Background:
Mohs micrographic surgery (MMS) has become the predominant modality of excision for non-melanoma skin cancers (NMSC). Patients are referred for MMS under the assumption that it is the most effective procedure for definitive removal of the cancer while also allowing for maximal tissue preservation to achieve optimal cosmesis. The objective of this study was to investigate outcomes of serial excision (SE) as an alternative excision modality for NMSC.
Methods:
Patients undergoing SE for basal cell carcinoma or squamous cell carcinoma by the senior author from 2009 to 2020 were retrospectively reviewed. Patient demographics, lesion characteristics, and excision characteristics were recorded. The primary outcome was the number of excisions required to achieve negative margins.
Results:
In total, 129 patients with 205 NMSC lesions were retrospectively reviewed. An estimated 69 lesions (33.7%) were located in high risk areas, as defined by the National Comprehensive Cancer Network. Negative margins were achieved in 191 (93.2%) lesions. In 88.3% of lesions (n = 181/205), negative margins were achieved in 2 or less excisions. 12 lesions (5.9%) were referred for MMS.
Conclusions:
Our results demonstrate that SE is an effective modality for definitive removal of NMSC. Recent research reveals that SE is much less expensive than MMS, and therefore places a smaller financial burden on the patient and the healthcare system as a whole. Relative to MMS, SE offers similar if not increased benefits for lower cost. Our findings highlight the need to critically reassess the select indications for MMS.
INTRODUCTION
Skin cancer is the most commonly diagnosed malignancy in the United States.1 Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), often referred to as non-melanoma skin cancers (NMSC) or keratinocyte carcinomas, comprise the vast majority of these diagnoses.1–3 The true incidence and mortality of NMSC is difficult to ascertain because NMSC data are typically excluded from cancer registries.1–3 It is estimated that 5.4 million cases of NMSC were diagnosed among 3.3 million individuals in the United States in 20124 and the incidence of NMSC continues to rise.4–8
The National Comprehensive Cancer Network establishes guidelines to guide management of NMSC lesions. Nonsurgical treatment options include curettage and electrodesiccation, cryotherapy, photodynamic therapy, radiation therapy, and/or topical or oral medications, whereas surgical treatment options include standard/serial excision (SE) or Mohs micrographic surgery (MMS).9,10 Findings from various studies suggest that SE and MMS have similar recurrence rates in a variety of settings.11–14
Although both MMS and SE are effective modalities for the removal of NMSC, reimbursement for MMS is much higher than SE.15 In their study investigating Medicare reimbursement, Chen et al found that reimbursements for MMS range from 120% to 370% more than reimbursements for surgical excision.15 In our experience, there are several factors of SE that may lend a superior cosmetic result when compared with MMS, including the ability to minimize scar length and optimally position the final scar as well as the ability to reduce tension on final closure. The decision to pursue SE or MMS should be based on several factors, including recurrence, cost, and cosmesis.
The aim of this study was to build upon the current understanding of the utility of SE as an effective surgical treatment modality for NMSC and to apply our findings to existing research regarding the cost of SE versus MMS to better understand the value of SE relative to MMS for the surgical management of NMSC. We hypothesize that SE is a clinically effective and cost-conscious alternative to MMS for the removal of NMSC.
METHODS
Retrospective Review and Primary Outcomes
Patients were identified by retrieving all pathology reports that were ordered by a single provider to rule out NMSC over a 10-year period. All consecutive patients treated for pathology-proven BCC or SCC by a single plastic surgeon were retrospectively reviewed. Data collected included patient demographics such as age, ethnicity, race, and median income by location of residence, as well as lesion-specific and encounter-specific data. Lesion-specific and encounter-specific data (including lesion dimensions and pathology findings) were also collected. Primary outcomes included (1) achievement of negative margins and (2) number of visits required to achieve negative margins. This study was approved by the Institutional Review Board (MHRI 2018-173).
