Abstract
BACKGROUND AND PURPOSE:
Indirect consequences of the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) pandemic include those related to failure of patients to seek or receive timely medical attention for seemingly unrelated disease. We report our experience with stroke code imaging during the early pandemic months of 2020.
MATERIALS AND METHODS:
Retrospective review of stroke codes during the 2020 pandemic and both 2020 and matched 2019 prepandemic months was performed. Patient variables were age, sex, hospital location, and severity of symptoms based on the NIHSS. We reviewed the results of CT of the head, CTA, CTP, and MR imaging examinations and classified a case as imaging-positive if any of the imaging studies yielded a result that related to the clinical indication for the study. Both year-to-year and sequential comparisons were performed between pandemic and prepandemic months.
RESULTS:
A statistically significant decrease was observed in monthly stroke code volumes accompanied by a statistically significant increased proportion of positive imaging findings during the pandemic compared with the same months in the prior year (P < .001) and prepandemic months in the same year (P < .001). We also observed statistically significant increases in average NIHSS scores (P = .045 and P = .03) and the proportion of inpatient stroke codes (P = .003 and P = .03).
CONCLUSIONS:
During our pandemic period, there was a significantly decreased number of stroke codes but simultaneous increases in positivity rates, symptom severity, and inpatient codes. We postulate that this finding reflects the documented reluctance of patients to seek medical care during the pandemic, with the shift toward a greater proportion of inpatient stroke codes potentially reflecting the neurologic complications of the virus itself.
The impact of the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) coronavirus disease 2019 (COVID-19) pandemic has reverberated throughout virtually all facets of daily life, with implications beyond those associated with the viral infection itself. Within radiology, overall imaging use initially dropped sharply, largely due to suspension of elective clinical practice.1 In addition, shifts in specific technique and subspecialty use have paralleled evolving recommendations regarding diagnosis, understanding of disease manifestations, and increasing recognition of delayed and chronic disease complications. For example, the role of chest CT during the pandemic underwent shifts from initial use for diagnosis, particularly when real-time polymerase chain reaction (RT-PCR) testing availability was limited, to later use primarily for assessment of patients with worsening or chronic respiratory failure.2 At the time of this writing, at least partial recovery of imaging volumes has occurred in many centers.3
In New York City, one of the early epicenters of COVID-19 in the world, this disease initially overtook all others in health care use, with concerns regarding the availability of hospital beds and supportive technology to accommodate the rapidly growing number of severely ill patients. Nevertheless, it was intuitively expected during the early stages of the pandemic that the frequency of other illnesses in the population would be unchanged by the presence of the virus. If anything, the multisystem strain of the disease seemed likely to exacerbate pre-existing morbidities, so that underlying neurologic, cardiovascular, metabolic, and other chronic conditions might worsen during viral infections, resulting in an increased incidence of acute events. In February, first reports of prothrombotic complications of SARS-CoV-2 were published, further supporting the likelihood of an increase in emergency presentation of vascular-related diseases such as pulmonary embolism, myocardial infarction, and stroke.4
