Abstract
CRISPR-Cas12a, a type-V CRISPR-Cas endonuclease, is an effective genome editing platform. To improve the gene editing efficiency of Cas12a, we rationally designed small molecule enhancers through a combined computational approach. First, we used extensive molecular dynamics (MD) simulations to explore the conformational landscape of Cas12a from Acidaminococcus (AsCas12a), revealing distinct conformational states that could be targeted by small molecules to modulate its genome editing function. We then identified 57 compounds that showed different binding behavior and stabilizing effects on these distinct conformational states using molecular docking. After experimental testing 6 of these 57 compounds, compound 1, quinazoline-2,4(1H,3H)-dione, was found particularly promising in enhancing the AsCas12a-mediated genome editing efficiency in human cells. Compound 1 was shown to act like a molecular “glue” at the interface between AsCas12a and crRNA near the 5′-handle region, thus specifically stabilizing the enzyme–crRNA complex. These results provide a new paradigm for future design of small molecules to modulate the genome editing of the CRISPR-Cas systems.
Graphical Abstract
The CRISPR-Cas systems are a complex molecular machinery consisting of multiple components including the Cas protein, CRISPR guide RNA (gRNA), and protospacer adjacent motif (PAM) in the targeting DNA molecules of the genome.1–7 Moreover, genome editing involves multistages of actions such as recruitment of gRNA, PAM detection, formation of a gRNA-DNA duplex inside the Cas protein, not to mention myriads of correction steps.7–14 Despite this complexity, the genome editing function is to a large extent governed by the three-dimensional structure and dynamics of the enzymes.8,12,13,15,16 Thus, modulation of the Cas protein structure and/or Cas protein–nucleic acid interactions with small molecules is a promising strategy for the improvement of the CRISPR-Cas mediated genome editing efficiency.
CRISPR-Cas12a (also known as Cpf1), a type-V CRISPR-Cas effector endonuclease, exhibits effective genome-editing capability in multiple cell and animal models.7,13,14,17–20 The guide RNA for Cas12a is a single CRISPR-RNA (crRNA) with a length of 44 nucleotides.6 Previously, several small molecules have been identified to improve the DNA repair process through blocking the nonhomologous end joining (NHEJ) or activating the abundance of proteins involved in homology directed repair (HDR)/damage-dependent signaling, after the Cas12a-mediated genome editing.21,22 Building upon the conventional structure-based drug design approach, we present here a strategy to discover genome editing enhancers by rationally designing small molecules that can bind AsCas12a and differentially stabilize specific functional conformations or states of AsCas12a.
RESULTS AND DISCUSSION
To thoroughly explore the conformational space of the protein in the different functional states, e.g., in the presence and the absence of crRNA and DNA, we performed microsecond molecular dynamics (MD) simulations of the AsCas12a ternary (T-State), binary (B-State), and apo (A-State) systems (Figure 1B), respectively (3 μs in total) (Table 1). We then clustered each simulation trajectory according to root-mean-squared deviation (RMSD) of Cα positions of the protein, from which 11 representative conformations were chosen, one from each of the most populated clusters (1 ternary, 6 binary, and 4 apo structures). These structures are expected to represent AsCas12a in its different functional states. We hypothesized that if a small molecule could bind AsCas12a and differentially stabilize these conformations along the genome cleavage pathway (Figure 1A), then this molecule would be able to affect AsCas12a’s activity through shifting the free energy equilibria among these conformations, thus modulating the enzyme’s genome editing efficiency.
Figure 1.
