Abstract
Background
The COVID-19 pandemic has heavily impacted elective and emergency surgery around the world. We aimed to confirm the incidence of perioperative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and associated mortality after surgery.
Methods
Analysis of routine electronic health record data from NHS hospitals in England. We extracted data from Hospital Episode Statistics in England describing adult patients undergoing surgery between January 1, 2020 and February 28, 2021. The exposure was SARS-CoV-2 infection defined by International Classification of Diseases (ICD)-10 codes. The primary outcome measure was 90 day in-hospital mortality. Data were analysed using multivariable logistic regression adjusted for age, sex, Charlson Comorbidity Index, Index of Multiple Deprivation, presence of cancer, surgical procedure type and admission acuity. Results are presented as n (%) and odds ratios (OR) with 95% confidence intervals (CI).
Results
We identified 2 666 978 patients undergoing surgery of whom 28 777 (1.1%) had SARS-CoV-2 infection. In total, 26 364 (1.0%) patients died in hospital. SARS-CoV-2 infection was associated with a much greater risk of death (SARS-CoV-2: 6153/28 777 [21.4%] vs no SARS-CoV-2: 20 211/2 638 201 [0.8%]; OR=5.7 [95% CI, 5.5–5.9]; P<0.001). Amongst patients undergoing elective surgery, 2412/1 857 586 (0.1%) had SARS-CoV-2, of whom 172/2412 (7.1%) died, compared with 1414/1 857 586 (0.1%) patients without SARS-CoV-2 (OR=25.8 [95% CI, 21.7–30.9]; P<0.001). Amongst patients undergoing emergency surgery, 22 918/582 292 (3.9%) patients had SARS-CoV-2, of whom 5752/22 918 (25.1%) died, compared with 18 060/559 374 (3.4%) patients without SARS-CoV-2 (OR=5.5 [95% CI, 5.3–5.7]; P<0.001).
Conclusions
The low incidence of SARS-CoV-2 infection in NHS surgical pathways suggests current infection prevention and control policies are highly effective. However, the high mortality amongst patients with SARS-CoV-2 suggests these precautions cannot be safely relaxed.
Keywords: anaesthesia, COVID-19, epidemiology, public policy, surgery
Editor's key points.
-
•
Patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection undergoing surgery have much higher risk of severe postoperative respiratory complications and death.
-
•
This study found that 1 in 100 surgical patients were infected by SARS-CoV-2 in the English NHS through 2020, most of whom had required emergency surgery.
-
•
Elective surgical patients who developed SARS-CoV-2 infection were 25 times more likely to die while in hospital.
-
•
Strict, additional infection prevention and control procedures are crucially important for perioperative care during a pandemic.
Surgery is an essential treatment modality with more than 330 million surgical procedures performed worldwide every year.1, 2, 3 However, the COVID-19 pandemic has led to substantial reductions in the volume of surgery performed.4 , 5 Recent estimates suggest that half of the 4.5 million expected surgical procedures in the English NHS were cancelled or postponed in 2020.4 This is partly driven by reallocation of resources to care for patients with COVID-19, but also by strict infection prevention and control procedures implemented to prevent patients becoming infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) while in hospital.6 Patient self-isolation before surgery, preoperative testing for SARS-CoV-2, creation of separate ‘green zones’ in hospitals to isolate non-infected patients, and personal protective equipment procedures all contribute to reductions in the efficiency of surgical care pathways, and substantial reductions in the volume of patients treated.7
Early reports have suggested that patients with SARS-CoV-2 infection who undergo surgery are at much greater risk of postoperative pulmonary complications and death.8 , 9 The COVIDSurg Collaborative undertook a large international study of outcomes for surgical patients infected with SARS-CoV-2 and reported mortality rates as high as 24% at the peak of the pandemic.8 However, more recent data suggest the mortality risk for surgical patients with SARS-CoV-2 may be lower than originally thought,9 especially for minor surgery in younger patients.10 It remains unclear how SARS-CoV-2 infection affects outcomes after surgery, and whether high mortality rates reported in some studies relate to a change in surgical case mix during the pandemic, or to SARS-CoV-2 infection itself. At present there are no large studies describing surgical outcomes for contemporaneous patient groups with and without SARS-CoV-2 infection. Given the extensive disruption to surgical services and the likely excess mortality owing to untreated cancer and other surgical diseases, the NHS is under significant pressure to relax infection prevention and control procedures to increase the volume of patients who can undergo surgery.
We used routine NHS electronic health record data to report the rate of SARS-CoV-2 infection amongst surgical patients during the pandemic, and the associated mortality and hospital stay.
Methods
Study design
Population-wide epidemiological study using routinely collected electronic health record data.
Setting
NHS hospitals in England.
Participants
All patients aged 18 yr or older who underwent a surgical procedure between January 1 and February 28, 2021.
Data source
We used pseudonymised record level Hospital Episode Statistics (HES) to identify eligible patients. This data source provides detailed data describing every episode of hospital care in England. Surgical procedures were identified using Office for Population Censuses Surveys version 4 (OPCS4) codes as described previously.2 , 4 These define procedures typically performed in an operating theatre or under general/regional anaesthesia (Appendix A). Individuals entered the cohort on the date of their first operative procedure and were followed until the point of hospital discharge. Hospital discharge date was determined based on the discharge from continuous in-patient stays, which were constructed by mapping continuous in-patient episodes.11 For patients remaining in hospital, their discharge date was right censored to February 28, 2021. The analysis was approved by the Health Research Authority (20/HRA/3121) and the NHS Digital Independent Group Advising on the Release of Data (DARS-NIC-375669-J7M7F).
