Abstract
INTRODUCTION:
Constipation is commonly treated with over-the-counter (OTC) products whose efficacy and safety remain unclear. We performed a systematic review of OTC therapies for chronic constipation and provide evidence-based recommendations.
METHODS:
We searched PubMed and Embase for randomized controlled trials of ≥4-week duration that evaluated OTC preparations between 2004 and 2020. Studies were scored using the US Preventive Services Task Force criteria (0–5 scale) including randomization, blinding, and withdrawals. The strengths of evidence were adjudicated within each therapeutic category, and recommendations were graded (A, B, C, D, and I) based on the level of evidence (level I, good; II, fair; or III, poor).
RESULTS:
Of 1,297 studies identified, 41 met the inclusion criteria. There was good evidence (grade A recommendation) for the use of the osmotic laxative polyethylene glycol (PEG) and the stimulant senna; moderate evidence (grade B) for psyllium, SupraFiber, magnesium salts, stimulants (bisacodyl and sodium picosulfate), fruit-based laxatives (kiwi, mango, prunes, and ficus), and yogurt with galacto-oligosaccharide/prunes/linseed oil; and insufficient evidence (grade I) for polydextrose, inulin, and fructo-oligosaccharide. Diarrhea, nausea, bloating, and abdominal pain were common adverse events, but no serious adverse events were reported.
DISCUSSION:
The spectrum of OTC products has increased and quality of evidence has improved, but methodological issues including variability in study design, primary outcome measures, trial duration, and small sample sizes remain. We found good evidence to recommend polyethylene glycol or senna as first-line laxatives and moderate evidence supporting fiber supplements, fruits, stimulant laxatives, and magnesium-based products. For others, further validation with more rigorously designed studies is warranted.
INTRODUCTION
Constipation is a common condition with individual studies reporting prevalence rates ranging from 2% to 39% (1–5). This wide variability seems to be related to both differences in populations studied and the criteria used to define constipation (6–8). One systematic review found that when ROME criteria were applied, prevalence ranged from 6.8% (ROME III) to 15.0% (ROME II), whereas rates of self-reported constipation were generally higher (3). Constipation is more common in women than in men, and its incidence increases with advancing age (2). Constipation has significant clinical, economic, and quality of life (QoL) impacts (4,9) and correlates with significantly higher rates of psychological distress (10). The QoL impact is similar to that of numerous other chronic conditions, including sciatica, dermatitis, and chronic allergies (9).
Approximately 40% of patients with constipation in the United States self-treat with laxatives (11), and in 2019, more than $1.5 billion dollars were spent on over-the-counter (OTC) agents (12). A claims-based analysis estimated the cost of gastrointestinal (GI) symptom management in patients with chronic constipation to be $1,500 per patient per year, inflation adjusted to 2020 dollars (5,13). There are many OTC preparations available to manage constipation, each with a different mechanism(s) of action. However, there is considerable variability in both the quality and quantity of evidence supporting their use. One of the authors (S.R.) previously reviewed the efficacy and safety data for OTC therapies in the management of adult patients with chronic constipation (date range 1966–2004), identifying that many of the treatment options lacked robust supporting evidence (14). Since then, numerous additional products have become available, and new trials have been conducted.
Our objective here was to perform an updated evidence-based systematic review of OTC treatment options for chronic constipation. We systematically reviewed new data published over the past 15 years and aimed to update the classification of products and provide new treatment recommendations based on levels and strength of evidence.
METHODS
Literature search
PubMed and Embase were searched from 2004 through July 2020 using the following search terms: (constipation OR opioid-induced constipation) AND (laxatives, stimulant OR laxatives, osmotic OR laxatives, irritant OR laxatives, bulk OR fecal softeners OR stool softener OR sorbitol OR magnesium OR milk of magnesia OR magnesium sulfate OR magnesium sulphate OR bisacodyl OR calcium polycarbophil OR polyethylene glycol OR PEG OR senna OR ispaghula OR bran OR celandin OR docusate OR aloevera OR aloe vera OR poloxalkol OR mineral oil OR glycerin OR glycerine OR psyllium OR methylcellulose OR herbal remedies OR traditional medicine OR Chinese herbal OR plantain OR doxinate OR prune OR kiwi OR fiber OR iberogast OR STW 5 OR sodium picosulfate OR macrogol OR sennosides OR inulin). Limits on the search were English language, randomized clinical trial, adults, and human.
Selection criteria
Abstracts of articles were screened, potentially relevant studies published in full were reviewed, and the following selection criteria were applied for inclusion: (i) randomized controlled trial (placebo or active comparator), (ii) parallel or cross over design, (iii) established definition of constipation (preferably ROME criteria), (iv) minimum duration of 4 weeks of active treatment, and (v) well-defined clinical endpoints. Studies evaluating colonic cleansing before colonoscopy or surgery, acute constipation indications (typically 1–2 days or weeks in duration), and patients with irritable bowel syndrome and/or evacuation disorders were excluded. Certain studies in patients with chronic comorbidities (i.e., chronic kidney disease [CKD]) were included.
Data extraction and analysis
Articles that met inclusion criteria were independently reviewed by both authors, and relevant data were extracted. This included therapeutic and control agent(s), study design, number of patients, mean age or age range, sex, study duration, outcome measures, efficacy, and safety outcomes.
Qualitative assessment of study methodology
Next, each study was independently scored by both authors for quality of evidence using the US Preventive Services Task Force criteria (15). Any discrepancies between authors were reconciled by mutual discussion and a second review of all relevant articles. Final scores were adjudicated by consensus. Individual study quality was determined using a 0- to 5-point scale summating individual scores for randomization, blinding, and completeness of follow-up:
Randomization was scored as 1 (simply described as randomized) or 2 (appropriate randomization technique and concealed allocation explicitly described)
Blinding was scored as 0 (not blind), 1 (described as double blind but no details provided), or 2 (both subjects and investigators were explicitly said to be blinded, and an identical placebo was used)
Withdrawals were scored as 0 (no statement) or 1 (number of withdrawals and reason was stated)
Levels of evidence classification of products and grading of recommendations
Current US Preventive Services Task Force (15) criteria were used to score the strength of evidence and grade recommendations. Once again, each investigator provided independent recommendations, and any differences were resolved by consensus. The level of evidence was graded as good (level I), fair (level II), or poor (level III). The recommendation was graded as A (good evidence in support), B (moderate evidence in support), C (poor evidence in support), D (moderate evidence against), or I (insufficient evidence). These criteria represent a slight modification of the grading criteria used in the previous systematic review (14). Detailed descriptions of the grading criteria and differences in the grading criteria between the previous review and the current review are summarized in Supplemental Table 1 (see Supplementary Digital Content 1, http://links.lww.com/AJG/B929).
After applying the selection criteria, we identified 41 studies that are included in this analysis. OTC products were grouped into the following 8 categories: osmotic laxatives, fiber laxatives, stimulant laxatives, magnesium-based laxatives, fruit-based laxative, foods with prebiotics, surfactants, and miscellaneous agents.
RESULTS
Studies
A total of 1,297 studies were identified from the Embase/PubMed literature searches. Of these, 110 were identified as randomized clinical trials evaluating treatments for constipation. Studies outside the selection criteria were excluded. In addition, we decided to limit the results to more readily available agents. Thus, some categories that were included in the search strategy (i.e., Chinese herbals, traditional medications, and probiotics) were not included in the results. Overall, a total of 40 studies were included in the analysis (Figure 1). In addition, a late-breaking study (16) published after our literature search was added, bringing the total number of qualifying studies to 41.
Figure 1.