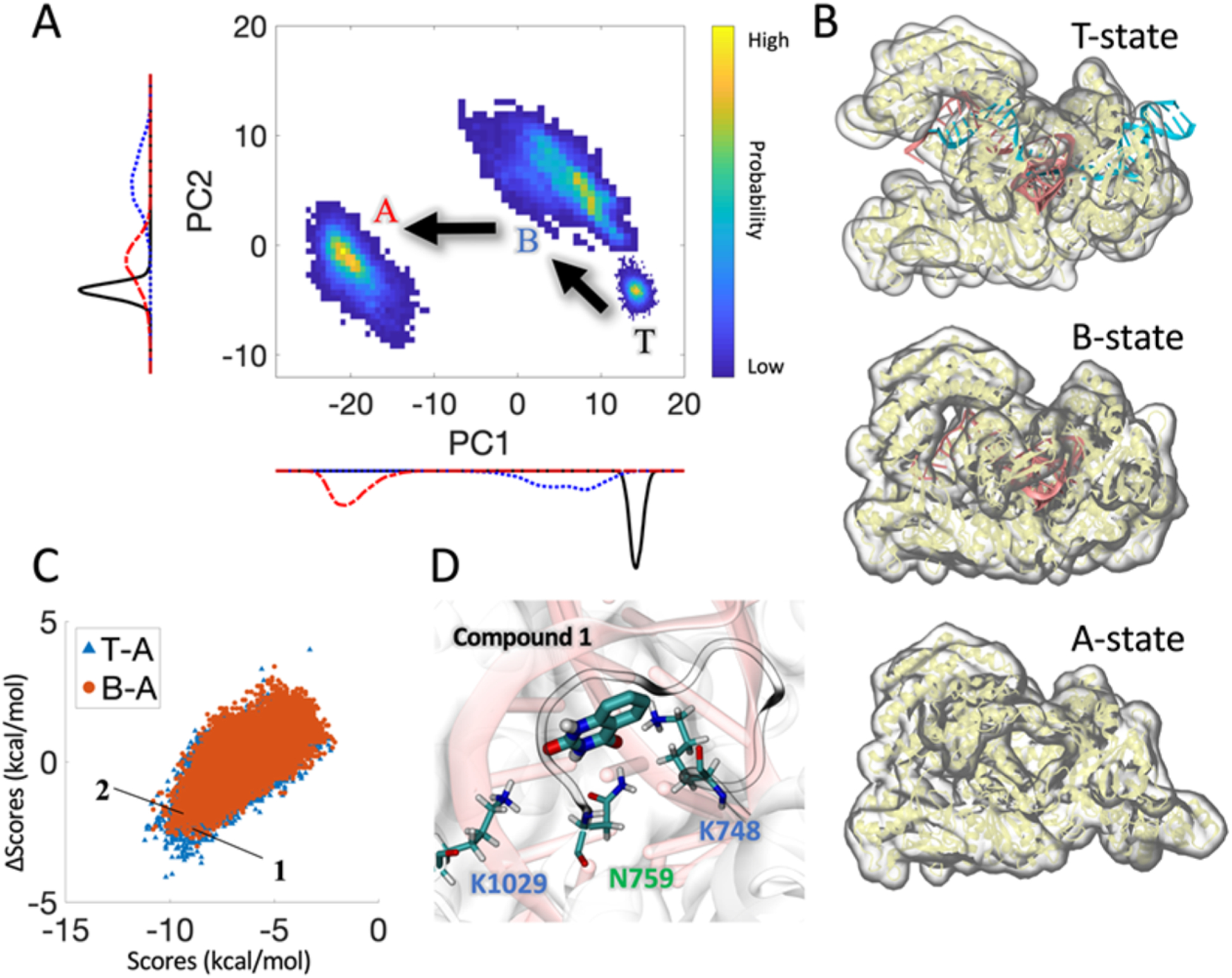
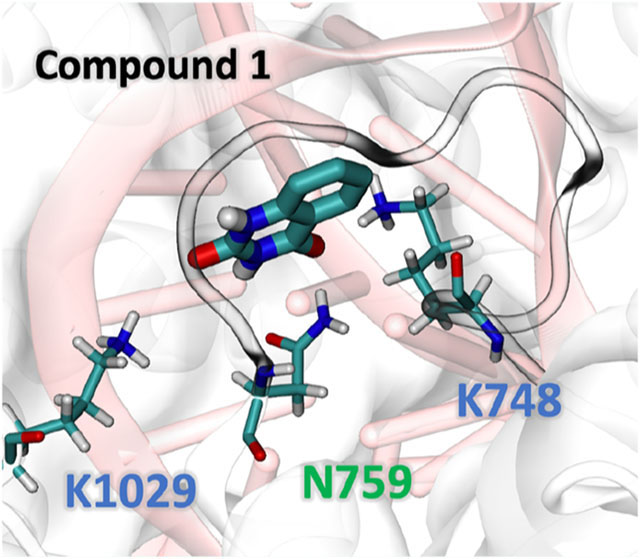
Rational design of small molecules differentially stabilizing AsCas12a at different functional states. (A) Conformational landscape of CRISPR-AsCas12a projected along the first two principal component (PC) modes sampled by microseconds of molecular dynamics simulations. The PCs of Cα atom fluctuations were computed using principal component analysis (PCA). (B) Snapshots of different functional states of CRISPR-AsCas12a. T: ternary; B: binary, and A: apo. (C) Scatter plot showing the docking score differences ΔScore vs the docking scores for all ~220,000 compounds. Ability of a small molecule to distinguish different functional states of the protein is reflected by a lower ΔScore. Compounds 1 and 2 are labeled. (D) Compound 1 bound at the protein–crRNA interface in the ternary AsCas12a system. Interacting residues were shown sticks. crRNA was shown in pink. Illustrations were made using VMD.23
Table 1.