Definitions
Ethnicity was categorized as White and other (African American, Hispanic/Spanish, or Alaska Native) due to the low representation of African Americans, Hispanic/Spanish, and Alaska Natives in the studied population. Age and median income were categorized based on quartiles.
Lesion locations were categorized as high, medium, and low risk according to the same characterizations set forth in the National Comprehensive Cancer Network practice guidelines for the management of BCC and SCC (Table 1).9,10
Table 1.
Lesion Risk according to Location
| Low Risk | Medium Risk | High Risk |
|---|---|---|
| Trunk Extremities |
Cheeks Forehead Scalp Neck Pretibial |
Central face Eyelids Eyebrows Periorbital area Nose Lips Chin Mandible Preauricular skin/sulci Postauricular skin/sulci Temple Ear Genitalia Hands Feet |
Adapted from National Comprehensive Cancer Network practice guidelines for the management of basal cell carcinoma and squamous cell carcinoma.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Basal Cell Skin Cancer. 2019. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Squamous Cell Skin Cancer. 2020. https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
Initial lesion size was extrapolated using tissue sample dimensions obtained from pathology reports as well as standard margins based on lesion location (Fig. 1). Lesions for which specimen dimensions were not included in pathology reports were excluded from size analysis. Similarly, lesions for which multiple specimens and therefore multiple dimensions were provided in pathology reports were also excluded from size analysis.
Fig. 1.
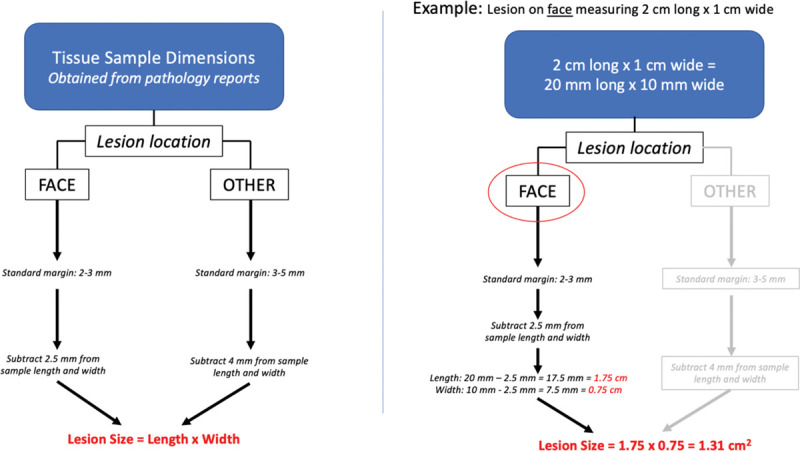
Calculation of lesion size.
For the purposes of this study, an “encounter” was defined as any patient encounter that (1) involved obtaining a tissue sample that was sent for pathology, (2) was a MMS procedure, (3) involved reconstruction, or (4) was an “other” procedure.
Inclusion Criteria
Patients who underwent MMS before being seen by a plastic surgeon were excluded from data collection and analysis. Similarly, as the purpose of this study was to investigate the utility of SE for the removal of NMSC, patients who were only managed by the plastic surgeon for reconstruction (as opposed to excision with or without reconstruction) were also excluded from analysis. Lastly, patient encounters that only entailed punch or shave biopsies were excluded and were not included for data collection or analysis.
Statistical Analysis
Continuous variables were described by means and SDs. Categorical variables were described by frequencies and percentages. Statistical analysis was performed using STATA v.16 (StataCorp, College Station, Tex.).
RESULTS
Demographics and Lesion Characteristics
Summary statistics are outlined in Table 2. An estimated 129 unique patients with 205 NMSC lesions were identified and retrospectively reviewed, for an average of 1.6 lesions per patient (range 1–10). In total, 50 patients were men (38.8%) and 79 patients were women (61.2%). An estimated 118 patients were White (91.5%), 3 patients were “other” (AA/Hispanic/Alaska Native), and 8 patients (6.2%) did not have an ethnicity documented in their medical record. Mean age at first presentation for lesion was 64.0 (SD 15.06). Mean number of office visits was 1.6 (range 1–9, SD 0.95).
