Background.
The 2013 HIV Organ Policy Equity Act has increased liver transplantation (LT) in HIV+ patients; however, transplant centers may remain reluctant to perform LT in HIV/hepatitis C virus (HCV)-coinfected patients due to inferior outcomes. We aimed to assess how direct-acting antivirals (DAAs) have impacted HIV+/HCV+-coinfected LT recipient outcomes.
Methods.
national data including 70 125 adult LT recipients between 2008 and 2019 were analyzed. Kaplan-Meier survival analysis and Cox proportional hazards model were used to analyze outcomes.
Results.
LT for HIV+ individuals increased in the DAA era from 28 in 2014 to 64 in 2019 (23 had HIV+/HCV+ coinfection). In the pre-DAA era, HIV+/HCV+-coinfected LT recipients had an increased risk of graft failure compared with HIV−/HCV−-uninfected LT recipients (hazard ratio [HR], 1.85; P < 0.001). In contrast, there was no difference in graft failure between HIV+/HCV+-coinfected versus HIV−/HCV−-uninfected LT recipients in the DAA era (HR, 1.24; P = 0.308). Among coinfected LT recipients in the DAA era, 1- and 3-y cumulative graft survivals were 88.6% and 81.7% compared with 76.3% and 58.0% in the pre-DAA era, respectively (P = 0.006). In Cox analysis, HCV coinfection was not associated with graft failure (HR, 1.00; 95% confidence interval, 0.53-1.89) among HIV+ LT recipients in the DAA era (n = 271). Black and Hispanic populations accounted for almost half of HIV+/HCV+ LTs in the DAA era.
Conclusions.
HIV+/HCV+-coinfected LT recipient outcomes have improved significantly in the DAA era. Our results should offer reassurance to transplant centers and encourage timely transplantation referral of HIV patients with decompensated cirrhosis, including patients coinfected with HCV.
INTRODUCTION
In the era of highly effective active antiretroviral therapy (HAART), there have been marked reductions in morbidity and mortality associated with HIV.1 In turn, a growing number of HIV+ patients are presenting with end-stage liver disease (ESLD) (~10% prevalence),2 predominantly from hepatitis C virus (HCV) due to shared routes of transmission.3 There are an estimated 2.3 million cases of HIV/HCV coinfection worldwide.4 Liver transplantation (LT) in select HIV+ patients with ESLD is effective with comparable outcomes to HIV− controls.5,6
The enactment of the HIV Organ Policy Equity Act (HOPE Act) in 2013 permitted the transplantation of organs from HIV+ donors into HIV+ recipients, thus expanding the donor pool for HIV+ patients. Although LT in HIV+ patients receiving HAART has been successful, the HOPE Act appears to be underused to date, with only 31 HOPE Act donor livers through 2018.7 This may be due to studies showing poor outcomes in HIV+/HCV+-coinfected LT recipients compared with matched HCV+-monoinfected patients with 5-y survival at 51%–54% (versus 71%–81%) in the pre–direct-acting antiviral (DAA) era.8-14 Graft loss due to recurrent HCV cirrhosis and the highly morbid fibrosing cholestatic hepatitis occurred at higher rates among HIV/HCV-coinfected patients.8-14 Moreover, HIV+ patients with hepatocellular carcinoma (HCC) had lower survival from time of waitlist listing and higher post-LT tumor recurrence compared with non–HIV-infected controls.15
In the DAA era, HCV+ patient outcomes have been transformed with high rates of sustained virologic response (SVR) (>90%), including HIV/HCV-coinfected patients.16,17 The success of DAA therapy has also translated into improved graft survival among HCV LT recipients.18,19 Treatment of pretransplant HCV patients with DAA therapy is often deferred to ensure that the patient’s MELD score remains competitive in the MELD-based organ allocation system and help mitigate any posttransplant insurance coverage issues that may arise with the second request of HCV treatment should a patient receive an HCV-viremic donor liver.20 The recommended treatment regimen is either glecaprevir–pibrentasvir or sofosbuvir glecaprevir–pibrentasvir velpatasvir with particular attention to potential drug–drug interactions such as high-dose proton pump inhibitors and amiodarone with sofosbuvir-inclusive regimens and certain statins (eg, atorvastatin) with other DAAs.21 A recent study from Europe and the United States showed that HIV-infected LT recipient outcomes had improved in the DAA era through 2015; however, HCV coinfection still conferred a significantly increased risk of graft failure and death.22 We hypothesized that as we move forward into the DAA era, HCV infection will no longer confer an increased risk for graft failure in HIV+ LT recipients. Thus, our study aim was to assess if the DAA era has improved HIV/HCV-coinfected LT recipient outcomes. Furthermore, we planned to ascertain the latest trends in HIV+ LT and assess the ongoing impact of the HOPE Act.