Paradoxically, however, reports in the cardiovascular literature showed a decrease in the incidence of diagnosed myocardial infarction during the initial weeks of the pandemic.5 At about the same time, reports in the media confirmed a growing suspicion that patients were choosing to stay home with cardiac and other acute symptoms that would have otherwise brought them to the emergency department due to fears of contracting the disease at health care facilities.6 Statistics compiled from the New York City Fire Department, which manages the city's 911 emergency response system, showed a striking increase of emergency calls that resulted in “refusals of medical aid” during March (118%) and early April (235%).7 Furthermore, emergency departments noted early drops in census followed by progressive increases, the latter composed primarily of patients with COVID-19-related illness.8
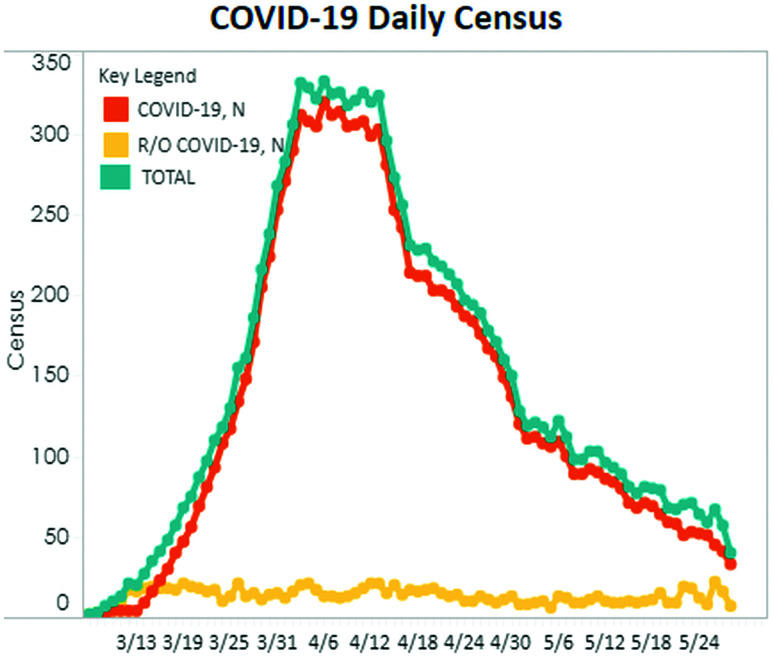
The first confirmed case of COVID-19 infection was diagnosed on RT-PCR testing at our 450-bed New York City hospital in early March 2020, with our peak occurring in early April (Fig 1). As both the emergency department and hospital censuses became dominated by patients positive for COVID-19, we noticed a trend toward a reduced frequency of stroke code–related imaging. This observed trend was later confirmed in a correspondence to the New England Journal of Medicine describing a concurrent 39% decrease in the use of the RApid processing of PerfusIon and Diffusion (RAPID; iSchemaView) software platform used at ours and many other US institutions to identify patients who might benefit from endovascular thrombectomy in the setting of acute stroke.9 Simultaneously, we began to note trends toward increased positivity rates of stroke code imaging, as well as shifts in patient demographics, including a greater proportion of stroke codes initiated in the inpatient setting. The purpose of this study was to retrospectively review our institution's stroke codes during the early COVID-19 pandemic (March 1 to April 30, 2020) to quantify imaging use and further analyze the positive imaging findings.
FIG 1.
Hospital census March 13, 2020, through May 24, 2020, shows the timing of the COVID-19 surge. The turquoise line indicates total hospital census; the orange line, patients positive for COVID-19; and the yellow line, patients under investigation for COVID-19. N indicates the number of patients in each category.
MATERIALS AND METHODS
In this retrospective Health Insurance Portability and Accountability Act–compliant, institutional review board–approved study, an initial query was performed at our academic teaching hospital's Radiology Information System for all “CT Head Stroke Protocol” studies performed between January 1, 2019, and April 30, 2019, and January 1, 2020, and April 30, 2020, to perform year-to-year comparisons. Typically, a stroke code is called when there is acute onset (<24 hours) of a neurologic deficit, though it may also be initiated if the duration is uncertain. A speaker announces “stroke code” and location to alert the stroke team; this is followed by emergent CT of the brain, typically followed by CTA of the neck and circle of Willis and CT perfusion studies when appropriate, for which results are dictated and communicated directly to the stroke team within 20 minutes of the patient being placed on the CT scanner. Patients were included if there was performance of, at minimum, the CT brain portion of the stroke code imaging evaluation. Exclusion criteria were cancellation of the code by the stroke team (typically because presentation was thought atypical for stroke) as well as stroke codes called in patients with known strokes (generally in patients transferred for treatment in whom changes in neurologic status were suspected).
Five board-certified radiologists, 4 with Certificates of Added Qualification in neuroradiology and with years of experience ranging from 2 to 26, reviewed the reports of each stroke code imaging study as well as the reports of any follow-up CT or brain MR imaging. We additionally reviewed the medical record and compiled relevant clinical information, including age, sex, patient service location (emergency department or inpatient setting), and NIHSS score at presentation.10 We included the COVID-19 RT-PCR-positivity status for March and April 2020, when these data were available.
