Abstract
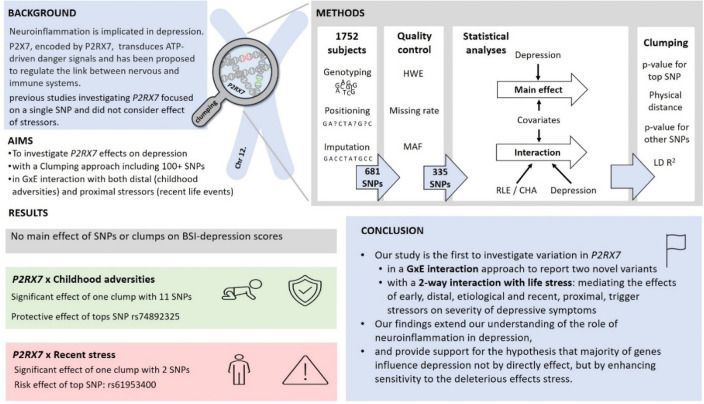
The P2X purinoceptor 7 (P2RX7) mediates inflammatory microglial responses and is implicated in neuroimmune mechanisms of depression and neurodegenerative disorders. A number of studies suggest that psychosocial stress may precipitate depression through immune activation. Genetic association studies of P2RX7 variants with depression have been inconclusive. However, nearly all studies have focused on only one single-nucleotide polymorphism (SNP) and have not considered interaction with psychosocial stress. We investigated the effect of several variations in P2RX7 gene using a clumping method in interaction with early adversities and recent stress on depression severity. 1752 subjects provided information on childhood adversities, recent life events, and current depression severity. Participants were genotyped for 681 SNPs in the P2RX7 gene, 335 of them passed quality control and were entered into linear regression models followed by a clumping procedure for main effect and interactions. No significant main effect was observed. Rs74892325 emerged as a top SNP for interaction with childhood adversities and rs61953400 for interaction with recent life events. Our study is the first to investigate several variants in the P2RX7 gene and in interaction with two types of stress, extending our understanding of neuroinflammation in depression, and supporting that the majority of genes influence depression by enhancing sensitivity to stressors.
Introduction
Depression has a high economic and social impact and leads to significant suffering, while 35% of patients are resistant to currently available treatments [1]. There is a clear need for better understanding of the neurobiology of depression, and more effective and novel diagnostics, treatment targets, and action mechanisms. Most antidepressants act on monoamine systems with a slow onset of action and partial response and remission is common. Furthermore, we still lack biological markers for underlying neural mechanisms of depression that can identify subtypes and personalise treatment. Recent years have seen a major shift in emphasis from a neuron-centric view of depression towards a recognition that neurons and glia act in partnership in mediating brain homeostasis and that abnormal neuron-glial interaction is central to the development of mood pathology [2–4].
Glial cells, and especially microglia have a crucial role in maintaining the immune health of the brain. They sense foreign proteins and signals of neuronal stress and respond by generating proinflammatory factors including TNF-α, IL-6 and IL-1β that amplify the protective immune response. In morbid states such as autoimmunity, neuroinflammation leads to neuronal dysfunction and damage. Increasing evidence suggests that dysfunctional neuroimmune activation plays a causal role in the onset and maintenance of depressive illness [2,5–8]. Most directly, several studies report increased in-vivo radioligand binding to the translocator protein, a marker of activated microglia, in depression [9,10]. Furthermore, meta-analyses suggest anti-inflammatory drugs, notably the antimicroglia inhibitor antibiotic minocycline [11], have antidepressant efficacy.
P2X purinoceptor 7 (P2RX7), showing abundant expression in microglia [6], is a cell surface sensor of danger signal activated by a sharp increase in extracellular ATP, a damage-associated molecular pattern (DAMP) indicative of neuronal damage. Hallmark downstream effects of P2RX7 activation include NLRP3 inflammasome complex mediated proinflammatory IL-1β and IL-18 release [2,6,12,13]. Stress is a major environmental etiological factor in mood disorders [14–16], and P2RX7 activation has been associated with an inflammatory and stress-mediated depression-like phenotype [2,17,18] with enhanced IL-1β release contributing to stress-induced depression [2]. The potential role of P2RX7 in depression is also reflected by the fact that its activity and hippocampal expression is selectively modulated by different antidepressants and by stress exposure [19].
Variation in the P2RX7 gene, encoding the P2X7 receptor, located on 12q24.31, has been implicated in both bipolar disorder (BD) and major depressive disorder (MDD) [20], but the majority of studies so far focused on a single SNP, rs2230912 [21–23], where the minor G allele is associated with a gain-of-function phenotype for increased IL-1β release in monocytes [24] and possibly also in microglia, leading to neuroinflammation and increased risk for mood disorders [23,25]. Furthermore, although the effects of the P2X7 receptor are manifested in transducing danger signals and regulating neurochemistry under stress [19] and it has been hypothesised that P2RX7 predisposes to depression in interaction with life stress [26], studies investigating the role of variation in P2RX7 in depression have not considered the effects of stress.
Thus, while rapidly emerging knowledge points to the regulatory role of purinergic signalling in mood-related behaviour, stress reactivity, and their pathological alterations, with novel brain-penetrant P2RX7 antagonists (JNJ-54175446, JNJ-55308942) in phase 2 and 3 clinical trials for the treatment of depression [2,26], we still lack proper understanding of the role of variation in P2RX7 in the background of depressive-like behaviours. Given our increasing understanding that the majority of genes implicated as etiological factors in the development of depression have a role in mediating response to stressors rather than directly influencing risk, P2RX7 may be an especially relevant target [27,28]. Furthermore, a more sophisticated insight on the role of stressors and gene x environment interactions is needed as life events may play both a predisposing role, leading to the development of a diatheses, in case of early, distal exposures, and a trigger role in case of recent, proximal exposures. Therefore, in our study, we focused on the effects of variation in the P2RX7 gene in interaction with early childhood adversities and recent life events on current depressive symptoms in a large general European sample.
