Summary
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are neuroendocrine tumors that express somatostatin receptors (SSTRs), a phenomenon that constitutes a basis for tumor imaging and treatment with somatostatin analogues and peptide receptor radionuclide therapy. We studied the immunohistochemical expression of SSTR1-5 in 151 primary tumors, including 14 metastasized and 16 SDHB-deficient tumors. SSTR2 and SSTR3 were most abundantly present in these tumors, whereas the tumors were mostly negative for SSTR1, SSTR4, and SSTR5. All metastasized PGLs (9/9), but only one metastasized PHEO (1/5), were strongly SSTR2 positive. SSTR3 expression was lower in metastatic tumors and tumors with a high proliferation rate (MIB1 ≥ 5%), but tumors had variable individual SSTR profiles. No correlation was found between SDE1B status and SSTR expression. Our results suggest that new SSTR analogues with affinity for several SSTRs could be relevant for a subgroup of patients with these tumors. Better knowledge of tumor SSTR profiles could open the door for personalized imaging and treatment in the future. Because SSTR profiles vary in PHEOs and PGLs, individual analysis is required for each tumor.
Keywords: Somatostatin receptors, Pheochromocytoma, Paraganglioma, Metastatic, Succinate dehydrogenase B, Immunohistochemistry
1. Introduction
PHEOs and PGLs are rare NETs that develop from the neural crest. PHEOs are intra-adrenal tumors, whereas extra-adrenal PGLs arise from the sympathetic or parasympathetic paraganglia [1]. All PHEOs and PGLs are thought to have some metastatic potential, and approximately 10% of PHEOs and 15% to 40% of PGLs metastasize [2–4]. Predicting primary tumor outcomes is uncertain and controversial; however, increased knowledge of the heterogeneous and variable genetic backgrounds of PHEOs and PGLs that are associated with different pathogenesis is promising for the prediction of prognosis and treatment effects. Nevertheless, the therapeutic options for metastasized PHEOs and PGLs remain limited.
SSTRs are peptide hormone and G-protein–coupled membrane receptors. Five different SSTR subtypes, SSTR1-5, can manifest in various combinations in human tumors [5]. In normal endocrine and neural tissues, such as the adrenal gland, pituitary gland, and pancreatic islets, SSTR expression is low [6]. SSTR overexpression has been observed in NETs, including PHEOs and PGLs [5,7]. SSTR2 demonstrates the highest expression in several NETs [6], and grade 3 neuroendocrine neoplasias can also express SSTR2 [8]. Overexpression of SSTRs constitutes a basis for the use of somatostatin analogues in imaging and therapeutic interventions for NETs [9,10]. The most commonly used somatostatin analogue, octreotide, has a high affinity for SSTR2 and a low affinity for SSTR3 and SSTR5 [6]. Somatostatin inhibits hormone secretion and exhibits antiproliferative and antiangiogenic effects in NETs [6]. New somatostatin analogues with affinity for several SSTRs have been tested and considered for clinical use. In addition, new specific monoclonal antibodies against SSTRs have been developed, which can help us obtain immunohistochemical information on tumor receptor status and receptor density [7,11].
The mitochondrial enzyme complex SDH participates both in the Krebs cycle and in the electron transport chain. The SDHx complex consists of 4 subunits, SDHA, SDHB, SDHC, and SDHD, and 2 assembly factors, SDHAF1 and SDHAF2 [12]. Five clinical syndromes, PGL syndromes 1-5, are known to associate with different SDHx mutations. SDHB mutation is associated with PGL syndrome 4. The risk of metastasis is approximately 30% in PGLs and PHEOs with SDHB mutations [13]. Thus, SDHB mutation is one of the strongest factors that predicts the metastatic potential of these tumors [14]. Mutation in some components of the SDHx complex causes false assembly, which leads to loss of the SDHB protein and provides a rationale to use IHC to detect mutations [12,15].
The aim of the study was to clarify the potential roles of 5 different SSTRs in PHEOs and PGLs, especially in metastasized and SDHB-negative tumors. There was additional interest in the correlations between immunohistochemical SSTR expression, SSTR-specific imaging findings, and the treatment response to SSTR radionucleotide therapy.
2. Materials and methods
2.1. Patients and samples
This study included 151 formalin-fixed, paraffin-embedded primary PHEO and PGL samples from 146 patients who were treated during the years 1973 to 2009 at Helsinki University Hospital. The mean follow-up time was 14.2 years and median was 13.9 years. Tissue samples were collected from the archives of the Department of Pathology. Clinical data were collected from hospital records and survival data from the Finnish Population Register Center. The cause of death was received from Statistics Finland. This study was approved by the local ethics committee (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013) and the National Supervisory Authority of Welfare and Health (TEO Dnro 10 041/06.01.03.01/2012).
Of 151 tumors, 127 were PHEOs and 24 were PGLs. Metastatic disease was observed in 13 patients with 14 tumors. Metastases were confirmed either histologically or radiologically via I-metaiodobenzylguanidine or somatostatin-receptor scintigraphy. Demographic data for the metastasized cases are shown in Table 1. One patient exhibited a thoracic and a retroperitoneal tumor and multiple lung metastases. Of the metastatic tumors, 5 were PHEOs, 7 were retroperitoneal PGLs, 1 was an intrathoracic PGL, and 1 was a carotid body PGL.
Table 1.