Table 2.
Summary Statistics
| Data for All Lesions | N | % | SD |
|---|---|---|---|
| Total no. lesions | 205 | 100% | |
| Mean no. excisions per lesion | 1.6 | Range: 1–9 | 0.95 |
| Mean age at first presentation for lesion | 64.0 | 15.06 | |
| # Lesions with subsequent recurrence | 3.0 | 1.5% | |
| Original diagnosis | |||
| BCC | 132 | 64.4% | |
| SCC | 46 | 22.4% | |
| BCC/SCC | 3 | 1.5% | |
| SCC in situ | 24 | 11.7% | |
| Lesion location | |||
| Face/neck | 103 | 50.2% | |
| Scalp | 11 | 5.4% | |
| Trunk | 37 | 18.0% | |
| Upper extremity, including the hands | 32 | 15.6% | |
| Lower extremity, including the feet | 22 | 10.7% | |
| Risk of lesion location | |||
| High risk | 69 | 33.7% | |
| Medium risk | 57 | 27.8% | |
| Low risk | 79 | 38.5% | |
| Lesions requiring Mohs (ever) | |||
| Yes | 12 | 5.9% | |
| No | 193 | 94.1% | |
| Negative margins achieved? | |||
| Yes | 191 | 93.2% | |
| No | 14 | 6.8% | |
| Unique patient data | n | % | SD |
| # Unique patients | 129 | ||
| Mean # lesions per patient | 1.6 | Range: 1-10 | |
| Gender | |||
| Men | 50 | 38.8% | |
| Women | 79 | 61.2% | |
| Race | |||
| White | 118 | 91.5% | |
| Other—Hispanic/Spanish, AA, Alaska Native | 3 | 2.3% | |
| Unknown | 8 | 6.2% | |
| Mean income (n = 127) | $129,202.50 | $45,248.02 |
Pathology reports identified BCC in 132 lesions (64.4%), SCC in 46 lesions (22.4%), SCC in situ in 24 lesions (11.7%), and combined BCC/SCC in 3 lesions (1.5%). 69 lesions (33.7%) were located in high-risk areas, 57 lesions (27.8%) were located in medium-risk areas, and 79 lesions (38.5%) were located in low-risk areas.
Achievement of Negative Margins
Table 3 outlines information regarding achievement of negative margins. A total of 295 standard excisions were performed in this study. Of these, 86.8% (n = 256) were performed in an outpatient clinic setting, and 13.2% (n = 39) were performed in the operating room. Negative margins were achieved in 93.2% of all lesions (n = 194). In 88.3% of all lesions, negative margins were achieved in 2 or less visits (n = 181/205). In the studied population, there were 3 excised lesions (1.5%), which subsequently recurred. 12 lesions (5.9%) undergoing SE were subsequently referred for definitive removal via MMS. Of note, all 12 of these lesions were located in high-risk areas, and 1 of these lesions underwent three serial excisions before MMS was ultimately pursued. The remainder of lesions (n = 193, 94.1%) were managed without MMS.
Table 3.
Negative Margins
| n | % | |
|---|---|---|
| Negative margins achieved | 191 | 93.2% |
| Negative margins not achieved | 14 | 6.8% |
| No. excisions required to achieve negative margins* | ||
| 1 visit | 111 | 54.2% |
| 2 visits | 70 | 34.1% |
| 3 visits | 9 | 4.4% |
| 4 visits | 2 | 1.0% |
| Mohs for lesions in which neg margins achieved (n = 194) | ||
| Yes | 12 | 6.2% |
| No | 179 | 93.7% |
*Does not include previous excisions or visits not explicitly documented in patient chart
Management Based on Lesion Location
Table 4 outlines data pertaining to management of lesions based on lesion location. Of note, all lesions requiring MMS in the studied population were in high-risk areas. Negative margins were achieved in 88.4%, 94.7%, and 96.2% of high, medium, and low risk lesions, respectively.