Materials and methods
Study population
This study was part of the NewMood study (New Molecules in Mood Disorders, Sixth Framework Program of the EU, LSHM-CT-2004-503474) and was funded by the European Union. Participants aged between 18–60 years were recruited from the general population through advertisements, a website, and general practices in greater Manchester and Budapest. 1752 non-related subjects of European white ethnic origin (501 males, 1251 females) provided self-reported data on gender, age, recent stress, childhood adversities, current depression among other variables by filling out a questionnaire pack, and provided genetic data by a saliva sampling kit. As NewMood focused on a general population with a continuum approach to affective symptoms and disorders, our sample included previously depressed, currently depressed, and never depressed participants as well. In the present study only information on current depressive symptom severity was included. More details about the population sample can be found in our previously published reports [29–31]. All participants provided written informed consent. The study was carried out in accordance with the Declaration of Helsinki, and it was approved by the Scientific and Research Ethics Committee of the Medical Research Council, Budapest, Hungary (ad.225/KO/2005.; ad.323-60/2005-1018EKU), and by the North Manchester Local Research Ethics Committee, Manchester, United Kingdom (REC reference number 05/Q1406/26).
Phenotypes
We applied a continuum approach in a general population sample to capture current severity of depressive symptoms, rather than ascertaining clinical levels of depression and employing a case-control design. The Brief Symptom Inventory (BSI) was used to measure current levels of depression [32]. Each item was scored between 0–4 depending on the distress caused. Depression score (BSI-depression) was calculated as the sum of depression and additional item scores divided by the number of completed items. The use of BSI for capturing current depressive symptoms has been validated in a previous study using a subsample of our study population with the Montgomery-Asberg Depression Rating Scale (MADRS) administered by trained interviewers [33].
In our study we measured two types of stress, early childhood adversity (CHA) and recent stressful life events (RLE). The childhood adversity measure was derived from the Childhood Trauma Questionnaire (CTQ) [34], including four items about emotional and physical abuse and emotional and physical neglect, and two items about the loss of parents. We had validated this short childhood adversity measure with the 28-item CTQ within a subpopulation of our sample, obtaining a high correlation between the original and derived measures [33]. The sum of item scores was used in the analyses. Recent stressful life events (RLE) occurring in the previous year related to financial difficulties, illnesses/injuries, personal problems, and intimate relationship or social network difficulties were ascertained by the List of Threatening Experiences [35,36]. The number of recent negative life events (RLEs) was used in the statistical analyses.
Following our analyses, solely for illustration purposes, RLE and CHA scores were both grouped into three categories to reflect severity of exposure (RLE: low = 0, medium = 1, high = 2 or more; and CHA: low = 0–3, medium = 4–6, high = 7 or more) based on our previous studies [33,37].
Genotyping
Participants provided buccal mucosa cells collected by a cytology brush (Cytobrush plus C0012, Durbin PLC). Genomic DNA was extracted according to the protocol of Freeman et al. [38]. Genotyping was performed by Illumina’s CoreExom PsychChip. All laboratory work was performed under the ISO 9001:2000 quality management requirements and was blinded with regard to phenotype.
Statistical analyses
We analysed all SNPs in the region of P2RX7 available in the NewMood database and surviving quality control steps using linear regression models, followed by a clumping procedure based on linkage disequilibrium (LD) estimates between the SNPs. Results of top SNPs from representing correlated SNPs in individual clumps are reported.
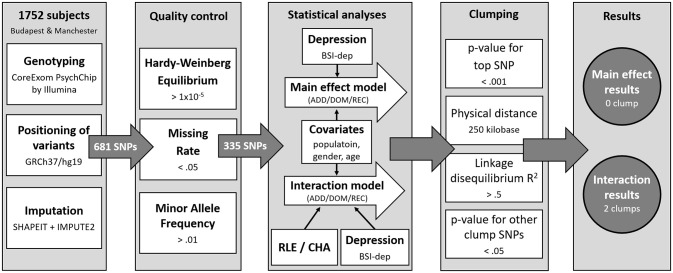
IBM SPSS Statistics 25 was used to calculate descriptive statistics and to run univariate general linear models solely for visualization purposes. Plink v1.90 was used to calculate missingness rate (MR), Hardy-Weinberg equilibrium (HWE) and minor allele frequency (MAF) as part of quality control steps, for clumping, and to build linear regression models on BSI-depression. Besides testing the main effects of genetic variants on BSI-depression, interaction models to test for gene x environment interactions with RLE and CHA were also run (Fig 1). Analyses were supported by scripts individually written in R 3.0.2 (R Core Team, 2013).
Fig 1. Methods of investigating the effects of variation in P2RX7 in interaction with childhood adversities and recent life events on current depression: Study population, quality control steps and statistical analyses.
RLE: recent life events; CHA: Childhood adversities; BSI-Dep–Brief Symptom Inventory depression score; ADD: additive model; DOM: dominant model; REC: recessive model.
First, we created (.map and.ped) files that contained only those SNPs which fulfilled the criteria of the quality control and only analysed those SNPs that survived this procedure. Genotyping provided a dataset incorporating 681 SNPs. Quality control entailed the calculation of Hardy-Weinberg Equilibrium (HWE; >1x10-5), missing rates (MR; < .05), and minor allele frequencies (MAF; >.01). After quality control, 335 SNPs remained to be entered into statistical analysis. In our Plink linear regression models population, sex and age were covariates in all analyses. When testing an SNP × CHA/RLE interaction effect, main effects of both the SNP and CHA/RLE were included as covariates in the model.