Exposure
The exposure of interest was a diagnosis of SARS-CoV-2 viral infection defined using the following International Classification of Diseases (ICD)-10 codes: U07.1 (Virus identified), U07.2 (Virus not identified) or B97.2 (Other coronavirus as the cause of diseases classified elsewhere). Patients with SARS-CoV-2 were categorised as symptomatic if they had ICD-10 codes for concomitant respiratory illness or OPCS-4 codes indicating respiratory support (Supplementary Table S1). Patients with SARS-CoV-2 were categorised as not symptomatic if there were no associated ICD-10 codes for respiratory illness or OPCS-4 codes for respiratory support. We divided the exposure into three time points: preoperative if SARS-CoV-2 codes were first recorded in an episode that finished within the 30 days before the index operation; perioperative if a code was first recorded in the same episode as the index operation; and postoperative if a code was first recorded in a subsequent healthcare episode before discharge within 30 days after the index operation.
Outcomes
The primary outcome was in-hospital death, censored at 90 days for patients remaining in hospital beyond this point. The secondary outcome was length of hospital stay, calculated as the number of days between initial operation date and discharge date of their continuous in-patient stay, censored at 90 days.
Derivation of variables
Age was defined as that recorded on the start date of the hospital episode including the first operative procedure. Charlson Comorbidity Index was derived according to the Royal College of Surgeons mapping, which includes a 1 yr look-back file to capture diagnoses from prior admissions.12 Classification of the type of surgical procedure was based on the first operative code.4 Where multiple operative codes were associated with surgery on a single day, the highest ranked code was considered the principal surgical procedure. Socioeconomic deprivation was defined according to lower super-output areas using the Index of Multiple Deprivation (IMD).13 We classified missing IMD as its own category.
Statistical methods
The incidence of SARS-CoV-2 infection among patients undergoing surgery was calculated by dividing the number of patients with an ICD-10 code indicating SARS-CoV-2 infection by the total number of patients in the cohort. Age-adjusted incidence was calculated using deciles of age.14 To test for associations between SARS-CoV-2 infection and in-hospital mortality, we used multivariable logistic regression analysis including the following covariates: age, sex, Charlson Comorbidity Index, IMD, presence of cancer, surgical procedure type, and admission acuity.15, 16, 17, 18 Results are presented as n (%), mean (standard deviation [sd]), median (inter-quartile range), or as odds ratios (OR) with 95% confidence intervals (CI) and P-values. The threshold for statistical significance was P<0.05. We undertook two pre-specified sub-group analyses. First, we assessed the risk of mortality among patients with SARS-CoV-2 infection stratified by urgency of surgery (elective surgery or emergency surgery). Second, we compared the risk of mortality among patients with SARS-CoV-2 infection between patients with and without respiratory symptoms. Analyses were conducted using Python (version 3.7.5) and graphs made in R (V4.0.2; R-project, Vienna, Austria).
Sensitivity analysis using laboratory data from NHS Wales
To check the completeness of ICD-10 coding for a positive SARS-CoV-2 diagnosis, we compared reported cases of SARS-CoV-2 infection using ICD-10 codes to the integrated central laboratory records system describing all SARS-CoV-2 test results in Wales, which includes results of tests conducted for Welsh residents from both NHS and private laboratories. SARS-CoV-2 swabs collected from between 30 days before to the end of the index surgical spell were used to identify perioperative SARS-CoV-2. This analysis used pseudonymised record level Patient Episode Database in Wales (PEDW) to identify eligible patients. The project was approved by the Secure Anonymised Information Linkage Databank (SAIL) independent Information Governance Review Panel (Project number: 0911).19
Post-hoc sensitivity analysis of hospital readmission and postoperative SARS-CoV-2 infection
Given the long incubation period of SARS-CoV-2 infection, we report the frequency of emergency hospital re-admission in England within 30 days of surgery, stratified by the presence of SARS-CoV-2 infection ICD-10 codes.
Sample size calculation
This was a population-wide cohort study including all patients who underwent surgery in England during the study period. A sample size of 2 million patients, with an allocation ratio of 0.01, and an alpha of 0.05 would give approximately 100% power to detect a 10% difference in the relative risk of mortality among patients with and without SARS-CoV-2 infection.
Results
Participants
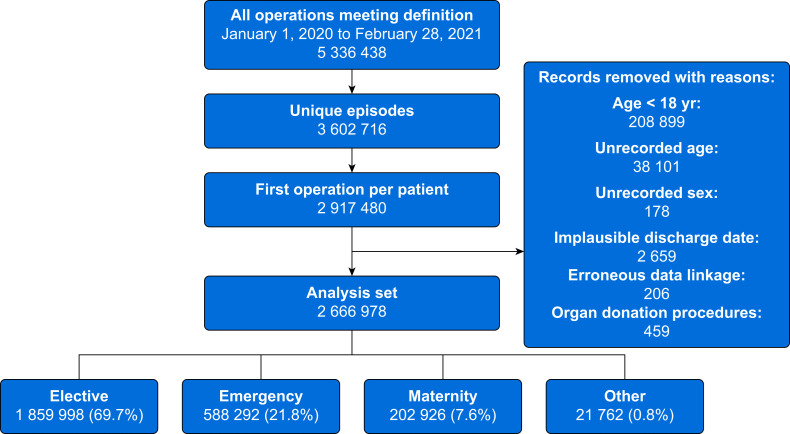
There were 5 336 438 surgical procedures among 2 917 480 patients in England between January 1, 2020 and February 28, 2021. After predefined exclusions (209 358; 7%) and exclusion of those with missing data (41 144; 1.4%), 2 666 978 patients undergoing surgery were included in the primary analysis (Fig. 1 ). Demographic data are presented in Table 1 . Data are presented according to surgical specialty in Table 2 . Demographic data of patients with missing age data are shown in Supplementary Table S2.
Fig 1.
Inclusion of patients in the analysis.
Table 1.