Demographic data and ICH staining results in 13 patients with metastatic disease
| Patient no. | Sex and age at the diagnosis (y) | Organ | SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 | SDHB-IHC (+/−) | Mutations tested | Location of the metastases |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/63 | Retroperitoneum | − | ++ | + | − | − | + | NA | Bone, lymph nodes, liver, lung (R) |
| 2 | F/57 | Retroperitoneum (pancreas) | + | ++ | ++ | − | − | + | No known mutations a | Liver, lymph nodes (R) |
| 3 | M/53 | Retroperitoneum | − | ++ | + | − | + | − | NA | Bone (H), pleural (R) |
| 4 | F/46 | Neck | − | ++ | − | − | − | − | NA | Lymph node, lung (H) bone (R) |
| 5 | F/24 | Thorax year 1973 | + | ++ | + | NA | − | − | SDHB | Lung (R), retroperitoneum (H) |
| The same patient as above | Retroperitoneum year 1998 | − | ++ | − | − | − | − | SDHB | Lung (R), retroperitoneum (H) | |
| 6 | M/31 | Retroperitoneum | − | ++ | ++ | − | − | − | SDHB | In the liver hilus, between the liver and the kidney (H) |
| 7 | M/48 | Retroperitoneum | + | ++ | − | − | − | − | NA | Bone (H) |
| 8 | M/39 | Retroperitoneum | + | ++ | ++ | − | − | − | SDHB | Bone (R), probably brain (H) |
| 9 | M/51 | Adrenal | − | ++ | + | − | − | + | NA | Lung, brain (R) |
| 10 | M/45 | Adrenal | + | − | − | − | − | + | NA | Lung (R), lymph nodes (H) |
| 11 | M/69 | Adrenal | − | − | ++ | − | − | + | NA | Lymph node (H) |
| 12 | M/19 | Adrenal | − | + | + | − | − | + | NF 1 | Bone, peritoneum, mesenterium (H) |
| 13 | M/28 | Adrenal | − | − | − | − | − | + | No (VHL, RET, SDHB) | Peritoneum, liver, omentum (H) bone (R) |
| SSTR scintigraphy | Time for the first metastasis (mo) | MIB-1 (%) | Alive/dead | Follow-up (y) | ||||||
| 111In-Octr SPECT-γ positive, bone, lymph nodes | 14 | 6 | Died of disease | 6 | ||||||
| 68Ga-Dotanoc-PET-CT positive liver, pancreas lymph nodes | 96 | 9 | Alive with the disease | 12 | ||||||
| Somatostatin γ-SPECT positive bone, pleura, left orbit, paravertebral | 0 | 12 | Died of the disease | 2,5 | ||||||
| 68Ga-Dotanoc-PET-CT positive neck, bone, lymph nodes, lung | 0 | 4 | Alive with the disease | 17 | ||||||
| NA | 430 | 3 | Died of the disease | 41 | ||||||
| NA | Please see above | 4 | Died of the disease | Please see above | ||||||
| NA | 106 | 1 | Died of other causes | 22 | ||||||
| NA | 0 | 8 | Died of other causes | 8 | ||||||
| NA | 14 | 1 | Died of the disease | 15 | ||||||
| NA | 0 | 8 | Died of the disease | 1 | ||||||
| NA | 57 | 3 | Died of the disease | 19 | ||||||
| NA | 0 | 10 | Died of the disease | 0 | ||||||
| NA | 92 | 10 | Died of the disease | 30 | ||||||
| 68Ga-Dotanoc PET-CT positive right adrenal, bone, omentum | 55 | 3 | Died of the disease | 17 | ||||||
2.2. TMA blocks
To prepare TMA blocks, hematoxylin and eosin slides were reevaluated and representative areas were selected from the primary tumor. Three 1-mm tumor punctures were drawn from the corresponding donor tissue blocks and inserted into a recipient paraffin block with a semiautomatic tissue microarrayer (MTABooster Version 1.01 for Beecher Manual Arrayer; Alphelys Beecher Instruments, Silver Spring, MD). One section was cut from each TMA block to obtain 3 spots per tissue sample for immunohistochemical analysis. Correct sampling was controlled histologically through hematoxylin and eosin and chromogranin A staining.
2.3. Immunohistochemistry
TMA slides (4 μm) were pretreated in a pretreatment module (LabVision UK Ltd, Suffolk, United Kingdom) with citrate buffer at pH 6.0 (SSTR1, SSTR3-5) or with Tris-HCl buffer at pH 8.5 (MIB-1, chromogranin A). For the SSTR2 antibody, pretreatment was performed using a Cell Conditioning Solution (CC1) in Benchmark XT (Roche, Tucson, AZ). Details regarding the antibodies, including dilutions, are shown in Table 2. Antigens were detected using the Envision peroxidase-conjugated polymer kit (Agilent, Santa Clara, CA) in an Autostainer 480 (LabVision Thermo Scientific, UK Ltd, Cheshire, United Kingdom), except for SSTR2, which was detected using the Optiview DAB kit in a Benchmark XT stainer (Roche, Tucson, AZ). The slides were counterstained with Mayer hematoxylin (Lillie’s Modification; Agilent) and mounted using Eukitt mounting medium (Sigma-Aldrich, St Louis, MO). SDH immunohistochemical staining was performed as previously described [16] with the SDHB-antibody 21A11 (Abcam, Cambridge, United Kingdom) and SDHA antibody 5A11 (Abcam).
Table 2.