MATERIALS AND METHODS
Study Population and Data Acquisition
HIV-infected and HIV-uninfected adult (≥18 y) LT recipients between January 1, 2008, and December 31, 2019 (followed through September 4, 2020) were identified from the United Network for Organ Sharing/Organ Procurement and Transplantation Network (OPTN) database. These data are prospectively collected from transplant programs and organ procurement organizations.23 Patients who underwent retransplantation or multiorgan transplantation apart from simultaneous liver-kidney (SLK) were excluded. HIV serostatus was recorded as positive, negative, not done, unknown, or missing. Patients with not done, unknown, or missing status were considered as missing and excluded from analysis. HCV infection was defined as anti-HCV+ serology or positive nucleic acid testing (NAT), as NAT testing was not performed on all transplant recipients. Donor livers were considered HIV+ if any of HIV antibody, HIV NAT, and HIV antigen/antibody combination test were positive, as OPTN allocates all donors with any positive HIV test result through the HOPE Act.7
The study cohort was divided into 4 groups that were subdivided into the pre-DAA era (January 1, 2008, to December 31, 2012) and the DAA era (January 1, 2014, to December 31, 2019): (1) HIV+/HCV+ (coinfected); (2) HIV+/HCV− (HIV monoinfected); (3) HIV−/HCV+ (HCV monoinfected); (4) HIV−/HCV− (uninfected) LT recipients. Simeprevir and sofosbuvir received regulatory approval in November 2013, among other less efficacious DAA agents available throughout 2013. Therefore, 2013 was excluded from the analysis as it was considered a transitional year.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges and categorical variables with frequencies and percentages. Continuous variables were compared using the 2-sample Wilcoxon rank test (all samples failed the Shapiro–Wilk normality test), while categorical variables were compared using the 2-sided chi-squared test. The primary comparison was the incidence of graft failure between HIV+/HCV+-coinfected, HIV-monoinfected, HCV-monoinfected, and HIV−/HCV−-uninfected LT recipients in respective DAA eras. A secondary comparison was graft failure between coinfected LT recipients in the DAA and pre-DAA eras. As per OPTN, graft failure was defined as the occurrence of either recipient death or retransplantation. We also examined patient survival as a secondary endpoint. Graft and patient survival rates were computed by the Kaplan–Meier method, with log-rank testing used to ascertain differences between groups.
Because of possible confounders between eras (eg, improved medical care, LT recipient selection changes), we initially used multivariable Cox proportional hazards regression models to analyze the study groups in respective eras (pre-DAA and DAA). These results are provided as hazard ratios (HRs) with 95% confidence intervals (CIs). Several model iterations were performed incorporating the study groups as an indicator variable (HIV−/HCV− LT group as the reference). Variables associated with graft failure in the medical literature were selected a priori as additional covariates, including the following donor variables: age, diabetes, cold ischemic time, and donation after circulatory death; and the following recipient variables: age, diabetes, gender, body mass index, Model for End-Stage Liver Disease (MELD) score, and life support requirement.18,19,24,25 The final model considered the strength of association of each covariate with graft failure and biological plausibility. The number of failure events was also considered to mitigate the risk of a type II error (“false negative”) that mandated us to limit the number of covariates, and model violations and assumptions were assessed including multicollinearity and proportional hazards.
We confirmed our results by assessing the association of HCV infection with graft failure in multivariable Cox proportional hazards regression analysis among all HIV LT recipients in the DAA era. The same covariates incorporated into the initial Cox model were used in subsequent analyses. A P value of <0.05 was considered statistically significant. Statistical analyses were completed using Stata (Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX). Our study qualified for institutional review board exemption, given the presence of de-identified data (IRB IRB20-0804).
RESULTS
Frequency and Demographics
Among 78 173 adult LTs during the study period, 4592 were excluded because of previous LT or multiorgan transplantation. Of the remaining 73 581, there were 3967 SLK transplants and 2985 living-donor liver transplants (LDLTs). HIV serostatus was not available for 3456 (4.7%) patients. The proportion with missing HIV serostatus was significantly less frequent over time, from 12.2% in 2008 to 1.9% in 2019 (P < 0.001). Among 70 125 LTs with known HIV serostatus, 416 (0.6%) were HIV+ LT recipients. A total of 5333 LTs were performed in 2013 and were thus omitted from analysis. The practice of LT for patients with HIV has increased since the HOPE Act, from 28 patients in 2014 to 64 patients in 2019 (of whom 23 had HIV+/HCV+ coinfection) (Figure 1). The Northeast (United Network for Organ Sharing regions 2 and 9) had the highest HIV+/HCV+ LT practice, which increased over time (Figure 1). For example, HIV+/HCV+ coinfection LTs made up 1.2% of all LTs in New York in 2019. Among the 138 LT centers who were active in the DAA era, less than half (65/138, 47%) performed HIV+ LTs, whereas 6 LT centers accounted for 39% (105/271) of all HIV+ LTs.
FIGURE 1.
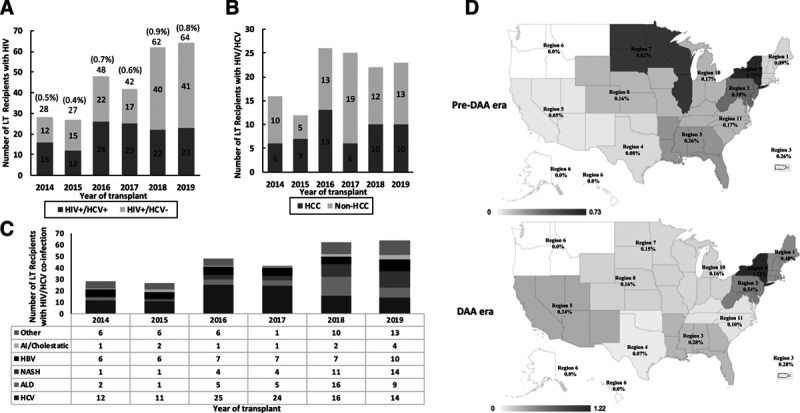
The practice of liver transplantation in patients with HIV/HCV in the DAA era in the United States. A, The annual number of LT recipients with HIV and as an overall percentage of all LTs is shown, further stratified by coinfection and HIV monoinfection. B and C, Stratification by HCC and cause of chronic liver disease, respectively. D, The geographic variation is demonstrated, by UNOS region, in LT practice for patients with HIV/HCV coinfection in the pre-DAA (2008–2012) and DAA (2014–2019) eras. The percentage of LTs in patients with HIV/HCV over all LTs in each respective region is shown. AI, autoimmune; ALD, alcohol-associated cirrhosis; DAA, direct-acting antivirals; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; LT, liver transplantation; NASH, nonalcoholic steatohepatitis; UNOS, United Network for Organ Sharing.
