAP, Whey acidic protein; WHO, World Health Organization
Abstract
Even given recent advances in nanomedicine development of breast cancer treatment in recent years and promising results in pre-clinical models, cancer nanomedicines often fail at the clinical trial stage. Limitations of conventional in vitro models include the lack of representation of the stromal population, the absence of a three-dimensional (3D) structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. Herein, we review those cell culture strategies that aim to overcome these limitations, including cell co-cultures, advanced 3D cell cultures, patient-derived cells, bioprinting, and microfluidics systems. The in vivo evaluation of nanomedicines must consider critical parameters that include the enhanced permeability and retention effect, the host's immune status, and the site of tumor implantation. Here, we critically discuss the advantages and limitations of current in vivo models and report how the improved selection and application of breast cancer models can improve the clinical translation of nanomedicines.
1. Introduction
The efficient clinical translation of anticancer nanomedicines requires the development of faithful pre-clinical models that recapitulate tumor characteristics to determine safety and efficacy and, perhaps even more importantly, to identify biomarkers for treatment response [1]. While widely-used conventional two-dimensional (2D) cell models of cancer have contributed tremendously to translational research in the field of nanomedicine, they exhibit significant differences to the real disease and, so, possess limited translational power. The primary deficiencies in 2D cancer cell models include the lack of cellular heterogeneity and the tumor microenvironment (TME), which significantly influences cancer development and treatment resistance [2]. More advanced models that adequately reflect the heterogeneity and complexity of the human disease should foster the development of relevant treatment strategies that will significantly improve patient outcomes.
Importantly, the development of pre-clinically relevant models of tumorigenesis (from localized to metastatic) must consider the evolution of the classic view of solid cancers as isolated masses of tumor cells to our current understanding, which places tumor cells within a TME comprised of stromal components, blood vessels, fibroblasts, and cells of the immune system. Indeed, the critical two-way interaction between the TME and tumor cells represents a crucial consideration when developing novel anticancer therapies [3], and targeting of TME components now represents an important therapeutic strategy. In this scenario, rationally-designed nanomedicines display a range of advantages [4], [5], [6], although we still require adequate in vitro and in vivo pre-clinical cancer models to properly evaluate their complex interactions within the TME.
A range of solid tumors are considered unmet clinical needs; specifically, breast cancer represents the most common form of cancer in women worldwide and suffers from high death rates even given the decades of research and development of small molecule drugs as treatment strategies [7]. Can the rational development of advanced nanomedicine formulations of such drugs provide a means to solve this problem? Treatment with anticancer nanomedicines allows for enhanced drug accumulation in the tumor, which improves direct effects and inhibits indirect effects by minimizing drug interactions in off-target tissue types [8], [9]. Preferential drug accumulation in tumors occurs both by passive mechanisms, due to the enhanced permeability and retention (EPR) effect [10], and active mechanisms, through the use of targeting moieties or the implementation of tumor-specific triggers for cargo delivery [11]. Given the sheer complexity of breast cancer [12], which we now understand to comprise a group of distinct diseases with varying molecular attributes [13], we require a full understanding of disease-specific characteristics to foster the design of effective targeting strategies and the development of novel single and combinatorial drug treatment strategies. Furthermore, this information will allow the development of advanced pre-clinical breast cancer models to accelerate clinical translation.
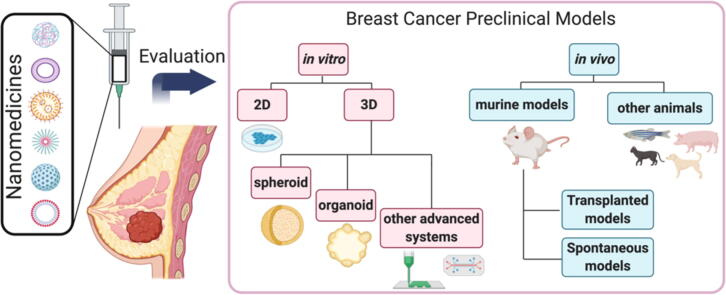
In this review, we hope to carefully consider currently-described models employed for the pre-clinical evaluation of nanomedicines for breast cancer treatment – taking in both in vitro (two- and three-dimensional [3D]) and in vivo models with increasing complexity (Fig. 1). Furthermore, we critically discuss the key characteristics to consider when evaluating a specific breast cancer subtype or a given rationally-designed anticancer nanomedicine at the pre-clinical level and explore both the challenges and opportunities that lie ahead in this area.
Fig. 1.
An overview of pre-clinical breast cancer models, including in vitro 2D conventional culture systems, 3D culture systems, and in vivo models, indicating the related nanomedicine characterization assays afforded by their intrinsic characteristics and complexity.
1.1. Why breast cancer? Breast cancer at a glance: progression and classification
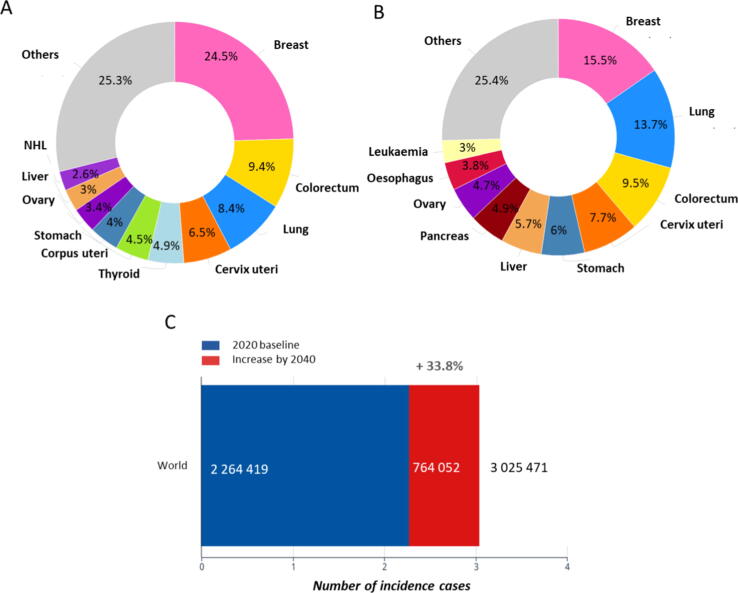
With 15.5% of total cancer cases in 2020, breast cancer currently represents the second most frequent cancer worldwide. In female patients, breast cancer is the most commonly diagnosed cancer type (24.5% of all cancers in 2020) and represents the most common cause of death, followed by lung and colorectal cancer (Fig. 2A and B). Unfortunately, the estimated incidence of cases and the mortality rate will increase worldwide by an estimated 33.8% until the year 2040 (Fig. 2C) [14], [15].
Fig. 2.
Estimated breast cancer incidence and mortality rate worldwide (2020). (A) Estimated number of new cases of different cancers in females in 2020. (B) Estimated number of deaths from various cancer types in females in 2020. (C) The estimated increase in breast cancer cases from 2020 to 2040 [15].
The World Health Organization (WHO) recently classified breast cancer into nineteen major histology subtypes [13]. Ductal carcinoma and lobular carcinoma represent the most frequently diagnosed subtypes with 70–75% and 10–14% of all cases, respectively. The seventeen rarer subtypes include tubular carcinoma, cribriform carcinoma, mucinous carcinoma, pleiomorphic lobular carcinoma, high-grade metaplastic carcinoma, micropapillary carcinoma, and inflammatory breast cancer [13].
All breast cancers arise in the terminal lobular units of the collecting duct, which contains a layer of epithelial and myoepithelial cells separated from the stroma, which itself comprises fibroblasts, myofibroblasts, macrophages, mast cells, neutrophils, and lymphocytes, by the basement membrane. Normal breast cells can become tumorigenic due to gain of function mutations in oncogenes such as PI3KCA (phosphatidylinositol 3-kinase) and HER2 (epidermal growth factor receptor 2), or a loss of function mutations in tumor suppressor genes such as BRCA1 (breast cancer type 1 susceptibility protein), BRCA2 (breast cancer type 2 susceptibility protein), ERS1 (estrogen receptor gene), ATM (ataxia-telangiectasia mutated), TP53 (tumor protein p53), PALB2 (partner and localizer of BRCA2), CHEK2 (Checkpoint kinase 2), PTEN (phosphatase and tensin homolog), STK11 (Serine/Threonine Kinase 11), or NF1 (Neurofibromin 1) [13], [16].
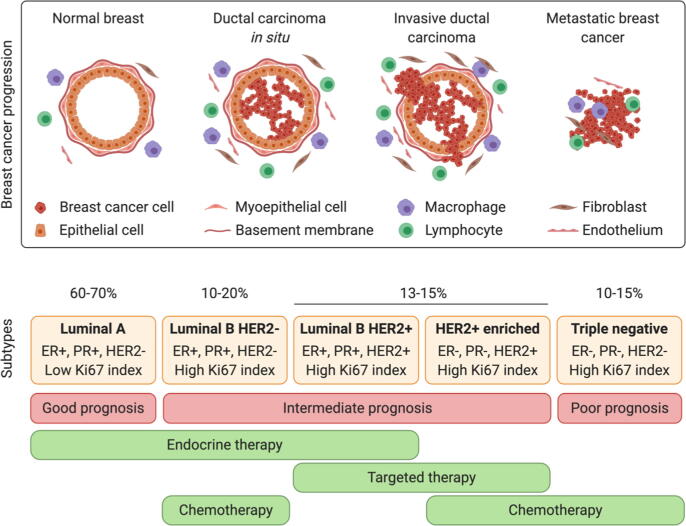
Breast cancer originates from an in-situ carcinoma characterized by epithelial cell proliferation with a complete myoepithelial cell layer and basement membrane, which progresses following myoepithelial cell layer disruption and basement membrane degradation to invasion into neighboring tissues (invasive ductal carcinoma) and metastasis. Breast cancer is considered metastatic when there exists the loss of the myoepithelial cell layer and basement membrane, stromal cell proliferation, angiogenesis, and the invasion of tumorigenic-epithelial cells to distant sites. After passage through the blood or lymphatic system, metastatic breast cancer cells' main target sites include the bones, liver, lungs, and brain (Fig. 3) [17], [18]. As metastatic events represent a significant cause of death in breast cancer patients, early disease detection will foster a better prognosis and reduce mortality [16], [19].
Fig. 3.
Breast cancer progression from the primary tumor to the metastatic stage [17] and breast cancer subtypes, prognosis, and most common current pharmacological treatment options in each case [13].
Breast cancer progression can be divided into five phases or stages (Table 1) by considering tumor location, tumor size, lymph node involvement, and metastatic progression. Together with the status of crucial molecular markers, these stages currently define patient stratification (Fig. 3).
Table 1.
Stages of breast cancer and representative clinical characteristics.
| Stage | Tumor size | Lymph node | Spreading | Survival Rate (5 years) |
|---|---|---|---|---|
| 0 | Small, Inside the glands | Non-affected | No | 100% |
| I | < 2 cm | Non-affected | No | 98% |
| II | 2–5 cm | Affected | No | 87% |
| III | > 5 cm | Affected | No | 61% |
| IV | Any size | Affected | Yes | 20% |
Estrogen receptor (ER), a steroid hormone receptor, is expressed in around 70% of invasive breast cancers and its activation prompts signaling through several oncogenic pathways. Additional important biomarkers include the progesterone receptor (PR), which is also involved in ER signaling, and epidermal growth factor receptor 2 (HER2), which is present in 20% of breast cancers and associates with poor prognosis. The status of hormone receptor expression (combined with the Ki67 proliferation marker) helps to divide breast cancer into five accepted clinical subtypes: Luminal A, HER2- Luminal B, HER2 + Luminal B, HER2-enriched, and triple negative breast cancer (TNBC) (Fig. 3).
Luminal A, the most common subtype (representing 60–70% of all breast cancer cases), is defined by high ER and PR and low HER2 and Ki67 expression. Luminal A tumors are considered low grade due to their slow growth and favorable prognosis due to a heightened response to therapy [20], [21]. The Luminal B subtype is defined by ER and PR expression and elevated Ki67 expression and can be either HER2 + or HER2-. Luminal B tumors are usually diagnosed in young patients and suffer from a less favorable prognosis than the Luminal A subtype due to accelerated tumor growth [13], [20], [21]. The HER2-enriched subtype is mainly characterized by high expression of HER2 and the absence of ER and PR expression (70%). HER2-enriched breast cancers display more rapid tumor growth, more aggressive development, and suffer from a poorer prognosis compared to the Luminal A and B subtypes [13], [20], [21]. The TNBC subtype represents about 10–15% of all breast cancer cases and is frequently present in young women (<40 years of age) with mutations in the BRCA1 gene. TNBC tumors, which are characterized by the lack of ER, PR, and HER2 expression and high expression of Ki67, frequently possess a more aggressive proliferative behavior, with the least favorable prognosis of the breast cancer subtypes. TNBC tumors also display lymphoplasmacytic inflammatory infiltration with visceral metastasis in the lungs, liver, and brain at later stages [20], [21], [22]. Notably, the lack of hormone receptor expression, which limits the effectiveness of conventional chemotherapeutic regimens, has hampered the development of effective anticancer therapeutics for TNBC.
1.2. Current therapeutic approaches for breast cancer
Patients diagnosed with non-metastatic breast cancer generally receive multimodal treatments that include surgical resection (in some cases a prior neo-adjuvance with chemotherapy), postoperative radiotherapy, and drug/chemotherapy, while chemotherapy represents the primary strategy for metastatic breast cancer patients. Immunotherapy is starting to gain importance also in a type of tumor considered immunologically “cold,” as suggest by some preclinical and recent clinical studies [13], [21], in particular with nanomedicines such as nab-paclitaxel (ClinicalTrials.gov Identifier: NCT02425891). The breast cancer subtype dictates the specific chemotherapeutic regimen employed, with the final aim to suppress cancer cell proliferation and diminish metastatic progression [21]. Tumors that express hormone receptors are treated with standard endocrine therapy, including oral antiestrogens such as tamoxifen and aromatase inhibitors such as exemestane, anastrozole, or letrozole. In some instances, patients with early-stage disease also receive chemotherapy to avoid recurrence, with adriamycin, docetaxel/cyclophosphamide, or cyclophosphamide/methotrexate/5-fluorouracil the main treatment options. In TNBC patients, chemotherapy currently represents the only US Food and Drug Administration (FDA)-approved therapeutic regime. Patients diagnosed with the HER2-enriched subtypes have been treated with targeted therapies that include the HER2-targeting trastuzumab (also known as Herceptin) or pertuzumab monoclonal antibodies (alone or in combination) with or without chemotherapy (Fig. 3) [21].
Unfortunately, many traditional therapies suffer from limitations that diminish their efficacy, including problems related to low solubility, inefficient tumor targeting, side toxicities in healthy tissues, and the development of drug resistance [23]. Advanced drug delivery technologies such as nanomedicines can overcome many of these limitations and improve breast cancer treatment. Importantly, nanomedicine formulations of traditional chemotherapeutics, including doxorubicin (Doxil®) and paclitaxel (Abraxane®), now represent routine first-line treatments for breast cancer [24], [25], [26], thereby providing the impetus for this treatment approach.
1.3. Currently used nanomedicine in breast cancer treatment
Liposomes, protein nanoparticles, polymeric nanoparticles, and immunoconjugates represent chemotherapeutic-bearing nanomedicines that have been FDA-approved for breast cancer treatment. All possess a clinically-demonstrated ability to reduce toxicity and improve efficacy compared to treatment with the parent drug (of “free” form of the drug) due to improvements regarding solubility, tumor targeting, and drug resistance, which prompt beneficial alterations to pharmacokinetics and whole-body biodistribution [27], [28], [29].
Liposomal nanoformulations of doxorubicin (Doxil®, Lipodox®, and Myocet®) reduce drug side effects by improving tumor specificity/inhibiting off-target toxicity [28], [30], [31], [32]. Liposomes have also been employed to deliver other critical chemotherapeutic drugs, including paclitaxel (Lipusu®, approved in China [33], [34]) or daunorubicin (DaunoXome®, currently in advanced clinical trials for metastatic breast cancer with expected approval on safety and efficacy grounds [34], [35]). Additional approaches include protein nanoparticles such as Abraxane® [36], [37], [38], polymeric micelles such as Genexol-PM® [39], [40] or Nanoxel® [41], [42], [43], which all represent nanoformulations of paclitaxel, and the antibody-drug conjugate Kadcyla (Trastuzumab emtansine) [44], [45], [46] (Table 2).
Table 2.
FDA-approved nanomedicines in routine clinical use for breast cancer treatment.
| Name/Manufacturer | Nanocarrier | Drug/Compound | Approval Date | Indication | References |
|---|---|---|---|---|---|
| Doxil® (Janssen Pharmaceutica) | PEGylated Liposome | Doxorubicin | 1995 | Metastatic | [30], [47], [48] |
| Lipodox® (Sun Pharma Global FZE) | PEGylated Liposome | Doxorubicin | 2013 | Metastatic | [28] |
| Myocet® (Sopherion Therapeutics) | Non-PEGylated Liposome | Doxorubicin | 2000 | Metastatic | [31], [32], [49] |
| Lipusu® (Sike Pharmaceutical Co. Ltd) | Liposome | Paclitaxel | 2006 | Non-metastatic | [33], [50], [51] |
| Abraxane® (Celgene) | Albumin | Paclitaxel | 2005 | Metastatic | [36], [37], [38], [52], [53] |
| Genexol-PM® (Samyang Biopharm) | mPEG-PDLLA | Paclitaxel | 2007 | Non-metastatic | [39], [40], [54], [55] |
| Nanoxel® (Fresenius Kabi India Pvt Ltd.) | NIPAM-VP | Paclitaxel | 2006 | Metastatic | [41], [42], [56] |
| Kadcyla® (Hoffmann-La Roche) | Antibody | Trastuzumab/DM1 | 2013 | Metastatic HER2+ | [44], [45], [46], [57] |
| 2019 | Early HER2+, residual disease |
Poly(ethylene glycol): PEG. Poly(ethylene glycol)-poly(lactide acid): mPEG-PDLLA. N-isopropyl acrylamide: NIPAM. Vinylpyrrolidone: VP. Emtansine: DM
Doxil®, the first FDA-approved anticancer nanomedicine for the treatment of Kaposi's sarcoma, comprises a polyethylene glycol-modified (“PEGylated”) liposomal formulation of around 80–90 nm in diameter containing around 15,000 doxorubicin molecules encapsulated in the core [30]. In addition to Kaposi's sarcoma, Doxil® also represents a second-line treatment for metastatic breast cancer, multiple myeloma, and ovarian cancer [30]. The liposomal formulation of doxorubicin promotes tumor accumulation, thanks to enhanced passive targeting, enhances tumor growth suppression, and increases overall survival [30]. Importantly, liposomal doxorubicin also significantly reduces the cardiotoxicity typically associated with doxorubicin treatment [30], [47]. Liposome PEGylation prevents recognition and clearance by the reticuloendothelial system and extends circulation time (~72 h) compared to the free form of doxorubicin (~5 min) to improve anti-tumor efficacy. Despite the clear benefits observed, Doxil® treatment induced oral mucositis and skin toxicity (palmar plantar erythrodysesthesia) as unwanted side effects due, in part, to the prolonged circulation time and a tendency for skin accumulation [47], [48]. Myocet®, approved in 2000, differs from Doxil® by the lack of PEGylation and, as a consequence, a shorter circulation time (~2.5 h) and a low incidence of side-effects [31]. While the antitumor activity and progression-free survival rate remained similar in patients treated with Myocet® and free doxorubicin in phase III clinical trials [32], Myocet® induced a lower incidence of cardiotoxicity (as with Doxil®). A combination of Myocet® and cyclophosphamide currently represents a first-line treatment for metastatic breast cancer in Europe and Canada [49]. Lipodox® was FDA-approved in 2013 as a generic form of Doxil® for the treatment of breast and ovarian cancer [28].
