Abstract
Objective
To evaluate the concordance between preoperative European Society for Medical Oncology (ESMO)-European Society of Gynaecological Oncology (ESGO)-European SocieTy for Radiotherapy and Oncology (ESTRO) risk classification in early-stage endometrial cancer (EC) assessed by biopsy and magnetic resonance imaging (MRI) with this classification based on histology of surgical specimen.
Methods
This bicentric retrospective study included women diagnosed with early-stage EC (≤stage II) who had a complete preoperative assessment and underwent a surgical management from January 2011 to December 2018. Patients were preoperatively classified into 3 degrees of risk of lymph node (LN) involvement based on biopsy and MRI. Based on final histological report, patients were re-classified using the preoperative classification. Concordance between the preoperative assessment and definitive histology was calculated with weighted Cohen's kappa coefficient.
Results
A total of 333 women were included and kappa coefficient of preoperative risk classification was 0.49. The risk was underestimated and overestimated in 37% and 10% of cases, respectively. Twenty-nine percent of patients had an incomplete LN staging according to the degree of risk of re-classification. The observed discordance in the risk classification was attributed to MRI in 75% of cases, to biopsy in 18% and in 7% to both (p<0.001). Kappa coefficient for concordance was 0.25 for MRI and 0.73 for biopsy.
Conclusion
Concordance between preoperative ESMO-ESGO-ESTRO risk classification and final histology is weak. Given that the risk was underestimated in the majority of patients wrongly classified, sentinel LN procedure instead of no LN dissection could be an option offered to preoperative low-risk patients to decrease the indication of second surgery for re-staging and/or to avoid toxicity of adjuvant radiotherapy.
Keywords: Endometrial Cancer, Biopsy, Cancer Staging, Magnetic Resonance Imaging, Sentinel Lymph Node, Risk Assessment
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignancy in high-income countries and the majority of patients are diagnosed at early stage with five-year survival rates over 95% [1]. Lymph node (LN) involvement is one of the main prognostic factors and guides adjuvant treatment [2]. According to European Society for Medical Oncology (ESMO), European Society of Gynaecological Oncology (ESGO) and European SocieTy for Radiotherapy and Oncology (ESTRO) clinical guidelines [2], preoperative criteria are used to classify patients with apparently early-stage EC into three degrees of risk of LN involvement [3]. This classification combines the histological analysis of an endometrial biopsy with uterine disease stage according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification [4] assessed by magnetic resonance imaging (MRI). According to the degree of risk of lymphatic involvement, the surgical strategy can vary from no LN dissection to sentinel LN (SLN) biopsy or systematic pelvic and paraaortic lymphadenectomy [2].
Data reporting the concordance of the preoperative ESMO-ESGO-ESTRO risk classification with the histological analysis of the surgical specimen is limited [5,6,7,8]. It is essential to correctly assess before surgery the degree of risk of LN involvement in order to avoid re-interventions which increase surgical morbidity [9], particularly in those women with multiple comorbidities [10,11].
The aim of our study was to evaluate the concordance of the preoperative ESMO-ESGO-ESTRO risk classification before and after surgery. The secondary objectives were 1) to report separately the concordance of endometrial biopsy and the concordance of MRI with final histological exam, 2) to identify the cause of misclassification and its clinical implications, and 3) to assess the accuracy of the preoperative assessment to identify patients at higher risk of LN involvement.
MATERIALS AND METHODS
1. Patients and study design
A computer-generated search within the institutional patients' database was carried out to retrospectively identify all hysterectomized women with a histologically confirmed EC, either on preoperative biopsy or on surgical specimen. All patients were treated at the Gynecologic Oncology Department of Liege University Hospital in Belgium and at the Oncopole Cancer University Institute of Toulouse in France, between January 1st, 2011 and December 31st, 2018. Institutional Review Board approval was obtained from both centers.
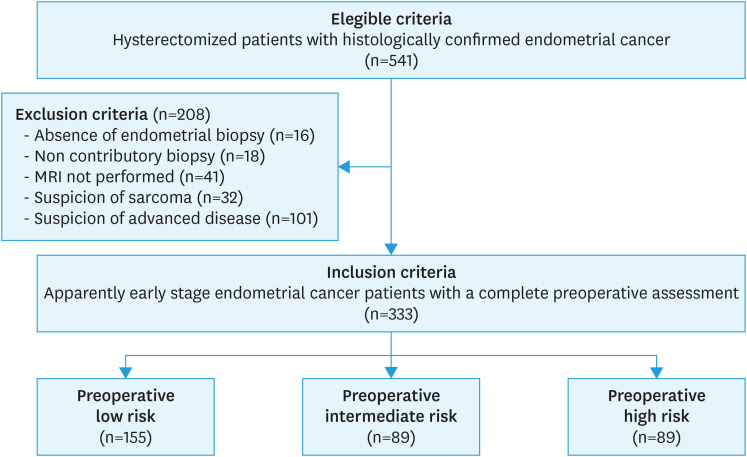
All patients met the following inclusion criteria: 1) available MRI and preoperative endometrial biopsy, 2) apparently early-stage EC (FIGO stage I or II), and 3) surgical management with at least hysterectomy with or without bilateral adnexectomy or LN assessment. Patients with any of the following criteria were excluded: 1) preoperative endometrial biopsy not performed or non-contributory due to insufficient material, 2) absence or poor-quality of MRI, 3) suspicion of uterine sarcoma, or 4) suspicion of LN involvement or advanced disease at MRI or computed tomography (>II stage FIGO).