After running the regressions, SNPs were clumped both for main effect and for interactions using the CLUMP function in Plink. The four parameters used for clumping were as follows: maximum p-value of the clump’s top SNP was set at 0.001; physical distance with it was 250 kilobase; minimum linkage disequilibrium R2 with it was 0.5; and maximum p-value for the clump’s other SNPs was 0.05. Data on all P2RX7 SNPs in the original NewMood database, including quality control and regression results are shown in S1 and S2 Tables.
All analyses were run according to additive, dominant and recessive models. Nominal significance threshold was p<0.05. To correct for multiple comparisons in analyses for each of the above outcome variables, Benjamini-Hochberg false discovery rate (FDR) Q-values were calculated. Results with a Q-value of ≤0.05 were considered as significant.
The data presented in this study are openly available in FigShare at 10.6084/m9.figshare.13483011 [39].
Results
Descriptive statistics
Descriptives of our study sample are provided in Table 1.
Table 1. Descriptive statistics of the study sample.
| Gender | n | % | ||
| Male | 501 | 28.6% | ||
| Female | 1251 | 71.4% | ||
| Minimum | Maximum | Mean | SEM | |
| Age | 18 | 60 | 32.56 | 0.25 |
| Depression score | 0 | 4 | 0.84 | 0.02 |
| Childhood adversity | 0 | 16 | 3.28 | 0.08 |
| Recent negative life events | 0 | 8 | 1.21 | 0.03 |
Depressive symptom scores were measured by the Brief Symptom Inventory (BSI). SEM: standard error of mean.
Effects of variation in P2RX7 on current depressive symptoms: Main effects and interaction with childhood adversities (CHA) and recent life events (RLE)
Our statistical analyses did not identify any SNPs with a nominally significant main effect on current depression (BSI-depression) thus clumps could not be identified. Our analyses for interaction either with childhood adversities (CHA) or recent life events (RLE) yielded one clump in each case. For interaction with CHA, the clump contained 10 SNPs, with rs74892325 as the top SNP, while for interaction with RLE the clump contained 2 SNPs with rs61953400 as the most significant SNP (Table 2).
Table 2. Significant clumps of P2RX7 SNPs interacting with early childhood adversities (CHA) and recent life events (RLE) on current depression symptoms.
| P2RX7 x CHA | ||||||
| ß | 95% C.I. | p | Hardy | MAF | Missing | |
| rs74892325 | -0.072 | -0.108 − -0.036 | <0.0001 | 0.830 | 0.058 | 0.010 |
| rs2686372 | -0.073 | -0.110 − -0.036 | 0.0001 | 1 | 0.055 | 0.012 |
| rs112265934 | -0.072 | -0.109 − -0.035 | 0.0001 | 1 | 0.054 | 0.008 |
| rs113760750 | -0.071 | -0.108 − -0.034 | 0.0002 | 1 | 0.055 | 0.007 |
| rs75009692 | -0.073 | -0.111 − -0.035 | 0.0002 | 0.813 | 0.052 | 0.014 |
| rs77594915 | -0.072 | -0.110 − -0.035 | 0.0002 | 1 | 0.054 | 0.010 |
| rs113810203 | -0.072 | -0.109 − -0.034 | 0.0002 | 1 | 0.054 | 0.010 |
| rs3751145 | -0.071 | -0.109 − -0.034 | 0.0002 | 1 | 0.054 | 0.010 |
| rs112956506 | -0.071 | -0.109 − -0.034 | 0.0002 | 1 | 0.054 | 0.009 |
| rs112803307 | -0.072 | -0.117 − -0.026 | 0.0020 | 0.466 | 0.035 | 0.021 |
| P2X7 x RLE | ||||||
| ß | 95% C.I. | p | Hardy | MAF | Missing | |
| rs61953400 | 0.209 | 0.087–0.331 | 0.0008 | 0.582 | 0.022 | 0.009 |
| rs61953398 | 0.182 | 0.070–0.293 | 0.0014 | 0.720 | 0.033 | 0.012 |
CHA: Childhood adversities; RLE: Recent life events. Bold type denotes top SNPs in each clump.
Both top P2X7R SNPs were in Hardy–Weinberg equilibrium in the sample (rs74892325: p = 0.8295, rs61953400: p = 0.5823). For rs74892325, T is the minor allele, with an allele frequency of 0.05771. For rs61953400, A is the minor allele, yielding an allele frequency of 0.0219.
Both top SNPs were tested in main effect models as well as for interaction with both CHA and RLE (Table 3). None of the top SNPs had a significant main effect on BSI-depression.
