Abstract
BACKGROUND
Implementation of new diagnostic criteria for gestational diabetes mellitus (GDM) are still a subject of debate, mostly due to concerns regarding the effects on the number of women diagnosed with GDM and the risk profile of the women newly diagnosed.
AIM
To estimate the impact of the World Health Organization (WHO) 2013 criteria compared with the WHO 1999 criteria on the incidence of gestational diabetes mellitus as well as to determine the diagnostic accuracy for detecting adverse pregnancy outcomes.
METHODS
We retrospectively analyzed a single center Dutch cohort of 3338 women undergoing a 75 g oral glucose tolerance test where the WHO 1999 criteria to diagnose GDM were clinically applied. Women were categorized into four groups: non-GDM by both criteria, GDM by WHO 1999 only (excluded from GDM), GDM by WHO 2013 only (newly diagnosed) and GDM by both criteria. We compared maternal characteristics, pregnancy outcomes and likelihood ratios for adverse pregnancy outcomes.
RESULTS
Retrospectively applying the WHO 2013 criteria increased the cohort incidence by 13.1%, from 19.3% to 32.4%. Discordant diagnoses occurred in 21.3%; 4.1% would no longer be labelled as GDM, and 17.2% were newly diagnosed. Compared to the non-GDM group, women newly diagnosed were older, had higher rates of obesity, higher diastolic blood pressure and higher rates of caesarean deliveries. Their infants were more often delivered preterm, large-for-gestational-age and were at higher risk of a 5 min Apgar score < 7. Women excluded from GDM were older and had similar pregnancy outcomes compared to the non-GDM group, except for higher rates of shoulder dystocia (4.3% vs 1.3%, P = 0.015). Positive likelihood ratios for adverse outcomes in all groups were generally low, ranging from 0.54 to 2.95.
CONCLUSION
Applying the WHO 2013 criteria would result in a substantial increase in GDM diagnoses. Newly diagnosed women are at increased risk for pregnancy adverse outcomes. This risk, however, seems to be lower than those identified by the WHO 1999 criteria. This could potentially influence the treatment effect that can be achieved in this group. Evidence on treatment effects in newly diagnosed women is urgently needed.
Keywords: Diagnostic criteria, Gestational diabetes, Glucose tolerance test, Incidence, Pregnancy outcome
Core Tip: The World Health Organization 2013 criteria would increase the number of women diagnosed with gestational diabetes mellitus to almost one third of the population tested. Our data confirm that the new criteria indeed identify women at risk, implying potential for treatment. However, we also show that implementation of the criteria would result in a great increase of women diagnosed with gestational diabetes mellitus, resulting in over half of the women to be subjected to unevaluated treatment, as evidenced by the treatment effect in this group is currently absent. This stresses the need for randomized trials to evaluate the new criteria prior to implementation.
INTRODUCTION
Gestational diabetes mellitus (GDM), defined as hyperglycemia first diagnosed in pregnancy that is not overt diabetes mellitus, is an increasing health problem worldwide and associated with substantial adverse maternal and fetal effects[1]. Adequate recognition, diagnosis and treatment of GDM improves health outcomes in mothers and their offspring[2-5]. Diagnostic criteria for GDM vary globally and are still much debated[6-9]. Based on the 2008 Hyperglycemia and Adverse Pregnancy Outcome study, new criteria were developed by the International Association of Diabetes in Pregnancy Study Groups, which were adopted by the World Health Organization (WHO) in 2013[1,10,11]. Uniform implementation of the revised criteria is currently held back due to ongoing concerns regarding their impact on prevalence and associated health care costs[12,13]. Previous studies have shown a substantial absolute increase of GDM prevalence ranging from 0.9% to as much as 25.9%, following conversion from the 1999 to the 2013 criteria, with limited data on their direct effect on pregnancy outcomes[12,14-28].