Patient characteristics. Numbers are presented as n (%) unless otherwise stated. ∗Numbers suppressed to maintain patient anonymity. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sd, standard deviation; IQR, inter-quartile range; IMD, Index of Multiple Deprivation.
| All patients | SARS-CoV-2 status |
|||||
|---|---|---|---|---|---|---|
| None | Preoperative | Perioperative | Postoperative | Any time | ||
| N | 2 666 978 | 2 638 201 (98.9%) | 18 484 (0.7%) | 2875 (0.1%) | 7418 (0.3%) | 28 777 (1.1%) |
| Age | ||||||
| Mean (sd) | 57 (20) | 57 (20) | 62 (18) | 64 (21) | 76 (15) | 65 (20) |
| Median (IQR) | 60 (40–74) | 60 (40–74) | 63 (50–75) | 68 (49–81) | 79 (68–87) | 68 (51–81) |
| Sex | ||||||
| Male | 1 182 896 (44.4%) | 1 168 372 (98.8%) | 4279 (0.4%) | 8829 (0.7%) | 1416 (0.1%) | 14 524 (1.2%) |
| Female | 1 483 913 (55.6%) | 1 469 660 (99%) | 3139 (0.2%) | 9655 (0.7%) | 1459 (0.1%) | 14 253 (1%) |
| Unspecified | 169 (0.0%) | 169 (0.0%) | ∗ (%∗) | ∗ (%∗) | ∗ (%∗) | ∗ (%∗) |
| Admission category | ||||||
| Elective | 1 859 998 (69.7%) | 1 857 586 (99.9%) | 809 (0%) | 1456 (0.1%) | 147 (0%) | 2412 (0.1%) |
| Emergency | 582 292 (21.8%) | 559 374 (96.1%) | 5543 (1%) | 14 721 (2.5%) | 2654 (0.5%) | 22 918 (3.9%) |
| Other | 21 762 (0.8%) | 20 568 (94.5%) | 634 (2.9%) | 491 (2.3%) | 69 (0.3%) | 1194 (5.5%) |
| Maternity | 202 926 (7.6%) | 200 673 (98.9%) | 432 (0.2%) | 1816 (0.9%) | ∗ (%∗) | 2253 (1.1%) |
| Inpatient/day-case | ||||||
| Inpatient | 1 343 864 (50.4%) | 1 315 606 (97.9%) | 7049 (0.5%) | 18 337 (1.4%) | 2872 (0.2%) | 28 258 (2.1%) |
| Day-case | 1 323 114 (49.6%) | 1 322 595 (100%) | 369 (0%) | 147 (0%) | ∗ (%∗) | 519 (0%) |
| Charlson Comorbidity Index | ||||||
| Score >0 | 1 238 969 (46.5%) | 1 217 783 (98.3%) | 5781 (0.5%) | 12 920 (1%) | 2485 (0.2%) | 21 186 (1.7%) |
| Index of Multiple Deprivation | ||||||
| 1 – most deprived | 514 727 (19.3%) | 507 170 (98.5%) | 2109 (0.4%) | 4818 (0.9%) | 630 (0.1%) | 7557 (1.5%) |
| 2 | 526 432 (19.7%) | 520 243 (98.8%) | 1680 (0.3%) | 3966 (0.8%) | 543 (0.1%) | 6189 (1.2%) |
| 3 | 532 351 (20.0%) | 526 934 (99%) | 1361 (0.3%) | 3486 (0.7%) | 570 (0.1%) | 5417 (1%) |
| 4 | 534 211 (20.0%) | 529 434 (99.1%) | 1152 (0.2%) | 3075 (0.6%) | 550 (0.1%) | 4777 (0.9%) |
| 5 – least deprived | 532 808 (20.0%) | 528 280 (99.2%) | 1059 (0.2%) | 2918 (0.5%) | 551 (0.1%) | 4528 (0.8%) |
| Missing IMD status | 26 449 (1.0%) | 26 140 (98.8%) | 57 (0.2%) | 221 (0.8%) | 31 (0.1%) | 309 (1.2%) |
Table 2.
Risk of in-hospital mortality stratified by anatomical location of primary procedure. Data presented as n (%). ∗Numbers suppressed to maintain patient anonymity. Risk of in-hospital mortality stratified by surgical specialty. Organ donation removed given low numbers. GI; gastrointestinal; HPB, hepatopancreatobiliary; LGU, lower genitourinary; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UGU, upper genitourinary.
| SARS-CoV-2 infection |
No SARS-CoV-2 infection |
|||
|---|---|---|---|---|
| n | In-hospital death | n | In-hospital death | |
| Bone | 3417 | 815 (23.9%) | 132 180 | 1909 (1.4%) |
| Breast | 43 | ∗ (%∗) | 56 450 | 12 (0.0%) |
| Cardiac | 2374 | 313 (13.2%) | 153 894 | 2609 (1.7%) |
| Cerebrovascular | 116 | 21 (18.1%) | 4769 | 211 (4.4%) |
| Ear | 56 | 10 (17.9%) | 27 756 | 28 (0.1%) |
| Endocrine | 46 | ∗ (%∗) | 14 778 | 32 (0.2%) |
| Female LGU | 48 | ∗ (%∗) | 24 587 | 19 (0.1%) |
| Female UGU | 348 | ∗ (%∗) | 128 201 | 51 (0.0%) |
| HPB | 752 | 120 (16.0%) | 74 445 | 723 (1.0%) |
| Joint | 3067 | 755 (24.6%) | 196 034 | 1860 (0.9%) |