Table 3. Main effects and interactions with childhood adversities (CHA) and recent life events (RLE) of P2RX7 tops SNPs rs74892325 and rs61953400 on current depression symptom severity.
| ADD | DOM | REC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß | 95% C.I. | p | FDR Q | ß | 95% C.I. | p value | FDR Q | ß | 95% C.I. | p | FDR Q | ||
| rs74892325 | Main effect | -0.102 | -0.227–0.023 | 0.1101 | 0.2202 | -0.114 | -0.244–0.016 | 0.0861 | 0.1968 | 0.130 | -0.638–0.898 | 0.7399 | 0.7892 |
| CHA interaction | -0.070 | -0.104 - -0.035 | <0.0001 | 0.0006 | -0.072 | -0.108 - -0.036 | <0.0001 | 0.0006 | -0.070 | -0.311–0.172 | 0.5713 | 0.7031 | |
| RLE interaction | -0.048 | -0.143–0.048 | 0.3263 | 0.4351 | -0.053 | -0.151–0.044 | 0.2843 | 0.4135 | 0.048 | -0.948–1.044 | 0.9241 | 0.9241 | |
| rs61953400 | Main effect | 0.126 | -0.073–0.326 | 0.2139 | 0.3422 | 0.138 | -0.066–0.341 | 0.1849 | 0.3287 | -0.429 | -2.151–1.293 | 0.6253 | 0.7146 |
| CHA interaction | 0.045 | -0.005–0.096 | 0.0752 | 0.1968 | 0.045 | -0.005–0.095 | 0.0795 | 0.1968 | |||||
| RLE interaction | 0.194 | 0.074–0.314 | 0.0015 | 0.0062 | 0.209 | 0.087–0.331 | 0.0008 | 0.0043 | |||||
Depressive symptom scores were measured by Brief Symptom Inventory (BSI). ADD, DOM, REC: additive, dominant and recessive heritability models. CHA: Childhood Adversity; FDR: false discovery rate; RLE: recent life stress. Bold type denotes significant P-values surviving correction for multiple testing (P<0.05, FDR Q<0.05).
In case of rs74892325, no significant interaction effect emerged with recent life events (ADD: p = 0.3263, DOM: p = 0.2843, REC: p = 0.9241), but a significant interaction with childhood adversity on current depressive symptoms was found in both additive (p<0.0001) and dominant (p<0.0001) but not in recessive (p = 0.5713) models which remained significant after correction for multiple testing (FDR Q = 0.0007 and FDR Q = 0.0007 for additive and dominant models respectively). Rs74892325 minor T allele carriers scored significantly lower on BSI-depression scale if they were exposed to moderate or severe childhood maltreatment reflecting a protective effect for the minor allele (Fig 2).
Fig 2. Linear regression analyses indicated a significant interaction between P2RX7 rs74892325 and exposure to childhood adversities on current depression, with the minor T allele as a protective allele.
Linear regression indicated a significant interaction between P2RX7 rs74892325 genotype and severe childhood adversities (CHA) on current depression scores (BSI-DEP) according to the additive (p<0.0001, FDR Q = 0.0006) and dominant (p<0.0001, FDR Q = 0.0006) models. Presence of the minor T allele was associated with lower depression scores in subjects exposed to severe recent life events conveying a protective effect. On the vertical axis weighted depression (BSI-Dep) scores are shown. The horizontal axis shows severity of childhood adversities (CHA) categorised as 1: low = 0–3, 2: medium = 4–6, 3: high = 7 or more. While regression models were used in the analyses, the figure depicts results from ANOVA solely for visualisation purposes. Mean and SEM values are displayed.
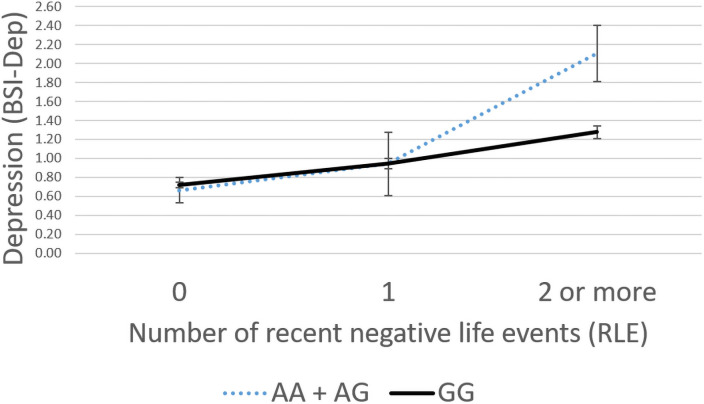
In case of rs61953400, no significant interaction effect with childhood adversities was observed (ADD: p = 0.0752, DOM: p = 0.0795), but a nominally significant interaction with recent life events was found in both additive (p = 0.0015) and dominant (p = 0.0008) models which remained significant after correction for multiple testing (FDR Q = 0.0062 and FDR Q = 0.0043 for additive and dominant models, respectively) (Table 3). Presence of the minor A allele was associated with increased BSI-depression scores in those exposed to severe recent life events (RLE) reflecting a risk effect (Fig 3). Because the particularly limited number of cases of homozygous carriers of the minor allele (A) in certain exposure groups, the recessive models could not be calculated and the results are not shown.
Fig 3. Linear regression analyses indicated a significant interaction between P2RX7 rs61953400 and exposure to recent life events on current depression, with the minor A allele as a risk allele.
Linear regression indicated a significant interaction between P2RX7 rs61953400 genotype and recent stressful life events (RLE) on current depression scores (BSI-DEP) according to the additive (p<0.0015, FDR Q = 0.0062) and dominant (p<0.0001, FDR Q = 0.0043) models. Presence of the minor A allele was associated with higher depression scores in subjects exposed to severe recent life events conveying a risk effect. On the vertical axis weighted depression (BSI-Dep) scores are shown. The horizontal axis shows recent life events (RLE) occurring within the past year as measured by the List of Threatening Experiences [36] categorised as 0 –low exposure (0 events reported) 1 –medium exposure (1 event reported) or 3 –high exposure (2 or more events reported). While regression models were used in the analyses, the figure depicts results from ANOVA solely for visualisation purposes. Mean and SEM values are displayed.
