The 12-month double-blind phase of the POISE trial had a primary endpoint of ALP of less than 1.67 times the ULN, a reduction of at least 15% in ALP from baseline, and total bilirubin of less than or equal to the ULN.1 After this initial phase, PBC patients were able to enroll in a 5-year, open-label extension.
An analysis presented in a poster at the AASLD 2020 Liver Meeting Digital Experience assessed the safety and efficacy of OCA treatment over 5 years by comparing responders and incomplete responders.2 The former were defined as patients who achieved the POISE primary endpoint after 1 year of OCA treatment and the latter were defined as patients who did not.
The researchers found that 95 responders (48%) and 101 incomplete responders (52%) had ALP of less than 1.67 times the ULN, 179 responders (91%) and 18 incomplete responders (9%) had total bilirubin of less than or equal to the ULN, and 131 responders (67%) and 65 incomplete responders (33%) experienced a decrease in ALP of at least 15%.
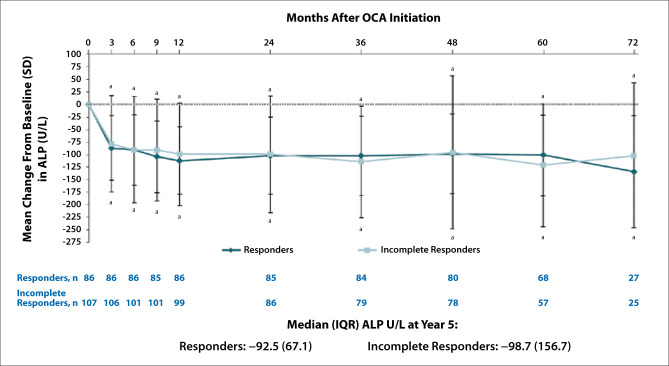
The researchers analyzed changes from baseline in ALP, ALT, AST, GGT, and bilirubin (total and direct) for responders and incomplete responders. Figure 3 shows the mean change from baseline in ALP for responders and incomplete responders. The mean change from baseline at year 5 for responders and incomplete responders was –19 U/L and –28 U/L, respectively, for ALT; –9 U/L and –16 U/L, respectively, for AST; and 155 U/L and 165 U/L, respectively, for GGT. In addition, responders and incomplete responders experienced similar mean changes from baseline in direct bilirubin over the 72 months of the extension study. Pruritus was the most common adverse event among both responders and incomplete responders, but caused few patients to discontinue treatment.
Figure 3.
Mean (SD) change from baseline in ALP levels through month 72 by responder subgroup. aP<.05 vs baseline. ALP, alkaline phosphatase; IQR, interquartile range; OCA, obeticholic acid; SD, standard deviation. Adapted from Hansen BE et al. AASLD abstract 1251. Hepatology. 2020;72(suppl 1).2
The researchers concluded that OCA improved important biochemical markers of PBC, regardless of meeting the POISE primary endpoint, following 1 year of treatment with OCA. Changes in biochemical markers over time were frequently similar between groups, suggesting that the POISE primary endpoint does not completely capture the benefit of OCA.
References
- 1.Nevens F, Andreone P, Mazzella G et al. POISE Study Group. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 2.Hansen BE, Jones D, Carbone M et al. Long-term efficacy and safety of obeticholic acid in primary biliary cholangitis: responder analysis of more than 5 years of treatment in the POISE trial [AASLD abstract 1251]. Hepatology. 2020;72(suppl 1) [PMC free article] [PubMed] [Google Scholar]