The impact of the widening of disease definitions, as is the case with the proposed changes in diagnostic criteria for GDM, has gained attention due to concerns regarding possible overdiagnosis, medicalization and overtreatment[12,13]. The WHO 2013 criteria propose a lower fasting, a higher post load glucose value and an additional one hour post load glucose value compared to the 1999 criteria. This leads to a newly diagnosed group of women based on their fasting glucose and a group of women no longer qualifying for the diagnosis based on their two-hour glucose value. Apart from an overall expected increase of the incidence of GDM as a result of the new criteria, the composition of the group of women labeled as GDM is therefore also likely to change. This is of interest in the assessment of incremental health benefits, potential harm and cost-effectiveness, which must be balanced for both individual patients and society prior to introduction of new criteria.
The aim of this study was to assess the potential impact of adopting the WHO 2013 criteria on the incidence of GDM. We sought to investigate the differences in patient characteristics of women with a discordant diagnosis of GDM between criteria, whether pregnancy outcomes in the newly diagnosed group differ from the nondiabetic population and to study the diagnostic value of the different criteria for adverse pregnancy outcomes.
MATERIALS AND METHODS
Setting and study population
We conducted a retrospective cohort study including all pregnant women at risk for GDM, booked for an oral glucose tolerance test (OGTT) and receiving obstetric care in the University Medical Center Utrecht from 1 August 2011–27 October 2016. This study was exempt from approval of the Medical Research Ethics Committee of the University Medical Center Utrecht (reference number 16-711/C), which granted a waiver after reviewing the protocol because the Dutch Medical Research Involving Human Subjects Act did not apply to this study. Therefore, no individual informed consent was required for this study. Data was anonymized and collectively analyzed to ensure there was no risk of disclosure of the identity of included subjects.
Women were identified using laboratory data, which were cross-referenced with obstetric patient files. Data was collected per pregnancy, meaning that women with OGTTs in multiple pregnancies during the study period were included as separate cases. Women undergoing an OGTT for another reason than to test for GDM were excluded.
Screening for GDM
Pregnant women with one or more predefined risk factors underwent screening between 24 and 28 wk of gestation by means of a 2-point 75 g OGTT[29]. Risk factors following Dutch national guidelines that prompted screening included a history of GDM, body mass index (BMI) > 30 kg/m2 at intake, previous delivery of a child with a birth weight > 95th centile or > 4500 g, a first-degree family member with diabetes, ethnic predisposition, history of unexplained stillbirth or polycystic ovarian syndrome[29]. Those with a history of GDM underwent screening at 16 wk of gestation, which was repeated at 24-28 wk if initially normal. Finally, women with clinical signs suggestive for GDM (e.g., polyhydramnios, suspected fetal macrosomia or polydipsia) could undergo OGTT testing at any gestational age.
Classification of OGTT results
Based on the OGTT results, we retrospectively classified the women into four groups: (1) no GDM according to both the WHO 1999 and WHO 2013 criteria: fasting glucose < 5.1 mmol/L and 2-h post load glucose < 7.8 mmol/L; (2) GDM according to the WHO 1999 criteria but not the WHO 2013 criteria: fasting glucose < 5.1 mmol/L and 2-h post load glucose ≥ 7.8 mmol/L but < 8.5 mmol/L; (3) GDM according to the WHO 2013 criteria but not the WHO 1999 criteria: fasting glucose ≥ 5.1 mmol/L but < 7.0 mmol/L and 2-h post load glucose < 7.8 mmol/L; and (4) GDM according to both criteria: either fasting glucose ≥ 7.0 mmol/L and/or 2-h post load glucose ≥ 8.5 mmol/L alone, or a combination of fasting glucose ≥ 5.1 mmol/L and 2-h post load glucose ≥ 7.8 mmol/L.
Groups 1 and 4 were groups where both the assessed criteria agree, and in groups 2 and 3 there is a discordance in diagnosis. For this study, group 1 was labelled non-GDM. Group 2 and 4 received treatment for GDM. Group 2 represents the group of women who would no longer be labelled as GDM with the new criteria, and group 3 consists of the newly added women that would switch from non-GDM to GDM. Some women had received more than one OGTT in a single pregnancy, for instance due to newly suspected macrosomia occurring after an initial negative routine screening. Results from all OGTTs were considered when classifying women into group 1 to 4 (Supplemental table 1). The 1-h post load glucose threshold of ≥ 10.0 mmol/L of the WHO 2013 criteria was not used in the classification, as these results were not available.
