Abstract
Background
We designed this single-centre clinical trial to assess the potential benefits of N-Acetylcysteine (NAC) in patients with COVID19-associated acute respiratory distress syndrome (ARDS).
Methods
Ninety-two patients with mild-to-moderate COVID19-associated ARDS were allocated to the placebo (45-cases) or NAC groups (47-cases). Besides standard-of-care treatment, the patients received either intravenous NAC at a dose of 40 mg/kg/day or the placebo for three consecutive days. The efficacy outcomes were overall mortality over 28-day, clinical status on day 28, based on the WHO Master Protocol, the proportion of patients requiring mechanical ventilation, changes in ARDS-severity (based on the PaO2/FiO2 ratio), and Sequential Organ Failure Assessment (SOFA) scores 48 and 96 h after intervention,
Results
No differences were found in the 28-day mortality rate between the two groups (25.5% vs. 31.1% in the NAC and placebo groups, respectively). Although the distribution of the clinical status at day 28 shifted towards better outcomes in the NAC-treated group, it did not reach a statistical significance level (p value = 0.83). Similar results were achieved in terms of the proportion of patients who required invasive ventilator support (38.3% vs. 44.4%), the number of ventilator-free days (17.4 vs. 16.6), and median time of ICU and hospital stay. Results regarding the change in PaO2/FiO2 ratio and SOFA scores also showed no significant differences between the groups.
Conclusions
Our pilot study did not support the potential benefits of intravenous NAC in treating patients with COVID-19-associated ARDS. More studies are needed to determine which COVID-19 patients benefit from the NAC administration.
Trial registration
The trial was registered at Clinicaltrials.gov (identifier code: IRCT20120215009014N355). Registration date: 2020-05-18.
Keywords: COVID-19, N-Acetylcysteine, Acute respiratory distress syndrome, Inflammation, Oxidative stress
Introduction
At the end of 2019, a new coronavirus was recognised as the cause of a cluster of pneumonia with extraordinarily high morbidity and mortality rates. Its rapid spread resulted in a global pandemic. The disease is named COVID-19 by the World Health Organisation (WHO), standing for coronavirus disease 2019 [1]. Although only mild or uncomplicated illness was developed in most people with COVID-19, about 14% had the severe disease requiring oxygen support and hospitalisation, and 5% needed admission to an intensive care unit (ICU) [2]. Acute respiratory distress syndrome (ARDS) is the major complication in patients with severe disease, revealing a substantial proportion of COVID-19 patients needing ICU admission [3]. It is a clinical syndrome marked by alveolar oedema, decreased lung compliance, and, ultimately, hypoxemia [4]. The exact mechanisms by which SARS-CoV-2 leads to ARDS and certain host factors conferring an increased risk of developing the severe disease are not still clear; however, available evidence suggests that uncontrolled host immune responses after viral infection, the hyper-inflammatory condition described as “cytokine storm,” and the development of oxidative stress at the inflammation site are important contributors of COVID19-associated ARDS [5].
Given the close connections between oxidative stress and inflammation [6], during severe viral infections, excessive reactive oxygen species (ROS) by activating the NF-κB pathway (nuclear factor kappa light chain enhancer of activated B cells) can amplify cytokine production [7]. Multiple studies found that the serum levels of inflammatory cytokines such as interleukin (IL)-6, IL-1β, IL-10, as well as Tumour Necrosis Factor-α (TNF-α) have a crucial role in a severe stage of COVID-19 [8, 9]. Altering immune function during cytokine storm results in immunopathogenic injuries and consequently leads to lung injury and multisystem organ dysfunction in severe COVID-19 patients [10]. Although mechanical ventilation is the main therapeutic intervention in the management of ARDS, it has been postulated that that anti-inflammatory and antioxidant agents have beneficial impacts to overcome both hyper inflammation and COVID19-associated ARDS. In this sense, multiple therapies aiming to mitigate the inflammatory and oxidative responses in the severe stage of COVID-19 are being investigated [9, 11], and at present, corticosteroids are recommended strongly as a treatment option for patients with severe and critical COVID-19 infection [12].
N-Acetylcysteine (NAC) is a precursor of the antioxidant glutathione classically utilised as an antidote for paracetamol overdose and as a mucolytic for chronic respiratory diseases with high mucus production. Based upon its broad range of mechanism of actions, including immune-modulating, anti-inflammatory, and antioxidant actions, as well as its well-established safety profile even at high doses [13], NAC also has attracted considerable attention for the prevention and treatment of a variety of other medical conditions [14]. Notably, in recent years it has been found that NAC can improve the immune system function, inhibit viral replication, and diminish virus-induced pro-inflammatory responses [15]; characteristics which make it as a potential therapeutic agent in acute viral respiratory infections like influenza and influenza‐like illnesses [16, 17]. Furthermore, due to antioxidant and anti-inflammatory effects, it has been shown that NAC can effectively prevent cytokine storm and oxidative stress-induced pulmonary oedema and respiratory failure in ARDS [18, 19]. In fact, as a potent antioxidant, NAC directly by inhibiting the ROS radicals and indirectly by increasing the intracellular glutathione level could effectively modulate inflammatory responses and, as a result, mitigates damage to molecules, cells, and tissues during oxidative burst and cytokine storm [20]. Considering this evidence, it is speculated that NAC target multiple mechanisms related to COVID-19 excessive inflammation. Thus, it is reasonable to assess NAC as a potential therapy for COVID-19. However, at present, clinical information on using NAC for COVID-19 treatment consists almost exclusively of single case reports [21–23] and one small case series study [24]. Hence, we designed this double-blind, placebo-controlled study to determine whether patients with mild-to-moderate COVID19-associated ARDS would benefit from intravenous NAC administration as adjuvant therapy to standard therapy.