In silico characterisation and functional prediction of identified top SNPs rs74892325 and rs61953400
Genomic location of significant SNPs identified in the clumping procedure are shown in Fig 4. Search in LitVar (https://www.ncbi.nlm.nih.gov/CBBresearch/Lu/Demo/LitVar/#!?query=), dbSNP (https://www.ncbi.nlm.nih.gov/snp/), and GWAS catalog database (https://www.ebi.ac.uk/gwas/) did not identify any relevant information regarding the two SNPs or any further SNPs in our clumps. FuncPred (www.funcpred.com) tool could also not identify any known functional effect of our top SNPs except for rs2686372 intronic variant (in the CHA interaction clump), where in function prediction we observed a 0.435 conservation score.
Fig 4. Genomic location of significant SNPs identified in the clumping procedure.
Significant SNPs identified in the analysis with an effect on current depression symptoms in interaction with childhood adversities are denoted as P2RX7 x CHA; significant SNPs identified in the analysis with an effect on current depression symptoms in interaction with recent life event are denoted as P2RX7 x RLE; top SNPs in the two analyses are marked with light blue. In the gene expression part of the image, yellow bars indicate expression in nervous tissues. UCSC Genome Browser on Human Feb. 2009 (GRCh37/hg19) was used to visualize the location of polymorphisms on P2RX7 gene.
Discussion
In our present study analysing variation along the P2RX7 gene using a linkage disequilibrium-based clumping procedure, we identified two significant clumps. One clump, with top SNP rs74892325 significantly interacted with early childhood adverse experiences conveying a protective effect, and the other clump with top SNP rs61953400 significantly interacted with recent stressful life events conveying a risk effect. As no main effect for any investigated variants were observed, our results support that variation in P2RX7 influences severity of current depressive symptoms in interaction with stress, mediating the effects of both early, distal stressors with an etiological effect and recent, proximal stressors, with a trigger effect. Our findings not only support previous results on the association of variation in this gene with depression, but emphasises its role in mediating the effects of stress (Fig 5).
Fig 5. A summary of the study on the effect of P2RX7 variation in interaction with childhood adversity and recent stress on the severity of depressive symptoms.
Besides expression in peripheral immune cells, P2X7 receptors display a wide distribution across the brain located in microglia, central (and peripheral) neurons, and also show inconsistent and controversial expression in astrocytes and oligodendrocytes [40]. As a nonselective cation channel activated by sharp increases of extracellular ATP typically occurring during stressful conditions, P2X7 receptors execute ATP-driven danger signal transduction with relevant pathological and clinical implications [41]. The P2X7 receptor has been implicated as a central hub of divergent brain disorders including neurodegenerative disorders, neuropsychiatric disorders such as depression and schizophrenia, and brain tumours, due to its role in mediating several key processes [42,43]. While effects mediated by P2X7 receptor occur mainly through neuroinflammatory response and inflammasome activation [44], it also interacts and interferes with other stress and depression-related mechanisms [19], including impaired serotonin, noradrenaline, glutamate, GABA and NO release and transmission, and reduced neurogenesis and neuroplasticity [19,45–51]. In fact, P2RX7 is now being investigated in major depressive disorder as a potential novel therapeutic target [2,26] with several brain-penetrating antagonists in phase 2 and 3 clinical trials.
P2X7 receptors are activated by sharp increases of extracellular ATP release typically triggered by environmental stress resulting in NLRP3 inflammasome activation, which is maintained by a regenerative circuit even following termination of exposure, causing reduced BDNF levels, diminished synaptogenesis and neurogenesis, and damage to emotion and mood-regulation-relevant brain circuits [19]. P2RX7 is thought to play a role especially in chronic stress, where IL-1β driven microglial activation and neuroinflammation is upregulated. Efficacy of P2RX7 selective brain penetrant antagonists in animal models of chronic stress [52,53] also support a stress-mediated ATP-driven activation of the P2RX7 –NLRP3 –IL-Iβ pathway leading to proinflammatory microglial activation and neuroinflammation [2,41].
Human highly polymorphic P2X7R is located on 12q24.31 and has been reported to be associated with both unipolar major depression and bipolar disorder [20]. However, in spite of the increased interest in purinergic signalling and especially P2RX7 in mood disorders, only a few studies focused on the role of variation in this gene. Most of these studies investigated one variant, rs2230912 of P2RX7, a nonsynonymous coding SNP causing an amino acid-exchange (Gln460Arg), associated with altered channel function enhancing Ca2+ influx, P2X7 dimerization, and further protein-protein interactions, thus influencing P2RX7-mediated signalling. Specifically, the G allele was associated with a gain-of-function phenotype for increased IL-1β release from monocytes in response to activation [24] suggesting that similar variation in P2RX7 in microglia may similarly modify cytokine release, leading to neuroinflammation and changing the functional state of neural networks leading to increased vulnerability for mood disorders [23,25], although it is not yet understood how such SNPs increase the risk of mood disorders or, maybe even more importantly, how a loss of function would protect against them [2].
Following initial reports of an association between this variant and major depressive disorder [21], a few controversial replication attempts have been published with lack of a significant association in one meta-analysis in bipolar and unipolar depression [22], but a positive association with MDD in a more recent one including a greater number of studies [23]. In spite of the failure of some of the case-control studies in various types of affective disorders, dimensional approaches yielded confirmation for an association between rs2230912 and severity of depressive symptoms in bipolar disorder or combined unipolar and bipolar patient samples [54,55] or in bipolar samples [56] as well as with course characteristics of depression in unipolar and bipolar clinical samples including longer depressive episodes and more time spent ill [57]. In the above study of Vereczkei et al., not only rs2230912 but a minor-allele containing rs1718119-A ~ rs2230912-G ~ rs1653625-A haplotype was associated with a significantly higher depressive symptom score compared to the most frequent G~A~C haplotype among psychiatric patients with a major depressive episode, and also among diabetic patients, who are at higher risk for developing mood disorders.