Routine care for GDM
Women started treatment if diagnosed with GDM according to the WHO 1999 criteria, comprising all women in groups 2 and 4. They received personalized dietary advice and were instructed to self-monitor fasting and postprandial glucose concentrations by finger stick at least twice a week. Target glucose values were fasting ≤ 5.3 mmol/L and 2-h postprandial ≤ 6.7 mmol/L. In case of insufficient glycemic regulation after 1 wk to 2 wk of dietary intervention, insulin treatment was initiated. Women with normal OGTT results according to the WHO 1999 criteria, e.g., groups 1 and 3, received care as usual.
Data collection
OGTT results, patient characteristics and pregnancy outcomes were collected from electronic patient files. Given that women without risk factors are not screened for GDM and that OGTT screening also took place in laboratories outside the University Medical Center Utrecht, we were not able to provide a population or regional incidence estimate of GDM but rather a cohort incidence.
Patient characteristics and outcomes
We calculated BMI using prepregnancy reported height and weight. Regarding ethnicity, Hindustan was used for women from the South Asian/Indian subcontinent or Surinamese descent. Mediterranean ethnicity comprised mostly women originating from Turkey and Morocco. Small-for-gestational-age (SGA) and large-for-gestational-age (LGA) were defined as neonatal birth weight < 10th and > 90th percentile for gestational age, respectively, using the Dutch reference curves[30]. Preterm birth was defined as a gestational age at birth < 37 wk, which was further divided in either spontaneous or indicated preterm birth. Individual diagnoses of gestational hypertension and preeclampsia were not available. As a proxy for hypertensive disorders during the pregnancy, the highest measured diastolic blood pressure was used for analysis.
Statistical analysis and reporting of data
The statistical methods of this study were reviewed by Christiana Naaktgeboren from the Department of Obstetrics and Gynaecology, Amsterdam University Medical Centers–Location AMC. GDM incidence in the cohort was calculated by applying both the WHO 1999 and 2013 criteria. We produced 2 × 2 contingency tables to display the number of women in groups 1 through 4, based on their OGTT results. Patient characteristics and pregnancy outcomes were reported and analyzed using groups 1 to 4 as determinants. Continuous variables were reported as mean ± SD or as median and interquartile range depending on distribution. Number and percentage were reported for categorical variables. Differences between groups were assessed using group 1 (non-GDM) as the reference group. We compared continuous variables using the independent t-test or Mann-Whitney U test and the χ-square test or Fisher’s exact test for categorical data. Furthermore, we assessed the diagnostic value of the OGTT by calculating positive and negative likelihood ratios for adverse obstetric and neonatal outcomes for groups 2 to 4, using group 1 as the reference group[31,32]. A P value < 0.05 was considered statistically significant. Analyses were performed using SPSS 25.0 for Windows (IBM SPSS, Chicago, IL, United States).
RESULTS
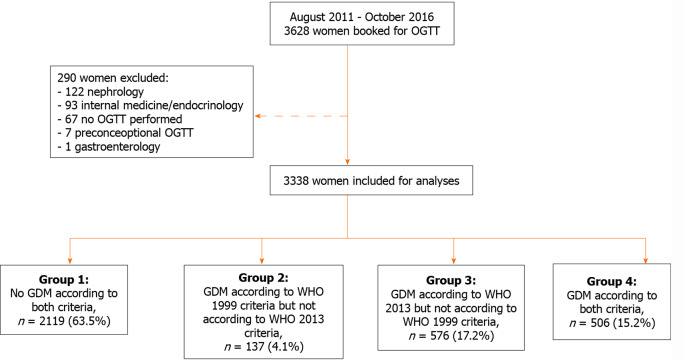
In the study period 3628 women were scheduled for an OGTT. Of these, 290 were excluded: 67 were planned for an OGTT but did not undergo the test and another 223 were excluded because they underwent an OGTT for other reasons than to test for GDM. In total we included 3338 women for analysis (Figure 1).
Figure 1.