Because our primary goal was to assess the yield of the stroke code for any acute neurologic event, we classified a case as imaging-positive if any of the stroke code CT or follow-up imaging studies yielded a result that related to the acute clinical indication for the study, whether or not it represented an ischemic stroke based on Trial of Org 10172 in Acute Stroke Treatment (TOAST) stroke classification.11 Examples of imaging-positive nonischemic diagnoses were hypertensive and traumatic intracranial hemorrhages as well as tumors and abscesses. However, chronic imaging findings such as encephalomalacia and vascular calcifications were not included as imaging-positive cases. In cases in which the initial reviewer was uncertain regarding positivity, this determination was made by consensus review.
Statistical Analysis
The dataset was split for statistical analyses into the following time periods: 1) 2020 COVID 3/4, representing the COVID-19 pandemic period from March 1 to April 30, 2020; 2) 2020 pre-COVID 1/2, representing the time period before the COVID-19 pandemic from January 1 to February 29, 2020; 3) 2019 pre-COVID 3/4, representing the same months during the COVID-19 pandemic in the prior year from March 1 to April 30, 2019; and 4) 2019 pre-COVID 1/2, representing the same months during the pre-COVID-19 time period in the prior year from January 1 to February 28, 2019. Multiple comparisons of the study groups were performed. Comparison of the 2020 COVID 3/4 and 2019 pre-COVID 3/4 was performed to assess differences during the COVID-19 pandemic compared with the same months in the prior year, 2019, to account for any monthly or seasonal variation in the data. Comparison of 2020 COVID 3/4 and 2020 pre-COVID 1/2 was performed to assess differences during the COVID-19 pandemic compared with the prior months in the same year to account for yearly variations in the data. Comparison of 2020 pre-COVID 1/2 and 2019 pre-COVID 1/2 was performed to assess whether differences existed in 2020 in the months before the pandemic, to assess other possible variations unknown in the data.
The χ2 and t tests were used to compare the demographic and clinical factors and imaging positivity rates among the study groups. P values < .05 were considered statistically significant. SAS, Version 9.4 (SAS Institute) was used for all statistical analyses.
RESULTS
There were 750 consecutive patients for whom a stroke code was called and who underwent, at minimum, a CT scan of the brain. Twenty-nine patients who were transferred with a known diagnosis of stroke and 8 patients for whom the stroke code was cancelled were excluded.
From January 1 to April 30, 2019, a total of 393 CT stroke codes were performed; and from January 1 to April 30, 2020, a total of 357 CT stroke codes were performed. A statistically significant difference was observed in both the monthly and bimonthly proportions of the stroke code volumes in 2020 compared with 2019 (P < .001, Table 1).
Table 1:
Comparison of the monthly stroke code volume in 2020 and 2019
| Month | 2020 No. (%) | 2019 No. (%) | P Value |
|---|---|---|---|
| January | 121 (34) | 84 (21) | <.001 |
| February | 113 (32) | 96 (24) | |
| March | 67 (19) | 105 (27) | |
| April | 56 (16) | 108 (28) | |
| January/February | 234 (66) | 180 (46) | <.001 |
| March/April | 123 (34) | 213 (54) |
Study Population Characteristics
The demographic and clinical characteristics of the study cohort are presented in Table 2. There were 45% (337/750) men and 55% (413/750) women, with an average age of 65.4 years (range, 18–98 years). No statistically significant differences were identified among the 3 comparisons with regard to patient age and sex. Furthermore, there were no statistically significant differences in the demographic and clinical characteristics and in the imaging-positive rates when comparing the 2020 COVID 1/2 and 2019 pre-COVID 1/2 periods.