Used antibody, clone, company, dilution, and scoring
| Antibody | Clone | Company | Dilution (incubation time) | Scoring |
|---|---|---|---|---|
| SSTR1 | sstr1 (monoclonal) | AbD Serotec, Oxford, United Kingdom | 1:500 (30 min) | 0, 0; 1, <10%, weak cytoplasmic positivity; 2, ≥10% weak cytoplasmic positivity; 3, moderate cytoplasmic positivity; 4, strong cytoplasmic positivity a |
| SSTR2 | UMB-1 (monoclonal) | Abcam | 1:300 (30 min) | 0, 0; 1, <10%, not totally circumferential membrane positivity; 2, ≥10% not totally circumferential membrane positivity; 3, strong circumferential membrane positivity in 10%-94% of tumor cells; 4, strong circumferential positivity in ≥95% of tumor cells b |
| SSTR3 | UMB-5 (monoclonal) | Abcam | 1:7000 (60 min) | Intensity of cytoplasmic staining 0, 0; 1, weak; 2, moderate; 3, strong If <10% of tumor cells positive, intensity downgraded by 1 grade c |
| SSTR4 | sstr4 (monoclonal) | AbD Serotec, Oxford, United Kingdom | 1:500 (30 min) | Same as SSTR1 |
| SSTR5 | sstr5 (monoclonal) | Bio-Rad, Oxford, United Kingdom | 1:1000 (30 min) | 0, 0; 1, <10%,weak cytoplasmic positivity; 2, ≥10% weak cytoplasmic positivity; 3, moderate cytoplasmic or some membrane positivity; 4, strong cytoplasmic positivity or membrane positivity a |
| Ki-67 | MIB1/M7240 (monoclonal) | Agilent | 1:100 (60 min) | Percentage of positive cells |
| Chromogranin A | Rabbit polyclonal | Agilent | 1:2000 (30 min) | Positive/negative |
| SDHB | 21A11 (mouse monoclonal) | Abcam | 1:1000 | Positive/negative |
| SDHA | 5A11 (mouse monoclonal) | Abcam | 1:1000 | Positive/negative |
For statistics, the scores for SSTR1, SSTR4, andSSTR5 were grouped into 3: negatives (score 0 and 1), intermediate positive (scores 2 and 3), and strongly positive (score 4).
For statistics, the SSTR2 scores were grouped into 3: negative (scores 0 and 1), intermediate positive (score 2), and strongly positive (scores 3 and 4).
For statistics, the SSTR3 scores were grouped into 3: negative (score 0), intermediate positive (score 1), and strongly positive (scores 2 and 3).
2.4. Scoring of IHC
The scoring for SSTR1-5 is in Table 2. For SSTR2, only membrane positivity was scored using a semiquantitative scheme that was modified from Elston et al [7] Körner et al [17].
SDHB and SDHA staining results were positive and consistent with an intact SDH complex when granular cytoplasmic staining was present and negative when the tumor cells stained negative. Endothelial cells served as the positive internal control.
The PI was determined via immunohistochemical staining with the MIB1 antibody. The proportion of positive tumor cells was calculated.
All scoring was done independently by 2 experienced pathologists (M. M. and H. L. for SDHB and SDHA and J. H. and H. L. for all other antibodies) without knowledge of the clinical data. The consensus of the results was excellent for the SDHB staining (κ value = 0.97) and good for SSTR stainings (κ value = 0.80-0.85).
2.5. Statistics
For statistics, the scores for SSTR1, SSTR4, and SSTR5 were grouped into 3 groups: negative (scores 0 and 1), intermediate positive (scores 2 and 3), and strongly positive (score 4). The SSTR2 scores were grouped as well into negative (scores 0 and 1), intermediate positive (score 2), and strongly positive (scores 3 and 4); the SSTR3 scores were grouped into negative (score 0), intermediate positive (score 1), and strongly positive (scores 2 and 3). For each tumor, the highest score out of 3 spots was selected for statistical analysis. The association of immunohistochemical and clinicopathological variables was assessed using the χ2 test or Fisher exact test when applicable. A P value of less than .05 was regarded as statistically significant. Statistical analyses were performed using SPSS software version 22 (SPSS, Chicago, IL).
3. Results
3.1. SSTR1-5 in PHEOs and PGLs
Examples of SSTR1-5 staining are visualized in Fig. 1. SSTR1 staining was cytoplasmic. Most tumors were negative (score 0 or 1) for SSTR1, including 66.7% (16/24) of PGLs and 85.8% (109/127) of PHEOs. Intermediate positivity was observed in 33.3% (8/24) of PGLs and 14.2% (18/127) of PHEOs. No strong SSTR1 positivity was observed. The greater abundance of SSTR1 positivity in PGLs than in PHEOs was statistically significant (P = .028).
Fig. 1.