Table 4.
Management Based on Lesion Location
| n | % | SD | |
|---|---|---|---|
| Face/Neck | 103 | 100.0% | |
| Average no. excisions | 1.8 | 1.1 | |
| Negative margins achieved | 93 | 90.3% | |
| Negative margins not achieved | 10 | 9.7% | |
| Mohs | 12 | 11.7% | |
| No Mohs | 91 | 88.3% | |
| Scalp | 11 | 100.0% | |
| Average no. excisions | 1.9 | 1.1 | |
| Negative margins achieved | 10 | 90.9% | |
| Negative margins not achieved | 1 | 9.1% | |
| No Mohs | 11 | 100.0% | |
| Trunk | 37 | 100.0% | |
| Average no. excisions | 1.5 | 0.6 | |
| Negative margins achieved | 37 | 100.0% | |
| No Mohs | 37 | 100.0% | |
| Upper extremity | 32 | 100.0% | |
| Average no. excisions | 1.4 | 0.7 | |
| Negative margins achieved | 30 | 93.8% | |
| Negative margins not achieved | 2 | 6.3% | |
| No Mohs | 32 | 100.0% | |
| Lower extremity | 22 | 100.0% | |
| Average no. excisions | 1.3 | 0.6 | |
| Negative margins achieved | 21 | 95.5% | |
| Negative margins not achieved | 1 | 4.5% | |
| No Mohs | 22 | 100.0% | |
| Management by lesion location risk | |||
| High risk | 69 | 100.0% | |
| Average no. excisions | 2.0 | 1.3 | |
| Negative margins achieved | 61 | 88.4% | |
| Negative margins not achieved | 8 | 11.6% | |
| Mohs | 12 | 17.4% | |
| No Mohs | 57 | 82.6% | |
| OR | 27 | 39.1% | |
| Never OR | 42 | 60.9% | |
| Medium risk | 57 | 100.0% | |
| Average no. excisions | 1.6 | 0.8 | |
| Negative margins achieved | 54 | 94.7% | |
| Negative margins not achieved | 3 | 5.3% | |
| Mohs | 0 | 0.0% | |
| No Mohs | 57 | 100.0% | |
| OR | 42 | 73.7% | |
| Never OR | 15 | 26.3% | |
| Low risk | 79 | 100.0% | |
| Average no. excisions | 1.4 | 0.6 | |
| Negative margins achieved | 76 | 96.2% | |
| Negative margins not achieved | 3 | 3.8% | |
| Mohs | 0 | 0.0% | |
| No Mohs | 79 | 100.0% | |
| OR | 7 | 8.9% | |
| Never OR | 72 | 91.1% | |
Management Based on Initial Lesion Size
Table 5 outlines data pertaining to management of lesions based on initial lesion size. In total, 190 lesions were included in the size analysis. Mean lesion size at first visit was 1.57 cm2. Mean size of high and low-risk lesions at first visit was 0.81 cm2 and 0.96 cm2, respectively.
Table 5.
