Abstract
Objective
This study investigated the characteristics of progestin-insensitive endometrioid endometrial cancer (EEC) and atypical endometrial hyperplasia (AEH) patients receiving fertility-sparing treatments and assessed the therapeutic effects of second-line fertility-preserving treatments.
Methods
Three hundred and thirty-eight patients with EEC (n=75) or AEH (n=263) receiving fertility-preserving treatment were retrospectively analyzed. ‘Progestin-insensitive’ was defined as meeting one of the following criteria: 1) presented with progressed disease at any time during conservative treatment, 2) remained with stable disease after 7 months of treatment, and/or 3) did not achieve complete response (CR) after 10 months of treatment. Clinical characteristics and treatment results of progestin-insensitive patients receiving second-line treatment and those of progestin-sensitive patients were compared.
Results
Eight-two patients (59 AEH and 23 EEC) were defined as progestin-insensitive and 256 as progestin-sensitive. In multivariate analysis, body mass index ≥28.0 kg/m2 (odds ratio [OR]=1.898) and lesion size >2 cm (OR=2.077) were independent predictors of progestin-insensitive status. Compared to AEH patients, progestin-insensitive EEC patients had poorer second-line treatment responses (28-week cumulative CR rate after changing second-line treatment, 56.3% vs. 85.4%, p=0.011). No statistical difference was found in CR rate among different second-line treatments.
Conclusion
Obesity and larger lesion size were independent risk factors associated with progestin-insensitive status. In progestin-insensitive patients receiving second-line treatment, EEC patients had lower CR rate comparing with AEH patients. Further study with larger sample size is needed to evaluate efficacy of different second-line treatments for progestin insensitive patients.
Keywords: Endometrial Hyperplasia, Endometrial Neoplasms, Conservative Treatment
INTRODUCTION
Fertility-preserving treatments for early stage endometrioid endometrial cancer (EEC) and atypical endometrial hyperplasia (AEH) in young women have received growing attention due to the increased incidence of these diseases. Although highly efficient progestin-based regimens have achieved satisfactory results [1,2], 10%–30% of patients are insensitive to progestin and become refractory cases [3,4].
Several clinical questions regarding progestin-insensitive cases remain unanswered. For instance, potential second-line treatments and their therapeutic effects have not been clarified. The prognosis of progestin-insensitive cases compared to that of progestin-sensitive patients has not been evaluated, and it remains unclear whether fertility-preserving treatments should be continued or whether definitive surgery should be indicated. Further, it remains unclear whether progestin-insensitive cases can be predicted prior to treatment initiation.
Although several reports on second-line treatments for progestin-insensitive patients exist [5,6,7], the reported case numbers were low, and there is an evident lack of studies comparing the effects of various second-line treatments. To address these lacunae in knowledge, we conducted a single-center retrospective study in EEC and AEH patients receiving fertility-preserving treatment. We divided our patients into progestin-insensitive and sensitive groups according to their response to first-line progestin-based treatment. The clinical characteristics and prognosis of progestin-insensitive patients were analyzed and compared with those of the progestin-sensitive group. Therapeutic effects of various second-line treatments were also investigated.
MATERIALS AND METHODS
1. Study population
Women with early-stage EEC and AEH receiving fertility-preserving treatment at the Obstetrics and Gynecology Hospital of Fudan University (OB&Gyn Hospital) from April 2012 to July 2018 were retrospectively investigated. This retrospective study was approved by the Ethics Committees of OB&Gyn Hospital (approval No. 2018-46).
All patients received standardized evaluation and treatment protocols. All data were recorded prospectively during the process of treatment and follow-up. The inclusion and exclusion criteria for fertility-sparing treatment [8] included: histologically proven AEH or well-differentiated EEC G1 without myometrial invasion; no signs of suspicious extrauterine involvement by image study; younger than 45 years old; strong willingness to preserve fertility; no contraindications for progestin treatment or pregnancy; not pregnant; good compliance for treatment. Written informed consent was obtained from all patients prior to initiating treatment.
All patients were pathologically diagnosed by endometrial biopsy through dilation and curettage (D&C) with or without hysteroscopy (HSC). If the patient was initially diagnosed by endometrial biopsy without hysteroscopy, hysteroscopic evaluation would be arranged within one month after endometrial biopsy before conservative treatment. Size of lesions were evaluated as previously reported [9]. Size of punctiform or polypoidal was evaluated by basal diameter rather than the diameter of lesions. The size of cluster or sheet-like lesion was evaluated with the maximum diameter. Pathologic diagnosis was confirmed by two experienced gynecological pathologists according to the World Health Organization pathological classification (2014) [10]. If their opinions differed, a discussion was held to determine the final diagnosis.
All patients were evaluated by ultrasound scan. Enhanced pelvic MRI and enhanced upper abdominal CT were performed for EEC patients to evaluate myometrial invasion and detect extrauterine metastasis.
2. Conservative treatment and evaluation
A total of 338 patients eligible for fertility-sparing treatment received treatment soon after diagnosis and comprehensive evaluation. As first-line treatment, 324 patients (95.9%) received oral megestrol acetate (MA) (160 mg/day) (n=319) or medroxyprogesterone acetate (MPA) (250 mg/day) (n=5). Of patients, 112 (112/293, 38.2%) also received metformin (1,500 mg/day). Of patients, 27 received MA combined with levonorgestrel intrauterine system (LNG-IUS) and 4 patients received MA combined with metformin and LNG-IUS. For the remaining 14 patients, 12 cases received LNG-IUS only, whereas the other 2 cases received ethinylestradiol cyproterone combined with metformin.