In addition to doxorubicin, taxanes (e.g., paclitaxel and docetaxel) represent one of the most important classes of approved anticancer drugs and form an integral part of breast cancer treatment. Abraxane®, a 130 nm albumin-bound nanoparticle formulation of paclitaxel developed by Abraxis BioScience (now Celgene), was FDA-approved in 2005 for the treatment of metastatic breast cancer [58]. This paclitaxel formulation allowed for safer administration thanks to the absence of Cremophor® as a surfactant and ethanol as a solubilizing agent [36]. Furthermore, Abraxane® promoted higher tumor accumulation of paclitaxel (~33%), inhibited elimination (4-fold decrease) [37], and demonstrated a superior overall response rate (34%) compared to paclitaxel (19%) in patients with advanced breast cancer [38], [58]. Importantly, combination therapies of Abraxane® with conventional chemotherapeutic agents and targeted therapies have also demonstrated safety and superior efficacy compared to single therapies in clinical trials [52], [53]. Lipusu® (China, 2003) was the first liposomal formulation of paclitaxel approved for the treatment of non-small lung cancer, ovarian, and breast cancer [41], [42], [51], [56]. Nanoxel® (Indian, 2006), an 80–100 nm-sized polymeric micelle nanoparticle comprising a pH-sensitive copolymer of N-isopropyl acrylamide and vinylpyrrolidone monomers that encapsulates paclitaxel, has also been approved for metastatic breast cancer treatment [41], [42], [56]. Finally, Genexol-PM® (South Korea, 2007), a 20–50 nm-sized polymeric micellar nanoparticle that employs an amphiphilic diblock copolymer (poly(ethylene glycol)-poly(lactide acid) or mPEG-PDLLA) to encapsulate paclitaxel [39], has been employed in the treatment of breast, lung, and ovarian cancer [54], [55]. Genexol-PM® demonstrated a prolonged circulation time (1.8-fold) and an improved overall response rate with fewer secondary effects than free paclitaxel treatment [40].
Advancing towards molecularly targeted therapies, the antibody-drug conjugate Kadcyla® was FDA-approved in 2013 to treat HER2 + metastatic breast cancer patients previously treated with trastuzumab and taxanes [57]. Kadcyla® comprises the chemotherapeutic agent DM1 (emtansine) covalently bound via a stable thioether linker to the trastuzumab monoclonal antibody. Overall, Kadcyla® was well-tolerated and prompted significant improvements to median survival and progression-free survival compared to a combination of lapatinib (dual tyrosine kinase inhibitor) and capecitabine (chemotherapeutic) in HER2 + metastatic breast cancer [44], [45]. The European Medicine Agency recently approved Kadcyla® as an adjuvant treatment for HER2 + breast cancer with residual disease in the breast and axillary lymph nodes after neoadjuvant therapy [46]. This approval was based on results obtained in a phase III clinical trial in which Kadcyla® treatment significantly diminished the risk of invasive breast cancer relapse compared with trastuzumab [46].
Despite their proven clinical benefits, the list of nanomedicines available for breast cancer treatment remains short, with most based around conventional chemotherapeutic drugs [28]. Why do we currently have such a poor armory of nanomedicines in the battle against breast cancer? The answers include a lack of known molecular targets, tumor heterogeneity, therapeutic resistance, and critical unaddressed translational aspects [59] that relate to both manufacturing/scale-up issues and, importantly, the lack of concordance between treatment outcomes in pre-clinical models and those observed in the clinic. This suggests that selecting appropriate pre-clinical models represents a crucial aspect that will accelerate the development and approval of safe and efficient nanomedicines to breast cancer treatment [60], [61].
2. Selecting in vitro breast cancer models for nanomedicine development
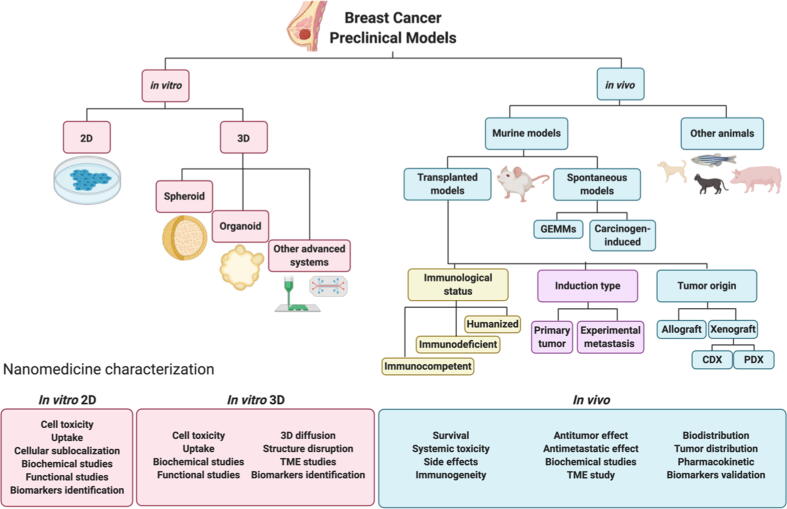
In general, each distinct phase of nanomedicine development requires a different model system, ranging from analysis in traditional 2D cell cultures to in vivo testing in small and large animal models. The selection of adequate model systems for each stage represents a tricky balancing act, with cost-effectiveness, difficulty, and ethical concerns “weighed up” against the potential for providing relevant data. Immortalized cell lines represent the most cost-effective and simplistic tumor models, and despite the alterations that cell lines undergo during their establishment and prolonged culture, breast cancer cell lines tend to maintain the major genetic alterations corresponding to the tumor subtype, thereby validating their implementation in tumor models [62], [63]. Even though a large number of human breast cancer cell lines exist for research purposes, studies tend to employ a core set that includes the human MCF7 (luminal A subtype) and MDA-MB-231 (TNBC subtype) [62] cell lines, and the mouse 4T1 (TNBC subtype) cell line [64].
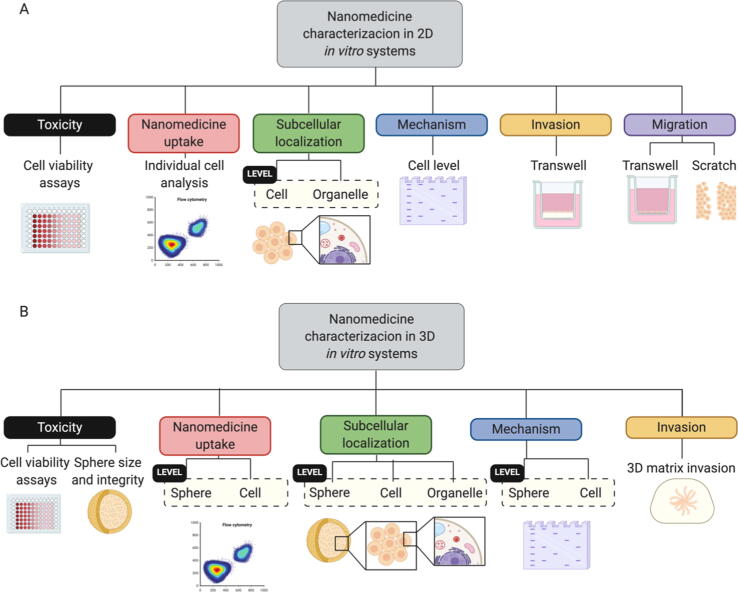
The evaluation of nanomedicines in conventional cell culture systems can provide information regarding toxicity, uptake, subcellular localization, mechanisms of action, and impact on certain biological processes (Fig. 4). Cell viability assays employed to assess nanomedicine toxicity include dye-exclusion, colorimetric, fluorometric, or luminometric assays; however, the colorimetric MTT assay represents the most widely employed cell viability assay [65], [66]. Nanomedicine uptake and subsequent subcellular localization in cell lines are traditionally evaluated using flow cytometry, fluorescence microscopy, and confocal microscopy. The mechanism of action of a given nanomedicine can be characterized by analyzing, for example, protein or RNA levels or through functional assays that predict their effects in vivo. These include migration (wound healing, motility assays, transwell) or invasion (matrix-coated transwells) assays.
Fig. 4.
Nanomedicine characterization assays in 2D (A) and 3D (B) in vitro systems.
Despite their utility, traditional 2D cell cultures lack the important tumor characteristics required to accurately predict nanomedicine treatment response. The limitations of homogeneous monolayer cultures include the presence of a single cell type, the lack of a 3D structure, and a poor representation of inter-tumor and intra-tumor heterogeneity. The ongoing development of novel cell culture strategies, including cell co-cultures, advanced 3D cell cultures, and patient-derived cells, aims to overcome these limitations. Of note, applying strategies in combination (e.g., 3D cell co-culture) to increase complexity can provide a more faithful disease model. In the following sections, we aim to provide a detailed review of relevant strategies and their implications in evaluating nanomedicines. Table 3 summarizes the main advantages, limitations, and applications of said in vitro models.
Table 3.
Summary of main advantages, limitations, and applications of cell culture strategies for nanomedicine in vitro evaluation.
| Strategy | Main advantages | Main limitations | Highlighted applications | Examples with nanomedicine |
|---|---|---|---|---|
| Monolayer cell culture | Inexpensive | Cell-line derived: clonal selection | Initial nanomedicine biological characterization. | [67] |
| Co-culture | Representation of different TME populations | Condition optimization for the growth of different cell lines | Cell type-targeted therapies | [69], [95] |
| Spheroid | Representation of: Cell-cell interactions Cell-matrix interactions In vivo-like gradients (over 400 µm diameter) Do not require highly specialized techniques |
Cell line-derived: clonal selection Higher costs than monolayer culture |
Tumor penetration studies | [75], [94], [95] |
| Organoid | Patient-derived Maintenance of intra- and inter- tumor heterogeneity |
High costs Complex establishment and maintenance compared to cell line-derived cultures |
Patient-tailored therapies | [87] |
| Tumor-on-chip | Supports microfluidics | High complexity Specific expertise and materials required |
High-throughput screening | [98] |
| Bioprinting | High spatial control | Specific expertise and materials required | Tissue and organ fabrication when patterning and precisely biologics placing is required | [103], [104] |
Tumor microenvironment: TME
2.1. Co-culture strategies
Co-culture techniques afford the study of tumor cells with cells of the TME, such as cancer-associated fibroblasts (CAFs), adipocytes, endothelial cells, and immune cells, such as dendritic cells, tumor-associated macrophages (TAMs), or lymphocytes [3]. This strategy can allow an understanding of differential cell uptake of nanomedicines, as exemplified by a study from Costa et al., who studied the uptake of chitosan-histidine-arginine nanoparticles encapsulating plasmid (p)DNA in a 2D co-culture of MCF7 cells and human fibroblasts [67]. Nanomedicines that modulate the TME generally target stromal cells, given their role in cancer cell growth and spread, or activate immune cells to then target cancer cells [68]. As a prime example, Zanganeh et al. proposed the use of Ferumoxytol®, an FDA-approved iron oxide nanoparticle, as a means to prevent breast cancer hepatic metastasis and potentiate TAM-associated immunotherapy for breast cancer [69]. They elucidated the mechanism underlying the effect of Ferumoxytol® using a co-culture of macrophages and breast cancer cells isolated from the MMTV-PyMT (mouse mammary tumor virus-polyoma middle tumor-antigen) mouse model. They discovered that Ferumoxytol® promoted the polarization of alveolar macrophages into pro-inflammatory macrophages that induce breast cancer cell apoptosis through reactive oxygen species (ROS) production [69], a finding that would have been missed in studies employing traditional single cell-type culture. However, even in co-culture, 2D in vitro models still display significant differences at the individual cell level (altered cell morphology and the deregulation of the cell cycle caused by monolayer growth) and at the entire culture level (the lack of nutrient/O2 gradients and cell–cell and cell-microenvironmental communication observed in tumors, points discussed in detail in following sections)[70], [71], [72]. Newly developed advanced 3D cell cultures hope to overcome said limitations and bridge the gap between 2D in vitro models and in vivo analysis (Fig. 1).
2.2. 3D breast cancer in vitro models: from spheroids to patient-derived organoids
3D cancer models aim to recapitulate clinical reality to a greater degree than traditional 2D models without entailing the problematic (complexity, cost, and ethics) implementation of animal models [73]. Therefore, in vitro 3D cell cultures represent exciting models regarding the evaluation of anticancer nanomedicines.
While there exists a huge variety of 3D cell culture systems (including spheroids and organoids, which can be combined with advanced techniques such as bioprinting and microfluidic-based tumors-on-chips), most share key characteristics that are crucial to the development of nanomedicines, which include the generation of gradients, the presence of the extracellular matrix (ECM), and the presence of cell–cell/cell-matrix interactions [70].
Similar to how the O2 and nutrient supply progressively diminishes with distance from tumor blood vessels, 3D models with diameters above 400 µm possess gradients of O2 and nutrients similar to those that exist in tumors, with O2 and nutrient supply progressively diminishing with distance from tumor blood vessels [70]. These gradients result in different environmental conditions (e.g., O2 concentrations or pH) depending on the distance from the cell culture core that significantly influences cell biology (e.g., metabolic state, proliferative capacity, drug resistance, and tumor potential).
Due to the depletion of O2 in tumor cores (hypoxia), cells switch to anaerobic metabolism, which is characterized by lactate production and the acidification of the TME [74]. The presence of an acidic pH (range 6.5–6.9) represents a well-known characteristic of tumors that nanomedicines containing pH-responsive functionalities can take advantage of to improve treatment outcomes [27]. For instance, Guo et al. designed a dual pH-responsive polycarbonate micelle with a tertiary amine surface coating that acted as a doxorubicin and lapatinib vehicle for breast cancer treatment [75]. The polycarbonate micelles (~112 nm of size) maintained a negative charge at pH 7.4, which favored prolonged blood circulation times; however, once accumulated in the tumor, a decrease in pH (at the TME or hypoxic core) prompted the protonation of tertiary amine groups, switching the surface charge from negative to positive, which enhanced tumor penetration and cell internalization [75], [76]. While this study physically entrapped lapatinib within the micelle, the authors conjugated doxorubicin to the carrier through a pH-responsive imine bond that enhanced intracellular drug release following endocytic internalization and limits any potential off-target side-effects [75]. Of note, this study highlighted micellar internalization at pH 7.4 and 6.8 by breast cancer cells in traditional 2D culture; however, in general, we lack an analysis of similar pH-responsive nanomedicines in more physiologically relevant in vitro 3D model systems. Hypoxic tumor cores also provide conditions conducive to the development of drug resistance mechanisms. For example, the stabilization of the hypoxia-inducible factor 1 alpha (HIF1α) prompts the increased activity of the HIF1 heterodimeric transcription factor, which regulates the expression of Multidrug Resistance Protein 1 (MDR1, also known as P-glycoprotein 1 or P-gp), an ATP-dependent efflux pump involved in the resistance to small drugs [74]. Notably, the internalization of nanomedicines via endocytic pathways may help to bypass this drug resistance mechanism [77], [78], [79]. Consequently, the study of possible drug resistance mechanisms in 3D models may provide more clinically relevant data than similar assessments in 2D models.
The characteristic loss of redox homeostasis in solid tumors prompts ROS generation, which can damage DNA, RNA, and proteins and promote tumor progression and metastasis during early-stage disease. Nevertheless, elevated ROS levels can also induce cancer cell apoptosis [80]. For these reasons, many rationally-design nanomedicines aim to take advantage of the high ROS levels in most cancer cells, either through specific drug delivery at the tumor site using ROS-responsive strategies or ROS-generating strategies to elevate levels above the toxic threshold [81]. In 3D cell culture systems, the modulation of oxygen tensions and metabolism in the hypoxic cores of tumors reduces ROS generation [74], which represents a significant limitation to such drug delivery strategies involving ROS. To solve the problem of low ROS levels in hypoxic cores, Wang et al. [82] combined platinum-cobalt (PtCo) nanozymes (which catalyze the oxidation cascade that leads to ROS generation) with MnO2 (catalyzes the decomposition of H2O2 to provide O2 as nanozyme substrate) allowing the increase of ROS in hypoxic conditions [82]. The resulting MnO2@PtCo “nanoflowers” induced ROS-mediated damage in most cells within 4T1 spheroids, while PtCo nanozymes alone only induced damage in the outer-layer cells of the spheroid.
As a direct consequence of the gradients present in tumors, and reflected in 3D models, cancer cells exist in a more proliferative state in the outer layers; however, cancer cells of the inner layers can exist in quiescent or necrotic states [83]. Quiescent cells within tumors exist in a reversible non-proliferative state [84] and represent a reservoir of chemotherapeutic-resistant cancer cells that may proliferate after a long period of inactivity, causing breast cancer relapse [85]. Thus, the more faithful representation of different cell states within in vitro 3D breast cancer models may allow for the development of novel nanomedicines targeted to quiescent tumor cells, the understanding of the limits of nanomedicines that target “bulk” proliferative cancer cells, or the construction of novel combination strategies.
Other than the above-described gradients, 3D breast cancer models more faithfully recapitulate the crucial physiological barriers facing an administered nanomedicine. A nanomedicine's ability to diffuse through the ECM represents one important factor limiting uptake by cancer cells. The size and physicochemical properties of a given nanomedicine can significantly influence outcomes due to interactions with the ECM, thereby supporting their evaluation using in vitro models that incorporate this crucial element [86]. Spheroids/organoids can be developed in suspension culture (scaffold-free cultures) or seeded within exogenous matrices of synthetic or natural origin (scaffold-based cultures). While the cancer and/or stromal cells employed naturally deposit their own ECM within scaffold-free systems, the exogenous matrix employed in scaffold-based cultures significantly influences the final ECM composition/characteristics. This can then influence mass transport, dosing, and particle distribution to the culture when evaluating nanomedicines. Therefore, employing a matrix with a similar composition and characteristics to the tumor stroma will provide a more faithful scenario for the evaluation of nanomedicines. Evidence for the importance of matrix choice includes the findings of a study from Astashkina et al., who observed that the strong interaction of gold nanoparticles with a hyaluronic acid-based hydrogel organoid matrix ultimately impeded any cell-based evaluations [87]. Matrix choice can also modulate the all-important interaction of cancer cells with the ECM and alter treatment responses. Lovitt et al. demonstrated that MDA-MB-231 cells cultured in a Matrigel-based 3D system gained resistance to doxorubicin compared to its 2D counterpart. Nevertheless, doxorubicin resistance could be partially reversed either by substituting Matrigel with PuraMatrix, a synthetic peptide hydrogel without ECM proteins (laminin, collagen IV, entactin), or by inhibiting ß1-integrin, a receptor involved in cell-matrix interactions, demonstrating the relevance of cell-ECM interactions in response to treatment [88]. Overall, the presence of a physiologically relevant matrix and relevant cell–cell and cell-matrix interactions in spheroids and organoids highlights their suitability for evaluating nanomedicines as breast cancer treatments [89], [90].
Implementing 3D cancer cell models for the evaluation of nanomedicines provides a range of advantages; they can provide more reliable information regarding the pharmacological effects (Fig. 4) at the cellular level (growth inhibition, apoptosis, migration, or other biochemical outputs [70], [90]) and allow an evaluation of how treatment can alter morphometric parameters (e.g., diameter, circularity, volume, cell density) or systemic integrity. Moreover, the application of 3D model systems can help to provide a more complete picture regarding the tumor distribution of a nanomedicine by combining the information about individual cell uptake with data regarding penetration and distribution through the whole system, which takes into account the effect of nanomedicine physico-chemical parameters such as the size, shape, deformability or Z potential on the penetration/diffusion process in the 3D culture [91].
While 3D models offer a better testing ground for nanomedicines than conventional 2D models, various approaches exist, with each suffering from inherent strengths and weaknesses related to the individual experiment/hypothesis in question.
2.2.1. Breast cancer spheroids
3D spheroids, multicellular aggregates formed under non-adherent conditions, can be classified as scaffold-free or scaffold-based [92]. In scaffold-free spheroid formation, cell lines form 3D structures induced through culture on non-adherent culture surfaces, culture in hanging drops or spinner flasks, or via external-force-driven aggregation [93]. Scaffold-based 3D spheroids are formed after seeding single-cell suspensions onto synthetic or natural matrices [93] that mimic the ECM and recapitulate cell-matrix signaling. Scaffold-free spheroids represent the most commonly employed option for nanomedicine development due to low associated costs and their easy manipulation.