| Lower GI | 2393 | 422 (17.6%) | 227 230 | 2369 (1.0%) |
| Major vessel | 457 | 112 (24.5%) | 19 330 | 819 (4.2%) |
| Male GU | 126 | 19 (15.1%) | 33 521 | 68 (0.2%) |
| Muscle | 157 | 26 (16.6%) | 56 427 | 84 (0.1%) |
| Nasal | 181 | 36 (19.9%) | 48 691 | 106 (0.2%) |
| Neurological | 1675 | 436 (26.0%) | 123 866 | 2654 (2.1%) |
| Obstetrics | 2489 | ∗ (%∗) | 215 432 | 11 (0.0%) |
| Ocular | 133 | ∗ (%∗) | 376 152 | 40 (0.0%) |
| Oral | 130 | 19 (14.6%) | 83 209 | 71 (0.1%) |
| Orthopaedics | 30 | ∗ (%∗) | 13 823 | 11 (0.1%) |
| Other | 395 | 111 (28.1%) | 32 119 | 564 (1.8%) |
| Pharynx | 45 | 9 (20.0%) | 15 182 | 48 (0.3%) |
| Skin | 1475 | 325 (22.0%) | 233 798 | 1059 (0.5%) |
| Skull and spine | 335 | 39 (11.6%) | 62 216 | 279 (0.4%) |
| Thoracic | 4722 | 1503 (31.8%) | 20 185 | 1122 (5.6%) |
| Upper GI | 999 | 258 (25.8%) | 33 128 | 1228 (3.7%) |
| Urological | 978 | 208 (21.3%) | 165 758 | 816 (0.5%) |
| Vascular | 1790 | 562 (31.4%) | 65 040 | 1408 (2.2%) |
Incidence of SARS-CoV-2 among surgical patients
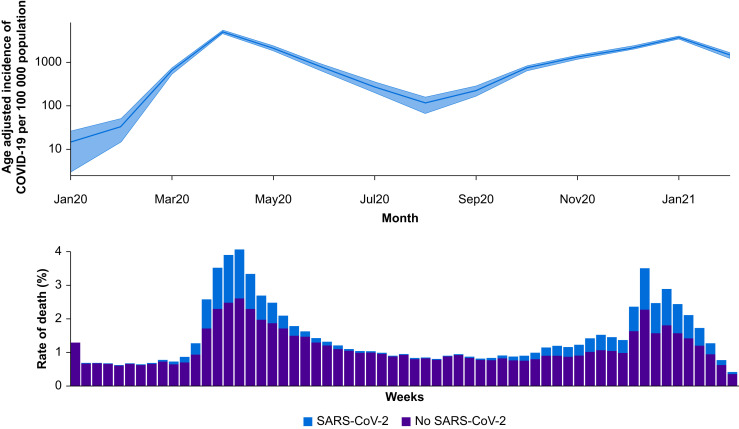
Among 2 666 978 adult patients who underwent surgery, 28 777 (1.1%) had SARS-CoV-2 (Table 1 and Fig. 2 ). Overall, 15 637/28 777 (54.3%) surgical patients with SARS-CoV-2 infection had respiratory symptoms. The age-adjusted incidence of SARS-CoV-2 infection among surgical patients was 934 infections (95% CI, 899–971) per 100 000.
Fig 2.
Top panel: age-adjusted incidence of SARS-CoV-2 infection amongst surgical patients, reported as the number of cases per 100 000 population (y axis on log scale). Bottom panel: weekly rate of death, stratified by SARS-CoV-2 infection status. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Patient outcomes
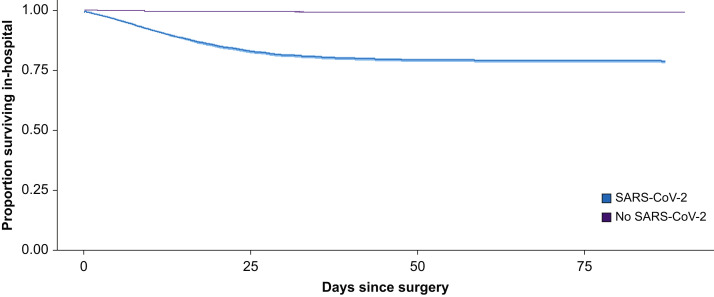
Overall, 26 364/2 666 978 (1.0%) patients who underwent a surgical procedure died. A total of 6153 of 28 777 (21.4%) surgical patients with SARS-CoV-2 infection died compared with 20 211/2 638 201 (0.8%) patients without SARS-CoV-2 infection (Table 3 ). The adjusted odds of death among surgical patients with SARS-CoV-2 infection compared with surgical patients without infection is 5.7 (95% CI, 5.5–5.9; P<0.001) (Table 4 ). Length of hospital stay among patients with and without SARS-CoV-2 infection is presented in Table 3. The survival curves of those with and without SARS-CoV-2 infection are presented in Figure 3 .
Table 3.
Mortality and hospital length of stay, stratified by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection status and acuity of surgery. Data are presented as median (inter-quartile range) or n (%).
| No SARS-CoV-2 | SARS-CoV-2 infection |
||||
|---|---|---|---|---|---|
| Any | Preoperative | Perioperative | Postoperative | ||
| All patients | |||||
| n | 2 638 201 | 28 777 | 7418 | 18 484 | 2875 |
| In-hospital death | 20 211 (0.8%) | 6153 (21.4%) | 1544 (20.8%) | 3755 (20.3%) | 854 (29.7%) |
| Length of hospital stay (days) | 0 (0–2) | 13 (4–26) | 9 (2–22) | 12 (4–25) | 23 (15–31) |
| Elective surgery | |||||
| n | 1 857 586 | 2412 | 809 | 1456 | 147 |
| In-hospital death | 1414 (0.1%) | 172 (7.1%) | 21 (2.6%) | 125 (8.6%) | 26 (17.7%) |
| Length of hospital stay (days) | 0 (0–1) | 4 (1–16) | 0 (0–3) | 7 (2–20) | 24 (14–33) |
| Emergency surgery | |||||
| n | 559 374 | 22 918 | 5543 | 14 721 | 2654 |
| In-hospital death | 18 060 (3.2%) | 5752 (25.1%) | 1414 (25.5%) | 3532 (24.0%) | 806 (30.4%) |
| Length of hospital stay (days) | 2 (1–7) | 15 (6–28) | 11 (4–24) | 15 (6–28) | 23 (15–31) |
Table 4.