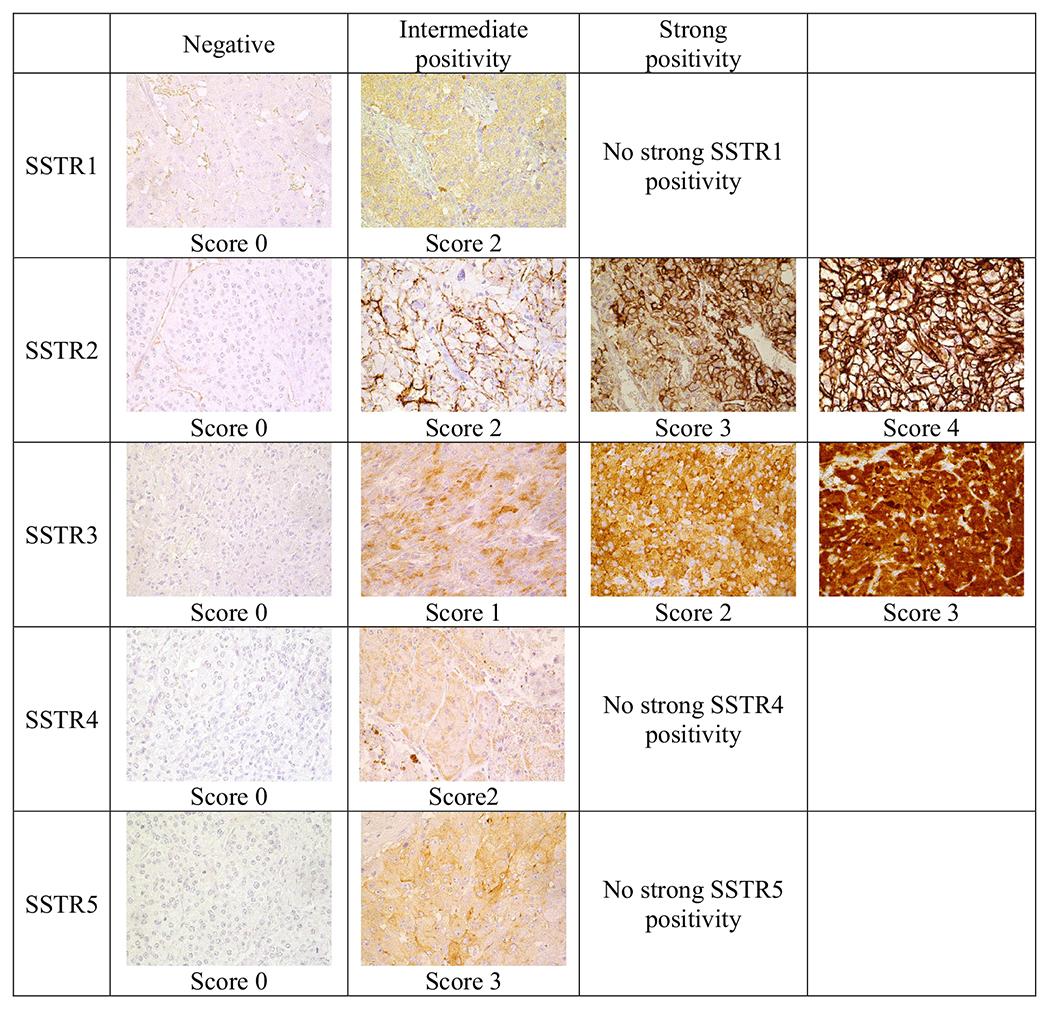
Examples of different SSTR1-5 scores. Original magnification ×400.
SSTR2 staining was nearly completely membranous. In addition, there was some diffuse cytoplasmic staining (Fig. 1). The intensity of staining and the proportion of stained cells varied. Most PGLs (66.7% [16/24]) and approximately half of PHEOs (54.3% [69/127]) exhibited strong (score 3 or 4) SSTR2 expression. Among all tumors, 74.8% (113/151) were intermediate or strongly SSTR2 positive. Three PGLs (12.5%) and 35 PHEOs (27.6%) were negative (score 0 or 1).
SSTR3 expression was mostly cytoplasmic and granular, and the intensity of staining and the proportion of stained cells varied (Fig. 1). Among PHEOs, 64.6% (82/127) were strongly positive (score 2 or 3), but 22.0% (28/127) of PHEOS were completely negative. Among PGLs, 62.5% (15/24) were strongly positive and 20.8% (5/24) were completely negative. Among all tumors, 64.2% showed strong SSTR3 positivity and 78.1% showed intermediate or strong SSTR3 positivity. More than half of all PGLs and PHEOs expressed strongly either SSTR3 or SSTR2. No statistically significant correlation was found between SSTR2 and SSTR3 expressions.
SSTR4 staining (only cytoplasmic) results were available for 147 tumors. Nearly all PHEOs (95.2% [119/125]) and all PGLs were negative for SSTR4. Among PHEOs, 4.8% (6/125) of tumors showed weak SSTR4 positivity in >10% of cells.
SSTR5 expression was nearly entirely cytoplasmic and scanty. Most PHEOs (83.5% [106/127]) and PGLs (91.7% [22/24]) were negative (score 0 or 1). Among all tumors, 15.2% exhibited intermediate SSTR5 positivity (score 2 or 3), including 16.5% (21/127) of PHEOs and 8.3% (2/24) of PGLs. The mean SSTR profiles for PHEOs and PGLs are visualized in Fig. 2. All SSTR scoring results are available in the Supplementary Table.
Fig. 2.
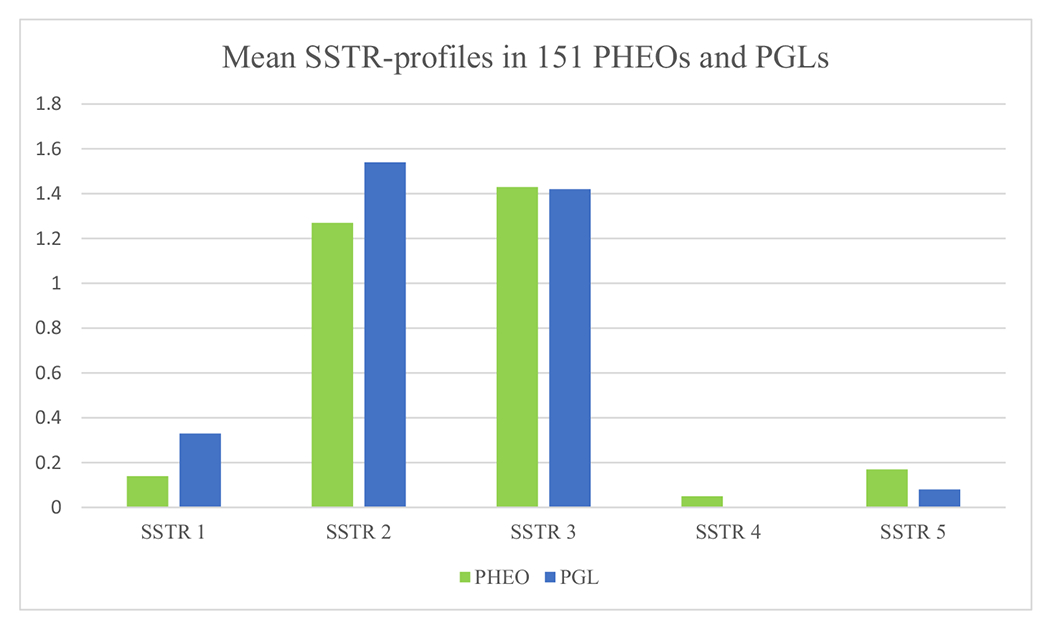
Mean SSTR1-5 profiles in 151 PHEOs and PGLs. Strong ICH staining, 2; intermediate, 1; and negative, 0.