Taresco et al. employed MDA-MB-231 homo-spheroids to establish the increased cytotoxicity of a poly(ethylene glycol)‐co‐poly(lactide)‐co‐poly(2‐((tert‐butoxycarbonyl)amino)‐3‐propyl carbonate) (PEG‐pLA‐pTBPC) conjugate of doxorubicin when compared to treatment with the free form of the drug [94]. Notably, a similar evaluation under 2D conditions failed to find any significant differences between the levels of cytotoxicity induced by free and conjugated doxorubicin. In a more complex scenario, Sethi et al. employed scaffold-free hetero-spheroids composed of 4T1 cells, CAFs, and endothelial cells to evaluate a combination treatment comprising radiation and liposomal nanoparticles carrying arsenic trioxide and cisplatin conjugated with Anginex, a Galactin-1 binding peptide [95]. Radiation exposure induced the endothelial cell expression of Galactin-1, thus promoting the targeting of the tumor stroma by the Anginex-conjugated nanoparticles. Excitingly, results from the hetero-spheroid culture resembled those observed during in vivo testing, providing evidence for the utility of this in vitro approach to nanomedicine evaluation that minimizes any requirement for animal testing. This study demonstrates how selecting a 3D model with appropriate cellular constituents can prove the effectiveness of a rationally designed stroma-targeted nanomedicine.
Unfortunately, the cell line origin of spheroids fails to recapitulate the intra-tumor and inter-tumor heterogeneity observed in vivo. Breast cancer cell lines are difficult to isolate from other cell populations from in situ tumors, and so, the majority of breast cancer cell lines derive from metastatic tumors and pleural effusions, and this leads to a poor representation of less-aggressive tumor subtypes and a loss of inter-tumor heterogeneity [62]. Furthermore, breast cancer cell lines adapt to culture conditions during their establishment, involving the clonal selection of those cells amenable to in vitro growth and the elimination of other less amenable but biologically relevant cell types [62], [96], resulting in a loss of intra-tumor heterogeneity. However, we can address some of these limitations through studies employing organoids, which maintain a similar heterogeneity to their tissue of origin [97].
2.2.2. Breast cancer organoids
Organoids, self-organizing 3D structures that arise from stem cells, possess organ-specific cell types [97] and, importantly, exhibit structural, functional, and molecular similarities to the tissue of origin [99]. Patient-derived organoids can be established from induced pluripotent stem cells (iPSCs) or tissue-resident adult stem cells. Patient-specific organoids derived from iPSCs represent a highly-utile tool for developmental studies; however, reprogramming coupled with differentiation and organoid establishment involves extended time scales, and they have less relevance with regards to cancer modeling [97]. Patient-specific organoids derived from adult stem cells represent a more rapid/easy means to model healthy tissues/organs like mammary tissue, stomach, liver, pancreatic duct, kidney tubule, or prostate. Cancer stem cells (CSCs) within tumor tissues contribute to the development of cancer organoids [99], [100], and the existence of a growing number of organoid biobanks, including 95 available breast cancer organoids [100], has helped to accelerate their experimental application [97]. Moreover, studies have also reported the development of tumor organoids in model animals such as mice, rats, and dogs [99].
Organoids can be in vitro expanded for long periods thanks to the self-renewal capacity of adult/cancer stem cells [99], and, unlike cell lines, they do not suffer from strong selection. Furthermore, they display levels of intratumor heterogeneity comparable to that of the original tumor tissue, as the heterogeneous genetic composition of the tumor is retained over time in organoid cultures [101]. Organoids can also be derived from tumors at distinct stages; therefore, they can recapitulate the diversity of human cancers without bias to high-grade tumors. Overall, cancer organoids provide a model system that maintains intra-tumor and inter-tumor heterogeneity and the characteristics (e.g., molecular footprints, histological grade, or differentiation status) of the original tumor. The growth of organoids in growth substrates such as Matrigel, a basement membrane extract, supports cell-ECM interactions. Moreover, the presence of specific growth factors in the culture medium allows the expansion of the epithelial tumor lining but does not support the growth of stromal cells naturally present in the TME. While co-cultures with CAFs and immune cells have been reported, examples remain scarce, perhaps due to a significant increase in technical difficulty and cost [99].
Due to the noted advantageous characteristics, organoids have been proposed as a “stepping-stone” between the evaluations of nanomedicines in traditional 2D culture and animal models [92]. Examples of the potential of normal tissue organoids in the pre-clinical evaluation of nanomedicines include a study from Astashkina et al., who employed murine kidney proximal tube-derived organoids to predict the toxicity of a hydroxylated 5th generation polyamidoamine (G5-OH PAMAM) dendrimer using a panel of hepatic toxicity biomarkers [87]. The authors observed significantly lower toxicity for G5-OH PAMAM dendrimers than for cisplatin, a nephrotoxic agent, and correlated their results with in vivo hepatic toxicity results from a previous study [102]. Overall, this study validated the use of murine kidney proximal tube organoids to assess kidney toxicity [87].
2.3. Advanced 3D modeling opportunities
Spheroids and organoids recreate some relevant aspects of breast tumors; however, other aspects, including fluid dynamics or the spatial control of cells, remain misrepresented. Organs-on-chips and 3D bioprinting approaches permit some control over those factors and can create complex model systems for the evaluation of nanomedicines [103], [104].
Organs-on-chips, microfluidic systems that contain living cells in perfused hollow microchannels, recapitulate multiple functional aspects of the modeled organ/tissue. These advanced devices model the 3D nature of the desired tissue by co-culturing different cell populations with tight spatial and temporal control of chemical gradients and biochemical forces [104], [105]. Organs-on-chips support flexibility in model generation, including implementing various sources of cells (cell lines, patient-derived cells, iPSCs), cell scaffolds (natural or synthetic hydrogels), and tunable linking patterns between microchannels [104].
The use of tumor cells (forming tumors-on-chips) provides additional advantages when compared with spheroids and patient-derived organoids, including compartmentalization and tight control over spatial organization. Human breast tumor-on-a-chip models, which have already been successfully implemented for drug discovery [106], can provide for in vivo-like gradients. In a bladder cancer tumor-on-chip model developed by Liu et al.[107], cancer cells were co-cultured with relevant stromal populations - macrophages, endothelial cells, and fibroblasts. While physically separating the four cultures in different chambers, a continuous cell culture medium flow allowed the interchange of paracrine factors, thereby faithfully recapitulating microenvironmental cell interactions that translated into TAM activation and migration and the induction of an in vivo-like organization of bladder cancer cells. Thus, tumor-on-chip microfluidics systems allow indirect co-culture conformations that recreate the signaling factors that cancer cells receive in vivo [107]. Importantly for the evaluation of immunotherapies, tumor-on-chips also support the generation of complex models featuring both cancer and immune cells that can be adapted for high-throughput screening. For instance, Jiang et al. [108] developed a tumor-on-chip system using MDA-MB-231 spheroids and Jurkat cells (immortalized T lymphocytes) to perform high-throughput studies of immune checkpoint interactions. The system included microwells for the formation of MDA-MB-231 spheroids, which were later co-cultured with activated T-cells. Initially, MDA-MB-231 cells inhibited T-cell growth by PD-1/PD-L1 interactions, although anti-PD1 mAb treatment reversed the inhibitory effect. This system also included antibody-coated micropillars that allowed the detection of IL-2 levels; interestingly, a simple switch of antibody can allow for the study of any other soluble biomarker of interest [108]. Also, tumor-on-chips can include a realistic vascular network in the tumor chamber with hypoxic areas [109]. As an example, Shirure et al. developed a microfluidics platform comprising a microvascular network of endothelial colony-forming cell-derived endothelial cells combined with MCF7, MDA-MD-231, or patient-derived organoids in an adjacent compartment. They demonstrated the feasibility of the evaluation of anticancer and antiangiogenic treatments by monitoring cell proliferation, cell migration, angiogenesis, and tumor cell intravasation [110].
Organ-on-chip platforms are especially relevant for modeling the different conditions and biological barriers that nanomedicines face during their voyage through the body towards tumor cells. The nanomedicine delivery process generally involves circulation in the bloodstream, extravasation in the tumor through “leaky” vasculature, passage through the tumor matrix, and tumor cell uptake [111]. While static 3D models can mimic biological barriers, such as the tumor stroma or the ECM, factors such as interstitial fluid pressure or passage through the endothelium are not well represented. Chen et al. developed a 3D breast-tumor-on-chip model for the evaluation of nanomedicines that comprised breast cancer spheroids (TNBC BT549 or non-TNBC T47D cells) cultured beyond a blood vessel wall-like biological barrier formed in a microchannel by an endothelial cell monolayer cultured on top of a basement membrane extract layer [98]. Interestingly, the authors demonstrated increased penetration and toxicity against TNBC BT549 spheroids following treatment with doxorubicin-containing folic acid-targeted PEGylated carbon dots (CDs-PEG-FA/DOX) due to the selective targeting of the folate receptor, which is upregulated in TNBC [98].
Spheroids, organoids, and organ-on-chip devices recapitulate various aspects of the TME; however, they lack precise control over the location and organization of the different cell types employed. To solve this problem, breast cancer modeling has also taken advantage of 3D bioprinting, the computer-controlled deposition of biological materials in different layers to create a 3D structure [103]. Using extrusion-based, laser-based, or droplet-based bioprinting, biological materials are deposited on a matrix that can be discarded (scaffold-free bioprinting) or maintained (scaffold-based bioprinting) after the generation of the model. While bioprinting technology remains in its infancy, Datta et al. recently published a review of breast cancer bioprinted models generated from established cell lines and primary cells [103]. As an example, Wang et al. bioprinted a breast cancer model comprising 21PT HER2 + breast cancer cells surrounded by a layer of primary adipose-derived mesenchymal stem cells, which represent a common stromal cell type within breast adipose tissue [112]. By altering the thickness of the mesenchymal stem cell layer, the authors concluded that these cells contributed to the resistance of 21PT cells to doxorubicin. While nanomedicine studies in bioprinted breast cancer models remain unreported, these highly organized models may form a central part of future studies. Bioprinting strategies for the evaluation of immunotherapies could support both the representation of all immune cell types implicated in the process and the precise modeling of their distribution [103]. Heinrich et al. [113] recently described a first of its kind bioprinted glioblastoma model that comprised both glioblastoma tumor cells and macrophages. In this study, the authors fully characterized gene expression profiles for both populations, finding that they resembled those observed in the clinical setting, and demonstrated the usefulness of this model in drug evaluation for both conventional chemotherapy and immunotherapy. They showed that carmustine, a chemotherapeutic for glioblastoma, had better performance in the macrophage-glioblastoma co-culture thanks to the higher tumor growth compared to monoculture, and also that the treatment with the immunomodulatory drug BLZ945, resulted in reduced tumor growth. To mimic in vivo metastasis, Cui et al. [114] developed a bioprinted breast-to-bone metastasis model that included the invasive breast cancer MDA-MB-231 cell line or the non-invasive MCF7 cell line, human fetal osteoblasts, and endothelial cells. In the future, this metastatic model could be applied to drug screening and could represent a significant advance for nanomedicine evaluation.
While these advanced 3D systems currently remain underused, due in part to high associated costs and relative difficulty, we believe that the application of organoid, 3D bioprinting, and organ-on-a-chip technology will support the pre-clinical evaluation of breast cancer nanomedicines, allow early-stage detection of predictive biomarkers and, foster the development of precise and personalized therapies for breast cancer patients [106], [115], [116]. Furthermore, the evolution of these technologies will help to implement the “3 R Principle” (reduction, refinement, and replacement) that aims to minimize the use of animals in the laboratory [117].
3. Pre-clinical breast cancer animal models
While obvious ethical concerns remain, animal models often represent the gold standard for understanding disease development and treatment response. At present, the development of anticancer nanomedicines requires evaluation in animal models to ensure efficacy and safety before moving to human trials as an understanding of nanomedicine fate, pharmacokinetics/pharmacodynamics, and whole-body biodistribution represent key features that guide the transfer of nanomedicines from the pre-clinical to the clinical scenario [118].
Evaluations in animal models allow an understanding of how a given nanomedicine can affect parameters such as tumor size, the number of metastases, and animal survival; however, these all-important measures must be understood in the context of other interactions, which include drug-tumor accumulation, targeting efficiency, pharmacokinetics (e.g., circulating drug concentration over time, the volume of distribution, mean clearance time, bioavailability) and pharmacodynamics (absorption, biodistribution, drug metabolism and excretion). Moreover, studies of systemic toxicity, hematocompatibility, and the maximum tolerated dose [119], [120] represent essential first steps in the potential for acute or chronic side effects [121], [122].
Mimicking human pathophysiology in animal models represents a critical aspect for any evaluation of a given nanomedicine. The choice of the animal model largely depends on the tumor type, the specific research aim, the ease of use, the cost, and the time required to develop the model. Furthermore, other parameters generally considered include the adequate recapitulation of different phases of the disease, the development of metastasis in a reasonable time-frame, immune-system status, and the overall resemblance to the human disease [123].
While there exists a substantial number of animal models of breast cancer, employing a range of animal species and related protocols [63], rats (Rattus norvergicus) and mice (Mus musculus) remain the most used model animals for the evaluation of nanomedicines due to the ease of management and physiological and genetic similarities with humans (98% genetic homology). Murine cancer models also provide cost-effective but highly reproducible results in a relatively short timeframe [123], [124], [125].
With this in mind, we now aim to critically discuss the most common pre-clinical breast cancer animal models employed in the evaluation of nanomedicines.
3.1. Pre-clinical murine models of breast cancer
Murine models of breast cancer (Fig. 1) are classically divided into two large groups based on how the tumor arises: via the transplantation of tumor cells or spontaneous tumorigenesis. Transplantation-based tumor models include syngeneic models (also known as allograft tumor models), where tumor cells and host are the same species, and xenograft models, where the tumor cells and host represent distinct species. Xenograft tumor models can be further subdivided into cell-derived xenografts (CDX) and patient-derived xenografts (PDX). The host's immunological status and the implantation site also represent crucial characteristics of transplanted murine models, so further subdivisions derive from these criteria. Spontaneous tumor models include genetically modified mouse models (GEMMs) and carcinogen-induced murine tumorigenesis models [124], [125], [126], [127] (Fig. 1).
3.1.1. Transplanted murine pre-clinical breast cancer models
3.1.1.1. Classification by implantation site
3.1.1.1.1. Generation of primary tumors
Transplantation-based tumor models involve the transplantation of tumor cells in suspension or solid tumors obtained from a donor [128]. The implantation site determines factors such as tumor growth rate, microenvironment composition, and vascularization, all of which represent factors that affect the response to nanomedicine treatment. Models are classified as orthotopic if tumor cell implantation occurs in the tissue where the tumor arose (i.e., breast cancer cells implanted into the mammary duct or fat pad) or heterotopic if tumor cell implantation occurs in another tissue (i.e., the subcutaneous implantation of breast cancer cells). Both these approaches are commonly employed for the establishment of primary breast tumors [129]. While the subcutaneous transplantation of breast cancer cells to generate breast tumors is technically straightforward, subsequent tumors lack a representative tumor stroma and fail to fully recapitulate expected metastatic patterns [130], [131]. In contrast, orthotopic breast cancer models represent a more faithful recapitulation of human tumorigenesis [132], [133], [134]. The generation of orthotopic breast cancer models via the injection of breast cancer cells inside the natural cavity of the mammary ducts (intraductal) provides for the histological and molecular features of the clinical setting, high implantation rates, and effective spontaneous metastasis [125], [130]. Unfortunately, the intraductal strategy remains technically challenging and has yet to find common use regarding nanomedicine evaluation [135], [136], [137], [138].
The injection of breast cancer cells into the mammary fat pad, which balances lower complexity with many of the advantages of orthotopic models, represents the more predominant strategy employed to generate orthotopic breast cancer models. Highlighting said advantages, Zhang et al. demonstrated the suitability of mammary fat pad orthotopic breast cancer models for studies involving the TME compared to subcutaneous models [131]. This study compared tumor growth and progression after 4T1 inoculation by subcutaneous or orthotopic injections in immunocompetent BALB/c mice. The orthotopic model displayed larger tumor sizes, elevated metastasis, and an increased invasive growth pattern with a more considerable number of CD31 + vessels and adipocytes within tumors than the subcutaneous model. In another example, Okano et al. found that orthotopic implantation led to enhanced grafting and more rapid tumor growth than subcutaneous implantation in breast cancer PDX models generated in immunodeficient NSG (NOD scid gamma) mice employing biopsies derived from eleven breast cancer patients [129].
Importantly, the EPR effect, a key feature for nanomedicine clinical performance [139], can be significantly affected by the selected implantation site in breast cancer transplantation models. Ho et al. sought to evaluate the EPR-mediated accumulation of nanomedicines in tumors formed by the subcutaneous and orthotopic inoculation of MDA-MB-231-H2N (HER2 transfected) breast cancer cells in NSG mice [132]. The evaluation of vessel permeability via the intravenous injection of a fluorescently labeled high molecular weight dextran (FITC-Dextran, 2 MDa, ~80 nm), used as a nanomedicine surrogate, demonstrated greater dextran accumulation and a more homogeneous growth profile in the orthotopic tumor. Furthermore, immunostaining revealed greater vascular density and thinner basement membranes in the orthotopic model, providing evidence that orthotopic breast cancer models may represent the optimal means of evaluating the influence of the EPR effect on nanomedicine accumulation and anti-tumor effect [132].
Parallel to the eradication of primary tumors, the identification and delivery of anticancer therapeutics to metastatic lesions also represents a significant challenge. Despite the numerous advantages regarding the primary tumor, orthotopic models generally only present metastases in the lymph nodes and lungs [64], with the liver affected occasionally. Metastatic spread tends not to reach the bones or the brain, which represent critical sites of metastasis in breast cancer patients [140], [141]. However, a recent study reporting metastasis to the bone from a 4T1-derived primary tumor [142] may provide a model system for the evaluation of bone-targeted anticancer nanomedicines or combination approaches with radiotherapy or photodynamic therapy (PDT) [143]. In general, the limitations regarding the generation of metastasis using subcutaneous and orthotopic models [64], [131] require the application of complementary experimental models to evaluate nanomedicines that target metastasis [130], [144], [145].
3.1.1.1.2. Generation of experimental metastasis
Experimental metastases in breast cancer models do not require the establishment of a primary tumor and are generally generated through the infusion of tumor cells, with the injection site determining the organ or tissue that harbors the metastasis. For example, tail vein injection commonly supports lung metastases, while intracardiac injection fosters bone and brain metastases [130]. As an example of the use of intravascular experimental metastatic models for nanomedicine evaluation, Guo et al. developed a dual complementary liposome containing doxorubicin coated with antibodies against intercellular adhesion molecule–1 (ICAM1) and epithelial growth factor receptor (EGFR) as TNBC-targeted treatment strategy [146]. They combined the use of an MDA-MB-231-Luc orthotopic model and metastasis induced by lateral tail vein injection to demonstrate the anti-tumor and anti-metastatic effect of their liposomal approach. Furthermore, they demonstrated that dual complementary liposomes displayed elevated tumor-targeting activity and antitumor efficacy compared to free doxorubicin in both orthotopic and lung metastasis models, indicating them as a suitable platform for the design of personalized nanomedicines for TNBC.
Experimental models of breast cancer metastasis have also been used in the study of nanomedicines that target TME components associated with metastatic spread, such as immune cells, endothelial cells, or CSCs [147]. A study by Kim et al. evaluated a doxorubicin-encapsulating liposome conjugated with double DNA aptamers (Dual-Apt-Dox) specific to the surface markers glycoprotein CD44 and transmembrane glycoprotein mucin 1 (MUC1) to target CSCs and tumor cells, respectively, represents a prime example [148]. The authors assessed the inhibitory effect of Dual-Apt-Dox on metastasis following the injection of equal amounts of bulk MCF7 cells and CD44+/ CD24− selected CSCs into the lateral tail vein of female BALB/c athymic nude mice, finding a reduction in lung metastasis when compared with treatment with either free doxorubicin or an untargeted doxorubicin-encapsulating liposome [148].
Other examples of metastatic models that mimic the clinical scenario of breast cancer include a study from Anders et al., who developed an intracranial breast cancer metastasis model to evaluate the efficacy of Doxil® compared to free doxorubicin treatment [149]. The generation of the model employed the injection of MDA-MB-231-BR cells (a subclone that commonly metastasizes to the brain) into the right caudate nucleus of the basal ganglia of athymic mice. The authors discovered that Doxil® treatment led to increased survival rates and higher plasma and intracranial tumor doxorubicin levels than free doxorubicin, most probably due to the enhanced stability of the nanoformulation in the blood. To note, a comparison of the most widely used orthotopic metastatic TNBC models - the immunocompetent (4T1) BALB/c and the immunosuppressed (MDA-MB-231) NOD/SCID model – revealed that the MDA-MB-231 model supported more significant EPR-dependent tumor accumulation, which the authors linked to high lipid content and lower cell density in the tumor stroma [64].