Material and methods
Study design
This is a single-centre, prospective, phase 2, randomised, double-blind, and pilot clinical trial comparing NAC to placebo in patients with mild-to-moderate COVID19-associated ARDS. The study was conducted from June 2020 until February 2021 in a tertiary referral hospital, with 30 intensive care unit beds affiliated to Hamadan University of Medical Sciences, Hamadan, Iran. The trial was performed according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines, and the Ethics Committee of Hamadan University of Medical Sciences approved the trial protocol (approval number: IR.UMSHA.REC.1399.153). The study patients or their legal representatives gave consent written informed consent. The trial was registered at the Iranian Registry of Clinical Trials (WWW.irct.ir); identifier code: IRCT20120215009014N355). The researchers, ICU nurses, physicians, and patients were blinded regarding the intervention, and the study drug (NAC or placebo) was prepared, hooded, and dispensed by Hospital Pharmacy Department.
Patient enrollment
No published data were available on NAC therapy in COVID-19 for an accurate sample size calculation at the trial design. Therefore, a sample size of 46 patients in each group was calculated based on Suter et al. study on non-COVID-19 ARDS to detect a difference of PiO2 between the two groups, while considering α = 0.05, 20% drop-out, and a power of 80% [25]. All Patients with a diagnosis of mild-to-moderate COVID19-associated ARDS admitted to the hospital were screened for enrolment in the study. Patients were eligible if they were: at least 18 years; had received a diagnosis of COVID-19 defined as either the positive SARS-CoV-2 on polymerase chain reaction testing (RT-PCR) in a respiratory tract specimen and/or clinical and radiological findings compatible with COVID-19 severe pneumonia, met the criteria for mild to moderate ARDS using the Berlin criteria [4] defined with oxygen saturation of less than 94% at rest on room air (i.e., with no supplemental oxygen) or the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2 ratio) of less than 300 mm Hg and more than 100 mm Hg, and less than 48 h from their hospital admission. Exclusion criteria included subjects with severe ARDS defined with a ratio PaO2/FiO2 ratio < 100 mm Hg and need for mechanical ventilation at the time of enrollment, on chronic home oxygen therapy, chronic renal or hepatic failure (aspartate aminotransferase or alanine aminotransferase higher than 5× the upper limit of the normal range, calculated glomerular filtration rate < 30 mL/min per 1·73 m2 utilising the Cockcroft-Gault formula); concurrent treatment with other agents outside the standard of care; using any other anti-inflammatory agents or antioxidant supplements outside the institutional protocol treatment; prior hypersensitivity to NAC; the occurrence of any adverse effect leading to the patients’ intolerance or complications; and pregnancy and lactation.
Intervention
Eligible patients by block randomisation method (1:1) were allocated into the placebo or the NAC groups. The randomisation was provided by an independent statistician not directly involved in the trial. Patients received either intravenous NAC or the equal volume of matching placebo (5% dextrose) for three consecutive days. The dose and duration of NAC in this study were chosen based on the former study performed by Moradi et al. on NAC in patients with mild-to-moderate acute lung injury [20]. Accordingly, NAC at a dose of 40 mg/kg/day diluted in 5% dextrose was administered as a continuous intravenous infusion for three consecutive days. A matching placebo was administered according to the same schedule and in the same volume as the active drug. All patients received supportive and/or COVID-19 standard treatments determined by their treating physician. In addition to optimal medical treatments for COVID-19 per the institutional protocol treatment, corticosteroids administration could be considered in the study patients if their need for supplemental oxygen was 6–8 L/min or more. Further, all the study patients per institutional protocol treatment received antioxidant supplements, including vitamin C (1000 mg bid), vitamin D3 (1000 IU bid), and Zinc (50 mg daily), through the study period. In addition, before intubation, a protocol of early awake prone positioning was promoted for all the study patients.