Cohort selection and flowchart of inclusions. OGTT: Oral glucose tolerance test; GDM: Gestational diabetes mellitus; WHO: World Health Organization.
Effect of WHO 2013 criteria on GDM incidence
Of the 3338 included women, 643 (19.3%) were diagnosed with GDM using the WHO 1999 criteria. Retrospectively applying the WHO 2013 criteria resulted in 1082 women diagnosed with GDM, corresponding to a cohort incidence of 32.4% (Figure 1). This is equivalent to a relative increase of the incidence of 68%. A total of 2219 women (63.5%) had normal glucose tolerance according to both criteria (group 1), 506 women (15.2%) were diagnosed with GDM by both criteria (group 4), 137 women (4.1%) were diagnosed by WHO 1999 criteria only (group 2) and 576 (17.2%) by the WHO 2013 criteria only (group 3) (Figure 1).
Maternal characteristics
Maternal characteristics are presented in Table 1. Compared to group 1 (non-GDM) women in group 3 (newly added by the WHO 2013 criteria) were older (33.0 vs 32.3 years, P = 0.002) and had a higher median prepregnancy BMI (29.0 kg/m2 vs 24.3 kg/m2, P < 0.001). BMI distribution was significantly different (P < 0.001), with a higher frequency of BMIs of 30-35 and ≥ 35 kg/m2 in the newly added group compared to the non-GDM women (24.4% vs 10.4% and 19.0% vs 5.9%, respectively). There were no differences in ethnicity between the groups. In group 3 smoking during pregnancy was reported more often (16.8% vs 13.3%, P = 0.031), and women were less often primiparous (29.7% vs 37.9%, P < 0.001).
Table 1.
Patient characteristics per group based on classification of oral glucose tolerance test results
| n (%) | Group 1: non-GDM | Group 2: GDM by WHO 1999 only1 | P value2 | Group 3: GDM by WHO 2013 only | P value2 | Group 4: GDM by both criteria1 | P value2 | |
| n (%) | 3338 | 2119 (63.5) | 137 (4.1) | 576 (17.2) | 506 (15.2) | |||
| Age, yr | 3336 | 32.3 (28.5-35.7) | 34.1 (30.4-38.0) | < 0.001 | 33.0 (29.0-36.7) | 0.002 | 33.6 (30.3-37.0) | < 0.001 |
| Age group, n (%) | 3336 | |||||||
| < 30 yr | 698 (33.0) | 31 (22.6) | 0.002 | 174 (30.2) | 0.031 | 117 (23.1) | < 0.001 | |
| 30-35 yr | 806 (38.1) | 51 (37.2) | 204 (35.4) | 185 (36.6) | ||||
| ≥ 35 yr | 613 (29.0) | 55 (40.1) | 198 (34.4) | 204 (40.3) | ||||
| Prepregnancy BMI, kg/m2 | 3156 | 24.3 (21.8-27.9) | 24.7 (22.7-28.7) | 0.072 | 29.0 (24.5-33.6) | < 0.001 | 28.0 (24.2-32.3) | < 0.001 |
| Prepregnancy BMI group, n (%) | 3156 | |||||||
| < 30 kg/m2 | 1669 (83.7) | 104 (80.6) | 0.598 | 309 (56.6) | < 0.001 | 314 (64.5) | < 0.001 | |
| ≥ 30-< 35 kg/m2 | 207 (10.4) | 18 (14.0) | 133 (24.4) | 102 (20.9) | ||||
| ≥ 35 kg/m2 | 118 (5.9) | 7 (5.4) | 104 (19.0) | 71 (14.6) | ||||
| Ethnicity, n (%) | 3248 | |||||||
| Caucasian | 1126 (54.8) | 69 (51.1) | 0.402 | 292 (51.7) | 0.378 | 241 (48.7) | 0.030 | |
| Mediterranean | 616 (30.0) | 40 (29.6) | 176 (31.2) | 166 (33.5) | ||||
| African, Caribbean | 72 (3.5) | 3 (2.2) | 19 (3.4) | 17 (3.4) | ||||
| Asian | 53 (2.6) | 7 (5.2) | 16 (2.8) | 22 (4.4) | ||||
| Hindustan | 37 (1.8) | 3 (2.2) | 18 (3.2) | 15 (3.0) | ||||
| Other | 150 (7.1) | 13 (9.5) | 44 (7.6) | 34 (6.7) | ||||