Multivariable logistic regression analysis for in-hospital mortality within 90 days. Patients with unspecified sex removed before analysis. CI, confidence interval; GI, gastrointestinal; HPB, hepatopancreatobiliary; LGU, lower genitourinary; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UGU, upper genitourinary.
| Odds ratio | Lower 95% CI | Upper 95% CI | P value | |
|---|---|---|---|---|
| Intercept | <0.01 | <0.01 | <0.01 | <0.001 |
| SARS-CoV-2 code present | 5.7 | 5.5 | 5.91 | <0.001 |
| Female compared with male | 0.81 | 0.79 | 0.83 | <0.001 |
| Age (yr) | 1.03 | 1.03 | 1.04 | <0.001 |
| Cancer present | 1.04 | 1.01 | 1.08 | 0.01 |
| Charlson Comorbidity Score: reference=0 | ||||
| Score 1 | 2.41 | 2.3 | 2.54 | <0.001 |
| Score 2 | 3.72 | 3.53 | 3.92 | <0.001 |
| Score 3 | 5.01 | 4.74 | 5.3 | <0.001 |
| Score 4 | 6.17 | 5.8 | 6.56 | <0.001 |
| Score 5 | 7.07 | 6.57 | 7.61 | <0.001 |
| Score ≥6 | 7.71 | 7.09 | 8.38 | <0.001 |
| Index of Multiple Deprivation: reference=quintile 1 (most deprived) | ||||
| Quintile 2 | 0.99 | 0.95 | 1.03 | 0.55 |
| Quintile 3 | 0.94 | 0.9 | 0.98 | <0.001 |
| Quintile 4 | 0.88 | 0.85 | 0.92 | <0.001 |
| Quintile 5 (least deprived) | 0.88 | 0.84 | 0.91 | <0.001 |
| Missing quintile | 1.17 | 1.03 | 1.33 | 0.016 |
| Admission category: reference=elective | ||||
| Emergency | 23.54 | 22.33 | 24.83 | <0.001 |
| Maternity | 2.07 | 0.78 | 5.46 | 0.14 |
| Other | 16.59 | 15.2 | 18.08 | <0.001 |
| Procedure grouping: reference=bone | ||||
| Breast | 0.23 | 0.14 | 0.38 | <0.001 |
| Cardiac | 0.97 | 0.91 | 1.02 | 0.24 |
| Cerebrovascular | 2.67 | 2.31 | 3.09 | <0.001 |
| Ear | 0.43 | 0.31 | 0.59 | <0.001 |
| Endocrine | 1.32 | 0.94 | 1.86 | 0.107 |
| Female LGU | 0.51 | 0.34 | 0.79 | 0.002 |
| Female UGU | 0.37 | 0.28 | 0.49 | <0.001 |
| HPB | 1.53 | 1.41 | 1.67 | <0.001 |
| Joint | 1.05 | 0.99 | 1.12 | 0.077 |
| Lower GI | 1.73 | 1.63 | 1.83 | <0.001 |
| Major vessel | 2.72 | 2.5 | 2.96 | <0.001 |
| Male GU | 0.69 | 0.55 | 0.87 | 0.001 |
| Muscle | 0.81 | 0.66 | 0.99 | 0.035 |
| Nasal | 0.46 | 0.38 | 0.54 | <0.001 |
| Neuro | 3.46 | 3.26 | 3.66 | <0.001 |
| Obstetrics | 0.31 | 0.12 | 0.77 | 0.012 |
| Ocular | 0.09 | 0.06 | 0.11 | <0.001 |
| Oral | 0.63 | 0.51 | 0.78 | <0.001 |
| Orthopaedics | 0.43 | 0.25 | 0.74 | 0.003 |
| Other | 2.52 | 2.3 | 2.77 | <0.001 |
| Pharynx | 1.71 | 1.3 | 2.26 | <0.001 |
| Skin | 0.83 | 0.77 | 0.89 | <0.001 |
| Skull and spine | 1.38 | 1.22 | 1.56 | <0.001 |
| Thoracic | 5.07 | 4.75 | 5.41 | <0.001 |
| Upper GI | 3.3 | 3.08 | 3.54 | <0.001 |
| Urological | 0.89 | 0.83 | 0.97 | 0.004 |
| Vascular | 1.85 | 1.74 | 1.98 | <0.001 |
Fig 3.
Kaplan–Meier survival curves stratified by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 status).
Urgency of surgery
Amongst patients undergoing elective surgery, 2412/1 859 998 (0.1%) had SARS-CoV-2 infection, of whom 172/2412 (7.1%) died compared with 1414/1 857 586 (0.1%) patients without SARS-CoV-2 infection (Table 3). Therefore, the incidence of death from SARS-CoV-2 infection among elective surgical patients was 172/1 859 998 (0.009%, or 1 in 10 814). The adjusted odds of death among patients undergoing elective surgery with SARS-CoV-2 around the time of surgery is 25.8 (95% CI, 21.7–30.9; P<0.001) (Supplementary Table S3). Amongst patients undergoing emergency surgery, 22 918/582 292 (3.9%) had SARS-CoV-2 infection, of whom 5752/22 918 (25.1%) died compared with 18 060/559 374 (3.2%) patients without SARS-CoV-2 infection. The adjusted odds of death among patients undergoing emergency surgery with SARS-CoV-2 around the time of surgery is 5.5 (95% CI, 5.3–5.7; P<0.001) (Supplementary Table S4).
Symptomatic SARS-CoV-2 infection
Among patients with SARS-CoV-2 infection and respiratory symptoms, 5265/15 637 (33.7%) died compared with 888/13 140 (6.8%) of patients with SARS-CoV-2 infection but no respiratory symptoms recorded (OR=5.5; 95% CI, 5.1–6.0; P<0.001) (Supplementary Tables S5 and S6).