Efficacy and safety assessment
All patients were assessed for a total of 28 days post-randomisation with daily monitoring of symptoms. Clinical data were gathered on paper case records by trained nurses and then double entered into an electronic database. As the primary efficacy outcomes, the following were compared between the two groups from baseline up to day 28 post-randomisation: (1) overall 28-day mortality, (2) clinical status on study day 28, using an 8-point ordinal scale of the WHO Master Protocol (V.3.0, 3 March 2020) 0 for uninfected, 1 for no limitation of activities (ambulatory), 2 for limitation of activities (ambulatory), 3 for hospitalised, no oxygen therapy (hospitalised mild disease), 4 for hospitalised, oxygen by mask or nasal prongs (hospitalised mild disease), 5 for hospitalised, noninvasive ventilation or high-flow oxygen (hospitalised severe disease), 6 for hospitalised, intubation and mechanical ventilation (hospitalised severe disease), 7 for hospitalised, ventilation plus additional organ support, pressors, renal replacement therapy, and ECMO (extracorporeal membrane oxygenation), and 8 = death, and proportion of patients who met recovery criteria which are defined with the WHO clinical status 1, 2, or 3. As the secondary efficacy outcomes, the following were compared between the two groups from baseline up today 28: (1) the proportion of patients needed mechanical ventilation and ventilator-free days, (2) mean change of ARDS severity based on the Berlin classification at 72 h and 96 h after intervention (defined as PaO2/FiO2 ratio 200–300 as mild ARDS, 100–200 as moderate ARDS, and < 100 as severe ARDS), (3) mean change of the Sequential Organ Failure Assessment (SOFA) scores within the range of 0–24, with higher scores representing higher organ dysfunction, at baseline, 72 and 96 h after intervention, (4) length of stay in ICU, (5) length of stay at the hospital, and (6) incidence of infusion-related adverse events. For patients who died, the number of ventilator-free days was 0, and for patients who were alive, the days not requiring mechanical ventilation during the 28-days were the ventilator-free days.
Statistical analyses
All analyses were done at 0.05 significance levels, using SPSS version 21. Data analyses were conducted for the intent-to-treat (ITT) population, and the last observation carried forward (LOCF) method was used for missing data handling. Normal distribution of continuous data was examined using Shapiro–Wilk test. The normally- and non-normally, continuous data were expressed as mean (Standard Deviation: SD) and median (Interquartile Range: IQR), respectively, while categorical data were expressed as numbers with percentages. Independent t test and Mann–Whitney U test were used to compare the mean (± SD) and median (± IQR) of continuous variables between the drug and placebo group. Chi-squared test was used to compare the proportions/test the association between groups. p values < 0.05 were considered statistically significant.
Results
Demographics and baseline characteristics
Figure 1 represents a flow chart of the trial process. During the study period, 175 patients were screened for eligibility. Of the 92 patients fulfilling the inclusion/exclusion criteria, 47 received IV NAC, and 45 received placebo and were followed for 28 days (Fig. 1). All participants received the entire 3-day treatment protocol, and no patients were excluded from the analysis. A comparison of the demographic and baseline clinical characteristics of the participants in the intervention and control groups is represented in Table 1. The patients’ average age was 57.6 ± 18.7 years, and 58.7% of the patients were men. The Median time from COVID-19 symptom onset to enrolment time was 7 (10–4) days, and all patients had bi-pulmonary ground-glass opacities on computed chest tomography imaging. As shown, at randomisation time, baseline characteristics, including general condition, the use of concomitant treatments, comorbidities, vital signs, and disease severity, were well-matched between the two groups. Thirty-three patients in the intervention group (70.21%) and 31 patients in the control group (68.9%) received dexamethasone. A protocol of early awake prone positioning was promoted in the non-intubated patients. Further, as shown in Table 2, none of the patients received extracorporeal membrane oxygenation (ECMO) therapy. The number of patients who underwent renal replacement therapy (RRT) and hemoperfusion therapy to day 28 was also comparable in the study groups.
Fig. 1.
The study flow diagram
Table 1.
Baseline demographics and clinical features of the intention-to-treat population
| Variable | NAC group (47 patients) | Placebo group (45 patients) | p value |
|---|---|---|---|
| Age, years, mean ± SD | 59.40 ± 21.10 | 55.49 ± 16.49 | 0.32 |
| Sex (M/F), n (%) | 15 (63.8/36.2) | 24/21 (53.3/46.7) | 0.40 |
| Median time (IQR) from symptom onset | 7 (10–4) | 7 (10–4.5) | 0.97 |
| General condition at baseline | |||
| Temperature, °C, mean ± SD | 37.6 (± 0.8) | 37.8 (± 0.9) | 0.41 |
| Heart rate, times/min, mean ± SD | 88.17 (± 12.89) | 92.27 (± 14.79) | 0.16 |
| Respiratory rate, times/min, median (IQR) | 27 (30–25) | 26 (29–24) | 0.7 |
| APACHE II score, mean ± SD | 8.3 (± 4.05) | 7.7 (± 2.3) | 0.4 |
| SOFA score, mean ± SD | 3.45 (± 2.06) | 2.84 (± 1. 35) | 0.1 |
| PaO2/FiO2 ratio (mmHg), mean ± SD | 143.8 (± 47.5) | 134.9 (± 42.1) | 0.34 |
| Comorbidities | |||
| Current smokers, n (%) | 10 (21.3) | 8 (17.8) | 0.79 |
| Cardiovascular diseases, n (%) | 16 (34.0) | 10 (22.2) | 0.25 |
| Diabetes, n (%) | 10 (21.3) | 12 (26.7) | 0.68 |
| Respiratory diseases, n (%) | 8 (17.0) | 6 (13.3) | 0.77 |
| Neurologic disorders, n (%) | 9 (19.1) | 11 (24.4) | 0.62 |
| Other, n (%) | 10 (21.3) | 4 (8.9) | 0.15 |
| Additional medication | |||
| Hydroxychloroquine, n (%) | 42 (89.4) | 41 (91.1) | 1.00 |
| Antiviral agents, n (%) | 23 (48.9) | 16 (35.6) | 021 |
| Azithromycin, n (%) | 21 (44.7) | 26 (55.3) | 0.44 |
| Dexamethasone, n (%) | 33 (70.21) | 31 (68.9) | 1.00 |
NAC N-Acetyl cysteine, SD standard deviation, IQR interquartile range, APACHE II acute physiology and chronic health evaluation II, SOFA Sequential Organ Failure Assessment, PaO2/FiO2 ratio ratio of arterial oxygen partial pressure to fractional inspired oxygen
Table 2.