After initiating fertility-preserving treatment, hysteroscopic evaluation was performed every 3 months until complete response (CR) or definitive hysterectomy [11]. The response to treatment was assessed histologically using specimens obtained during each hysteroscopic evaluation. CR was defined as the absence of hyperplasia or carcinoma. Partial response (PR) was defined as pathological improvement. Stable disease (SD) was defined as the persistence of lesions as originally diagnosed. Progressed disease (PD) was defined as evidence of endometrial cancer for AEH patients or evidence of higher pathological grade, myometrial invasion, or extra-uterine metastasis for EEC patients.
Definitive hysterectomy was suggested for patients presenting with PD during conservative treatment, those presenting with SD after 7 months of treatment, or those who did not achieve CR after 10 months of treatment. For patients who insisted on receiving fertility-preserving treatment, a multidisciplinary discussion was held for individual cases, and second-line treatment was administered after careful consultation and informed consent.
Second-line treatments included MA (160 mg/day) combined with metformin (1,500 mg/day) (MA + metformin group, only for those who used MA/MPA alone as first-line treatment); ethinylestradiol cyproterone (Diane-35®) one pill per day for 21 days of a 28-day cycle combined with metformin (1,500 mg/day) (ethinylestradiol cyproterone + metformin group, for those who used MA plus metformin as first-line treatment); LNG-IUS, gonadotropin-releasing hormone agonist (GnRH-a), and/or letrozole alone or in combination with other regimens. The choice of second-line treatment was at the discretion of the treating doctor.
3. Maintenance treatment and follow-up
Once the patient achieved CR, the same regimen was administered for another 2–3 months for consolidation. Another hysteroscopy was performed 3 months after the first diagnosis of CR for confirmation. Progestin-based maintenance therapy, including cyclic ethinylestradiol cyproterone one pill per day for 21 days of a 28-day cycle, cyclic MPA 10 mg per day for 15 days, or LNG-IUS was recommended for patients without a recent pregnancy plan. For those who desired pregnancy, assisted reproduction technology (ART) under close surveillance was strongly encouraged. Patients were advised to initiate regular follow-up 6 months after delivery, although definitive surgery after childbirth was always advised.
CR patients were followed up every 3 to 6 months. Ultrasound and endometrial biopsy by Pipelle were used to evaluate the endometrium. Enhanced pelvic MRI, serum CA-125 were used when indicated.
Adverse effects were recorded throughout treatment and subsequent follow-up, including weight gain, thrombosis, lactic acidosis, impaired liver and renal function, and other complaints. All patients were followed up until July 2019.
4. Definition of ‘progestin-insensitive’
We defined ‘progestin-insensitive’ patients as meeting one of the following criteria: 1) presented with PD at any time during conservative treatment; 2) remained with SD after 7 months of first-line treatment; 3) did not achieve CR after 10 months of first-line treatment.
The reason for this definition is explained in the discussion part.
5. Data collection
All blood samples were collected before initiating fertility-preserving treatment and examined in the laboratory of OB&Gyn Hospital. Fasting blood glucose (FBG), fasting insulin (FINS), sex hormones, CA-125 and HE-4 were assessed. Body mass index (BMI) and the homeostasis model assessment-insulin resistance (HOMA-IR) index were calculated, and metabolic syndrome (MS) criteria were evaluated as reported previously [11]. Obesity was defined as BMI ≥28 kg/m2 following the criteria for Chinese adults. HOMA-IR index (FBG [mmol/L]×FINS [μU/mL]/22.5) was used to evaluate insulin resistant (IR) status. When HOMA-IR was ≥2.95, we considered the patient to be IR.
6. Statistical analysis
Duration to achieve CR was measured from the time point of initiating conservative treatment to pathological diagnosis of CR. The time point of achieving CR was defined as the initial time of pathological diagnosis of CR.
Continuous variables are summarized as medians and ranges. Categorical variables are presented as frequencies and percentages. The intra-group differences for continuous variables were compared using a Student's t-test or the Mann-Whitney U-test as appropriate. Frequency distributions were compared using the χ2 test or Fisher's exact test as appropriate. Therapeutic duration was estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Univariate and multivariate logistic regression was used to explore risk factors in progestin-insensitive patients. The p-values <0.05 in two-sided tests were considered statistically significant. All statistical analyses were performed using SPSS (version 24.0; Armonk, New York, NY, USA).
RESULTS
The clinical and pathological characteristics are summarized in Table 1 and Supplementary Table 1. A total of 338 (263 AEH and 75 EEC) patients were evaluated. Of patients, three with age ≥45 years old (47, 48, and 49 years old, respectively) who insisted on uterus preservation were included after being fully informed. The median age at diagnosis was 32 years old (range, 20–49 years old), and median BMI was 24.18 kg/m2 (range, 15.63–44.06 kg/m2). The median follow-up time from the date of initiating treatment to the last follow-up was 27 months (range, 3–94 months). The median follow-up time from the date of achieving CR to the last follow-up was 25 months (range, 0–86 months). In total, 323 patients (323/338, 95.6%) achieved CR with a median treatment duration to CR of 6.0 months. The 28-week CR rate in EEC and AEH was 41.3% and 54.2% respectively (p=0.049) and the 40-week CR rate was 69.3% and 79.5% respectively (p=0.063) (Supplementary Fig. 1A).