3.1.1.1.3. Classification by host immunological state
The host's immune status significantly affects parameters directly relevant to the evaluation of nanomedicines [150]. The more faithful recapitulation of the in vivo TME through the inclusion of immune system components will support the accurate prediction of clinical responses to a novel therapeutic [151]. Moreover, the immune system itself represents a crucial target for rationally designed nanomedicines, which can either activate the immune system against the tumor (e.g., nanovaccines) or eliminate those immune cells that support tumor growth and metastasis [152], [153], [154].
With a focus on the immunological state of murine models, hosts can be classified as immunocompetent, immunocompromised, or humanized [155]. Immunocompetent hosts present a complete immune system, with C57BL/6, BALB/c, and FVB the most commonly employed mice strains for murine cell transplantation, carcinogen-induced tumor generation, and the generation of GEMMs [156]. Additionally, immunocompetent Sprague Dawley rats are commonly used for carcinogen-induced models. Of note, the murine immune system exhibits differences from the human immune system, including an altered balance of leukocyte subsets and expression of Toll-like receptors, antibody subsets, and cytokines [126], [157], [158], [159]. Moreover, different immunocompetent mouse strains display varying proportions of critical immune system components [63], directly impacting nanomedicine evaluations. For example, Korangath et al. [151] evaluated amine-functionalized starch-coated ionized nanoferrite nanoparticles labeled with trastuzumab, a HER2-targeted antibody, and found that iron uptake correlated with their HER2 expression in vitro. However, during in vivo analyses, the results differed depending on the immune status of the mouse strain employed. They observed nanoparticle accumulation in HER2-positive or HER2-negative tumors generated by orthotopic transplantation in three mouse strains: the highly immunocompromised NSG, the slightly immunocompromised nude, and the immunocompetent FVB/N strain. Interestingly, trastuzumab enhanced tumor retention regardless of the HER2 state of the tumor. Moreover, the authors discovered strong, subtle, and non-existent correlations between HER2 levels and nanoparticle accumulation in the tumor in the NSG, nude, and FVB/N models, respectively, and encountered the accumulation of nanoparticles in cancer-associated immune cells in the immunocompetent FVB/N model. This study highlights the critical role of the immune system in nanoparticle uptake and retention and how the unrealistic immunocompromised setting could lead to misleading results that do not represent the potential effect in patients [151].
As another example, Type 1 T helper (Th1) cell-dominant mouse strains such as C57BL/6 exhibit slow clearance rates of cylindrical PEGylated hydrogel nanoparticles compared to Type 2 T helper (Th2) cell-dominant mouse strains [160]. T helper cells adopt distinct identities during immune responses and secrete specific cytokines/chemokines that instruct a wide variety of immune cells, including macrophages [161]. While Th1-associated cytokines prompt the polarization of macrophages into a pro-inflammatory M1 phenotype, Th2 responses induce the polarization of macrophages into an anti-inflammatory/pro-regenerative M2 phenotype [162]. Notably, an increased proportion of M1 macrophages correlated with lower particle uptake by macrophages in Th1-dominant strains, while increased M2 macrophage polarization prompts higher particle uptake in Th2-dominant strains, highlighting the importance of the immune system on nanomedicines [163].
Human-derived tumor models are limited to the use of immunodeficient or humanized mouse strains to avoid host rejection [155]. Humanized models, in which the grafting of human bone marrow-derived hematopoietic stem/progenitor cells replaces the ablated murine hematopoietic system, provides a means to support the implantation of human cancer cells within a model that possesses a human-like immune system. The elevated costs and complexity involved have generally limited the implementation of this approach [164]; therefore, the generation of tumors from human cells commonly employs immunocompromised mouse strains that lack essential molecular compartments of the immune system to reduce the murine immunological response and increase cell engraftment [165].
Immunodeficient murine models can be subdivided according to their immune profile. In breast cancer, commonly employed immunodeficient mouse models include nude (athymic), SCID (severe combined immunodeficient), NOD-SCID (non-obese diabetic-severe combined immunodeficient), RAG (Rag-deficient), NOG (NOD/Shi-scid/γc−/− null), and NSG (NOD/SCID/γc−/−) strains (Table 4) [165], [166]. Each immunodeficient mouse strain exhibits differences regarding primary tumor establishment and growth and metastatic potential. For example, Puchalapalli et al. compared orthotopic breast tumor growth and metastasis in nude and NSG models using ER- breast cancer cell lines (MDA-MB-231, SUM1315, CN34BrM) in both nude and NSG mice and an ER + cell line (T47D) in NSG mice [165]. They discovered that NSG mice were more permissive for primary breast tumor growth and metastasis than nude mice, with a metastatic profile (presence in lungs, liver, bones, brain, and residual lymph nodes) similar to that observed in human patients. Overall, these data suggest that NSG models represent an exciting model system for the evaluation of nanomedicines aimed at treating metastatic breast cancer. Peng et al. also employed the NSG strain to analyze the effect of different nanomaterials in metastatic breast cancer [167]. The authors demonstrated that intravenously injected titanium dioxide, silica, and gold nanoparticles significantly accelerated the intravasation and extravasation of breast cancer cells, thereby increasing the extent of existing metastasis (lungs) and promoting the appearance of new metastatic sites (liver bone and spleen). This study emphasized the importance of evaluating a given nanomaterial's interactions within an appropriate biological environment to evaluate beneficial/harmful effects.
Table 4.
Immunodeficient mouse strains and their applications for nanomedicine evaluation. Code: +++ immune components present, + residual components, and - absent components in each model.
| Model | Immunological Profile | Innate components | Adaptative components | Applications | Examples of Tested Nanomedicines. |
|---|---|---|---|---|---|
| NUDE | Foxn1nu mutation. Athymic and T cell-deficient. Intact innate immunity. |
C5 complement +++ Macrophages +++ Granulocytes +++ Natural killer cells +++ |
Dendritic cells+++ Antibodies +++ B cells+ T cells- |
Engraftment of cancer cell lines. Easy evaluation of tumor growth. Not suitable for primary cells |
Doxil® [149] |
| RAG | Rag deficient. T and B cells depleted. Intact innate immunity. |
C5 complement +++ Macrophages +++ Granulocytes +++ Natural killer cells + |
Dendritic cells+++ Antibodies- B cells- T cells- |
Most commonly used genetic background. Radiation tolerant. Poor host for primary cells. |
– |
| SCID | Prkdcscid mutation. T and B cells depleted. Intact innate immunity. |
C5 complement +++ Macrophages +++ Granulocytes +++ Natural killer cells +++ |
Dendritic cells+++ Antibodies- B cells- T cells- |
Engrafts hematopoietic cancer cells and some primary cells. NK activity limits engraftment. Poor radiation tolerance. |
Ag/Au bimetallic nanoparticles [168] |
| NOD-SCID | Lacks mature B and T cells. Reduced innate immunity. |
C5 complement- Macrophages+ Granulocytes+++ Natural killer cells+ |
Dendritic cells+++ Antibodies- B cells- T cells- |
Engrafts hematopoietic cancer cells and some primary cells. Residual natural killer cell activity limits engraftment. Poor radiation tolerance. |
Polymeric micelles loaded with Zileuton® [169] |
| NOG | NOD-SCID mice crossed with IL2γ receptor null mice resulted from a truncation of the intracellular signaling domain in the NOD/ShiJic-Prkdcscid mouse. The receptors can bind cytokines IL-2, 4, 7, 9, 15, and 21 but do not become active. Lacks mature B and T cells. Impaired innate immunity. |
C5 complement- Macrophages+ Granulocytes+++ Natural killer cells- |
DC+ Antibodies- B cells- T cells- |
Enhanced engraftment of primary cells, tissues, and tumors. Efficient host for human hematopoiesis and immunity. Optimal strain for humanized models. Permits long-term experiments. Poor radiation tolerance. |
– |
| NSG | NOD-SCID mice crossed with IL2γ receptor null mice resulted from a complete null mutation in the NOD/ShiLtSz-Prkdcscid mouse. Complete knockout of receptors for cytokines IL-2, 4, 7, 9, 15, and 21. Lacks mature B and T cells. Impaired innate immunity. |
C5 complement- Macrophages+ Granulocytes+++ Natural killer cells- |
DC+ Antibodies- B cells- T cells- |
Enhanced engraftment of primary cells, tissues, and tumors. Efficient host for human hematopoiesis and immunity. Optimal strain for humanized models. Permits long-term experiments. Poor radiation tolerance. |
Titanium dioxide, silica, and gold nanoparticles. [167] |
Abbreviations: dendritic cells (DC), natural killer (NK), athymic nude (Nude), severe combined immunodeficiency (SCID), non-obese diabetic severe combined immunodeficiency (NOD-SCID), Rag-deficient (RAG), NOD/Shi-scid/γc−/− null (NOG) and NOD/SCID/γc−/− (NSG) strains.
3.1.1.2. Classification by origin of implanted cells
3.1.1.2.1. Syngeneic mouse models
Syngeneic mouse models (murine tumor host and donor) are widely used in nanomedicine studies in breast cancer due to their easy management, rapid establishment, and their experimentally reproducible nature [126]. As they present a more human-like metastatic profile and support the presence of immune components, syngeneic models represent a valuable resource for evaluating a wide range of nanomedicines [170].
The most widely used syngeneic breast cancer model for the evaluation of nanomedicines is the orthotopic TNBC 4 T1 model [171], [172], which exhibits several advantageous characteristics, including the well-vascularized nature of the tumor [173], the high metastatic capacity in the lungs and lymph nodes [64], and adequate TME representation [174].
One of the main advantages of including TME components in pre-clinical models relates to the study of immunotherapies. For example, the most prominent advancement made in the clinic for the treatment of patients with unresectable, locally advanced, or metastatic TNBC expressing the anti-programmed death-ligand 1 (PD-L1), is the combination of Abraxane® and the new PD-L1 inhibitor, atezolizumab (Tecentriq®) [175], [176]. Lesniak et al. demonstrated the influence of the TME in PD-L1 expression by tumor cells by linking low endogenous PD-L1 expression 4 T1 cell with low atezolizumab uptake in vitro. However, the in vivo 4 T1 model exhibited greater tumor uptake of atezolizumab due to the presence of an inflammatory TME that induced higher PD-L1 expression in tumor cells [177]
Factors present in the unique TMEs can also be considered endogenous stimuli that can aid the rational design of tumor-specific nanomedicines [178]. As an example, we can design nanomedicines with bioresponsive linkers that release their cargo in the presence of microenvironmental factors associated with tumorigenesis, such as acidic pH, elevated ROS, glutathione (GSH), hypoxia, H2O2, the elevated expression of proteases (including matrix metallopeptidases (MMP) and cathepsins), or other overexpressed proteins [4], [179], [180], [181]. The orthotopic TNBC 4 T1 metastatic model is often employed in the study of polymer-based combination conjugates, where the polymer-drug(s) linker design is a crucial feature ruling final therapeutic output at the primary tumor and metastasis level. Polyglutamate-based conjugates bearing a synergistic ratio of doxorubicin and the aromatase inhibitor aminoglutethimide (AGM) were prepared firstly using protease-cleavable drug linkers [182]. While the co-delivery of both drugs led to improved antitumor effects compared to the single counterparts, we also established the importance of a small and flexible glycine linker in modifying the spatial conformation of the polymer–drug conjugate, which promoted the release of both drugs and the significant improvement in biological activity [182]. TNBC tumors display unique microenvironmental characteristics, including an acidic pH and high GSH concentration [183], especially compared to the Luminal A subtype, which can be taken advantage of to inhibit metastases. Therefore, in a follow-up study, we incorporated a pH-labile hydrazone linker for the conjugation of doxorubicin combined with the selected Gly-AGM as means to further potentiate the inhibition of metastasis [184]. We demonstrated that a lower loading of doxorubicin and shorter hydrazone linkers triggered a more controlled and sustained doxorubicin already at the TME, producing optimal antitumor and antimetastatic effects in vivo [184].
As an example of the importance of the 4 T1 TNBC mouse model at the clinical level, Biancacci et al. employed this model to evaluate the biodistribution and target site accumulation of PEG-b-poly(N-(2-hydroxypropyl)methacrylamide)(pHPMA)-lactate core-crosslinked block copolymer micelle (CCPM), the CriPec® platform developed by Cristal Therapeutics [185]. Based on this platform, CPC634 is a core-crosslinked micelle with covalently bound docetaxel that induced the complete regression of breast tumors after a single injection in mice and is currently in Phase I/II clinical trials for various tumor types (NCT03742713) [186], [187]. Using advanced imaging techniques, the authors differentiated between the accumulation of the CCPMs in the tumor and stroma, obtained detailed insight into the interactions between CCPMs and cancer, endothelial, and immune cells within the tumor, liver, and spleen, and reported extensive tumor and immune cell accumulation in all three organs [185]. These results suggest the utility of PEG-b-pHPMA-lactate CCPMs to target therapeutic agents to tumor and TME cells as an improved breast cancer treatment strategy.
Despite the extensive use of syngeneic models in the evaluation of nanomedicines, the implementation of human cancer cells in CDX and PDX breast cancer models allows for the more faithful recapitulation of the phenotypic and genetic characteristics of tumors.
3.1.1.2.2. Cell-derived xenografts
CDX breast tumor models involve the transplantation of human breast cancer cells into an immunodeficient animal model [165], [170]. While the use of human cells brings closer the recapitulation of the human disease and CDX models represent an amenable model system for the analysis of nanomedicines, the lack of an immune system represents a crucial limitation. There exist many examples of the pre-clinical evaluation of nanomedicines in CDX models, with MDA-MB-231 (TNBC) and MCF7 (luminal A) cells commonly employed.
For example, targeting CSCs represents a prominent application of nanomedicines. Gener et al. employed an MCF7-derived tumor to evaluate polymeric micelles loaded with Zileuton®, an active inhibitor of arachidonate 5-lipoxygenase gene (ALOX5), a critical regulator of CSCs in chronic myeloid leukemia [169]. Phase II randomized trials studying the efficacy of Zileuton® combined with standard treatments have yet to yield positive results, perhaps due to this compound's hydrophobicity and the associated high IC50 value; however, the nanoformulation of Zileuton® provided for a significant reduction of intratumoral and circulating CSC levels [169].
The implementation of MDA-MB-231 cells generally provides for a more invasive, metastatic, and experimentally reproducible model than MCF7 cells, in agreement with the behavior of the relevant tumor types in the clinical scenario [188], [189], [190]. Furthermore, the use of estrogen-dependent breast cancer cells such as MCF7 requires the administration of additional supplements such as estradiol to promote tumor growth initiation and growth, thereby adding additional complexity [165], [191]. Examples of MDA-MB-231-based CDX models for the evaluation of nanomedicines include a study from Zheng et al., who employed MDA-MB-231 tumor-bearing nude mice to demonstrate the antitumor efficacy of a superparamagnetic iron oxide nanoparticle (SPIO) containing paclitaxel as the active agent [192]. The authors reported enhanced tumor accumulation and antitumor efficacy following the added use of a pH-responsive peptide (H7K(R2)2) to target the TME. In another example, Liu et al. evaluated the antitumor efficacy of a folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green in subcutaneous MDA-MB-231-derived tumors in BALB/c nude mice [193]. Laser-mediated activation permitted the controlled release of paclitaxel in areas of high folate-receptor densities, which demonstrated high antitumor efficacy.
Even given their immunodeficient status, CDX models can be effectively used to evaluate immunotherapies [194]. Only 20% of TNBCs tumors express PD-L1, which correlates with the presence of tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy [195], [196]. LesniakWojciech et al. demonstrated high levels of PD-L1 expression in the MDA-MB-231 cell line and correlated this to an increase in atezolizumab uptake in vitro and in vivo when compared to models with lower PD-L1 expression [177].
Although CDX models have many advantages, the use of cell lines entails notable limitations that include the loss of tumor heterogeneity or the use of a clonal population of cells that suffer from genetic drift associated with ongoing culture (Section 2) [197]. Thus, CDX tumors can be used to provide proof of principle in a simple manner, but they may not accurately reflect the clinical setting. The development of PDX models represents a solution, providing a better reflection of intratumoral and intertumoral heterogeneity. PDX tumors, unlike CDX models, retain the architecture and stromal components of the original tumor and more accurately represent the complex biochemical and physical interactions between cancer cells and their microenvironment. Therefore, PDX models represent a better scenario for predicting the outcome of nanomedicines mainly those targeting TME [198].
3.1.1.2.3. Patient-derived xenografts
PDX models are generated by transplanting human primary tumor cells, tumor fragments [123], or patient-derived tumor organoids (generating patient-derived organoid-derived xenografts, PDOX) into immunocompromised model animals with the hope of recapitulating the original heterogeneity of the tumor tissue [100]. While subcutaneous PDX transplantation models have been used to measure primary tumor growth in breast cancer, orthotopic PDX transplantation models are more suitable for mechanistic studies of metastasis and therapeutic resistance [125], [170], [199], [200]. DeRose et al. reported that directly implanting breast tumor tissues derived from patients into mouse mammary fat pads resulted in a remarkable recapitulation of human breast cancer [201]. These tumor grafts closely resembled the original tumors regarding their clinical markers and histopathology, hormone dependence/independence, gene expression and DNA copy number variations, and metastatic profile. Interestingly, the authors also found that co-engraftment of primary human mesenchymal stem cells alongside tumor grafts helped maintain phenotypic stability and improve growth by promoting angiogenesis and reducing necrosis.
Overall, PDX models offer notable advantages regarding the pre-clinical evaluation of nanomedicines and the identification and validation of new targets and functional biomarkers and can be used to evaluate personalized treatments on a patient-to-patient basis [124], [202]. A recent study from Miller-Kleinhenz et al. employed orthotopic human breast cancer PDX models established from surgically resected residual chemoresistant breast cancer patient biopsies to study possible nanomedicine treatments [203]. The authors noted the prevalence of CD44high/CD24low CSCs, which has been linked to therapeutic resistance and poor prognosis [204], and the overexpression of biomarkers associated with increased tumorigenicity, metastasis, and resistance to chemotherapy (LRP6 and urokinase plasminogen activator receptor, or uPAR) via the upregulation of Wnt/β-catenin pathway signaling [205], [206] within biopsies derived from doxorubicin-resistant tumors. In vitro treatment with a doxorubicin-loaded magnetic iron oxide nanoparticle (IONP) conjugated with peptides that targeted LRP6 and uPAR suppressed breast cancer cell invasion by inhibiting Wnt/β-catenin signaling and reducing the level of stemness-associated markers. Systemic administration of this dual-targeted nanomedicine led to enhanced tumor targeting and more robust tumor growth inhibition compared to non-targeted or single-targeted IONPs carrying doxorubicin, thereby suggesting the efficacy of this approach to chemoresistant breast cancer [203].
PDX breast cancer models display certain disadvantages that limit their routine pre-clinical use, including difficulties regarding their procurement and establishment in the laboratory [207], a requirement for immunodeficient models, and a lack of growth of some human breast cancer subtypes in mice. Strategies employed to overcome these problems and enhance tumor engraftment include supplementation with estradiol, a proven approach for both ER-positive and -negative breast cancer. Even though ER-negative breast tumors do not directly respond to estradiol stimulation, an ER-dependent mechanism involving myeloid cells promotes angiogenesis and tumor growth in said breast cancer subtypes [208]. Additionally, the requirement for human tissue limits the amount of starting material, while the use of immunocompromised or humanized mouse strains increases costs. Despite these difficulties, their human origin, complexity, structural heterogeneity, and overall similarity to the clinical situation make PDX breast cancer models a highly valuable platform for the evaluation of personalized nanomedicines [209].
3.1.2. Spontaneous murine breast cancer models
Spontaneous breast cancer models (GEMMs and carcinogen-induced models) fully recapitulate the tumorigenic process - from early-stage disease development to the generation of metastasis. Notably, such models possess a competent immune system and a naturally evolved TME and, therefore, faithfully mimic human disease [125].