2. Preoperative assessment and risk classification
The endometrial biopsy was obtained either by hysteroscopy plus curettage or by Cornier pipelle biopsy. Histological subtypes were classified according to World Health Organization (WHO) 2014 classification [4]. A histopathological review of the endometrial biopsies performed at external hospitals was systematically done by specialized gynecological pathologists in our centers when clear cell carcinoma or carcinosarcoma subtypes were suspected.
A preoperative MRI was performed according to protocols of European Society of Urogenital Imaging to assess myometrial and cervical invasion [12], and to classify patients in stage IA, IB or II according to FIGO 2009 classification before surgery [4]. If imaging was externally done, it was reviewed by the specialized radiologists from our institutions during preoperative multidisciplinary tumor board. Patients with MRI not complying with current protocols were excluded.
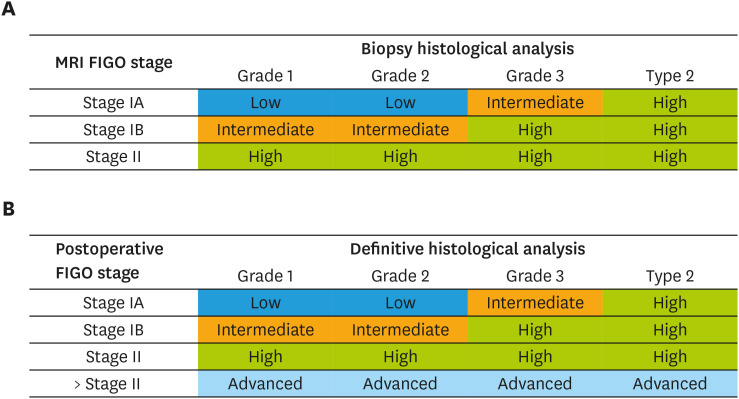
According to the ESMO-ESGO-ESTRO Consensus Conference on EC [2], combined assessment of histological examination of endometrial biopsy -histological subtype and grade- and MRI -preoperative FIGO staging- allowed to classify patients into three categories of risk of LN involvement: low (stage IA grade 1–2 endometrioid adenocarcinoma, theoretical risk of positive LN of 1%–2%), intermediate (stage IB grade 1–2 endometrioid or stage IA grade 3 endometrioid adenocarcinoma, theoretical risk of positive LN of 10%),) and high (stage IB grade 3 endometrioid, all non-endometrioid subtypes or stage II, theoretical risk of positive LN of 30%) (Fig. 1A) [2,3].
Fig. 1. ESMO-ESGO-ESTRO classification of risk of lymph node involvement. (A) Classification based on MRI and preoperative endometrial biopsy. (B) Re-classification based on definitive histological analysis of surgical specimen.*.
ESGO, European Society of Gynaecological Oncology; ESMO, European Society for Medical Oncology; ESTRO, European SocieTy for Radiotherapy and Oncology; FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging.
*The authors modified ESMO-ESGO-ESTRO classification and added a fourth category (advanced) to the original classification.
3. Surgical management
After discussion at multidisciplinary tumor board, surgical strategy to assess LN status was adapted according to the ESMO-ESGO-ESTRO preoperative risk of involvement. Total hysterectomy was systematically performed in all patients. Women at low-risk for LN involvement underwent either no LN dissection or optional SLN biopsy. In selected patients, at surgeon's discretion, pelvic LN dissection was performed in case of SLN detection failure. Women at intermediate risk underwent either SLN procedure or pelvic lymphadenectomy in case of SLN detection failure. In case of significant medical comorbidities or technical difficulties during surgery, no LN dissection was performed in intermediate risk patients. Women at high-risk underwent pelvic and paraaortic LN dissection, but, in the presence of comorbidities or surgical difficulties, no LN dissection or exclusive pelvic lymphadenectomy was performed.
4. Histological evaluation and re-classification of risk
Definitive histological evaluation of the surgical specimen was performed in all patients. Histological subtypes were determined according to WHO 2014 classification [4] and final postoperative stage was defined according to FIGO 2009 classification.
After the initial surgical procedure, all cases were re-discussed at the multidisciplinary tumor board. The need of a nodal re-staging to further assess LN status (pelvic, paraaortic or pelvic plus paraaortic LN dissection) was evaluated. The decision to perform re-staging surgery depended on the co-morbidities, surgical difficulties and the impact of LN status on adjuvant treatment. The indication of adjuvant treatment according to the definitive histological analysis of surgical specimens was also discussed.