Table 1 outlines the demographic and clinical characteristics among the 4 DAA era groups and the HIV+/HCV+-coinfected pre-DAA group. Missing data comprised <1% of this final dataset. Table 2 provides a comparative analysis of the HIV+/HCV+-coinfected pre-DAA group with other pre-DAA groups. In the DAA era, there were 124 HIV+/HCV+-coinfected LT recipients, 147 HIV-monoinfected LT recipients, 11 231 HCV-monoinfected LT recipients, and 29 052 HIV−/HCV−-uninfected LT recipients, while there were 68 HIV+/HCV+-coinfected LT recipients in the pre-DAA era. HIV+/HCV+-coinfected LT recipients in the DAA era were older, had longer waitlist times, and received more HIV+ donors compared with HIV+/HCV+-coinfected LT recipients in the pre-DAA era. There have been shifts in racial group representation among LT recipients with HIV+/HCV+ coinfection from 2014 to 2019: a decrease was seen among White (61.8% to 46.8%) and Asian (4.6% to 3.2%) race, whereas an increase was observed among Black (23.5% to 25.8%) and Hispanic (14.7% to 20.2%) populations (P = 0.047). American Indians and other Pacific Islanders made up 4.0% of the HIV+/HCV+ coinfection LT DAA group, having 0% representation in the pre-DAA era. In the DAA era, HIV-monoinfected LT recipients had reduced waitlist time and higher MELD scores, as compared with HIV-monoinfected LT recipients in the pre-DAA era (Table 1).
TABLE 1.
Baseline characteristics and comparative analysis of liver transplant recipients with HIV+/HCV+ coinfection in the United States in the DAA era (2014–2019), and monoinfected and uninfected DAA recipients and HIV+/HCV+-coinfected pre-DAA recipients
| DAA (2014–2019) (N = 40 554) | Pre-DAA (2008–2012) | ||||
|---|---|---|---|---|---|
| HIV+/HCV+a (n = 124) | HIV+/HCV− (n = 147) | HIV−/HCV+ (n = 11 231) | HIV−/HCV−(n = 29 052) | HIV+/HCV+ (n = 68) | |
| Donor | |||||
| Age (y) | 39.0 (28.0–51.5) | 38.0 (25.0–53.0) | 40.0 (28.0–53.0) | 41.0 (28.0–54.0) | 40.5 (24.5–51.5) |
| BMI (kg/m2) | 25.8 (23.5–30.0) | 27.1 (23.3–32.9) | 27.0 (23.6–31.2) | 27.0 (23.5–31.3) | 25.7 (22.2–30.9) |
| Male gender | 76 (61.3) | 93 (63.3) | 6815 (60.7) | 17 273 (59.5) | 40 (58.8) |
| Caucasian race | 73 (58.9) | 88 (59.9) | 7518 (66.9) | 18 758 (64.6) | 38 (55.9) |
| Diabetes | 6 (4.8) | 13 (8.8) | 1333 (11.9)* | 3350 (11.5)* | 8 (11.8) |
| HIV+b (HOPE Act) | 24 (19.4) | 19 (12.9) | 2 (0.01)*** | 1 (0.01)*** | 0 (0.0)*** |
| DCD | 8 (6.5) | 12 (8.2) | 815 (7.3) | 2038 (7.0) | 4 (5.9) |
| Cold ischemic time (h) | 6.1 (4.8–7.9) | 5.5 (4.5–7.0) | 5.8 (4.5–7.2) | 5.6 (4.3–7.0)** | 6.0 (5.1–7.3) |
| DRIc | 1.76 (1.55–2.06) | 1.71 (1.51–2.09) | 1.71 (1.51–2.04) | 1.74 (1.53–2.10) | 1.71 (1.57–1.91) |
| Recipient | |||||
| Age (y) | 57.0 (52.5–62.0) | 55.0 (49.0–60.0)* | 60.0 (56.0–64.0)*** | 57.0 (48.0–64.0) | 54.0 (49.5–57.0)*** |
| Male gender | 92 (74.2) | 120 (81.6) | 8422 (75.0) | 17 856 (61.5)** | 52 (76.5) |
| Caucasian race | 58 (46.8) | 82 (55.8) | 7570 (67.4)*** | 21 118 (72.7)*** | 42 (61.8)* |
| BMI (kg/m2) | 26.7 (23.6–31.9) | 26.1 (23.2–30.3) | 28.0 (24.7–31.9) | 28.2 (24.5–32.6)* | 26.6 (23.4–30.0) |
| Posttransplant LOS (d) | 10.0 (7.0–14.0) | 10.0 (7.0–19.0) | 9.0 (6.0–15.0) | 10.0 (7.0–17.0) | 9.0 (7.0–13.0) |
| Waiting list time (d) | 230.5 (48.0–522.0) | 91.0 (17.0–243.0)*** | 196.0 (45.0–215.0) | 78.0 (13.0–264.0)*** | 99.0 (37.5–256.5)** |
| Diabetes | 27 (21.8) | 42 (28.6) | 3001 (26.7) | 8794 (30.3)* | 12 (17.7) |
| MELD score | 17.0 (10.0–24.0) | 22.0 (14.0–31.0)** | 16.0 (10.0–25.0) | 24.0 (16.0–33.0)*** | 18.0 (12.0–24.0) |
| Life support requirement | 3 (2.4) | 15 (10.2)* | 592 (5.3) | 2923 (10.1)** | 1 (1.5) |
| ICU | 6 (4.8) | 18 (12.2)* | 934 (8.3) | 4759 (16.4)** | 4 (5.9) |
| Dialysis requirement | 12 (9.7) | 18 (12.2) | 1296 (11.5) | 5150 (17.7)* | 2 (2.9) |
| Ascites (mild or worse) | 77 (62.1) | 105 (71.4) | 7232 (64.4) | 22 228 (76.5)*** | 51 (75.0) |