Table 2:
Comparison of the demographic and clinical characteristics of the study cohort stratified by time periods
| 2020 Pre-COVID 1/2 | 2019 Pre-COVID 1/2 | P Value | 2020 COVID 3/4 | 2019 Pre-COVID 3/4 | P Value | 2020 COVID 3/4 | 2020 Pre-COVID 1/2 | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| No. | 234 | 180 | 123 | 213 | 123 | 234 | |||
| Mean age (range) (yr) | 66.1 (18–98) | 65.7 (22–98) | .81 | 66.4 (25–97) | 63.8 (20–101) | .18 | 66.4 | 66.1 | .87 |
| Sex (No.) (%) | |||||||||
| Male | 102 (44) | 73 (41) | .54 | 67 (54) | 95 (45) | .08 | 67 (54) | 102 (44) | .05 |
| Female | 132 (56) | 107 (59) | 56 (46) | 118 (55) | 56 (46) | 132 (56) | |||
| Location (No.) (%) | |||||||||
| ED | 192 (82) | 157 (87) | .15 | 89 (72) | 182 (85) | .003 | 89 (72) | 192 (82) | .03 |
| Inpatient | 42 (18) | 23 (13) | 34 (28) | 31 (15) | 34 (28) | 42 (18) | |||
| Mean NIHSS | 4.7 | 4.4 | .65 | 7.3 | 5.2 | .045 | 7.3 | 4.8 | .01 |
| Positive imaging findings (No.) (%) | 42 (18) | 32 (18) | .96 | 42 (34) | 37 (17) | <.001 | 42 (34) | 42 (18) | <.001 |
Note:—ED indicates emergency department; NA, not available.
Eighty-three percent (620/750) of stroke codes were called in the emergency department, and 17% (130/750), on inpatient floors. Statistically significant differences were identified in the incidence of inpatient-versus–emergency department location in comparisons of 2020 COVID 3/4 with either 2019 pre-COVID 3/4 or 2020 pre-COVID 1/2 periods (P = .0034 and P = .0335, respectively).
With regard to the NIHSS, statistically significant differences in symptom severity were identified in the comparison of 2020 COVID 3/4 and both 2020 pre-COVID 1/2 (P = .014) and 2019 pre-COVID 3/4 (P = .045) periods, with a higher proportion of patients presenting with worse NIHSS scores during the COVID-19 pandemic.
Of 123 stroke codes performed during the COVID-19 months of March and April 2020, twenty-seven percent (33/123) of patients were diagnosed as COVID-19-positive based on RT-PCR testing, 28% (35/123) were negative, and 45% (55/123) were not tested. Fourteen of the 33 (42%) stroke codes in the COVID-19-positive population were performed on inpatients. This represented the only statistically significant (P = .0380) frequency change in our measured variables between COVID-19-positive and -negative patients during this period.
Stroke Code Imaging Yield
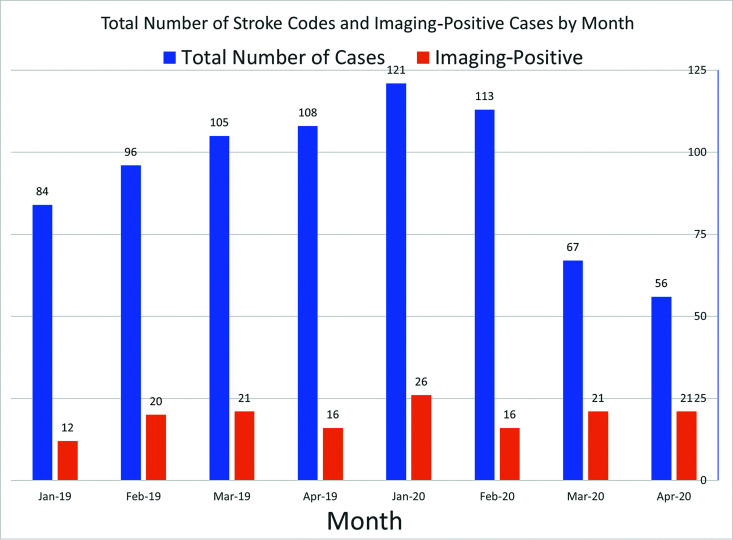
Table 2 reveal a statistically significantly increased proportion of imaging-positive cases during the 2020 COVID 3/4 period compared with the 2019 pre-COVID 3/4 (P < .001) and 2020 pre-COVID 1/2 (P < .001) periods. Figure 2 illustrates the total number of stroke codes and imaging-positive cases by month.