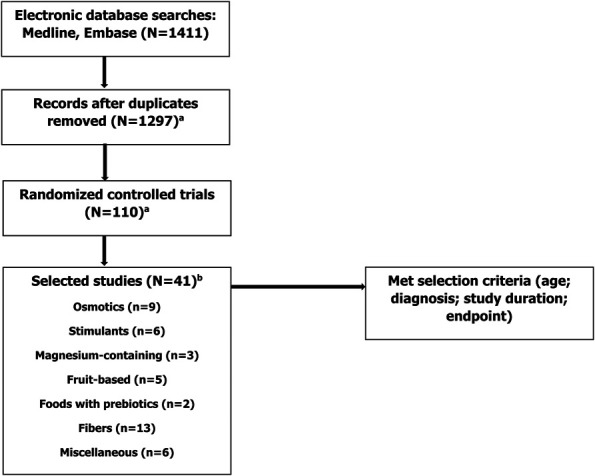
Flow diagram of study. aOne study added after literature search. bThree studies were included in 2 categories.
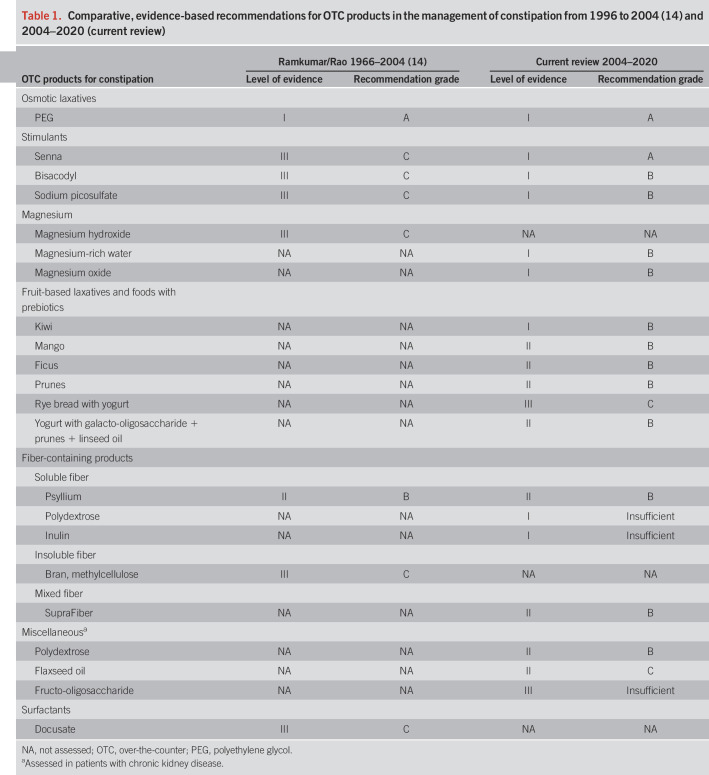
There was considerable variability in the quality of studies, the patient populations who were enrolled, and outcomes evaluated. A comparison of our current recommendations and those previously reported is summarized in Table 1. Study details and results for each product included in this review are summarized in Tables 2–7 and discussed in detail below. Studies that included products from more than one treatment category were included in both tables. Treatment categories are organized according to the quality of evidence, and those with the strongest evidence are discussed first.
Table 1.
Comparative, evidence-based recommendations for OTC products in the management of constipation from 1996 to 2004 (14) and 2004–2020 (current review)
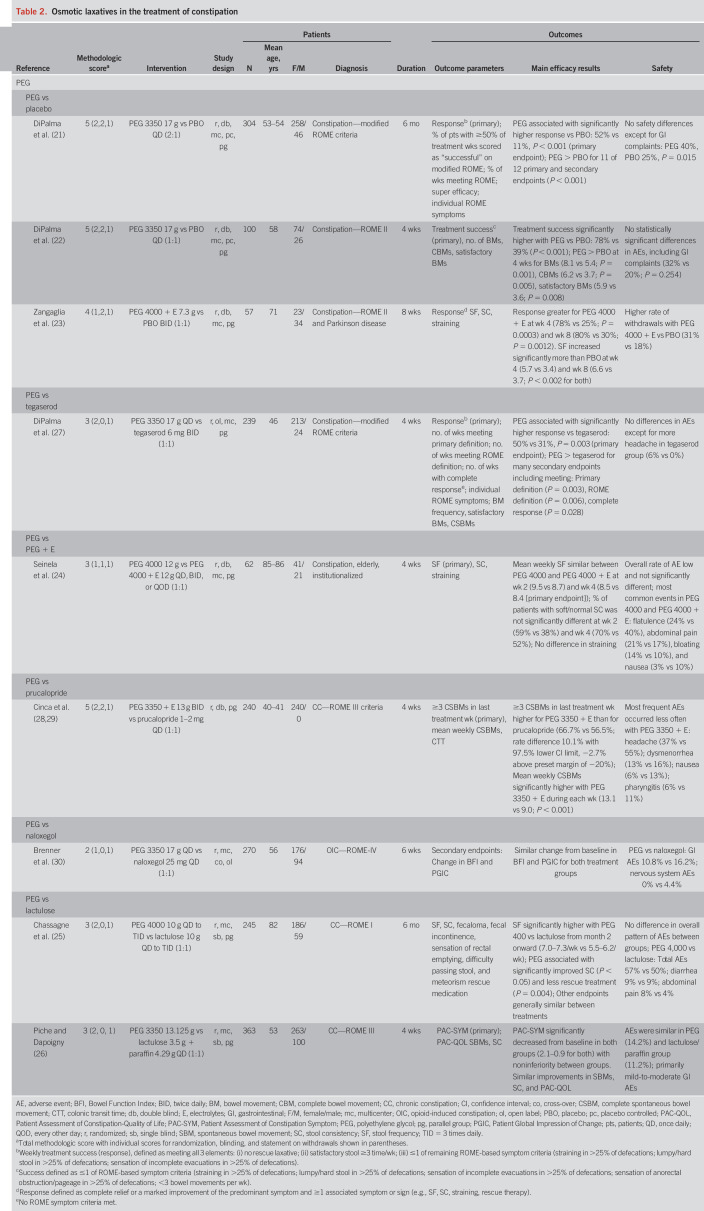
Table 2.
Osmotic laxatives in the treatment of constipation
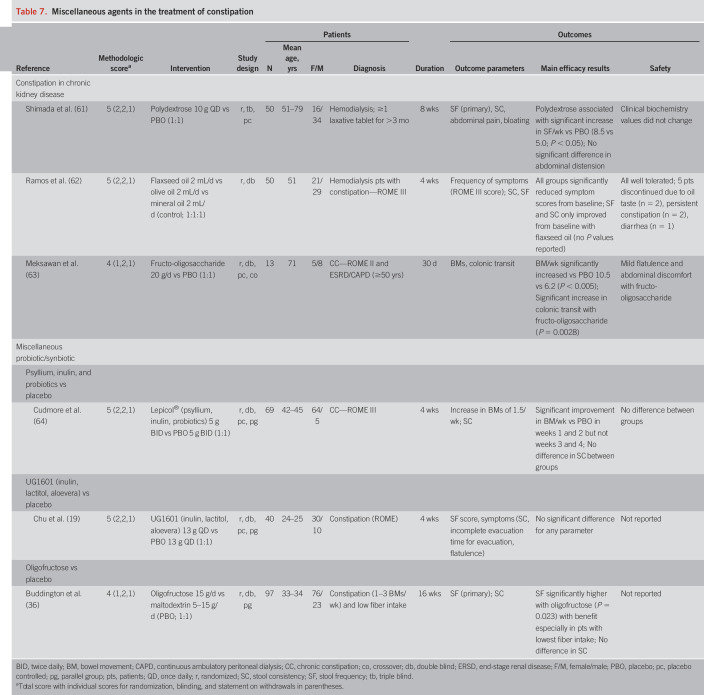
Table 7.
