Abstract
Background:
Recurrent adrenocortical carcinoma (ACC) has a poor prognosis with minimal clinical and biochemical factors to guide management. The aim of this study was to evaluate the prognostic significance of systemic inflammatory response in patients with recurrent ACC.
Methods:
Patients who underwent resection for recurrent ACC were retrospectively analyzed. Preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio (LMR), and mean platelet volume were calculated.
Results:
Twenty-five patients (age at operation 52.2 ± 9.5 years) were identified. We observed a statistically significant shorter disease-specific survival (DSS) in patients with LMR less than 4 (41 ± 7.4 months vs 71 ± 12.3, P = .023) and male sex (26.6 ± 4.2 months vs 57.6 ± 9.5 months, P = .079), while time-to-recurrence (TTR) less than 12 months (40 ± 7.7 months vs 70.3 ± 13.1 months, P = .059) had a trend on univariate analysis for worse DSS. On multivariable analysis, LMR < 4 (hazard ratio [HR] 4.18; 95% confidence interval [CI]: 1.18-14.76; P = .027) and TTR less than 12 months (HR 2.77 95% CI: 1-7.62; P = .049) were found to be significantly associated with worse DSS.
Conclusion:
Preoperative LMR greater than 4 and TTR greater than 12 months are associated with longer DSS. Patients with LMR greater than 4 and TTR greater than 12 months may benefit from a more aggressive therapeutic approach and may require less frequent surveillance.
Keywords: adrenocortical cancer, lymphocyte, monocyte, recurrence
1 ∣. INTRODUCTION
Adrenocortical carcinoma (ACC) is a rare cancer with an estimated incidence of 0.72 to 2 cases per 1 million people per year.1,2 The median age at the time of diagnosis is between 55 and 56 years.3,4 Prognosis for patients with ACC remains poor, with an overall 5-year survival between 16% to 47%.2,4 Several prognostic factors have been identified to be associated with a poor disease course, including a tumor size greater than 12 cm, an elevated Ki-67 index or mitotic rate, the presence of tumor necrosis on histologic examination,2,5-7 and metabolic parameters on 18fluoro-deoxyglucose positron emission tomography/computed tomography(18F-FDG-PET/CT), such as whole body metabolic volume, total lesion glycolysis (TLG), and maximum standardized uptake value (SUV).8 However, preoperative prognostic markers are needed as they may alter therapeutic choices. Recently, both an elevated preoperative neutrophil-to-lymphocyte ratio (NLR) and an elevated platelet-to-lymphocyte ratio (PLR) were found to be associated with worse disease-specific survival (DSS) and recurrence-free survival (RFS) in patients with ACC who underwent the initial operation for primary tumors.9
Prognostic indicators are especially needed for patients with recurrent ACC after curative resection, to identify patients who may benefit from reoperation. Recurrence after R0 resection is very common in ACC, occurring in 74.4% of patients.10 There are few preoperative prognostic markers available, including elevated serum IGFBP2,11 time-to-recurrence (TTR) more than 12 months,12 and metabolic features on FDG-PET scan.8 Preoperative systemic inflammatory markers can be measured readily with a routine blood sample, are inexpensive and their value in predicting survival has been described for many malignancies.13 Several systemic inflammatory markers have been described to be associated with survival, including NLR, PLR, lymphocyte-to-monocyte ratio (LMR), mean platelet volume (MPV), and neutrophil-to-albumin ratio (NAR).9,14
Systemic inflammatory markers have been shown to carry prognostic value in patients with primary ACC at the time of initial surgery, and therefore the purpose of this study was to identify whether these markers are prognostic in patients undergoing reoperation for recurrent ACC.
2 ∣. METHODS
Patients who underwent surgical resection for recurrent ACC (n = 28) were enrolled in a prospective protocol (NCI NCT01005654) at the National Institutes of Health Clinical Center (NIH, Bethesda, MD). The protocol was approved by the Institutional Review Board and written informed consent was obtained from all participants. Patients who had an operation at the NIH Clinical Center for recurrent adrenocortical carcinoma between December of 2004 and October of 2012 were included (follow-up of median 32.5 months; range 0-119.6 months). Two patients had an active infection with neutrophilic leukocytosis throughout the preoperative period and were excluded from the analysis. One patient died intraoperatively and was also excluded.