In our study, based on the definitive histological findings, patients were postoperatively re-classified according to the ESMO-ESGO-ESTRO classification into the three risk categories defined preoperatively: low, intermediate and high [3]. Patients in whom the histological findings upstaged the disease above FIGO stage II were classified as ‘advanced’ (Fig. 1B).
5. Study data
Patient demographic data, histological subtype and grade of endometrial biopsy, MRI findings, histological analysis of surgical specimen (histological subtype, grade, myometrial/cervical invasion, lymphovascular space invasion, LN involvement, and extrauterine disease spread), as well as the indication and completion of second surgery for re-staging were retrieved after a careful and thorough examination of medical records.
6. Statistical analysis
Continuous variables were summarized using medians and ranges, showing both minimum and maximum values, while categorical variables were shown with their absolute and relative frequencies, expressed as percentages. Proportions were compared using Fisher's exact test. The concordance between the preoperative ESMO-ESGO-ESTRO classification—and its individual components—and the re-classification carried out after surgery was assessed using weighted Cohen's kappa coefficient and interpreted following McHugh's criteria [13]. The evaluation of the concordance was performed for the classification of risk, for the histological evaluation and for the MRI. Finally, diagnostic accuracy of the preoperative classification and its components was assessed by calculating the sensitivities, specificities and predictive values, of which the 95% confidence intervals (CI) were obtained with Wilson's method. The analysis was performed using Stata 13.0 (StataCorp, College Station, TX, USA) and statistical significance threshold was established at 0.05.
RESULTS
1. Patients' characteristics and preoperative classification
During the study period, 333 women were included (Fig. 2). Median age was 69.4 years (range, 39.3–93.4 years) and median body mass index was 28.0 kg/m2 (range, 14.6–55.9 kg/m2). Endometrioid adenocarcinoma was preoperatively identified in 265 patients (79.6%), including 231 patients with a grade 1–2 and 34 with a grade 3 disease, and 68 patients (20.4%) had a non-endometrioid subtype. Patients were referred with a biopsy performed in an external center in 138 cases (41.4%). MRI classified 216 patients (64.9%) as stage IA, 104 (31.2%) as stage IB and 13 (3.9%) as stage II (Table 1). According to these assessments, the preoperative risk of LN involvement was classified as low in 155 patients (46.6%), intermediate in 89 (26.7%) and high-risk in 89 women (26.7%).
Fig. 2. Chart representing the flow of eligible patients within the study.
MRI, magnetic resonance imaging.
Table 1. Histological and magnetic resonance imaging findings (n=333).
| Preoperative assessment | No. (%) | Definitive histological analysis after surgery | ||
|---|---|---|---|---|
| Endometrial biopsy | ||||
| Type 1 (Endometrioid) | 265 (79.6) | 253 (76.0) | ||
| Grade 1 | 150 (45.0) | 113 (33.9) | ||
| Grade 2 | 81 (24.3) | 99 (29.7) | ||
| Grade 3 | 34 (10.2) | 41 (12.3) | ||
| Type 2 (Non endometrioid) | 68 (20.4) | 80 (24.0) | ||
| Serous | 21 (6.3) | 20 (6.0) | ||
| Clear cell | 7 (2.1) | 6 (1.8) | ||
| Carcinosarcoma | 25 (7.5) | 30 (9.0) | ||
| Mixed | 15 (4.5) | 24 (7.2) | ||
| FIGO stage by MRI finding | ||||
| IA | 216 (64.9) | 162 (48.6) | ||
| IB | 104 (31.2) | 105 (31.5) | ||
| II | 13 (3.9) | 21 (6.3) | ||
| IIIA | 0 (0.0) | 14 (4.2) | ||
| IIIB | 0 (0.0) | 1 (0.3) | ||
| IIIC1 | 0 (0.0) | 15 (4.5) | ||
| IIIC2 | 0 (0.0) | 9 (2.7) | ||
| IVA | 0 (0.0) | 0 (0.0) | ||
| IVB | 0 (0.0) | 6 (1.8) | ||
Values are presented as number (%).
MRI, magnetic resonance imaging.
2. Surgical data
All the included patients underwent a total hysterectomy, and 204 patients (61.3%) had LN assessment during the first surgery. Among the 155 women in the preoperative low-risk group, 98 patients (63.2%) did not have any LN assessment, 46 patients (29.7%) underwent from SLN detection and 11 patients (7.1%) had a pelvic LN dissection. Out of the 89 women with preoperative intermediate risk, 12 patients (13.5%) did not have any LN assessment because of comorbidities and/or surgical difficulties, 28 patients (31.5%) had SLN detection and 49 patients (55.0%) had a pelvic LN dissection. Among the 89 women in the preoperative high-risk group, 19 patients (21.3%) did not have any LN assessment due to comorbidities and/or surgical difficulties, 12 patients (13.5%) had a pelvic LN dissection, and 58 patients (65.2%) had a pelvic and paraaortic LN dissection.