Table 1. General characteristics of the study population.
| Variables | Total | Progestin-insensitive patients | Progestin-sensitive patients | p-value* | |
|---|---|---|---|---|---|
| Patient (No.) | 338 | 82 | 256 | ||
| Age (yr) | 32 (20–49) | 32 (20–43) | 32 (21–49) | 0.981 | |
| Initial pathological diagnosis | |||||
| EEC | 75 (22.2) | 23 (28.0) | 52 (20.3) | 0.142 | |
| AEH | 263 (77.8) | 59 (72.0) | 204 (79.7) | ||
| Hypertension | 63 (18.6) | 15 (18.3) | 48 (18.8) | 0.926 | |
| Diabetes mellitus† | 22 (6.5) | 5 (6.1) | 17 (6.7) | 0.857 | |
| Nulliparous† | 257 (76.0) | 65 (79.3) | 192 (75.0) | 0.495 | |
| BMI (kg/m2) | 24.18 (15.63–44.06) | 25.82 (15.63–44.06) | 23.62 (15.94–37.65) | 0.003 | |
| BMI≥28 | 93 (27.5) | 34 (41.5) | 59 (23.0) | 0.001 | |
| BMI<28 | 245 (72.5) | 48 (58.5) | 197 (77.0) | ||
| HOMA-IR | 2.26 (0.44–16.50) | 2.59 (0.44–16.50) | 2.20 (0.44–13.03) | 0.017 | |
| HOMA-IR≥2.95 | 129 (38.2) | 38 (46.3) | 91 (35.5) | 0.080 | |
| HOMA-IR<2.95 | 209 (61.8) | 44 (53.7) | 165 (64.5) | ||
| Waist-hip ratio | 0.86 (0.65–1.12) | 0.87 (0.72–1.05) | 0.85 (0.65–1.12) | 0.009 | |
| ≥0.85 | 190 (56.2) | 55 (67.1) | 135 (52.7) | 0.023 | |
| <0.85 | 148 (43.8) | 27 (32.9) | 121 (47.3) | ||
| CA125 (U/mL)† | 17.50 (1.37–819.50) | 17.09 (4.66–72.80) | 17.50 (1.37–819.50) | 0.965 | |
| HE-4 (pmol/mL)† | 44.70 (2.04–107.00) | 46.05 (2.04–102.90) | 43.20 (3.50–107.00) | 0.038 | |
| Estradiol (pg/mL)† | 54 (1–1,237) | 52 (3–778) | 54 (1–1,237) | 0.535 | |
| Progesterone (ng/mL)† | 0.43 (0.01–67.50) | 0.43 (0.01–12.85) | 0.45 (0.01–67.50) | 0.288 | |
| Total testosterone (ng/mL)† | 0.39 (0.01–45.00) | 0.35 (0.01–1.19) | 0.40 (0.01–45.00) | 0.215 | |
| MS† | 140 (41.4) | 32 (39.0) | 108 (42.4) | 0.595 | |
| Lesion size (cm)† | |||||
| ≤2 | 135 (47.7) | 25 (34.2) | 110 (52.4) | 0.008 | |
| >2 | 148 (52.3) | 48 (65.8) | 100 (47.6) | ||
| Treatment options | 0.567 | ||||
| MA/MPA | 181 (53.6) | 42 (51.2) | 139 (54.3) | ||
| MA/MPA + metformin | 112 (33.1) | 30 (36.6) | 82 (32.0) | ||
| MA/MPA + LNG-IUS | 27 (8.0) | 4 (4.9) | 23 (9.0) | ||
| LNG-IUS | 12 (3.6) | 4 (4.9) | 8 (3.1) | ||
| Others | 6 (1.8) | 2 (2.4) | 4 (1.6) | ||
| Median treatment duration to CR (mo)‡ | 6.0 (5.5–6.5) | 14.0 (12.9–15.1) | 5.0 (4.6–5.4) | ||
| 1-year cumulative CR rate (%)‡ | 84.2 | 33.1 | 100 | <0.001 | |
| 2-year cumulative CR rate (%)‡ | 98.6 | 94.1 | 100 | <0.001 | |
| Median follow-up duration after CR (mo) | 25 (0–86) | 16 (0–56) | 27 (0–86) | <0.001 | |
| 1-year relapse-free survival after CR (%)‡ | 92.5 | 85.1 | 93.8 | 0.061 | |
| Pregnancy rate§ | 60/127 (47.2) | 9/23 (39.1) | 51/104 (49.0) | 0.389 | |
| Live birth rate | 48/60 (80.0) | 7/9 (77.8) | 41/51 (80.4) | 0.857 | |
| Method of conception | 0.081 | ||||
| Spontaneous conception | 37/178 (20.7) | 6/31 (19.4) | 31/147 (21.1) | ||
| Ovulation induction | 36/178 (20.2) | 2/31 (6.5) | 34/147 (23.3) | ||
| In-vitro fertilization | 105/178 (59.0) | 23/31 (74.2) | 82/147 (55.8) | ||
Data are shown as number (%) or median (range); treatment duration is shown as median, range. p-value: comparison between progestin-insensitive and progestin-sensitive group.
