Abstract
Background
Non-pharmaceutical interventions (NPIs) are the cornerstone of infectious disease outbreak response in the absence of effective pharmaceutical interventions. Outbreak strategies often involve combinations of NPIs that may change according to disease prevalence and population response. Little is known with regard to how costly each NPI is to implement. This information is essential to inform policy decisions for outbreak response.
Objective
To address this gap in existing literature, we conducted a systematic review on outbreak costings and simulation studies related to a number of NPI strategies, including isolating infected individuals, contact tracing and quarantine, and school closures.
Methods
Our search covered the MEDLINE and EMBASE databases, studies published between 1990 and 24 March 2020 were included. We included studies containing cost data for our NPIs of interest in pandemic, epidemic, and outbreak response scenarios.
Results
We identified 61 relevant studies. There was substantial heterogeneity in the cost components recorded for NPIs in outbreak costing studies. The direct costs of NPIs for which costing studies existed also ranged widely: isolating infected individuals per case: US$141.18 to US$1042.68 (2020 values), tracing and quarantine of contacts per contact: US$40.73 to US$93.59, social distancing: US$33.76 to US$167.92, personal protection and hygiene: US$0.15 to US$895.60.
Conclusion
While there are gaps and heterogeneity in available cost data, the findings of this review and the collated cost database serve as an important resource for evidence-based decision-making for estimating costs pertaining to NPI implementation in future outbreak response policies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-021-00659-z.
Key Points for Decision Makers
| There are gaps in existing non-pharmaceutical intervention cost data literature both geographically and by intervention. |
| Publishing costs for the SARS-CoV-2 pandemic outbreak responses will help fill these gaps. |
Introduction
The SARS-CoV-2 pandemic has put unprecedented strain on health systems around the world and brought to the fore the importance of establishing effective infectious disease outbreak response strategies to protect population health. Countries have had to implement non-pharmaceutical interventions (NPIs) in the absence of suitable vaccines and other medical interventions as part of their outbreak mitigation or suppression strategies [1]. NPIs often come with a direct and socioeconomic cost, as in addition to administration costs or lost wages, they often require changes in behavioural patterns, which in turn, have wider impacts such as productivity losses or reduced consumption.
Considering that NPIs have been adopted at scale by nearly all countries globally as a response to SARS-CoV-2 in 2020, and for prolonged periods of time, discussion regarding the burden brought by the costs associated with NPIs has become commonplace [2]. Countries were making decisions on suppression and mitigation strategies early on in the pandemic while ignoring the costs associated with these interventions when implemented on a large scale. As the costs and scale of interruption associated with the SARS-CoV-2 pandemic and control interventions are becoming apparent, the current pandemic also acts as a prompt to consider the costs of NPIs associated with outbreak response strategies generally. Knowing the costs of NPIs would help countries to make informed evidence-based decisions when deciding on NPIs for future outbreaks, leading to more resilient health systems. This being said, NPI costs remain relevant for SARS-CoV-2 as although vaccines are being rolled out, it will likely still be many months before populations are vaccinated at a level that would allow for NPIs to be completely lifted around the globe.
Previous literature reviews on NPIs have focussed on particular pathogens or NPIs. Examples of such reviews include school closures for influenza pandemics, or travel bans, [3, 4]. To our knowledge, a comprehensive systematic review covering all the literature on costs for all settings and pathogens for community-based NPIs does not yet exist. There is a great need for this review, as we need to map what is known about the costs of these community-based NPIs for different settings and for different pathogens so that knowledge gaps can be identified and filled to improve the evidence available, and to inform future strategies relating to outbreak response in cases where pharmaceutical interventions are not available or feasible.
The aim of this review is to provide a comprehensive overview of the existing literature on the costs of community-based NPIs. We cover the costs of NPIs relating to isolating infected individuals, contact tracing and quarantine, travel and flight restrictions, social distancing, point-of-entry measures, and personal protection and hygiene in relation to outbreaks in non-hospital settings. We include studies that are both presenting outbreak response costs as well as simulation studies.
Methods
The objective of this literature review was to capture the literature on costs of community-based NPIs for different types of outbreak settings. Studies of interest were separated into two categories: outbreak costing studies, and simulation studies. We define outbreak costing studies as studies which contain observed primary costs for components of NPI implementation in outbreak response scenarios, which could be used in economic models and future policy decisions. Simulation studies, on the other hand, are more useful for identifying relevant literature on applying different NPI modelling strategies, or for policy-making purposes where comparative costs between different strategies are considered.
Inclusion and Exclusion Criteria
Table 1 presents the inclusion and exclusion criteria for our review. We considered outbreaks affecting the human population (excluding outbreaks in animals) in any location published from 1990 onwards for any non-chronic infectious disease. We only included original articles or reviews published or accepted in a peer-reviewed journal or published reports from official public health bodies, such as the Centers for Disease Control, published in English. We focused on interventions in the community, as these are most likely to provide useful information to inform response strategies for larger outbreaks, such as SARS-CoV-2. Studies involving hospital employees were included if the hospital was within a community outbreak (e.g., costs of home isolation of infected healthcare workers during community-wide H1N1 influenza outbreak), otherwise we excluded hospital-based studies as we deemed them to not be representative of a general community outbreak scenario. Studies for which pharmaceutical intervention costs could not be separated from non-pharmaceutical intervention costs were excluded.
Table 1.
Inclusion and exclusion criteria for the literature review
| Inclusion | Exclusion |
|---|---|
| Contains cost data of defined interventionsa of interest or on items relating to these interventions in pandemic, epidemic, or outbreak scenarios related to humans | Does not contain cost data on direct OR socio-economic costs of defined interventionsa in pandemic, epidemic, or outbreak scenarios |
| Original articles or reviews published or accepted in a peer-reviewed journal or reports | Intervention done to animals |
| Modelling studies estimating costs for defined interventionsa | Cost data for diseases in endemic settings or chronic illnesses |
| Duplicates | |
| Not in English | |
| Editorials, commentaries, letters, conference abstracts. (items that are not original articles or reviews published or accepted in a peer-reviewed journal or reports) |
aDefined interventions: isolation of infected individuals, contact tracing and quarantine, travel and flight bans, social distancing, measures at point-of-entry, personal protection and hygiene, community stay at home orders
Non-pharmaceutical Interventions of Interest
We considered NPIs that related to isolating infectious individuals or contacts, or included community interventions aiming to reduce community contacts through social distancing, such as curfews, school closures, workplace contact reductions (through closure, workplace or school absenteeism, or remote working), and wider crowd avoidance measures such as avoiding public transport and events. We also included stricter community-wide social distancing interventions, such as community-wide or country-wide stay-at-home orders. Additionally, we included travel restrictions and border closures and measures at points of entry, focussing on scans or screens done when individuals are entering or exiting a country or region. For personal protection measures, we included community-based usage of face masks, gloves, hand hygiene measures, and sanitisation protocols of contaminated surfaces. Table 2 presents a full list of NPIs considered.
Table 2.
A list of non-pharmaceutical interventions considered in this literature review
| Non-pharmaceutical intervention | Sub-categories of intervention |
|---|---|
| Isolation of infected individuals | Non-hospital case quarantine |
| Tracing and quarantine of contacts |
Contact tracing Non-hospital contact quarantine Household quarantine |
| Social distancing |
Curfew School closure Workplace closure Workplace absenteeism Working from home Crowd avoidance |
| Strict social distancing |
Community stay-at-home orders Country stay at home orders |
| Travel & flight bans | Any sort of travel restriction, ban, or border closure |
| Measures for persons at point-of-entry | Scans/screens done when entering/exiting a country/region |
| Personal protection & hygiene |
Face masks Hand hygiene (hand washing, sanitising, etc.) Sanitising contaminated surfaces Using gloves |
Intervention Costs of Interest
For outbreak costing studies, we extracted costs incurred by the individual affected by the NPI (e.g., wages lost due to home quarantine), costs incurred by the government, business, or public health body due to administering the NPI (e.g., contact tracing activities, face masks), and information relating to labour (e.g., number of hours spent on contact investigation per contact). We did not extract costs that were linked to pharmaceutical interventions that were combined with an NPI (e.g., vaccine administration costs) or case management in hospitals. For simulation studies, we included studies which presented costs separately from pharmaceutical costs. We covered simulation studies presenting any kind of financial impact, from cost calculations to reductions in gross domestic product.
For the quarantine of infectious individuals and their contacts, we considered cost or labour data relating to quarantine in a non-hospital setting. We excluded the costs of quarantine in hospital settings, as we considered them to not be representative of the costs relating to a community-based quarantine intervention due to the additional costs of components such as medical staff and hospital beds. We included costs relating to testing for infection only if testing was a component of the case identification and contact tracing protocol. With regard to contact tracing, we were interested in the community investigation costs and not pharmaceutical intervention costs. This meant that studies which did not separate non-pharmaceutical contact investigation costs from the vaccine or prophylactic treatment costs were excluded.