Miscellaneous agents in the treatment of constipation
Osmotic agents
Osmotic agents draw fluid into the intestine, soften stool, and increase luminal water retention, and the ensuing luminal distention secondarily increases colonic peristalsis and causes laxation (17,18). PEG is an osmotic agent that is approved by the US Food and Drug Administration (US FDA) for the treatment of occasional constipation (19). It is poorly absorbed (<0.28%), and nearly 100% of PEG is excreted in the feces (20). No other osmotic agents meeting current inclusion criteria were identified (magnesium-based laxatives are discussed separately).
Nine PEG studies satisfied the selection criteria in this updated analysis (Table 2), with methodological scores of 5 (n = 3), 4 (n = 1), 3 (n = 3), and 2 (n = 2). Three were placebo controlled (21–23), one compared PEG with PEG plus electrolytes (24), and 6 compared PEG with another active product. Two studies compared PEG with lactulose (25,26), whereas single studies compared PEG with tegaserod (27), prucalopride (28,29), and naloxegol (30). The 3 placebo-controlled trials were well designed, included large numbers of patients, and had appropriate clinically relevant endpoints. In these studies, PEG formulations demonstrated significantly greater responses versus placebo on primary (mainly ROME-based response criteria) and secondary endpoints following both short- (4 weeks) and long-term administration (6 months) (21–23). PEG preparations also demonstrated significantly greater efficacy across various endpoints compared with tegaserod (27), prucalopride (28,29), and lactulose (25) in patients with ROME-defined constipation. In patients with opioid-induced constipation, patient preferences for PEG were equivalent to naloxegol (30). In a comparative trial, iso-osmotic and hypo-osmotic formulations of PEG demonstrated similar efficacy and safety, indicating that the addition of electrolytes to PEG formulations may not yield significant clinical advantages in the context of constipation (24).
Consistent with their lack of significant systemic absorption and low rates of metabolism, PEG formulations were well tolerated with low incidences of adverse events. Most events were GI and mild to moderate in intensity and included abdominal distension, diarrhea, loose stools, flatulence, and nausea (21,22,24).
Overall, the data support PEG as an effective treatment with minimal side effects. Response rates were superior to psyllium and prescription agents and similar to naloxegol for the treatment of chronic and opioid-induced constipation, respectively. These data confirm and support PEG as a first-line agent for the treatment of chronic constipation.
PEG: Level I Evidence, Grade A Recommendation
Stimulant agents
Stimulant laxatives can be subdivided into 2 categories: diphenylmethane derivatives (e.g., bisacodyl and sodium picosulfate) and plant-based anthraquinones (e.g., senna, aloe, and cascara). All act locally at the nerve plexus of smooth muscle in the intestine to stimulate colonic motility.
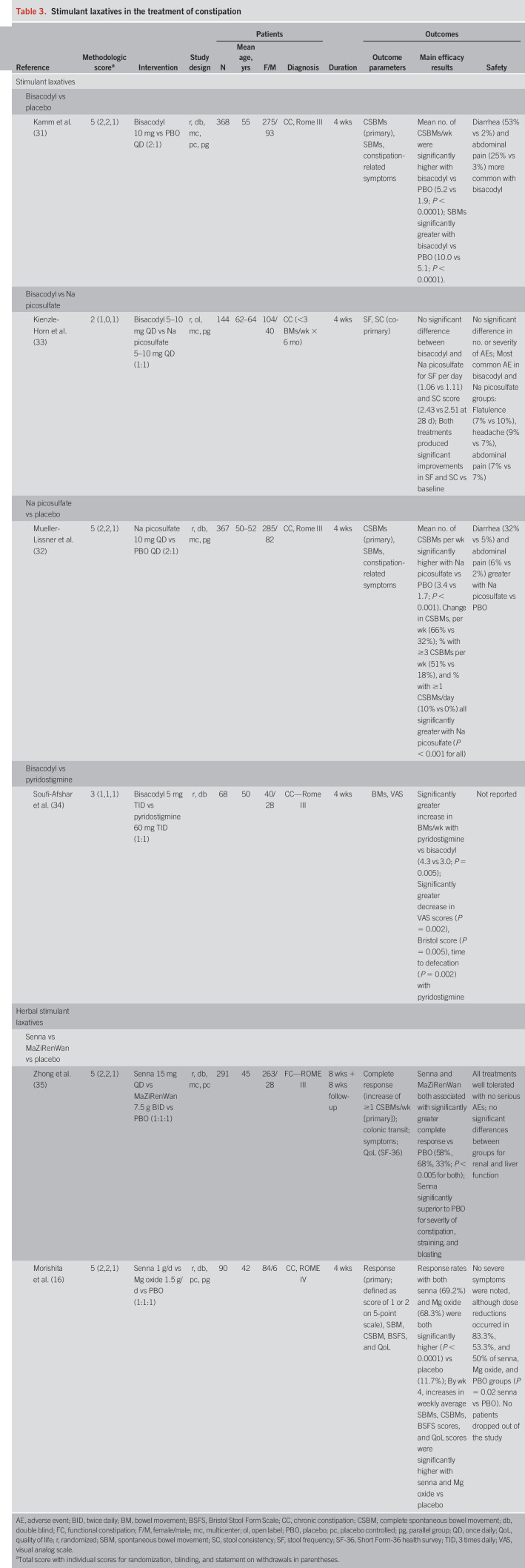
Four trials using diphenylmethane derivatives (Table 3) were identified in the current analysis. Two (1 bisacodyl, 1 sodium picosulfate) were placebo controlled. Both were large, rigorously designed studies (methodological scores = 5), and active treatment with both agents was associated with significant increases in mean complete spontaneous bowel movements (CSBMs)/week (the primary endpoint in both trials) compared with placebo (31,32). In a comparative trial, bisacodyl and sodium picosulfate demonstrated similar efficacy (i.e., number of bowel movements [BMs]/stool consistency) and safety (33). Bisacodyl proved inferior to the cholinesterase inhibitor pyridostigmine in the final study based on BM frequency and a visual analog scale assessing pain on a 0- to 10-point scale (34).
Table 3.
Stimulant laxatives in the treatment of constipation
Two studies also evaluated the anthraquinone senna (methodological score = 5; Table 3). In the first, senna was superior to placebo and had similar efficacy to a Chinese herbal preparation used for constipation (MaZiRenWan) as assessed by complete response rates (i.e., increase of ≥1 CSBM/week) (35). Senna was also superior to placebo for secondary endpoints, including frequency of CSBMs and spontaneous BM (SBMs), severity of constipation, and sensation of straining. In a recently published study from Asia, senna (at a starting dose of 1 g/d, which could subsequently be reduced) was superior to placebo in improving overall symptoms (primary endpoint), stool frequency, and QoL; however, the doses of senna consumed were significantly greater than those used in clinical practice in the United States (16).
Most adverse events were GI in nature owing to the irritant properties of stimulants. Increased rates of diarrhea (32%–53% vs 2%–5%) and abdominal pain (6%–25% vs 2%–3%) in comparison to placebo may limit the tolerability of bisacodyl (31) and sodium picosulfate (32). Senna was well tolerated in 1 study (35), but in the more recent study, 83.3% of subjects requested dose reduction of senna because of abdominal pain and diarrhea but completed the 4-week trial (16).
Overall, these data indicate that senna, bisacodyl, and sodium picosulfate are effective for the treatment of chronic constipation, although they are associated with increased potential for dose reduction or intolerance.
