Abstract
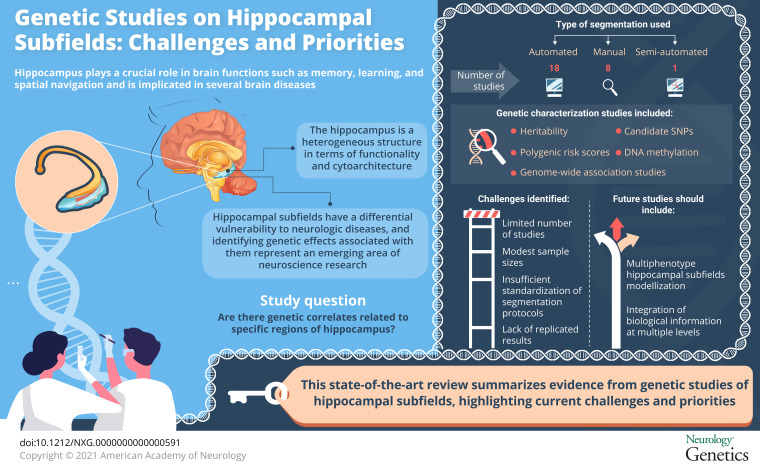
There is clear evidence that hippocampal subfield volumes have partly distinct genetic determinants associated with specific biological processes. The identification of genetic correlates of hippocampal subfield volumes may help to elucidate the mechanisms of neurologic diseases, as well as aging and neurodegenerative processes. However, despite the emerging interest in this area of research, the current knowledge of the genetic architecture of hippocampal subfields has not yet been consolidated. We aimed to provide a review of the current evidence from genetic studies of hippocampal subfields, highlighting current priorities and upcoming challenges. The limited number of studies investigating the influential genetic effects on hippocampal subfields, a lack of replicated results and longitudinal designs, and modest sample sizes combined with insufficient standardization of protocols are identified as the most pressing challenges in this emerging area of research.
It is well known that certain genes confer increased risk to the development of neurologic diseases up to the point that some are considered to be causative.1,2 However, for some pathologies, genetic association findings do not explain their entire genetic architecture.3-5 Identifying genes linked to disease-specific intermediate phenotypes, like brain areas known to be affected by a particular disorder, can improve our understanding of the biological mechanisms underlying these neurologic-related phenotypes. In addition, this can aid future functional genetic studies because such genetic effects are likely to be stronger than and independent of clinical status of the disease. Thus, through studying the genetics of the brain, it is possible to capture disease mechanisms more objectively and accurately than by clinical diagnosis.6-8
Neuroimaging studies based on neurologic-related processes usually focus on specific brain structures, such as the hippocampus. The hippocampus plays a central role on several brain functions such as memory, learning, and spatial navigation9,10 and is often found to be a highly vulnerable brain target.11 However, the hippocampus is a heterogeneous structure in terms of functionality and cytoarchitecture. Therefore, the hippocampus as a whole structure may be too simplistic to fully explain its role in cognition and in disease, thus limiting the sensitivity and specificity for differential diagnosis. The hippocampal structure can be subdivided into distinct regions, also called subfields. Hippocampal subfields have unique functional properties and differential vulnerability to complex diseases.
Current evidence suggests that analysis at the hippocampal subfield level may improve the sensitivity of the detection of effects compared with the analysis of the hippocampus taken as a whole.12,13 In this review, we aim to summarize genetic studies published on hippocampal subfields and to describe the priorities, challenges, and future application of this area of research.
Methods
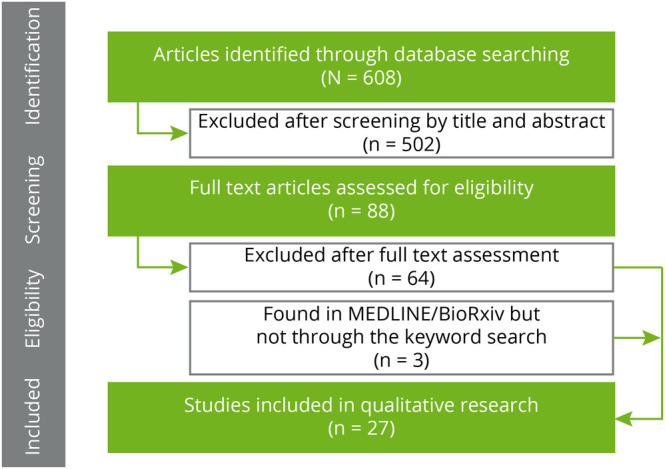
The selection process is summarized in figure 1. A total of 608 articles were identified in PubMed. We additionally screened titles and abstracts to identify eligible articles based on the following inclusion criteria: (1) the article must be focused on the combined analysis of genetic data and hippocampal subfield volumes and (2) the article must analyze human data. As a result of this screening, we evaluated 88 full-text articles. In total, 65 articles were excluded after full-text assessment for not meeting the inclusion criteria. In addition, 2 additional studies were found in PubMed but not through the keyword search and were added. As a result, a total of 26 articles were chosen for full-text evaluation. The final list of selected articles is cataloged in table e-1, links.lww.com/NXG/A418, along with key information regarding the study samples, specific hippocampal subfields analyzed, omics data assessed, processing software, the field strength of the scanner, the acquired magnetic resonance pulse sequences with their main parameters, and a summary of the main results and characteristics of each study.