Simulation Summary
| index | system | Time (μs) | size | atom number | clusters identified |
|---|---|---|---|---|---|
| 1 | Ternary | 1.0 | 216 × 163 × 163 | 520,620 | 1 |
| 2 | Binary | 1.0 | 203 × 153 × 153 | 430,664 | 6 |
| 3 | Apo | 1.0 | 203 × 153 × 153 | 430,624 | 4 |
We obtained 11 representative structures corresponding to the different functional states of AsCas12a from the above MD simulations. Subsequently, a virtual screening of ~220,000 compounds against all these 11 AsCas12a structures was carried out, which yielded 57 compounds that showed reasonably strong binding to at least one functional state of AsCas12a (i.e., binding score ≤ −9.0 kcal/mol) as well as preferential binding for the ternary or binary structure vs the apo structure (i.e., ΔScore ≥ 2.7 kcal/mol). By performing a global docking, we were able to identify small molecules that bind to unique sites only present in distinct functional states of AsCas12a. Many small molecules identified appear to act as a molecular “glue”, which enhances the interactions between individual components of the system, i.e., between the AsCas12a and the crRNA in the ternary and binary systems. Finally, based on some practical considerations (e.g., molecular weight < 500 g/mol, 0 < CLogP < 2.0 and availability), six compounds were selected for experimental validation (Table 2).
Table 2.
Chemical Compound Information
| ZINC | CAS No. | Chemical structure | Compound code |
|---|---|---|---|
| ZINC00150158 | 86-96-4 | 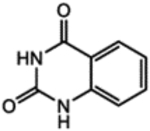 |
1 |
| ZINC04430890 | 13548-68-0 | 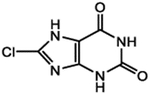 |
2 |
| ZINC00155806 | 17647-60-8 | 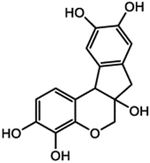 |
3 |
| ZINC45373881 | 1038549-25-5 | 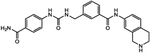 |
4 |
| ZINC04764008 | 1009315-76-7 | 5 | |
| ZINC09600261 | 868981-42-4 | 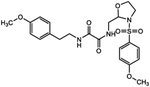 |
6 |
We first examined the cytotoxicity of these compounds in human HEK293T cells by an MTT assay. Minimal cytotoxicity was observed in cells treated with 5 μM and 10 μM of each compound (Figure S1). The increasing concentrations of compounds 3 and 6 led to higher cytotoxicity such as 69% and 73% cell death, respectively, at the concentration of 50 μM. Then, we used human HEK293T cells to evaluate these six compounds for their effects on genome editing efficiency of crRNA targeting DNMT1 (crDNMT1). Compounds 1, 2, 4, and 6 significantly increased genome editing efficiency (Table 2, Figures 2A and S2A). Then, we studied their effects on the HEK293-AsCas12a cells, stably expressing AsCas12a. Compound 1 significantly enhanced the gene-editing efficiency of crDNMT1, while compound 2 did not (Figures 2B and S2B). Similarly, compound 1 increased the gene-editing efficiency of crDNMT1 in U87 cells (Figures 2C and S3A). We then investigated the crRNAs targeting FANCF-2 (crFANCF-2). Consistent enhancement of gene editing was found with the treatment of compound 1 in HEK293-AsCas12a cells (Figures 2D, S3B, and S3C). Increase of compound 1 concentrations further improved the gene-editing efficiency of crDNMT1 in 293T-AsCas12a cells in comparison to the control group (Figure 2E). To further characterize on-target and off-target effects, we conducted targeted deep sequencing of the DNA samples isolated from the experiments in Figure 2E. The results showed that compound 1 increased the gene-editing efficiency by 25%, without affecting off-target sites compared with the control group (Figure S4). Taken together, compound 1 was found to particularly enhance the AsCas12a-mediated genome editing efficiency in human cells. It enhances the genome editing directly without affecting the DNA repair process, which may also decrease the off-targeting effect.