Study clinical outcomes up to day 28
| Variable | NAC group (47 patients) | Placebo group (45 patients) | p value |
|---|---|---|---|
| Proportion of patients needed mechanical ventilation, n (%) | 18 (38.3) | 20 (44.4) | 0.67 |
| Ventilator-free days, mean ± SD | 17.4 (± 12.6) | 16.6 (± 13.0) | 0.76 |
| 28-day mortality, n (%) | 12 (25.5) | 14 (31.1) | 0.64 |
| Proportion of patients who met the recovery criteria | 22 (46.8) | 17 (37.8) | 0.41 |
| ICU stay, days, median time (IQR) | 8 (13–6) | 10 (17–6) | 0.48 |
| Hospital stay, days, median time (IQR) | 10 (28–7) | 15 (28–8) | 0.31 |
| SOFA score at baseline, mean ± SD | 3.45 (± 2.06) | 2.84 (± 1. 35) | 0.10 |
| SOFA score at 48 h, mean ± SD | 3.02 (± 2.45) | 3.33 (± 1.93) | 0.53 |
| SOFA score at 96 h, mean ± SD | 3.13 (± 2.83) | 3.95 (± 3.15) | 0.18 |
| PaO2/FiO2 ratio at baseline (mmHg), mean ± SD | 143.8 (± 47.5) | 134.9 (± 42.1) | 0.34 |
| PaO2/FiO2 ratio at 48 h, mean ± SD | 175.4 (± 51.4) | 162.6 (± 59.2) | 0.27 |
| PaO2/FiO2 ratio at 96 h, mean ± SD | 217.6 (± 102.3) | 209.0 (± 104.4) | 0.69 |
| Additional advanced life support modalities | |||
| ECMO | 0 (0%) | 0 (0%) | 1.00 |
| RRT | 3 (6.4) | 4 (8.9%) | 0.71 |
| Hemoperfusion therapy | 5 (10.6) | 7 (15.6) | 0.55 |
NAC N-Acetyl cysteine, SD standard deviation, IQR interquartile range, SOFA Sequential Organ Failure Assessment, PaO2/FiO2 ratio ratio of arterial oxygen partial pressure to fractional inspired oxygen, ECMO extracorporeal membrane oxygenation, RRT renal replacement therapy
Clinical efficacy outcomes
Table 2 and Fig. 2 show the main clinical efficacy outcomes of the trial. According to the obtained results, although the overall 28-day mortality rate was lower in the NAC group than to the placebo group [12 of 47 patients (25.5%) in the NAC group vs. 14 of 45 patients (31.1%) in the control group], no statistically significant differences were found between the two groups (p value = 0.64; Table 2). Results regarding the comparison of the distribution of the clinical status at day 28, using the 8-point ordinal scale of the WHO, are shown in Fig. 2. Although the distribution of the clinical status at day 28 in the NAC-treated group shifted towards better outcomes than did placebo-treated group, it did not reach a statistical significance level (p value = 0.83). The same result was found, considering the proportion of patients who met recovery criteria at day 28, which was defined with the WHO clinical status 1, 2, or 3 (p value = 0.41). The proportion of patients achieving recovery criteria until day 28 was 46.8% in the NAC-treated group (22 out of 47 patients) vs. 37.8% (17 out of 45 patients) in the placebo-treated group (Table 2). Eighteen of 47 patients in the NAC-treated group (38.3%) and 20 of 45 patients in the placebo-treated group (44.4%) required invasive ventilatory support, and both groups had a comparable need for mechanical ventilation (p value = 0.83). Further, the mean number of ventilator-free days was 17.4 (± 12.6) in the NAC-treated patients and was 16.6 (± 13.0) in the placebo-treated patients, which was not a statistically significant difference (p value = 0.76). The PaO2/FiO2 ratio was similar on randomisation day in the two treatment groups [134.9 (± 42.1) and 143.8 (± 47.5) in the placebo and NAC treatment groups, respectively; p value = 0.34]. The mean PaO2/FiO2 ratio rose from 134.9 (± 42.1) to 209.0 (± 104.4) in the placebo group and from 143.8 (± 47.5) to 217.6 (± 102.3) in the NAC group on day 5 after treatment. So, the PaO2/FiO2 ratio significantly improved in both groups on day 5 after treatment, and no difference was found between the two groups (Table 2). On the first day of the intervention, the mean SOFA score was 3.45 (± 2.06) in the NAC-treated group and 2.84 (± 1. 35) in the placebo-treated group that did not differ significantly (p value = 0.10). The mean SOFA score rose from 2.84 to 3.94 in the placebo group and from 3.02 to 3.13 in the NAC group on day 5 after treatment. As shown in Table 2, no statistically significant differences were found in SOFA scores between the two groups on the third and fifth days after intervention (p value = 0.53 and 0.18, respectively). The median time (IQR) of ICU stay was 8 days (13–6) in the patients treated with NAC and was 10 days (17–6) in the patients treated with placebo, but this difference was not statistically significant (p value = 0.48). The study groups also showed no differences in terms of the median time (IQR) of hospitalisation length [NAC = 10 days (28–7); placebo = 15 days (28–8); p value = 0.31].