Figure 1. Selection Process of the Articles.
Results
Hippocampal Subfield Assessment
MR Pulse Sequences, Voxel Size, and Magnetic Field Strength
Eighteen studies used T1-weighted (T1-w) MRI sequences to perform hippocampal subfields segmentation. Most of them used an isotropic voxel resolution of 1.0 mm or lower voxel size. Besides, at least 8 studies used T2-weighted (T2-w) MRI sequences using 0.4 mm × 0.4 mm in-plane voxel high resolution with 2 mm of thickness. Finally, 1 study used proton density weighted. In addition, almost all the studies used scanners at field strengths of at least 3-T (23 studies). Three studies used a magnetic field strength of 1.5 T, whereas 3 more studies combined 1.5 and 3 T scans.
Segmentation Software
The distribution of studies depending on the segmentation software used to quantify hippocampal subfields volumes is showed in figure e-1, links.lww.com/NXG/A416. Classification showed that 18 studies used automated segmentation tools, 8 studies used manual segmentation, and 1 study used a semi-automated approach.
Within the studies using automated segmentation, 16 studies used FreeSurfer software.13 Eight studies used the new ex vivo atlas-based segmentation from FreeSurfer version 6.0, and 2 used FreeSurfer version 5.3.14,15 Four used previous versions (<v. 5.3), and 2 studies used different versions or did not specify the version used.
Segmentation of hippocampal subfields in FreeSurfer v.6.0 is driven by a probabilistic atlas, built from 2 manually delineated data sets. The first data set comprised 15 autopsy samples, including 4 with Alzheimer disease (AD), from ex vivo high-resolution 7-T MRI (0.13 mm isotropic voxels on average), and a second that comprised 39 T1-weighted images (1 mm isotropic voxels) from 29 controls and 10 individuals with mild dementia. In FreeSurfer v.5.3, the atlas is built in vivo scans at 0.38 × 0.38 × 0.80 mm3 resolution (without manual delineation), yielding voxels larger than the ex vivo protocol (version 6.0).16
Moreover, 2 studies used MAGeT-Brain (Multiple Automatically-GEnerated Templates).17,18 In MAGeT, hippocampal subfield segmentation is driven by propagating atlas segmentations (optimal number of atlases, 9) to a template library, formed from a subset of target images (with several types of image acquisition and disease populations), via transformations estimated by nonlinear image registration.
Finally, none of the studies included segmentation based on automatic segmentation of hippocampal subfields (ASHS),19 although this method is used extensively in nongenetic hippocampal subfield studies. In ASHS, hippocampal subfields are labeled using a pipeline that combines multiatlas label fusion and learning-based error correction from T1-weighted and T2-weighted MRI sequences.
Hippocampal Subfield Volumes
Segmentation using FreeSurfer returns raw volumes of 12 hippocampal subregions (per hemisphere). These subfields were the cornu ammonis (CA) regions CA1, CA2/CA3, and CA4 of the hippocampus proper, the granule cell and molecular layer of the dentate gyrus (DG), the subiculum, presubiculum and parasubiculum, the hippocampal fissure, the hippocampus-amygdala-transition-area, the hippocampal tail, the hippocampal fimbria, and the molecular layer of the DG. Segmentation using MAGeT yields 5 hippocampal subregions. These subregions are CA1, CA2/3, CA4/DG, subiculum, and stratum radiatum/stratum lacunosum/stratum moleculare (SRLM). Finally, segmentation using ASHS yields 8 subregions (5 hippocampal and 3 peripheral areas from the medial temporal lobe; figure e-2, links.lww.com/NXG/A417).
Genetic Characterization of Hippocampal Subfields
Heritability
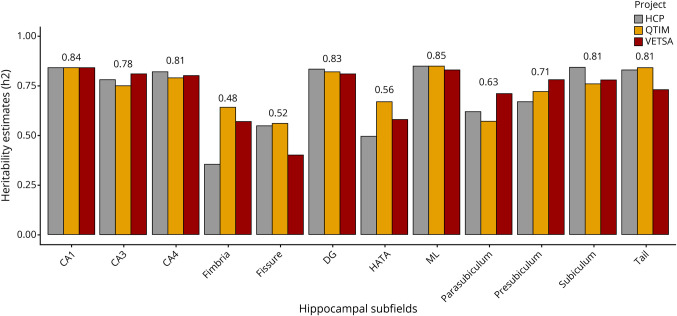
Four studies were performed in a sample of cognitively unimpaired twins, investigating the heritability of hippocampal subfield volumes.12,20-22 Three of them evaluated narrow-sense heritability (h2) (figure 2). Whelan et al (2015) reported univariate estimates ranged from 0.67 to 0.91 using data from the Queensland Twins Imaging study. In order of decreasing estimate, molecular layer, CA1, hippocampal tail, and DG showed higher heritability (0.82–0.88). Similarly, Greenspan et al (2016) reported univariate and bivariate heritability estimates for hippocampal subfields ranged from 0.42 to 0.87 using data from the HCP (Human Connectome Project), of which, in order of decreasing estimate, CA1, subiculum, hippocampal tail, CA2/3, DG, and molecular layer showed higher heritability (0.81–0.87). Elman et al (2019) further explore narrow-sense heritability using data from the HCP project as well as the Vietnam Era Twin Study of Aging. This study reported similar heritability estimations in subfields ranging from 0.37 to 0.89. Finally, Patel et al (2017) calculated broad-sense heritability (H2) for the different subfields in each hemisphere. In general, subfields from the right hemisphere showed higher heritability compared with the left hemisphere. In complement to these studies, 1 study23 accounted for DNA-based unrelated heritability estimates through a genome-wide complex trait analysis using the GCTA (Genome-wide Complex Trait Analysis).24 The DNA-based heritability estimates for all subfields ranged from 0.14 to 0.27 and were comparable to those reported in previous multicenter studies of subcortical structures.25
Figure 2. Twin-Based Heritability Estimates (Narrow-Sense Heritability) of Hippocampal Subfields From Different Twin-Study Projects (Average Measurement).