Management Based on Initial Lesion Size
| n | % | SD | |
|---|---|---|---|
| Total no. lesions included in final size analysis* | 190 | 92.7% | |
| Mean lesion size at first visit (cm2) | 1.57 | 9.27 | |
| Lesion size percentiles | n | Min | Max |
| 25%ile | 49 | −0.04 | 0.06 |
| 50%ile | 46 | 0.0675 | 0.3375 |
| 75%ile | 48 | 0.3675 | 0.9375 |
| 100%ile | 47 | 0.96 | 126.3125 |
| Size and lesion risk | n | Mean (cm2) | SD |
| High risk | 63 | 0.81 | 1.13 |
| Medium risk | 51 | 3.41 | 17.68 |
| Low risk | 76 | 0.96 | 2.01 |
| Management by lesion size | |||
| Bottom 25%ile (smallest lesions) | 49 | 100.0% | |
| Average no. visits | 1.98 | 1.36 | |
| Negative margins achieved | 46 | 93.9% | |
| Negative margins not achieved | 3 | 6.1% | |
| Mohs | 7 | 14.3% | |
| No Mohs | 42 | 85.7% | |
| OR | 11 | 22.4% | |
| Never OR | 38 | 77.6% | |
| 25%ile–50%ile | 46 | 100.0% | |
| Average no. visits | 1.65 | 0.71 | |
| Negative margins achieved | 43 | 93.5% | |
| Negative margins not achieved | 3 | 6.5% | |
| Mohs | 2 | 4.3% | |
| No Mohs | 44 | 95.7% | |
| OR | 7 | 15.2% | |
| Never OR | 39 | 84.8% | |
| 50%ile–75%ile | 48 | 100.0% | |
| Average no. visits | 1.56 | 0.80 | |
| Negative margins achieved | 46 | 95.8% | |
| Negative margins not achieved | 2 | 4.2% | |
| Mohs | 2 | 4.2% | |
| No Mohs | 46 | 95.8% | |
| OR | 8 | 16.7% | |
| Never OR | 40 | 83.3% | |
| Top 25%ile (largest lesions) | 47 | 100.0% | |
| Average no. visits | 1.49 | 0.81 | |
| Negative margins achieved | 43 | 91.5% | |
| Negative margins not achieved | 4 | 8.5% | |
| Mohs | 0 | 0.0% | |
| No Mohs | 47 | 100.0% | |
| OR | 18 | 38.3% | |
| Never OR | 29 | 61.7% |
*Based on lesion dimensions provided in pathology report after first excision. 19 lesions were excluded from analysis: 8 excisions with multiple specimens for a single lesion were excluded from analysis. 11 lesions without dimensions in pathology report excluded.
DISCUSSION
Effectiveness and Recurrence
As of 2018, the American Academy of Dermatology recommends MMS for any high-risk BCC or SCC, which includes (1) regions ≥ 2 cm on the trunk or extremities, (2) ≥ 1 cm on the cheeks, forehead, scalp, neck, and pretibial areas, and (3) any lesion in the “H zone” (central face, eyelids, eyebrows, periorbital skin, nose, lips, chin, mandible, preauricular and postauricular skin/sulci, temple, ear, genitalia, hands, and feet).16,17 Our results demonstrate that SE is an effective alternative to MMS for the removal of NMSC, including on high-risk areas. In the studied population, there were 57 lesions in high-risk areas that were never managed with MMS. Negative margins were achieved in 86.0% of these lesions, and on average, 1.65 excisions were required to achieve negative margins in this high-risk group.