NLR, PLR, LMR, MPV, and NAR were calculated preoperatively for all patients. For each patient, blood samples drawn up to 30 days preoperatively were considered, of which the maximum values for each biomarker were considered. DSS was calculated from the date of reoperation. Both patients with locoregional and distant recurrence were considered. Documentation of recurrence was based on imaging findings CT, magnetic resonance imaging or 18FDG-PET/CT, and pathologic confirmation for sites that were resected. R status refers to the reoperation. Maximum SUVs, metabolic tumor volume, and TLG were calculated based on preoperative 18FDG-PET/CT imaging, as previously described.8
2.1 ∣. Statistical analyses
Receiver operating characteristic (ROC) curve analysis and the Youden's J statistic (ie sensitivity + specificity-1)15 were used to identify optimal cut-offs for each biomarker. Kaplan–Meier curves were constructed and univariate analysis was performed using the log-rank test using the optimal cut-offs for each biomarker. Multivariable analysis was performed by the Cox proportional hazards model using backwards selection. All variables with a P < .1 by univariate analysis were included in multivariable analysis. A two-tailed t test and analysis of variance were used to identify differences between groups. Statistical analysis was performed on SPSS Version 21.0. (IBM Corp, Armonk, NY). A P ≤ .05 was considered significant and a P >.5 and < .1 was considered to be a trend.
3 ∣. RESULTS
3.1 ∣. Clinical characteristics and demographics
Twenty-five patients with recurrent ACC underwent an operation. Twenty-one of the 25 patients were female, and the median age at the time of operation was 54 years (range: 33-64 years). Patients were followed for a median follow-up period of 32.6 months (range: 15.6-119.6 months). Twenty-two (88%) patients underwent abdominal resections and three (12%) patients underwent pulmonary resections with all systemic therapies held at least 4 weeks before surgery. In addition, 23 (92%) patients received adjuvant chemotherapy after the initial resection. Detailed demographic and clinical characteristics are summarized in Table 1. ROC curves were created to identify optimal cut-offs. The optimal cut-offs that were identified were NLR greater than 3.7, LMR less than 4, PLR greater than 208.5, MPV greater than 10.7, and NAR less than 1.1 (Table 2).
TABLE 1.
Demographic, clinical, and pathological characteristics of the study cohort (n = 25)
| Variable | Number (%) |
|---|---|
| Age at diagnosis, median | 54 y (range 33-64) |
| Sex | |
| Male | 4 (16%) |
| Female | 21 (84%) |
| Median BMI (kg/m2) | 29.3 (range 17.7-43.8) |
| Past medical history | |
| Hypertension | 11 (44%) |
| Diabetes mellitus | 1 (4%) |
| Depression | 4 (16%) |
| Asthma | 2 (8%) |
| Breast cancer | 1 (4%) |
| Hyperlipidemia | 4 (16%) |
| Hypothyroidism | 2 (8%) |
| Hyperthyroidism | 1 (4%) |
| Li-Fraumeni syndrome | 1 (4%) |
| Ki-67 index >20% | 25 (100%) |
| Maximum SUV (median) | 9.4 (range: 4.5-45) |
| MTV (median) | 47.5 mL (range: 4.9-416.9 mL) |
| TLG (median) | 197 SUVlbm*mL (range: 3.9-2557.9 SUVlbm*mL) |
| Operation performed | |
| Locoregional resection | 10 (40%) |
| Hepatic resection | 7 (28%)a |
| Pulmonary resection | 4 (16%)b |
| Other intra-abdominal resection | 4 (16%)c |
| R status | |
| R0-1 | 12 (48%) |
| R2 | 13 (52%) |
| Adjuvant chemotherapy after reoperation | 6 (24%) |
| Tariquidar, doxorubicin, vincristine, etoposide, mitotane | 1 (4%) |
| Gefitinib, EDP, metyrapone | 1 (4%) |
| EDP, mitotane | 1 (4%) |
| EDP | 1 (4%) |
| AT-101, mitotane | 1 (4%) |
| Mitotane | 1 (4%) |
| Received chemotherapy before resection | 20 (80%) |
| Time-to-recurrence >12 mo | 12 (48%) |
Abbreviations: BMI, body mass index, EDP, etoposide, doxorubicin, cisplatin; MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis.