Fig. 2.
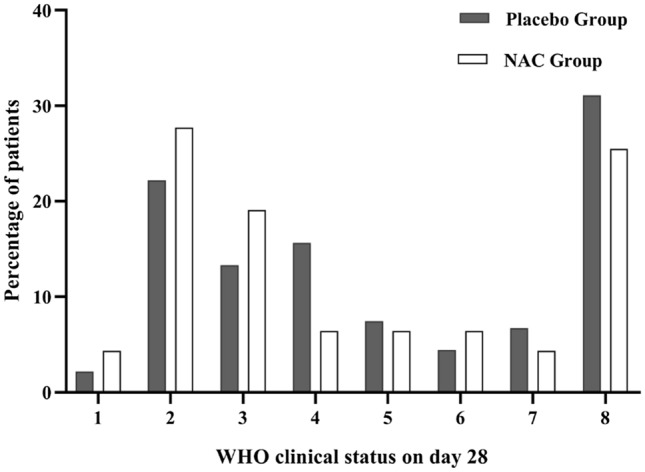
Comparison of the distribution of the clinical status of two groups at study day 28, using the 8-point ordinal scale of the WHO (p value = 0.83)
Safety and adverse events
During the study, no intolerable or severe adverse events were caused by the NAC infusion, and no patients dropped out of the study due to the intolerable adverse events during NAC administration.
Discussion
To our knowledge, it was the first prospective randomised, placebo-controlled study examining the efficacy and safety of intravenous NAC in patients with COVID19-associated acute respiratory distress syndrome. Results of this pilot trial showed that adding intravenous NAC to the standard-of-care treatment did not affect the clinical outcomes of patients with mild to moderate COVID19-associated ARDS.
It has been previously well-documented that NAC has excellent anti-inflammatory, anti-oxidative, and immune system enhancing actions. NAC, through a variety of potential mechanisms, including extracellular scavenging reactive oxygen species, replenishing intracellular glutathione (GSH), suppression of the overreaction of the innate immune response and overshooting inflammation, and promoting the adaptive immune activity and T cell response can mitigate destructive inflammation and tissue injury resulting from an overreaction of the innate immune response [26]. Due to these multifactorial pharmacological actions, recently, the potential utility of NAC as adjuvant therapy in treating severe infectious diseases associated with the destructive inflammation and overreaction of innate immune responses has been investigated. In this context, current studies revealed some benefits of NAC in treating septic shock [27].
Beyond these mechanisms, accumulated evidence indicates that NAC also has antiviral properties and suppresses viral replication. RNA viruses, including coronaviruses, need active NF-κB pathway support within host cells to replicate, and it has been found antioxidant and anti-inflammatory agents such as NAC through regulatory effects on NF-κB expression and activation can have indirect antiviral effects [28]. Further, it has been hypothesised that NAC, through other mechanisms, including binding to specific cell-surface receptors and inhibiting viral penetration or uncoating, inhibiting viral DNA polymerase, inhibiting viral protein synthesis, and blocking late stages of virus assembly might also prevent viral replication [15]. Apart from these mechanisms, considering that the overload of reactive oxygen species and inflammatory cytokines is associated with the compromised of immune system function in fighting viral infections, NAC, by maintaining adequate endogenous antioxidant defences can potentially boost our immune system responses and, as a result, indirectly might increase viral eradication [29]. In this context, in experimental studies, both in-vitro and in-vivo, the beneficial effects of NAC against viral infections with noticeable results have been reported [29–31].
Given that the large body of data demonstrating COVID-19 adverse outcomes is usually related to hyper-inflammatory state and immunity–inflammatory damage [32], it is thought that NAC may be helpful in targeting COVID-19 excessive immune/inflammatory processes. Thus, NAC has been proposed by an increasing number of publications to prevent and treat SARS-CoV-2 infections, in particular COVID19-associated ARDS [33–35]. ARDS is the most devastating complication of SARS‐CoV‐2, which is associated with oxidative stress and inflammatory cytokine production following viral infection, a condition causing diffuse alveolar damage with endothelial and epithelial apoptosis, pulmonary fibrinolysis, and dysregulated coagulation [36, 37]. It is speculated that NAC, through the inhibitory effects on viral replication, modulating the pro-inflammatory cytokines production, and inhibition of oxidative damage, could reduce acute lung damage during COVID-19 [38].