Based on final histological type and grade, FIGO stage and presence of lymph-vascular space invasion (LVSI; 154 patients), a second surgery for re-staging would have been theoretically indicated in 95 women (28.5%). It was finally performed in 31 of the 95 patients (32.6%) as a result of the decision made by the tumor board. Among the re-staging procedures, no pelvic LN involvement was found, and 6 patients presented paraaortic metastases, including 4 patients with pelvic involvement after first surgery.
3. Histological evaluation and postoperative re-classification
In the final anatomopathological analysis, 253 women (76.0%) had an endometrioid subtype and 80 (24.0%) had a non-endometrioid subtype. Including the results of second surgery for re-staging, the re-classification stratified 115 patients in low-risk (34.5%), 88 patients with intermediate risk (26.4%), 85 patients with high-risk (25.5%) and 45 patients (13.5%) with advanced disease (15 locally advanced disease [stage IIIA–IIIB], 24 LN metastases [IIIC] and 6 carcinomatosis [IVB] among whom 3 had LN metastases) (Table 1).
4. Concordance assessment of preoperative ESMO-ESGO-ESTRO classification
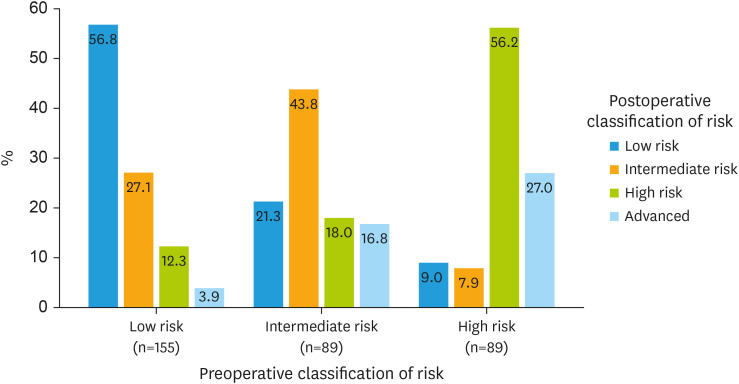
Cohen's kappa coefficient for the concordance between preoperative classification assessed by endometrial biopsy and MRI with the re-classification established after histological analysis of the surgical specimen was 0.49 (95% CI=0.41–0.57), with an overall agreement of 53.2%. The risk was underestimated and overestimated respectively in 36.6% (122/333) and 10.2% (34/333) of the cases. Among the 156 misclassified women, the discordance was attributed to MRI in 117 patients (75.0%), to the biopsy in 28 patients (17.9%), and in 11 patients (7.1%) both MRI and biopsy were responsible of the misclassification. MRI was accountable for the inaccuracy in a significantly higher number of patients compared to the biopsy (p<0.001). In the preoperative low-risk group, 67/155 women (43.2%) had an underestimated risk. Among the preoperative intermediate risk group, 19/89 women (21.3%) had an overestimated risk, while 31/89 women (34.8%) were underestimated. Finally, in the preoperative high-risk group, 15/89 women (16.9%) had an overestimated risk and 24/89 women (27.0%) had an unexpected advanced EC (Table 2). These results are graphically represented in Fig. 3.
Table 2. Concordance of preoperative assessment with definitive histological analysis of surgical specimen.
| Preoperative assessment | Final histological analysis | Total | |||
|---|---|---|---|---|---|
| Risk of LN involvement (Overall agreement: 53.2%, weighted kappa: 0.49 [95% CI 0.41–0.57]) | |||||
| Low risk | Intermediate risk | High risk | Advanced disease | ||
| Low risk | 88 (56.8) | 42 (27.1) | 19 (12.3) | 6 (3.9) | 155 |
| Interm risk | 19 (21.3) | 39 (43.8) | 16 (18.0) | 15 (16.8) | 89 |
| High risk | 8 (9.0) | 7 (7.9) | 50 (56.2) | 24 (27.0) | 89 |
| Total | 115 | 88 | 85 | 45 | 333 |
| Endometrial biopsy (Overall agreement: 69.4%, weighted kappa: 0.73 [95% CI 0.65–0.80]) | |||||
| Type 1 grade 1 | Type 1 grade 2 | Type 1 grade 3 | Type 2 | ||
| Type 1 grade 1 | 95 (63.3) | 40 (26.7) | 4 (2.7) | 11 (7.3) | 150 |
| Type 1 grade 2 | 15 (18.5) | 53 (65.4) | 8 (9.9) | 5 (6.2) | 81 |
| Type 1 grade 3 | 0 | 3 (8.8) | 25 (73.5) | 6 (17.7) | 34 |
| Type 2 | 3 (4.4) | 3 (4.4) | 4 (5.9) | 58 (85.3) | 68 |
| Total | 113 | 99 | 41 | 80 | 333 |
| MRI finding (Overall agreement: 57.7%, weighted kappa: 0.26 [95% CI 0.18–0.34]) | |||||
| Stage IA | Stage IB | Stage II | >Stage II | ||
| Stage IA | 136 (63.0) | 52 (24.1) | 9 (4.2) | 19 (8.8) | 216 |
| Stage IB | 24 (23.1) | 51 (49.0) | 7 (6.7) | 22 (21.2) | 104 |
| Stage II | 2 (15.4) | 2 (15.4) | 5 (38.5) | 4 (30.8) | 13 |
| Total | 162 | 105 | 21 | 45 | 333 |
LN, Lymph node; CI, 95% confidence interval; MRI, magnetic resonance imaging.