CA = cornu ammonis; CA1 = CA region 1; CA2 = CA region 2; CA3 = CA region 3; CA4 = CA region 4; DG = dentate gyrus; fissure = hippocampal fissure; HATA = hippocampus-amygdala-transition-area; HCP = Human Connectome Project; ML = molecular layer of the DG; QTIM = Queensland Twins Imaging; tail = hippocampal tail; VETSA = Vietnam Era Twin Study of Aging.
Candidate Single Nucleotide Polymorphism Studies
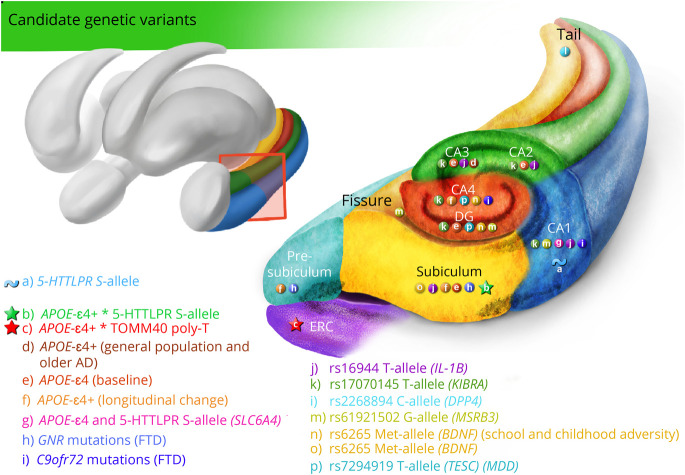
Studies that used automated segmentation were largely based on candidate single nucleotide polymorphisms (SNPs) within genes related to memory, cognition, and diseases such as major depressive disorder (MDD), schizophrenia, bipolar disorder, and AD. A summary of the results can be found in figure 3.
Figure 3. Summary of Candidate Genetic Variants Associated With Hippocampal Subfields.
AD = Alzheimer disease; CA = cornu ammonis; CA1 = CA region 1; CA2 = CA region 2; CA3 = CA region 3; CA4 = CA region 4; ERC = entorhinal cortex; E-legend: links.lww.com/NXG/A420. Circle, main genetic effect; star, interactive effect; wave, significant activation.
APOE Gene
Six studies assessed the association between hippocampal subfield volumes and the APOE (apolipoprotein) ε4 allele.26-31 One study focused on cognitively unimpaired individuals; Burggren et al (2008) demonstrated that middle-aged APOE ε4 homozygous carriers (57 years ± 7.8) had reduced cortical thickness percentage rates compared with non-ε4 carriers (57.7 years ± 9.6) in ERC (β = −14.8%, p = 1 × 10E-06) and the subiculum (β = −12.6%, p = 6.8 × 10E-05). In contrast, another study on middle-aged individuals (45.4 years ± 19) suggested no relationship between APOE ε4 status and subfield volume.31 The most recent study examined the annual rate of subfield volume change comparing ε4+ (73.02 years ± 4.98) and ε4 (73.9 years ± 5.38) elderly individuals.30 In line with previous studies, results indicated no volumetric differences at baseline. However, over the follow-up interval (5 time points over 1.5 years), the ε4 carriers showed a greater rate of volume loss in the right CA2/3 (−0.7%), CA4/DG (−0.4%), presubiculum (−0.4%), and subiculum (−0.5%) compared with the non-ε4 carriers.
In addition, 3 studies combined samples of cognitively unimpaired individuals and patients with dementia and impaired episodic memory. One study from Mueller et al (2008) showed significant effects of APOE ε4 on the CA3/DG in older individuals (aged 61–85 years) but not in younger individuals (aged 28–60 years), indicating that APOE ε4 carriers have smaller CA3/DG volumes than noncarriers (β = −20.3, p = 0.042). Significant effects for APOE ε4 on the CA3/DG were also found in a subgroup of older subjects with AD. Specifically, AD with APOE ε4 presented smaller CA3/DG volumes than AD without ε4 allele (β = −48.0, p = 0.027). An update of this study also showed a significant volume loss on CA3/DG for APOE ε4 carriers in healthy controls and AD. In addition, Kerchner et al (2014) showed a significant relationship between APOE ε4 allele and smaller CA1-SRLM volumes after controlling for dementia severity in a sample of elderly individuals.