Incidence of SARS-CoV-2 infection among surgical patients in Wales
We identified 116 531 patients undergoing their first surgical procedure in Wales between January 1, 2020 and February 28, 2021. In Wales, a higher proportion of patients underwent emergency surgery (29.4%) than in England (21.8%). Using ICD-10 codes alone, 1834/116 531 (1.5%) surgical patients in Wales had SARS-CoV-2 infection. Using SARS-CoV-2 polymerase chain reaction (PCR) tests resulted in a marginally decreased incidence of 1515/116 531 (1.3%). Using either ICD-10 code or SARS-CoV-2 PCR definitions, the incidence rose to 2182/116 531 (1.8%). Among elective surgical patients in Wales, SARS-CoV-2 infection ICD-10 codes were recorded amongst 166/72 262 (0.23%) patients. Adding positive SARS-CoV-2 PCR test results resulted in an increased incidence of 271/72 262 (0.38%) (Supplementary Table S7).
Post-hoc sensitivity analysis of emergency hospital readmission
A total of 2 587 789 patients were discharged alive before 30 days, and so eligible for hospital readmission. We identified 155 717 hospital readmissions within 30 days of surgery (6.1%), of which 6381 had a SARS-CoV-2 code recorded (0.2%). The rate of emergency hospital readmission with SARS-CoV-2 was 0.8% (4352/516 209) amongst patients who had emergency surgery, and 0.1% (1784/1 852 651) amongst patients undergoing elective surgery (Supplementary Table S8).
Discussion
The principal finding of this population-wide epidemiological study is that the incidence of SARS-CoV-2 infection among NHS surgical patients in England through 2020 and up to February 28, 2021 was 1.1%. Among those who were discharged, one in 405 were readmitted with SARS-CoV-2 within 30 days. Most hospital admissions with SARS-CoV-2 within 30 days of surgery were among patients who had emergency surgery. Where perioperative SARS-CoV-2 infection did occur, it was associated with more than a 5-fold increase in risk of in-hospital mortality. Meanwhile, the very low risk of in-hospital death (one in 1000) among patients undergoing elective surgery who have not been infected with SARS-CoV-2 suggests that infection prevention and control procedures to create COVID-19-free ‘green’ surgical pathways have been highly effective during the pandemic.20 , 21 Surgical patients with symptomatic SARS-CoV-2 infection were five times more likely to die compared with infected patients without respiratory symptoms.
The incidence of SARS-CoV-2 infection requiring hospitalisation and subsequent deaths reported in this study represent the measured population incidences, rather than an estimate from a population sample. Our findings are consistent with previous estimates of perioperative mortality associated with SARS-CoV-2 infection.22, 23, 24, 25, 26, 27 The largest of these is the COVIDSurg international multicentre observational cohort study of 1128 surgical patients with confirmed SARS-CoV-2 infection, which was conducted during the first quarter of 2020.8 It reported a 30-day mortality rate of 23.8%. However, the cohort consisted of only patients with confirmed SARS-CoV-2, so it was not possible to infer the relative risk of mortality associated with perioperative SARS-CoV-2 infection compared with a contemporaneous non-infected comparator. Our data contextualise these findings from early in the pandemic by reporting the population risk of mortality among surgical patients with SARS-CoV-2 in England using a cohort of patients with SARS-CoV-2 infection that is more than 10 times larger. Because we included all patients undergoing surgery in England, we were able to compare the risk of mortality among patients with SARS-CoV-2 infection to patients without infection to provide an estimate of the relative risk of death associated with SARS-CoV-2 infection. This important finding, which until now was unknown, could inform shared decision-making about surgical care of patients with SARS-CoV-2.
The volume of surgical activity during the pandemic was greatly reduced for three main reasons.4, 5, 6 , 28 , 29 First, the reduced capacity for elective surgery as a result of reallocation of staff and resources to the care of hospitalised patients with COVID-19 pneumonia.30 Second, the reorganisation of care pathways and the introduction of necessary, but onerous, infection control procedures have slowed down the delivery of care and patient throughput.21 , 31 Third, the reluctance of some clinicians to operate on patients at high risk of complications should they suffer a nosocomial infection with SARS-CoV-2.5 , 32 This is coupled with a reluctance of some patients to undergo surgery because of fears of acquiring SARS-CoV-2 in hospital at the time of surgery. Measures to prevent nosocomial infection include preoperative testing, household isolation, and dedicated ‘green’ pathways for patients who are known to be SARS-CoV-2 negative. Patients and clinicians should be reassured that the population incidence of SARS-CoV-2 among surgical patients is low, and the overall risk of death from SARS-CoV-2 for a patient undergoing elective surgery in a ‘green’ pathway is less than 1 in 11 000.20 Our data confirm that the volume of surgical activity during 2020 is about 25% lower than expected compared with previous years.1 , 2 The predicted delays to surgical treatments as a result of the pandemic are substantial, with an estimated 5 million cases outstanding by March 2021.4 Although the morbidity and mortality associated with cancelled or postponed surgery is unknown, it is clearly not desirable to have a large and rapidly expanding waiting list for treatments.5 , 31 Our data confirm that although it is possible to undertake surgery safely during the pandemic, the risk of mortality among surgical patients with SARS-CoV-2 is substantial, and all efforts should be made to prevent nosocomial infection.
This study has several strengths. We included data from all patients undergoing surgery in England during the COVID-19 pandemic in England; thus our results represent the true population incidence of SARS-CoV-2 infection and associated mortality. We included data from more than 2 million surgical patients, which represents one of the largest observational cohorts of surgical patients during the COVID-19 pandemic. These data will be generalisable to other high-income countries. A major strength of our analysis is that we controlled for changing surgical case mix during the pandemic, and potential factors that could confound association between SARS-CoV-2 infection and mortality. We used the contemporaneous comparator of patients undergoing surgery during the pandemic who did not have SARS-CoV-2 infection. This allowed us to control for unexpected changes in surgical case mix associated with changes in population behaviour and healthcare delivery. We explored the potential for misclassification of SARS-CoV-2 status using a sample of patients from Wales with detailed SARS-CoV-2 testing data. The incidence of SARS-CoV-2 defined using ICD-10 codes in this cohort was higher than that in England, possibly because of a higher proportion of emergency surgical patients. The paradoxically lower incidence of SARS-CoV-2 recorded on PCR tests is likely attributable to the reduced availability of tests during the first wave, where clinical suspicion was required for diagnosis. Adding SARS-CoV-2 PCR tests increased the incidence compared with ICD-10 codes alone by about 15%. However, among elective surgical patients, the incidence of SARS-CoV-2 infection remained very low (<0.4%).