Previously, multiple studies have investigated the use of NAC in treating ARDS. However, to date, the clinical studies on NAC in ARDS have given conflicting results. Some clinical studies with NAC in ARDS have provided promising results. In this regard, the studies by Moradi et al. [39] and Suter et al. [25] revealed that intravenous NAC in patients with mild-to-moderate acute lung injury not only enhanced systemic oxygenation and decreased the need for ventilatory support but also slightly reduced the mortality rate. Another promising study by Ortolani et al. demonstrated that intravenous NAC administration as monotherapy and in combination with rutin standard treatment through improving antioxidant capacity could have protective effects on the lung epithelium’s lipid peroxidation in the early stage of ARDS [40]. Despite this promising clinical evidence, in Domenighetti et al.’ clinical study on ARDS associated with various underlying aetiologies, intravenous NAC treatment for 72 h had no benefits on systemic oxygenation and reduction in the mechanical ventilation need. Further, the intravenous NAC did not demonstrate mortality benefits [41]. Jepsen et al., also in their study, found no significant difference in terms of oxygenation, ventilator support, and mortality rate between intravenous NAC and placebo treatment in ARDS patients [42]. The results of these studies were consistent with our findings. Zhang et al. at 2017 in a meta-analysis of the available randomised controlled trials on NAC treatment in ARDS patients, found that although NAC treatment may reduce the period of ICU stay in such patients, it does not significantly reduce short-term and 30-day mortality and does not improve the PaO2/FiO2 ratio [43]. However, it is worth noting that there is yet no clinical evidence that using NAC in ARDS is associated with an increased rate of negative adverse outcomes.
At present, clinical evidence about using NAC in COVID‐19 is sparse. In this line, two cases with severe COVID-19-associated respiratory failure who had received standard of care and routine treatment of severe COVID-19 had been treated successfully with intravenous or inhalation administration of NAC [21, 22]. In another case report, IV and oral glutathione, alpha-lipoic acid, and NAC successfully treated two COVID-19 patients with dyspnea [23]. Further, in a small case series from the USA reported by Ibrahim et al., intravenous NAC administration to ten respirator-dependent COVID-19-infected patients that of whom one subject had Glucose–6–phosphate dehydrogenase (G6PD) deficiency significantly decreased the inflammatory markers, like ferritin and C-reactive protein (CRP). It also showed beneficial effects on lung functions in these cases [24]. In another report by Alamdari et al., the combination of methylene blue-vitamin C-NAC (1 mg/kg methylene blue, 1500 mg/kg vitamin C, 1500 mg/kg NAC), as last resort therapy, was administrated to five critically ill COVID-19 patients with high serum level of nitrite, nitrate, methemoglobin, and oxidative stress. Results of this small case serious also are promising, as four out of five patients responded well to treatment and recovered [44]. However, the results of our study are in discordance with these reports. There are several possible explanations for our findings. First, because in our study, a significant percentage of patients in both groups received dexamethasone, it is possible that dexamethasone as a potent anti-inflammatory modality treatment in COVID-19-associated respiratory failure obscured the effects of NAC and NAC adds nothing further to its effect. Second, although other medications used for the treatment of COVID-19 did not differ between the patients of the two groups, the permitted use of these medications may also influence the effects of NAC treatment on clinical outcomes of the study populations. Third, the sample size of our study was relatively small and thus only powered to detect large effects and would be incapable of detecting small or medium effects. Fourth, since inflammatory response profiles changed at different disease stages of COVID-19, and while regulated activation of the innate immune system is responsible for both viral elimination and recovery from the infection, exaggerated inflammatory responses are implicated in the disease’s progression to a more severe and lethal process, the timing of anti-inflammatory drugs administration could potentially determine treatment outcomes [45, 46]. So, although NAC administration in our study patients with mild to moderate ARDS did not demonstrate statistically significant benefits, maybe treatment with it if used in the earlier or later phases of the disease process showed different effects on the COVID-19 patients’ clinical outcomes. Further, our study was a single-centre investigation. Thus, based on the results of a single-centre study, it is impossible to make definitive conclusions about the efficacy of the NAC as adjunctive therapy in patients with COVID19-associated ARDS, and larger, multicenter studies are still needed to assess its potential usefulness in such patients.
However, although the current clinical data are conflicting, there is a rationale to study NAC as an adjunct therapy to prevent and treat COVID-19 infection. Research has revealed that despite the potent antioxidant and anti-inflammatory effects of NAC, unlike glucocorticoids with intrinsic immunosuppressive drawbacks, NAC is not immunosuppressive. In fact, NAC not only does not increase the risk of infections but also, through regulatory actions on the immune system, may decrease the risk of infections [33]. Furthermore, it is well-known that NAC against acute cardiovascular and neurological events, such as myocardial infarction and stroke, has protective effects [47, 48]; this effect may also be helpful for some patients at an increased risk of cardiovascular and neurological complications associated with COVID-19 [49, 50]. Moreover, one of the main properties of NAC that makes it a proper candidate in preventing and treating COVID-19 is its excellent safety and tolerability even when used in high doses. Although compared to oral NAC, a higher incidence of adverse reactions has been reported with intravenous administration of NAC, and in some cases, life-threatening anaphylactoid reactions were reported by its intravenous administration, most of these reactions are mild in nature and easily manageable by a slower infusion rate [51]. It is to be noted that the only promising result in our trial was that intravenous NAC administration was safe in the study patients and did not cause any intolerable or severe side effects. It should be mentioned that NAC at high-serum concentration (10 mM or more) displays its maximum antioxidant and immunomodulatory effects in severe immunopathological conditions such as ARDS [52]. Such high-serum concentrating can achieve quickly by the IV formula of NAC. Due to the extensive first-pass metabolism, the oral formula of NAC has low bioavailability [53]. Thus, high doses of oral NAC that should be administrated to achieve such high serum concentration are associated with intolerable gastrointestinal adverse effects such as nausea, vomiting, and diarrhoea [54].