| Hepatic encephalopathy (grade 1 or worse) | 56 (45.2) | 92 (62.6)** | 6067 (54.0)* | 19 155 (65.9)*** | 40 (58.8) |
| PVT | 13 (10.5) | 28 (19.0)* | 1627 (14.5) | 4014 (13.8) | 6 (8.8) |
| HCC | 52 (41.9) | 32 (21.7)*** | 6387 (56.9)** | 5803 (20.0)*** | 29 (42.6) |
aHIV+/HCV+ group in DAA era are compared with all other groups through pairwise comparisons of P value from chi-squared test for categorical variables and 2-sample Wilcoxon rank test for continuous variables (all continuous variables failed the Shapiro-Wilk normality test).
bAs per positive HIV antibody, HIV NAT, or HIV antigen/antibody combination tests, by which OPTN allocate donors through the HOPE Act. All data were completely apart from the following missing data: donor variables = diabetes, n = 2105; cold ischemic time, n = 147; BMI, n = 53. Recipient variables = diabetes, n = 35; BMI, n = 9; length of stay, n = 491; days on waitlist, n = 1; ICU, n = 1; life support requirement, n = 1; portal vein thrombosis, n = 173; hepatic encephalopathy, n = 1; dialysis, n = 114; ascites, n = 1. Missing values of continuous variables were ignored, while missing values of categorical variables were assumed to be negative.
cCalculated by the DRI formula provided by Feng et al.24
Values are n (%) or median (interquartile range).
Significance codes: ***P < 0.001; **0.001 < P ≤ 0.01; *0.01 < P ≤ 0.05 (all other P > 0.05).
BMI, body mass index; DAA, direct-acting antiviral; DCD, donation after circulatory death; DRI, donor risk index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HOPE, HIV Organ Policy Equity; ICU, intensive care unit; LOS, length of stay; MELD, Model For End-Stage Liver Disease; NAT, nucleic acid test; OPTN, Organ for Procurement and Transplantation Network; PVT, portal vein thrombosis.
TABLE 2.
Baseline characteristics and comparative analysis of liver transplant recipients with HIV+/HCV+ coinfection in the United States in the pre-DAA era (2008–2012)
| Pre-DAA (2008–2012) (N = 24 238) | ||||
|---|---|---|---|---|
| HIV+/HCV+a (n =68) | HIV+/HCV− (n = 49) | HIV−/HCV+ (n = 10 451) | HIV−/HCV− (n = 13 670) | |
| Donor | ||||
| Age (y) | 40.5 (24.5–51.5) | 45.0 (31.0–53.0) | 42.0 (27.0–52.0) | 43.0 (27.0–56.0) |
| BMI (kg/m2) | 25.7 (22.2–30.9) | 25.9 (24.2–30.0) | 26.4 (23.2–30.5) | 26.3 (23.1–30.3) |
| Male gender | 40 (58.8) | 23 (46.9) | 6248 (59.8) | 7987 (58.4) |
| Caucasian race | 38 (55.9) | 26 (53.1) | 6871 (65.7) | 9084 (66.5) |
| Diabetes | 8 (11.8) | 4 (8.2) | 1116 (10.7) | 1558 (11.4) |
| DCD | 4 (5.9) | 0 (0) | 570 (5.5) | 624 (4.6) |
| Cold ischemic time (h) | 6.0 (5.1–7.3) | 7.2 (5.0–8.6)* | 6.0 (4.7, 8.0) | 6.1 (4.7–8.0) |
| DRIb | 1.71 (1.57–1.91) | 1.80 (1.59–2.01) | 1.71 (1.53–1.98) | 1.75 (1.55–2.10) |
| Recipient | ||||
| Age (y) | 54.0 (49.5–57.0) | 51.0 (46.0–57.0) | 56.0 (52.0–60.0)*** | 56.0 (48.0–62.0)* |
| Male gender | 52 (76.5) | 41 (83.7) | 7790 (74.5) | 8333 (61.0)** |
| Caucasian race | 42 (61.8) | 33 (67.3) | 7166 (68.6) | 10 004 (73.2)* |
| BMI (kg/m2) | 26.6 (23.4–30.0) | 24.1 (23.2–26.5) | 27.9 (24.7–31.7)** | 27.6 (24.1–32.3)* |
| Posttransplant LOS (d) | 9.0 (7.0–13.0) | 11.0 (7.0–16.5) | 9.0 (7.0–16.0) | 10.0 (7.0–17.0) |
| Waiting list time (d) | 99.0 (37.5–256.5) | 70.0 (7.0–274.0) | 110.0 (29.0, 298.0) | 69.0 (13.0–235.0) |
| Diabetes | 12 (17.7) | 11 (22.4) | 2361 (22.6) | 3733 (27.3) |
| MELD score | 18.0 (12.0–24.0) | 24.0 (13.0–34.0)* | 18.0 (12.0–26.0) | 22.0 (15.0–30.0)** |
| Life support requirement | 1 (1.5) | 5 (10.2)* | 488 (4.7) | 1159 (8.5)* |
| ICU | 4 (5.9) | 7 (14.3) | 811 (7.8) | 1895 (13.9) |
| Dialysis requirement | 2 (2.9) | 6 (12.2)* | 981 (9.4) | 1772 (13.0)* |
| Ascites (mild or worse) | 51 (75.0) | 30 (61.2) | 7804 (74.7) | 10 473 (76.6) |
| Hepatic encephalopathy (grade 1 or worse) | 40 (58.8) | 27 (55.1) | 6306 (60.3) | 8778 (64.2) |
| PVT | 6 (8.8) | 8 (16.3) | 979 (9.4) | 4014 (13.8) |
| HCC | 29 (42.6) | 18 (36.7) | 4811 (46.0) | 2697 (19.7)*** |