Figure 2.
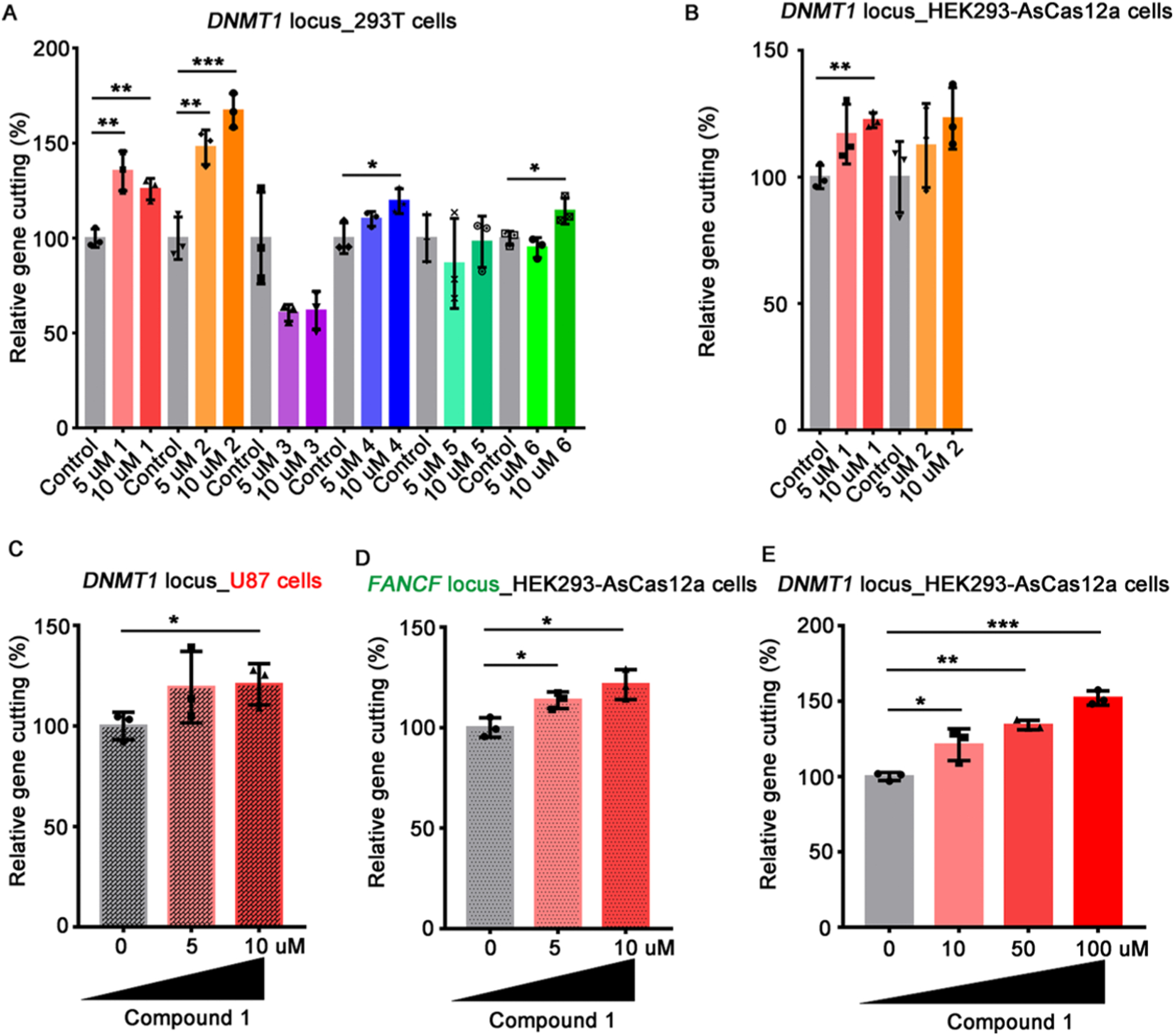
Chemical compounds affect the genome editing efficiency of the AsCas12a-crRNAs system. (A) Effects of chemical compounds on gene-editing efficiency of AsCas12a-crDNMT1–3 in 293T cells. (B) Effects of compounds 1 and 2 on gene-editing efficiency of crDNMT1–3 in HEK293-AsCas12a cells. (C) Compound 1 increased gene-editing efficiency of AsCas12a-crDNMT1–3 in U87 cells. (D) Compound 1 increased the gene-editing efficiency of crFANCF-2 in HEK293-AsCas12a cells. (E) Effects of increased concentrations of compound 1 on gene-editing efficiency of crDNMT1–3 in HEK293-AsCas12a cells. Data are shown as mean ± SD (n = 3); *P < 0.05; **P < 0.01; ***P < 0.001, two-tailed t test.