While the use of these pre-clinical models can overcome the limitations associated with transplanted tumor models, including cell selection (syngeneic and CDX), high costs technical complexity (PDX), or the requirement for immunocompromised animals (xenografts) [210], spontaneous breast cancer models also display certain limitations. These include the extended period of time required to establish each model (up to a year, depending on the model) [127], thereby suggesting their implementation in a validation step during the analysis of a novel nanomedicine rather than as the first choice for in vivo evaluations. Due to this limitation, there exist few reported examples of the application of spontaneous models in the nanomedicine field; however, their implementation as validation tools may foster rapid clinical translation.
3.1.2.1. Genetically engineered mouse models
Tumors in GEMMs spontaneously arise in immunocompetent mice due to the presence of introduced genetic alterations (i.e., knock-in of potential oncogene or knock-out of potential tumor suppressor gene). The most common genetic manipulations in breast cancer models include the overexpression of mammary oncogenes or the mammary gland-specific deletion of tumor suppressor genes, such as the p53-null “T11” model [170], [211]
The overexpression of mammary oncogenes (e.g., cyclin D1, PyMT, neu/ErbB2/ HER2, Myc, Ras, SV40 Tag, and Wnt 1) via regulatory sequences that include the mouse mammary tumor virus (MMTV) long terminal repeat (LTR) promoter (for several oncogenes such as cyclin D1, neu/ErbB2/HER2, Myc, Wnt 1 and PyMT), the whey acidic protein (WAP) promoter (for the Ras oncogene), or the 5′ flanking region of the C3(1) component of the rat prostate steroid-binding protein gene (commonly used to drive expression of the SV40 large T-antigen - C3Tag) [125], [212], [213]. The administration of factors such as estrogen, lactogenic hormones, or steroid hormones to engineered mice activates the oncogene in question and leads to tumorigenesis (Table 5) [214].
Table 5.
GEMMs of Breast Cancer.
| Promoter | Activator | Mammary oncogene | Latency (week) | Metastasis |
|---|---|---|---|---|
| MMTV-LTR | Steroid hormones | neu/ErbB2 gene PyMT viral oncogene Cyclin D1 gene Myc gene Wnt1 gene |
30 4–8 88 16–76 32 |
Lungs Lungs & lymph nodes Non-metastatic Non-metastatic Non-metastatic |
| WAP | Lactogenic hormones | Ras gene | 24 | Lungs |
| C(3)1 | Estrogen | SV40 Tag viral oncogene | 21 | Lungs & lymph nodes |
| P53 null “T11” | Steroid hormones | p53 | 24–48 | Peritoneal cavity |
(Adapted from [214])
As GEMMs display the critical histopathological, molecular, and genetic characteristics of cancer, as well as de novo disease and full disease development (including the formation of blood vessels and a natural TME), they represent important tools regarding our understanding of how specific genetic elements can influence breast cancer tumorigenesis and metastasis, the evaluation of the immunogenicity of therapies [122], [215], and the definition of the molecular mechanisms of drug resistance [216].
The ability to explore drug resistance mechanisms and evaluate possible nanomedicines represents key advantage for GEMMs, especially given the problem of acquired chemoresistance in TNBC patients [217]. For example, Bowerman et al. employed the C3Tag breast cancer model that recapitulates basal-like subtypes of TNBC and displays resistance to chemotherapeutics from the taxane family (i.e., paclitaxel and docetaxel) [218]. This study aimed to improve docetaxel's efficacy in taxane-resistant TNBC treatment through its incorporation into biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticles. The subsequent pharmacokinetic analysis demonstrated that delivery of PLGA-docetaxel nanoparticles increased docetaxel circulation time and provided similar docetaxel exposure to the tumor compared to the clinical formulation of docetaxel, thereby increasing antitumor efficacy.
The main limitations of GEMMs include the extended periods of time required for model development and the low and heterogeneous rate of metastasis formation, which requires the use of large cohorts of animals to accurately measure metastatic disease and the therapeutic efficacy of treatments [125], [212]. Moreover, mice are normally sacrificed before the development of macroscopic metastases due to primary tumor burden. This problem can be overcome by orthotopic transplantation of GEMM‐derived tumor fragments, which maintain the intratumoral heterogeneity of donor tumors, followed by surgical resection to allow the development of clinically overt metastatic disease [212]. While the MMTV-PyMT and MMTV-Erbb2 models spontaneously metastasize to the lungs and lymph node [219] and have found use in the study of metastatic disease, few models mimic the clinical scenario of metastasis to the brain and bones [197].
Recent advances in CRISPR-based gene editing [220] and the orthotopic transplantation of spontaneous tumors into recipient mice have permitted improvements that have accelerated model development and improved homogeneity of metastatic spread. For example, Kim et al. employed MMTV-neu mouse-derived tumors for the in vivo evaluation of peptide-targeted liposomal doxorubicin nanoparticles with enhanced selectivity for HER2-positive breast tumor cells [221]. The authors cryopreserved portions of any spontaneously-arising tumors and then orthotopically transplanted them into female NOD-SCID mice. Overall, they demonstrated that HER2-targeted nanoparticles achieved 90% tumor growth inhibition compared to free doxorubicin. In another example, Song et al. employed FVB/n mice carrying a transgene for C3(1)-T-antigen and BALB/c mice carrying a p53-null T11 mouse-derived tumor to compare the efficacy of Doxil® and free doxorubicin treatment [6]. Overall, they demonstrated an increased efficacy for Doxil® compared to free doxorubicin in both models.
3.1.2.2. Carcinogen-induced models
Carcinogen-induced models develop spontaneous tumors after exposure to carcinogens such as metals (i.e., arsenic, cadmium, and lead), hormones, chemicals, radiation, or viruses [222]. Rats have been widely employed in this sense, as commonly used carcinogens induce hormone-dependent tumors that display histopathological characteristics and genetic alterations similar to those described in humans [223], [224], [225]. In mice, carcinogen-induced tumors tend to be hormone-independent [226].
The most common chemical carcinogens employed to develop breast cancer models are 7,12-dimethylbenzantracene (DMBA) and N-methyl-n-nitrosourea (NMU). DMBA, a classic polycyclic aromatic hydrocarbon, forms adducts in DNA after cytochrome P-450 bioactivation in the liver, while NMU is an alkylating agent that methylates guanine nucleosides and prompts AT:GC transition mutations. While NMU tends to generate more aggressive breast tumors with spontaneous metastases, DMBA generally generates less aggressive tumors that fail to metastasize [227]. Carcinogen-induced models develop a natural TME and mimic the multistage process of human mammary carcinogenesis; however, the main limitations include the extended times required to develop the model and the unpredictability in terms of time, location, and the number of tumors and metastasis formation [119].
Given these factors, carcinogen-induced models are commonly employed to study cancer development; however, very few examples of nanomedicine evaluations have been reported. Dagar et al. demonstrated the increased antitumor activity of a sterically-stabilized mixed phospholipid nanomicelle (SSMM) encapsulating paclitaxel and decorated with vasoactive intestinal peptide (VIP) as a targeting ligand when compared to free paclitaxel or an untargeted paclitaxel-SSMM [228]. MNU-induced breast tumors display approximately five-times more VIP receptors than the surrounding normal breast tissue [229] and are considered the optimal pre-clinical model to achieve proof of concept.
3.2. Additional animal models - from small to large animals
Despite the advantages of using murine models to study nanomedicines in breast cancer, the success rate regarding the translation of therapies into clinical practice remains relatively low [230]. Possible mediating factors include the fact that murine models can tolerate higher drug doses than human patients, as their bone marrow displays less sensitivity to many cytotoxic drugs and the reduced blood volume (~2 ml) limits analysis. Therefore, the involvement of other animal models may be required to generate sufficient pre-clinical-clinical data [231].
Additional pre-clinical animal models currently under development to study breast cancer therapies include zebrafish (Danio rerio), which are considered less complex than murine models but with interesting properties as an intermediate tool for nanomedicine screening [232]. Their advantages include rapid development, large numbers of offspring, and the transparency of embryos and larvae, which, importantly, allows a more straightforward and cost-effective evaluation of some aspects of nanomedicine biodistribution/pharmacology using diverse imaging techniques [232], [233]. Zebrafish provide a powerful intermediate model for the first screening of anticancer nanomedicines as various studies on angiogenesis [234], tumor growth, or metastasis have been reported for different types of nanomedicines [235], [236]. While these data generally lack robustness and reproducibility, the development of zebrafish as an accepted model by regulatory agencies may provide an interesting alternative/complement to murine pre-clinical models [232]. Zebrafish models may also find use in determining parameters including lethal dose, acute toxicity, teratogenicity, and organ-specific toxicity, which all represent established applications [237]. For example, Calienni et al. employed zebrafish to compare the in vivo toxicity and teratogenicity of doxorubicin-loaded mixed micelles (MMDOX) composed of D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and Tetronic® T1107 with free doxorubicin and Doxil® [238]. MMDOX displayed lower levels of lethality, morphological alterations, and neurotoxic effects compared to free doxorubicin; however, but Doxil® performed better overall. In this case, drug administration employed the oral or transdermal routes, and, therefore, these results require confirmation following intravenous injection in an in vivo mammal cancer model. The precision required for the automated injection of nanomedicines into the circulation currently remains a major unmet challenge that represents a significant barrier to the implementation of as a cost-effective model system [232].
Larger animal models, including companion animals [239], [240], non-human primates [241], or pigs [242], have been employed in pre-clinical breast cancer research. Companion animals such as cats and dogs have been employed given the closer clinical, biological, and genetic similarities of their tumors to the human disease than murine models [243], [244]. Both cats and dogs possess the same oncogenes and tumor suppressors that contribute to cancer development in humans and present with greater sequence homology than mice [245], [246], [247]. While age and environmental stressors influence the spontaneous development of cancer, dogs develop tumors at twice the frequency of humans and cats at half the frequency of humans. Cat and dog models possess an intact immune system, and heterogeneous tumors can develop into a recurrent, drug-resistant form of the disease and metastasize to distant sites [248]. Several studies have already highlighted the value of companion animal models in nanomedicine development [249], [250], [251]. Ali et al. treated cats and dogs with spontaneous breast cancer with gold nanorod (AuNR)-assisted plasmonic photothermal therapy (PPTT) as an adjunct to surgical approaches for solid tumor treatment [251]. In PPTT, exposure to near-infrared (NIR) laser light following the injection of AuNRs into tumors creates localized heat that induces tumor necrosis and apoptosis [252]. Encouragingly, the authors discovered that PPTT before surgery prompted tumor regression; furthermore, the impact on the blood vessels decreased blood loss during surgery and decreased the risk of metastasis.
Despite the similarities of breast cancer development in dogs and cats with humans, several disadvantages inhibit their widespread use as routine pre-clinical models, including elevated costs, extended timescales, and difficulties connected to gathering the minimum number of animals necessary for a single study [253]. Moral/ethical concerns perhaps represent the most critical problem, as research use is not well accepted by the general public. In the same manner, strict regulatory and ethical concerns significantly limit the use of non-human primates; however, pigs have been traditionally domesticated as a food source, and therefore, their use as research models raises fewer moral concerns [254]. Pigs display many anatomical and physiological similarities to humans and, consequently, have routinely been used to study the effect of nutrition, new surgical approaches, imaging modalities, and organ transplantation techniques. Pigs and humans exhibit similar pharmacokinetics, and pig cancer models have been employed to study disease progression [255] and regression upon treatment [256]. Importantly, their use to monitor cardiac function is well-recognized [257].
In the realm of nanomedicines, pig models have been used to evaluate cardioprotection [258], chemotherapy-associated cardiotoxicity [259], and hypersensitivity/infusion reactions (complement activation-related pseudoallergy) [260], [261]. For example, Gyöngyösi et al. compared the cardiotoxicity of doxorubicin and Myocet® in pigs by monitoring factors such as survival, body weight, and cardiac function [262]. In agreement with the clinical information available, the authors confirmed a reduced level of cardiac damage following Myocet® treatment due to a lower level of cardiac accumulation; furthermore, they proposed the specific-upregulation of interferon-stimulated genes in Myocet®-treated animals as a cardioprotective mechanism [262]. Many marketed nanomedicine products can induce life-threatening infusion reactions, including liposomes (Doxil® and Ambisome®), polymeric micelles (Taxol® and Taxotere®), or inorganic nanoparticles (Feraheme®) and pigs represents a suitable model system for the pre-clinical prediction of possible infusion reactions [260]. Examples include the evaluation of complement activation-related pseudoallergy following treatment with PEGylated liposomal-based nanomedicines such as Doxil® [260]; however, there do exist significant differences between the human and porcine immune responses. Porcine pulmonary intravascular macrophages (PIMs), which are not present in the lungs of humans or other pre-clinical models [260], rapidly extract nanomedicines from blood, thereby compromising the reliability of results [263]. Finally, attempts have been made to establish porcine GEMM breast cancer models [254], including those carrying BCRA1 mutations [264]; however, a representative pig breast cancer model has yet to be established [254], [264].
4. Current challenges and future opportunities for the use of pre-clinical breast cancer models in the clinical translation of nanomedicines
The clinical translation of nanomedicines for breast cancer treatment remains a slow and inefficient process [60], with very few examples currently employed on a routine clinical basis (Table 2). While factors such as difficult manufacturing, low reproducibility, high costs, and a lack of “quality by design” (QbD) implementation [209], [266], [267] contribute to the generally poor bench-to-bedside translation of nanomedicines, the suboptimal selection of pre-clinical models must be considered of prime importance, as <10% of anticancer nanomedicines with promising pre-clinical data have gained clinical approval [59].
Compared with other types of drug products, the lack of predictability regarding the performance of anticancer nanomedicines derives from the strong influence of pharmacokinetics, whole-body biodistribution, tumor accumulation and penetration, and drug release kinetics at the target site, among other important parameters. Furthermore, all these parameters vary significantly when comparing animal models to human patients [59].
Long-term cultured immortalized human cancer cell lines routinely used at pre-clinical stages possess growing concerns regarding their true identity [268]. Therefore, careful validation of the cell lines used for the evaluation of nanomedicines and the implementation of standardized culture conditions (e.g., passage number, serum supplementations, and confluence) represent essential steps to ensure experimental reproducibility [60]. In vitro 3D models that recapitulate the TME should replace traditional 2D cultures to allow an enhanced understanding of cell toxicity and uptake mechanisms and to accurately identify the molecular basis of the disease and the mechanism of action of the given nanomedicine. If possible, cells should be obtained from patient-derived biopsies to provide heterogeneity
Nevertheless, the relevance of nanomedicine evaluation in vitro will depend on the mechanism employed that one hopes will provide advantages over conventional therapies. For example, in vitro evaluations may suffice for the early development of nanomedicines with cell-specific targeting moieties or those that carry a combination of synergistic drugs or deliver nucleic acids. Conversely, the development of nanomedicines that rely on the alteration of parameters that function at a whole-organism level (biodistribution, toxicity, or clearance, among others) may require in vivo analysis to reveal their full potential (or lack thereof).
Nanomedicines selected from advanced in vitro testing should then quickly move to in vivo evaluation to fully understand pharmacokinetic/pharmacodynamic parameters following the selection of an appropriate model (see Table 6). Preferably, the implementation of orthotopic and immunocompetent hosts with adequate genetic backgrounds (Table 4) will ensure a high degree of correlation with the human disease. This approach represents the only real means to identify adverse effects, such as immune suppression or activation [59]. Whenever possible, humanized models and human cells (such as PDXs) should be employed. While this approach remains complicated and costly and the engraftment of many breast cancer subtypes is limited, the information generated from humanized and PDX-based studies provides highly relevant and valuable evidence to support the clinical translation of personalized nanomedicines. Current and future research efforts should be devoted to the development of better animal models of human immunity as a translational tool existing between pre-clinical models and the reality of human tumor immunology.
Table 6.
Examples of selected nanomedicines discussed within the manuscript, pre-clinical models used in their evaluation, and the main readouts in vitro and in vivo.