AEH, atypical endometrial hyperplasia; BMI, body mass index; CR, complete response; EEC, endometrioid endometrial cancer; HE-4, human epididymis protein 4; HOMA-IR, homeostasis model assessment-insulin resistance; LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate; MPA, medroxyprogesterone acetate; MS, metabolic syndrome.
*P value for comparison between progestin-insensitive patients and progestin-sensitive patients. †All variables were analyzed among 341 patients except for diabetes mellitus, nulliparous, CA125, HE-4, estradiol, progesterone, total testosterone and MS. Missing data for 5 cases for diabetes mellitus, 2 for Nulliparous, 31 for CA125, 51 for HE-4, 7 for estradiol, 11 for progesterone, 12 for total testosterone, 1 for MS and 55 for Lesion size. ‡Median treatment duration to CR, 1-year cumulative CR rate, 2-year cumulative CR rate and 1-year relapse-free rate after CR were performed by Kaplan-Meier analysis. And median treatment duration to CR is presented as the treatment duration when the estimated CR rate is 50% and 95% confidence interval. §Pregnant rate within 16 months after CR.
1. Clinical-pathological characteristics of progestin-insensitive group
Our classification identified 82 progestin-insensitive cases and 256 progestin-sensitive cases. Compared to progestin-sensitive patients, progestin-insensitive patients had higher BMI (25.82 kg/m2 vs. 23.62 kg/m2, p=0.003), higher HOMA-IR index (2.59 vs. 2.20, p=0.017), higher waist-hip ratio (WHR, 0.87 vs. 0.85, p=0.009), higher HE-4 levels (46.05 pmol/mL vs. 43.20 pmol/mL, p=0.038), and larger lesion size (>2 cm, 65.8% vs. 47.6%, p=0.008) (Table 1). As initial pathological diagnosis may affect treatment outcomes, patients were stratified into AEH and EEC groups. In the AEH group, progestin-insensitive patients were more likely to have higher BMI (p=0.007), higher HOMA-IR index (p=0.012), WHR ≥0.85 (p=0.0499), higher HE-4 levels (p=0.047), and larger lesion size (p=0.037) (Supplementary Table 1). However, no significant differences were observed in clinical characteristics between progestin-sensitive and insensitive EEC patients, which could be due to low case numbers.
For progestin-insensitive patients, the median treatment time to achieve CR was 14.0 months, and the 1-year and 2-year cumulative CR rates were 33.1% and 94.1%, respectively (Table 1, Supplementary Fig. 2A). A trend for lower 1-year relapse-free survival after CR was observed in the progestin-insensitive group when compared to the progestin-sensitive group (85.1% vs. 93.8%, p=0.061). The median follow-up duration after CR for progestin sensitive patients was 27 months, and 16 months for insensitive patients (p<0.001). To adjust for this bias, we calculated pregnancy rate within 16 months after CR. The pregnancy rate within 16 months after CR was 49.0% (51/104) and 39.1% (9/23) in progestin sensitive group and insensitive group respectively (p=0.389). Among these pregnant women, 41 out of 51 (80.4%) in progestin sensitive group and 7 out 9 (77.8%) in progestin insensitive group achieved live birth (p=0.857). Pregnancy was lost in 10 out of 51 (19.6%) patients in progestin sensitive group and 2 out 9 (22.2%) in progestin insensitive group.
2. Risk factors associated with progestin-insensitive status
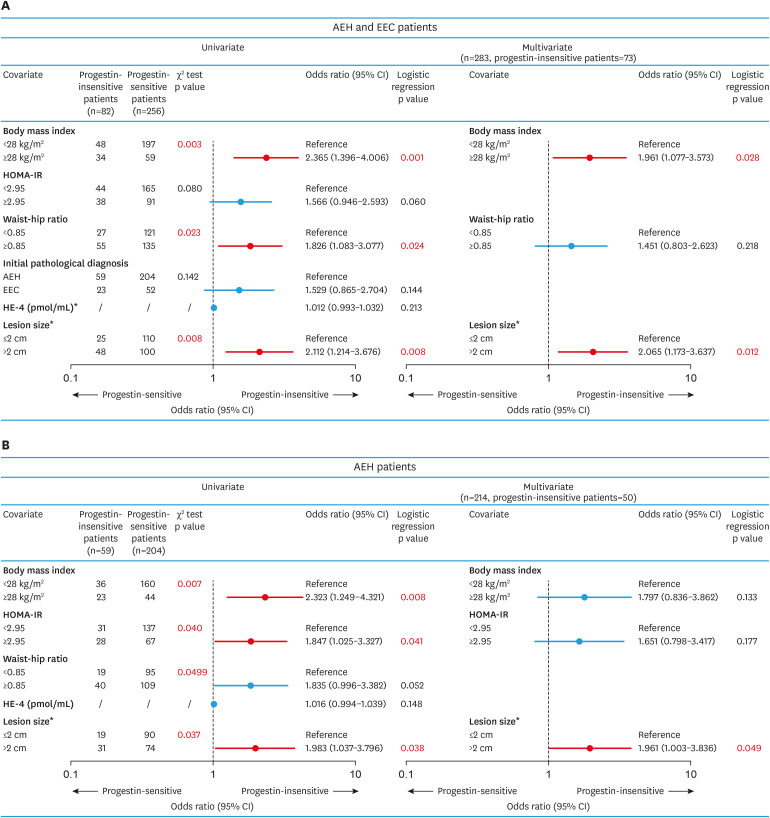
We then explored possible risk factors associated with progestin-insensitive status. Univariate analysis revealed that BMI ≥28.0 kg/m2, WHR ≥0.85, and lesion size >2 cm were associated with progestin-insensitivity (Fig. 1A). In multivariate analysis, BMI ≥28.0 kg/m2 (odds ratio [OR]=1.961; 95% CI=1.077–3.573; p=0.028) and lesion size >2 cm (OR=2.065; 95% CI=1.173–3.637; p=0.012) remained independent predictors of progestin-insensitive status. Similar findings were also seen in AEH patients (Fig. 1B). Consistently, patients with BMI ≥28.0 kg/m2 had lower 28 weeks CR rate (38.0% vs. 55.5%, p=0.003) and lower 40 weeks CR rate (65.2% vs. 81.8%, p=0.001) compared to those with BMI<28 kg/m2 (Supplementary Fig. 1B). And lesion size >2 cm was also associated with lower 28 weeks CR rate (39.0% vs. 59.3%, p=0.001) and lower 40 weeks CR rate (69.9% vs. 82.8%, p=0.011) compared to lesion size <2 cm (Supplementary Fig. 1C).