Senna: Level I Evidence, Grade A Recommendation
Bisacodyl: Level I Evidence, Grade B Recommendation
Sodium Picosulfate: Level I Evidence, Grade B Recommendation
Magnesium-containing agents
Magnesium-based compounds are laxatives that act by retaining water in the intestinal lumen, resulting in bulking and softening of stool (36). Magnesium salts have historically been classified as osmotic, saline, or mixed osmotic-saline laxatives with categorization dependent on the magnesium compound used. Although magnesium citrate is a stronger formulation with more characteristically osmotic properties, other agents including magnesium hydroxide, magnesium gluconate, and magnesium oxide or magnesium enriched water act as more gentle saline laxatives. Given the breadth of new studies evaluating different magnesium-based formulations, these results have been categorized and addressed separately from the other osmotic agents. This review identified 4 new relevant studies using magnesium-based regimens, 3 evaluating magnesium-rich water vs placebo (Table 4) with methodological scores of 5 (n = 2) and 4 (n = 1) and one evaluating magnesium oxide versus placebo or senna (16). The amount of elemental magnesium consumed in the 3 magnesium water studies varied from 105 mg/d (1,000 mL/d of water containing 105 mg/L Mg) (37), 60 or 119 mg/d (500 or 1,000 mL/d of water containing 119 mg/L Mg) (38) to 500 mg/d (500 mL/d of water containing 1,000 mg/L of Mg) (39). None of the trials demonstrated a statistically significant difference versus placebo for their primary endpoints (based on stool frequency per week or ROME II–based response), although various secondary endpoints, including stool frequency at alternative time points and stool consistency, were significantly improved (37–39). Magnesium-containing mineral water preparations were well tolerated with low rates of adverse events (diarrhea and abdominal distention). In the magnesium oxide study, subjects were administered a total of 1.5 g in 3 divided doses and reported significant overall improvements in constipation, SBMs, CSBMs, and QoL when compared with placebo but no differences compared with senna (16). Although not explicitly stated, abdominal pain and diarrhea were adverse events, necessitating a dose reduction in 53.3% of subjects. None of the studies reported hypermagnesemia as a potential adverse event.
Magnesium-containing Agents: Level I Evidence, Grade B Recommendation
Table 4.
Magnesium-based laxatives in the treatment of constipation
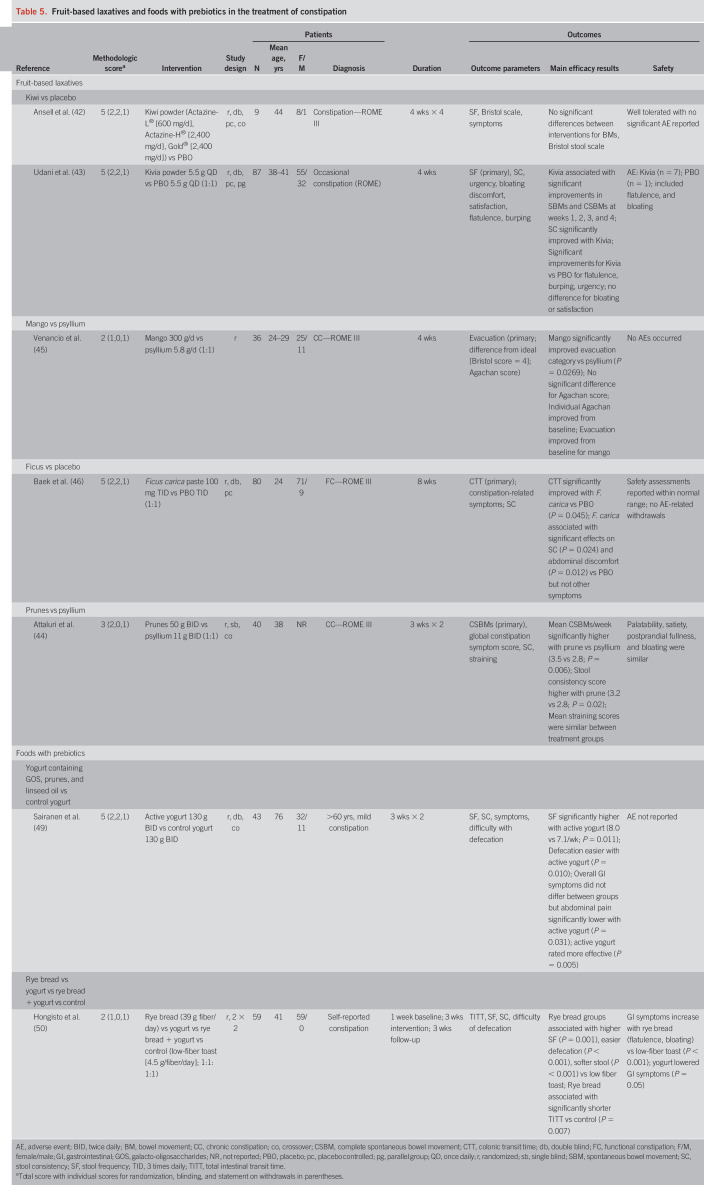
Fruit-based laxatives
Fruits contain varying proportions of soluble and insoluble dietary fiber (including nonfermentable, slowly fermentable, and rapidly fermentable fibers), sugars (e.g., fructose and sucrose), and sorbitol (40). Fruit-based laxatives increase intestinal water retention and colonic volume, resulting in increased stool frequency and softer stools (41). In this updated review, 5 studies were identified (Table 5). These trials evaluated preparations of kiwi (n = 2), mango (n = 1), ficus (n = 1), and prune (n = 1). The methodological scores of these studies were 5 (n = 3), 3 (n = 1), and 2 (n = 1).
Table 5.
Fruit-based laxatives and foods with prebiotics in the treatment of constipation
In a small study (N = 9), a kiwi fruit–based supplement had no significant effect on stool frequency or stool consistency versus placebo (42), although a second larger study (N = 87) found that a kiwi-derived powder was associated with significant improvements in the frequency of CSBMs, SBMs, and stool consistency compared with placebo-treated patients (43). Prunes were associated with significantly greater improvements in mean CSBMs per week (primary outcome) and stool consistency compared with psyllium in 40 patients with chronic constipation (44). A mango-based supplement was associated with significantly improved evacuation categorization (based on stool consistency and shape) compared with psyllium in a pilot study in 36 patients with chronic constipation (45). A ficus carica paste significantly improved colonic transit time (primary endpoint), stool consistency, and abdominal discomfort but had no effect on stool frequency, defecation time, abdominal pain, effort for evacuation, and sensation of incomplete evacuation (46).
Fruit-based laxatives were very well tolerated with few (mild GI-related events) or no adverse events reported. Based on the small numbers of patients enrolled in these trials, the data suggest that fruit-based laxatives are a well-tolerated and promising option for the treatment of constipation. Additional well-designed trials are required to confirm their efficacy.
Kiwi-based Laxatives: Level I Evidence, Grade B Recommendation
Mango-based Laxatives: Level II Evidence, Grade B Recommendation
Ficus-based Laxatives: Level II Evidence, Grade B Recommendation
Prune-based Laxatives: Level II Evidence, Grade B Recommendation
Foods with prebiotics
Prebiotics are nondigestible fibers (oligosaccharides such as oligofructose, galacto-oligosaccharides, inulin, and lactulose) that are fermented by and support the growth of beneficial intestinal bacteria (e.g., bifidobacteria and lactobacillus) (47). It is hypothesized that intestinal microbiota increase colonic peristalsis via a number of potential mechanisms and that prebiotics augment this process by supporting a healthy microbiome (48).
Two studies are included in the current analysis. One study (methodologic score = 5) found that a yogurt containing galacto-oligosaccharides, prunes, and linseed oil was associated with significantly greater stool frequency, easier defecation, and softer stools compared with a control yogurt in elderly patients with mild constipation (Table 5) (49). Another study (methodological score = 2) evaluated rye bread, with or without Lactobacillus GG–containing yogurt versus yogurt alone and control (low-fiber toast) in patients with self-reported constipation (50). The rye bread–containing groups experienced shortened intestinal transit time, increased stool frequency, softened stool, and easier defecation compared with low-fiber toast but were associated with increased GI side effects (flatulence and bloating) (50).