| Nanomedicine | In vitro model(s) | Most relevant parameters in vitro | In vivo model(s) | Most relevant parameters in vivo | REF |
|---|---|---|---|---|---|
| Nanomedicines in Clinics / Clinical Trials | |||||
| Doxil® | 2D: MDA-MB-231-BR (brain-seeking subclone) | Cell toxicity | Experimental metastasis by MDA-MB-231-BR intracerebrally injected in athymic nude mice. | Antitumor efficacy, biodistribution, pharmacokinetics | [149] |
| Doxil® | – | – | GEMM FVB/n strain carrying a transgene for C3(1)-T-antigen (C3-TAg) and tumors derived from BALB/c T11/TP53Null orthotopically transplanted into BALC/c mice. | Antitumor efficacy, pharmacokinetics, macrophage levels, microvessel density | [6] |
| Unloaded CPC634 (Cristal Therapeutics) | – | – | 4 T1 orthotopically injected in BALB/c | Biodistribution, tumor accumulation, stromal distribution, uptake, ex vivo imaging | [185] |
| Ferumoxytol® (Feraheme®, AMAG Pharmaceuticals Inc., Cambridge, MA, USA) | 2D: MDA-MB-468, HUVEC 2D co-culture: RAW264.7 + MMTV-PyMT-derived cells // murine bone-marrow-derived macrophages + MMTV-PyMT-derived cells |
Cell toxicity, ROS generation, apoptosis | MMTV-PyMT-derived cancer cells orthotopically injected on female FVB/N mice. Experimental metastasis with intravenously injected KPI-GFP-Luc in NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ mice. Metastatic to lung and liver. |
Antitumor efficacy, macrophages population study, metastasis | [69] |
| Myocet® | – | – | Domestic pigs | Cardiac safety |
[262] |
| Inorganic Systems | |||||
| MnO2-Platinum-Cobalt nanozymes | 2D: 4 T1, mouse embryonic fibroblasts NIH3T3 3D: 4 T1 cells on agarose-precoated wells |
Cell toxicity (2D and 3D), uptake (2D), ROS generation (2D and 3D), apoptosis, lipid peroxidation (2D), DNA double-strand breaks (2D), lysosomal disruption (2D) | 4 T1 subcutaneously injected in BALB/c | Antitumor efficacy, biodistribution, safety, pharmacokinetics | [82] |
| Titanium dioxide, silica, and gold NPs | 2D: Human microvascular endothelial cells, Human mammary microvascular endothelial cells, MDA-MB-231, MCF7 | Cell toxicity, migration assays, adhesion, uptake | MDA-MB-231-Luc orthotopically injected in NSG. Metastasis in lungs, liver, bone, and spleen | Antitumor efficacy, biodistribution, metastasis | [167] |
| PEGylated gold NPs | – | – | 4 T1 orthotopically injected in BALB/c, U87-MG subcutaneously injected in CD1 Nude, MMTV-PyMT GEMM in FVC/N strain, and TNBC PDX orthotopically injected in NOD-SCID | Tumor vasculature analysis, EPR effect, biodistribution, | [265] |
| Gold nanorod-assisted plasmonic photothermal therapy | – | – | Pet cats and dogs with spontaneous breast cancer tumors | Antitumor efficacy | [251] |
| Targeted inorganic systems | |||||
| Trastuzumab-labeled amine-functionalized starch-coated bionized nanoferrite nanoparticles | 2D: MCF7, MCF7/Neo, MCF/HER2, HCC1954, MDA-MB-231, BT474, SKBR3, RAW264.7 | Uptake, macrophage activation | MDA-MB-231, MCF7/Neo, MCF7/HER2, BT474, or HCC1954 cells orthotopically injected in athymic nude or NSG mice. Transgenic HuHER2-FVB/N mice-derived cells orthotopically implanted in athymic nude, NSG, or FVB/N mice. |
Antitumor efficacy, tumor accumulation | [151] |
| SPIO NPs containing a pH-responsive peptide H7K(R2)2 and paclitaxel | 2D: MDA-MB-231 | Cell toxicity, uptake | MDA-MB-231 subcutaneously injected in BALB/c nude mice | Antitumor efficacy, biodistribution | [192] |
| Magnetic IO NP-dually targeted peptides to Wnt/LRP5/6 and uPAR | 2D: MDA-MB-231 | Cell uptake, cell proliferation assay, invasion assay, cell cycle analysis, Western blotting | Orthotopic human breast cancer PDX in SCID mice | Antitumor efficacy, biodistribution | [98], [203] |
| PEG-coated carbon dots conjugated to folic acid encapsulating doxorubicin (CDs-PEG-FA/Dox) | 2D: HUVECs, T47D, and BT549 Microfluidics: HUVEC monolayer on channel A, T47D, or BT549 spheroids on channel C |
Cell toxicity, uptake, | – | – | |
| Liposomes | |||||
| Liposome containing doxorubicin and coated with anti-ICAM1 and anti- EGFR antibody | 2D: MDA-MB-231, MDA-MB-436, MDA-MB-157 and MCF10A | Cell toxicity, uptake, wound healing | MDA-MB-231-Luc orthotopically injected in nude. Experimental metastasis by MDA-MB-231-Luc lateral tail vein injection in nude mice, resulting in lung metastasis. |
Antitumor efficacy, safety, biodistribution, metastasis | [146] |
| HER2 peptide-targeted liposomal doxorubicin | 2D: BT-474, SK-BR-3, and MCF7 | Cell toxicity, uptake | Breast tumors generated by MMTV-neu mice were transplanted orthotopically in NOD-SCID mice. | Antitumor efficacy, biodistribution | [221] |
| Anginex-conjugated liposomal nanoparticles (nanobins) carrying arsenic trioxide and cisplatin | 3D: 4T1mCherry in hanging drop method. 3D co-culture: 4T1mCherry + C166-GFP + MEFs in hanging drop method (TTA: tumor tissue analogs). |
Cell toxicity, uptake | 3D 4T1mCherry or TTA orthotopically injected in athymic nude mice. Metastasis in the lungs. | Antitumor efficacy, tumor accumulation, metastasis | [95] |
| Anti-MUC1/CD44 dual-aptamer-conjugated liposomes encapsulating Dox | 2D: MCF7, MCF7-CSC. 3D: MCF7 on Matrigel layer. |
Cell toxicity (2D and 3D), Uptake (2D and 3D) | Experimental metastasis by MCF7-CSC and MCF7 lateral tail vein injection in athymic nude mice. Metastasis in lung and liver. | Metastasis | [148] |
| Polymeric Nanoparticles | |||||
| FA-PEG-PLGA-paclitaxel@indocyanine Green-Perfluorohexane NPs | 2D: MDA-MB-231, HUVEC | Cell toxicity, uptake | MDA-MB-231 orthotopically injected in BALC/c nude mice | Antitumor efficacy, biodistribution, safety | [193] |
| Rod-shaped PLGA nanoparticles encapsulating docetaxel | 2D: MD-MBA-231 | Cell toxicity | Taxane-resistant GEMMs: FVN/NJ strain carrying a transgene for C3(1) SV40 T-antigen | Antitumor efficacy, biodistribution, pharmacokinetics, dosing | [218] |
| Polymeric Micelles | |||||
| Lapatinib-loaded polycarbonate-doxorubicin conjugate micelles | 2D: 4 T1 | Cell toxicity, uptake, wound healing assay, invasion assay | 4 T1 subcutaneously injected in BALB/c | Antitumor efficacy, biodistribution, safety, | [75] |
| Polymeric micelles loaded with Zileuton® | 2D: MCF7 and MDA-MB-231. 3D: MCF7 and MDA-MB-231 in low attachment plates. |
Cell toxicity, CSC-resistance assay, cell transformation assay | MCF7 and MDA-MB-231 orthotopically injected in NOD-SCID mice | Antitumor efficacy, biodistribution, safety | [169] |
| Doxorubicin-loaded mixed micelles composed of TPGS and Tetronic® T1107 | – | – | Wild-type adult zebrafish | Biodistribution, safety | [238] |
| Polymer Conjugates | |||||
| PEG‐pLA‐pTBPC-Dox | 2D: MDA‐MB‐231, MCF7, HCC70, MDA‐MB‐468, MCF‐10A. 3D: MDA‐MB‐231 spheroids hanging drop method. |
Uptake (2D and 3D) Cytotoxicity (2D and 3D) | MDA-MB-231 orthotopically injected in CD-1 Nude mice | Antitumor efficacy, biodistribution, histology | [94] |
| PGA-Dox-(Glycine-AGM) | 2D: MCF7 and 4 T1 | Cell toxicity, Uptake | 4 T1 orthotopically injected in BALB/c | Antitumor efficacy, biodistribution, pharmacokinetics, | [182] |
| PGA-(Hydrazone-Dox)-(Glycine-AGM) | 2D: 4 T1 | Cell toxicity | 4 T1 orthotopically injected in BALB/c | Antitumor efficacy, metastasis, | [184] |
| High molecular weight dextran conjugated to a fluorophore (FITC-Dextran) | – | – | MDA-MB-231 orthotopically and subcutaneously injected in NSG | Tumor volume, Tumor accumulation, Vascularization, EPR effect | [132] |
| Additional Systems | |||||
| Chitosan-histidine-arginine/ plasmid DNA | 2D co-culture: MCF7 + primary normal human dermal fibroblasts | Uptake | – | – | [67] |
| Sterically stabilized mixed phospholipid nanomicelle encapsulating paclitaxel and decorated with VIP | 2D: MCF7 | Cell toxicity | Sprague Dawley rats intravenously injected with NMU | Antitumor efficacy, biodistribution, systemic arterial blood pressure |
[228] |
Abbreviations: AGM = aminoglutethimide; DOX = doxorubicin; EPR = enhanced permeability and retention; FA = folic acid; FITC = fluorescein isothiocyanate; GEMM = genetically modified mouse model; HPMA = hydroxypropyl methacrylamide; IHC = immunohistochemistry; IO = iron oxide; MEF = mouse embryonic fibroblasts; MMTV = mouse mammary tumor virus; NOD = non-obese diabetic; NP = nanoparticle; NSG = NOD scid gamma; pDNA = plasmid DNA; PDX = patient-derived xenograft; PEG = polyethylene glycol; PGA = poly-L-glutamic acid; pLA = poly(lactide); PLGA = poly(lactide-co-glycolic acid); PyMT = polyoma middle tumor-antigen; pTBPC = poly(2‐((tert‐butoxycarbonyl)amino)‐3‐propyl carbonate; SCID = severe combined immunodeficient; SPIO = Superparamagnetic iron oxide; TPGS = D-α-tocopheryl poly-ethylene glycol 1000 succinate
Lessons learned from those anticancer nanomedicines in current clinical use indicate that a deeper understanding of tumor pathology will dictate not only the incorporation of crucial nanomedicine design features (i.e., responsiveness, size, shape, and targeting moiety choice) [179] but will allow the establishment and implementation of pre-clinical models that provide reliable pharmacological and toxicological data. The foundations for “rational design” should also consider well-recognized aspects that impact the clinical performance of nanomedicines, including tumor vascularization (EPR effect) [269], the expression of disease-associated cell surface markers that allow cancer cell recognition and uptake, the TME, and the presence infiltrating immune cells at the target site [179].
In vivo pre-clinical breast cancer models for each given patient subtype should not only carefully recapitulate the molecular basis of the pathology but also - the presence of disrupted tumor-associated vasculature, ECM density, stromal composition [270], adequate levels of selected endogenous triggers (i.e., enzyme levels or GSS/GSH ratio), and the presence of TME conditions that impact nanomedicine anti-tumor efficacy (e.g., the host immunological status [269], [271] or the activation-deactivation of the angiogenic switch [139], [272]). Notably, different vascular extravasation pathways vary with the type or stage of the tumor and interact differently according to the specific physico-chemical characteristics of a given nanomedicine, a factor that must be considered. Consequently, while the influence of the EPR effect on nanomedicine performance has been demonstrated at the clinical level [139], other important competing/complementary mechanisms [265], [273] will likely be involved, as demonstrated with PEGylated AuNPs in four different breast cancer models [265]. The careful choice of a pre-clinical model may allow us to understand the contribution of non-EPR mechanisms.
We also require the full validation of active-targeting strategies for nanomedicines, which requires a detailed characterization of nanoparticle distribution within the target tissues and those organs at risk for non-specific accumulation. Novel label-free imaging modalities that do not alter the structure of nanomedicines (such as imaging mass spectrometry [274] represent emerging tools that will support pharmacokinetic/pharmacodynamic studies and allow a better understanding of the tissue biodistribution and fate of administered nanomedicines.
All the discussed aspects can vary from patient to patient and, more importantly, within a single tumor [9]; therefore, we require the implementation of functional biomarkers and companion diagnostics [1]. At the pre-clinical stage, a focus on biomarkers in model systems may improve predictability and foster the more rapid clinical translation of anticancer nanomedicines [1].
Acknowledgments
Acknowledgments
The authors acknowledge Stuart P. Atkinson for critical English editing and the use of Biorender.com in the Graphical abstracts and Fig. 1, Fig. 3, Fig. 4.
Funding
This work was supported by the European Research Council (ERC-CoG-2014-648831 ‘MyNano’) (MJV), European Commission Program H2020-INFRADEV-03-2018-2019 (EU-Openscreen-Drive, Proposal number: SEP-210496506) (MJV and MO), the Spanish Ministry of Science and Innovation (Refs: SAF2017-84689-R-B (MCUI/AEI/FEDER, UE) (MO); PID2019-108806RB-I00 (MJV) and PhD grant awarded to PB Ref: FPU18/00386), Centro de Investigación Príncipe Felipe (CIPF) PhD grant awarded to PS and the Generalitat Valenciana (PROMETEO/2019/065 (MO)).
Contributor Information
Paz Boix-Montesinos, Email: pboix@cipf.es.
Paula M. Soriano-Teruel, Email: psoriano@cipf.es.
Ana Armiñán, Email: aarminan@cipf.es.
Mar Orzáez, Email: morzaez@cipf.es.
María J. Vicent, Email: mjvicent@cipf.es.
References
- 1.Atkinson S.P., Andreu Z., Vicent M.J. Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. J Pers Med. 2018;8 doi: 10.3390/jpm8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denayer T., Stöhr T., Van Roy M. Animal models in translational medicine: Validation and prediction. New Horiz Trans Med. 2014;2:5–11. [Google Scholar]
- 3.Soysal S.D., Tzankov A., Muenst S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology. 2015;82:142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Tang H., Liu Z., Chen B. Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. Int J Nanomedicine. 2017;12:8483–8493. doi: 10.2147/IJN.S148359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Liu X., Yuan H., Yang Z., von Roemeling C.A., Qie Y. Therapeutic Remodeling of the Tumor Microenvironment Enhances Nanoparticle Delivery. Adv Sci. 2019;6:6. doi: 10.1002/advs.201802070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song G., Darr D.B., Santos C.M., Ross M., Valdivia A., Jordan J.L. Effects of Tumor Microenvironment Heterogeneity on Nanoparticle Disposition and Efficacy in Breast Cancer Tumor Models. Clin Cancer Res. 2014;20:6083. doi: 10.1158/1078-0432.CCR-14-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. Ca-Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 8.Wolfram J., Ferrari M. Clinical cancer nanomedicine. Nano today. 2019;25:85–98. doi: 10.1016/j.nantod.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J.M., Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 11.Huang K.-W., Hsu F.-F., Qiu J.T., Chern G.-J., Lee Y.-A., Chang C.-C. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci Adv. 2020;6:eaax5032. doi: 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedard P.L., Hansen A.R., Ratain M.J., Siu L.L. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P. Breast cancer. Nat Rev Dis Primers. 2019;5:019–0111. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 14.F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin. 68 (2018) 394-424. [DOI] [PubMed]
- 15.International Agency for Research on Cancer. Global Cancer Observatory. https://gco.iarc.fr/.
- 16.Sun Y.S., Zhao Z., Yang Z.N., Xu F., Lu H.J., Zhu Z.Y. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017;13:1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S., Desmedt C., Larsimont D., Sotiriou C., Goormaghtigh E. Change in the microenvironment of breast cancer studied by FTIR imaging. Analyst. 2013;138:4058–4065. doi: 10.1039/c3an00241a. [DOI] [PubMed] [Google Scholar]
- 18.Khamis Z.I., Sahab Z.J., Sang Q.X. Active roles of tumor stroma in breast cancer metastasis. Int J Breast Cancer. 2012;574025:19. doi: 10.1155/2012/574025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y., Spezia M., Huang S., Yuan C., Zeng Z., Zhang L. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waks A.G., Winer E.P. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 22.Peddi P.F., Ellis M.J., Ma C. Molecular Basis of Triple Negative Breast Cancer and Implications for Therapy, Int. J Breast Cancer. 2012 doi: 10.1155/2012/217185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Reilly E.A., Gubbins L., Sharma S., Tully R., Guang M.H., Weiner-Gorzel K. The fate of chemoresistance in triple negative breast cancer (TNBC) BBA Clin. 2015;3:257–275. doi: 10.1016/j.bbacli.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan R., Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 25.Wang M., Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Hartshorn C.M., Bradbury M.S., Lanza G.M., Nel A.E., Rao J., Wang A.Z. Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano. 2018;12:24–43. doi: 10.1021/acsnano.7b05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D., Si M., Xue H.Y., Wong H.L. Nanomedicine applications in the treatment of breast cancer: current state of the art. Int J Nanomedicine. 2017;12:5879–5892. doi: 10.2147/IJN.S123437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.S. Aulic, D. Marson, E. Laurini, M. Fermeglia, S. Pricl. 16 - Breast cancer nanomedicine market update and other industrial perspectives of nanomedicine. In: Thorat ND, Bauer J, editors. Nanomedicines for Breast Cancer Theranostics: Elsevier; 2020. p. 371-404.
- 29.Thorat N.D., Bauer J. 1 - Nanomedicine: next generation modality of breast cancer therapeutics. In: Thorat N.D., Bauer J., editors. Nanomedicines for Breast Cancer Theranostics. Elsevier; 2020. pp. 3–16. [Google Scholar]
- 30.Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Waterhouse D.N., Tardi P.G., Mayer L.D., Bally M.B. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24:903–920. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 32.Harris L., Batist G., Belt R., Rovira D., Navari R., Azarnia N. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q., Huang X.E., Gao L.L. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed Pharmacother. 2009;63:603–607. doi: 10.1016/j.biopha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 34.O'Byrne K.J., Thomas A.L., Sharma R.A., DeCatris M., Shields F., Beare S. A phase I dose-escalating study of DaunoXome, liposomal daunorubicin, in metastatic breast cancer. Br J Cancer. 2002;87:15–20. doi: 10.1038/sj.bjc.6600344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute (NCI), https://clinicaltrials.gov/ct2/show/NCT00004207.
- 36.Li Y., Chen N., Palmisano M., Zhou S. Pharmacologic Sensitivity of Paclitaxel to Its Delivery Vehicles Drives Distinct Clinical Outcomes of Paclitaxel Formulations. Mol Pharmaceutics. 2015;12:1308–1317. doi: 10.1021/acs.molpharmaceut.5b00026. [DOI] [PubMed] [Google Scholar]
- 37.Gardner E.R., Dahut W.L., Scripture C.D., Jones J., Aragon-Ching J.B., Desai N. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai N., Trieu V., Yao Z., Louie L., Ci S., Yang A. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 39.Lim W.T., Tan E.H., Toh C.K., Hee S.W., Leong S.S., Ang P.C.S. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol. 2010;21:382–388. doi: 10.1093/annonc/mdp315. [DOI] [PubMed] [Google Scholar]
- 40.Lee K.S., Chung H.C., Im S.A., Park Y.H., Kim C.S., Kim S.B. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 41.Ranade A.A., Joshi D.A., Phadke G.K., Patil P.P., Kasbekar R.B., Apte T.G. Clinical and economic implications of the use of nanoparticle paclitaxel (Nanoxel) in India. Ann Oncol. 2013;6 doi: 10.1093/annonc/mdt322. [DOI] [PubMed] [Google Scholar]
- 42.Brahmachari B., Hazra A., Majumdar A. Adverse drug reaction profile of nanoparticle versus conventional formulation of paclitaxel: An observational study. Indian J Pharmacol. 2011;43:126–130. doi: 10.4103/0253-7613.77341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi P., Miyata K., Kataoka K., Cabral H. Clinical Translation of Self-Assembled Cancer Nanomedicines. Adv Ther. n/a. 2020:2000159. [Google Scholar]
- 44.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Minckwitz G., Huang C.-S., Mano M.S., Loibl S., Mamounas E.P., Untch M. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2018;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien M.E., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 48.Ansari L., Shiehzadeh F., Taherzadeh Z., Nikoofal-Sahlabadi S., Momtazi-Borojeni A.A., Sahebkar A. The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther. 2017;24:189–193. doi: 10.1038/cgt.2017.9. [DOI] [PubMed] [Google Scholar]
- 49.Lao J., Madani J., Puértolas T., Alvarez M., Hernández A., Pazo-Cid R. Liposomal Doxorubicin in the treatment of breast cancer patients: a review. J Drug Delivery. 2013 (2013):456409-. doi: 10.1155/2013/456409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F., Porter M., Konstantopoulos A., Zhang P., Cui H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J Control Release. 2017;267:100–118. doi: 10.1016/j.jconrel.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koudelka Š., Turánek J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322–334. doi: 10.1016/j.jconrel.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Tezuka K., Takashima T., Kashiwagi S., Kawajiri H., Tokunaga S., Tei S. Phase I study of nanoparticle albumin-bound paclitaxel, carboplatin and trastuzumab in women with human epidermal growth factor receptor 2-overexpressing breast cancer. Mol Clin Oncol. 2017;6:534–538. doi: 10.3892/mco.2017.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nahleh Z.A., Barlow W.E., Hayes D.F., Schott A.F., Gralow J.R., Sikov W.M. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat. 2016;158:485–495. doi: 10.1007/s10549-016-3889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.A. Samyang Biopharm. https://www.samyangbiopharm.com/eng/ProductIntroduce/injection01.
- 55.Ventola C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 56.Singh A.T., Jaggi M., Khattar D., Awasthi A., Mishra S.K., Tyagi S. A novel nanopolymer based tumor targeted delivery system for paclitaxel. J Clin Oncol. 2008;26:11095. doi: 10.1007/s12094-012-0883-2. [DOI] [PubMed] [Google Scholar]
- 57.US Center for Drug Evaluation and Research. Kadcyla approval letter. February 22, August 2020.
- 58.Gradishar W.J., Tjulandin S., Davidson N., Shaw H., Desai N., Bhar P. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 59.Metselaar J.M., Lammers T. Challenges in nanomedicine clinical translation. Drug Deliv and Transl Res. 2020;10:721–725. doi: 10.1007/s13346-020-00740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ioannidis J.P.A., Kim B.Y.S., Trounson A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat Biomed Eng. 2018;2:797–809. doi: 10.1038/s41551-018-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabizon A.A., de Rosales R.T.M., La-Beck N.M. Translational considerations in nanomedicine: The oncology perspective. Adv Drug Deliv Rev. 2020;158:140–157. doi: 10.1016/j.addr.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Dai X., Cheng H., Bai Z., Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8:3131–3141. doi: 10.7150/jca.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa E., Ferreira-Gonçalves T., Chasqueira G., Cabrita A.S., Figueiredo I.V., Reis C.P. Experimental Models as Refined Translational Tools for Breast Cancer Research. Sci Pharm. 2020;88:32. [Google Scholar]
- 64.Arroyo-Crespo J.J., Armiñán A., Charbonnier D., Deladriere C., Palomino-Schätzlein M., Lamas-Domingo R. Characterization of triple-negative breast cancer preclinical models provides functional evidence of metastatic progression. Int J Cancer. 2019;145:2267–2281. doi: 10.1002/ijc.32270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fornaguera C., Solans C. Methods for the In Vitro Characterization of Nanomedicines-Biological Component Interaction. J Pers Med. 2017;7 doi: 10.3390/jpm7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ö.S. Aslantürk, In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages, Genotoxicity. In: Soloneski MLLaS, editor. A Predictable Risk to Our Actual World: IntechOpen; 2017.
- 67.Costa E.C., Gaspar V.M., Marques J.G., Coutinho P., Correia I.J. Evaluation of nanoparticle uptake in co-culture cancer models. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J., Burgess D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu H., Stenzel M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small. 2018;14:1702858. doi: 10.1002/smll.201702858. [DOI] [PubMed] [Google Scholar]
- 71.Baker B.M., Chen C.S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edmondson R., Broglie J.J., Adcock A.F., Yang L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A.Q. Khan, K. Rashid, S.S. Raza, R. Khan, F. Mraiche, S. Uddin, Role of 3D tissue engineering models for human cancer and drug development. 2019.