SLC6AC, TOMM40, and APOE ε4
Besides the previous studies exploring the effect of APOE on hippocampal subfields, other studies based on cognitively unimpaired individuals evaluated the synergistic effect of the poly-T sequence polymorphism rs10524523 (TOMM40, translocase of outer mitochondrial membrane 40) and the 5-HTTLPR (SLC6A4, solute carrier family 6 member 4) polymorphism depending on APOE ε4 status.32,33 Garret et al (2015) proposed a fMRI study in which elderly participants with the s allele of the 5-HTTLPR had significantly different right CA1 activation during face-name recognition tasks (p = 0.019). In addition to activation differences, they investigated volumetric differences without finding main significant effects. However, volumes of the right subiculum showed a significant interaction between 5-HTTLPR and APOE ε4 alleles, suggesting that carriers of the l allele who were also carriers of the ε4 allele had larger subiculum volumes, whereas no effects were found in the s allele carriers. On the other hand, Burggren et al (2017) found a significant relationship between the rs10524523-L allele carriers (TOMM40) and thickness of the ERC only in subjects who did not carry the APOE ε4 allele: the L/L group showed significantly reduced thickness compared with both the S/L and S/S groups (β = −0.03, p < 0.001).
KIBRA Gene
Two studies analyzed the rs17070145 SNP within the KIBRA (kidney and brain) gene.34,35 Notably, Palombo et al. (2013) provided evidence that T-allele carriers were associated with larger CA1 (β = 65, p = 0.007) and DG/CA2/3 (β = 70, p = 0.049) in cognitively healthy young adults. In line with these results, Witte et al (2016) reported that T-allele carriers exhibited larger subfields than non–T-allele carriers within CA2/3 (β = 45.9, p = 0.008) and CA4/DG (β = 25.4, p = 0.008) in elderly cognitively healthy adults. In addition, higher microstructural integrity of the DG/CA4 dependent on T allele was found. T-allele carriers showed significant lower mean diffusivity values compared with non–T-carriers (β = −0.05, p = 0.005).
BDNF Gene
Three studies specifically explored the association between hippocampal subfield volumetry and BDNF (brain-derived neurotrophic factor) Val66Met (rs6265) polymorphism.36-38 Frodl et al (2014) showed significant interaction effects between BDNF Val66Met genotypes and childhood adversity within hippocampal subfields in a sample involving individuals diagnosed with MDD. On the one hand, the study highlighted that Met allele carriers with childhood adversity had smaller subfield volumes than Val allele homozygotes, where the most reduced volumes were specific for CA2/3 (β = −0.28, p = 0.015). In addition, Met-carrying healthy controls had larger volumes compared with Val/Val homozygotes in CA4/DG (β = −0.19, p = 0.036) and CA2/3 (β = −0.25, p = 0.006). In a similar line, Aas et al (2014) demonstrated that Met carriers reporting high levels of childhood trauma exhibited significantly reduced DG, CA2/3, and CA4 in a sample of patients with a broad DSM-IV schizophrenia spectrum disorder or bipolar disorder. Finally, Vilor-Tejedor et al (2020) found a significant effect of Met allele carriers on subiculum volumes in cognitively unimpaired individuals (β = 19.4, p = 0.013).
TESC Gene
One study found statistically significant associations between the intergenic rs7294919 (regulatory variant of TESC [tescalcin] gene) and the diagnosis of MDD in the volumes of CA4 (p = 0.004) and DG (p = 0.006).39 Specifically, C allele carriers diagnosed with MDD had smaller volumes of CA4 and DG than healthy C allele carriers. Moreover, no significant differences in volumes were observed between patients with MDD and healthy controls, who were T-allele carriers.
IL Genes
One study explored differential associations between hippocampal subfield volumetry and candidate proinflammatory genetic variants rs16944 (IL-1β, interleukin 1 beta), rs1800795 (IL-6, interleukin 6), and APOE ε4 in healthy cognitively adults.40 The results suggested that rs16944-T carriers exhibited significantly smaller CA1/2, CA3-DG, and subiculum compared with rs16944-C carriers, who were subsequently more likely to have reduced proinflammatory activity.
Frontotemporal Dementia Genes (MAPT, C9orf72, and GRN)
An additional study investigated differential patterns of hippocampal volume reduction in 3 different samples of patients who were symptomatic carriers of autosomal mutations of frontotemporal dementia.41 They found that the group carrying mutations at the MAPT (microtubule-associated protein tau) had the most atrophied hippocampal proper (region that comprises the subfields CA1/2/3/4) with an average volume loss of around 25% compared with a sample of controls. Moreover, the group with mutations at C9orf72 (guanine nucleotide exchange) showed the most atrophic patterns in CA4 (−6%), CA1 (−9%), and DG (−4%), and the group with mutations at the GRN (granulin precursor) showed the largest differences from controls in the presubiculum (−14%) and subiculum (−10%).