Fig. 1. Possible risk factors related to progestin-insensitive status (versus progestin-sensitive cases).
Univariate and multivariate logistic regression models were used to identify risk factors associated with progestin-insensitive status in all patients or AEH patients (A, B).
*Missing data for 51 cases for HE-4 and 55 for lesion size.
AEH, atypical endometrial hyperplasia; CI, confidence interval; EEC, endometrioid endometrial carcinoma; HE-4, human epididymis protein 4; HOMA-IR, homeostasis model assessment-insulin resistance.
3. Results of second-line treatments for progestin-insensitive patients
In order to avoid interference of different first-line regimens, we only included patients receiving MPA/MA with or without metformin as initial treatment to compare therapeutic effects of various second line treatment. And we restricted our analysis to second-line treatment regimens with more than five cases. Patients who underwent hysterectomy within 32 weeks of receiving second-line treatment were also excluded. The effects of second-line treatment in progestin-insensitive patients were analyzed in a final total of 57 patients (Table 2, Supplementary Tables 2 and 3). No significant differences were observed in the reasons for second-line treatments (p=0.098) (Supplementary Table 4). A significant difference was noted in the distribution of second-line treatment regimens between EEC and AEH patients. More EEC patients received MA plus LNG-IUS with or without metformin as a second-line treatment (37.5%, 6/16), whereas more AEH patients received ethinylestradiol cyproterone with metformin (51.2%, 21/41) as a second-line treatment (p=0.008) (Supplementary Table 4).
Table 2. Results of second-line treatments for progestin-insensitive patients (n=57).
| Initial treatments | Second-line treatments | No. | Treatment duration to achieve CR* (wk) (median, range) | 28-week cumulative CR* rate, % | Remained PR* after 28 weeks of second-line treatment, % (No.) | Remained SD* after 28 weeks of second-line treatment, % (No.) | 28-week cumulative PD* rate, % |
|---|---|---|---|---|---|---|---|
| MA/MPA | Prolonged treatment duration† | 7 | 12 (8–32) | 71.4 | 14.3 (1/7) | 14.3 (1/7) | 0.0 |
| MA/MPA + metformin | Prolonged treatment duration† | 5 | 20 (12–28) | 100.0 | 0.0 (0/5) | 0.0 (0/5) | 0.0 |
| MA/MPA | MA + metformin | 12 | 23 (9–100) | 66.7 | 25.0 (3/12) | 8.3 (1/12) | 0.0 |
| MA/MPA ± metformin | Ethinylestradiol cyproterone + metformin | 26 | 19 (6–41) | 83.2 | 11.5 (3/26) | 3.8 (1/26) | 7.7 |
| MA/MPA ± metformin | MA + LNG-IUS ± metformin | 7 | 27 (12–56) | 57.1 | 42.9 (3/7) | 0.0 (0/7) | 0.0 |
Treatment duration is shown as median (range).
*Treatment duration for these progestin insensitive patients was defined as from the time point of initiating second line treatment to the time point indicated in each column. †Kept on original treatment.
CR, complete response; LNG-IUS, levonorgestrel-releasing intrauterine system; MA, megestrol acetate; MPA, medroxyprogesterone acetate; PD, progressed disease; PR, partial response; SD, stable disease.