Includes small bowel and colon resections for metastatic disease.
Three hepatic resections were performed for single lesions and four hepatic resections for multiple lesions. Five out of seven patients recurred late ( > 12 mo).
All pulmonary resections were performed for multiple lesions. Three out of four patients recurred early (<12 mo).
Includes small bowel and colon resections for metastatic disease.
TABLE 2.
Systemic inflammatory markers in the study cohort
| Mean ± SD | Range | Optimal cut-offa | |
|---|---|---|---|
| NLR | 3.2 ± 1.9 | 0.92-8.28 | 3.7 |
| LMR | 6.5 ± 14.2 | 1.67-74 | 4 |
| PLR | 179.5 ± 67.7 | 109-462 | 208.5 |
| MPV | 10.4 ± 0.84 | 9.4-12 | 10.7 |
| NAR | 1.16 ± 0.47 | 0.6-2.39 | 1.1 |
Abbreviations: LMR, lymphocyte-to-monocyte ratio; MPV, mean platelet volume; NAR, neutrophil-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SD, standard deviation.
Based on receiver operating characteristic (ROC) curve analysis and the Youden's J statistic.
3.2 ∣. Long-term DSS
The median DSS of the entire cohort was 35.5 ± 10.9 months. The 1-, 3-, and 5-year DSS for the cohort were 100%, 40%, and 16%, respectively. On univariate analysis, LMR less than 4 (41 ± 7.4 months vs 71 ± 12.3, P = .023) and male sex (26.6 ± 4.2 months vs 60.4 ± 9.5 months, P = .05) were associated with worse DSS, while TTR less than12 months (40 ± 7.7 months vs 70.3 ± 13.1 months, P = .059) had a trend on univariate analysis for worse DSS. On multivariable analysis, including LMR less than 4, TTR less than 12 months and sex, LMR less than 4 (hazard ratio [HR] 4.18; 95% confidence interval [CI]: 1.18-14.76; P = .027) and TTR less than12 months (HR 2.77; 95%CI: 1-7.62; P = .049) were independently associated with worse DSS (Table 3). Among all preoperative measurements during the 30-day postoperative period, eight patients had one blood draw, 12 patients had two blood draws and five patients had three blood draws. The variation of preoperative LMR was assessed by subtracting the minimum from the maximum LMR. The median variation of preoperative LMR was 0.4 (interquartile range, 0-0.99). This demonstrates that LMR did not change considerably during the preoperative period in patients that had multiple blood draws.
TABLE 3.
Results of univariate and multivariable analysis for DSS
| Univariate P value |
Multivariable adjusted HR (95% CI) |
P value | |
|---|---|---|---|
| Age >54 y | 0.94 | ||
| Sex | 0.05 | .929 | |
| Male | 1.06 (0.27-4.18) | ||
| Female | Ref | ||
| BMI >29.3 kg/m2 | 0.458 | ||
| Distant metastasis | 0.745 | ||
| Adjuvant chemotherapy | 0.862 | ||
| Preoperative chemotherapy | 0.803 | ||
| Maximum SUV >9.4 | 0.186 | ||
| MTV >47.5 mL | 0.623 | ||
| TLG >197 SUVlbm*mL | 0.346 | ||
| Type of operation | 0.186 | ||
| Distant resection | 0.105 | ||
| R status | 0.303 | ||
| TTR <12 mo | 0.059 | 2.77 (1-7.62) | .049 |
| NLR >3.7 | 0.119 | ||
| PLR >208.5 | 0.287 | ||
| LMR <4 | 0.023 | 4.18 (1.18-14.76) | .027 |
| MPV >10.7 | 0.595 | ||
| NAR <1.1 | 0.392 |
Abbreviations: BMI, body mass index, CI, confidence interval; DSS, disease-specific survival; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; MPV, mean platelet volume; MTV, metabolic tumor volume; NAR, neutrophil-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SUV, standardized uptake value; TLG, total lesion glycolysis; TTR, time to recurrence.