All costs from the outbreak-costing studies were converted to 2020 USD (mid-year, June) by first inflating the cost in its original reported currency to 2020 and then converting the value to USD [5]. The initial consumer price index was matched to the month when the intervention occurred, or the mid-point of the intervention timing if it lasted for a longer time-frame. The method of inflation adjustment followed the following formula:
The Consumer Price Index used was that of the International Monetary Fund [6]. Bloomberg’s currency conversion charts were used for currency conversion to USD [7].
The outputs of simulation studies were not converted as they are often the outcome of multiple inputs and assumptions, meaning that converting their outcomes would not be appropriate.
Search Strategy
We searched the MEDLINE and EMBASE databases for studies pertaining to the NPIs described in Table 1 on 24 March 2020. The search strategy, including the search strings, can be found in the supplement file called “Search strategy”. The two databases were chosen, as they are the major databases that cover literature on pandemics, epidemics, and outbreaks, leading us to believe that other databases would have likely only added duplicate references.
The literature review was conducted systematically, meaning that at both title and abstract screening and full text screening, each paper was examined by two reviewers of the review team, these included ABH, ALS, HAS, JS, JWEO, LC, LD, MX, and SSW. Conflicts were resolved in conflict resolution meetings between two members of the review team (JS, JWEO). We followed a first-degree snowball approach for the relevant reviews identified in our screening process, where studies in the identified review were evaluated for inclusion, but second-degree references (references of references) were not. We enquired about full texts of difficult-to-find studies through the British Library.
We adapted the British Medical Journal guidelines for assessing economic studies [8]. Our quality assessment contained 26 points, some of which were exclusive only to simulation studies. We categorised studies as low, medium, or high quality based on the proportion of “Yes” scores to the total number of points that were applicable to the study. Studies of low quality covered 25 % or fewer of the points, studies of moderate quality covered between > 25 % and < 75 % of the points, while studies of high quality covered ≥ 75 % of the points. See supplementary spreadsheet for individual quality assessment scores for each study.
We registered the literature review on PROSPERO (review ID CRD42020177418).
Results
Studies Identified
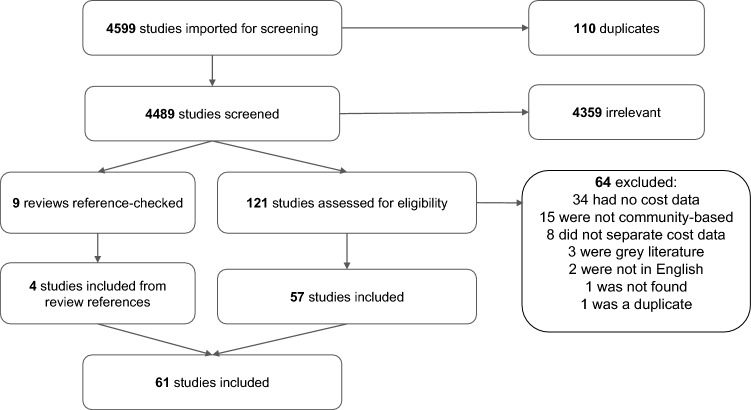
We identified 4599 studies for title and abstract screening, 4359 of which were excluded and 121 studies were assessed for eligibility during full-text screening. Additionally, nine reviews were reference checked. Consequently, we identified a total of 61 relevant studies with cost information on relevant NPIs (27 costing studies and 34 simulation studies). Of these 61 studies, 4 were identified through reference-checking reviews relevant to the NPIs of interest, while the remaining 57 were identified directly through the MEDLINE and EMBASE search (see Fig. 1). At the full-text screening phase, there was disagreement between reviewers regarding inclusion for 27 (22.3%) studies. Of the included studies, 1.6% (1/61) were assessed as being of low quality, 44.2% (27/61) were assessed as being of moderate quality, and 54.1% (33/61) were assessed as being of high quality (see supplementary spreadsheet for full quality assessment for each study).
Fig. 1.
Flow diagram of literature review and studies identified, included, and excluded at each stage of the review process
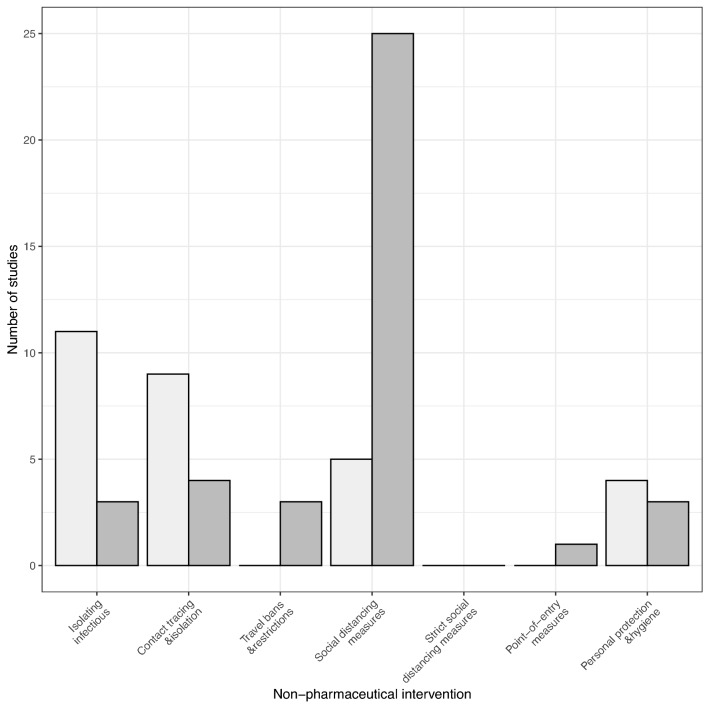
In the following sections, we present the identified cost and simulation evidence for each category of NPI (see Fig. 2 for number of studies by intervention). Due to the heterogeneity of costs recorded for the implementation strategies, it was deemed inappropriate to pool cost estimates. Hence, here we present the range of costs identified when there are comparable intervention components.
Fig. 2.
Bar plot of the number of studies that contain cost data for each non-pharmaceutical intervention for outbreak costing studies (light grey) and simulation studies (dark grey)
Non-hospital Isolation of Infected Individuals
We identified 11 outbreak costing studies relating to isolating infected individuals at home or in a hotel in outbreak scenarios [9–19], and three simulation studies that explored the costs of isolating infected individuals [20–22]. Table 3 summarises the available cost information from these studies in 2020 USD (US$) converted to unit costs where applicable for the outbreak costing studies, and Table 4 summarises the simulation studies in the reported currencies (see supplementary spreadsheet for original extracted data in its original currencies and units). The available studies were focused largely in Europe, North America, and China, with few studies from low- and middle-income countries (LMICs). The pathogens were vaccine-preventable diseases (measles, pertussis), diarrhoeal pathogens (norovirus, Escherichia coli), or respiratory pathogens (H1N1 influenza, SARS).
Table 3.