The literature also demonstrates that SE can be performed with a high degree of success without increasing the risk of recurrence relative to MMS. In a retrospective review of over 1500 patients occurring over the course of 18 years, van der Eerden found that patients undergoing conventional excision and MMS for NMSC removal had similar rates of disease recurrence (7/709 versus 6/795, respectively; p=0.78).11 In their prospective cohort study, Chren et al found no statistically significant difference in tumor recurrence, after adjusting for risk factors, between patients who underwent MMS versus SE (2.1% versus 3.5%, respectively) for removal of primary NMSC lesions.13 The first randomized control trial comparing outcomes of MMS versus serial excision for the treatment of primary and recurrent facial BCC found a trend toward lower recurrence rates with MMS relative to SE for both primary and recurrent lesions but these findings did not reach statistical significance.14 Subsequent 5-year18 and 10-year12 updates to this study found significantly lower rates of recurrence at both time points when MMS was used to treat recurrent facial BCC lesions. Recurrence rates were not significantly different at either time point between MMS and SE for the treatment of primary facial BCC lesions. There is a notable paucity of randomized control studies and prospective studies comparing recurrence rates after MMS and SE for the treatment of cutaneous SCC lesions.16
Cost Considerations
In addition to having a similar profile with respect to effectiveness and recurrence rates, SE offers a distinct benefit from a cost perspective when compared with MMS. Between 2002 and 2006, the annual cost of skin cancer averaged $3.6 billion.15 This average annual cost increased by 126.2% to $8.1 billion between 2007 and 2011.15 Between 1992 and 2009, utilization of MMS for the treatment of NMSC increased by 700%, whereas SE utilization only increased by 20%.15 Similarly, the number of providers billing for MMS has risen: in 2009, 1800 providers billed Medicare for MMS, and by 2012, that number had increased to 3209.15
The increase in cost for the management of NMSC can be explained in part by the growing incidence of NMSC, but we also know from existing research that the management of NMSC with MMS is much more expensive than SE. Medicare reimbursement for a first-stage MMS removal of a lesion on the head, neck, hands, feet, or genitalia is $424 ± $90 ($156.70–$639.70). For subsequent stages, the amount is $306.90 ± $48.87 ($140.20–$395.20).15 The average Medicare reimbursement for a first-stage MMS excision of the trunk, arm, or leg is $412.30 ± $83.87 ($179–$578). For subsequent stages in this area of the body, the amount is $294.30 ± $39.62 ($137–$365).15 Importantly, these reimbursement rates do not include the complex closures that are often required for defects after MMS. In our experience, these complex closures often entail either local tissue transfer or full thickness skin graft and can add an additional 10–15 RVUs, which translates to at least an additional $1000 to the total cost of surgical management of NMSC with MMS.
SE of a malignant lesion on the face, eyes, ears, and neck with margins ranges from $131.90 ± $23.11 ($75.90–$187.00) for 0.5- to 1-cm margins up to $240.50 ± $73.02 ($101.90–$360.90) for 4-cm margins. As expected, 4-cm margins are uncommon, with only 50 excisions requiring such aggressive margins performed by all plastic surgeons in 2012. The pathology fee is $68.37 ± $11.00 ($24.00–$90.70).15 Primary closure is used in the vast majority of patients undergoing SE, and this procedure is included in the original CPT for the SE itself for lesions that are <1 cm. In contrast, complex closures required for MMS defects are billed under a CPT code that is separate from the lesion removal and these complex closures are typically more expensive than the primary closures used for SE.
Based on the Medicare reimbursement rates for SE published by Chen et al15 in conjunction with the results of our study, the average Medicare reimbursement to achieve negative margins for SE, including pathology fees, in our studied population was $279.38. Based on historical data reported by Krishnan et al19 regarding the average number of stages required to achieve negative margins via MMS, the average Medicare reimbursement for MMS excision of a lesion in the H-zone is $651.12—which is well over twice the price of SE (Fig. 2). Previous studies have demonstrated similar price premiums for MMS relative to SE: Smeets et al found that total operative costs of MMS were almost double those of SE for the management of primary BCC (total operative costs were €405.79 for MMS and €216.86 for SE).14
Fig. 2.
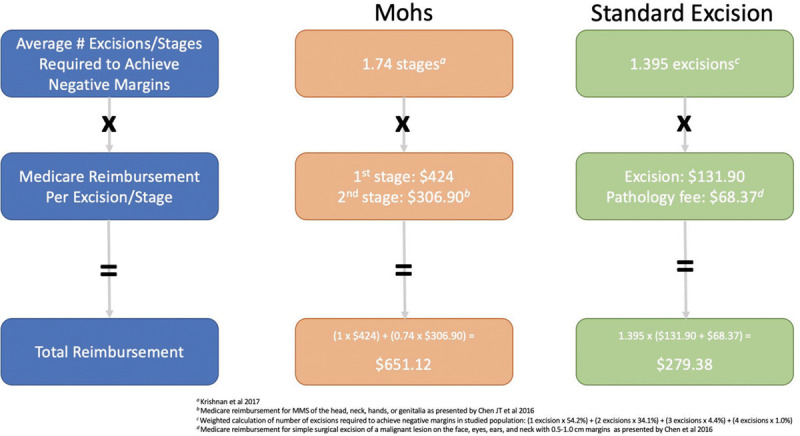
Medicare reimbursement of Mohs micrographic surgery versus serial excision.