Other Candidate Genetic Variants
Finally, 1 study examined the top genetic variants associated with whole hippocampal volume for influence on hippocampal subfields in healthy adults from the general population.42 They found that the rs77956314-C allele presented the greatest effect in the right hippocampal tail (β = −13.36, p = 1.27E-08). In addition, the rs61921502 T allele showed strong lateral effects in the right hippocampal fissure (β = 3.97, p = 6.45E-09) and in the right CA1 (β = 10.47, p = 7.34E-09). The rs7020341-C allele showed the greatest bilateral effects in the subiculum (right: β = −4.94, 1.42E-08; left: β = −5.13, p = 1.59E-08). They did not find additional significant effects considering a p value threshold of 10E-08.
Genome-wide Association Studies
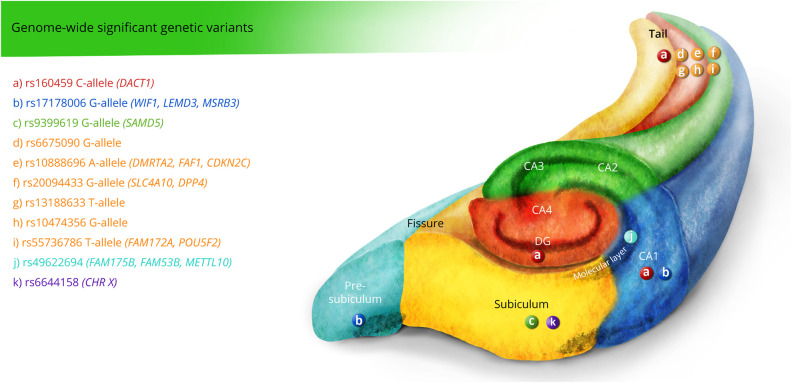
To date, only 1 genome-wide association study (GWAS) specifically based on hippocampal subfields has been assessed (N = 21,297 individuals, 16 cohorts),23 whereas none of the genetic candidate variants evaluated in the previous studies were reported as genome-wide significant (figure 4). They found 10 genome-wide significant loci among 6 subfields that were associated with neuronal differentiation, motor behavior, schizophrenia, and AD. Notice that none of the significant loci in Hibar et al (2017) were replicated in van der Meer et al (2018). In addition, a recent study using the UKBiobank data43 conducted a multimodal brain imaging genetic study (autosomal chromosome GWAS, sex-specific X-chromosome GWAS, and cluster analysis by chromosome) for 3,144 brain features.44 Interestingly, a peak association between rs6644158-A (chromosome X) was associated with the left subiculum (β = 0.018, p replication = 0.026).
Figure 4. Summary of Genome-wide Significant Genetic Variants Associated With Hippocampal Subfields.
E-legend: links.lww.com/NXG/A420. Circle, main genetic effect; star, interactive effect; wave, significant activation.
Polygenic Risk Scores
We found 1 study assessing the influence of the genetic risk of schizophrenia on hippocampal subfield volumes, which report nonsignificant results.45 Another study explored the association between PRS for 4 reproductive hormones (estradiol, progesterone, prolactin, and testosterone) and subfield volumetry in a sample of MDD with an average age of 50 ± 8.1.46 The study reported that PRS for estradiol predicted reduced volumes of the right and left subiculum (right: β = −2,394.58, p = 0.034; left: β = −2,284.7, p = 0.049), DG (right: β = −2,398.3, p = 0.034; left: β = −3,062.294, p = 0.011), molecular layer of the DG (right: β = −2,690.30, p = 0.034, left: β = −2,774.238, p = 0.013), and CA4 (right: β = −2,451.74, p = 0.034; left: β = −2,860, p = 0.013).
Finally, one study47 proposed a genetic risk score based on the number of risk alleles of rs11136000 (C allele) (CLU; clusterin), rs3851179 (T allele) (PICALM; phosphatidylinositol binding clathrin assembly protein gene), and the number of APOE ε4 alleles weighted by family history of AD (GRS-AD) on a sample of individual from the general population. Although GRS-AD correlated strongly with the percentage change in thickness across the whole hippocampal complex (β = −0.25, p = 0.048) and ERC (β = −0.35, p = 0.009), no significant results were found between the risk scores and thickness in any specific hippocampal subfield.
DNA Methylation
Finally, 1 study investigated the association between DNA methylation in the peripheral blood of nonpsychotic outpatients with MDD and that of healthy controls in the promoter region of the glucocorticoid receptor gene (NR3C1) and hippocampal subfield volumes.48
The results indicated that patients with MDD exhibited significant correlations of NR3C1 promoter methylations with bilateral CA2/3 and CA4/DG, whereas in healthy controls, methylations exhibited significant correlations with the subiculum and presubiculum. In addition, in patients with MDD, they observed that volumes of the right CA2/3 and hippocampal fissure were significantly correlated with the methylation in 2 CpG sites of the rs7294919 region.
Discussion
We provide a review of the state of the art on genetic studies of hippocampal subfields. Thanks to current brain imaging techniques for acquisition and processing, studies involving hippocampal subfields are becoming increasingly popular. Through the discovery of biologically meaningful genetic risk variants, such studies aim at disentangling their specific role in neurodevelopmental and neurodegenerative processes.