Identified outbreak costing studies that contained cost or labour information on non-pharmaceutical interventions
| 1st author, Publication year [Reference] | Year of intervention | Country | Pathogen | Target group | Intervention characteristic | Cost measured | Cost |
|---|---|---|---|---|---|---|---|
| Isolating infectious individuals | |||||||
| Christie, 1995 [9] | 1993 | USA | Pertussis | Healthcare workers during pandemic influenza | Furloughing isolated infected individuals | Cost per case | 971.26 |
| Case confirmation | Laboratory testing (per sample) | 71.42 | |||||
| Wahl, 2011 [10] | 2009 | Norway | Escherichia coli | Parents of children in child-care | Isolating infected children | Work-days lost by parents per infected case | 25.38 |
| Ma, 2017 [11] | 2015 | China | Measles | Office workers | Isolating infected | Mean work-days lost | 8.7 (95 % CI 8.5–8.9) |
| Mean wages lost | 593.14 (95 % CI: 546.03–640.24) | ||||||
| Galante, 2012 [12] | 2009–2010 | Spain | H1N1 | Community | Isolating infected | Cost of work absenteeism | 672.05 |
| Cost of work absenteeism due to caregiving responsibilities | 57.51 | ||||||
| Mota, 2011 [13] | 2009 | Brazil | H1N1 | Physician in community outbreak | Isolating infected | Staff replacement (cost per day) | 276.66 |
| Productivity loss (cost per day) | 122.85 | ||||||
| Nurse in community outbreak | Isolating infected | Staff replacement (cost per day) | 82.84 | ||||
| Productivity loss (cost per day) | 98.98 | ||||||
| Nurse assistant in community outbreak | Isolating infected | Staff replacement (cost per day) | 53.85 | ||||
| Productivity loss (cost per day) | 50.65 | ||||||
| Sugerman, 2010 [14] | 2008–2009 | USA | Measles | Children | Isolating infected children | Mean cost per case | 946.57 |
| Case confirmation | Laboratory work (hours per confirmed case) | 322 | |||||
| Laboratory materials and work (cost per confirmed case) | 641.35 | ||||||
| Gallagher, 2013 [15] | 2009 | USA | Escherichia coli | Parents of isolating children | Isolating infected children | In-home childcare cost | 1814.05 |
| Ooi, 2005 [16] | 2003 | Singapore | SARS | Community | Quarantine enforcement and surveillance | Cost per case | 340.23 |
| Quarantine command centre | Cost per case | 71.63 | |||||
| Quarantine allowance | Cost per case | 322.32 | |||||
| Emergency call centre and ambulance | Cost per case | 71.63 | |||||
| Wang, 2012 [17] | 2009 | China | H1N1 | Community | Isolation of infected | Inspection cost per case | 29.48 |
| Disinfectant cost per case | 20.95 | ||||||
| Home medical observation cost per case | 90.75 | ||||||
| Coleman, 2012 [18] | 2010 | USA | Measles | Community | Case confirmation | Labour hours (per sample) | 0.5 |
| Labour costs (per sample) | 17.86 | ||||||
| Screening kit cost (per sample) | 141.9 | ||||||
| Quarantine of infected individuals | Labour hours per case at quarantine stations | 4 | |||||
| Labour costs per case at quarantine stations | 330.28 | ||||||
| Bownds, 2003 [19] | 1998 | USA | Hepatitis A | Community | Case quarantine | Cost of laboratory tests and procedures (per sample) | 20.05 |
| Productivity loss due to staying at home when ill (per case) | 4038.23 | ||||||
| Tracing and quarantine of contacts | |||||||
| Wang, 2012 [17] | 2009 | China | H1N1 | Community | Contact quarantine | Quarantine at home (per person) | 40.73 |
| Quarantine at hospital (per person) | 724.94 | ||||||
| Quarantine in hotel (per person) | 1062.32 | ||||||
| Contact observation | Isolated observation | 4778.33 | |||||
| Laboratory costs | Network laboratory | 140.33 | |||||
| Specimen collection | 26.41 | ||||||
| Virus isolation and identification | 237.65 | ||||||
| Nucleic acid detection | 528.1 | ||||||
| Serology tests | 66.01 | ||||||
| Parker, 2006 [23] | 2005 | USA | Measles | Community | Contact tracing | Investigation hours | 11.9 |
| Laboratory work hours | 9.33 | ||||||
| Pike, 2020 [24] | 2016–2017 | USA | Mumps | Community | Contact tracing for outbreak containment | Overall costs (total) | 941104.38 |
| Labour costs (total) | 503687.63 | ||||||
| Travel costs (total) | 88927.80 | ||||||
| Personnel hours (total) | 12585 | ||||||
| Laboratory costs | Tests (per sample) | 18.53 | |||||
| Supplies and equipment (total) | 114861.53 | ||||||
| Rosen, 2018 [25] | 2013 | USA | Measles | Community | Contact tracing activities | Community outreach (h per identified contact) | 0.29 |
| Administration (h per identified contact) | 0.13 | ||||||
| Advertising (h per identified contact) | 8.63 | ||||||
| Laboratory | Laboratory personnel (h per sample) | 57.63 | |||||
| Laboratory supplies and testing ($ per sample) | 214.96 | ||||||
| Sugerman, 2010 [14] | 2008 | USA | Measles | Children | Contact tracing | Investigation (h per contact) | 0.49 |
| Dayan, 2005 [26] | 2004 | USA | Measles | Community | Contact tracing | Investigation (h per contact) | 0.75 |
| Public information (cost per contact) | 1.80 | ||||||
| Flego, 2013 [27] | 2011 | Australia | Measles | Community | Contact tracing | Personnel cost (per contact) | 23.96 |
| Personnel time (mean h per contact) | 0.63 | ||||||
| Laboratory (cost per tested contact) | 25.88 | ||||||
| Telephone (cost per contact call) | 0.51 | ||||||
| Stationery and mail (cost per contacted contact) | 2.8 | ||||||
| Ma, 2017 [11] | 2015 | China | Measles | Contact tracing and surveillance | Cost (per contact) | 42.99 | |
| Time (h per contact) | 2.12 | ||||||
| Field investigation | Cost of contact tracing and sample collection (per contact) | 1.01 | |||||
| Hours taken to contact trace (per contact) | 0.07 | ||||||
| Laboratory testing of contacts | Cost of laboratory work (per sample) | 11.83 | |||||
| Hours of laboratory work (per sample) | 101.91 | ||||||
| Cost of kit (per sample) | 37.76 | ||||||
| Gallagher, 2013 [15] | 2009 | USA | Escherichia coli | Children | Laboratory testing of contacts | Cost (per sample) | 183.15 |
| Social distancing | |||||||
| Borse, 2011 [28] | 2009 | USA | H1N1 | Parents of elementary school children | School closure | Households where at least 1 adult took time off work (%) | 17 |
| Households where no adults took time off work (%) | 83 | ||||||
| Chen, 2011 [29] | 2009 | Taiwan | H1N1 | Parents of elementary school children | School closure | Average income loss (per household) | 33.76 |
| Gift, 2010 [30] | 2009 | USA | H1N1 | Parents of elementary school children | 1 week school closure | % of households where 0 days of work were lost | 78.5 |
| % of households where 1 days of work were lost | 6.1 | ||||||
| % of households where 2 days of work were lost | 3.3 | ||||||
| % of households where 3 days of work were lost | 1.9 | ||||||
| % of households where 4 days of work were lost | 1.9 | ||||||
| % of households where 5 days of work were lost | 8.4 | ||||||
| Johnson, 2008 [31] | 2006 | USA | Influenza B | Households with elementary school children | 2-week school closure | Households where adults missed at least 1 day of work (%) | 3.2 |
| Russell, 2016 [32] | 2013 | USA | ILI | Households with school children | 4 work-day school closure | Cost of childcare for households that required it (median, min-max) | 111.95 (34.70–167.92) |
| Personal protection and hygiene measures | |||||||
| Tracht, 2012 [33] | 2009 | USA | H1N1 | Community | N95 mask | Cost per mask | 2.14 |
| Ma, 2017 [11] | 2015 | China | Measles | Office workers | Disposable mask | Cost per mask | 0.32 |
| Hand sanitiser | Cost per bottle | 5.09 | |||||
| Mukerji, 2017 [34] | 2008–2010 | China | Influenza | Healthcare workers during community transmission | Medical mask | Cost per mask | 0.15 |
| N95 mask | Cost per mask | 0.87 | |||||
| Baracco, 2015 [35] | 2013 | USA | Influenza | Healthcare workers during community transmission | N95 mask | Min/max cost per mask | 0.28–0.73 |
| Reusable mask | Min/max cost per mask | 27.99-55.97 | |||||
| Set of filters for reusable mask | Cost per set | 2.8 | |||||
| Air-purifying device | Min/max cost per device | 559.75–895.60 | |||||
| Air-purifying device battery | Cost per battery | 279.87 | |||||
| Additional hood for purifier | Cost per hood | 33.58 | |||||
| Additional tubes for purifier | Cost per tube | 33.58 | |||||
All costs converted to 2020 USD unless indicated otherwise, original costs presented in supplementary spreadsheet
AUD Australian Dollars, CAD Canadian Dollars, CGE Computable General Equilibrium, GDP Gross Domestic Product, h hours, ICER incremental cost-effectiveness ratio, R0 basic reproduction number, SEIR susceptible-exposed-infected-recovered, SEIQR susceptible-exposed-infected-quarantined-recovered, SI susceptible-infected, SIR susceptible-infected-recovered
Table 4.