Although the literature has demonstrated similar recurrence rates in MMS versus SE on multiple occasions,11,13,14,18 even if MMS did offer superior recurrence rates, providers must consider whether the purported benefits of MMS are worth the cost. Essers et al performed a cost-effectiveness analysis using data from the randomized control trial by Smeets et al and found that the additional cost of preventing 1 recurrence by employing MMS for the removal of primary BCC was €29,231. In essence, MMS is more expensive than SE, but it offers only a marginally improved recurrence rate (0.0091 at 30 months).20 Our findings are consistent with those of Essers et al and should call to question the broadened use of MMS in recent years. MMS should be reserved for clinical scenarios in which its superior effectiveness has been clearly proved.12
Cosmesis
MMS advocates cite studies that show MMS leaves the patient with a smaller defect after NMSC excision and infer that a smaller defect results in better cosmesis; however, these studies compare the 2 approaches when removing all of the lesions in 1 excision. In these studies, MMS leaves a smaller (more cosmetic) defect when compared with a single excision with standard margins because it removes the minimal amount of normal tissue to obtain a free margin. However, the majority of early presenting lesions of NMSC have a very low risk of metastasis: the literature cites overall metastatic rates between 0.0028% and 0.55%21–24 for BCC and 4%25 for SCC. Therefore, the surgeon has the luxury of not having to remove the entire lesion in 1 procedure without violating oncologic principles. In pediatric plastic surgery, patients present with congenital nevi that could be removed in 1 stage but rarely are. SE is the optimal approach in plastic surgery to minimize scar length, optimally position the final scar, and reduce tension on final closure. These advantages make SE aesthetically superior to an approach where the entire lesion is removed in 1 stage. SE is the preferred approach to benign cutaneous lesions because it results in superior aesthetic outcomes compared with other mechanisms of removal.26–28 The only reason to excise a lesion in 1 stage would be to reduce recurrence of disease, but as the previously cited studies show, MMS does not offer an advantage over SE in efficacy of disease management. Therefore, it is the authors’ conclusion that because there is no difference in efficacy between MMS and SE, and SE of large lesions has long been considered the ideal aesthetic management of lesion removal in plastic surgery, SE is actually cosmetically superior to MMS.
It should be emphasized that there are certainly instances in which MMS is the preferable excision modality. One such instance is for the removal of recurrent BCC on the face. The only prospective randomized trial comparing MMS to standard excision has found significantly less recurrence rates at 5-year18 and 10-year12 follow up when MMS is used for the excision of recurrent facial BCC lesions. MMS should also be used on specific cosmetically sensitive regions of the body.
Limitations
The findings of this study represent the experience of a single plastic surgeon and may not be generalizable to other practice settings. Furthermore, the patient population studied only included patients who were at some time managed by a plastic surgeon for NMSC. As a result, this study does not include a comparison group that was managed solely by MMS or dermatology and its findings should not be interpreted as if they were part of a comparison study. Future studies should include longer-term follow up to further assess the cost effectiveness of SE versus MMS.
CONCLUSIONS
Our findings and other supporting evidence suggest that SE is an effective excision modality for many NMSC lesions that can be performed at a lower relative cost with excellent cosmesis. When providing care to patients, we must consider the value of a given treatment: that is, we must consider its benefits and costs. In many cases of NMSC, SE offers equal if not greater benefits (by way of similar recurrence rates with excellent cosmetic outcomes) at a lower cost and should therefore be considered the preferred treatment modality in many instances. The incidence of NMSC is expected to rise as the American population continues to age. The cost to manage NMSC lesions will also grow, representing a growing burden on Medicare and our healthcare system as a whole. This study highlights the critical importance of reevaluating the select indications of MMS to ensure the most judicious allocation of healthcare resources.