The majority of studies investigating the role of P2RX7 in depression have focused on rs2230912, but more recently it has been suggested that tagging all variations could help determine the extent of the role P2RX7 plays in mood disorders [23], and understanding the role of other variations may shed further light on the association between P2RX7 and disease susceptibility/protection [41]. Furthermore, given the role of P2X7 in mediating the effects of danger signals and stress, one possible reason for the above contradictory findings may be that pathological conditions as depression result from interaction between genes and the environment [23] yet studies failed to consider the interacting effects of life events.
The present study investigated the effects of several variants in P2RX7 using a linkage disequilibrium-based clumping method, and focused on the interacting effects of stress with a complex approach, including distal and more etiological stressors in the form of early childhood adversities as well as proximal stressors playing a triggering role, in the form of recent negative life events occurring in the previous year. Furthermore, we also investigated severity of depressive symptoms using a dimensional approach. Several aspects of our findings are noteworthy. First of all, we identified two clumps with novel lead variants which have previously not been reported. None of the investigated variants have main effects on depression symptom score, that is, their effects became observable only in those exposed to moderate or severe stress in case of childhood adversities or severe stress in case of recent life events. This finding is in line not only with the supposed activation of P2RX7 by stress and danger signals, but also with findings in our previous study, where using a Bayesian network analysis approach we reported that none of several variants previously associated with depression in seven genes including rs758311 in P2RX7 showed relevance with respect to a complex depressive phenotype in the absence of stress, while the majority of them gained relevance in those exposed to moderate or severe recent life events [28]. Furthermore, our findings in detecting the effect of P2RX7 variation on depression only in interaction with stress are in line with rodent models investigating humanized hP2Rx7 mice in a GxE scenario reporting that rs2230912 heterozygous mice exposed to chronic social defeat show higher anhedonia and anxiety compared to homozygotes [58], and concluding that P2RX7 plays a role specifically in stress-related pathologies [26]. This conclusion is also supported by findings of enhanced P2RX7 expression in the frontal cortex and hippocampus of mice exposed to chronic unpredictable stress or chronic restraint stress [59,60], while knockout mice studies suggest that absence of P2RX7 confers both increased stress resilience and a phenotype with decreased depression-like characteristics [19]. Synthesising findings from several animal studies it has been hypothesised that P2RX7 in interaction with stress predisposes to depression via profound effects on several processes interacting with environmental factors through the life span [26,58]. In summary, both pharmacological and genetic studies suggest that P2X7 receptor stimulation during stress leads to increased vulnerability and subsequent development of behavioural abnormalities associated with the neurobiology of depression [19,61,62]. Our finding showing the interaction of P2RX7 variation with early and current stressors confirms these results.
The present study has several limitations. First, childhood adversity and recent life events were assessed retrospectively, and based on self-report of the subjects but not ascertained by other informants, which may contribute to distortion from several sources, including recall bias. Second, in a similar way, current depression severity was also based on a self-reported measure. Third, our population sample was relatively small, consisted in approximately two thirds of female subjects and limited to European white participants. Fourth, scoring childhood adversity and counting the number of recent negative life events does not take into consideration the differing severity of individual life events. Also, we considered objective occurrence of these life events and not their subjective meaning. Nevertheless, our study also has several strengths, including considering several hundred variants along the P2RX7 gene with a clump method rather than just a single candidate SNP, employing a dimensional approach to capture current depression symptom severity, and using a GxE paradigm with two etiologically different types of stressors.
Conclusions
In conclusion, our study is the first to investigate variation along the P2RX7 gene in a large sample using a linkage disequilibrium-based clumping method and also a GxE interaction approach to report two clumps of variants with novel lead SNPs mediating the effects of distal and proximal stressors on the severity of depressive symptoms. Besides specifically focusing on the role of P2RX7, our findings extend our understanding of the role of neuroinflammation in depression, and also provide further support for the hypothesis that a great majority of genes involved in the background of depression do not act by directly leading to mood symptoms, but by enhancing sensitivity to the deleterious effects of different types of stressors.
Supporting information
(XLSX)
(XLSX)
Data Availability
The data underlying this study are available from FigShare (DOI: 10.6084/m9.figshare.13483011) and within the manuscript and its Supporting information files.