Identified simulation studies that contained cost or labour information on non-pharmaceutical interventions
| 1st author, Publication year [Reference] | Year of intervention | Country | Pathogen | Intervention type | Intervention characteristic | Cost measured | Cost | Model type |
|---|---|---|---|---|---|---|---|---|
| Isolating infectious individuals | ||||||||
| Agusto, 2013 [20] | NA | NA | Avian influenza | Isolation of infected | Total cost and ICER of isolating infectious individuals | Avian strain (total cost in theoretical units [ICER]) | 89648 (0.18411) | Deterministic compartmental SI transmission model + incremental cost-effectiveness ratio |
| Mutant strain (total cost in theoretical units [ICER]) | 71133 (−0.08633) | |||||||
| Both strains (total cost in theoretical units [ICER]) | 16441 (−0.68322) | |||||||
| Yarmand, 2010 [21] | NA | USA | H1N1 | Isolation of infected | Cost effectiveness of isolating infectious individuals vs vaccination | Compares various percentages of isolation against a vaccination policy in a theoretical manner—no single cost reported | compartmental SEIR transmission model + linear function of costs, optimising cost-effectiveness of a response consisting of isolation and vaccination | |
| Mubayi, 2010 [22] | NA | Hong Kong | SARS | Isolation of infected | Cost effectiveness of case isolation for a contact tracing strategy that has a per-capita rate independent of number infected | Presents a variation of costs for multiple contact-tracing parameter values | Compartmental SEIQR transmission model + linear cost function to model cost-effectiveness, incremental cost-effectiveness also evaluated | |
| Cost effectiveness of case isolation for a contact tracing strategy that has a per-capita rate that is proportional to number infected | Presents a variation of costs for multiple contact-tracing parameter values | |||||||
| Cost effectiveness of case isolation for a contact tracing strategy that has a per-capita rate that is finite and saturates | Presents a variation of costs for multiple contact-tracing parameter values | |||||||
| Tracing and quarantine of contacts | ||||||||
| Li, 2013 [36] | 2009 | China | H1N1 | 1-week contact quarantine for a 60-day intervention period | Contact tracing and contact quarantine in hotel | Total cost (USD 2009) | 2560000 | deterministic compartmental SEIR transmission model + cost-effectiveness with a counterfactual of no contact quarantine |
| Orset, 2018 [37] | NA | France | Pandemic influenza | Contact quarantine | Contact quarantine at home | Presents a variety of hypothetical thresholds and compliance levels | Cost-benefit analysis and probit model | |
| Gupta, 2005 [38] | NA | Canada | SARS | Contact quarantine | Total cost of quarantine | Total cost of primary wave (million CAD 2003) | 12.2 | simple population level transmission model + analysis of cost savings with a counterfactual of no contact quarantine |
| Total cost of secondary wave (million CAD 2003) | 13 | |||||||
| Total cost of tertiary wave (million CAD 2003) | 17 | |||||||
| Total savings due to quarantine | Total saving in primary wave (million CAD 2003) | 279 | ||||||
| Total savings in secondary wave (million CAD 2003) | 274 | |||||||
| Total savings in tertiary wave (million CAD 2003) | 232 | |||||||
| Mubayi, 2010 [22] | NA | Hong Kong | SARS | Contact quarantine | Cost effectiveness of contact quarantine per-capita rate independent of number infected | Presents a variation of costs for multiple contact-tracing parameter values | Compartmental SEIQR transmission model + linear cost function to model cost-effectiveness, incremental cost-effectiveness also evaluated | |
| Cost effectiveness of contact quarantine per-capita rate that is proportional to number infected | Presents a variation of costs for multiple contact-tracing parameter values | |||||||
| Cost effectiveness of contact quarantine per-capita rate that is finite and saturates | Presents a variation of costs for multiple contact-tracing parameter values | |||||||
| Travel and flight bans and restrictions | ||||||||
| Epstein, 2007 [39] | NA | USA | Pandemic influenza | Air travel restrictions | Cost of air travel restrictions | Cost of shutting down major airlines (billion USD per annum) | 93–100 | Network-based individual-based SEIR transmission model + costs associated with epidemic and intervention along with benefits, counterfactual: no travel restriction |
| Cost of shutting down major airlines (% GNP) | 0.8 | |||||||
| Labour cost of shutting down major airlines (billion USD per annum) | 6 | |||||||
| Prager, 2017 [40] | NA | USA | Seasonal influenza | Travel restrictions | Travel restrictions' direct impact on GDP | Inbound international travel (% change in GDP) | −2.425 | CGE model |
| Outbound international travel ( % change in GDP) | −2.425 | |||||||
| Domestic travel (% change in GDP) | −0.063 | |||||||
| Pandemic influenza | Travel restrictions | Travel restrictions' direct impact on GDP | Inbound international travel (% change in GDP) | −19.833 | ||||
| Outbound international travel (% change in GDP) | −19.833 | |||||||
| Domestic travel (% change in GDP) | −3.125 | |||||||
| Boyd, 2017 [41] | NA | New Zealand | Influenza | Border closure | Net costs and social benefits of border closure | Costs and benefits for multiple scenarios given for 12 week closer, 26-week closure, failed border closure | Transmission model + net costs and net societal benefits society calculated, counterfactual: cost of pandemic with no border closure | |
| Social distancing | ||||||||
| Andradottir, 2011 [42] | NA | Canada | Pandemic influenza | School closure | 5-day school closure and social distancing with 20 % contact limitation | Total cost (million CAD 2008) | 125 | Individual-based compartmental transmission model + cost calculation for interventions |
| Araz, 2012 [43] | NA | USA | Pandemic influenza | School closure | School closure for low transmission and low severity | 12-week school closure cost (USD) | 2,560,372,2 19 | SEIR transmission model + cost calculation for interventions |
| 24-week school closure cost (USD) | 5,120,744,439 | |||||||
| School closure for high transmission and high severity | 12-week school closure cost (USD) | 2,560,372,219 | ||||||
| 24-week school closure cost (USD) | 5,120,744,439 | |||||||
| Brown, 2011 [44] | 2009 | USA | H1N1 | School closure | 1-, 4-, and 8-week school closure | Present costs for school closure alone, the cost of school closure combined with costs of disease, and net costs of school closure (accounting for averted cases) for pandemics with an R0 of 1.2, 1.6 and 2.0 | Agent-based transmission model + Monte Carlo cost-benefit simulation model | |
| Halder, 2011 [45] | NA | Australia | H1N1 | School closure | 2-week school closure | Total cost (million USD 2010 per 100,000 population) | 5.9 | Individual-based transmission model + cost analysis |
| 4-week school closure | Total cost (million USD 2010 per 100,000 population) | 6.6 | ||||||
| 8-week school closure | Total cost (million USD 2010 per 100,000 population) | 11.6 | ||||||
| Continuous school closure | Total cost (million 2010 USD per 100,000 population) | 34.1 | ||||||
| 2-week school closure + 4-week 50 % workplace closure + 50 % community contact reduction | Total cost (million USD 2010 per 100,000 population) | 21 | ||||||
| 2-week school closure + 4-week 50 % workplace closure | Total cost (million USD 2010 per 100,000 population) | 21.1 | ||||||
| 2-week school closure + 50 % community contact reduction | Total cost (million USD 2010 per 100,000 population) | 5.7 | ||||||
| 2-week school closure + 2-week 50 % workplace closure | Total cost (million USD 2010 per 100,000 population) | 13.6 | ||||||
| Continuous school closure + continuous 50 % workplace closure | Total cost (million USD 2010 per 100,000 population) | 103 | ||||||
| Jones, 2013 [46] | NA | NA | Influenza | Social distancing | Contact reduction through social distancing | Presents costs for both transmission models and cost functions | Investigates two transmission models and linear and exponential increases in intervention costs when optimising non-pharmaceutical interventions | |
| Kelso, 2013 [47] | NA | Australia | Influenza A | Social distancing | School closure, 50 % workplace reduction, 50 % community contact reduction | Presents intervention costs for five pandemic severities, with varying combinations of the three social distancing measures | SEIR compartmental model + cost analysis including direct healthcare costs and productivity loss | |
| Keogh-Brown, 2010 [48] | NA | UK, France, Belgium, Netherlands | Influenza | Social distancing measures | School closure | GDP loss due to school closure (% GDP loss min-max) | 1.32–3.20 | unspecified transmission model and one-country CGE model |
| Prophylactic absenteeism | GDP loss due to prophylactic absenteeism (% GDP min-max) | 0.94–2.34 | ||||||
| Lempel, 2009 [49] | NA | USA | Pandemic influenza | Worker absenteeism due to school closure | Length of school closure | 2 weeks (base cost (low-high cost) in billion USD 2008) | 21.3 (5.2–23.6) | Economic cost calculation based on weekly earnings of caretakers multiplied by school closure length, no transmission model |
| 4 weeks (base cost (low-high cost) in billion USD 2008) | 42.6 (10.6–47.1) | |||||||
| 6 weeks (base cost (low-high cost) in billion USD 2008) | 63.9 (15.6–70.7) | |||||||
| 12 weeks (base cost (low-high cost) in billion USD 2008) | 127.8 (31.3–141.3) | |||||||
| Weekly cost per student (base cost (low-high cost) in billion USD 2008) | 142 (35–157) | |||||||
| Maharaj, 2012 [50] | NA | NA | Social distancing | Reduction of contacts | Presented for a range of infectiousness levels and show its effect on the net economic benefit of social distancing | Compartmental SIR transmission model with and without small-world interactions with calculation of net economic benefit | ||
| Milne, 2013 [51] | NA | Australia | Pandemic influenza | School closure, workplace closure, community contact reduction | Continuous school closure + continuous workplace closure | Total cost per member of population (AUD, low severity-high severity) | 1217–4804 | Individual-based transmission model + costing model to determine economic cost to society |
| Continuous school closure + 4-week 50 % community contact reduction | Total cost per member of population (AUD, low severity-high severity) | 519–3826 | ||||||