Despite this strong scientific evidence, at percent, there is insufficient clinical evidence to recommend NAC for COVID-19 in routine clinical practice and strong evidence of its benefit in preventing and treating COVID-19 is still lacking. However, several ongoing clinical trials to investigate the potential effectiveness of NAC in patients with COVID‐19 are being implemented (NCT04545008; NCT04370288; NCT04419025; NCT04455243; NCT04374461; and NCT04466657). These studies provide more evidence on the potential utility of NAC in treating COVID-19 patients. We did not assess the changes in the serum levels of oxidative stress and inflammatory biomarkers in the present study. So, it did not allow knowing the regulatory effects of NAC on immunoinflammatory mediators as the main mechanism of its action in COVID-19 patients. So, it is also necessary to address this issue in-depth in future studies.
Conclusion
In conclusion, these preliminary results did not support the potential beneficial effects of intravenous NAC as an adjunct therapy in treating patients with mild-to-moderate COVID-19 associated ARDS. Although basic science suggests a rationale for NAC’s use in preventing and treating COVID-19, based on current clinical data, NAC’s benefits in treating COVID-19 remains uncertain, and its use should be considered experimental. Future studies are needed to determine which COVID-19 patients benefit from the NAC treatment, and the optimal timing of NAC administration in these patients is also determined.
Acknowledgements
We thank all patients and medical staff of Farshchian (Sina) Hospital who contributed to this study.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care unit
- IL-6
Interleukin 6
- TNF-α
Tumour Necrosis Factor-α
- NAC
N-Acetylcysteine
- PaO2/FiO2 ratio
Ratio of arterial oxygen partial pressure to fractional inspired oxygen
- ECMO
Extracorporeal membrane oxygenation
- SOFA
Sequential Organ Failure Assessment
- GSH
Glutathione
- NF-κB
Nuclear factor kappa light chain enhancer of activated B cells
- G6PD
Glucose–6–phosphate dehydrogenase
- CRP
C-reactive protein
- SD
Standard deviation
- IQR
Interquartile range
- ECMO
Extracorporeal membrane oxygenation
- RRT
Renal replacement therapy
Author contributions
Conceptualisation: AT, MM; methodology: AT, MM, JP; data acquisition: AT, LS, ML, FR-B; statistical analysis: MM, JP; writing—original draft preparation: LS; writing—review and editing: MM, AT; all authors contributed to the interpretation of the results and read and approved the final manuscript.
Funding
This research was supported by funding from the vice-chancellor for research and technology, Hamadan University of Medical Sciences, Hamadan, Iran (No: 9903271878). This grant was not assigned to the manuscript writing, editing, and publication fee.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request up to 2 years after publication.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The trial protocol was according to the Declaration of Helsinki as revised in 1989, and the study protocol was approved by the research and ethics committee at Hamadan University of Medical Sciences (IR.UMSHA.REC.13999.153).
Consent for publication
All authors have given consent for publication.
Contributor Information
Abbas Taher, Email: t_anesthesia@yahoo.com.
Marjan Lashgari, Email: marjan0084@gmail.com.
Ladan Sedighi, Email: l.sedighie@yahoo.com.
Farshid Rahimi-bashar, Email: fr_rahimibashar@yahoo.com.
Jalal Poorolajal, Email: poorolajal@yahoo.com.
Maryam Mehrpooya, Email: m_mehrpooya2003@yahoo.com, Email: m.mehrpoya@umsha.ac.ir.