3.2. SSTR2 and SSTR3 in metastasized versus nonmetastasized tumors
Most metastasized tumors (71.4% [10/14]) were strongly SSTR2 positive, but 3 metastasized PHEOs were SSTR2 negative (21.4%). Among nonmetastatic tumors, 54.7% (75/137) showed strong SSTR2 positivity and 25.5% (35/137) were negative. No correlation was found in SSTR2 positivity between metastatic and nonmetastatic tumors. The mean SSTR profiles for metastasized tumors are visualized in Fig. 3.
Fig. 3.
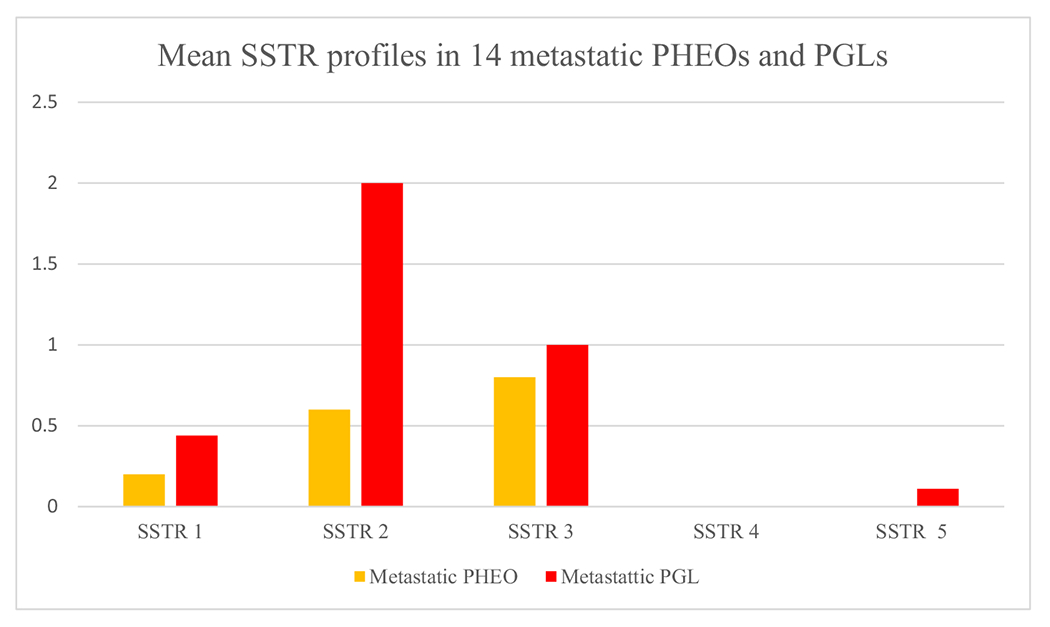
Mean SSTR1-5 profiles in 14 metastatic PHEOs and PGLs. Strong ICH staining, 2; intermediate, 1; and negative, 0.
Metastatic tumors (n = 14) exhibited significantly less (P = .005) SSTR3 positivity than did nonmetastatic tumors (n = 137). Among metastatic tumors, 42.9% (6/14) were completely SSTR3 negative, whereas 19.7% (27/137) of nonmetastasized tumors showed no SSTR3 positivity.
3.3. SSTR profiles in metastasized tumors
The SSTR results for all metastasized tumors are shown in Table 1. SSTR profiles differed considerably among tumors. Intermediate SSTR1 positivity was observed in 35.7% (5/14, 1 PHEOs and 4 PGLs) of metastatic tumors. SSTR2 expression was different in metastasized PGLs than in PHEOs. SSTR2 was strongly expressed in all metastatic PGLs but in only one metastatic PHEO, a case in which retroperitoneal origin could not be excluded. Among metastatic PHEOs, 3 of 5 were SSTR2 negative. The difference in SSTR2 expression was statistically significant between metastatic PHEOs and PGLs (P = .005).
Strong SSTR3 expression was observed in 28.6% (4/14) of metastatic tumors, in 1 PHEO and 3 PGLs. No SSTR4 positivity was found, and no widespread SSTR5 positivity was observed in metastatic tumors.
3.4. Correlation of SSTR expression with MIB1 and among SSTRs
PHEOs and PGLs that exhibited MIB1 ≥ 5% were more often negative or intermediate SSTR3 positive than tumors with MIB1 < 5% (P < .001). There was no correlation between SSTR1, SSTR2, SSTR4, and SSTR5 expression and proliferation. There was a correlation between SSTR2 and SSTR5. All SSTR2-negative tumors were also SSTR5 negative, and most SSTR5-negative tumors were SSTR2 positive (P = .003). Otherwise, there was no correlation among different SSTRs.
3.5. SDHB and SDHA staining results and correlation with SSTR1-5
SDHB-negative tumors (16/151) were all PGLs; 12 were retroperitoneal, 3 were thoracic PGLs, and 1 was a neck PGL. One patient had 2 SDHB-negative tumors; thus, 15 of 146 patients had SDHB-negative tumors (10.3%), and 6 of these had metastatic disease (40%). Patients with SDHB-negative tumors were younger. The median age of SDHB-negative patients was 36 years at diagnosis (range, 22-53 years), and that of SDHB-positive patients was 53 years (range, 17-87 years). SBHA-negative tumors were not found in this cohort.