| Continuous school closure + 4-week workplace closure and 50 % community contact reduction | Total cost per member of population (AUD, low severity-high severity) | 654–3882 | ||||||
| Continuous school closure + continuous 50 % community contact reduction | Total cost per member of population (AUD, low severity-high severity) | 447–2275 | ||||||
| Continuous school closure, workplace closure, and 50 % community contact reduction | Total cost per member of population (AUD, low severity-high severity) | 1116–2603 | ||||||
| Morin, 2014 [52] | NA | NA | Community contact reduction | Varied reduction percentages | Presents interface between numbers infected and susceptible where community contact reduction is considered worth the cost for each transmission model | Three transmission models: SI, SIR, SEIR and costs to society | ||
| Nishiura, 2014 [53] | NA | Japan | Pandemic influenza | School closure | Varying lengths of school closure (0–50 days) | ICER presented for varying lengths of school closure and varying infectiousness | Renewal process transmission model + incremental cost effectiveness ratio | |
| Perlroth, 2010 [54] | NA | USA | Pandemic influenza | Social distancing | Adult and child social distancing + school closure | Total cost per person in a setting with R0 of 2.1 and case fatality rate 1 %) (USD 2009) | 1400 | Agent-based network model of transmission with calculation of costs |
| Total cost per person in a setting with R0 of 1.6 and case fatality rate 0.25 % (USD 2009) | 1370 | |||||||
| Adult and child social distancing | Total cost per person in a setting wit R0 of 2.1 and case fatality rate 1 % (USD 2009) | 490 | ||||||
| Total cost per person in a setting with R0 of 1.6 and case fatality rate 0.25 % (USD 2009) | 290 | |||||||
| Quarantine | Total cost per person in a setting with R0 of 2.1 and case fatality rate 1 % (USD 2009) | 720 | ||||||
| Total cost per person in a setting with R0 of 1.6 and case fatality rate 0.25 % (USD 2009) | 510 | |||||||
| School closure | Total cost per person in a setting with R0 of 2.1 and case fatality rate 1 % (USD 2009) | 1330 | ||||||
| Total cost per person in a setting with R0 of 1.6 and case fatality rate 0.25 % (USD 2009) | 1510 | |||||||
| Prager, 2017 [40] | NA | USA | Pandemic influenza | Social distancing | Social distancing measures’ impact on GDP | Public transportation (% impact on GDP) | −3.125 | CGE model |
| Workplace absenteeism (% impact on GDP) | −0.125 | |||||||
| Parents keeping children from school (school avoidance + workplace absenteeism) (% impact on GDP) | − 0.012 | |||||||
| Reduction in school attendance (% impact on GDP) | − 0.167 | |||||||
| Seasonal influenza | Social distancing | Social distancing measures’ impact on GDP | Public transportation (% impact on GDP) | −0.063 | ||||
| Workplace absenteeism (% impact on GDP) | −0.038 | |||||||
| Parents keeping children from school (school avoidance + workplace absenteeism) (% impact on GDP) | −0.006 | |||||||
| Reduction in school attendance (% impact on GDP) | −0.083 | |||||||
| Reluga, 2010 [55] | NA | NA | NA | Social distancing | Contact reduction | Presents total costs and savings for varying social distancing efficiencies | SIR transmission model + cost calculation | |
| Sadique, 2008 [56] | NA | UK | Pandemic influenza | School closure | 2- 12-week school closure | Presents a range of school closure policies' effects on GDP with different labour impact assumptions | No transmission model, lost income calculated with human capital method | |
| Sander, 2009 [57] | NA | USA | Pandemic influenza | School closure | 26-week closure | Total cost per 1000 population (million USD) | 2.72 | Discrete time stochastic transmission model + cost calculation |
| Saunders-Hastings, 2017 [58] | NA | Canada | Pandemic influenza | Social distancing + personal hygiene | Community contact reduction + personal protective measures + voluntary isolation | Cost per life-year saved compared to no intervention (CAD) | 6671 | Discrete time population-level stochastic transmission model + cost calculation |
| School closure + community contact reduction + personal protective measures + voluntary isolation and quarantine | Cost per life-year saved compared to no intervention (CAD) | 260472 | ||||||
| Smith, 2013 [59] | NA | Thailand, South Africa, Uganda | pandemic influenza | School closure | 1-week school closure | Presents cost per capita (in USD) of closure for 9 disease severity scenarios and % impact on GDP for each country for each scenario | CGE model | |
| Smith, 2011 [60] | NA | UK | Pandemic influenza | Social distancing | School closure | Presents % impact on GDP for three disease severities | CGE model | |
| Prophylactic absenteeism | Presents % impact on GDP for three disease severities | |||||||
| Smith, 2009 [61] | NA | UK | Pandemic influenza | School closure | School closure | Presents % impact on GDP for 3 case fatality rate scenarios and 3 clinical attack rate scenarios | CGE model | |
| Wang. 2008 [62] | NA | NA | Community contact reduction | Closure of public spaces | Presents a theoretical interface of closure policy cost optimisation for outbreak scenarios | Scale-free SIR transmission model + cost calculation | ||
| Wong, 2016 [63] | NA | Hong Kong | H1N1 | School closure | 1- to 16-week closures and 3 different closure modes | Presents mean cost incurred for each closure scenario | SEIR compartmental transmission model + cost calculation | |
| Xue, 2012 [64] | NA | Norway | Pandemic influenza | School closure | Various lengths of school closure | Presents costs and productivity losses for multiple lengths of school closure for 3 reproduction numbers | SEIR compartmental transmission model + cost-benefit calculation | |
| Yaesoubi, 2016 [65] | NA | NA | School closure | Various lengths of school closure | Presents an interface of costs due to school closure | mathematical decision model with transmission dynamics (including SIR compartmental type structure) + cost optimisation | ||
| Measures for persons at point-of-entry | ||||||||
| Jacobson, 2016 [66] | NA | USA | Ebola | Point-of-entry screening | Screening cost per passenger | Costs for three different monitoring levels under two different policies (CDC and an alternative policy) are presented | Linear cost function applied to different scenarios | |
| Personal protection and hygiene measures | ||||||||
| Jones, 2013 [46] | NA | NA | Influenza | Hygiene | Hygiene measures | Presents costs for both transmission models and cost functions | Investigates two transmission models and linear and exponential increases in intervention costs when optimising non-pharmaceutical interventions | |
| Sardar, 2013 [67] | 2008–2011 | Zimbabwe | Cholera | Hygiene | Hand hygiene | Presents the optimal cost for 9 Zimbabwean locations for hygiene measures | Compartmental transmission model + cost function | |
| Tracht, 2012 [33] | 2009 | USA | H1N1 | Face masks | N95 face masks (10 %, 25 %, and 50 % usage) | Show savings gained by percentage of population who are using masks by age group | Compartmental SEIR transmission model + cost-benefit analysis | |
Costs are presented in their original currencies. The years of intervention and the country modelled are indicated where possible, but when no particular year or location is mentioned, they are specified as not applicable (NA)
AUD Australian Dollars, CAD Canadian Dollars, CGE Computable General Equilibrium, GDP Gross Domestic Product, h hours, ICER incremental cost-effectiveness ratio, R0 basic reproduction number, SEIR susceptible-exposed-infected-recovered, SEIQR susceptible-exposed-infected-quarantined-recovered, SI susceptible-infected, SIR susceptible-infected-recovered
The costs covered by the 11 studies were highly heterogeneous, and included case confirmation costs, wages and productivity lost due to being in quarantine, costs of taking care of quarantined children at home. One study considered the costs incurred to the government due to isolating infected individuals during the SARS pandemic response in Singapore, and reported the costs of quarantine enforcement (US$340.23 [2020 values] per case), quarantine command centres (US$71.63 per case), quarantine allowance (US$322.32 per case), and emergency call centres (US$71.63 per case) [16]. There was one cost component, laboratory costs relating to case confirmation, that was covered by multiple studies. The ranges of laboratory costs are presented in section 3.8. The three simulation studies presented heterogeneous cost-related outputs, including the total cost of isolating infectious individuals, cost effectiveness of an isolation intervention versus vaccination, and cost effectiveness of isolating infectious individuals given different levels of contact tracing.
Tracing and Quarantine of Contacts
We identified nine cost studies [11, 14, 15, 17, 23–27], and four simulation studies relating to contact tracing and contact quarantine in outbreak scenarios [22, 36–38]. Tables 3 and 4 summarise the cost information from these studies (the original extracted data in original currencies and units can be found in the supplementary spreadsheet). The studies were focussed on respiratory diseases (SARS and influenza) and vaccine-preventable diseases (measles and mumps). Much the same as isolation of infected individuals, the identified contact tracing papers were from North America and China.
As with case isolation, there was substantial heterogeneity in the types of costs recorded by the outbreak costing studies. Ranges of costs relating to laboratory testing are presented in section 3.8. The average hours spent on contact tracing was reported by five studies on measles outbreaks, and ranged from 0.5 to 11.9 hours [11, 14, 23, 26, 27]. The four simulation studies presented costs of contact tracing and quarantine at home and in a hotel.
Travel and Flight Bans
We did not identify any outbreak costing studies on travel and flight bans or restrictions. However, we did identify three simulation studies [39–41], see Table 4 for further details and original extracted costs in the supplementary spreadsheet. All studies were on influenza, two were located in the USA and one in New Zealand. The two USA studies simulated the costs and GDP impacts of air travel restrictions, while the New Zealand study covered the full border closure.