Fig. 3. Concordance between preoperative risk classification assessed by endometrial biopsy and magnetic resonance imaging with the final histological analysis of the surgical specimen.
Kappa coefficient for the concordance between preoperative biopsy and final histological analysis was 0.73 (95% CI=0.65–0.80), with an overall agreement of 69.4% (Table 2). The concordance between MRI findings and final FIGO postoperative stage had a kappa coefficient of 0.25 (95% CI=0.18–0.34], with an overall agreement of 57.7% (Table 2).
5. Accuracy analysis
The preoperative classification showed a sensitivity and a specificity to detect patients with intermediate or high-risk of LN involvement or advanced disease of 69.3% and 76.5%, respectively. Sensitivity and specificity of endometrial biopsy to identify high-risk disease—endometrioid grade 3 and type 2 tumors—were 76.9 and 95.8%, respectively. To detect exclusively type 2 EC, sensitivity and specificity were 72.5% and 96.0%. Among patients with type 1 EC, sensitivity and specificity to detect grade 3 endometrioid subtype was 67.6% and 98.5%, respectively. This sensitivity increased from 58.3% when the biopsy was performed externally to 72.0% when it was performed internally. Sensitivity and specificity of MRI to diagnose ≥IB stage disease were 53.2% and 84.0%, respectively (Table 3).
Table 3. Accuracy analyses of preoperative classification, endometrial biopsy and MRI.
| Characteristics | Sensivity (%) | Specificity (%) | PPV (%) | NPV (%) | Efficiency (%) | Total | ||
|---|---|---|---|---|---|---|---|---|
| Preoperative classifcation | ||||||||
| ≥High-risk | 56.9 (48.3–65.1) | 92.6 (88.2–95.5) | 83.1 (74.0–89.5) | 77.0 (71.4–81.9) | 78.7 (74.0–82.7) | 333 | ||
| ≥Intermediate risk | 69.3 (62.9–75.0) | 76.5 (68.0–83.3) | 84.8 (78.8–89.4) | 56.8 (48.9–64.3) | 71.8 (66.7–76.3) | 333 | ||
| Biopsy | ||||||||
| G3* | 67.6 (51.5–80.4) | 98.5 (95.8–99.5) | 89.3 (72.8–96.3) | 94.4 (90.5–96.8) | 93.8 (90.1–96.2) | 243 | ||
| Type 2 | 72.5 (61.9–81.1) | 96.0 (92.9–97.8) | 85.3 (75.0–91.8) | 91.7 (87.8–94.5) | 90.4 (86.7–93.1) | 333 | ||
| G3 and type 2 | 76.9 (68.6–83.5) | 95.8 (92.1–97.8) | 91.2 (84.1–95.3) | 87.9 (83.0–91.5) | 88.9 (85.1–91.8) | 333 | ||
| Internal biopsy | ||||||||
| G3* | 72.0 (52.4–85.7) | 98.2 (93.7–99.5) | 90.0 (69.9–97.2) | 94.0 (88.2–97.1) | 93.4 (88.0–96.5) | 137 | ||
| Type 2 | 76.0 (62.6–85.7) | 94.5 (89.5–97.2) | 82.6 (69.3–90.3) | 91.9 (86.5–95.3) | 89.7 (84.7–93.3) | 195 | ||
| G3 and type 2 | 77.2 (66.8–85.1) | 94.8 (89.2–97.6) | 91.0 (81.8–95.8) | 85.9 (78.9–90.9) | 87.7 (82.3–91.6) | 195 | ||
| External biopsy | ||||||||
| G3* | 58.3 (32.0–80.7) | 98.9 (94.2–99.8) | 87.5 (52.9–97.8) | 94.9 (88.6–97.8) | 94.3 (88.2–97.4) | 106 | ||
| Type 2 | 66.7 (48.8–80.8) | 98.1 (93.5–99.5) | 90.9 (72.2–97.5) | 91.4 (84.9–95.3) | 91.3 (85.4–95.0) | 138 | ||
| G3 and type 2 | 76.2 (61.5–86.5) | 96.9 (91.2–98.9) | 91.4 (77.6–97.0) | 90.3 (83.0–97.0) | 90.6 (84.5–94.4) | 138 | ||
| MRI | ||||||||
| ≥IB stage FIGO | 53.2 (45.7–60.5) | 84.0 (77.5–88.8) | 77.8 (69.4–84.4) | 63.0 (56.3–69.1) | 68.2 (63.0–72.9) | 333 | ||
Values are presented as 95% confidence interval.