In all these studies, statistical power becomes a critically limiting factor as genetic effects on hippocampal subfields are typically small and on the edge of detection. This situation is further aggravated in GWASs due to the need to correct for multiple comparisons that negatively influences statistical power. Larger samples are the most straightforward way to increase statistical power and may be achieved by joining efforts in global consortia (e.g., The Enhancing NeuroImaging Genetics through Meta-Analysis Consortium),49 the UNITED Consortium,50 or by using publicly available data sets, such as the Alzheimer's Disease Neuroimaging Initiative51 and data from biobanks.52
Statistical power can also be enhanced by improving the accuracy and precision of hippocampal subfield volume estimates. This underlines the important role of automated segmentation methods, and their harmonization, in particular to foster comparability across studies.53 It is important to note that several studies also tested the reliability of automated segmentation methods (FreeSurfer) within and across scanners, reporting stable and reliable estimations of hippocampal subfields volumes.54,55 However, the accuracy of hippocampal subfield volume estimates also depends on MRI quality, resolution, and the use of multiple pulse sequences.
Likewise, a lack of sequence harmonization and optimization would lead to inconsistent delineation of subfield boundaries and/or yield poor statistical power to detect any existing effects.56 Moreover, several concerns regarding the utility and validity of hippocampal subfields segmentation in 1 × 1 × 1 mm3 MRI for volumetric studies have been reported.57
This effect may partially explain some of the mixed results found in the literature. Moreover, because the image quality of an MRI scan depends on signal and magnetic field strength, the quantification of hippocampal subfields also depends on the magnetic field strength of the MRI scan. For instance, this applies to CA4, which lies within the DG. This is also the case of CA2/3, which are usually considered jointly due to lack of contrast in most MRIs.
Furthermore, it is important to note that only 2 of the published studies have exclusively focused on longitudinal changes influenced by genetic component; one explored changes on hippocampal subfield volumes over a 5-year follow-up period,30 whereas the other investigated complex gray matter thickness changes in hippocampal subfields at 2 points in time.47 Longitudinal models allow us to estimate not only the mean values of hippocampal subfield volumes at the baseline but also their rates of change because of aging or other factors, including a group-age interaction. As shown in Ref. 58, longitudinal models help extract true biological variance, specifically in hippocampal volume. This might provide unique insights into temporal dynamics of the biological characterization of neurodegenerative diseases, which, in turn, represents a closer link to underlying pathologic processes.
Another point to emphasize is the importance of the population under study. First, because certain genetic patterns could have selective vulnerability depending on distinct pathologies, which in turn are not expected in healthy individuals, and second, because pathology can impact on hippocampal subfields by other nongenetic mechanisms.
Also relevant is that twin/family-based heritability estimates of hippocampal subfields tend to be higher than DNA-based estimates. These results are in line with the previous literature and suggest the influence of additional factors. For instance, the effect of aging, nongenetic factors, and/or causal variants not captured on the genotyping array or through imputation. Moreover, although no statistically significant, some studies showed greater heritability for subfields in the right hemisphere. Thus, future work on hippocampal subfields would benefit from providing information on each hemisphere even if laterality is not the focus of the study.
Moreover, all the studies included in this review were performed on individual hippocampal subfield volumes avoiding the correlation structure among them. This strategy may produce a loss in power. Future work could benefit from jointly analyzing hippocampal subfields in multiple-phenotype genetic association studies.
An additional critical challenge in genetic studies of hippocampal subfields consists in building models of genetic effects with greater complexity. To date, none of the reviewed studies has jointly examined a single pair of sources of genetic data. The integration of multimodal sources of biological information at multiple levels may facilitate a better understanding of the molecular function underlying hippocampal subfields volumes (e.g., GWAS, gene expression, and methylation), holding the potential to amplify the synergistic value of IG studies.59 Yet, this represents an extra burden on data dimensionality and may therefore require the development of novel analytical methods.60
Following a similar line of reasoning, designs focused on the integration of imaging gene-environmental interactions open up new perspectives of analysis potentially leading to a better understanding of the conditional mechanisms through which genes and environment interact to exert an impact on the hippocampal subfields and may develop further into neurodegenerative diseases.61 Such designs represent an opportunity, not only to integrate different sources of omics and imaging features but also to integrate target environmental sources relevant to the brain's structure and function and in particular in hippocampal subfields.62
Study Funding
N. Vilor-Tejedor is funded by a postdoctoral grant, Juan de la Cierva Programme (FJC2018-038085-I), Ministerio de Ciencia, Innovación y Universidades Spanish State Research Agency. In addition, N. Vilor-Tejedor has been partially supported by the European Molecular Biology Organization (EMBO) Postdoctoral Fellowships. H.H. Adams was supported by ZonMW grant number 916.19.151. J.M. González-de-Echávarri holds a Ramón y Cajal fellowship (RYC-2013-13054). All CRG authors acknowledge the support of the Spanish Ministry of Science, Innovation and Universities to the EMBL partnership, the Centro de Excelencia Severo Ochoa, and the CERCA Programme/Generalitat de Catalunya. All BBRC authors research received the support of la Caixa Foundation (LCF/PR/GN17/10300004) and the Health Department of the Catalan Government (Health Research and Innovation Strategic Plan [PERIS] 2016-2020 grant SLT002/16/00201). This research has been also supported by EU COST Action 15120 Open Multiscale Systems Medicine (OpenMultiMed) and the Alzheimer Nederland Project (WE.15-2019-09).