Social Distancing
We identified five costing [28–32] and 25 simulation studies on social distancing measures [40, 42–65], see Tables 3 and 4, respectively. Again, studies largely focussed on North America and Europe. All studies on a specified disease were on respiratory infections (various strains of influenza).
All costing studies reported only on school closures, and presented heterogenous costs, including days of work lost by parents, income loss due to lost work, and cost of childcare due to school closure. The simulation studies largely focussed on school closures and workplace absenteeism or closure, with many studies also considering combinations of community contact-reducing interventions.
Measures for Persons at Point-of-entry
We identified one simulation study on NPI measures at point-of-entry [66]. This USA-based study simulated the costs per airline passenger of point-of-entry screening for Ebola for three different monitoring levels (Table 4).
Personal Protection and Hygiene
While personal protection and hygiene measures in hospital settings for hospital-based outbreaks and nosocomial transmission were well documented, studies involving community-based outbreaks or community usage were rarer (see Tables 3 and 4 for costing and simulation studies, respectively). We identified four costing [11, 33–35] and three simulation studies on personal protection and hygiene measures [33, 46, 67]. The countries covered were USA, China and Zimbabwe. Most studies were on influenza, with one on measles and another on cholera. Face masks and hand sanitiser were the most covered interventions.
Three costing studies reported the costs of N95 face masks, which ranged from US$0.28 to US$2.14 [33–35]. Three simulation studies covered the savings due to different N95 face mask usage levels, and costs of general hygiene and hand hygiene measures.
Laboratory Testing in Conjunction with Non-pharmaceutical Interventions
We included only studies where laboratory testing was combined with another NPI. We identified 11 costing studies that involved laboratory cost data, 4 of which were related to isolation of infectious cases [9, 14, 18, 19] and 7 were related to contact tracing (Table 3) [11, 15, 17, 23–25, 27]. We also identified one simulation study on laboratory testing in conjunction with an NPI, which was a cost-benefit analysis of an E. coli surveillance system in Colorado, USA (Table 4) [68].
The diseases covered were vaccine-preventable (measles, mumps, pertussis, hepatitis A), respiratory (H1N1), and E. coli. The only pathogen for which there were costs reported for more than one study was measles, where six studies contained information [11, 14, 18, 23, 25, 27]. For the measles studies, the reported costs of testing ranged from US$25.88 to US$641.00 per sample and data on hours ranged from 0.5 to 101.9 hours per sample. The reporting of components included in laboratory cost calculations were not consistent, as some studies reported cost of labour as part of laboratory costs and others did not.
Discussion
In this study, we have reviewed the existing published literature on the NPIs of interest, covering both outbreak costing studies, which contain primary costs relating to NPIs in outbreak response, and simulation studies, which estimate costs of NPIs in outbreak response. Cost data are essential components of any evidence-based policy process and provide valuable information to be used alongside evidence of effectiveness to inform analyses pertaining to projected or actual estimates of the cost effectiveness and budget impact of implementation of different NPI strategies. There is variability in the levels of representation amongst the different NPI categories. Case isolation, contact tracing measures, and social distancing measures (in particular school closures) were well represented while travel restrictions, point-of-entry measures, and personal hygiene measures were less represented. Wider and stricter social distancing measures, such as community-wide measures, had not been covered in published literature before March 2020. Labour costs were often the most expensive component of isolating infected individuals and contact tracing, while laboratory costs also contributed greatly to the overall cost. There were nine papers that included NPIs and their costs, but did not present these costs separately from pharmaceutical (often vaccines and/or antivirals) interventions, and as such were excluded as the costs of the two different types of intervention could not be separated.
While we identified multiple costing studies that contained cost information for NPIs, providing meaningful and comparable summary statistics for them is difficult, as studies covered multiple locations and recorded different cost components relating to the community-based NPIs. Having a database of available cost information from outbreak costing studies is nonetheless useful for ease of locating relevant studies and cost components in future applications, such as for model parameterisation or in scenarios where policy-makers must compare the costs of different potential interventions. Studies covering the costs of travel bans and measures at point-of-entry would be a valuable addition to the existing literature. As many countries closed their borders or restricted entry into the country in the first months of 2020, this knowledge gap in cost data may be covered to an extent in literature that has been published since then [69]. The simulation studies also provided a range of model outputs, ranging from the total cost of implementing an NPI to the estimated impact on a country’s GDP. The database of simulation studies can act as a starting point for estimating the costs of a community-based NPI during an outbreak.
Published literature on the costs of NPIs for outbreaks in low-income settings was sparse. The majority of the studies identified were focused on North America, Europe, or Australia and New Zealand. While this may, in part, be by the exclusion of non-English studies and grey literature, this alone is likely not the only reason for the trend. In order to make well-informed pandemic response decisions, it is important that costings studies focus on low-income settings. The ongoing SARS-CoV-2 pandemic offers an opportunity for countries to collect outbreak response cost data for low-income settings to help fill this knowledge gap. We found that many studies were excluded from this review because they did not disentangle NPI costs from pharmaceutical intervention costs. It would be helpful if studies would present these costs separately to provide a clearer view of how each intervention contributes to the total cost of outbreak response.
The results published in this study are limited by the scope and extent of the literature review. This review covered literature that had been published by 24 March 2020. This necessarily limits the identification of publications to only those published up to the very beginnings of the SARS-CoV-2 pandemic. We did not identify any studies that recorded costs or simulated costs of strict social distancing measures (i.e., community stay-at-home orders) that are now commonplace across the globe for controlling the SARS-CoV-2 pandemic, due to this early cut-off point, which is a limitation of this study. This study does provide a broad review of the available epidemic- and pandemic-related research until COVID-19, and future research relating to COVID-19 outbreak costing and simulation studies can build on it. Extensive future research is indeed warranted to capture the cost of implementing NPIs, including strict social distancing, in relation to this unprecedented and devastating outbreak [70–72]. This review only covered studies from the MEDLINE and EMBASE databases, which publish studies on outbreaks. Studies that might have been exclusively available in the grey literature would not have been identified in this study.
This review presents the existing literature pertaining to the direct costs of implementing NPIs. There are important additional socioeconomic costs associated with the implementation of NPIs, such as the cost of businesses closing due to the intervention or the effects the NPIs have on mental health, the literature for which has not been covered by this review. Additionally, this review does not comprehensively summarise the cost effectiveness of all possible NPIs in outbreak response. Furthermore, as this review is focussed on the costs of public health measures, the costs of policies such as stimulus packages are beyond the scope of this review. The results of this study can be used for information purposes to provide a narrative summary of the cost of implementing historical NPI strategies, and to inform conversations around future planning for implementation of NPIs for pandemic response. The results of this study are also highly useful to inform future research, where numerous gaps or incomplete data were identified.
During the SARS-CoV-2 pandemic, community-based NPIs such as community-wide social distancing measures have been applied rapidly in countries across the globe, with little evidence available for estimating the costs of such an intervention a priori. Having easily accessible collated cost information on community-based NPI strategies will provide a valuable resource for informing future outbreak response policies, where cost data represent a vital component of any cost-effectiveness assessment of NPI options under consideration for implementation. Literature in this field will likely continue to accrue rapidly over the following months. Additional care should also be taken to collect and publish costs for low-income settings for future planning of pandemic financing. Maintaining a database summarising published literature on NPI costs in relation to outbreak response could be valuable for model parameterisation and outbreak response planning purposes.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary document: Literature review search strategy (PDF 40 kb)
Supplementary spreadsheet: Full information on costs presented in review papers and study quality (XLSX 323 kb)
Acknowledgements
We would like to thank Dr Kris Murray and Dr Laura Anselmi for their suggestions for improvement for the written presentation of this review.
Declarations
Funding
The authors acknowledge funding from The Wellcome Trust, and from the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union; and acknowledges funding by Community Jameel. ABH acknowledges support from her Imperial College Research Fellowship. KH was additionally supported by the NIHR HPRU in Modelling and Health Economics, a partnership between PHE, Imperial College London and LSHTM (grant code NIHR200908) JWEO acknowledges support from his MRC Skill development fellowship (reference MR/T025409/1). JS acknowledges PhD funding from the Wellcome Trust (reference 215163/Z/18/Z).
Conflicts of interest
ABH reports personal fees from the World Health Organization, outside the submitted work. ABH was previously engaged by Pfizer Inc to advise on modelling RSV vaccination strategies for which she received no financial compensation, outside the submitted work. ALS, HAS, JS, JWEO, KH, LC, LD, MX, and SSW report no conflicts of interest.
Availability of data and material
Data collected for this literature review is included in the Supplementary spreadsheet that accompanies this manuscript.
Author contributions
Title and abstract screening, full-text screening, preliminary data extraction: JS, LD, JWEO, LC, ABH, ALS, SSW, HAS, MX. Search string testing and planning: JS, JWEO. Data extraction protocol planning, data extraction check and finalisation, data quality assessment, manuscript writing: JS. Comments and edits on manuscript: LD, KH, SWEO, LC, ABH, ALS, SSW, HAS, MX.