FIGO, International Federation of Gynecology and Obstetrics; G3, endometrioid grade 3 adenocarcinoma; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value.
*Accuracy of grade 3 endometrioid subtype was evaluated in patients with an endometrioid subtype in preoperative biopsy and in final histological evaluation.
DISCUSSION
The combined assessment of MRI and histological biopsy classifies women with early-stage EC into three preoperative risk groups of LN involvement [3]. This risk classification allows to guide the surgical strategy of nodal staging [2]. LN status is a key variable to indicate adjuvant therapy [14,15,16]. Due to the frequent comorbidities of EC patients [10,11], it is essential to correctly assess preoperatively the degree of risk of LN involvement to avoid unnecessary staging procedures or additional morbidity of second surgeries for re-staging [9,17]. However, to our knowledge, there is no data available in the literature evaluating the concordance between the preoperative risk classification with that determined on histological analysis of the surgical specimen, using a statistical tool designed for this issue (Cohen's kappa coefficient) [5,6,7,8].
In our study, Cohen's kappa coefficient for the concordance of the preoperative classification was 0.49 with an overall agreement of 53%. According to McHugh's criteria, these results are considered as a ‘weak’ concordance [13]. The degree of risk was underestimated in 37% of cases and overestimated for 10% of the patients. These results are in line with previous reports such as the study performed by Body et al. [5] who described, in a smaller cohort, an underestimation of the preoperative risk in more than 30% of cases and an overestimation in 8% of patients. Similar rates are reported by Groff et al. [6] in a cohort of 169 women, but only 53% of women had a complete preoperative assessment with biopsy and MRI.
Regarding the accuracy analysis of preoperative classification, sensitivity and specificity to identify disease with intermediate risk or higher in our study were 69% and 77%, respectively, which is concordant with the two previous studies assessing this issue [5,6]. Groff et al. [6] found a sensitivity and specificity of the classification to detect intermediate or high-risk disease of 60% and 77%, respectively. Body et al. [5] reported slightly higher values with a sensitivity and a specificity of 70% and 82%, respectively, to detect intermediate or high-risk patients. As well, in this last study, the sensitivity and specificity to detect specifically high-risk disease were of 48% and 94% [5], which is in line with our findings (57% and 93%).
Multiple studies have compared the correlation between the histological grade and type of the preoperative biopsy with the final histology in the surgical specimen [5,6,7,18,19]. Our series reported a ‘moderate’ concordance of the histological biopsy—grade and type—with a kappa coefficient of 0.73 and an overall agreement of 69% [13]. A similar value (kappa=0.69) was reported by Huang et al. [19] in a study of 360 women. In contrast, in a recent meta-analysis, Visser et al. [18] found a ‘weak’ concordance for the histological grade (kappa=0.45), with an overall agreement of 67%. However, the overall agreement for type 1 and type 2 EC were 95% and 81%, respectively [18]. Regarding high grade disease, the sensitivity and specificity to detect type 2 EC in our study were 73% and 96%. Our findings are slightly better than Body et al. [5] study who reported a sensitivity and specificity to detect non-endometrioid EC of 64% and 95%. Furthermore, the sensitivity to detect grade 3 disease was higher in our series (68% vs. 30%) [5]. This sensitivity decreased when the biopsies were performed and analyzed in an external center for referred patients, which highlights the importance of a systematic review of external biopsies by specialized pathologists.
In the present series, the concordance of MRI for assessing myometrial invasion was ‘minimal’ with a kappa of 0.26 and an overall agreement of 58%. Zamani et al. [20] described a ‘moderate’ concordance with a kappa of 0.72 in a small cohort of 54 women. Regarding the accuracy of MRI to detect ≥IB stage disease in our series, the sensitivity was 53% and specificity was 84%. Data available in the literature regarding this issue are controversial. Previous series report a sensitivity ranging from 33% to 82% and a specificity varying from 35% to 100% [5,6,7,20,21].
LN status is crucial to tailor adjuvant treatment [2,14,15,16]. However, the role of systematic lymphadenectomy is still questioned [22,23] as no survival benefit has been demonstrated and it is associated to high morbidity [17,24,25]. According to the recent European consensus conference, LN dissection is not recommended for patients with low-risk EC, it can be considered in intermediate risk and it should be recommended for high-risk disease [2]. For this reason, women who were inadequately classified as preoperative low-risk and did not have LN assessment should theoretically undergo a second surgery to complete LN staging [2,14,16,26]. In our study, the degree of risk was underestimated in 37% of patients and re-staging would have been indicated in almost 30% of these patients, but it was only performed in one third of them. Our results are in keeping with a multicentric study which showed a suboptimal surgical staging in 35% of the patients [7,9]. The decision to perform or not re-staging surgeries has to consider the potential benefit of this re-intervention—as it can modify the adjuvant treatment—[2,14,15,16] and the increased surgical morbidity [9,17].