aHIV+/HCV+ group in pre-DAA era are compared with all other groups through pairwise comparisons of P value from chi-squared test for categorical variables and 2-sample Wilcoxon rank test for continuous variables (all continuous variables failed the Shapiro–Wilk normality test). All data were completely apart from the following missing data: donor variables = DCD, n = 783; diabetes, n = 890; cold ischemic time, n = 362; BMI, n = 50. Recipient variables = diabetes, n = 262; BMI, n = 8; length of stay, n = 323; days on waitlist, n = 5; portal vein thrombosis, n = 147; hepatic encephalopathy, n = 5; dialysis, n = 47; ascites, n = 5. Missing values of continuous variables were ignored, while those of categorical variables were assumed to be negative.
bCalculated by the DRI formula provided by Feng et al.24
Values are n (%) or median (interquartile range).
Significance codes: ***P < 0.001; **0.001 < P ≤ 0.01; *0.01 < P ≤ 0.05 (all other P > 0.05).
BMI, body mass index; DAA, direct-acting antiviral; DCD, donation after circulatory death; DRI, donor risk index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICU, intensive care unit; LOS, length of stay; MELD, Model For End-Stage Liver Disease; NAT, nucleic acid test; PVT, portal vein thrombosis.
HOPE Act Organs
There were 46 HIV+ organs transplanted under the HOPE Act, and these 46 recipients had a 1- and 3-y cumulative graft survival rate of 85.7% and 82.1%, respectively. Given the low numbers, a more in-depth analysis was not possible. In 2018, 18 HIV+ donor livers were transplanted, which was the highest annual number of HIV+ organs since the HOPE Act was introduced; however, this number decreased to 14 in 2019.
Posttransplant Outcomes
Graft Survival
In the pre-DAA era, the HIV+/HCV+-coinfected group had a significantly increased risk of graft failure compared with the HIV−/HCV− uninfected group (HR, 1.85; 95% CI, 1.31-2.59) (Table 3), adjusting for donor age, recipient age, and donation after circulatory death graft use. In contrast, there was no difference in graft failure between the HIV+/HCV+-coinfected group and the HIV−/HCV−-uninfected group in the DAA era (HR, 1.24; 95% CI, 0.81-1.89).
TABLE 3.
Multivariate Cox proportional hazards regression of associationsa with graft failure in liver transplant recipients in the pre-DAA (2008–2012) and DAA (2014–2019) eras
| Pre-DAA era (ref. HIV−/HCV−) | HR (95% CI) | P | DAA era (ref. HIV−/HCV−) | HR (95% CI) | P |
|---|---|---|---|---|---|
| HIV+/HCV+ | 1.85 (1.31-2.59) | <0.001 | HIV+/HCV+ | 1.24 (0.81-1.89) | 0.308 |
| HIV+/HCV− | 1.21 (0.77-1.91) | 0.840 | HIV+/HCV− | 1.23 (0.81-1.88) | 0.324 |
| HIV−/HCV+ | 1.24 (1.19-1.29) | <0.001 | HIV−/HCV+ | 1.01 (0.96-1.07) | 0.709 |
aAdjusted for donor and recipient age and DCD graft use.
CI, confidence interval; DAA, direct-acting antiviral; DCD, donation after circulatory death; HCV, hepatitis C virus.
Among HIV+/HCV+-coinfected LT recipients, 1- and 3-y cumulative graft survival rates were 88.6% and 81.7% in the DAA era compared with 76.3% and 58.0% in the pre-DAA era, respectively (P = 0.006) (Figure 2). Within the HIV+/HCV+-coinfected DAA-era group, there were 9 patients with a positive HCV NAT at transplant, 27 were HCV NAT negative, and 88 did not have HCV NAT status recorded. The 9 patients with positive HCV NAT had a graft survival of 100% over a median follow-up of 656 (275–1073) d. For the HIV-monoinfected, HCV-monoinfected, and HIV−/HCV−-uninfected DAA era groups, 1-y cumulative graft survivals were 88.3%, 91.1%, and 90.3%, while 3-y cumulative graft survival rates were 80.1%, 83.4%, and 84.3%, respectively. These rates were not significantly different from the HIV+/HCV+-coinfected DAA-era group (Figure 2) (all pairwise comparison P > 0.05). In the DAA era, there was no difference in acute rejection episodes (within 1 y of transplant) between the HIV+/HCV+-coinfected and the HIV-monoinfected groups (9.7% versus 8.3%, respectively; P = 0.710).