SDHB-negative tumors did not exhibit significantly higher SSTR2 positivity than did SDHB-positive tumors. Of the 16 SDHB-deficient tumors, 12 showed strong SSTR2 staining and 2 showed intermediate SSTR2 staining; together, 87.5% (14/16) of the tumors expressed SSTR2. Among SDHB-sufficient tumors, 99 (73.3%) of 135 were strongly or intermediate positive for SSTR2. No correlations were detected among SSTR1, SSTR3, SSTR4, SSTR5, and SDHB staining. Among SDHB-negative tumors, strong SSTR3 positivity was seen in 9 (56.3%) and intermediate SSTR3 positivity in 3 (18.8%). Of SDHB-positive tumors, 65.2% showed strong SSTR3 positivity and 13.3% showed intermediate SSTR3 positivity.
3.6. Correlation between SSTR IHC status and SSTR scintigraphy or PRRT
Five patients with metastatic tumors underwent SSTR scintigraphy that showed positive imaging with metastases and possible tumor residue. Four of these showed strong SSTR2 IHC positivity in the primary tumor tissue.
Only 2 patients received PRRT therapy (Table 1, patients 1 and 4). Both patients had SSTR2-positive metastasized PGLs; however, only patient 4, who got 4 times Lu-177 DOTATATE therapy with 7.4 GBq:n activity, exhibited a good response in bone and lymph node metastases when lung metastases and primary tumor remained stable. Patient 1 got 2 times Lu-177 DOTATATE therapy with reduced dose without response.
4. Discussion
Our consecutive series of 151 PHEOs and PGLs with a long follow-up time, including 14 metastasized tumors, showed the most abundant immunohistochemical positivity with SSTR2 and SSTR3. The staining patterns varied; however, all metastasized PGLs were strongly positive for SSTR2, whereas most metastasized PHEOs were negative. SSTR3 was found less frequently in the metastatic cases, both in PHEOs and in PGLs. No correlation was observed between SSTRs and SDHB status.
Choice of scoring system for IHC is essential for reliable results. Atleast3 systems have been published [7,17,18], which are based on membranous staining of the SSTR2 antibody. No broad consensus for scoring SSTR1, 3-5 exist in the literature. Modified Elston scoring was used in our work because Elston [7] studied PHEOs and PGLs and they used same SSTR2 and SSTR3 antibodies as we did.
Positivity for SSTR2 in PHEOs and PGLs is consistent with previous reports [7,19,20]. Regarding SSTR3, we found mostly cytoplasmic and granular positivity in 78% of PHEOs and PGLs. Mundschenk et al [21] studied PHEOs and extra-adrenal PGLs from 36 patients and found SSTR3 immunopositivity in 90.4% with a similar staining pattern. However, other works have presented moderate or strong SSTR3 positivity in 26% of PHEOs and PGLs [7] and in 35% of PGLs [20]. In most reports, SSTR4 expression in PHEOs and PGLs is scant [7,20,21], which is consistent with our practically negative SSTR4 result. We found that 17% of tumors were intermediate SSTR1 positive and 15% were intermediate SSTR5 positive, whereas in previous reports, SSTR positivity varied from 8% to 89% for SSTR1 and 1% to 47% for SSTR5 [7,20,21]. This difference in results could be partly explained by the difference in the scoring systems. We used the same monoclonal antibody as Elston et al [7] and Kaemmerer et al [20] for SSTR2 and SSTR3 but different antibodies for the other SSTR subtypes. Differences in the antibodies used may also partly explain the difference in results. The patient series in all of these works are not very large because of the rarity of these tumors, and the possibility that the tumor materials have different profiles cannot be excluded. Kaemmerer et al studied only PGLs, whereas other studies included both PHEOs and PGLs. Nevertheless, the discrepancies among different reports emphasize the importance of antibody validation and standardization and call for further analysis and multicenter biobank research in this field.
Individual SSTR profiles in metastatic cases are of interest owing to their possible roles in imaging and the treatment of metastatic disease. Metastatic PGLs showed SSTR1-3 positivity in various combinations, which could open the door for imaging and individual targeted treatment with PRRT with affinity for several SSTRs. One PHEO showed only strong SSTR3 positivity without significant positivity for other SSTRs, and this patient might have benefited from treatment with PRRT based on SSTR analogues with SSTR3 affinity. Imaging and IHC do not necessarily correlate directly. One PHEO (patient 13) with no known VHL, SDHB, or RET mutation was practically negative for all 5 SSTRs, according to IHC; however, SSTR scintigraphy showed a positive result. It is possible that SSTR scintigraphy is even more sensitive than IHC. Studies that combine the results of somatostatin scintigraphy, the response to somatostatin analogue–based therapy, and SSTR-ICH results in a large patient cohort would be valuable. In this case, one cannot totally exclude a technical error, for example, a fixation problem with the specimen; however, such an error is unlikely because the tumor stained positive for MIB1, chromogranin A and SDHB. The number of patients treated with PPRT was too small to draw any conclusions.