Yogurt with Galacto-Oligosaccharides + Prune + Linseed Oil: Level II Evidence, Grade B Recommendation
Rye Bread with Yogurt: Level III Evidence, Grade C Recommendation
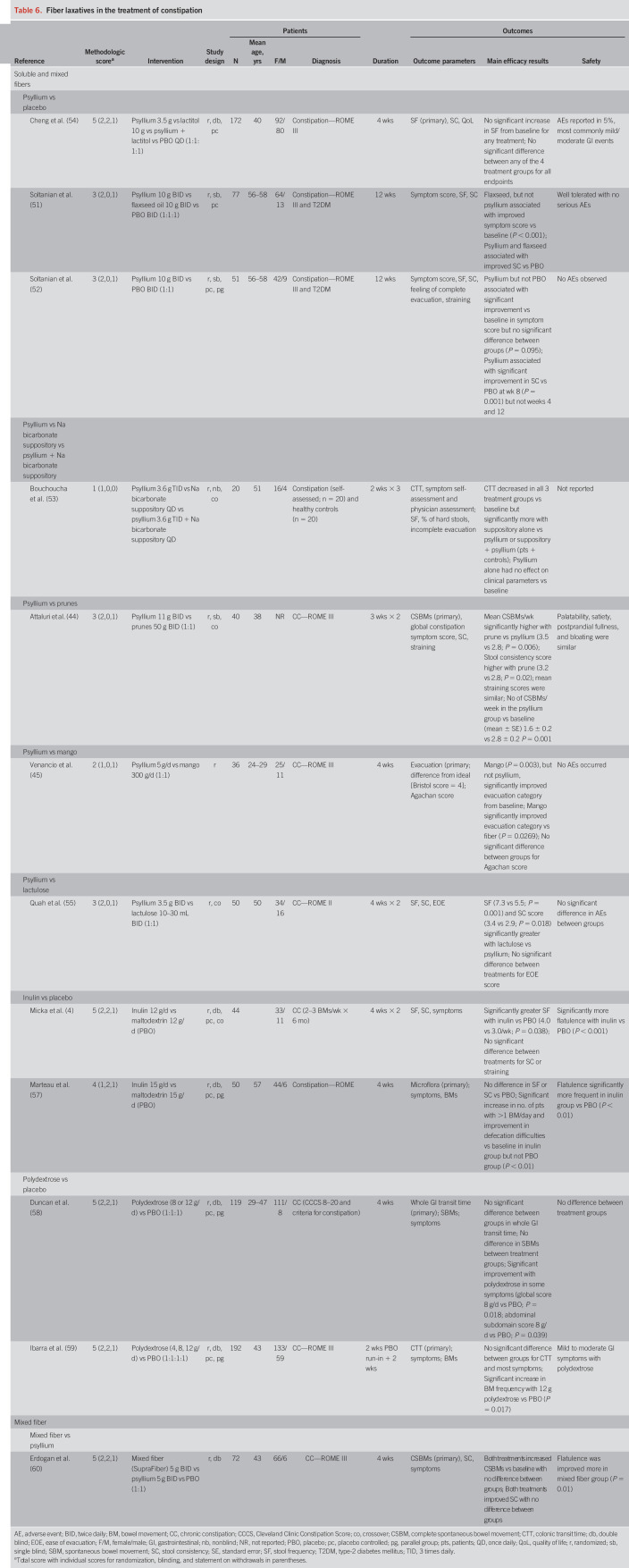
Fiber-containing agents
Fiber laxatives work by increasing the weight and water-absorbent properties of stool, thereby increasing stool bulk and softening stool consistency. Fiber products can be classified based on solubility (soluble vs insoluble), viscosity (viscous vs nonviscous), and fermentability (fermentable vs nonfermentable) (40). Soluble fiber (e.g., psyllium, gums, and pectins) blends with water, forming a gel-like substance, whereas insoluble fiber (e.g., cellulose, lignin, and oligosaccharides) remains unchanged as it passes through the GI tract. Fermentable fibers such as gums, inulin, oligosaccharides, and wheat dextrin can be digested by gut bacteria.
Soluble and mixed fibers.
Psyllium (also known as ispaghula) is a soluble fiber derivative of the husk of Plantago ovata. We identified 9 new studies that evaluated psyllium (Table 6), with methodological scores of 5 (n = 2), 3 (n = 4), 2 (n = 2), and 1 (n = 1). The doses of psyllium varied substantially in these studies, ranging from 3.5 g/d to 11 g twice daily. Three studies included a placebo arm, and 7 studies included an active comparator (lactitol, flaxseed oil, sodium bicarbonate suppository, lactulose, prunes, mango, and mixed fiber [n = 1 each]). Whereas some studies used an active comparator, in 2 placebo-controlled studies, no differences between psyllium and placebo in global constipation symptom scores were identified, but more patients receiving psyllium had a greater than 2-point symptom improvement from baseline (5-point Likert scale) (51–53). Psyllium was also associated with improved stool consistency at various time points. In the third placebo-controlled trial, which also included lactitol and psyllium + lactitol as comparators, there were no significant differences between any of the 4 groups in terms of stool frequency (primary endpoint), stool consistency, QoL, or patient-assessed symptoms in subjects with self-reported constipation (<3 BMs/week) (54). In other comparative trials, lactulose (55), mango (45), and prunes (44) significantly outperformed psyllium in stool frequency and consistency.
Table 6.
Fiber laxatives in the treatment of constipation
Two placebo-controlled trials evaluated inulin (56,57), receiving methodological scores of 5 and 4, respectively. In the first study, inulin was associated with increased stool frequency but showed no differences in stool consistency or straining compared with placebo (56). In the second study, no differences in stool frequency or consistency were detected between the inulin and placebo cohorts. Inulin was associated with increased numbers of patients achieving >1 BM/day and fewer defecation difficulties (not defined) compared with baseline (57).
Two placebo-controlled trials (methodological scores = 5) evaluated the potential for polydextrose to improve GI transit time. In both studies, polydextrose proved no more effective than placebo (58,59). One study did identify an increase in SBM frequency (59), but this response was not corroborated by the second (58).
One study (methodological score = 5) compared mixed fiber (plum-derived soluble/insoluble fiber) with psyllium over 4 weeks. Within-group comparisons from baseline identified increased mean numbers of CSBMs per week (primary endpoint), improved stool consistency, and reduced straining but no significant differences between the groups. Mixed fiber was associated with a significantly greater improvement in flatulence compared with psyllium (60).
Soluble fibers (psyllium, inulin, and polydextrose) seem safe and well tolerated. Abdominal distension/pain and flatulence were the most common adverse events and were mild to moderate in nature.
Overall, considering the differences in products tested, dosages used, and variability in study design, the current data suggest that both soluble fiber, psyllium, and mixed fiber (SupraFiber) have modest efficacy for treating constipation. The data are most robust for psyllium. However, it is worth noting that the highest graded placebo-controlled psyllium study (54) revealed no significant benefit over placebo and head-to-head trials revealed that psyllium is less effective than comparator agents (e.g., PEG, lactulose, and fruits).
Psyllium: Level II Evidence, Grade B Recommendation
Polydextrose: Level I Evidence, Grade I (Insufficient) Recommendation
Inulin: Level I Evidence, Grade I (Insufficient) Recommendation
Mixed Fiber: Level II Evidence, Grade B Recommendation
Miscellaneous agents
Table 7 summarizes studies evaluating miscellaneous patient groups and therapeutic agents. Three high-quality studies evaluated constipated patients with CKD (methodological scores of 5 [n = 2] or 4 [n = 1]) (61–63). Polydextrose (61) and fructo-oligosaccharide (63) both demonstrated significant increases in stool frequency versus placebo. In another study, flaxseed oil, olive oil, and mineral oil (control) all significantly reduced the frequency of symptom scores (ROME criteria) from baseline (62).