3.3 ∣. Combination of prognostic factors
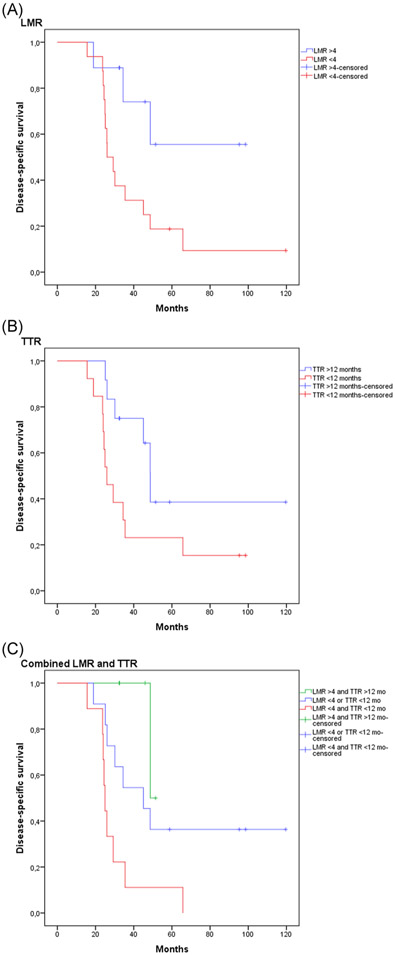
LMR and TTR were analyzed in combination to better define prognosis. The combination of LMR less than 4 and TTR less than 12 months was found to have the worst prognosis with a median survival of 25 months compared to patients with an LMR greater than 4 and TTR greater than 12 months with a median survival of 48.7 months. Prognosis was intermediate when either LMR less than 4 or TTR less than 12 months (45.1 months; P = .005; Figure 1).
FIGURE 1.
Kaplan–Meier curves for: A, Lymphocyte-to-monocyte ratio (LMR) less than 4, (B) time-to-recurrence (TTR) less than 12 months, and (C) LMR greater than 4 and TTR greater than 12 months, LMR less than 4 or TTR less than 12 months, LMR less than 4, and TTR less than 12 months
4 ∣. DISCUSSION
Determining the prognosis of patients with recurrent ACC is difficult. In this study, a LMR less than 4 and TTR less than 12 months were found to be independently associated with worse DSS. In addition, the combination of LMR less than 4 and TTR less than 12 months carried the worst prognosis compared to patients with both a LMR greater than 4 and TTR greater than 12 months. These patients with the best prognosis may benefit from aggressive surgical treatment.
Multiple studies have demonstrated that systemic inflammatory markers may be used to assess prognosis in patients with several types of malignancies. Lower LMR has been associated with worse survival in multiple types of cancer, including pancreatic cancer,16 stage II-III gastric cancer,17 hepatocellular carcinoma,18 and non--small cell lung cancer.19 In this study, preoperative LMR less than 4 was associated with worse DSS in patients undergoing resection for recurrent ACC. The prognostic utility of LMR may be attributed to the two distinct areas of cellular interaction between the immune system and tumor cells. Tumor-associated macrophages (M2 macrophages) enhance tumor growth, invasion, metastasis, and angiogenesis, as well as suppress the immune response, thereby contributing to a worse clinical course.20,21 On the other hand, the presence of tumor-infiltrating lymphocytes is associated with improved prognosis, due to their anticancer activity and multiple immunotherapeutic strategies have successfully applied this role in clinical practice22,23 Therefore, at the tumor level, this may represent the underlying mechanism for the use of LMR as a prognostic indicator. The lack of immune infiltration in ACC,24 however, reduces the likelihood of this mechanism. The second etiologic mechanism is the contribution of circulating lymphocytes and macrophages to the intravascular survival and extravasation of circulating tumor cells. More specifically, circulating natural killer cells recognize circulating tumor cells and promote their apoptosis via perforin and granzyme B, while circulating monocytes assist in the extravasation of circulating tumor cells.25 Therefore, a lower LMR may reflect lower apoptosis and higher extravasation of circulating tumor cells that can lead to faster tumor progression and worse prognosis.