The primary location of the tumor can impact SSTR status. All metastatic PGLs were strongly SSTR2 positive. One metastatic PHEO showed strong SSTR2 positivity, 1 showed intermediate SSTR2 positivity, and 3 other metastatic PHEOs were negative. Unger et al [22] studied SSTR1-5 expression in 8 metastasized and 7 nonmetastasized PHEOs. All metastasized PHEOs were SSTR2 negative. The strongly SSTR2-positive metastatic PHEO in our cohort was, in fact, a large tumor (19 cm in diameter) in the vicinity of the right adrenal. It is impossible to be certain of the primary location of such a large tumor with infiltration and decompression of the surrounding tissues: it might be either an adrenal tumor or a PGL arising close to the adrenal gland. Nevertheless, the difference in SSTR2 expression was significant between metastatic PHEOs and PGLs. The number of metastasized PHEOs was low; however, the result raised some questions, such as follows: are SSTR2-negative PHEOs more aggressive? In studies with gastroenteropancreatic NETs, SSTR2 and SSTR5 expressions have been associated with better survival [23,24]. Differences in genetic background and pathogenesis of PHEOs and PGLs can explain differences between SSTR2 expressions in metastatic tumors. Somatotroph pituitary adenomas with low immunohistochemical AIP expression have been shown to express less SSTR2 [25]. Because the cohort in this study was quite old, genetic testing was not a common practice; however, 7 of 9 metastatic PGLs were SDHB negative in IHC. One patient with metastatic PHEO was known to have NF1; in 1 patient, VHL, RET, and SDHB mutations were excluded, and all 5 were positive for SDHB, according to IHC.
The SSTR3 staining results warrant discussion, as SSTR3 expression was lower in metastasized tumors or tumors with a higher PI (MIB1 ≥ 5%). In a previous work, no correlation was found between SSTRs and PI [20], but Unger et al [22] found less SSTR3 positivity in metastasized PHEOs than in nonmetastasized. One explanation for our result could be that SSTR3 disappears as tumors dedifferentiate. In gastroenteropancreatic NETs, SSTR2 expression has been shown to decrease in more aggressive diseases [26]. However, 4 metastatic tumors showed strong SSTR3 positivity. Such tumors may benefit from imaging and treatment based on somatostatin analogues with SSTR3 affinity. Thus, individual assessment protocols are needed for patients with metastatic PHEOs and PGLs.
We could not confirm the result of Elston et al [7], who presented increased SSTR2A and SSTR3 expression in SDHB-negative PGLs and PHEOs. In their work, 29 of 32 SDHB-negative tumors and 72 of 147 SDHB-positive tumors were moderately or strongly SSTR2 positive. From our SDHB-negative group, 14 of 16 of the cases were SSTR2 positive and 12 of 16 were SSTR3 positive; however, in the SDHB-positive group, 99 of 135 were SSTR2-positive and 106 of 135 were SSTR3 positive. No significant difference was found among these groups. One explanation could be the low number of SDHB-negative cases in our series. However, Kaemmerer et al [20] also did not find any difference between their SSTR results and SDHB staining results. Most tumors in our series were PHEOs or sympathetic PGLs. Of 146 patients, 15 were SDHB negative and 40% had metastatic disease, which is concordant with other works [13].
PHEOs and PGLs, especially metastatic tumors, are rare tumors. The strength of our study lies in the large size of our consecutive cohort of rare tumors, which include clinical follow-up data. In addition, the antibodies used are validated. However, the number of metastatic and SDHB-negative tumors was low. Further research on a larger tumor series is needed to determine the prognostic role of SSTR subtype expression. Our tumor series was collected over a long period because of the rarity of these tumors. Some of the tumors are from a time when genetic testing was not a common practice; however, the immunohistochemical SDHB status is known for all of the tumors. Some tissue material was old, and there may be variations in fixation time among the tumors, which can impact the staining results. Many methodological and technical problems and errors are possible in IHC.
The PHEOs and PGLs constitute a rare and heterogeneous group of genetically, histologically, and molecularly different tumors with variations in prognosis, which makes diagnosis and treatment challenging. The metastatic potential of primary PHEOs or PGLs still cannot be unequivocally predicted, even if knowledge of this tumor group has increased considerably in recent years [27]. Limited treatment options are available for metastatic PHEOs and PGLs. Prognostic and predictive markers, as well as new treatment options, are needed. Today, new somatostatin analogues with an affinity for several SSTR receptors have been tested and considered for clinical use. For example, the ligand DOTA-NOC has a high affinity for SSTR2, SSTR3, and SSTR5 [28,29]. The long-acting analogue SOM230 (pasireotide) has a high affinity for SSTR1, SSTR2, SSTR3, and SSTR5 [30]. Peptide analogues with an affinity for SSTRs can be coupled with radioisotopes such as 68Ga, 99mTc, and 111In and used for imaging. By combining suitable radioisotopes such as 177Lu and 90Y with peptide analogues, it is possible to achieve targeted PRRT [6]. Radionuclide treatment exhibits fewer side effects and is less toxic than conventional cytotoxic treatments for metastatic PHEOS and PGLs [31].
In conclusion, in our cohort, PHEOs and PGLs most abundantly express SSTR2 and SSTR3. However, tumors have individual SSTR profiles. Therefore, tumor-specific SSTR IHC, which is a readily available and low-cost technique in pathology laboratories, can assist in selecting an optimal imaging method or therapy in the future. Immunohistochemical analyses of tumors are especially useful if no imaging has been conducted before operation. Some SSTRs may be prognostic or predictive; however, clarifying such features would require large studies combining broad clinical and follow-up information. The development of new broad-spectrum SSTR agonists, monoclonal validated antibodies and the ability to identify tumors with different individual SSTR profiles could provide us with the possibility of offering personalized targeted therapy for metastatic PHEOs and PGLs in the future.
Supplementary Material
Funding/Support:
This work was supported by the Finnish Awarded Special State Subsidy EVO (Grant No. TYH 2014203), the Finnish Cancer Foundation (Grant No. 4703666), and the Sigrid Jusélius Foundation and Finska Läkaresällskapet.