In other studies evaluating miscellaneous synbiotics or prebiotics, a combination synbiotic of psyllium, inulin, and probiotics significantly increased stool frequency versus baseline at weeks 1 and 2 (but not weeks 3 or 4) and had no effect on stool consistency (64). A prebiotic combination of inulin, lactitol, and aloevera had no significant benefit for any outcome parameter (19).
Polydextrose: Level II Evidence, Grade B Recommendation (patients with CKD)
Flaxseed Oil: Level II Evidence, Grade C Recommendation (patients with CKD)
Fructo-Oligosaccharide: Level III Evidence, Grade I (Insufficient) Recommendation (patients with CKD)
Surfactants
Docusate is an anionic surfactant that is purported to lower the surface tension at the oil–water interface of stools, allowing water and lipids to penetrate, thereby hydrating and softening stool. Although docusate is one of the most commonly used OTC agents for the treatment of constipation, inconsistent clinical data have led to questions regarding its efficacy. There have been no additional studies since 2004 that met the inclusion criteria for this new analysis. We conclude that despite docusate's frequent use in constipated patients, there is little clinical evidence to support its use.
Surfactants: Level III Evidence, Grade I (Insufficient) Recommendation
DISCUSSION
We have systematically reviewed new evidence supporting the use of OTC laxatives because the previous review was published in 2005. In the previous review, PEG had level I evidence and a grade-A recommendation, psyllium had level II evidence with a grade-B recommendation, and stimulant laxatives, magnesium hydroxide (milk of magnesia), and docusate all had level III evidence with grade C recommendations. Fruit-based laxatives and foods with prebiotics were not assessed (14). In general, the spectrum of OTC products that have been tested has increased and the quality of evidence has improved. Today, there are a greater number of placebo-controlled trials of higher quality, but there remains considerable variability in trial design. Definitions of constipation have become more standardized, with most studies in this analysis using ROME-based criteria (30/41 [73%]). The remainder did not use these criteria, which limited their quality. The biggest hinderance to evaluating these studies is a lack of consistent outcome measures between trials. Stool frequency (CSBMs, SBMs, or BMs) and stool consistency were the most common outcomes used, but the manner in which these were defined, measured, and the intervals of measurement varied.
The current FDA responder criteria for chronic idiopathic constipation trials are based on a CSBM weekly response (i.e., ≥3 CSBMs/week plus an increase of ≥1 CSBM/week from baseline). These were not used in any of the studies. Other measures of outcome included response rates (varying definitions), ease of defecation, straining, symptoms, transit times, and patient-reported outcomes (PROs) such as the Patient Global Impression of Change, the Patient Assessment of Constipation Symptoms, and the Patient Assessment of Constipation-Quality of Life. It should be noted that PRO measures have become increasingly important clinical tools, and the US FDA developed a draft guidance statement on their use for GI conditions (65). Another limitation was that there were only single studies available for many of the treatment categories. Consequently, the lack of confirmatory studies resulted in both a lower level of evidence category and a lower grade of recommendation. Most studies were of very short duration (typically 4 weeks) except for PEG studies, which ranged up to 6 months. Given that constipation is often a chronic problem, it behooves all investigators to consider longer-term (3- to 12-month) studies to provide confidence regarding durability of response. If the indication of an OTC product is for occasional and/or short-term use, then a 4- to 6-week study design may be appropriate, with stricter appraisal of changes in daily stool habits, ideally using validated paper form stool diary (66) or a recently validated electronic constipation stool APP diary (67).
Overall, PEG was the OTC laxative with the most robust clinical evidence (i.e., 3 placebo-controlled trials with quality scores of 5, 5, and 4), and it and senna are the only OTC agents with level I evidence and grade A recommendations. For PEG, this recommendation remains unchanged with newer studies providing further evidence supporting its use as a first-line treatment for chronic idiopathic constipation. PEG is highly effective with similar or superior efficacy to other OTC and prescription therapies and is well tolerated with long-term administration. In comparison studies, adverse events associated with PEG were similar in pattern and frequency to those of lactulose (25,26) and tegaserod (increased rates of headaches with tegaserod) (27) and lower in frequency compared with prucalopride (68% vs 85%) (28,29) and naloxegol (17% vs 24%) (30).
Psyllium and SupraFiber both have level II evidence and grade B recommendations. Despite the addition of 9 new studies, psyllium seems to have only modest efficacy and in head-to-head trials seems inferior to some other agents (e.g., PEG, lactulose, and prunes). Thus, its level of evidence and grade recommendation remains unchanged. Other fiber laxatives (polydextrose, inulin, and insoluble fibers) have insufficient evidence to recommend for or against their use in the treatment of constipation.
Magnesium-containing agents seem to be effective. Four high-quality placebo-controlled trials demonstrated the efficacy of both magnesium-containing mineral water and, more recently, magnesium oxide, resulting in level I evidence and a grade B recommendation. However, there was a lack of standardization of magnesium content in the evaluated preparations. The starting dose of magnesium oxide was 1.5 g daily, but 53.3% of subjects required a dose reduction during the 4-week trial. Allowance for dose reduction resulted in a lack of any withdrawals. No further studies evaluating magnesium hydroxide (milk of magnesia) were identified. More recent studies using stimulant laxatives (bisacodyl, sodium picosulfate, and senna) have yielded improved levels of evidence and grade recommendations. Although all 3 had level III evidence with grade C recommendations in the previous systematic review, the current review identified level I evidence with grade B recommendations for both bisacodyl and sodium picosulfate and level I evidence and a grade A recommendation for senna. However, diphenylmethane derivatives are associated with a significantly greater incidence of GI adverse events (diarrhea and abdominal pain) which may limit their clinical utility. Senna was tested in 2 randomized placebo-controlled studies with both yielding improvements in constipation. The most recent study with senna used a starting dose of 1 g/d, and although it was significantly more efficacious than placebo and comparable with magnesium oxide, dose reductions were required in 83.3% of subjects. Thus, whether a smaller dose of this product is as effective and better tolerated, especially for the occasional constipation, merits further study. Of the fruit-based laxatives evaluated, kiwi was the only one with Level I evidence (i.e., 2 randomized controlled trials), whereas mango, prunes, and ficus all had level II evidence (1 trial each). These products were efficacious (grade B recommendation), but because of the few studies with limited numbers of patients, they were afforded lower Grade recommendations.
Despite docusate's wide use for constipation, there have been no new studies evaluating its efficacy. In the previous review, docusate had level III evidence and a grade C recommendation, based on trials that found docusate to be no more effective than placebo and significantly inferior to psyllium (14). Given the current literature or lack thereof, we are unable to provide a recommendation for docusate.
Overall, the OTC products analyzed in this review seemed to be safe and well tolerated, with no reports of serious adverse events, although some studies failed to report any adverse events. GI symptoms (abdominal pain, cramps, bloating, diarrhea, and nausea) were the most common adverse events reported with a variable incidence for each product, and headache was the chief non-GI adverse event reported.
The limitations of our systematic review include that only studies published in the English literature were assessed, and given that constipation is a common global problem, it is possible that there are some remedies that have been tested and published in other languages that have been excluded in this review. We did exclude studies of less than 4 weeks' duration because we believed that a product that is likely to benefit a chronic condition should demonstrate both efficacy and safety over at least a one-month period. Although there are several methods of categorizing the basis of evidence for drug efficacy and safety, we chose the USPSTF criteria to provide meaningful comparative data with our previous analysis (14).