Glossary
- AD
Alzheimer disease
- ASHS
automatic segmentation of hippocampal subfields
- CA
cornu ammonis
- DG
dentate gyrus
- GWAS
genome-wide association study
- MAGeT
Multiple Automatically-GEnerated Templates
- MDD
major depressive disorder
- SNP
single nucleotide polymorphism
- SRLM
subiculum and stratum radiatum/stratum lacunosum/stratum moleculare
Appendix. Authors
Disclosure
N. Vilor-Tejedor, T.E. Evans, H.H. Adams, and J.M. González-de-Echávarri report no disclosures. J.L. Molinuevo has served/serves as a consultant or at advisory boards for the following for-profit companies or has given lectures in symposia sponsored by the following for-profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, and ProMIS Neurosciences. R. Guigo, J.D. Gispert, and G. Operto report no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.García JC, Bustos RH. The genetic diagnosis of neurodegenerative diseases and therapeutic perspectives. Brain Sci. 2018;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santiago JA, Bottero V, Potashkin JA. Dissecting the molecular mechanisms of neurodegenerative diseases through network biology. Front Aging Neurosci. 2017;9:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer MC, Hall PH, Morgan JC. Accuracy of clinical diagnosis of Alzheimer's disease in Alzheimer's Disease Centers (ADCS). Alzheimers Dement. 2017;13:P800-P801. [Google Scholar]
- 4.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hustad E, Skogholt AH, Hveem K, Aasly JO. The accuracy of the clinical diagnosis of Parkinson disease. The HUNT study. J Neurol. 2018;265:2120-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818-827. [DOI] [PubMed] [Google Scholar]
- 8.Munafò MR, Flint J. The genetic architecture of psychophysiological phenotypes. Psychophysiology. 2014;51:1331-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggleton JP. Looking beyond the hippocampus: old and new neurological targets for understanding memory disorders. Proc Biol Sci. 2014;281:20140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisman J, Buzsáki G, Eichenbaum Het al. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017;20:1434-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu H, Hardy J, Duff KE. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21:1350-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elman JA, Panizzon MS, Gillespie NAet al. Genetic architecture of hippocampal subfields on standard resolution MRI: how the parts relate to the whole. Hum Brain Mapp. 2019;40:1528-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iglesias JE, Augustinack JC, Nguyen Ket al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desikan RS, Ségonne F, Fischl Bet al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968-980. [DOI] [PubMed] [Google Scholar]
- 15.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050-11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sämann PG, Iglesias JE, Gutman B, et al. FreeSurfer-based segmentation of hippocampal subfields: a review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum Brain Mapp. Published online December 27, 2020. doi: 10.1002/hbm.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarty MM, Steadman P, van Eede MCet al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp. 2013;34:2635-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pipitone J, Park MT, Winterburn Jet al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 2014;101:494-512. [DOI] [PubMed] [Google Scholar]
- 19.Yushkevich PA, Pluta JB, Wang Het al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015;36:258-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenspan KS, Arakelian CR, van Erp TGM. Heritability of hippocampal formation sub-region volumes. J Neurol Neurosci. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S, Park MTM, Devenyi GAet al. Heritability of hippocampal subfield volumes using a twin and non-twin siblings design. Hum Brain Mapp. 2017;38:4337-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelan CD, Hibar DP, van Velzen LS, et al. Heritability and reliability of automatically segmented human hippocampal formation subregions. Hum Brain Mapp. 2016;128:125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Meer D, Rokicki J, Kaufmann T, et al. Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatry. 2018;25:3053-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roshchupkin GV, Gutman BA, Vernooij MWet al. Heritability of the shape of subcortical brain structures in the general population. Nat Commun. 2016;7:13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burggren AC, Zeineh MM, Ekstrom ADet al. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41:1177-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerchner GA, et al. APOE 4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology. 2014;82:691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. Neuroimage. 2008;42:42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiter K, Nielson KA, Durgerian Set al. Five-year longitudinal brain volume change in healthy elders at genetic risk for Alzheimer's disease. J Alzheimers Dis. 2017;55:1363-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voineskos AN, Winterburn JL, Felsky Det al. Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan. Hum Brain Mapp. 2015;36:3020-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burggren AC, Mahmood Z, Harrison TMet al. Hippocampal thinning linked to longer TOMM40 poly-T variant lengths in the absence of the APOE ε4 variant. Alzheimers Dement. 2017;13:739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrett A, Gupta S, Reiss ALet al. Impact of 5-HTTLPR on hippocampal subregional activation in older adults. Transl Psychiatry. 2015;5:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palombo DJ, Amaral RS, Olsen RKet al. KIBRA polymorphism is associated with individual differences in hippocampal subregions: evidence from anatomical segmentation using high-resolution MRI. J Neurosci. 2013;33:13088-13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witte AV, Köbe T, Kerti L, Rujescu D, Flöel A. Impact of KIBRA polymorphism on memory function and the Hippocampus in older adults. Neuropsychopharmacology. 2016;41:781-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aas M, Haukvik UK, Djurovic Set al. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. J Psychiatr Res. 2014;59:14-21. [DOI] [PubMed] [Google Scholar]