For patients with preoperative low-risk disease finally re-classified in intermediate risk group—42 women in our study—an adequate LN staging would have potentially reduced the rate of unnecessary adjuvant pelvic radiotherapy recommended in case of positive LVSI [2,26] and its associated toxicity [27]. In order to avoid second surgeries, an option would be to broaden the practice of SLN procedure in case of preoperative low-risk disease [28,29]. With such an approach, in case of postoperative re-classification to intermediate risk, a re-staging surgery for LN assessment could be avoided. Moreover, SLN procedure has already been validated in patients with low and intermediate risk EC in multiple studies [28,29].
The same issue should be discussed for preoperative high-risk disease. Due to the overestimation of risk in our series (10%), SLN could also be offered to patients with preoperative high-risk disease to decrease the morbidity of an extended LN dissection. A recent meta-analysis assessing the role of SLN strategy in patients with high-risk EC has reported promising results with high detection rate and a sensitivity and specificity of almost 90% and 96%, respectively [30].
Other preoperative stratification systems to assess the risk of EC have been proposed by several authors, but most of these models also include histological and MRI findings such as tumor size [31,32]. Another exciting perspective described by The Cancer Genome Atlas is the molecular classification. ProMisE trial proposes a molecular classification to determine four profile subtypes of EC [33]. Stello et al. [34] described the importance of these molecular factors that will probably be in the future of paramount importance to guide the therapeutic surgical strategy. This molecular classification may be used to establish an individualized management not considering LN status, hence, avoiding over- or under-treatment of EC patients [34].
To the best of our knowledge, we report the largest series evaluating the concordance between the preoperative ESMO-ESGO-ESTRO risk classification assessed by endometrial biopsy and MRI findings with this classification based on histology of the surgical specimen. The main strength of our study was a large and homogeneous cohort of patients who had a complete preoperative assessment. Moreover, it was a bicentric study including two expert centers. There was a systematic histological review of rare endometrial subtypes and the accuracy of the preoperative endometrial biopsy was assessed separately for internal and external biopsies. A limitation of our work is its retrospective nature with the inherent selection bias. Moreover, differences of concordance between the biopsies performed by hysteroscopy or Cornier Pipelle were not evaluated. Nevertheless, this issue has been previously assessed without significant differences between the 2 techniques [5,18,19]. Another flaw of our study is that most patients with preoperative low-risk did not have surgical LN assessment and were thereby considered as node negative, which may have introduced bias by increasing the concordance of the classification.
In conclusion, the preoperative combined assessment of histological biopsy and MRI has a weak concordance with final risk profile determined on the basis of the histological exam. Approximately one third of patients have an underestimated risk and an incomplete LN staging. MRI assessment is accountable for the misclassification of a significantly higher proportion of patients compared with endometrial biopsy. These results support the principle to apply SLN procedure for patients with preoperative low-risk disease with the aim to decrease the indication of either nodal surgical re-staging or adjuvant pelvic radiation and their associated morbidity.
Footnotes
Funding: Martina Aida Angeles acknowledges the grant support from “la Caixa” Foundation, Barcelona (Spain), ID 100010434. The fellowship code is LCF/BQ/EU18/11650038.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: D.M., A.A.M., M.A., F.G., K.F.
- Data curation: D.M., M.F.
- Formal analysis: M.F.
- Investigation: D.M.
- Methodology: D.M., A.A.M., M.F., K.A., M.G.C., D.K., M.E., T.S., G.E., D.M., G.F., M.A., F.G., K.F.
- Validation: D.M., A.A.M.
- Visualization: D.M., A.A.M.
- Writing - original draft: D.M., A.A.M., K.F.