In conclusion, PEG and senna are the only OTC laxatives with level I evidence and grade A recommendations for the treatment of constipation, although PEG is the only one supported by both short- and long-term studies. Other OTC laxatives with a grade B recommendation include psyllium, SupraFiber, magnesium-rich water, magnesium oxide, diphenylmethane stimulants (bisacodyl and sodium picosulfate), fruit-based laxatives (kiwi, mango, prunes, and ficus), and yogurt plus galacto-oligosaccharide, prunes, and linseed oil. There was insufficient evidence (grade I) for polydextrose, inulin, and fructo-oligosaccharide. For these and other alternative products, there is a clear need for more rigorous, high-quality studies using standardized endpoints. Docusate lacks well-controlled trials demonstrating its efficacy and has poor evidence to support its use in clinical practice.
CONFLICTS OF INTEREST
Guarantor of the article: Darren M. Brenner, MD.
Specific author contributions: S.S.C.R. and D.M.B. conceived the project and developed the search criteria and parameters for the systematic review, reviewed the literature independently, and provided independent recommendations regarding the quality of studies, strength of evidence, and recommendation grading. Both authors equally contributed to the writing of the manuscript and provided extensive revisions.
Financial support: Medical writing support and literature search was provided by BioCentric, Inc. The development of this manuscript was supported by funding from the Bayer US LLC.
Potential competing interests: SSCR has served as an advisory board member and consultant for Bayer Pharmaceuticals and previously received unrestricted research grant support from Sun Sweets corporation and California dried plums grower's association.DMB has received consulting fees from Bayer Pharmaceuticals and is supported in research by an unrestricted gift from the Irene D. Pritzker Foundation.
Study Highlights.
WHAT IS KNOWN
✓ Chronic constipation is a common condition that significantly affects quality of life.
✓ Approximately 40% of individuals with constipation self-treat with over-the-counter (OTC) laxatives.
✓ Multiple classes of OTC therapies are available for treating chronic constipation.
✓ Polyethylene glycol was the only OTC therapy to receive a strong recommendation based on high levels of evidence in a previous systematic review published in 2005.
WHAT IS NEW HERE
✓ The spectrum of OTC products that have been tested has increased and the quality of evidence has improved.
✓ There is now good evidence based on high-quality trials supporting the use of polyethylene glycol and senna for constipation.
✓ Moderate evidence supports the use of psyllium, fruits, magnesium-containing compounds, bisacodyl, and sodium picosulfate for the treatment of constipation.
✓ There is a clear need for more rigorous, high-quality studies using standardized endpoints.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B929
REFERENCES
- 1.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: Definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol 2001;96:3130–7. [DOI] [PubMed] [Google Scholar]
- 2.Higgins PD, Johanson JF. Epidemiology of constipation in North America: A systematic review. Am J Gastroenterol 2004;99(4):750–9. [DOI] [PubMed] [Google Scholar]
- 3.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–91. [DOI] [PubMed] [Google Scholar]
- 4.Pinto Sanchez MI, Bercik P. Epidemiology and burden of chronic constipation. Can J Gastroenterol 2011;25(Suppl B):11b–15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q, Buono JL, Spalding WM, et al. Healthcare costs among patients with chronic constipation: A retrospective claims analysis in a commercially insured population. J Med Econ 2014;17:148–58. [DOI] [PubMed] [Google Scholar]
- 6.Werth BL, Williams KA, Fisher MJ, et al. Defining constipation to estimate its prevalence in the community: Results from a national survey. BMC Gastroenterol 2019;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SSC, Rattanakovit K, Parcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 2016;13:295–305. [DOI] [PubMed] [Google Scholar]
- 8.Rao SSC, Camilleri M. Approach to the patient with constipation. In: Podolsky DK, Camilleri M, Fitz JG, et al. (eds). Yamada's Textbook of Gastroenterology, 6th edn. Wiley-Blackwell: Hoboken, NJ,: 2016, pp 757–72. [Google Scholar]
- 9.Belsey J, Greenfield S, Candy D, et al. Systematic review: Impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 2010;31(9):938–49. [DOI] [PubMed] [Google Scholar]
- 10.Rao SSC, Seaton K, Miller MJ, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosomatic Res 2007;63;441–9. [DOI] [PubMed] [Google Scholar]
- 11.Wald A, Scarpignato C, Mueller-Lissner S, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther 2008;28:917–30. [DOI] [PubMed] [Google Scholar]
- 12.Consumer Health Products Association. OTC Sales by Category 2015-2019 (https://www.chpa.org/OTCsCategory.aspx). Accessed Sept 8 2019. [Google Scholar]
- 13.US Bureau of Labor Statistics. CPI Inflation Calculator (https://www.bls.gov/data/inflation_calculator.htm). Accessed September 10 2020. [Google Scholar]
- 14.Ramkumar D, Rao SSC. Efficacy and safety of traditional medical therapies for chronic constipation: Systematic review. Am J Gastroenterol 2005;100:936–71. [DOI] [PubMed] [Google Scholar]
- 15.US Preventive Services Task Force. Grade definitions (https://www.uspreventiveservicestaskforce.org/uspstf/grade-definitions) (2018). Accessed September 29 2020.
- 16.Morishita D, Tomita T, Mori S, et al. Senna versus magnesium oxide for the treatment of chronic constipation: A randomized, placebo-controlled trial. Am J Gastroenterol 2021;116(1):152–61. [DOI] [PubMed] [Google Scholar]
- 17.Tack J, Müller-Lissner S. Treatment of chronic constipation: Current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol 2009;7:502–8. [DOI] [PubMed] [Google Scholar]
- 18.Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol 2011;25(Suppl B:16B–21B. [PMC free article] [PubMed] [Google Scholar]
- 19.Chu JR, Kang SY, Kim SE, et al. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J Gastroenterol 2019;25:6129–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelham RW, Nix LC, Chavira RE, et al. Clinical trial: Single- and multiple-dose pharmacokinetics of polyethylene glycol (PEG-3350) in healthy young and elderly subjects. Aliment Pharmacol Ther 2008;28:256–65. [DOI] [PubMed] [Google Scholar]
- 21.DiPalma JA, Cleveland MvB, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007;102:1436–41. [DOI] [PubMed] [Google Scholar]
- 22.DiPalma JA, Cleveland MB, McGowan J, et al. A comparison of polyethylene glycol laxative and placebo for relief of constipation from constipating medications. South Med J 2007;100:1085–90. [DOI] [PubMed] [Google Scholar]
- 23.Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson's disease. A randomized placebo-controlled study. Mov Disord 2007:22:1239–44. [DOI] [PubMed] [Google Scholar]
- 24.Seinelä L, Sairanen U, Laine T, et al. Comparison of polyethylene glycol with and without electrolytes in the treatment of constipation in elderly institutionalized patients. Drugs Aging 2009;26:703–13. [DOI] [PubMed] [Google Scholar]
- 25.Chassagne P, Ducrotte P, Garnier P, et al. Tolerance and long-term efficacy of polyethylene glycol 4000 (Forlax®) compared to lactulose in elderly patients with chronic constipation. J Nutr Health Aging 2017;21:429–39. [DOI] [PubMed] [Google Scholar]
- 26.Piche T, Dapoigny M. Comparative efficacy and safety of lactulose plus paraffin vs polyethylene glycol in functional constipation: A randomized clinical study. United Eur Gastroenterol J 2020;8:923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiPalma JA, Cleveland MvB, McGowan J, et al. A randomized, multicenter comparison of polyethylene glycol laxative and tegaserod in treatment of patients with chronic constipation. Am J Gastroenterol 2007;102:1964–71. [DOI] [PubMed] [Google Scholar]
- 28.Cinca R, Chera D, Gruss H-J, et al. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation-a comparison in a controlled environment. Aliment Pharmacol Ther 2013;37:876–86. [DOI] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov. Comparator study of PEG 3350 versus prucalopride in females with chronic constipation (https://clinicaltrials.gov/ct2/show/NCT01251822) (2012). Accessed October 19, 2020.