Disrupting protein–protein interactions by small molecules has become an increasingly popular strategy in drug discovery. However, the design of small molecules that stabilize protein–protein interactions is under appreciated, whereas the proteolysis targeting chimera (PROTAC) technique that uses heterobifunctional small molecules to promote transient interactions between a target protein and an E3 ligase to degrade the protein has recently attracted a lot of attention.24,25 An essential premise of this work is that the AsCas12a function can be modulated by the design of small molecules that selectively stabilize the different functional states of the enzyme complex.
We first employed large-scale MD simulations to thoroughly explore the conformational space of AsCas12a in the different functional states. The enzyme was found to adopt distinct conformations, which are believed to correspond to different functional states of the enzyme along the genome cleavage pathway (Figure 1A). We thus obtained representative structures of AsCas12a from the simulations and then computationally docked two libraries of ~220,000 compounds to these selected structures. The “hit” compounds were chosen based on the docking score difference between the binding of a molecule to two different functional states of AsCas12a in addition to the conventional scoring criteria. This filter resulted in 57 small molecules that can bind differentially to the different states of AsCas12a. After experimental testing of 6 of these compounds, we confirmed compound 1, quinazoline-2,4(1H,3H)-dione, as a small molecule enhancer of AsCas12a. Compound 1 was found to act like a molecular “glue” at the interface between AsCas12a and crRNA near the 5′-handle region (Figure 1D) to stabilize the complex structure. It forms extensive electrostatic interactions with positively charged residues Lys748, Lys1029 and polar residue Asn759 (Figure 1D). Its aromatic rings stacking against the nearby nucleotides may further strengthen the “gluing” effect.
CONCLUSION
Overall, our molecular dynamics simulations revealed distinct conformations in AsCas12a that could be targeted by small molecules to modulate its genome editing function (Figure 1C), which provided a unique approach to understand the CRISPR-Cas systems and rationally design new agents to modulate the dynamic function of the CRISPR-Cas systems.
Supplementary Material
ACKNOWLEDGMENTS
All the computation was performed using the computing resources at the Ohio Supercomputer Center.
Funding
Y.D. acknowledges the support from the NIH through the National Heart, Lung, and Blood Institute (grant R01HL136652), as well as the start-up fund from the College of Pharmacy at The Ohio State University. X.C. acknowledges the start-up fund from the College of Pharmacy and the Discovery Themes at The Ohio State University.
ABBREVIATIONS
- CRISPR
clustered regularly interspaced short palindromic repeats
- NHEJ
homologous end joining
- HDR
directed repair
- MD
molecular dynamics
- RMSD
root-mean squared deviation
- PC
principal component
- PROTAC
proteolysis targeting chimera
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.bioconjchem.0c00062.
Materials and methods: molecular dynamics (MD); molecular docking; cell culture; MTT assay; AsCas12a mRNA and crRNAs; gene editing in cells; MiSeq library preparation for deep sequencing; statistical analysis; and associated figures (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.bioconjchem.0c00062
The authors declare the following competing financial interest(s): Authors RT and MAB are employees of Integrated DNA Technologies, Inc., (IDT) which offers reagents for sale similar to some of the compounds described in the manuscript. The authors receive compensation in the form of salary from IDT. MAB owns equity in DHR, the parent company of IDT.
Contributor Information
Wenqing Li, Division of Pharmaceutics & Pharmacology, College of Pharmacy, The Ohio State University, Columbus, Ohio 43210, United States.
Chun Chan, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, Columbus, Ohio 43210, United States.
Chunxi Zeng, Division of Pharmaceutics & Pharmacology, College of Pharmacy, The Ohio State University, Columbus, Ohio 43210, United States.
Rolf Turk, Integrated DNA Technologies, Inc., Coralville, Iowa 52241, United States.
Mark A. Behlke, Integrated DNA Technologies, Inc., Coralville, Iowa 52241, United States
Xiaolin Cheng, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, Columbus, Ohio 43210, United States.