FIGURE 2.
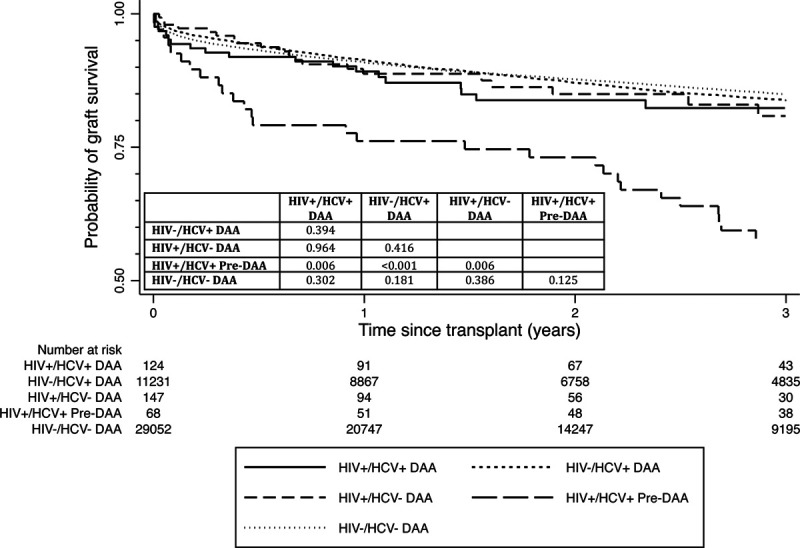
The 3-y cumulative graft survival rate for HIV/HCV coinfected LT recipients in the DAA era was 82% compared with 58% in the pre-DAA era (P = 0.006), while there were no statistical differences in graft survival when compared with the other control groups in the DAA era (all P > 0.05). DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LT, liver transplant.
Among the entire cohort of HIV LT recipients in the DAA era (n = 271), the 1- and 3-y cumulative graft survival rates were 88.4% and 81.4%, respectively, which were not significantly different from corresponding graft survival rates among HIV−/HCV−-uninfected LT recipients (adjusted HR, 1.23, 95% CI, 0.92-1.66; P = 0.160).
Patient Survival
Among HIV+/HCV+-coinfected LT recipients, the 3-y cumulative patient survival rates were 84.0% and 62.5% in the DAA and pre-DAA era, respectively (adjusted P = 0.002). For the HIV-monoinfected, HCV-monoinfected, and HIV−/HCV−-uninfected groups in the DAA era, 3-y cumulative patient survivals were 81.2%, 84.7%, and 86.7%, respectively (all pairwise comparison P values >0.05 versus HIV+/HCV+-coinfected recipients in DAA-era). Among all HIV LT recipients in the DAA era, the 3-y cumulative patient survival was 82.7%, which was not significantly different from HIV−/HCV−-uninfected LT recipients (adjusted P = 0.250).
HCC, LDLT, and SLK Outcomes in HIV/HCV-coinfected LT DAA Era Recipients
The presence of HCC (versus no HCC) was not associated with graft failure among HIV+/HCV+-coinfected LT recipients in the DAA era (adjusted HR, 0.71; 95% CI, 0.28-1.75; P = 0.46). The cause of graft failure among HIV+/HCV+-coinfected LT recipients with HCC did not differ significantly, although there was a higher number of recurrent hepatitis C observed in the pre-DAA group (P = 0.390) (6 graft failures in the DAA era: 1 cardiovascular-related, 1 infection-related, 3 cancer-related, and 1 trauma-related; 7 graft failures in the pre-DAA era: 1 cardiovascular-related, 3 recurrent hepatitis, 1 infection-related, and 2 cancer-related).
The 16 HIV+/HCV+-coinfected DAA-era patients who underwent SLK had a 3-y graft survival of 86.7% after a median follow-up of 716 d (interquartile range, 356–1105). In multivariable Cox analysis, SLK was not associated with graft failure among the HIV+/HCV+-coinfected DAA group (HR, 0.82; 95% CI, 0.19-3.64; P = 0.800). The 5 LDLTs among the HIV+/HCV+-coinfected LT recipients in the DAA era had a 3-y graft survival of 75.0% after a median follow-up of 349 d (interquartile range, 189–1003).
Sensitivity Analysis
In a Cox proportional hazards model limited to HIV+ LT recipients in the DAA era (n = 271), HCV infection was not associated with graft failure (HR, 0.96; 95% CI, 0.49-1.89; P = 0.920). In addition to covariates used in the previously described models, the presence of an HIV+ organ was also included and found not to be associated with graft failure (HR, 1.62; 95% CI, 0.68-3.85; P = 0.270). Among all the LT recipients in the DAA era (n = 40 554), HIV infection was not significantly associated with graft failure (HR, 1.23; 95% CI, 0.92-1.66; P = 0.160).