References
- 1.Imai N, Gaythorpe KAM, Abbott S, Bhatia S, van Elsland S, Prem K, et al. Adoption and impact of non-pharmaceutical interventions for COVID-19. Wellcome Open Res. 2020;5:59. doi: 10.12688/wellcomeopenres.15808.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demirguc-Kunt A, Lokshin M, Torre I. The sooner, the better: the early economic impact of non-pharmaceutical interventions during the COVID-19 Pandemic. 2020. https://papers.ssrn.com/abstract=3611386. Accessed 7 Oct 2020.
- 3.Bin Nafisah S, Alamery AH, Al Nafesa A, Aleid B, Brazanji NA. School closure during novel influenza: a systematic review. J Infect Public Health. 2018;11:657–661. doi: 10.1016/j.jiph.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Errett NA, Sauer LM, Rutkow L. An integrative review of the limited evidence on international travel bans as an emerging infectious disease disaster control measure. Am J Disaster Med. 2019;14:193–200. doi: 10.5055/ajdm.2019.0331. [DOI] [PubMed] [Google Scholar]
- 5.Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22:1026–1032. doi: 10.1016/j.jval.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 6.International Monetary Fund. Country Indexes and Weight. 2020. https://data.imf.org/regular.aspx?key=61015892. Accessed 23 Sep 2020.
- 7.Bloomberg. Currencies. 2020. https://www.bloomberg.com/markets/currencies. Accessed 23 Sep 2020
- 8.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie CD, Glover AM, Willke MJ, Marx ML, Reising SF, Hutchinson NM. Containment of pertussis in the regional pediatric hospital during the Greater Cincinnati epidemic of 1993. Infect Control Hosp Epidemiol. 1995;16:556–563. doi: 10.2307/30141094. [DOI] [PubMed] [Google Scholar]
- 10.Wahl E, Vold L, Lindstedt BA, Bruheim T, Afset JE. Investigation of an Escherichia coli O145 outbreak in a child day-care centre—extensive sampling and characterization of eae- and stx 1-positive E. coli yields epidemiological and socioeconomic insight. BMC Infect Dis. 2011 doi: 10.1186/1471-2334-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma R, Lu L, Suo L, Li X, Yang F, Zhou T, et al. An expensive adult measles outbreak and response in office buildings during the era of accelerated measles elimination, Beijing, China. Vaccine. 2017;35:1117–1123. doi: 10.1016/j.vaccine.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Galante M, Garin O, Sicuri E, Cots F, García-Altés A, Ferrer M, et al. Health services utilization, work absenteeism and costs of pandemic influenza A (H1N1) 2009 in Spain: a multicenter-longitudinal study. PLoS ONE. 2012;7:e31696. doi: 10.1371/journal.pone.0031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mota NVVP, Lobo RD, Toscano CM, Pedrosode Lima AC, Souza Dias MB, Komagata H, et al. Cost-effectiveness of sick leave policies for health care workers with influenza-like illness, Brazil, 2009. Emerg Infect Dis. 2011;17:1421–1429. doi: 10.3201/eid1708.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125:747–755. doi: 10.1542/peds.2009-1653. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher L, Soyemi K, Conover C, Austin C, Saathoff-Huber L, Nelson S, et al. Outbreak of Escherichia coli O157:H7 in a child care center in Cook County, Illinois, with prolonged shedding and household transmission. Am J Infection Control. 2013 doi: 10.1016/j.ajic.2013.03.312. [DOI] [PubMed] [Google Scholar]
- 16.Ooi PL, Lim S, Chew SK. Use of quarantine in the control of SARS in Singapore. Am J Infect Control. 2005;33:252–257. doi: 10.1016/j.ajic.2004.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Xie J, Fang P. Is a mass prevention and control program for pandemic (H1N1) 2009 good value for money? Evidence from the Chinese Experience. Iran J Public Health. 2012;41:34–43. [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman MS, Garbat-Welch L, Burke H, Weinberg M, Humbaugh K, Tindall A, et al. Direct costs of a single case of refugee-imported measles in Kentucky. Vaccine. 2012;30:317–321. doi: 10.1016/j.vaccine.2011.10.091. [DOI] [PubMed] [Google Scholar]
- 19.Bownds L, Lindekugel R, Stepak P. Economic impact of a hepatitis A epidemic in a mid-sized urban community: the case of Spokane, Washington. J Commun Health. 2003;28:233–246. doi: 10.1023/A:1023981924010. [DOI] [PubMed] [Google Scholar]
- 20.Agusto FB. Optimal isolation control strategies and cost-effectiveness analysis of a two-strain avian influenza model. Biosystems. 2013;113:155–164. doi: 10.1016/j.biosystems.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Yarmand H, Ivy JS, Roberts SD, Bengtson MW, Bengtson NM. Cost-effectiveness analysis of vaccination and self-isolation in case of H1N1. In: Proceedings of the 2010 Winter Simulation Conference. 2010. pp. 2199–210.
- 22.Mubayi A, Zaleta CK, Martcheva M, Castillo-Chávez C. A cost-based comparison of quarantine strategies for new emerging diseases. Math Biosci Eng. 2010;7:687–717. doi: 10.3934/mbe.2010.7.687. [DOI] [PubMed] [Google Scholar]
- 23.Parker AA, Staggs W, Dayan GH, Ortega-Sánchez IR, Rota PA, Lowe L, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355:447–455. doi: 10.1056/NEJMoa060775. [DOI] [PubMed] [Google Scholar]
- 24.Pike J, Marin M, Guo A, Haselow D, Safi H, Zhou F. 2016–2017 Arkansas mumps outbreak in a close-knit community: assessment of the economic impact and response strategies. Vaccine. 2020;38:1481–1485. doi: 10.1016/j.vaccine.2019.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen JB, Arciuolo RJ, Khawja AM, Fu J, Giancotti FR, Zucker JR. Public health consequences of a 2013 measles outbreak in New York City. JAMA Pediatr. 2018;172:811–817. doi: 10.1001/jamapediatrics.2018.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayan GH, Ortega-Sánchez IR, LeBaron CW, Quinlisk MP. Iowa Measles Response Team. The cost of containing one case of measles: the economic impact on the public health infrastructure–Iowa, 2004. Pediatrics. 2005;116:e1–4. doi: 10.1542/peds.2004-2512. [DOI] [PubMed] [Google Scholar]
- 27.Flego KL, Belshaw DA, Sheppeard V, Weston KM. Impacts of a measles outbreak in Western Sydney on public health resources. Commun Dis Intell Q Rep. 2013;37:E240–E245. doi: 10.33321/cdi.2013.37.36. [DOI] [PubMed] [Google Scholar]
- 28.Borse RH, Behravesh CB, Dumanovsky T, Zucker JR, Swerdlow D, Edelson P, et al. Closing schools in response to the 2009 pandemic influenza A H1N1 virus in New York City: economic impact on households. Clin Infect Dis. 2011;52(Suppl 1):S168–S172. doi: 10.1093/cid/ciq033. [DOI] [PubMed] [Google Scholar]
- 29.Chen W-C, Huang AS, Chuang J-H, Chiu C-C, Kuo H-S. Social and economic impact of school closure resulting from pandemic influenza A/H1N1. J Infect. 2011;62:200–203. doi: 10.1016/j.jinf.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Gift TL, Palekar RS, Sodha SV, Kent CK, Fagan RP, Archer WR, et al. Household effects of school closure during pandemic (H1N1) 2009, Pennsylvania, USA. Emerg Infect Dis. 2010;16:1315–1317. doi: 10.3201/eid1608.091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson AJ, Moore ZS, Edelson PJ, Kinnane L, Davies M, Shay DK, et al. Household responses to school closure resulting from outbreak of influenza B, North Carolina. Emerg Infect Dis. 2008;14:1024. doi: 10.3201/eid1407.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell ES, Zheteyeva Y, Gao H, Shi J, Rainey JJ, Thoroughman D, et al. Reactive school closure during increased influenza-like illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infectious Diseases. Oxford University Press; 2016. https://academic.oup.com/ofid/article-abstract/3/3/ofw113/2593258. Accessed 25 Nov 2020. [DOI] [PMC free article] [PubMed]
- 33.Tracht SM, Del Valle SY, Edwards BK. Economic analysis of the use of facemasks during pandemic (H1N1) 2009. J Theor Biol. 2012;300:161–172. doi: 10.1016/j.jtbi.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukerji S, MacIntyre CR, Seale H, Wang Q, Yang P, Wang X, et al. Cost-effectiveness analysis of N95 respirators and medical masks to protect healthcare workers in China from respiratory infections. BMC Infect Dis. 2017;17:464. doi: 10.1186/s12879-017-2564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baracco G, Eisert S, Eagan A, Radonovich L. Comparative cost of stockpiling various types of respiratory protective devices to protect the health care workforce during an influenza pandemic. Disaster Med Public Health Prep. 2015;9:313–318. doi: 10.1017/dmp.2015.12. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Geng W, Tian H, Lai D. Was Mandatory Quarantine Necessary in China for Controlling the 2009 H1N1 Pandemic? Int J Environ Res Public Health. 2013 doi: 10.3390/ijerph10104690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orset C. People’s perception and cost-effectiveness of home confinement during an influenza pandemic: evidence from the French case. Eur J Health Econ. 2018;19:1335–1350. doi: 10.1007/s10198-018-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta AG, Moyer CA, Stern DT. The economic impact of quarantine: SARS in Toronto as a case study. J Infect. 2005;50:386–393. doi: 10.1016/j.jinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein JM, Goedecke DM, Yu F, Morris RJ, Wagener DK, Bobashev GV. Controlling pandemic flu: the value of international air travel restrictions. PLoS ONE. 2007;2:e401. doi: 10.1371/journal.pone.0000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prager F, Wei D, Rose A. Total economic consequences of an influenza outbreak in the United States. Risk Anal. 2017;37:4–19. doi: 10.1111/risa.12625. [DOI] [PubMed] [Google Scholar]