Our analysis also has several limitations. It possible that our data underestimate the true incidence of SARS-CoV-2 infection among surgical patients, particularly amongst those undergoing day case procedures, which would lead to under-representation of asymptomatic patients who are less likely to die. We determined the proportion of patients requiring emergency hospital admission with SARS-CoV-2 within 30 days of surgery in England and found this was very low, particularly among elective surgical patients. We only included in-hospital deaths, and our findings will therefore underestimate the true mortality risk. Early in the pandemic, testing may have only been available to patients with more severe disease leading to an over-representation of these cases. SARS-CoV-2 may cause asymptomatic disease, and our case definition would miss these patients. Our sensitivity analysis indicates that ICD-10 codes under-report SARS-CoV-2 infections by about 15% compared with SARS-CoV-2 PCR results. However, the overall incidence of SARS-CoV-2 infection remained low, particularly amongst elective surgical patients. Administrative data sets are dependent on coding quality. Data are coded by trained professionals according to standardised methods.33, 34, 35 Reports indicate HES are accurate, particularly for procedures and important diagnoses. The influence of the pandemic on coding quality is unclear, but our sensitivity analysis results suggest that the quality of SARS-CoV-2 coding is good. At the date of our data extract, some patients will have remained in hospital so the true impact of the second wave will not be fully captured.
In conclusion, we have shown that the prevalence of SARS-CoV-2 infection among surgical patients is low and the risk of postoperative mortality among patients without SARS-CoV-2 infection is very small. However, where it occurs, SARS-CoV-2 infection among surgical patients is associated with a very high risk of death.
Authors' contributions
Study design: TA, AF, TD, RP
Data collection: AF, GD, AA
Data analysis: AF, TA, JG
Data interpretation: AF, TD, RP, TA
Writing of the first draft of the manuscript: TA, RP
All authors revised the manuscript for important intellectual content and approved the final version. AJF and TA had full access to the data and act as guarantors.
Acknowledgements
This work uses data provided by patients and collected by the NHS as part of their care and support. We acknowledge all data providers who make anonymised data available for research and the collaborative partnership that enabled acquisition and access to the de-identified data, which led to this output. The analysis was approved by the Health Research Authority (20/HRA/3121) and the NHS Digital Independent Group Advising on the Release of Data (DARS-NIC-375669-J7M7F). The analysis included Welsh data that was generated through a collaboration led by the Swansea University Health Data Research UK team under the direction of the Welsh Government Technical Advisory Cell (TAC) and includes the following groups and organisations: the Secure Anonymised Information Linkage (SAIL) Databank, Administrative Data Research (ADR) Wales, NHS Wales Informatics Service (NWIS), Public Health Wales, NHS Shared Services and the Welsh Ambulance Service Trust (WAST). All research conducted has been completed under the permission and approval of the SAIL independent Information Governance Review Panel (IGRP) project number 0911.
Handling editor: Paul Myles
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.05.018.
Declarations of interest
All authors have completed the Unified Competing Interest form and declare: AJF holds a National Institute for Health Research Doctoral Research fellowship (DRF-2018-11-ST2-062). TDD reports funding from the Welsh Clinical Academic Training (WCAT) Fellowship. IW reports active grants from the American Association of Plastic Surgeons and the European Association of Plastic Surgeons; is an editor for Frontiers of Surgery, associate editor for the Annals of Plastic Surgery, editorial board of BMC Medicine and numerous other editorial board roles. RP has received honoraria, research grants, or both from Edwards Lifesciences, Intersurgical, and GlaxoSmithkline within the past 5 yr and holds editorial roles with the British Journal of Anaesthesia, the British Journal of Surgery and BMJ Quality and Safety. TA is a member of the associate editorial board of the British Journal of Anaesthesia and has received consultancy fees from MSD unrelated to this work. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 yr, no other relationships or activities that could appear to have influenced the submitted work.
Transparency declaration
TA affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination declaration
The results of this study have been shared with representatives from NHS England before publication, in order to inform patient care.
Patient and public involvement
A patient representative was consulted in the design of this study.
Funding
This study was funded by a grant from Barts Charity (MGU0545). The Welsh data source was supported by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006) funded by the UK Medical Research Council (MRC), Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation (BHF) and the Wellcome Trust. The study was also supported by a separate grant from the MRC. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing the report.