Funding Statement
The study group (not individual authors) was supported by the Sixth Framework Programme of the European Union (NewMood, LSHM-CT-2004-503474); by the Hungarian Brain Research Program (Grants KTIA_13_NAPA-II/14 and 2017-1.2.1-NKP-2017-00002), and the National Development Agency (Grant KTIA_NAP_13-1-2013-0001); by the Hungarian Academy of Sciences, Hungarian National Development Agency, Semmelweis University and the Hungarian Brain Research Program (Grant KTIA_NAP_13-2-2015-0001, MTA-SE-NAP B Genetic Brain Imaging Migraine Research Group); by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group); by OTKA 119866 and 131629; by TAMOP-4.2.1.B-09/1/KMR-2010-0001; by the National Research, Development and Innovation Office, Hungary (2019-2.1.7-ERA-NET-2020-00005), under the frame of ERA PerMed (ERA-PERMED2019-108); and by the Thematic Excellence Programme (Tématerületi Kiválósági Program, 2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Neurology and Translational Biotechnology thematic programmes of the Semmelweis University and the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska Curie grant agreement No. 766124. Ms. Sara Sutori was supported by ÚNKP-19-1-I-PPK, Dr. Nora Eszlari by ÚNKP-20-4-II-SE-9 and Mr. Daniel Baksa by ÚNKP-20-3-II-SE-51, by the New National Excellence Program of The Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya A, Jones DNC. Emerging role of the P2X7-NLRP3-IL1beta pathway in mood disorders. Psychoneuroendocrinology. 2018;98:95–100. doi: 10.1016/j.psyneuen.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 3.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6(11):e946. doi: 10.1038/tp.2016.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ongur D, Bechtholt AJ, Carlezon WA Jr., Cohen BM. Glial abnormalities in mood disorders. Harv Rev Psychiatry. 2014;22(6):334–7. doi: 10.1097/HRP.0000000000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–42. doi: 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64(10):1772–87. doi: 10.1002/glia.23001 [DOI] [PubMed] [Google Scholar]
- 7.Pfau ML, Menard C, Russo SJ. Inflammatory Mediators in Mood Disorders: Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2018;58:411–28. doi: 10.1146/annurev-pharmtox-010617-052823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzer R, Cohen S, Russo SJ, Dinan TG. Resilience and immunity. Brain Behav Immun. 2018;74:28–42. doi: 10.1016/j.bbi.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8(1):57. doi: 10.1186/s13550-018-0401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7(12):1064–74. doi: 10.1016/S2215-0366(20)30255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler-Forsberg O, C NL, Hjorthoj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404–19. doi: 10.1111/acps.13016 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170(3):624–40. doi: 10.1111/bph.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42(11):1753–6. doi: 10.1016/j.biocel.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS, Gardner CO. Monozygotic twins discordant for major depression: a preliminary exploration of the role of environmental experiences in the aetiology and course of illness. Psychol Med. 2001;31(3):411–23. doi: 10.1017/s0033291701003622 [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiat. 1999;156(6):837–41. doi: 10.1176/ajp.156.6.837 [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, KarkowskiShuman L. Stressful life events and genetic liability to major depression: Genetic control of exposure to the environment. Psychol Med. 1997;27(3):539–47. doi: 10.1017/s0033291797004716 [DOI] [PubMed] [Google Scholar]
- 17.Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198(1):83–90. doi: 10.1016/j.bbr.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Otrokocsi L, Kittel A, Sperlagh B. P2X7 Receptors Drive Spine Synapse Plasticity in the Learned Helplessness Model of Depression. Int J Neuropsychopharmacol. 2017;20(10):813–22. doi: 10.1093/ijnp/pyx046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro DE, Roncalho AL, Glaser T, Ulrich H, Wegener G, Joca S. P2X7 Receptor Signaling in Stress and Depression. Int J Mol Sci. 2019;20(11). doi: 10.3390/ijms20112778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuffin P, Knight J, Breen G, Brewster S, Boyd PR, Craddock N, et al. Whole genome linkage scan of recurrent depressive disorder from the depression network study. Hum Mol Genet. 2005;14(22):3337–45. doi: 10.1093/hmg/ddi363 [DOI] [PubMed] [Google Scholar]
- 21.Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15(16):2438–45. doi: 10.1093/hmg/ddl166 [DOI] [PubMed] [Google Scholar]
- 22.Feng WP, Zhang B, Li W, Liu J. Lack of association of P2RX7 gene rs2230912 polymorphism with mood disorders: a meta-analysis. Plos One. 2014;9(2):e88575. doi: 10.1371/journal.pone.0088575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czamara D, Muller-Myhsok B, Lucae S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:272–7. doi: 10.1016/j.pnpbp.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010;24(8):2916–27. doi: 10.1096/fj.09-150862 [DOI] [PubMed] [Google Scholar]
- 25.Bennett MR. Synaptic P2X7 receptor regenerative-loop hypothesis for depression. Aust N Z J Psychiatry. 2007;41(7):563–71. doi: 10.1080/00048670701399994 [DOI] [PubMed] [Google Scholar]
- 26.Deussing JM, Arzt E. P2X7 Receptor: A Potential Therapeutic Target for Depression? Trends Mol Med. 2018;24(9):736–47. doi: 10.1016/j.molmed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Gonda X, Petschner P, Eszlari N, Baksa D, Edes A, Antal P, et al. Genetic variants in major depressive disorder: From pathophysiology to therapy. Pharmacol Ther. 2019;194:22–43. doi: 10.1016/j.pharmthera.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Gonda X, Hullam G, Antal P, Eszlari N, Petschner P, Hokfelt TG, et al. Significance of risk polymorphisms for depression depends on stress exposure. Sci Rep. 2018;8(1):3946. doi: 10.1038/s41598-018-22221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juhasz G, Chase D, Pegg E, Downey D, Toth ZG, Stones K, et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology. 2009;34(8):2019–27. doi: 10.1038/npp.2009.19 [DOI] [PubMed] [Google Scholar]
- 30.Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, et al. New evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biol Psychiatry. 2008;64(6):498–504. doi: 10.1016/j.biopsych.2008.03.030 [DOI] [PubMed] [Google Scholar]
- 31.Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 Pathway in Depression: Multiple Gene-Cognition-Environment Interactions. Biological psychiatry. 2011;69(8):762–71. doi: 10.1016/j.biopsych.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 32.Derogatis LR. BSI: Brief Symptom Inventory: Administration, Scoring, and Procedures Manual Minneapolis: National Computer Systems 1993.