Footnotes
Published online 10 June 2021.
Disclosure: All the authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Published 2020 . Accessed February 6, 2021.
- 2.American Cancer Society. Key Statistics for Basal and Squamous Cell Skin Cancers. Published 2020. Available at https://www.cancer.org/cancer/basal-and-squamous-cell-skin-cancer/about/key-statistics.html. Accessed February 6, 2021.
- 3.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005; 353:2262–2269. [DOI] [PubMed] [Google Scholar]
- 4.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015; 151:1081–1086. [DOI] [PubMed] [Google Scholar]
- 5.Guy GP, Jr, Machlin SR, Ekwueme DU, Yabroff R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011 gery. Am J Prev Med. 2015; 48:183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012; 166:1069–1080. [DOI] [PubMed] [Google Scholar]
- 7.Jean Bolognia, et al. Actinic Keratosis, Basal Cell Carcinoma, and Squamous Cell Carcinoma. 4th edn. Elsevier Ltd; 2012. [Google Scholar]
- 8.Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017; 92:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Basal Cell Skin Cancer. 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Squamous Cell Skin Cancer. Available at https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Published 2020.
- 11.van der Eerden PA, Prins ME, Lohuis PJ, et al. Eighteen years of experience in Mohs micrographic surgery and conventional excision for nonmelanoma skin cancer treated by a single facial plastic surgeon and pathologist. Laryngoscope. 2010; 120:2378–2384. [DOI] [PubMed] [Google Scholar]
- 12.van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014; 50:3011–3020. [DOI] [PubMed] [Google Scholar]
- 13.Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013; 133:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004; 364:1766–1772. [DOI] [PubMed] [Google Scholar]
- 15.Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of medicare payment data. Plast Reconstr Surg Glob Open. 2016; 4:e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018; 78:560–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018; 78:540–559. [DOI] [PubMed] [Google Scholar]
- 18.Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008; 9:1149–1156. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan A, Xu T, Hutfless S, et al. ; the American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017; 153:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essers BA, Dirksen CD, Nieman FH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006; 142:187–194. [DOI] [PubMed] [Google Scholar]
- 21.Paver K, Poyzer K, Burry N, et al. Letter: the incidence of basal cell carcinoma and their metastases in Australia and New Zealand. Australas J Dermatol. 1973; 14:53. [DOI] [PubMed] [Google Scholar]
- 22.Ganti AK, Kessinger A. Systemic therapy for disseminated basal cell carcinoma: an uncommon manifestation of a common cancer. Cancer Treat Rev. 2011; 37:440–443. [DOI] [PubMed] [Google Scholar]
- 23.Fram BR, Strony J, Jagannathan G, et al. Cutaneous basal cell carcinoma with bone metastases: an orthopaedic case report. Case Rep Orthop. 2019; 2019:1628980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakshmipathi T, Hunt KM. Metastasizing basal-cell carcinoma. Br J Dermatol. 1967; 79:267–270. [DOI] [PubMed] [Google Scholar]
- 25.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008; 9:713–720. [DOI] [PubMed] [Google Scholar]
- 26.Mutti LA, Mascarenhas MRM, Paiva JMG, et al. Giant congenital melanocytic nevi: 40 years of experience with the serial excision technique. An Bras Dermatol. 2017; 92:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassanein AH, Rogers GF, Greene AK. Management of challenging congenital melanocytic nevi: outcomes study of serial excision. J Pediatr Surg. 2015; 50:613–616. [DOI] [PubMed] [Google Scholar]
- 28.Arneja JS, Gosain AK. Giant congenital melanocytic nevi of the trunk and an algorithm for treatment. J Craniofac Surg. 2005; 16:886–893. [DOI] [PubMed] [Google Scholar]