DISCUSSION
Since the enactment of the HOPE Act, the practice of LTs for HIV+ patients in the United States has increased over time from 28 patients in 2014 to 64 patients in 2019 (over a third had HIV+/HCV+ coinfection). There have been 46 HIV+ liver grafts transplanted during this time frame, although a decrease in frequency was observed in 2019 compared with 2018, which requires monitoring in the coming years. The vast majority of HIV+ LT recipients received non-HIV organs suggesting ongoing underutilization of the HOPE Act.
Racial representation in HIV/HCV LT practice has evolved, with more Black and Hispanic recipients in the DAA era, possibly signaling movements in addressing racial inequity considering HIV infection incidence has decreased in these racial groups. The HIV incidence decreased from 80 per 100 000 individuals in 2014 to 72 per 100 000 in 2018, and from 23 per 100 000 in 2014 to 22 per 100 000 in 2018, in Black and Hispanic populations, respectively.26 The geographic distribution of HIV+/HCV+ LT practice has remained relatively stable in the United States, apart from a slight increase in prevalence in the Northeast and a decline in the Midwest. Of concern is the limited number of transplant centers participating in HIV+ LT practice, with 6 centers accounting for 40% of the practice, despite the HIV burden affecting all 50 states.26 This may reflect a lack of confidence in HIV+ LT practice in transplant centers or additional factors such as access to care. The HIV+ LTs described in our study are unlikely to be fully representative of the HIV+ population with ESLD who may benefit from LT, suggesting that this patient population remains underserved.
A recent multicenter study from Europe and the United States showed that HCV infection conferred an increased risk of graft failure in HIV+ LT recipients transplanted between 2008 and 2015.22 Our data suggest that as the DAA era has continued, the presence of HCV infection among HIV+ LT candidates does not confer increased risk of graft failure. Moreover, there was no difference in graft failure between HIV+/HCV+-coinfected LT recipients compared with HIV−/HCV−-uninfected LT recipients in the DAA era. Although there is a risk of residual confounding, this improvement compared with the pre-DAA era is likely due to improved outcomes in HCV treatment. With the success of DAA therapies, graft and patient survival rates among HIV+/HCV+-coinfected LT recipients are comparable with HIV-monoinfected, HCV-monoinfected, and HIV−/HCV−-uninfected groups. These results were consistent across special populations including those with HCC, SLK, and LDLT. Although long-term studies are needed, these encouraging results support the practice of LT in HIV+/HCV+-coinfected patients. Our results also reinforced the excellent outcomes HIV+ LT recipients have, regardless of HCV status.
In the pre-DAA era, a multicenter study demonstrated that the cumulative incidence of acute rejection was higher in coinfected patients compared with monoinfected patients (39% versus 24%, respectively; P = 0.01).12 It has been hypothesized that this higher rate of acute rejection may be due to various reasons including a higher misdiagnosis of acute rejection (compared with recurrent HCV), an overly cautious approach to immunosuppression due to concerns for exacerbating HIV- or HCV-related diseases, antiretroviral drugs and calcineurin inhibitor interactions, or immune dysregulation associated with HIV infection.12 In recent years, DAAs have been highly efficacious in achieving SVR in both de novo and recurrent HCV infection in post–LT recipients, including HIV+/HCV+-coinfected patients with fewer drug interactions and excellent tolerability.17,19 Our results confirm that there is likely no difference in acute rejection rates when compared between HIV+/HCV+-coinfected LT recipients and HIV-monoinfected individuals.
In addition to perceived transplant outcome differences between HIV+/HCV+-coinfected and HCV-monoinfected patients, HIV+ patients also face unique barriers to transplantation. Although most transplant surgeons appear willing to transplant hepatitis B and C candidates, only one-third considered HIV+ patients to be appropriate candidates in the US survey.27 Most surgeons underestimated the transmission risk from exposure to HBV- and HCV-infected blood, whereas they overestimated the transmission risk associated with HIV. Stigma surrounding HIV and lack of awareness surrounding the HOPE Act may explain the discrepancy in willingness to transplant HIV+ patients. Ongoing efforts to address these barriers and overcome HIV-related stigma will be essential to improving HIV+ patients’ access to organs. The results from our study should offer further reassurance to transplant centers and to encourage timely referral of ESLD patients with coinfection for consideration of transplantation.
The strengths of this study include the large sample size of 70 125 LT recipients, including 271 HIV+ recipients in the DAA era. We analyzed the groups using multiple analytic models strengthening study validity. Unfortunately, OPTN does not collect HIV status at candidate listing precluding analysis of waitlist outcomes and lacks posttransplant HCV therapy data. However, it has already been established in the literature that sustained virologic response at 12 wk (SVR12) can be obtained safely in HCV+/HIV+-coinfected LT recipients, so the absence of these data is likely less important. We acknowledge that a small proportion of HIV+ donor livers acquired under the HOPE Act may have been false+.28 Finally, the proportion of missing data was extremely small in this large data set, mitigating the potential for bias.
In conclusion, HIV+/HCV+-coinfected LT recipient outcomes have improved significantly in the DAA era. Our results should offer reassurance to transplant centers and to encourage timely referral of HIV patients with decompensated cirrhosis for transplantation evaluation, including patients coinfected with HCV.
Footnotes
Published online 9 June, 2021.
A.G.S. has received grant/research support from Gilead. M.C. has received grant/research support from Gilead, Conatus, and Galectin and consultant fees from Gilead, Metacrine, Enterome, Novartis, AbbVie, Intercept, and NGM Bio. The other authors declare no conflicts of interest.