FIG 2.
Total number of stroke codes and imaging-positive cases by month.
Neurologic diagnoses designated as imaging-positive that did not fall into the TOAST classification of ischemic stroke were the following: intraparenchymal hematoma (n = 6), subdural hematoma (n = 5), subarachnoid hemorrhage (n = 5), primary brain tumor (n = 4), metastatic disease (n = 3), Guillain-Barré syndrome (n = 1), encephalitis (n = 1), and posterior reversible encephalopathy syndrome (n = 1). No statistically significant differences were identified in the proportion of nonischemic diagnoses during the 2020 COVID-19 pandemic compared with the same months in the prior year and pre-COVID-19 months in the same year.
DISCUSSION
Our data confirm previous reports of diminished numbers of stroke presentations during the early phases of the COVID-19 pandemic. Kansagra et al9 reported a 39% drop in RAPID software use from their defined prepandemic period of February 1 through February 29, 2020, compared with the pandemic period from March 26 through April 8, 2020, and de Havenon et al12 showed an approximately 18% drop in hospitalizations with a discharge diagnosis of stroke. In an analysis of data from a national repository of electronic health records from visits to Veterans Affairs facilities, Baum et al13 found a 41.9% reduction in overall admissions, with a 51.9% decrease in patients admitted with a principal diagnosis of stroke. It is likely that this drop is multifactorial in origin, though it seems probable that the reluctance of patients to seek medical aid was a primary contributor. A nationwide examination of emergency department visits performed by the Centers for Disease Control and Prevention showed a 42% drop during the pandemic period.14 In a nationwide survey with >1000 responders by the Society for Cardiovascular Angiography and Interventions, 61% of responders thought they were likely to acquire COVID-19 in a hospital, and half were more afraid of contracting the disease than experiencing a heart attack or stroke.15 Reduced availability of health care resources, including ambulances, likely contributed to reduced emergency department presentations, as well. Additionally, it is possible that in the early phase of the pandemic, many providers in emergency departments overwhelmed with patients with COVID-19 were less likely to activate a stroke code for less compelling clinical presentations. Recommendations regarding “protected” hyperacute stroke management using personal protective equipment and other safety measures did not emerge until later in the pandemic, and fear of nosocomial infection may have similarly impacted the provider threshold for initiating a stroke code for perceived lesser-yield presentations.16
We observed a statistically significant increase in the rate of imaging-positive stroke codes during the 2020 COVID-19 months compared with 2019 and the 2020 pre-COVID-19 months, accompanied by a simultaneous statistically significant increase in the proportion of patients presenting with severe stroke symptoms (based on the NIHSS). These findings suggest that patients who presented to the hospital with stroke-like symptoms during the pandemic period were more likely to have more severe symptomatology and to have an attributable acute neurologic event. Of note, a report by Paliwal et al17 of patients presenting with acute stroke symptoms before and during the pandemic showed that while there was a significant decline in acute stroke activations during the pandemic, the median NIHSS score was unchanged.