- 30.Brenner DM, Hu Y, Datto C, et al. A randomized, multicenter, prospective, crossover, open-label study of factors associated with patient preferences for naloxegol or PEG 3350 for opioid-induced constipation. Am J Gastroenterol 2019;114:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011;9:57–83. [DOI] [PubMed] [Google Scholar]
- 32.Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am J Gastroenterol 2010;105:897–903. [DOI] [PubMed] [Google Scholar]
- 33.Kienzle-Horn S, Vix J-M, Schuijt C, et al. Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr Med Res Opin 2007;23:691–9. [DOI] [PubMed] [Google Scholar]
- 34.Soufi-Afshar I, Moghadamnia A, Bijani A, et al. Comparison of pyridostigmine and bisacodyl in the treatment of refractory chronic constipation. Caspian J Intern 2015;7:19–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong LLD, Cheng C-w, Kun W, et al. Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with functional constipation in a randomized controlled trial. Clin Gastroenterol Hepatol 2019:17:1303–10. [DOI] [PubMed] [Google Scholar]
- 36.Buddington RK, Kapadia C, Neumer F, et al. Oligofructose provides laxation for irregularity associated with low fiber intake. Nutrients 2017;9:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naumann J, Sadaghiani C, Alt F, et al. Effects of sulfate-rich mineral water on functional constipation: A double-blind, randomized, placebo-controlled study. Forsch Komplementmed 2016;23(6):356–63. [DOI] [PubMed] [Google Scholar]
- 38.Dupont C, Campagne A, Constant F. Efficacy and safety of magnesium sulfate-rich natural mineral water for patients with functional constipation. Clin Gastroenterol Hepatol 2014;12:1280–7. [DOI] [PubMed] [Google Scholar]
- 39.Bothe G, Cob A, Auinger A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. Eur J Nutr 2017:56:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams BA, Mikkelsen D, Flanagan BM, et al. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J Anim Sci Biotech 2019;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson-Smith V, Dellschaft N, Ansell J, et al. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment Pharmacol Ther 2019;59:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansell J, Butts CA, Paturi G, et al. Kiwifruit-derived supplements increase stool frequency in healthy adults: A randomized, double-blind, placebo-controlled study. Nutr Res 2015;35:401–8. [DOI] [PubMed] [Google Scholar]
- 43.Udani JK, Bloom DW. Effects of kivia powder on gut health in patients with occasional constipation: A randomized, double-blind, placebo-controlled study. Nutr J 2013;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: Dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther 2011;33:822–8. [DOI] [PubMed] [Google Scholar]
- 45.Venancio VP, Kim H, Sirven MA, et al. Polyphenol-rich mango (Mangifera indica L.) ameliorate functional constipation symptoms in humans beyond equivalent amount of fiber. Mol Nutr Food Res 2018;62:1701034. [DOI] [PubMed] [Google Scholar]
- 46.Baek HI, Ha KC, Kim HM, et al. Randomized, double-blind, placebo controlled trial of Ficus carica paste for the management of functional constipation. Asia Pac J Clin Nutr 2016;25:487–96. [DOI] [PubMed] [Google Scholar]
- 47.Malaguarnera M, Vacante M, Condorelli G, et al. Probiotics and prebiotics in the management of constipation in the elderly. Acta Med Mediterranea 2013;29:791–8. [Google Scholar]
- 48.Dimidi E, Christodoulides S, Scott SM, et al. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv Nutr 2017;8:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sairanen U, Piirainen L, Nevala R, et al. Yoghurt containing galacto-oligosaccharides, prunes and linseed reduces the severity of mild constipation in elderly subjects. Eur J Clin Nutr 2007;61:1423–8. [DOI] [PubMed] [Google Scholar]
- 50.Hongisto SM, Paajanen L, Saxelin M, et al. A combination of fibre-rich rye bread and yoghurt containing Lactobacillus GG improves bowel function in women with self-reported constipation. Eur J Clin Nutr 2006;60:319–24. [DOI] [PubMed] [Google Scholar]
- 51.Soltanian N, Janghorbani M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin Nutr ESPEN 2019;29:41–8. [DOI] [PubMed] [Google Scholar]
- 52.Soltanian N, Janghorbani M, Adibi P. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Compl Ther Med 2018:40:1–7. [DOI] [PubMed] [Google Scholar]
- 53.Bouchoucha M, Faye A, Savarieau B, et al. Effect of an oral bulking agent and a rectal laxative administered alone or in combination for the treatment of constipation. Gastroenterol Clin Biol 2004;28:438–43. [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Tennila J, Stenman L, et al. Influence of lactitol and psyllium on bowel function in constipated Indian volunteers: A randomized, controlled trial. Nutrients 2019;11:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quah HM, Ooi BS, Seow-Choen F, et al. Prospective randomized crossover trial comparing fibre with lactulose in the treatment of idiopathic chronic constipation. Tech Coloproctol 2005;10:111–4. [DOI] [PubMed] [Google Scholar]
- 56.Micka A, Siepelmeyer A, Holz A, et al. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: A randomized, double-blind, placebo-controlled trial. Int J Food Sci Nutr 2017;68:82–9. [DOI] [PubMed] [Google Scholar]
- 57.Marteau P, Jacobs H, Cazaubiel M, et al. Effects of chicory inulin in constipated elderly people: A double-blind controlled trial. Int J Food Sci Nutr 2011;62:164–70. [DOI] [PubMed] [Google Scholar]
- 58.Duncan PI, Enters-Weijnen CF, Emami N, et al. Short-term daily intake of polydextrose fiber does not shorten intestinal transit time in constipated adults: A randomized controlled trial. Nutrients 2018;10:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibarra A, Pelipyagina T, Rueffer M, et al. Efficacy of polydextrose supplementation on colonic transit time, bowel movements, and gastrointestinal symptoms in adults: A double-blind, randomized, placebo-controlled trial. Nutrients 2019;11:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erdogan A, Rao S, Thiruvaiyaru D, et al. Randomized clinical trial: Soluble/insoluble fiber or psyllium for chronic constipation. Aliment Pharmacol Ther 2016;44:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada M, Nagano N, Goto S, et al. Effect of polydextrose intake on constipation in Japanese dialysis patients: A triple-blind, randomized, controlled trial. J Nutr Sci Vitaminol 2015;61:345–53. [DOI] [PubMed] [Google Scholar]
- 62.Ramos CI, de Lima AFA, Grilli DG, et al. The short-term effects of olive oil and flaxseed oil for the treatment of constipation in hemodialysis patients. J Ren Nutr 2015;25:50–6. [DOI] [PubMed] [Google Scholar]
- 63.Meksawan K, Chaotrakul C, Leephorn N, et al. Effects of fructo-oligosaccharide supplementation on constipation in elderly continuous ambulatory peritoneal dialysis patients. Peritoneal Dial Internat 2016;35:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cudmore S, Doolan A, Lacey S, et al. A randomized, double-blind, placebo-controlled clinical study: The effects of a synbiotic, lepical, in adults with chronic, functional constipation. Internat J Food Sci Nutr 2017;68:366–7. [DOI] [PubMed] [Google Scholar]
- 65.FDA. Guidance for industry irritable bowel syndrome—clinical evaluation of drugs for treatment (https://www.fda.gov/media/78622/download) (2012). Accessed September 10, 2020.
- 66.Meduri K, Brown CK, Attaluri A, et al. Validation of a prospective stool diary for assessment of constipation. Neurogastroenterol Motil 2011;23(Suppl 1):12. [Google Scholar]
- 67.Yan Y, Jimenez E, Sharma A, et al. How useful is constipation stool APP compared to paper stool diary-randomized study of constipation and healthy subjects. Gastroenterology 2020;158(6 Suppl 1):S–400. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.