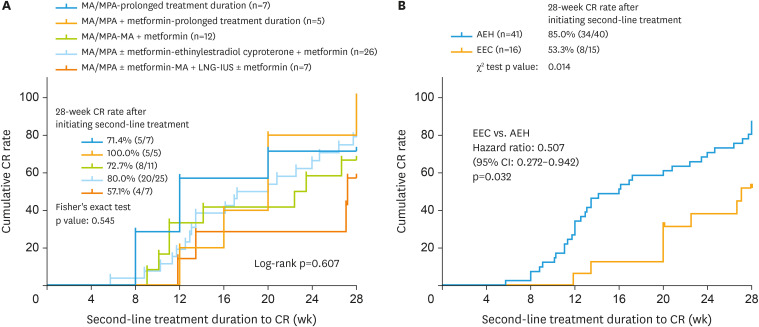
The 28-week CR rate after initiating second-line treatment (CRsec rate) was 71.4%, 100%, 72.7%, 80.0%, and 57.1% for prolonged MA/MPA, prolonged MA/MPA plus metformin, MA/MPA plus metformin, ethinylestradiol cyproterone plus metformin, and MA plus LNG-IUS with or without metformin treatment groups, respectively (Fig. 2A). No significant differences were observed in the 28-week CRsec rate among treatment groups (p=0.545) (Fig. 2A) in AEH and EEC patients or AEH patients alone (p=0.664) (Supplementary Fig. 3A). However, the 28-week CRsec rate was lower in EEC patients than in AEH patients (53.3% vs. 85.0%, p=0.014) (Fig. 2B).
Fig. 2. The 28-week CR rate in progestin-insensitive patients with different second-line options (A) and the 28-week CR rate after initiating second-line treatment (B) of AEH and EEC patients.
AEH, atypical endometrial hyperplasia; CI, confidence interval; CR, complete response; EEC, endometrioid endometrial carcinoma; LNG-IUS, levonorgestrel-releasing intrauterine system; MA, megestrol-acetate; MPA, Medroxyprogesterone-acetate.
DISCUSSION
Our research proposed a preliminary definition of progestin-insensitive cases of endometrial cancer and precancerous lesions for fertility-sparing treatment and summarized possible second-line treatment options for progestin-insensitive patients. We observed that progestin-insensitive status was associated with obesity and lesion size >2 cm. We observed that second-line treatment using either prolonged progestin treatment or metformin in combination with MA/MPA or ethinylestradiol resulted in satisfactory treatment outcomes in progestin-insensitive patients. Therapeutic outcomes of second-line treatments were poorer in EEC patients than in AEH patients. Our study also identified that progestin-insensitive patients exhibited a trend for lower RFS although this did not reach statistical significance.
Although progestin has been used for fertility-preserving treatment in AEH and EEC patients for 50 years, a definitive consensus has yet to be reached regarding the definition of ‘progestin-insensitive’ patients. We defined ‘progestin-insensitive’ based on the following rationale: 1) the appearance of disease progression at any time during progestin treatment should be undeniably considered as progestin-insensitive; 2) In our practice, patients were suggested to receive pathological evaluation of endometrium every 3 months during treatment. However, it is a little bit hard for patients to receive pathological evaluation on exactly 12th week, 24th week or 36th week of treatment. Many patients will delay their evaluation because of various reasons, such as vaginitis. Most patients finish their pathological evaluation needed within 7 months or 10 months of treatment. This is the main reason we decided to use seven months and ten months as cut off value; 3) Several studies reported that the median treatment duration to achieve CR is 6–7 months for both AEH and EEC patients [7,9,12]. And NCCN guideline recommends the patients not achieving CR after 6-12 months of treatment to receive hysterectomy [8]. We thus decided that patient remained SD after 7 months of treatment as progestin insensitive; 4) According to our experience [9], and other reports [7,13], more than 70%–80% of AEH or EEC patients achieved CR after 9 to 10 months of treatment. We considered it reasonable to regard the patients not achieving CR after 10 months of treatment as ‘progestin insensitive.’
The optimal second-line treatment regimen and its effects on progestin-insensitive AEH and EEC patients remain unclear. Various regimens have been proposed as alternative options for treating AEH and EEC, like prolonged treatment duration, Diane-35, metformin, LNG-IUS, GnRH-a, aromatase inhibitors (such as letrozole), etc. [5,14,15,16]. Several studies have reported improved therapeutic effects through prolonging treatment duration [17]. A latest study demonstrated that the CR rate after continuing medical treatment (oral MPA 500 mg daily or MA 650 mg daily with or without LNG-IUS) was 72.5% (37/51) in patients who had not responded to progestin therapy at 9 months [18]. This is similar with our findings that more than 70% of progestin insensitive patients continuing original treatment achieved CR after another 28 weeks of treatment. A KGOG 2002 study reported that a higher dose of MPA (≥500 mg/day) did not result in superior therapeutic effects [19]. Thus, we did not use a higher dose of progestin to treat progestin-insensitive patients. Li et al. [5] reported that the CR rate in five patients with comorbid EEC and polycystic ovarian syndrome (PCOS) who received ethinylestradiol cyproterone plus metformin was 100% (5/5). We also added metformin as part of fertility preserving regimen. Because clinical observation and lab research has shown possible cancer inhibiting effect of metformin which might help improve the fertility preserving outcome in EEC and AEH patients [14,20,21,22,23,24,25]. Our prospective clinical trial demonstrated that metformin plus MA was associated with an improved 16-week CR rate when compared to MA alone in AEH patients [14]. LNG-IUS provides doses of progestin to the local endometrium that are several times higher than that provided by oral progesterone and have less systemic effects [26]. LNG-IUS combined with MPA (500 mg) has been used as a treatment option for patients with grade 2 Stage IA endometrial cancer, with 12-month CR rates of 80% [15]. Taken together, the current study and published reports did not reveal differences in the efficacy of second-line treatments. Further study with larger sample size is needed. However, the present evidence suggested that different second-line treatments might be considered for progestin insensitive patients individually depending on the patient's situation. Nevertheless, our study demonstrated that progestin-insensitive status exhibited a trend of higher recurrence rate. We suggest that caution should be exercised when recommending continuing conservative treatment for progestin-insensitive patients.