- Writing - review & editing: D.M., A.A.M., K.A., M.G.C., D.K., M.E., T.S., G.E., D.M., G.F., M.A., F.G., K.F.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Querleu D, Darai E, Lecuru F, Rafii A, Chereau E, Collinet P, et al. Primary management of endometrial carcinoma. Joint recommendations of the French society of gynecologic oncology (SFOG) and of the French college of obstetricians and gynecologists (CNGOF) Gynecol Obstet Fertil Senol. 2017;45:715–725. doi: 10.1016/j.gofs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 5.Body N, Lavoué V, De Kerdaniel O, Foucher F, Henno S, Cauchois A, et al. Are preoperative histology and MRI useful for classification of endometrial cancer risk? BMC Cancer. 2016;16:498. doi: 10.1186/s12885-016-2554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groff B, Pouget O, Stoll F, Mathelin C, Baldauf JJ, Akladios CY. Pertinence of the preoperative exploration in the evaluation of the risk of lymph node metastasis in endometrial cancer. Gynecol Obstet Fertil. 2014;42:92–96. doi: 10.1016/j.gyobfe.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Raimond E, Canlorbe G, Bendifallah S, Hudry D, Selvi F, Ballester M, et al. Endometrial carcinoma. Application of the guidelines of 2010: multicentre trial. Bull Cancer. 2014;101:703–713. doi: 10.1684/bdc.2014.1955. [DOI] [PubMed] [Google Scholar]
- 8.Lavaud P, Fedida B, Canlorbe G, Bendifallah S, Darai E, Thomassin-Naggara I. Preoperative MR imaging for ESMO-ESGO-ESTRO classification of endometrial cancer. Diagn Interv Imaging. 2018;99:387–396. doi: 10.1016/j.diii.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Arsène E, Bleu G, Merlot B, Boulanger L, Vinatier D, Kerdraon O, et al. Implications of a two-step procedure in surgical management of patients with early-stage endometrioid endometrial cancer. J Gynecol Oncol. 2015;26:125–133. doi: 10.3802/jgo.2015.26.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45:28–36. doi: 10.1007/s12020-013-9973-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers. 2014;29:e21–9. doi: 10.5301/jbm.5000047. [DOI] [PubMed] [Google Scholar]
- 12.Kinkel K, Forstner R, Danza FM, Oleaga L, Cunha TM, Bergman A, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19:1565–1574. doi: 10.1007/s00330-009-1309-6. [DOI] [PubMed] [Google Scholar]
- 13.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 14.Creutzberg CL. GOG-99: ending the controversy regarding pelvic radiotherapy for endometrial carcinoma? Gynecol Oncol. 2004;92:740–743. doi: 10.1016/j.ygyno.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273–1285. doi: 10.1016/S1470-2045(19)30395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatebe K, Hasan Y, Son CH. Adjuvant vaginal brachytherapy and chemotherapy versus pelvic radiotherapy in early-stage endometrial cancer: outcomes by risk factors. Gynecol Oncol. 2019;155:429–435. doi: 10.1016/j.ygyno.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017;10:CD007585. doi: 10.1002/14651858.CD007585.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser NCM, Reijnen C, Massuger LFAG, Nagtegaal ID, Bulten J, Pijnenborg JMA. Accuracy of endometrial sampling in endometrial carcinoma: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:803–813. doi: 10.1097/AOG.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 19.Huang GS, Gebb JS, Einstein MH, Shahabi S, Novetsky AP, Goldberg GL. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am J Obstet Gynecol. 2007;196:243.e1–243.e5. doi: 10.1016/j.ajog.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Zamani F, Goodarzi S, Hallaji F, Zamiri A, Deilami T, Malek M, et al. Diagnostic value of pelvic MRI for assessment of the depth of myometrial invasion and cervical involvement in endometrial cancer: comparison of new versus old FIGO staging. Iran J Radiol. 2012;9:202–208. doi: 10.5812/iranjradiol.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol. 2012;67:2–12. doi: 10.1016/j.crad.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Suh DH, Kim MK, Chung HH, Park NH, Song YS. Systematic lymphadenectomy for survival in patients with endometrial cancer: a meta-analysis. Jpn J Clin Oncol. 2012;42:405–412. doi: 10.1093/jjco/hys019. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 25.Barton DPJ, Naik R, Herod J. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Int J Gynecol Cancer. 2009;19:1465. doi: 10.1111/IGC.0b013e3181b89f95. [DOI] [PubMed] [Google Scholar]
- 26.Sharma C, Deutsch I, Lewin SN, Burke WM, Qiao Y, Sun X, et al. Lymphadenectomy influences the utilization of adjuvant radiation treatment for endometrial cancer. Am J Obstet Gynecol. 2011;205:562.e1–562.e9. doi: 10.1016/j.ajog.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol. 2018;36:2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 29.Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 30.Lecointre L, Lodi M, Faller É, Boisramé T, Agnus V, Baldauf JJ, et al. Diagnostic accuracy and clinical impact of sentinel lymph node sampling in endometrial cancer at high risk of recurrence: a meta-analysis. J Clin Med. 2020;9:3874. doi: 10.3390/jcm9123874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendifallah S, Canlorbe G, Collinet P, Arsène E, Huguet F, Coutant C, et al. Just how accurate are the major risk stratification systems for early-stage endometrial cancer? Br J Cancer. 2015;112:793–801. doi: 10.1038/bjc.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuomi T, Pasanen A, Leminen A, Bützow R, Loukovaara M. Prediction of lymphatic dissemination in endometrioid endometrial cancer: comparison of three risk-stratification models in a single-institution cohort. Gynecol Oncol. 2017;144:510–514. doi: 10.1016/j.ygyno.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29:1180–1188. doi: 10.1093/annonc/mdy058. [DOI] [PubMed] [Google Scholar]
- 34.Stelloo E, Nout RA, Osse EM, Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22:4215–4224. doi: 10.1158/1078-0432.CCR-15-2878. [DOI] [PubMed] [Google Scholar]