- 74.Nunes A.S., Barros A.S., Costa E.C., Moreira A.F., Correia I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019;116:206–226. doi: 10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 75.Guo Z., Liang E., Sui J., Ma M., Yang L., Wang J. Lapatinib-loaded acidity-triggered charge switchable polycarbonate-doxorubicin conjugate micelles for synergistic breast cancer chemotherapy. Acta Biomater. 2020;10:051. doi: 10.1016/j.actbio.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 76.Foroozandeh P., Aziz A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Research Letters. 2018;13:339. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seymour L.W., Ferry D.R., Kerr D.J., Rea D., Whitlock M., Poyner R. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 78.Donahue N.D., Acar H., Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Markman J.L., Rekechenetskiy A., Holler E., Ljubimova J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9 doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwon S., Ko H., You D.G., Kataoka K., Park J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc Chem Res. 2019;52:1771–1782. doi: 10.1021/acs.accounts.9b00136. [DOI] [PubMed] [Google Scholar]
- 82.Wang Z., Zhang Y., Ju E., Liu Z., Cao F., Chen Z. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat Comm. 2018;9:3334. doi: 10.1038/s41467-018-05798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.I. Mó, I.J. Sabino, D.d. Melo-Diogo, R. Lima-Sousa, C.G. Alves, I.J. Correia, The importance of spheroids in analyzing nanomedicine efficacy, Nanomedicine. 15 (2020) 1513-25. [DOI] [PubMed]
- 84.Terzi M.Y., Izmirli M., Gogebakan B. The cell fate: senescence or quiescence. Mol Biol Rep. 2016;43:1213–1220. doi: 10.1007/s11033-016-4065-0. [DOI] [PubMed] [Google Scholar]
- 85.Quayle L.A., Ottewell P.D., Holen I. Chemotherapy resistance and stemness in mitotically quiescent human breast cancer cells identified by fluorescent dye retention. Clin Exp Metastasis. 2018;35:831–846. doi: 10.1007/s10585-018-9946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barua S., Mitragotri S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Astashkina A.I., Jones C.F., Thiagarajan G., Kurtzeborn K., Ghandehari H., Brooks B.D. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials. 2014;35:6323–6331. doi: 10.1016/j.biomaterials.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 88.Lovitt C.J., Shelper T.B., Avery V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18:017–3953. doi: 10.1186/s12885-017-3953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kenny P.A., Lee G.Y., Myers C.A., Neve R.M., Semeiks J.R., Spellman P.T. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazzari G., Couvreur P., Mura S. Multicellular tumor spheroids: a relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym Chem. 2017;8:4947–4969. [Google Scholar]
- 91.Grainger S.J., Serna J.V., Sunny S., Zhou Y., Deng C.X., El-Sayed M.E.H. Pulsed Ultrasound Enhances Nanoparticle Penetration into Breast Cancer Spheroids. Mol Pharmaceutics. 2010;7:2006–2019. doi: 10.1021/mp100280b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ranga A., Gjorevski N., Lutolf M.P. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69–70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Cui X., Hartanto Y., Zhang H. Advances in multicellular spheroids formation. J R Soc, Interface. 2017;14:20160877. doi: 10.1098/rsif.2016.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taresco V., Abelha T.F., Cavanagh R.J., Vasey C.E., Anane-Adjei A.B., Pearce A.K. Functionalized Block Co-Polymer Pro-Drug Nanoparticles with Anti-Cancer Efficacy in 3D Spheroids and in an Orthotopic Triple Negative Breast Cancer Model. Adv Ther. n/a. 2020:2000103. [Google Scholar]
- 95.Sethi P., Jyoti A., Swindell E.P., Chan R., Langner U.W., Feddock J.M. 3D tumor tissue analogs and their orthotopic implants for understanding tumor-targeting of microenvironment-responsive nanosized chemotherapy and radiation. Nanomedicine. 2015;11:2013–2023. doi: 10.1016/j.nano.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vincent K.M., Findlay S.D., Postovit L.M. Assessing breast cancer cell lines as tumour models by comparison of mRNA expression profiles. Breast Cancer Res. 2015;17:114. doi: 10.1186/s13058-015-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schutgens F., Clevers H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Ann Rev Pathl: Mech Dis. 2020;15:211–234. doi: 10.1146/annurev-pathmechdis-012419-032611. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y., Gao D., Wang Y., Lin S., Jiang Y. A novel 3D breast-cancer-on-chip platform for therapeutic evaluation of drug delivery systems. Anal Chim Acta. 2018;7:97–106. doi: 10.1016/j.aca.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 99.Fujii M., Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 2021;20:156–169. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- 100.Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 2018;172 doi: 10.1016/j.cell.2017.11.010. 373 86.e10. [DOI] [PubMed] [Google Scholar]
- 101.Drost J., Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 102.Perumal O., Khandare J., Kolhe P., Kannan S., Lieh-Lai M., Kannan R.M. Effects of branching architecture and linker on the activity of hyperbranched polymer-drug conjugates. Bioconjugate Chem. 2009;20:842–846. doi: 10.1021/bc800526z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Datta P., Dey M., Ataie Z., Unutmaz D., Ozbolat I.T. 3D bioprinting for reconstituting the cancer microenvironment, npj Precis. Oncol. 2020;4:18. doi: 10.1038/s41698-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sontheimer-Phelps A., Hassell B.A., Ingber D.E. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019;19:65–81. doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- 105.Low L.A., Mummery C., Berridge B.R., Austin C.P., Tagle D.A. Organs-on-chips: into the next decade. Nat Rev Drug Discov. 2020;10:020–0079. doi: 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- 106.Subia B., Dahiya U.R., Mishra S., Ayache J., Casquillas G.V., Caballero D. Breast tumor-on-chip models: From disease modeling to personalized drug screening. J Control Release. 2021;331:103–120. doi: 10.1016/j.jconrel.2020.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu P.F., Cao Y.W., Zhang S.D., Zhao Y., Liu X.G., Shi H.Q. A bladder cancer microenvironment simulation system based on a microfluidic co-culture model. Oncotarget. 2015;6:37695–37705. doi: 10.18632/oncotarget.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang X., Ren L., Tebon P., Wang C., Zhou X., Qu M. Cancer-on-a-Chip for Modeling Immune Checkpoint Inhibitor and Tumor Interactions. Small. 2021;17:27. doi: 10.1002/smll.202004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pradhan S., Smith A.M., Garson C.J., Hassani I., Seeto W.J., Pant K. A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy. Sci. Rep. 2018;8:3171. doi: 10.1038/s41598-018-21075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shirure V.S., Bi Y., Curtis M.B., Lezia A., Goedegebuure M.M., Goedegebuure S.P. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip. 2018;18:3687–3702. doi: 10.1039/c8lc00596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ozcelikkale A., Moon H.R., Linnes M., Han B. In vitro microfluidic models of tumor microenvironment to screen transport of drugs and nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:14. doi: 10.1002/wnan.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y., Shi W., Kuss M., Mirza S., Qi D., Krasnoslobodtsev A. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater Sci Eng. 2018;4:4401–4411. doi: 10.1021/acsbiomaterials.8b01277. [DOI] [PubMed] [Google Scholar]
- 113.Heinrich M.A., Bansal R., Lammers T., Zhang Y.S., Michel Schiffelers R., Prakash J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv Mater. 2019;31:31. doi: 10.1002/adma.201806590. [DOI] [PubMed] [Google Scholar]
- 114.Cui H., Esworthy T., Zhou X., Hann S.Y., Glazer R.I., Li R. Engineering a Novel 3D Printed Vascularized Tissue Model for Investigating Breast Cancer Metastasis to Bone. Adv Healthc Mater. 2020;9:17. doi: 10.1002/adhm.201900924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.E. Garreta, R.D. Kamm, S.M. Chuva de Sousa Lopes, M.A. Lancaster, R. Weiss, X. Trepat, et al., Rethinking organoid technology through bioengineering, Nat Mater. 16 (2020) 020-00804. [DOI] [PubMed]
- 116.Brancato V., Oliveira J.M., Correlo V.M., Reis R.L., Kundu S.C. Could 3D models of cancer enhance drug screening? Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119744. [DOI] [PubMed] [Google Scholar]
- 117.Doke S.K., Dhawale S.C. Alternatives to animal testing: A review. Saudi Pharm J. 2015;23:223–229. doi: 10.1016/j.jsps.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.S.S. Rohiwal, A.P. Tiwari. 14 - Consensus protocols for animal experimentation and nanomedicine trials at clinical stage in breast cancer. In: Thorat ND, Bauer J, editors. Nanomedicines for Breast Cancer Theranostics: Elsevier; 2020. p. 331-49.
- 119.Dawidczyk C.M., Russell L.M., Searson P.C. Recommendations for Benchmarking Preclinical Studies of Nanomedicines. Cancer Res. 2015;75:4016. doi: 10.1158/0008-5472.CAN-15-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dawidczyk C.M., Kim C., Park J.H., Russell L.M., Lee K.H., Pomper M.G. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J Control Release. 2014;187:133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan H. What ADME tests should be conducted for preclinical studies? ADMET & DMPK. 2013;1:19–28. [Google Scholar]
- 122.Valcourt D.M., Kapadia C.H., Scully M.A., Dang M.N., Day E.S. Best Practices for Preclinical In Vivo Testing of Cancer Nanomedicines. Adv Healthcare Mater. 2020;9:2000110. doi: 10.1002/adhm.202000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ireson C.R., Alavijeh M.S., Palmer A.M., Fowler E.R., Jones H.J. The role of mouse tumour models in the discovery and development of anticancer drugs. Br J Cancer. 2019;121:101–108. doi: 10.1038/s41416-019-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manning H.C., Buck J.R., Cook R.S. Mouse Models of Breast Cancer: Platforms for Discovering Precision Imaging Diagnostics and Future Cancer Medicine. J Nucl Med. 2016;1 doi: 10.2967/jnumed.115.157917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holen I., Speirs V., Morrissey B., Blyth K. In vivo models in breast cancer research: progress, challenges and future directions. Dis Models Mech. 2017;10:359. doi: 10.1242/dmm.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olson B., Li Y., Lin Y., Liu E.T., Patnaik A. Mouse Models for Cancer Immunotherapy Research. Cancer Discovery. 2018;8:1358. doi: 10.1158/2159-8290.CD-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakamoto K., Schmidt J.W., Wagner K.-U. Mouse Models of Breast Cancer. In: Eferl R., Casanova E., editors. Mouse Models of Cancer: Methods and Protocols. New York, NY; Springer, New York: 2015. pp. 47–71. [Google Scholar]
- 128.Vargo-Gogola T., Rosen J.M. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 129.Okano M., Oshi M., Butash A., Okano I., Saito K., Kawaguchi T. Orthotopic Implantation Achieves Better Engraftment and Faster Growth Than Subcutaneous Implantation in Breast Cancer Patient-Derived Xenografts. J Mammary Gland Biol Neoplasia. 2020;25:27–36. doi: 10.1007/s10911-020-09442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gómez-Cuadrado L., Tracey N., Ma R., Qian B., Brunton V.G. Mouse models of metastasis: progress and prospects. Dis Model Mech. 2017;10:1061–1074. doi: 10.1242/dmm.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y., Zhang G.L., Sun X., Cao K.X., Ma C., Nan N. Establishment of a murine breast tumor model by subcutaneous or orthotopic implantation. Oncol Lett. 2018;15:6233–6240. doi: 10.3892/ol.2018.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ho K.S., Poon P.C., Owen S.C., Shoichet M.S. Blood vessel hyperpermeability and pathophysiology in human tumour xenograft models of breast cancer: a comparison of ectopic and orthotopic tumours. BMC Cancer. 2012;12:1471–2407. doi: 10.1186/1471-2407-12-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W., Fan W., Rachagani S., Zhou Z., Lele S.M., Batra S.K. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci Rep. 2019;9:019–47308. doi: 10.1038/s41598-019-47308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taurin S., Nehoff H., van Aswegen T., Greish K. Tumor Vasculature, EPR Effect, and Anticancer Nanomedicine: Connecting the Dots. Cancer Target. Drug Deliv. 2013:207–239. [Google Scholar]
- 135.Sflomos G., Dormoy V., Metsalu T., Jeitziner R., Battista L., Scabia V. A Preclinical Model for ERα-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell. 2016;29:407–422. doi: 10.1016/j.ccell.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Atiya H.I., Dvorkin-Gheva A., Hassell J., Patel S., Parker R.L., Hartstone-Rose A. Intraductal Adaptation of the 4T1 Mouse Model of Breast Cancer Reveals Effects of the Epithelial Microenvironment on Tumor Progression and Metastasis. Anticancer Res. 2019;39:2277–2287. doi: 10.21873/anticanres.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo X.L., Lin L., Hu H., Hu F.L., Lin Y., Luo M.L. Development and characterization of mammary intraductal (MIND) spontaneous metastasis models for triple-negative breast cancer in syngeneic mice. Sci Rep. 2020;10:020–61679. doi: 10.1038/s41598-020-61679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fiche M., Scabia V., Aouad P., Battista L., Treboux A., Stravodimou A. Intraductal patient-derived xenografts of estrogen receptor α-positive breast cancer recapitulate the histopathological spectrum and metastatic potential of human lesions. J Pathol. 2019;247:287–292. doi: 10.1002/path.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020 doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 140.Lee Y.-T.N. Breast carcinoma: Pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 141.Kutasovic J.R., McCart Reed A.E., Males R., Sim S., Saunus J.M., Dalley A. Breast cancer metastasis to gynaecological organs: a clinico-pathological and molecular profiling study. J Pathol Clin Res. 2019;5:25–39. doi: 10.1002/cjp2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monteran L., Ershaid N., Sabah I., Fahoum I., Zait Y., Shani O. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci Rep. 2020;10:020–70788. doi: 10.1038/s41598-020-70788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Calixto G.M., Bernegossi J., de Freitas L.M., Fontana C.R., Chorilli M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules. 2016;21 doi: 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu L., Valiente M. Preclinical Models of Brain Metastasis. 2020:37–51. [Google Scholar]
- 145.Mustafa D.A.M., Pedrosa R.M.S.M., Smid M., van der Weiden M., de Weerd V., Nigg A.L. T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathologica. 2018;135:581–599. doi: 10.1007/s00401-018-1806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo P., Yang J., Liu D., Huang L., Fell G., Huang J. Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y., Humphries B., Yang C., Wang Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer-Challenges and Opportunities. Nanomaterials. 2018;8 doi: 10.3390/nano8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim D.-M., Kim M., Park H.-B., Kim K.-S., Kim D.-E. Anti-MUC1/CD44 Dual-Aptamer-Conjugated Liposomes for Cotargeting Breast Cancer Cells and Cancer Stem Cells. ACS Appl Bio Mater. 2019;2:4622–4633. doi: 10.1021/acsabm.9b00705. [DOI] [PubMed] [Google Scholar]
- 149.Anders C.K., Adamo B., Karginova O., Deal A.M., Rawal S., Darr D. Pharmacokinetics and Efficacy of PEGylated Liposomal Doxorubicin in an Intracranial Model of Breast Cancer. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dobrovolskaia M.A., Shurin M., Shvedova A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol Appl Pharmacol. 2016;299:78–89. doi: 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Korangath P., Barnett J.D., Sharma A., Henderson E.T., Stewart J., Yu S.-H. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. Sci Adv. 2020;6:eaay1601. doi: 10.1126/sciadv.aay1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shi Y., Lammers T. Combining Nanomedicine and Immunotherapy. Acc Chem Res. 2019;52:1543–1554. doi: 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu H.-J., De Geest B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol Sin. 2020;41:879–880. doi: 10.1038/s41401-020-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moura L.I.F., Malfanti A., Peres C., Matos A.I., Guegain E., Sainz V. Functionalized branched polymers: promising immunomodulatory tools for the treatment of cancer and immune disorders. Mater Horizons. 2019;6:1956–1973. [Google Scholar]
- 155.Chulpanova D.S., Kitaeva K.V., Rutland C.S., Rizvanov A.A., Solovyeva V.V. Mouse Tumor Models for Advanced Cancer Immunotherapy. Int. J. Mol. Sci. 2020;21:4118–4122. doi: 10.3390/ijms21114118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brekke T.D., Steele K.A., Mulley J.F. Inbred or Outbred? Genetic Diversity in Laboratory Rodent Colonies. G3: Genes|Genomes|Genetics. 2018;8:679. doi: 10.1534/g3.117.300495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mestas J., Hughes C.C. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 158.Calbo J., van Montfort E., Proost N., van Drunen E., Beverloo H.B., Meuwissen R. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–256. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 159.Bonnotte B., Gough M., Phan V., Ahmed A., Chong H., Martin F. Intradermal injection, as opposed to subcutaneous injection, enhances immunogenicity and suppresses tumorigenicity of tumor cells. Cancer Res. 2003;63:2145–2149. [PubMed] [Google Scholar]
- 160.La-Beck N.M., Gabizon A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murphy K.M., Reiner S.L. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 162.Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 163.Jones S.W., Roberts R.A., Robbins G.R., Perry J.L., Kai M.P., Chen K. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest. 2013;123:3061–3073. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.-E., Greiner D.L., Brehm M.A. Humanized Mouse Models of Clinical Disease. Ann Rev Pathol: Mech Dis. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Puchalapalli M., Zeng X., Mu L., Anderson A., Hix Glickman L., Zhang M. NSG Mice Provide a Better Spontaneous Model of Breast Cancer Metastasis than Athymic (Nude) Mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yong K.S.M., Her Z., Chen Q. Humanized Mice as Unique Tools for Human-Specific Studies. Arch Immunol Ther Exp. 2018;66:245–266. doi: 10.1007/s00005-018-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peng F., Setyawati M.I., Tee J.K., Ding X., Wang J., Nga M.E. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat Nanotechnol. 2019;14:279–286. doi: 10.1038/s41565-018-0356-z. [DOI] [PubMed] [Google Scholar]
- 168.Katifelis H., Mukha I., Bouziotis P., Vityuk N., Tsoukalas C., Lazaris A.C. Ag/Au Bimetallic Nanoparticles Inhibit Tumor Growth and Prevent Metastasis in a Mouse Model. Int J Nanomedicine. 2020;15:6019–6032. doi: 10.2147/IJN.S251760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gener P., Montero S., Xandri-Monje H., Díaz-Riascos Z.V., Rafael D., Andrade F. Zileuton™ loaded in polymer micelles effectively reduce breast cancer circulating tumor cells and intratumoral cancer stem cells. Nanomedicine. 2020;24 doi: 10.1016/j.nano.2019.102106. [DOI] [PubMed] [Google Scholar]
- 170.Park M.K., Lee C.H., Lee H. Mouse models of breast cancer in preclinical research. Lab Anim Res. 2018;34:160–165. doi: 10.5625/lar.2018.34.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu T., Romanova S., Wang S., Hyun M.A., Zhang C., Cohen S.M. Alendronate-Modified Polymeric Micelles for the Treatment of Breast Cancer Bone Metastasis. Mol Pharm. 2019;16:2872–2883. doi: 10.1021/acs.molpharmaceut.8b01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.B.A. Pulaski, S. Ostrand-Rosenberg, Mouse 4T1 Breast Tumor Model, Curr Protoc Immunol. 39 (2000) 20.2.1-.2.16. [DOI] [PubMed]