Consistent with past findings, our study demonstrated that obesity was an independent risk factor for poorer fertility-preserving treatment results in EEC and AEH patients [11,27,28]. Park proposed that overweight was an important predictor of poor treatment response and high recurrence rate [29]. The reason of obesity causing poor fertility preserving outcome might be due to various mechanisms, such as promoting estrogen production and down-regulating SHBG level [30,31], inducing chronic inflammation and insulin resistance [32,33,34] which in turn enhance estrogen sensitivity of EC cells [35] and promote EC cell proliferation [36]. However, correlation with BMI and progestin insensitivity was not shown in EEC patients in our study. We suggest that this is caused by limited EEC case number in our study. We propose that obese EEC and AEH patients should be consulted carefully before initiation of fertility-preserving treatment to avoid poor treatment outcomes. Although study has showed that changes in body weight during progestin treatment had minimal effects on CR, relapse, pregnancy, and live birth rate [29], we still recommend weight reduction in obese patients for the sake of general health. And, prospective study is needed to investigate the role of weight reduction on fertility preserving treatment of AEH and EEC patients.
In our study, we evaluated the treatment response by hysteroscopy every three months during treatment. We concerned that blind D&C might miss endometrial lesion, and also harm normal endometrium which is precious for these women desiring for fertility. Hysteroscopic comprehensive evaluation and precise removal of suspected lesion would help reach our fertility preservation goal to the best extend. Our previous study has reported that comprehensive hysteroscopic evaluation and lesion resection plus progestin therapy seem to be an effective and safe fertility sparing therapy for patients with EAH or EEC [9].
Our study has several limitations. Firstly, we acknowledge that our definition of ‘progestin insensitive’ is arbitrary. However, since there has been no definition for ‘progestin insensitive’ in EEC or AEH patients receiving fertility preserving treatment, we tried to provide this preliminary definition for reference and welcome all comments and appropriate revision. Secondly, its retrospective nature and small sample size may limit the power of the results. We were only able to qualitatively describe the results of second-line treatment in EEC patients. The small sample size and unevenly distributed data limited our analysis of the treatment efficacy of different second-line regimens. Finally, there might be difference in the definition of progestin insensitivity between AEH and EEC patients. Our preliminary results showed that EEC patients and AEH patients showed the same trend of response to first line progestin treatment, and the differences of characteristics between progestin sensitive and insensitive patients in either AEH or EEC groups was similar. Therefore, we suggest that the definition of progestin insensitivity could be applied to both AEH and EEC patients. However, further study using lager sample size of EEC patients should be carried out to testify the application of our definition of progestin insensitivity.
In conclusion, our study provided a preliminary definition of ‘progestin-insensitive’ for AEH and EEC patients receiving fertility-preserving treatment. Obesity and larger lesion size were independent risk factors associated with progestin-insensitive status. In progestin-insensitive patients receiving second-line treatment, EEC patients had lower CR rate comparing with AEH patients. The current study did not reveal differences in the efficacy of second-line treatments. Further study with larger sample size is needed. However, the results of our study suggested that different second-line treatments might be considered for progestin insensitive patients individually depending on the patient's situation.
ACKNOWLEDGEMENTS
We are grateful for the data work of Hongwei Zhang, Jiongbo Liao, Qin Zhu and Xuezhen Luo.
Footnotes
Funding: This study was supported by the National Key Technology R&D Program of China (Grant No 2019YFC1005200 and 2019YFC1005203), National Natural Science Foundation of China (Grant No 81671417 and 81370688), Shanghai Medical Centre of Key Programs for Female Reproductive Diseases (Grant No. 2017ZZ010616), Shanghai Science and Technology Development medical guide project (Grant No 17411961000, 134119a4500, 19411960400), and Municipal Human Resources Development Program for Outstanding Leaders in Medical Disciplines in Shanghai (Grant No. 2017BR035).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.X., Y.B.
- Formal analysis: Z.S., X.Z., Y.B., G.J.
- Funding acquisition: C.X.
- Investigation: Z.S., X.Z., G.J., S.W., S.Y.
- Methodology: Z.S., X.Z., C.X.
- Resources: Z.S., X.Z., Y.B., S.W., S.Y.
- Software: X.Z., G.J.
- Supervision: C.X., Y.B.
- Validation: Z.S., X.Z.
- Writing - original draft: Z.S., X.Z.
- Writing - review & editing: C.X., B.Y.
SUPPLEMENTARY MATERIALS
General characteristics of the study population classified by pathological diagnosis
Results of second-line treatments for progestin-insensitive patients with EEC (n=16)
Results of second-line treatments for progestin-insensitive patients with AEH (n=41)
Reasons for second-line treatment
The effect of initial pathological diagnosis, BMI and lesion size on treatment response.
Cumulative CR rate of progestin-insensitive and sensitive patients. Progestin-insensitive patients had poor 1-year and 2-year cumulative CR rate and needed longer treatment duration to achieve CR compared to progestin-sensitive patients.
The 28-week CR rate in progestin-insensitive AEH patients with different second-line options.