| Gravidity | 3336 | 2 (1-3) | 2 (1-3) | 0.368 | 2 (2-4) | < 0.001 | 2 (2-3) | 0.045 |
| Parity, n (%) | 3323 | |||||||
| 0 | 803 (37.9) | 46 (33.6) | 0.102 | 171 (29.7) | < 0.001 | 169 (33.4) | 0.010 | |
| 1 | 785 (37.0) | 48 (35.0) | 231 (40.1) | 183 (36.2) | ||||
| ≥ 2 | 519 (24.9) | 43 (31.4) | 173 (30.0) | 152 (30.0) | ||||
| Smoking during pregnancy, n (%) | 3270 | 277 (13.3) | 15 (11.1) | 0.470 | 94 (16.8) | 0.031 | 79 (16.1) | 0.104 |
| Conception spontaneous, n (%) | 3277 | 1866 (89.8) | 120 (88.9) | 0.748 | 496 (87.9) | 0.216 | 431 (86.4) | 0.029 |
| Multiple pregnancy, n (%) | 3336 | 84 (4.0) | 9 (6.6) | 0.138 | 10 (1.7) | 0.010 | 33 (6.5) | 0.012 |
| Gestational age at OGTT, wk | 3336 | 27.1 (25.1-29.6) | 28.1 (25.4-32.7) | 0.041 | 27.0 (24.6-29.1) | < 0.001 | 27.0 (23.9-29.7) | < 0.001 |
| OGTT fasting plasma glucose value, mmol/L | 3336 | 4.6 (4.4-4.8) | 4.7 (4.5-4.8) | 0.087 | 5.2 (5.1-5.4) | < 0.001 | 5.4 (5.0-5.8) | < 0.001 |
| OGTT 2-h post load glucose value, mmol/L | 3298 | 5.7 (4.9-6.4) | 8.0 (7.8-8.2) | < 0.001 | 6.4 (5.7-6.9) | < 0.001 | 8.7 (8.1-9.6) | < 0.001 |
Continuous variables are presented as mean ± SD or median (interquartile range). Percentages may not add up to 100% due to rounding.
Women in this group received treatment for gestational diabetes mellitus.
P value reported compared to group 1: non-gestational diabetes mellitus.
GDM: Gestational diabetes mellitus; BMI: Body mass index; OGTT: Oral glucose tolerance test; WHO: World Health Organization.
The women in group 2 (GDM according to the WHO 1999 but not the WHO 2013 criteria) had a higher median age compared to the non-GDM group (34.1 vs 32.3 years, P < 0.001) and more often were ≥ 35 years of age (40.1% vs 29.0%, P = 0.002). Median BMI and BMI distribution as well as other baseline characteristics were similar to the non-GDM group.
The women in group 4 (GDM according to both criteria) had a significantly higher median age, were more often ≥ 35 years of age, had a higher prepregnancy BMI and had higher rates of obesity compared to the non-GDM women. In this group Caucasian ethnicity was less frequent, while Asian ethnicity was more frequent.
Pregnancy outcomes
Maternal and neonatal outcomes per group are presented in Table 2. Compared to the non-GDM group, women in group 3 were more likely to have a highest diastolic blood pressure ≥ 90 mmHg (18.6% vs 13.9%, P = 0.007) and had similar rates of induction of labor, assisted vaginal deliveries and emergency caesarean section but were more likely to deliver by planned caesarean section (15.5% vs 11.1%, P = 0.006). Indicated preterm birth occurred more often (1.2% vs 0.3%, P = 0.024). Also, women in group 3 gave birth to children with higher median birth weight (3598 vs 3490 g, P < 0.001), had higher rates of birth weight > 4000 g (22.3% vs 16.2%, P < 0.001), LGA (16.2% vs 10.2%, P < 0.001) and higher rates of 5-min Apgar score < 7 (3.9% vs 2.0%, P = 0.01) compared to the offspring of women in the reference group 1 with normal glucose tolerance. SGA occurred less frequently in group 3 (5.4% vs 8.0%, P < 0.001).