Data sharing
The data used in this study were derived from two data sources. It is not possible to share the raw patient-level data provided by NHS Digital describing NHS patients in England. Regarding data from NHS patients in Wales, the data used are available in the SAIL Databank at Swansea University, Swansea, UK, but as restrictions apply they are not publicly available. All proposals to use SAIL data are subject to review by an independent Information Governance Review Panel (IGRP). Before any data can be accessed, approval must be given by the IGRP. The IGRP gives careful consideration to each project to ensure proper and appropriate use of SAIL data. When access has been granted, it is gained through a privacy protecting safe haven and remote access system referred to as the SAIL Gateway. SAIL has established an application process to be followed by anyone who would like to access data via SAIL at https://www.saildatabank.com/application-process.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fowler A.J., Abbott T.E.F., Prowle J., Pearse R.M. Age of patients undergoing surgery. Br J Surg. 2019;106:1012–1018. doi: 10.1002/bjs.11148. [DOI] [PubMed] [Google Scholar]
- 2.Abbott T.E.F., Fowler A.J., Dobbs T.D., Harrison E.M., Gillies M.A., Pearse R.M. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth. 2017;119:249–257. doi: 10.1093/bja/aex137. [DOI] [PubMed] [Google Scholar]
- 3.Weiser T.G., Haynes A.B., Molina G. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385:S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 4.Fowler A.J., Dobbs T.D., Wan Y.I. Estimated surgical requirements in England after COVID-19: a modelling study using hospital episode statistics. Br J Surg. 2020;108:97–103. [Google Scholar]
- 5.CovidSurg Collaborative. Nepogodiev D., Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVIDSurg Collaborative Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020;109:1097–1103. doi: 10.1002/bjs.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler A., Abbott T.E.F., Pearse R.M. Can we safely continue to offer surgical treatments during the COVID-19 pandemic? BMJ Qual Saf. 2020;30:268–270. doi: 10.1136/bmjqs-2020-012544. [DOI] [PubMed] [Google Scholar]
- 8.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doglietto F., Vezzoli M., Gheza F. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean B.J.F., Corona Hands Collaborative Mortality and pulmonary complications in patients undergoing upper extremity surgery at the peak of the SARS-CoV-2 pandemic in the UK: a national cohort study. BMJ Qual Saf. 2020;30:283–291. doi: 10.1136/bmjqs-2020-012156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busby J., Purdy S., Hollingworth W. Calculating hospital length of stay using the Hospital Episode Statistics; a comparison of methodologies. BMC Health Serv Res. 2017;17:347. doi: 10.1186/s12913-017-2295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armitage J.N., van der Meulen J.H., Royal College of Surgeons Co-morbidity Consensus G Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97:772–781. doi: 10.1002/bjs.6930. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Housing, Communities and Local Government . HMG; UK: 2019. English indices of deprivation 2019. [Google Scholar]
- 14.National Cancer Institute . 2006. Calculating age-adjusted rates.https://seer.cancer.gov/seerstat/tutorials/aarates/step1.html Available from: [Google Scholar]
- 15.International Surgical Outcomes Study group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearse R.M., Harrison D.A., James P. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearse R.M., Moreno R.P., Bauer P. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–1065. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y.I., McGuckin D., Fowler A.J., Prowle J.R., Pearse R.M., Moonesinghe S.R. Socioeconomic deprivation and long-term outcomes after elective surgery: analysis of prospective data from two observational studies. Br J Anaesth. 2021;126:642–651. doi: 10.1016/j.bja.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Lyons J., Akbari A., Torabi F. Understanding and responding to COVID-19 in Wales: protocol for a privacy-protecting data platform for enhanced epidemiology and evaluation of interventions. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-043010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffa D.J., Judson B.L., Billingsley K.G. Results of COVID-minimal surgical pathway during surge-phase of COVID-19 pandemic. Ann Surg. 2020;272:e316–e320. doi: 10.1097/SLA.0000000000004455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasbey J.C., Nepogodiev D., Simoes J.F.F. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39:66–78. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K.C., Xiao R., Cheung Z.B., Barbera J.P., Forsh D.A. Early mortality after hip fracture surgery in COVID-19 patients: a systematic review and meta-analysis. J Orthop. 2020;22:584–591. doi: 10.1016/j.jor.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abate S.M., Mantefardo B., Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:37. doi: 10.1186/s13037-020-00262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De C., Wignall A., Giannoudis V. Peri-operative outcomes and predictors of mortality in COVID-19 positive patients with hip fractures: a multicentre study in the UK. Indian J Orthop. 2020;54:1–11. doi: 10.1007/s43465-020-00272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps D.L., Saso S., Ghaem-Maghami S. Analysis of worldwide surgical outcomes in COVID-19-infected patients: a gynecological oncology perspective. Future Sci OA. 2020;6:FS0629. doi: 10.2144/fsoa-2020-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahlberg A., Mascia D., Bellosta R. Vascular surgery during COVID-19 emergency in hub hospitals of Lombardy: experience on 305 patients. Eur J Vasc Endovasc Surg. 2021;61:306–315. doi: 10.1016/j.ejvs.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clement N.D., Hall A.J., Makaram N.S. IMPACT-Restart: the influence of COVID-19 on postoperative mortality and risk factors associated with SARS-CoV-2 infection after orthopaedic and trauma surgery. Bone Joint J. 2020;102-B:1774–1781. doi: 10.1302/0301-620X.102B12.BJJ-2020-1395.R2. [DOI] [PubMed] [Google Scholar]
- 28.Clarke J., Murray A., Markar S.R., Barahona M., Kinross J., PanSurg Collaborative New geographic model of care to manage the post-COVID-19 elective surgery aftershock in England: a retrospective observational study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cano-Valderrama O., Morales X., Ferrigni C.J. Reduction in emergency surgery activity during COVID-19 pandemic in three Spanish hospitals. Br J Surg. 2020;107:e239. doi: 10.1002/bjs.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CRC COVID Research Collaborative Colorectal cancer services during the COVID-19 pandemic. Br J Surg. 2020;107:e255–e256. doi: 10.1002/bjs.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVIDSurg Collaborative Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg. 2020;107:e601–e602. doi: 10.1002/bjs.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soreide K., Hallet J., Matthews J.B. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. 2020;107:1250–1261. doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert A., Wijlaars L., Zylbersztejn A., Cromwell D., Hardelid P. Data resource profile: Hospital Episode Statistics Admitted Patient Care (HES APC) Int J Epidemiol. 2017;46:1093–i. doi: 10.1093/ije/dyx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas D.S., Gentry-Maharaj A., Ryan A. Colorectal cancer ascertainment through cancer registries, hospital episode statistics, and self-reporting compared to confirmation by clinician: a cohort study nested within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Cancer Epidemiol. 2019;58:167–174. doi: 10.1016/j.canep.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommerlad A., Perera G., Singh-Manoux A., Lewis G., Stewart R., Livingston G. Accuracy of general hospital dementia diagnoses in England: sensitivity, specificity, and predictors of diagnostic accuracy 2008–2016. Alzheimers Dement. 2018;14:933–943. doi: 10.1016/j.jalz.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.