References
- 1.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 2.Gressel GM, Parkash V, Pal L. Management options and fertility-preserving therapy for premenopausal endometrial hyperplasia and early-stage endometrial cancer. Int J Gynaecol Obstet. 2015;131:234–239. doi: 10.1016/j.ijgo.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Kim MK, Seong SJ, Kim JW, Jeon S, Choi HS, Lee IH, et al. Management of endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: a Korean Gynecologic-Oncology Group Study. Int J Gynecol Cancer. 2016;26:711–715. doi: 10.1097/IGC.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 4.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Guo YR, Lin JF, Feng Y, Billig H, Shao R. Combination of Diane-35 and metformin to treat early endometrial carcinoma in PCOS women with insulin resistance. J Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol. 2008;112:465–467. doi: 10.1097/AOG.0b013e3181719b92. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuhashi A, Habu Y, Kobayashi T, Kawarai Y, Ishikawa H, Usui H, et al. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J Gynecol Oncol. 2019;30:e90. doi: 10.3802/jgo.2019.30.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning C, et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. 2019;153:55–62. doi: 10.1016/j.ygyno.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Zaino R, Carinelli SG, Ellenson LH, Eng C, Katabuchi H, Konishi I, et al. Tumours of the uterine corpus: epithelial tumours and precursors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: IARC; 2014. [Google Scholar]
- 11.Yang B, Xie L, Zhang H, Zhu Q, Du Y, Luo X, et al. Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J Gynecol Oncol. 2018;29:e35. doi: 10.3802/jgo.2018.29.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol. 2016;27:262–266. doi: 10.1093/annonc/mdv539. [DOI] [PubMed] [Google Scholar]
- 14.Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG. 2020;127:848–857. doi: 10.1111/1471-0528.16108. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW, Yun BS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. 2017;27:738–742. doi: 10.1097/IGC.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. 2017;27:1178–1182. doi: 10.1097/IGC.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhou R, Wang H, Liu H, Wang J. Impact of treatment duration in fertility-preserving management of endometrial cancer or atypical endometrial hyperplasia. Int J Gynecol Cancer. 2019;29:699–704. doi: 10.1136/ijgc-2018-000081. [DOI] [PubMed] [Google Scholar]
- 18.Cho A, Lee SW, Park JY, Kim DY, Suh DS, Kim JH, et al. Continued medical treatment for persistent early endometrial cancer in young women. Gynecol Oncol. 2021;160:413–417. doi: 10.1016/j.ygyno.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur J Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Lee TY, Martinez-Outschoorn UE, Schilder RJ, Kim CH, Richard SD, Rosenblum NG, et al. Metformin as a therapeutic target in endometrial cancers. Front Oncol. 2018;8:341. doi: 10.3389/fonc.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–120. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Dong L, Sui L, Yang Y, Liu X, Yu Y, et al. Metformin reverses progestin resistance in endometrial cancer cells by downregulating GloI expression. Int J Gynecol Cancer. 2011;21:213–221. doi: 10.1097/IGC.0b013e318207dac7. [DOI] [PubMed] [Google Scholar]
- 23.Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, Guerra EN, et al. Effects of metformin on endometrial cancer: Systematic review and meta-analysis. Gynecol Oncol. 2017;147:167–180. doi: 10.1016/j.ygyno.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 24.Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31–43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 25.Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–480. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Trimble CL, Method M, Leitao M, Lu K, Ioffe O, Hampton M, et al. Management of endometrial precancers. Obstet Gynecol. 2012;120:1160–1175. doi: 10.1097/aog.0b013e31826bb121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKintosh ML, Derbyshire AE, McVey RJ, Bolton J, Nickkho-Amiry M, Higgins CL, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer. 2019;144:641–650. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol. 2017;146:39–43. doi: 10.1016/j.ygyno.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 31.Simó R, Saez-Lopez C, Lecube A, Hernandez C, Fort JM, Selva DM. Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology. 2014;155:2820–2830. doi: 10.1210/en.2014-1072. [DOI] [PubMed] [Google Scholar]
- 32.Perrotta F, Nigro E, Mollica M, Costigliola A, D'Agnano V, Daniele A, et al. Pulmonary hypertension and obesity: focus on adiponectin. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Wang N, Ma Y, Wen D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390. doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Xie BY, Lv QY, Ning CC, Yang BY, Shan WW, Cheng YL, et al. TET1-GPER-PI3K/AKT pathway is involved in insulin-driven endometrial cancer cell proliferation. Biochem Biophys Res Commun. 2017;482:857–862. doi: 10.1016/j.bbrc.2016.11.124. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Wang J. The role of metabolic syndrome in endometrial cancer: a review. Front Oncol. 2019;9:744. doi: 10.3389/fonc.2019.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General characteristics of the study population classified by pathological diagnosis
Results of second-line treatments for progestin-insensitive patients with EEC (n=16)
Results of second-line treatments for progestin-insensitive patients with AEH (n=41)
Reasons for second-line treatment
The effect of initial pathological diagnosis, BMI and lesion size on treatment response.
Cumulative CR rate of progestin-insensitive and sensitive patients. Progestin-insensitive patients had poor 1-year and 2-year cumulative CR rate and needed longer treatment duration to achieve CR compared to progestin-sensitive patients.
The 28-week CR rate in progestin-insensitive AEH patients with different second-line options.