- 33.Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biol Psychiatry. 2011;69(8):762–71. doi: 10.1016/j.biopsych.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 34.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- 35.Brugha T, Bebbington P, Tennant C, Hurry J. The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. 1985;15(1):189–94. doi: 10.1017/s003329170002105x [DOI] [PubMed] [Google Scholar]
- 36.Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand. 1990;82(1):77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x [DOI] [PubMed] [Google Scholar]
- 37.Juhasz G, Gonda X, Hullam G, Eszlari N, Kovacs D, Lazary J, et al. Variability in the effect of 5-HTTLPR on depression in a large European population: the role of age, symptom profile, type and intensity of life stressors. Plos One. 2015;10(3):e0116316. doi: 10.1371/journal.pone.0116316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33(1):67–72. doi: 10.1023/a:1021055617738 [DOI] [PubMed] [Google Scholar]
- 39.Gonda X. Raw data. 2020.
- 40.Illes P, Khan TM, Rubini P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J Neurosci. 2017;37(30):7049–62. doi: 10.1523/JNEUROSCI.3103-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharya A. Recent Advances in CNS P2X7 Physiology and Pharmacology: Focus on Neuropsychiatric Disorders. Front Pharmacol. 2018;9:30. doi: 10.3389/fphar.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Wang H, Gao H, Zhang H, Wang Q, Sun Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020;10:28. doi: 10.1186/s13578-020-00388-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, Glaser T, Arnaud-Sampaio VF, Lameu C, et al. The P2X7 Receptor: Central Hub of Brain Diseases. Front Mol Neurosci. 2020;13:124. doi: 10.3389/fnmol.2020.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheffer A, Castillo ARG, Correa-Velloso J, Goncalves MCB, Naaldijk Y, Nascimento IC, et al. Purinergic system in psychiatric diseases. Mol Psychiatry. 2018;23(1):94–106. doi: 10.1038/mp.2017.188 [DOI] [PubMed] [Google Scholar]
- 45.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–93. doi: 10.1038/nrn1867 [DOI] [PubMed] [Google Scholar]
- 46.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–23. doi: 10.1016/j.conb.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, et al. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109(3):846–57. doi: 10.1111/j.1471-4159.2009.06008.x [DOI] [PubMed] [Google Scholar]
- 48.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78(6):327–46. doi: 10.1016/j.pneurobio.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 49.Goloncser F, Baranyi M, Balazsfi D, Demeter K, Haller J, Freund TFF, et al. Regulation of Hippocampal 5-HT Release by P2X7 Receptors in Response to Optogenetic Stimulation of Median Raphe Terminals of Mice. Front Mol Neurosci. 2017;10:325. doi: 10.3389/fnmol.2017.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Csölle C, Baranyi M, Zsilla G, Kittel A, Gölöncsér F, Illes P, et al. Neurochemical Changes in the Mouse Hippocampus Underlying the Antidepressant Effect of Genetic Deletion of P2X7 Receptors. PLoS One. 2013;8(6):e66547. doi: 10.1371/journal.pone.0066547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papp L, Balazsa T, Kofalvi A, Erdelyi F, Szabo G, Vizi ES, et al. P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J Pharmacol Exp Ther. 2004;310(3):973–80. doi: 10.1124/jpet.104.066712 [DOI] [PubMed] [Google Scholar]
- 52.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol Psychiatry. 2016;80(1):12–22. doi: 10.1016/j.biopsych.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 53.Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14(1):102. doi: 10.1186/s12974-017-0865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hejjas K, Szekely A, Domotor E, Halmai Z, Balogh G, Schilling B, et al. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(2):295–9. doi: 10.1002/ajmg.b.30799 [DOI] [PubMed] [Google Scholar]
- 55.Vereczkei A, Abdul-Rahman O, Halmai Z, Nagy G, Szekely A, Somogyi A, et al. Association of purinergic receptor P2RX7 gene polymorphisms with depression symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:207–16. doi: 10.1016/j.pnpbp.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 56.Halmai Z, Dome P, Vereczkei A, Abdul-Rahman O, Szekely A, Gonda X, et al. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J Affect Disord. 2013;150(1):104–9. doi: 10.1016/j.jad.2013.02.033 [DOI] [PubMed] [Google Scholar]
- 57.Soronen P, Mantere O, Melartin T, Suominen K, Vuorilehto M, Rytsala H, et al. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(4):435–47. doi: 10.1002/ajmg.b.31179 [DOI] [PubMed] [Google Scholar]
- 58.Metzger MW, Walser SM, Dedic N, Aprile-Garcia F, Jakubcakova V, Adamczyk M, et al. Heterozygosity for the Mood Disorder-Associated Variant Gln460Arg Alters P2X7 Receptor Function and Sleep Quality. J Neurosci. 2017;37(48):11688–700. doi: 10.1523/JNEUROSCI.3487-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su WJ, Zhang T, Jiang CL, Wang W. Clemastine Alleviates Depressive-Like Behavior Through Reversing the Imbalance of Microglia-Related Pro-inflammatory State in Mouse Hippocampus. Front Cell Neurosci. 2018;12:412. doi: 10.3389/fncel.2018.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan S, Wang Y, Chen K, Long Z, Zou J. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol Pharm Bull. 2017;40(8):1260–7. doi: 10.1248/bpb.b17-00131 [DOI] [PubMed] [Google Scholar]
- 61.Pereira VS, Casarotto PC, Hiroaki-Sato VA, Sartim AG, Guimaraes FS, Joca SR. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: involvement of nitric oxide. Eur Neuropsychopharmacol. 2013;23(12):1769–78. doi: 10.1016/j.euroneuro.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 62.Burnstock G. Purinergic Signalling and Neurological Diseases: An Update. CNS Neurol Disord Drug Targets. 2017;16(3):257–65. doi: 10.2174/1871527315666160922104848 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
The data underlying this study are available from FigShare (DOI: 10.6084/m9.figshare.13483011) and within the manuscript and its Supporting information files.