Any evaluation of stroke during the pandemic era must consider the now well-documented increased incidence of acute cerebrovascular disease in these patients. Early reports of an increased incidence of acute ischemic stroke, venous sinus thrombosis, and cerebral hemorrhage in SARS-CoV-2-infected patients emerged from China in early April 2020,18 with a pooled analysis of the literature published in late April demonstrating an approximately 2.5-fold increased incidence of stroke in patients with severe COVID-19.19 Postulated etiologies include sepsis-induced coagulopathy associated with high D-dimer and fibrinogen levels as well as endothelial damage related to binding of the spike protein of the virus to angiotensin-converting enzyme 2 receptors on the vascular endothelium.20 While 33 of 123 stroke codes during the 2020 COVID-19 months were performed on patients who tested positive for SARS-CoV-2, an even greater number (n = 55) were not tested during this period, reflecting the limited availability of testing during the early pandemic months and precluding precise quantification of the contribution of SARS-CoV-2-related stroke to our observed increased yield. Fourteen of the 33 stroke codes in the COVID-19-positive population (44%) were performed on inpatients, contributing to the statistically significant increase in overall frequency of inpatient codes performed during the COVID-19 months. We postulate that this increase in the relative frequency of inpatient stroke codes may reflect a shift toward sicker inpatients, as the hospital census shifted toward mostly COVID-19-positive patients and specifically those sick enough to require admission and away from those undergoing elective procedures and other less serious causes of hospitalization.
The implications of our findings with regard to these first and subsequent waves of the COVID-19 pandemic, as well as to future pandemics, are numerous. At the time of this report, excess mortality during the pandemic related to both COVID-19-related illness and unrelated disease has only begun to be quantified. On May 11, 2020, the New York City Department of Health and Mental Hygiene COVID-19 response team published a preliminary estimate of excess mortality during the COVID-19 outbreak and found that 22% (5293) of excess deaths between March 11 and May 2 were not identified as either laboratory-confirmed or probable COVID-19-associated deaths.21 Numerous factors make precise attribution of these deaths impossible, including-but-not-limited-to the vast array of potential etiologies and testing being limited and inaccurate in early stages of the pandemic. However, the authors stated, “Social distancing practices, the demand on hospitals and health care providers, and public fear related to COVID-19 might lead to delays in seeking or obtaining lifesaving care.”21 Certainly, our findings of decreased numbers of stroke codes with increased positivity rates suggest that many patients with transient or mild neurologic events may have not obtained appropriate early or comprehensive testing and intervention and are at high risk of subsequent morbidity and mortality. It is hoped that with the knowledge gained during the initial experience with this disease, practice will be modified to prepare for any future waves, and, specifically, for a larger proportion of more serious neurologic presentations in both emergency and inpatient departments.
Limitations of this study include limited and sometimes inaccurate testing for SARS-CoV-2 during the pandemic months as well as an inability to establish direct causality of the SARS-CoV-2 virus to those strokes occurring in COVID-19-positive patients. However, the main purpose of the analyses was not to compare COVID-19-positive and -negative groups in this study. Given that this is a retrospective study, the statistical analysis included patients with missing data variables. Additionally, all associations between our observed changes in stroke code use and reported reluctance of patients to present to the hospital and other potential causes represent conjecture and are unlikely to be proved with certainty. There are competing pressures between these effects and the expected increase in the frequency of stroke and other neurologic manifestations related to SARS-CoV-2 infection, and the contributions of each to overall change cannot be accurately quantified.
CONCLUSIONS
During the March and April 2020 COVID-19 surge in New York City, we observed a decrease in the number of stroke codes and an increase in imaging-positivity rates and the severity of presenting symptoms. While the etiologies of these shifts cannot be determined with certainty, we postulate that our findings reflect an observed reluctance of patients to seek medical care due to fears of contracting SARS-CoV-2, potentially coupled with other factors such as the overwhelming demand on health care workers during this period. A simultaneous shift toward a greater proportion of inpatient stroke codes may reflect the documented thromboembolic and other neurologic complications of the virus itself, a competing pressure on stroke code volumes that suggests an even greater reduction in new presentations to the emergency department. It seems likely that our findings represent a contributing factor toward the observed excess mortality beyond that directly associated with SARS-CoV-2 infection and may portend additional excess mortality from cerebrovascular causes during the recovery period and beyond.