- 37.Frodl T, Skokauskas N, Frey EMet al. BDNF Val66Met genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp. 2014;35:5776-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilor-Tejedor N, Operto G, Evans TE, et al. Effect of BDNF Val66Met on hippocampal subfields volumes and compensatory interaction with APOE-ε4 in middle-age cognitively unimpaired individuals from the ALFA study. Brain Struct Funct. 2020;225:2331-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han KM, Won E, Kang Jet al. TESC gene-regulating genetic variant (rs7294919) affects hippocampal subfield volumes and parahippocampal cingulum white matter integrity in major depressive disorder. J Psychiatr Res. 2017;93:20-29. [DOI] [PubMed] [Google Scholar]
- 40.Raz N, Daugherty AM, Bender AR, Dahle CL, Land S. Volume of the hippocampal subfields in healthy adults: differential associations with age and a pro-inflammatory genetic variant. Brain Struct Funct. 2015;220:2663-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bocchetta M, Iglesias JE, Scelsi MAet al. Hippocampal subfield volumetry: differential pattern of atrophy in different forms of genetic frontotemporal dementia. J Alzheimers Dis. 2018;64:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibar DP, Adams HHH, Jahanshad Net al. Novel genetic loci associated with hippocampal volume. Nat Commun. 2017;8:13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bycroft C, Freeman C, Petkova Det al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, Douaud G, Chen W, et al. Enhanced brain imaging genetics in UK biobank. bioRxiv doi: 10.1101/2020.07.27.223545. [DOI]
- 45.Papiol S, Popovic D, Keeser Det al. Polygenic risk has an impact on the structural plasticity of hippocampal subfields during aerobic exercise combined with cognitive remediation in multi-episode schizophrenia. Transl Psychiatry. 2017;7:e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smeeth DM, Dima D, Jones Let al. Polygenic risk for circulating reproductive hormone levels and their influence on hippocampal volume and depression susceptibility. Psychoneuroendocrinology. 2019;106:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison TM, Mahmood Z, Lau EP, et al. An Alzheimer's disease genetic risk score predicts longitudinal thinning of hippocampal complex subregions in healthy older adults. eNeuro. 2016;3;ENEURO.0098-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Na KS, Chang HS, Won Eet al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One. 2014;9:e85425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson PM, Stein JL, Medland SEet al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams HH, Evans TE, Terzikhan N. Call for collaborators in the UNITED consortium: uncovering neurodegenerative insights through ethnic diversity. Lancet Neurol. 2019;18:P915. [DOI] [PubMed] [Google Scholar]
- 51.Weiner MW, Veitch DP, Aisen PSet al. The Alzheimer's Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement. 2017;13:561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott LT, Sharp K, Alfaro-Almagro Fet al. Genome-wide association studies of brain imaging phenotypes in UK biobank. Nature. 2018;562:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giuliano A, Donatelli G, Cosottini Met al. Hippocampal subfields at ultra high field MRI: an overview of segmentation and measurement methods. Hippocampus. 2017;27:481-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quattrini G, Pievani M, Jovicich Jet al. Amygdalar nuclei and hippocampal subfields on MRI: test-retest reliability of automated volumetry across different MRI sites and vendors. Neuroimage. 2020;218:116932. [DOI] [PubMed] [Google Scholar]
- 55.Brown EM, Pierce ME, Clark DCet al. Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage. 2020;210:116563. [DOI] [PubMed] [Google Scholar]
- 56.Wisse LEM, Daugherty AM, Olsen RK, et al. A harmonized segmentation protocol for hippocampal and parahippocampal subregions: why do we need one and what are the key goals? Hippocampus. 2017;27:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wisse LEM, Chételat G, Daugherty AM, et al. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: a note of caution. Hum Brain Mapp. 2021;42:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams HHH. Complex Neurological Diseases: Insights From Genetics and Neuroimaging—Complexe neurologische ziekten: Inzichten vanuit de genetica en beeldvorming van de hersenen. Erasmus University Rotterdam; 2016. hdl.handle.net/1765/94114 [Google Scholar]
- 59.Meyer-Lindenberg A. The future of fMRI and genetics research. Neuroimage. 2012;62:1286-1292. [DOI] [PubMed] [Google Scholar]
- 60.Vilor-Tejedor N, Alemany S, Cáceres A, et al. Strategies for integrated analysis in imaging genetics studies. Neurosci Biobehav Rev. 2018;93:57-70. [DOI] [PubMed] [Google Scholar]
- 61.Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene–environment interactions. Trends Cogn Sci. 2011;15:417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabl U, Meyer BM, Diers Ket al. Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci. 2014;34:9917-9926. [DOI] [PMC free article] [PubMed] [Google Scholar]