Yizhou Dong, Division of Pharmaceutics & Pharmacology, College of Pharmacy and Department of Biomedical Engineering, The Center for Clinical and Translational Science, The Comprehensive Cancer Center, Dorothy M. Davis Heart & Lung Research Institute, Department of Radiation Oncology, The Ohio State University, Columbus, Ohio 43210, United States.
REFERENCES
- (1).Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gasiunas G, Barrangou R, Horvath P, and Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. U. S. A 109, E2579–E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, et al. (2013) Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mali P, Yang LH, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, and Church GM (2013) RNA-Guided Human Genome Engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Van der Oost J, Westra ER, Jackson RN, and Wiedenheft B (2014) Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat. Rev. Microbiol 12, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, et al. (2015) Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 163, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fonfara I, Richter H, Bratovic M, Le Rhun A, and Charpentier E (2016) The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521. [DOI] [PubMed] [Google Scholar]
- (8).Stella S, Mesa P, Thomsen J, Paul B, Alcon P, Jensen SB, Saligram B, Moses ME, Hatzakis NS, and Montoya G (2018) Conformational Activation Promotes CRISPR-Cas12a Catalysis and Resetting of the Endonuclease Activity. Cell 175, 1856–1871. [DOI] [PubMed] [Google Scholar]
- (9).Yamano T, Zetsche B, Ishitani R, Zhang F, Nishimasu H, and Nureki O (2017) Structural Basis for the Canonical and Non-canonical PAM Recognition by CRISPR-Cpf1. Mol. Cell 67, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Swarts DC, van der Oost J, and Jinek M (2017) Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol. Cell 66, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Stella S, Alcon P, and Montoya G (2017) Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature 546, 559–563. [DOI] [PubMed] [Google Scholar]
- (12).Palermo G, Ricci CG, Fernando A, Basak R, Jinek M, Rivalta I, Batista VS, and McCammon JA (2017) Protospacer Adjacent Motif-Induced Allostery Activates CRISPR-Cas9. J. Am. Chem. Soc 139, 16028–16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yamano T, Nishimasu H, Zetsche B, Hirano H, Slaymaker IM, Li Y, Fedorova I, Nakane T, Makarova KS, Koonin EV, et al. (2016) Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 165, 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dong D, Ren K, Qiu X, Zheng J, Guo M, Guan X, Liu H, Li N, Zhang B, Yang D, et al. (2016) The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532, 522–526. [DOI] [PubMed] [Google Scholar]
- (15).Swarts DC, and Jinek M (2019) Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol. Cell 73, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Palermo G, Miao YL, Walker RC, Jinek M, and McCammon JA (2017) CRISPR-Cas9 conformational activation as elucidated from enhanced molecular simulations. Proc. Natl. Acad. Sci. U. S. A 114, 7260–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Li B, Zhao W, Luo X, Zhang X, Li C, Zeng C, and Dong Y (2017) Engineering CRISPR-Cpf1 crRNAs and mRNAs to maximize genome editing efficiency. Nature biomedical engineering 1, 0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Li B, Zeng C, and Dong Y (2018) Design and assessment of engineered CRISPR-Cpf1 and its use for genome editing. Nat. Protoc 13, 899–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kleinstiver BP, Sousa AA, Walton RT, Tak YE, Hsu JY, Clement K, Welch MM, Horng JE, Malagon-Lopez J, Scarfo I, et al. (2019) Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol 37, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chow RD, Wang GC, Ye LP, Codina A, Kim HR, Shen L, Dong MB, Errami Y, and Chen SD (2019) In vivo profiling of metastatic double knockouts through CRISPR-Cpf1 screens. Nat. Methods 16, 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ma X, Chen X, Jin Y, Ge W, Wang W, Kong L, Ji J, Guo X, Huang J, Feng XH, et al. (2018) Small molecules promote CRISPR-Cpf1-mediated genome editing in human pluripotent stem cells. Nat. Commun 9, 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Riesenberg S, and Maricic T (2018) Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun 9, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Humphrey W, Dalke A, and Schulten K (1996) VMD: visual molecular dynamics. J. Mol. Graphics 14, 33–38. [DOI] [PubMed] [Google Scholar]
- (24).Cromm PM, and Crews CM (2017) Targeted Protein Degradation: from Chemical Biology to Drug Discovery. Cell chemical biology 24, 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Paiva SL, and Crews CM (2019) Targeted protein degradation: elements of PROTAC design. Curr. Opin. Chem. Biol 50, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