The relationship between systemic inflammatory markers and ACC has previously been demonstrated by Bagante et al,9 who identified elevated NLR and PLR to be prognostic markers of recurrence-free and DSS. Interestingly, the present study did not find an association between NLR or PLR and DSS in patients with recurrent ACC. The discrepancies in the results of the two studies may also be attributed to potential differences in prognostic indicators between primary and recurrent ACC, with LMR being a more suitable prognostic biomarker for recurrent rather than primary ACC. Important clues towards the underlying inflammatory signaling pathways in ACC have been observed by Gara et al,26 who identified the pathway of oncostatin M, an important inflammatory cytokine of the interleukin-6 group, to be upregulated in aggressive recurrent ACC. This study further supports these results by demonstrating the presence of a relationship between inflammatory response and DSS in patients undergoing surgery for recurrent ACC. These findings, linking the presence of systemic inflammation with worse prognosis, may provide clues towards the pathogenetic mechanisms of tumor progression in ACC, as well as future therapies targeting inflammatory signaling pathways.
There is a need for prognostic indicators in patients with recurrent ACC. There are few available treatment modalities, including reoperation and chemotherapy. Proper patient selection for reoperation lies on the availability of preoperative prognostic indicators that may indicate favorable prognostic outcomes after surgery. Few studies have examined this issue. Erdogan et al12 identified TTR greater than 12 months and an R0 resection as indicators of favorable prognosis after reoperation.12 Dy et al10 also identified patients with a disease-free interval greater than 6 months to benefit from surgical resection.10 Tran et al27 found that favorable prognosis after reoperation may be expected when at least two of the following factors are present, namely: a solitary tumor, disease-free interval greater than 12 months and locoregional or pulmonary recurrence. Finally, Patel et al11 identified elevated serum IGFBP2 to be associated with improved survival after reoperation in recurrent ACC. The results of this study demonstrate that inflammatory markers may also be used during decision-making, since patients with a LMR greater than 4 had improved prognosis. Patients with both LMR greater than 4 and TTR greater than 12 months may benefit from aggressive surgical resection of recurrent ACC and more intensive surveillance program. However, patients with both LMR less than 4 and TTR greater than 12 months had an unfavorable prognosis and thus surgical resection may not be the most appropriate treatment option, especially if the patient has a poor functional status. These patients may benefit from clinical trials or aggressive chemotherapy.
There are several limitations to this study. The relatively small size may limit the applicability of this study. However, ACC is a rare cancer, and surgery for recurrent ACC is only rarely performed in major tertiary centers. In addition, there is a selection bias due to the inclusion only of patients that underwent an operation, who may have a more favorable outcome. There is also variation in terms of both the type of operation performed (ie locoregional vs hepatic vs pulmonary) and the completeness of resection. Patients with unresectable metastatic disease were excluded, and therefore may change the LMR value that would predict DSS. Therefore, this data should only be applied to patients who are surgical candidates. The values of preoperative systemic inflammatory markers are greatly influenced by multiple factors and thus small elevations or reductions in these values may affect prognostic estimations. For this reason, application of this marker should be used with caution and clinicians should be aware of these weaknesses. However, none of the clinical factors that were studied was significantly associated with elevated or reduced levels of LMR. RFS could not be reliably ascertained since many patients in this cohort an R2 resection, but the primary endpoint of disease-specific survival, which is arguably more important, was calculated. The initial surgical approach, laparoscopic or open approach, was not available for all patients and may have influenced the disease-specific survival. However, it should be noted that the association between approach and survival is controversial. The presence of metastasis and R status were not associated with worse survival in patients, and this may have been a type II error due to a relatively small cohort of a rare disease.
5 ∣. CONCLUSIONS
In conclusion, preoperative markers of systemic inflammation may be useful as prognostic indicators in patients with recurrent ACC who undergo an operation. This study identified patients with an LMR greater than 4 and TTR greater than 12 months to be associated with improved DSS. These two factors may be used as indicators of favorable prognosis to identify patients that are likely to benefit from surgical treatment.