Abbreviations
- IHC
immunohistochemistry
- NET
neuroendocrine tumor
- PGL
paraganglioma
- PHEO
pheochromocytoma
- PI
proliferation index
- PRRT
peptide receptor radionuclide therapy
- SDH
succinate dehydrogenase
- SSTR
somatostatin receptor
- TMA
tissue microarray
Footnotes
Competing interests: The authors declare no conflict of interests.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.humpath.2018.11.020.
References
- [1].Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med 2008;132:1272–84. [DOI] [PubMed] [Google Scholar]
- [2].Harari A, Inabnet WB III. Malignant pheochromocytoma: a review. Am J Surg 2011;201:700–8. [DOI] [PubMed] [Google Scholar]
- [3].Choi YM, Sung TY, Kim WG, et al. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: a single institution experience. J Surg Oncol 2015;112:815–21. [DOI] [PubMed] [Google Scholar]
- [4].Tischler AS, de Krijger RR, Gill A, et al. Pheochromocytoma, extra-adrenal paragangliomas. In: Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: IARC; 2017. p. 180–95. [Google Scholar]
- [5].Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24:389–427. [DOI] [PubMed] [Google Scholar]
- [6].Korner M Specific biology of neuroendocrine tumors: peptide receptors as molecular targets. Best Pract Res Clin Endocrinol Metab 2016;30:19–31. [DOI] [PubMed] [Google Scholar]
- [7].Elston MS, Meyer-Rochow GY, Conaglen HM, et al. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase—deficient pheochromocytomas and paragangliomas. Hum Pathol 2015;46:390–6. [DOI] [PubMed] [Google Scholar]
- [8].Korner M, Waser B, Reubi JC. Does somatostatin or gastric inhibitory peptide receptor expression correlate with tumor grade and stage in gut neuroendocrine tumors? Neuroendocrinology 2015;101:45–57. [DOI] [PubMed] [Google Scholar]
- [9].Cives M, Strosberg J. The expanding role of somatostatin analogs in gastroenteropancreatic and lung neuroendocrine tumors. Drugs 2015;75:847–58. [DOI] [PubMed] [Google Scholar]
- [10].Xu C, Zhang H. Somatostatin receptor based imaging and radionuclide therapy. Biomed Res Int 2015;2015:917968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Casar-Borota O, Heck A, Schulz S, et al. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and longterm effects of octreotide. J Clin Endocrinol Metab 2013;98:E1730–9. [DOI] [PubMed] [Google Scholar]
- [12].Pillai S, Gopalan V, Smith RA, Lam AK. Updates on the genetics and the clinical impacts on phaeochromocytoma and paraganglioma in the new era. Crit Rev Oncol Hematol 2016;100:190–208. [DOI] [PubMed] [Google Scholar]
- [13].Benn DE, Robinson BG, Clifton-Bligh RJ. 15 Years of paraganglioma: Clinical manifestations of paraganglioma syndromes types 1-5. Endocr Relat Cancer 2015;22:T91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Papathomas TG, de Krijger RR, Tischler AS. Paragangliomas: update on differential diagnostic considerations, composite tumors, and recent genetic developments. Semin Diagn Pathol 2013;30:207–23. [DOI] [PubMed] [Google Scholar]
- [15].Papathomas TG, Oudijk L, Persu A, et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a multinational study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol 2015;28:807–21. [DOI] [PubMed] [Google Scholar]
- [16].Miettinen M, Sarlomo-Rikala M, McCue P, et al. Mapping of succinate dehydrogenase losses in 2258 epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Korner M, Waser B, Schonbrunn A, Perren A, Reubi JC. Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am J Surg Pathol 2012;36:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007;20:1172–82. [DOI] [PubMed] [Google Scholar]
- [19].de Herder WW, Hofland LJ. Somatostatin receptors in pheochromocytoma. Front Horm Res 2004;31:145–54. [DOI] [PubMed] [Google Scholar]
- [20].Kaemmerer D, Sanger J, Arsenic R, et al. D’Haese CXCR4 chemokine and endothelin a receptor expression in a large set of paragangliomas. Oncotarget 2017;8:89958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mundschenk J, Unger N, Schulz S, et al. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab 2003;88:5150–7. [DOI] [PubMed] [Google Scholar]
- [22].Unger N, Serdiuk I, Sheu SY, et al. Immunohistochemical localization of somatostatin receptor subtypes in benign and malignant adrenal tumours. Clin Endocrinol 2008;68:850–7. [DOI] [PubMed] [Google Scholar]
- [23].Qian ZR, Li T, Ter-Minassian M, et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 2016;45:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Wang W, Jin K, et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett 2017;13:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iacovazzo D, Carlsen E, Lugli F, et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. Eur J Endocrinol 2016;174:241–50. [DOI] [PubMed] [Google Scholar]
- [26].Kaemmerer D, Trager T, Hoffmeister M, et al. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015;6:27566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Roman-Gonzalez A, Jimenez C. Malignant pheochromocytoma-paraganglioma: pathogenesis, TNM staging, and current clinical trials. Curr Opin Endocrinol Diabetes Obes 2017;24:174–83. [DOI] [PubMed] [Google Scholar]
- [28].Ambrosini V, Fani M, Fanti S, Forrer F, Maecke HR. Radiopeptide imaging and therapy in Europe. J Nucl Med 2011;52(Suppl. 2):42S–55S. [DOI] [PubMed] [Google Scholar]
- [29].Wild D, Schmitt JS, Ginj M, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 2003;30:1338–47. [DOI] [PubMed] [Google Scholar]
- [30].Chan JA, Ryan DP, Zhu AX, et al. Phase I study of pasireotide (SOM 230) and everolimus (RAD001) in advanced neuroendocrine tumors. Endocr Relat Cancer 2012;19:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gunawardane PTK, Grossman A. Phaeochromocytoma and paraganglioma. Adv Exp Med Biol 2017;956:239–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