References
- 1.WHO . WHO director-general’s remarks at the media briefing on 2019-nCoV on 11 February 2020. WHO; 2020. [Google Scholar]
- 2.CPERE Novel The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Force ADT, Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E. Acute respiratory distress syndrome. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 7.Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;102:39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapenna D Antioxidant therapy in COVID-19: the crucial role of early treatment and antioxidant typology. Clin Infect Dis. 2021. 10.1093/cid/ciab055. [DOI] [PMC free article] [PubMed]
- 12.WHO . Corticosteroids for COVID-19: living guidance, 2 September 2020. World Health Organization; 2020. [Google Scholar]
- 13.Zafarullah M, Li W, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 15.Dass E. Brief review of N-acetylcysteine as antiviral agent: potential application in COVID-19. J Biomed Pharm Res. 2020;9(3–):69–73. [Google Scholar]
- 16.De Flora S, Grassi C, Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 17.Mata M, Morcillo E, Gimeno C, Cortijo J. N-acetyl-l-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV) Biochem Pharmacol. 2011;82:548–555. doi: 10.1016/j.bcp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 19.Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, Khajavi MR, Rouini MR, Moradi M, et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;26:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- 20.Mohanty R, Padhy B, Das S, Meher B. Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence. Eur Rev Med Pharmacol Sci. 2021;25:2802–2807. doi: 10.26355/eurrev_202103_25442. [DOI] [PubMed] [Google Scholar]
- 21.Puyo C, Kreig D, Saddi V, Ansari E, Prince O. Case report: Use of hydroxychloroquine and N-acetylcysteine for treatment of a COVID-19 positive patient. F1000Research. 2020;9:491. [Google Scholar]
- 22.Liu Y, Wang M, Luo G, Qian X, Wu C, Zhang Y, et al. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: a case report. Medicine. 2020;99(42):e22577. doi: 10.1097/MD.0000000000022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasi A, McArdle S, Gaudernack G, Westman G, Melief C, Rockberg J, et al. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim H, Perl A, Smith D, Lewis T, Kon Z, Goldenberg R, et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter PM, Domenighetti G, Schaller M-D, Laverrière M-C, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 26.Radomska-Leśniewska DM, Skopiński P. Review paper N-acetylcysteine as an anti-oxidant and anti-inflammatory drug and its some clinical applications. Cent Eur J Immunol. 2012;37:57–66. [Google Scholar]
- 27.Chertoff J. N-acetylcysteine’s role in sepsis and potential benefit in patients with microcirculatory derangements. J Intensive Care Med. 2018;33:87–96. doi: 10.1177/0885066617696850. [DOI] [PubMed] [Google Scholar]
- 28.Poppe M, Wittig S, Jurida L, Bartkuhn M, Wilhelm J, Müller H, et al. The NF-κB-dependent and-independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells. PLoS Pathog. 2017;13:e1006286. doi: 10.1371/journal.ppat.1006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol. 2004;17:99–102. doi: 10.1177/039463200401700114. [DOI] [PubMed] [Google Scholar]
- 30.Mata M, Sarrion I, Armengot M, Carda C, Martinez I, Melero JA, et al. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS ONE. 2012;7:e48037. doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiler J, Michaelis M, Naczk P, Leutz A, Langer K, Doerr H-W, et al. N-acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Flora S, Balansky R, La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34:13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal N, Bhatnagar M, Shah H. N-acetylcysteine: a potential therapeutic agent in COVID-19 infection. Med Hypotheses. 2020;144:110133. doi: 10.1016/j.mehy.2020.110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Hecke O, Lee J. N-acetylcysteine: a rapid review of the evidence for effectiveness in treating COVID-19. 2020. https://hdl.handle.net/20.500.12663/1089.
- 36.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Z, Puyo CA. N-acetylcysteine to combat COVID-19: an evidence review. Ther Clin Risk Manag. 2020;16:1047. doi: 10.2147/TCRM.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, et al. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med. 2009;103:434–441. doi: 10.1016/j.rmed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Ortolani O, Conti A, De Gaudio AR, Masoni M, Novelli G. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock. 2000;13:14–18. doi: 10.1097/00024382-200013010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Domenighetti G, Suter PM, Schaller M-D, Ritz R, Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care. 1997;12:177–182. doi: 10.1016/s0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 42.Jepsen S, Herlevsen P, Knudsen P, Bud MI, Klausen N. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med. 1992;20:918–923. doi: 10.1097/00003246-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Ding S, Li C, Wang Y, Chen Z, Wang Z. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2017;14:2863–2868. doi: 10.3892/etm.2017.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alamdari DH, Moghaddam AB, Amini S, Keramati MR, Zarmehri AM, Alamdari AH, et al. Application of methylene blue-vitamin C–N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur J Pharmacol. 2020;885:173494. doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lariccia V, Magi S, Serfilippi T, Toujani M, Gratteri S, Amoroso S. Challenges and opportunities from targeting inflammatory responses to SARS-CoV-2 infection: a narrative review. J Clin Med. 2020;9:4021. doi: 10.3390/jcm9124021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know?! J Am Coll Cardiol. 2002;39:1422–1428. doi: 10.1016/s0735-1097(02)01797-7. [DOI] [PubMed] [Google Scholar]
- 48.Sabetghadam M, Mazdeh M, Abolfathi P, Mohammadi Y, Mehrpooya M. Evidence for a beneficial effect of oral N-acetylcysteine on functional outcomes and inflammatory biomarkers in patients with acute ischemic stroke. Neuropsychiatr Dis Treat. 2020;16:1265. doi: 10.2147/NDT.S241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samidurai A, Das A. Cardiovascular complications associated with COVID-19 and potential therapeutic ~ strategies. Int J Mol Sci. 2020;21:6790. doi: 10.3390/ijms21186790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meri W, Koutsogiannis Z, Kerr D, Kelly. How safe is intravenous N-Acetylcysteine for the treatment of paracetamol poisoning?. Hong Kong J Emerg Med 2007, 14(4):198–203.
- 52.Sadowska AM, Manuel-y-Keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, et al. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res. 2006;53:216–225. doi: 10.1016/j.phrs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kearns SR, O’Briain DE, Sheehan KM, Kelly C, Bouchier-Hayes D. N-acetylcysteine protects striated muscle in a model of compartment syndrome. Clin Orthop Relat Res. 2010;468:2251–2259. doi: 10.1007/s11999-010-1287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request up to 2 years after publication.
Not applicable.