T.G.C. participated in study concept and design; acquisition, analysis, interpretation of data; and drafting and critical revision of article for important intellectual content. J.W., M.A.O., N.E.R., S.R.L., A.A., J.A.M., A.G.S., M.C.M., and M.C. participated in critical revision of article for important intellectual content. J.F. participated in study concept and design and critical revision of article for important intellectual content. All authors approved final version to be published.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998; 338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Castellares C, Barreiro P, Martín-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat. 2008; 15:165–172. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006; 166:1632–1641. [DOI] [PubMed] [Google Scholar]
- 4.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 5.Fung J, Eghtesad B, Patel-Tom K, et al. Liver transplantation in patients with HIV infection. Liver Transpl. 2004; 10:S39–S53. [DOI] [PubMed] [Google Scholar]
- 6.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008; 8:355–365. [DOI] [PubMed] [Google Scholar]
- 7.Wilk AR, Hunter RA, McBride MA, et al. National landscape of HIV+ to HIV+ kidney and liver transplantation in the United States. Am J Transplant. 2019; 19:2594–2605. [DOI] [PubMed] [Google Scholar]
- 8.de Vera ME, Dvorchik I, Tom K, et al. Survival of liver transplant patients coinfected with HIV and HCV is adversely impacted by recurrent hepatitis C. Am J Transplant. 2006; 6:2983–2993. [DOI] [PubMed] [Google Scholar]
- 9.Pineda JA, Romero-Gómez M, Díaz-García F, et al. ; Grupo Andaluz para el Estudio de las Enfermedades Infecciosas; Grupo Andaluz para el Estudio del Hígado. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005; 41:779–789. [DOI] [PubMed] [Google Scholar]
- 10.Miro JM, Stock P, Teicher E, et al. Outcome and management of HCV/HIV coinfection pre- and post-liver transplantation. A 2015 update. J Hepatol. 2015; 62:701–711. [DOI] [PubMed] [Google Scholar]
- 11.Sawinski D, Goldberg DS, Blumberg E, et al. Beyond the NIH multicenter HIV transplant trial experience: outcomes of HIV+ liver transplant recipients compared to HCV+ or HIV+/HCV+ coinfected recipients in the United States. Clin Infect Dis. 2015; 61:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terrault NA, Roland ME, Schiano T, et al. ; Solid Organ Transplantation in HIV: Multi-Site Study Investigators. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012; 18:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miro JM, Montejo M, Castells L, et al. ; Spanish OLT in HIV-Infected Patients Working Group investigators. Outcome of HCV/HIV-coinfected liver transplant recipients: a prospective and multicenter cohort study. Am J Transplant. 2012; 12:1866–1876. [DOI] [PubMed] [Google Scholar]
- 14.Duclos-Vallée J-C, Féray C, Sebagh M, et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2007; 47:407–417. [DOI] [PubMed] [Google Scholar]
- 15.Vibert E, Duclos-Vallée JC, Ghigna MR, et al. Liver transplantation for hepatocellular carcinoma: the impact of human immunodeficiency virus infection. Hepatology. 2011; 53:475–482. [DOI] [PubMed] [Google Scholar]
- 16.Wyles DL, Sulkowski MS, Dieterich D. Management of hepatitis C/HIV coinfection in the era of highly effective hepatitis C virus direct-acting antiviral therapy. Clin Infect Dis. 2016; 63:S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015; 313:1223. [DOI] [PubMed] [Google Scholar]
- 18.Cotter TG, Paul S, Sandikçi B, et al. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct-acting antiviral era. Liver Transpl. 2019; 25:598–609. [DOI] [PubMed] [Google Scholar]
- 19.Cotter TG, Paul S, Sandikçi B, et al. Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)-viremic donors into HCV-negative recipients: outcomes following liver transplant of HCV-viremic donors. Hepatology. 2019; 69:2381–2395. [DOI] [PubMed] [Google Scholar]
- 20.Cotter TG, Aronsohn A, Reddy KG, et al. Liver transplantation of HCV-viremic donors into HCV-negative recipients in the USA: increasing frequency with profound geographic variation. Transplantation 2020[Epub ahead of print. June 29, 2020]. doi:10.1097/TP.0000000000003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020; 71:686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos-Varela I, Dodge JL, Berenguer M, et al. Temporal trends and outcomes in liver transplantation for recipients with HIV infection in Europe and United States. Transplantation. 2020; 104:2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health & Human Services. Organ for Procurement and Transplantation Network: Scientific Registry of Transplant Recipients. The SRTR Database: Overview. Available at https://optn.transplant.hrsa.gov. Accessed November 15, 2020. [Google Scholar]
- 24.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006; 6:783–790. [DOI] [PubMed] [Google Scholar]
- 25.Haddad L, Cassenote AJ, Andraus W, et al. Factors associated with mortality and graft failure in liver transplants: a hierarchical approach. PLoS One. 2015; 10:e0134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV Surveillance Supplemental Report 2020; 25(No. 1). Available at http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed December 10, 2020. [Google Scholar]
- 27.Halpern SD, Asch DA, Shaked A, et al. Determinants of transplant surgeons’ willingness to provide organs to patients infected with HBV, HCV or HIV. Am J Transplant. 2005; 5:1319–1325. [DOI] [PubMed] [Google Scholar]
- 28.Durand CM, Halpern SE, Bowring MG, et al. Organs from deceased donors with false-positive HIV screening tests: an unexpected benefit of the HOPE act. Am J Transplant. 2018; 18:2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]