- 173.Mei K.-C., Bai J., Lorrio S., Wang J.T.-W., Al-Jamal K.T. Investigating the effect of tumor vascularization on magnetic targeting in vivo using retrospective design of experiment. Biomaterials. 2016;106:276–285. doi: 10.1016/j.biomaterials.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie H.-Y., Shao Z.-M., Li D.-Q. Tumor microenvironment: driving forces and potential therapeutic targets for breast cancer metastasis. Chin J Cancer. 2017;36:36. doi: 10.1186/s40880-017-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmid P., Rugo H.S., Adams S., Schneeweiss A., Barrios C.H., Iwata H. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 176.Adams S., Diamond J.R., Hamilton E., Pohlmann P.R., Tolaney S.M., Chang C.W. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019;5:334–342. doi: 10.1001/jamaoncol.2018.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lesniak W.G., Chatterjee S., Gabrielson M., Lisok A., Wharram B., Pomper M.G. PD-L1 Detection in Tumors Using [64Cu]Atezolizumab with PET. Bioconjugate Chem. 2016;27:2103–2110. doi: 10.1021/acs.bioconjchem.6b00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F. Analysis of nanoparticle delivery to tumours. Nat Rev Mat. 2016;1:16014. [Google Scholar]
- 179.Nino-Pariente A., Nebot V.J., Vicent M.J. Relevant Physicochemical Descriptors of “Soft Nanomedicines” to Bypass Biological Barriers. Curr Pharm Des. 2016;22:1274–1291. doi: 10.2174/1381612822666151216152143. [DOI] [PubMed] [Google Scholar]
- 180.Li Y., An L., Lin J., Tian Q., Yang S. Smart nanomedicine agents for cancer, triggered by pH, glutathione, H(2)O(2), or H(2)S. Int J Nanomedicine. 2019;14:5729–5749. doi: 10.2147/IJN.S210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Feng L., Dong Z., Tao D., Zhang Y., Liu Z. The acidic tumor microenvironment: a target for smart cancer nano-theranostics. Nat Sci Rev. 2018;5:269–286. [Google Scholar]
- 182.Arroyo-Crespo J.J., Deladriere C., Nebot V.J., Charbonnier D., Masiá E., Paul A. Anticancer Activity Driven by Drug Linker Modification in a Polyglutamic Acid-Based Combination-Drug Conjugate. Adv Funct Mater. 2018;28:1800931. [Google Scholar]
- 183.Yu T., Di G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin J Cancer Res. 2017;29:237–252. doi: 10.21147/j.issn.1000-9604.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arroyo-Crespo J.J., Armiñán A., Charbonnier D., Balzano-Nogueira L., Huertas-López F., Martí C. Tumor microenvironment-targeted poly-L-glutamic acid-based combination conjugate for enhanced triple negative breast cancer treatment. Biomaterials. 2018;186:8–21. doi: 10.1016/j.biomaterials.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 185.Biancacci I., Sun Q., Möckel D., Gremse F., Rosenhain S., Kiessling F. Optical imaging of the whole-body to cellular biodistribution of clinical-stage PEG-b-pHPMA-based core-crosslinked polymeric micelles. J Control Release. 2020;30:30562–30569. doi: 10.1016/j.jconrel.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.F. Atrafi, H. Dumez, R.H.J. Mathijssen, C.W. Menke van der Houven van Oordt, C.J.F. Rijcken, R. Hanssen, et al., A phase I dose-escalation and pharmacokinetic study of a micellar nanoparticle with entrapped docetaxel (CPC634) in patients with advanced solid tumours, J Control Release. 325 (2020) 191-7. [DOI] [PubMed]
- 187.Hu Q., Rijcken C.J., Bansal R., Hennink W.E., Storm G., Prakash J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials. 2015;53:370–378. doi: 10.1016/j.biomaterials.2015.02.085. [DOI] [PubMed] [Google Scholar]
- 188.Sun N., Xu H.N., Luo Q., Li L.Z. Potential Indexing of the Invasiveness of Breast Cancer Cells by Mitochondrial Redox Ratios. Adv Exp Med Biol. 2016;923:121–127. doi: 10.1007/978-3-319-38810-6_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Y.L., Chou C.K., Kim M., Vasisht R., Kuo Y.A., Ang P. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci Rep. 2019;9:018–37625. doi: 10.1038/s41598-018-37625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mu Q., Wang H., Zhang M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin Drug Deliv. 2017;14:123–136. doi: 10.1080/17425247.2016.1208650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gottardis M.M., Robinson S.P., Jordan V.C. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: A model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30:311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 192.Zheng X.C., Ren W., Zhang S., Zhong T., Duan X.C., Yin Y.F. The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2018;13:1495–1504. doi: 10.2147/IJN.S157082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu F., Chen Y., Li Y., Guo Y., Cao Y., Li P. Folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green for photoacoustic/ultrasound imaging and chemo/photothermal therapy. Int J Nanomedicine. 2018;13:5139–5158. doi: 10.2147/IJN.S167043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reguera-Nuñez E., Xu P., Chow A., Man S., Hilberg F., Kerbel R.S. Therapeutic impact of Nintedanib with paclitaxel and/or a PD-L1 antibody in preclinical models of orthotopic primary or metastatic triple negative breast cancer. J. Exp. Clin. Cancer Res. 2019;38:16. doi: 10.1186/s13046-018-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wimberly H., Brown J.R., Schalper K., Haack H., Silver M.R., Nixon C. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol Res. 2015;3:326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murayama T., Gotoh N. Patient-Derived Xenograft Models of Breast Cancer and Their Application. Cells. 2019;8 doi: 10.3390/cells8060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lwin T.M., Hoffman R.M., Bouvet M. Advantages of patient-derived orthotopic mouse models and genetic reporters for developing fluorescence-guided surgery. J Surg Oncol. 2018;118:253–264. doi: 10.1002/jso.25150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eyre R., Alférez D.G., Spence K., Kamal M., Shaw F.L., Simões B.M. Patient-derived Mammosphere and Xenograft Tumour Initiation Correlates with Progression to Metastasis. J Mammary Gland Biol Neoplasia. 2016;21:99–109. doi: 10.1007/s10911-016-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X., Taftaf R., Kawaguchi M., Chang Y.-F., Chen W., Entenberg D. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019;9:96. doi: 10.1158/2159-8290.CD-18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.DeRose Y.S., Wang G., Lin Y.-C., Bernard P.S., Buys S.S., Ebbert M.T.W. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Que Z., Luo B., Zhou Z., Dong C., Jiang Y., Wang L. Establishment and characterization of a patient-derived circulating lung tumor cell line in vitro and in vivo. Cancer Cell Int. 2019;19:21. doi: 10.1186/s12935-019-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miller-Kleinhenz J., Guo X., Qian W., Zhou H., Bozeman E.N., Zhu L. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 2018;152:47–62. doi: 10.1016/j.biomaterials.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kakarala M., Wicha M.S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu C.C., Prior J., Piwnica-Worms D., Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.DiMeo T.A., Anderson K., Phadke P., Feng C., Perou C.M., Naber S. A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer. Cancer Res. 2009;69:5364. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.DeRose Y.S., Gligorich K.M., Wang G., Georgelas A., Bowman P., Courdy S.J. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol. 2013;14 doi: 10.1002/0471141755.ph1423s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dobrolecki L.E., Airhart S.D., Alferez D.G., Aparicio S., Behbod F., Bentires-Alj M. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2016;35:547–573. doi: 10.1007/s10555-016-9653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 210.Usary J., Darr D.B., Pfefferle A.D., Perou C.M. Overview of Genetically Engineered Mouse Models of Distinct Breast Cancer Subtypes. Curr Protoc Pharmacol. 2016;72:1–14. doi: 10.1002/0471141755.ph1438s72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jerry D.J., Kittrell F.S., Kuperwasser C., Laucirica R., Dickinson E.S., Bonilla P.J. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 212.Kersten K., de Visser K.E., van Miltenburg M.H., Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9:137–153. doi: 10.15252/emmm.201606857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Greenow K.R., Smalley M.J. Overview of Genetically Engineered Mouse Models of Breast Cancer Used in Translational Biology and Drug Development. Curr Protoc Pharmacol. 2015;70:1–14. doi: 10.1002/0471141755.ph1436s70. [DOI] [PubMed] [Google Scholar]
- 214.G. Kumar, V. Rao, N. Kumar. 13 - Development in efficacy assessment in relevant oncology models for breast cancer nanomedicine. In: Thorat ND, Bauer J, editors. Nanomedicines for Breast Cancer Theranostics: Elsevier; 2020. p. 299-329.
- 215.DuPage M., Jacks T. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr Opin Immunol. 2013;25:192–199. doi: 10.1016/j.coi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hanahan D., Wagner E.F., Palmiter R.D. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007;21:2258–2270. doi: 10.1101/gad.1583307. [DOI] [PubMed] [Google Scholar]
- 217.Nedeljković M., Damjanović A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells. 2019;8 doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bowerman C.J., Byrne J.D., Chu K.S., Schorzman A.N., Keeler A.W., Sherwood C.A. Docetaxel-Loaded PLGA Nanoparticles Improve Efficacy in Taxane-Resistant Triple-Negative Breast Cancer. Nano Lett. 2017;17:242–248. doi: 10.1021/acs.nanolett.6b03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kabeer F., Beverly L.J., Darrasse-Jèze G., Podsypanina K. Methods to Study Metastasis in Genetically Modified Mice. Cold Spring Harb Protoc. 2016;1 doi: 10.1101/pdb.top069948. [DOI] [PubMed] [Google Scholar]
- 220.Ideno N., Yamaguchi H., Okumura T., Huang J., Brun M.J., Ho M.L. A pipeline for rapidly generating genetically engineered mouse models of pancreatic cancer using in vivo CRISPR-Cas9-mediated somatic recombination. Lab Invest. 2019;99:1233–1244. doi: 10.1038/s41374-018-0171-z. [DOI] [PubMed] [Google Scholar]
- 221.Kim B., Shin J., Wu J., Omstead D.T., Kiziltepe T., Littlepage L.E. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for HER2-positive breast cancer cells to achieve enhanced in vivo efficacy. J Control Release. 2020;322:530–541. doi: 10.1016/j.jconrel.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Y., Yin T., Feng Y., Cona M.M., Huang G., Liu J. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. 2015;5:708–729. doi: 10.3978/j.issn.2223-4292.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shavi G.V., Nayak U.Y., Maliyakkal N., Deshpande P.B., Raghavendra R., Kumar A.R. Nanomedicine of anastrozole for breast cancer: Physicochemical evaluation, in vitro cytotoxicity on BT-549 and MCF-7 cell lines and preclinical study on rat model. Life Sci. 2015;141:143–155. doi: 10.1016/j.lfs.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 224.Clemens J.A., Bennett D.R., Black L.J., Jones C.D. Effects of a new antiestrogen, keoxifene (LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin levels. Life Sci. 1983;32:2869–2875. doi: 10.1016/0024-3205(83)90323-5. [DOI] [PubMed] [Google Scholar]
- 225.Constantinou A.I., Lantvit D., Hawthorne M., Xu X., van Breemen R.B., Pezzuto J.M. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001;41:75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- 226.Zarbl H. Toxicogenomic analyses of genetic susceptibility to mammary gland carcinogenesis in rodents: implications for human breast cancer. Breast Dis. 2007;28:87–105. doi: 10.3233/bd-2007-28109. [DOI] [PubMed] [Google Scholar]
- 227.Perse M., Cerar A., Injac R., Strukelj B. N-methylnitrosourea induced breast cancer in rat, the histopathology of the resulting tumours and its drawbacks as a model. Pathol Oncol Res. 2009;15:115–121. doi: 10.1007/s12253-008-9117-x. [DOI] [PubMed] [Google Scholar]
- 228.Dagar A., Kuzmis A., Rubinstein I., Sekosan M., Onyuksel H. VIP-targeted Cytotoxic Nanomedicine for Breast Cancer. Drug Deliv Transl Res. 2012;2:454–462. doi: 10.1007/s13346-012-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dagar S., Sekosan M., Rubinstein I., Önyüksel H. Detection of VIP Receptors in MNU-Induced Breast Cancer in Rats: Implications for Breast Cancer Targeting. Breast Cancer Res Treat. 2001;65:49–54. doi: 10.1023/a:1006406617497. [DOI] [PubMed] [Google Scholar]
- 230.Mak I.W., Evaniew N., Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- 231.Cekanova M., Rathore K. Animal models and therapeutic molecular targets of cancer: utility and limitations. Drug Des Devel Ther. 2014;8:1911–1921. doi: 10.2147/DDDT.S49584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sieber S., Grossen P., Bussmann J., Campbell F., Kros A., Witzigmann D. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv Drug Deliv Rev. 2019;151–152:152–168. doi: 10.1016/j.addr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 233.Shive H.R. Zebrafish models for human cancer. Vet Pathol. 2013;50:468–482. doi: 10.1177/0300985812467471. [DOI] [PubMed] [Google Scholar]
- 234.Cheng J., Gu Y.J., Wang Y., Cheng S.H., Wong W.T. Nanotherapeutics in angiogenesis: synthesis and in vivo assessment of drug efficacy and biocompatibility in zebrafish embryos. Int J Nanomedicine. 2011;6:2007–2021. doi: 10.2147/IJN.S20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou Q., Li Y., Zhu Y., Yu C., Jia H., Bao B. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J Control Release. 2018;275:67–77. doi: 10.1016/j.jconrel.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 236.Wu Q., Deng S., Li L., Sun L., Yang X., Liu X. Biodegradable polymeric micelle-encapsulated quercetin suppresses tumor growth and metastasis in both transgenic zebrafish and mouse models. Nanoscale. 2013;5:12480–12493. doi: 10.1039/c3nr04651f. [DOI] [PubMed] [Google Scholar]
- 237.Lee K.Y., Jang G.H., Byun C.H., Jeun M., Searson P.C., Lee K.H. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: promoting preclinical applications. Biosci Rep. 2017;37:30. doi: 10.1042/BSR20170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Calienni M.N., Cagel M., Montanari J., Moretton M.A., Prieto M.J., Chiappetta D.A. Zebrafish (Danio rerio) model as an early stage screening tool to study the biodistribution and toxicity profile of doxorubicin-loaded mixed micelles. Toxicol Appl Pharmacol. 2018;357:106–114. doi: 10.1016/j.taap.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 239.Alvarez C.E. Naturally occurring cancers in dogs: insights for translational genetics and medicine. Ilar J. 2014;55:16–45. doi: 10.1093/ilar/ilu010. [DOI] [PubMed] [Google Scholar]
- 240.MacEwen E.G. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–136. doi: 10.1007/BF00046339. [DOI] [PubMed] [Google Scholar]
- 241.Appleby E.C., Keymer I.F., Hime J.M. Three cases of suspected mammary neoplasia in non-human primates. J Comp Pathol. 1974;84:351–364. doi: 10.1016/0021-9975(74)90009-7. [DOI] [PubMed] [Google Scholar]
- 242.Perleberg C., Kind A., Schnieke A. Genetically engineered pigs as models for human disease. Dis Model Mech. 2018;11 doi: 10.1242/dmm.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rowell J.L., McCarthy D.O., Alvarez C.E. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Park J.S., Withers S.S., Modiano J.F., Kent M.S., Chen M., Luna J.I. Canine cancer immunotherapy studies: linking mouse and human. J ImmunoTher Cancer. 2016;4:97. doi: 10.1186/s40425-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lindblad-Toh K., Wade C.M., Mikkelsen T.S., Karlsson E.K., Jaffe D.B., Kamal M. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 246.Gardner H.L., Fenger J.M., London C.A. Dogs as a Model for Cancer. Annu Rev Anim Biosci. 2016;4:199–222. doi: 10.1146/annurev-animal-022114-110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gray M., Meehan J., Martínez-Pérez C., Kay C., Turnbull A.K., Morrison L.R. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Paoloni M., Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 249.Khanna C., Rosenberg M., Vail D.M. A Review of Paclitaxel and Novel Formulations Including Those Suitable for Use in Dogs. J Vet Intern Med. 2015;29:1006–1012. doi: 10.1111/jvim.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ali M.R., Ibrahim I.M., Ali H.R., Selim S.A., El-Sayed M.A. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int J Nanomedicine. 2016;11:4849–4863. doi: 10.2147/IJN.S109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ali M.R.K., Farghali H.A.M., Wu Y., El-Sayed I., Osman A.H., Selim S.A. Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats. Cancers (Basel). 2019;11:851. doi: 10.3390/cancers11060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pérez-Hernández M., del Pino P., Mitchell S.G., Moros M., Stepien G., Pelaz B. Dissecting the Molecular Mechanism of Apoptosis during Photothermal Therapy Using Gold Nanoprisms. ACS Nano. 2015;9:52–61. doi: 10.1021/nn505468v. [DOI] [PubMed] [Google Scholar]
- 253.Abdelmegeed S.M., Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15:8195–8205. doi: 10.3892/ol.2018.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kalla D., Kind A., Schnieke A. Genetically Engineered Pigs to Study Cancer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21020488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Flisikowska T., Kind A., Schnieke A. Pigs as models of human cancers. Theriogenology. 2016;86:433–437. doi: 10.1016/j.theriogenology.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 256.Roth W.J., Kissinger C.B., McCain R.R., Cooper B.R., Marchant-Forde J.N., Vreeman R.C. Assessment of juvenile pigs to serve as human pediatric surrogates for preclinical formulation pharmacokinetic testing. AAPS J. 2013;15:763–774. doi: 10.1208/s12248-013-9482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Crisóstomo V., Sun F., Maynar M., Báez-Díaz C., Blanco V., Garcia-Lindo M. Common swine models of cardiovascular disease for research and training. Lab Animal. 2016;45:67–74. doi: 10.1038/laban.935. [DOI] [PubMed] [Google Scholar]
- 258.Tejedor S., Dolz-Pérez I., Decker C.G., Hernándiz A., Diez J.L., Álvarez R. Polymer Conjugation of Docosahexaenoic Acid Potentiates Cardioprotective Therapy in Preclinical Models of Myocardial Ischemia/Reperfusion Injury. Adv. Healthcare Mat. 2021:2002121. doi: 10.1002/adhm.202002121. [DOI] [PubMed] [Google Scholar]
- 259.Watson A.L., Carlson D.F., Largaespada D.A., Hackett P.B., Fahrenkrug S.C. Engineered Swine Models of Cancer. Front Genet. 2016;7 doi: 10.3389/fgene.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Szebeni J., Simberg D., González-Fernández Á., Barenholz Y., Dobrovolskaia M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat Nanotechnol. 2018;13:1100–1108. doi: 10.1038/s41565-018-0273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Szebeni J. Hemocompatibility testing for nanomedicines and biologicals: predictive assays for complement mediated infusion reactions. Eur J Nanomed. 2012;4:33–53. [Google Scholar]
- 262.Gyöngyösi M., Lukovic D., Zlabinger K., Spannbauer A., Gugerell A., Pavo N. Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc Res. 2020;116:970–982. doi: 10.1093/cvr/cvz192. [DOI] [PubMed] [Google Scholar]
- 263.Moghimi S.M., Simberg D. Translational gaps in animal models of human infusion reactions to nanomedicines. Nanomedicine. 2018;13:973–975. doi: 10.2217/nnm-2018-0064. [DOI] [PubMed] [Google Scholar]
- 264.Donninger H., Hobbing K., Schmidt M.L., Walters E., Rund L., Schook L. A porcine model system of BRCA1 driven breast cancer. Front Genet. 2015;6 doi: 10.3389/fgene.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 266.Wu L.-P., Wang D., Li Z. Grand challenges in nanomedicine. Mater Sci Eng C. 2020;106 doi: 10.1016/j.msec.2019.110302. [DOI] [PubMed] [Google Scholar]
- 267.Greish K., Mathur A., Bakhiet M., Taurin S. Nanomedicine: is it lost in translation? Ther Deliv. 2018;9:269–285. doi: 10.4155/tde-2017-0118. [DOI] [PubMed] [Google Scholar]
- 268.Masters J.R. Cell-line authentication: End the scandal of false cell lines: Nature. 2012;492:186. doi: 10.1038/492186a. [DOI] [PubMed] [Google Scholar]
- 269.Golombek S.K., May J.-N., Theek B., Appold L., Drude N., Kiessling F. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi: 10.1016/j.addr.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shi Y., van der Meel R., Chen X., Lammers T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy: Theranostics. 2020;10:7921–7924. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lucas A.T., White T.F., Deal A.M., Herity L.B., Song G., Santos C.M. Profiling the relationship between tumor-associated macrophages and pharmacokinetics of liposomal agents in preclinical murine models. Nanomedicine. 2017;13:471–482. doi: 10.1016/j.nano.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 272.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 273.Moghimi S.M., Simberg D. Nanoparticle transport pathways into tumors. J Nanopart Res. 2018;20:169. doi: 10.1007/s11051-018-4273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dasgupta A., Biancacci I., Kiessling F., Lammers T. Imaging-assisted anticancer nanotherapy. Theranostics. 2020;10:956–967. doi: 10.7150/thno.38288. [DOI] [PMC free article] [PubMed] [Google Scholar]