- 41.Boyd M, Baker MG, Mansoor OD, Kvizhinadze G, Wilson N. Protecting an island nation from extreme pandemic threats: proof-of-concept around border closure as an intervention. PLoS ONE. 2017;12:e0178732. doi: 10.1371/journal.pone.0178732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andradóttir S, Chiu W, Goldsman D, Lee ML, Tsui K-L, Sander B, et al. Reactive strategies for containing developing outbreaks of pandemic influenza. BMC Public Health. 2011;11(Suppl 1):S1. doi: 10.1186/1471-2458-11-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araz OM, Damien P, Paltiel DA, Burke S, van de Geijn B, Galvani A, et al. Simulating school closure policies for cost effective pandemic decision making. BMC Public Health. 2012;12:449. doi: 10.1186/1471-2458-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown ST, Tai JHY, Bailey RR, Cooley PC, Wheaton WD, Potter MA, et al. Would school closure for the 2009 H1N1 influenza epidemic have been worth the cost?: a computational simulation of Pennsylvania. BMC Public Health. 2011;11:353. doi: 10.1186/1471-2458-11-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halder N, Kelso JK, Milne GJ. Cost-effective strategies for mitigating a future influenza pandemic with H1N1 2009 characteristics. PLoS ONE. 2011;6:e22087. doi: 10.1371/journal.pone.0022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones RM, Adida E. Selecting nonpharmaceutical interventions for influenza. Risk Anal. 2013;33:1473–1488. doi: 10.1111/j.1539-6924.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- 47.Kelso JK, Halder N, Postma MJ, Milne GJ. Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories. BMC Public Health. 2013;13:211. doi: 10.1186/1471-2458-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keogh-Brown MR, Smith RD, Edmunds JW, Beutels P. The macroeconomic impact of pandemic influenza: estimates from models of the United Kingdom, France, Belgium and The Netherlands. Eur J Health Econ. 2010;11:543–554. doi: 10.1007/s10198-009-0210-1. [DOI] [PubMed] [Google Scholar]
- 49.Lempel H, Epstein JM, Hammond RA. Economic cost and health care workforce effects of school closures in the US. PLoS Curr. 2009;1:RRN1051. doi: 10.1371/currents.RRN1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maharaj S, Kleczkowski A. Controlling epidemic spread by social distancing: Do it well or not at all. BMC Public Health. 2012;12:679. doi: 10.1186/1471-2458-12-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milne GJ, Halder N, Kelso JK. The cost effectiveness of pandemic influenza interventions: a pandemic severity based analysis. PLoS ONE. 2013;8:e61504. doi: 10.1371/journal.pone.0061504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morin BR, Perrings C, Levin S, Kinzig A. Disease risk mitigation: the equivalence of two selective mixing strategies on aggregate contact patterns and resulting epidemic spread. J Theor Biol. 2014;363:262–270. doi: 10.1016/j.jtbi.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishiura H, Ejima K, Mizumoto K, Nakaoka S, Inaba H, Imoto S, et al. Cost-effective length and timing of school closure during an influenza pandemic depend on the severity. Theor Biol Med Model. 2014;11:5. doi: 10.1186/1742-4682-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perlroth DJ, Glass RJ, Davey VJ, Cannon D, Garber AM, Owens DK. Health outcomes and costs of community mitigation strategies for an influenza pandemic in the United States. Clin Infect Dis. 2010;50:165–174. doi: 10.1086/649867. [DOI] [PubMed] [Google Scholar]
- 55.Reluga TC. Game theory of social distancing in response to an epidemic. PLoS Comput Biol. 2010 doi: 10.1371/journal.pcbi.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadique MZ, Adams EJ, Edmunds WJ. Estimating the costs of school closure for mitigating an influenza pandemic. BMC Public Health. 2008;8:135. doi: 10.1186/1471-2458-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sander B, Nizam A, Garrison LP, Jr, Postma MJ, Halloran ME, Longini IM., Jr Economic evaluation of influenza pandemic mitigation strategies in the United States using a stochastic microsimulation transmission model. Value Health. 2009;12:226–233. doi: 10.1111/j.1524-4733.2008.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saunders-Hastings P, Quinn Hayes B, Smith R, Krewski D. Modelling community-control strategies to protect hospital resources during an influenza pandemic in Ottawa, Canada. PLoS ONE. 2017;12:e0179315. doi: 10.1371/journal.pone.0179315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith RD, Keogh-Brown MR. Macroeconomic impact of pandemic influenza and associated policies in Thailand, South Africa and Uganda. Influenza Other Respir Viruses. 2013;7:64–71. doi: 10.1111/irv.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith RD, Keogh-Brown MR, Barnett T. Estimating the economic impact of pandemic influenza: an application of the computable general equilibrium model to the UK. Soc Sci Med. 2011 doi: 10.1016/j.socscimed.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith RD, Keogh-Brown MR, Barnett T, Tait J. The economy-wide impact of pandemic influenza on the UK: a computable general equilibrium modelling experiment. BMJ. 2009;339:b4571. doi: 10.1136/bmj.b4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Szeto KY, Leung FC-C. Effectiveness of closure of public places with time delay in disease control. J Integr Bioinform. 2008 doi: 10.2390/biecoll-jib-2008-96. [DOI] [PubMed] [Google Scholar]
- 63.Wong ZS-Y, Goldsman D, Tsui K-L. Economic evaluation of individual school closure strategies: the Hong Kong 209 H1N1 pandemic. PLoS ONE. 2016;11:e0147052. doi: 10.1371/journal.pone.0147052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue Y, Kristiansen IS, de Blasio BF. Dynamic modelling of costs and health consequences of school closure during an influenza pandemic. BMC Public Health. 2012;12:962. doi: 10.1186/1471-2458-12-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yaesoubi R, Cohen T. Identifying cost-effective dynamic policies to control epidemics. Stat Med. 2016 doi: 10.1002/sim.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson SH, Yu G, Jokela JA. A double-risk monitoring and movement restriction policy for Ebola entry screening at airports in the United States. Prev Med. 2016;88:33–38. doi: 10.1016/j.ypmed.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 67.Sardar T, Mukhopadhyay S, Bhowmick AR, Chattopadhyay J. An optimal cost effectiveness study on Zimbabwe Cholera Seasonal Data from 2008–2011. PLoS ONE. 2013;8:e81231. doi: 10.1371/journal.pone.0081231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elbasha E. Costs and benefits of a subtype-specific surveillance system for identifying Escherichia coli O157:H7 outbreaks. Emerging Infectious Dis. 2000 doi: 10.3201/eid0603.000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng C, Barceló J, Hartnett AS, Kubinec R, Messerschmidt L. COVID-19 Government Response Event Dataset (CoronaNet v.1.0) Nat Hum Behav. 2020;4:756–768. doi: 10.1038/s41562-020-0909-7. [DOI] [PubMed] [Google Scholar]
- 70.Eilersen A, Sneppen K. Estimating cost-benefit of quarantine length for COVID-19 mitigation. Epidemiology. medRxiv; 2020. https://www.medrxiv.org/content/10.1101/2020.04.09.20059790v2?rss=1
- 71.Wang Q, Shi N, Huang J, Cui T, Yang L, Ai J, et al. Effectiveness and cost-effectiveness of public health measures to control COVID-19: a modelling study. Epidemiology. medRxiv; 2020. https://www.medrxiv.org/content/10.1101/2020.03.20.20039644v2. Accessed 25 Nov 2020.
- 72.Ugarov A. Inclusive Costs of NPI Measures for COVID-19 Pandemic: Three Approaches. Health Economics. MedRxiv; 2020. https://www.medrxiv.org/content/10.1101/2020.03.26.20044552v1. Accessed 25 Nov 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary document: Literature review search strategy (PDF 40 kb)
Supplementary spreadsheet: Full information on costs presented in review papers and study quality (XLSX 323 kb)