ACKNOWLEDGMENT
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Part of this study was presented at the 2018 American College of Surgeons Clinical Congress, Boston, October 201
Funding information
NIH Clinical Center; Center for Cancer Research, National Cancer Institute, National Institutes of Health
REFERENCES
- 1.Kebebew E, Reiff E, Duh Q-Y, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872–878. 10.1007/s00268-005-0329-x [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23(2):273–289. 10.1016/j.beem.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Kerkhofs TMA, Verhoeven RHA, Van der Zwan JM, et al. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11): 2579–2586. 10.1016/j.ejca.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States. Cancer. 2008;113(11):3130–3136. 10.1002/cncr.23886 [DOI] [PubMed] [Google Scholar]
- 5.Assié G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2007;92(1):148–154. 10.1210/jc.2006-0706 [DOI] [PubMed] [Google Scholar]
- 6.Morimoto R, Satoh F, Murakami O, et al. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr J. 2008;55(1):49–55. [DOI] [PubMed] [Google Scholar]
- 7.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20(4):941–950. 10.1200/JCO.2002.20.4.941 [DOI] [PubMed] [Google Scholar]
- 8.Satoh K, Patel D, Dieckmann W, Nilubol N, Kebebew E. Whole body metabolic tumor volume and total lesion glycolysis predict survival in patients with adrenocortical carcinoma. Ann Surg Oncol. 2015;22(S3):714–720. 10.1245/s10434-015-4813-8 [DOI] [PubMed] [Google Scholar]
- 9.Bagante F, Tran TB, Postlewait LM, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. J Surg Oncol. 2015;112(2):164–172. 10.1002/jso.23982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dy BM, Wise KB, Richards ML, et al. Operative intervention for recurrent adrenocortical cancer. Surgery. 2013;154(6):1292–1299. 10.1016/j.surg.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Patel D, Ellis R, Howard B, et al. Analysis of IGF and IGFBP as prognostic serum biomarkers for adrenocortical carcinoma. Ann Surg Oncol. 2014;21(11):3541–3547. 10.1245/s10434-014-3768-5 [DOI] [PubMed] [Google Scholar]
- 12.Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):181–191. 10.1210/jc.2012-2559 [DOI] [PubMed] [Google Scholar]
- 13.Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717. 10.1038/s41598-017-16955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaitanidis A, Patel D, Nilubol N, Tirosh A, Sadowski S, Kebebew E. Markers of systemic inflammatory response are prognostic factors in patients with pancreatic neuroendocrine tumors (PNETs): a prospective analysis. Ann Surg Oncol. 2018;25:122–130. 10.1245/s10434-017-6241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 16.Ji J-J, Gao B-Q, Xu H-W, Li G-J, Yang F. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085. 10.2147/OTT.S96707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Du Y, Xu J, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumor Biol. 2014;35(11):11659–11666. 10.1007/s13277-014-2504-x [DOI] [PubMed] [Google Scholar]
- 18.Lin Z-X. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21(38):10898. 10.3748/wjg.v21.i38.10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H, Sun Z, Deng L, Zhu D, Wang D. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with stage i non-small cell lung cancer undergoing complete resection. Cancer Invest. 2016;34(8):378–384. 10.1080/07357907.2016.1213276 [DOI] [PubMed] [Google Scholar]
- 20.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- 21.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410. 10.1016/j.imbio.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. 10.1200/JCO.2010.30.5425 [DOI] [PubMed] [Google Scholar]
- 23.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Haas GP, Solomon D, Rosenberg SA. Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol Immunother. 1990;30(6):342–350. 10.1007/BF01786883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32(3):282–293. 10.1016/j.ccell.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 26.Gara SK, Wang Y, Patel D, et al. Integrated genome-wide analysis of genomic changes and gene regulation in human adrenocortical tissue samples. Nucleic Acids Res. 2015;43(19):9327–9339. 10.1093/nar/gkv908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran TB, Maithel SK, Pawlik TM, et al. Clinical score predicting long-term survival after repeat resection for recurrent adrenocortical carcinoma. J Am Coll Surg. 2016;223(6):794–803. 10.1016/j.jamcollsurg.2016.08.568 [DOI] [PMC free article] [PubMed] [Google Scholar]