Seladelpar is currently under investigation as a second-line treatment for PBC.1 It is a potent and selective peroxisome proliferator-activated receptor (PPAR)-delta agonist, targeting a receptor found in hepatocytes, cholangiocytes, Kupffer cells, macrophages, and stellate cells— cell types that play a key role in liver disease. PPAR-delta agonism with seladelpar is both anti-inflammatory and antifibrotic. This approach also reduces bile acids and increases lipid metabolism.
The phase 3 ENHANCE study investigated the use of seladelpar in patients with PBC who did not respond to first-line treatment, and findings were presented by Dr Gideon M. Hirschfield at the AASLD 2020 Liver Meeting Digital Experience.2 Patients diagnosed with PBC were randomized to 1 of 3 treatment arms: seladelpar 10 mg (80 patients), seladelpar 5 mg for 26 weeks followed by an additional 26 weeks of either 5 mg or 10 mg (80 patients), or placebo (80 patients). The primary endpoint was a composite response by month 3 that included ALP of less than 1.67 times the ULN, a 15% or greater decrease in ALP, and total bilirubin at or below the ULN. The researchers also looked at whether ALP was normalized by month 3 and at the change from baseline in pruritus at month 3, and evaluated all of these measures at month 6.
An unexpected histologic finding in a clinical trial of seladelpar for non-alcoholic steatohepatitis led to early termination of the ENHANCE study. The finding turned out to be unrelated to the drug, but rather due to preexisting circumstance. The investigators conducted a blinded analysis following termination, as well as a safety analysis that included all patients who received at least 1 dose of seladelpar.
ABSTRACT SUMMARY Durability of Treatment Response After 1 Year of Therapy With Seladelpar in Patients With Primary Biliary Cholangitis: Final Results of an International Phase 2 Study.
In this phase 2, open-label, uncontrolled, dose-finding study, 112 patients with PBC with an inadequate response to UDCA received seladelpar at a dose of 2 mg (11 patients), 5 mg (49 patients), or 10 mg (52 patients), with doses potentially increased up to 10 mg after 12 weeks, depending on biochemical response for 1 year (EASL abstract FRI133). Patients were mostly female and had an average age of 58 years. After a year of treatment, the mean decrease in ALP in the 5/10-mg group was 40%, and 45% in the 10-mg group. In the 5-mg group that escalated to 10 mg, 53% met the composite endpoint, as did 69% of patients who received 10 mg from the start.
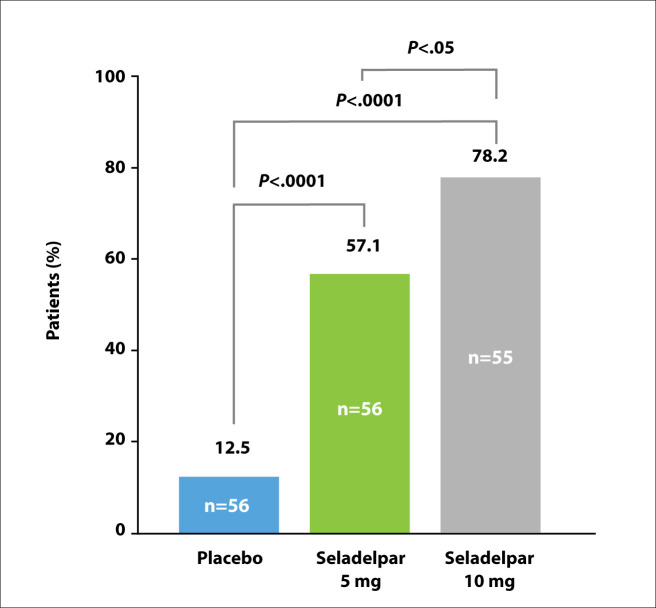
A composite response was achieved by 78.2% of patients in the 10-mg arm, 57.1% of patients in the 5-mg arm, and 12.5% of patients in the placebo arm (Figure 4). The response rates for the 5- and 10-mg arms were both statistically significantly higher than the rate for the placebo arm. The secondary endpoint of ALP normalization was achieved by 27.3% of patients in the 10-mg arm, 5.4% of patients in the 5-mg arm, and no patients in the placebo arm. The investigators reported an absolute reduction in ALP of nearly 45% with the 10-mg dose, a decrease of approximately 122 units. Other serum liver tests reflected a similar benefit from seladelpar.
Figure 4.
Primary composite endpoint achieved at 3 months with seladelpar. P values by Cochran-Mantel-Haenszel test. CymaBay, data on file 2020. Adapted from Hirschfield GM et al. AASLD abstract LO11. Hepatology. 2020;72(suppl 1).2
Adverse events were mild to moderate. The most common issues were pruritus (13% in the placebo arm, 3% in the 5-mg arm, and 11% in the 10-mg arm) and abdominal pain (3%, 9%, and 7%, respectively). A 52-week phase 3 study of seladelpar is scheduled to begin in early 2021.
References
- 1.Jones D, Boudes PF, Swain MG et al. Seladelpar (MBX-8025), a selective PPAR-delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2(10):716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Kowdley KV, Shiffman ML et al. ENHANCE: safety and efficacy of seladelpar in patients with primary biliary cholangitis—a phase 3 international, randomized, placebo-controlled study [AASLD abstract LO11]. Hepatology. 2020;72(suppl 1) [PMC free article] [PubMed] [Google Scholar]