ABBREVIATIONS:
- COVID-19
coronavirus disease 2019
- RT-PCR
real-time polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronavirus 2
Footnotes
Disclosures: Christopher G. Filippi—UNRELATED: Consultancy: Syntactx, Comments: interpretation of clinical trials of brain MR imaging examinations; Grants/Grants Pending: Foundation of the American Society of Neuroradiology grant and National MS Society Grant; Payment for Manuscript Preparation: article in the journal Topics in Magnetic Resonance Imaging (April 2020)*; Stock/Stock Options: minority stakeholder in start-up Avicenna.ai. Pina C. Sanelli—UNRELATED: Grants/Grants Pending: Siemens, Comments: research partnership.* *Money paid to the institution.
References
- 1. Naidich JJ, Boltyenkov A, Wang JJ, et al. Impact of the COVID-19 pandemic on imaging case volumes. J Am Coll Radiol 2020;17:865–72 10.1016/j.jacr.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 from the Fleischner Society. Chest 2020;158:106–16 10.1016/j.chest.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang M, Yeung T, Mendoza DP, et al. Imaging volume trends and recovery during the COVID-19 pandemic: a comparative analysis between a large urban academic hospital and its affiliated imaging centers. Acad Radiol 2020;27:1353–62 10.1016/j.acra.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020;75:2871–72 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Revive Health. COVID-19 consumer survey findings report: Part 3. https://www.thinkrevivehealth.com/covid-19/survey-findings-part-3. Accessed April 30, 2020
- 7. New Yorkers are refusing to be hospitalized over fear of getting coronavirus. New York Post April 12, 2020. https://nypost.com/2020/04/12/coronavirus-fears-have-new-yorkers-avoiding-hospitals-in-nyc/?utm_source=email_sitebuttons&utm_medium=site%20buttons&utm_campaign=site%20button. Accessed April 30, 2020
- 8. Comelli I, Scioscioli F, Cervellin G. Impact of the COVID-19 epidemic on census, organization and activity of a large urban emergency department. Acta Biomed 2020;91:45–49 10.23750/abm.v91i2.9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kansagra AP, Goyal MS, Hamilton S, et al. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med 2020;383:400–01 10.1056/NEJMc2014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke—A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019:50:10–31 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 11. Adams HP Jr, Biller J. Classification of subtypes of ischemic stroke; history of the Trial of Org 10172 in acute stroke treatment classification. Stroke 2015;46:e114–17 10.1161/STROKEAHA.114.007773 [DOI] [PubMed] [Google Scholar]
- 12. de Havenon A, Ney J, Callaghan B, et al. A rapid decrease in stroke, acute coronary syndrome, and corresponding interventions at 65 United States hospitals following emergence of COVID-19. medRxiv 2020. May 11. [Epub ahead of print] 10.1101/2020.05.07.20083386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baum A, Schwartz MD. Admissions to Veterans Affairs Hospitals for emergency conditions during the COVID-19 pandemic. JAMA 2020;324:96–99 10.1001/jama.2020.9972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartnett KP, Kite-Powell A, DeVies J, et al. ; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits: United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:699–704 10.15585/mmwr.mm6923e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grines CL. Consumer Survey Comparing Fear of COVID-19 Versus Heart Attack or Stroke. September 4, 2020. https://scai.org/consumer-survey-comparing-fear-covid-19-versus-heart-attack-or-stroke. Accessed October 1, 2020 [DOI] [PMC free article] [PubMed]
- 16. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke 2020;51:1891–95 10.1161/STROKEAHA.120.029838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paliwal PR, Tan BY, Leow AS, et al. Impact of the COVID-19 pandemic on hyperacute stroke treatment: experience from a comprehensive stroke centre in Singapore. J Thromb Thrombolysis 2020;50:596–603 10.1007/s11239-020-02225-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aggarwal G, Lippi G, Henry BM. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke 2020;15:385–89 10.1177/1747493020921664 [DOI] [PubMed] [Google Scholar]
- 20. Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res 2020;11:322–25 10.1007/s12975-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. New York City Department of Health and Mental Hygiene (DOHMH) COVID-19 Response Team. Preliminary estimate of excess mortality during the COVID-19 outbreak: New York City, March 11-May 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:603–05 10.15585/mmwr.mm6919e5 [DOI] [PubMed] [Google Scholar]