Table 2.
Pregnancy outcomes per group based on classification of oral glucose tolerance test results
| n | Group 1: non-GDM | Group 2: GDM by WHO 1999 only1 | P value2 | Group 3: GDM by WHO 2013 only | P value2 | Group 4: GDM by both criteria1 | P value2 | |
| n (%) | 3338 | 2119 (63.5) | 137 (4.1) | 576 (17.2) | 506 (15.2) | |||
| Maternal | ||||||||
| Highest diastolic blood pressure, mmHg | 3103 | 75 (70-82) | 80 (70-85) | 0.060 | 80 (75-85) | < 0.001 | 80 (75-85) | < 0.001 |
| Highest diastolic blood pressure, n (%) | 3103 | |||||||
| < 90 mmHg | 1691 (86.1) | 111 (84.1) | 0.520 | 429 (81.4) | 0.007 | 379 (79.0) | < 0.001 | |
| ≥ 90 mmHg | 273 (13.9) | 21 (15.9) | 0.520 | 98 (18.6) | 0.007 | 101 (21.0) | < 0.001 | |
| ≥ 110 mmHg | 31 (1.6) | 3 (2.3) | 0.541 | 4 (0.8) | 0.156 | 7 (1.5) | 0.849 | |
| Induction of labor, n (%) | 3044 | 335 (17.4) | 38 (28.8) | 0.001 | 97 (19.0) | 0.390 | 155 (32.6) | < 0.001 |
| Planned caesarean, n (%) | 3044 | 213 (11.1) | 17 (12.9) | 0.520 | 79 (15.5) | 0.006 | 81 (17.1) | < 0.001 |
| Assisted vaginal delivery, n (%) | 3059 | 124 (6.4) | 9 (6.8) | 0.853 | 29 (5.6) | 0.517 | 17 (3.6) | 0.018 |
| Emergency caesarean, n (%) | 3059 | 223 (11.5) | 12 (9.1) | 0.394 | 65 (12.6) | 0.492 | 65 (13.6) | 0.205 |
| Blood loss > 1000 cc, n (%) | 3019 | 173 (9.1) | 15 (11.4) | 0.374 | 35 (6.9) | 0.131 | 41 (8.7) | 0.813 |
| Neonatal | ||||||||
| Gestational age at delivery, wk | 3070 | 40.0 (39.0-40.9) | 39.4 (38.3-40.6) | 0.001 | 39.9 (38.7-41.0) | 0.515 | 39.1 (38.1-40.0) | < 0.001 |
| Preterm birth, n (%) | 3070 | |||||||
| Spontaneous | 79 (4.1) | 7 (5.3) | 0.491 | 26 (5.0) | 0.337 | 33 (6.9) | 0.008 | |
| Indicated | 6 (0.3) | 1 (0.8) | 0.370 | 6 (1.2) | 0.024 | 7 (1.5) | 0.006 | |
| Birth weight, grams | 3186 | 3490 (3090-3840) | 3449 (3041-3839) | 0.654 | 3598 (3216-3943) | < 0.001 | 3420 (2969-3800) | 0.056 |
| Birth weight, percentile | 3165 | 50.0 (24.8-75.8) | 52.0 (28.8-76.4) | 0.388 | 59.9 (32.6-81.6) | < 0.001 | 58.7 (29.5-82.1) | < 0.001 |
| Birth weight > 4000 grams, n (%) | 3186 | 325 (16.2) | 21 (15.0) | 0.719 | 117 (22.3) | < 0.001 | 81 (15.9) | 0.896 |
| Small-for-gestational-age (< 10th percentile), n (%) | 3165 | 160 (8.0) | 6 (4.3) | 0.117 | 28 (5.4) | 0.043 | 34 (6.7) | 0.336 |
| Large-for-gestational-age (> 90th percentile), n (%) | 3165 | 203 (10.2) | 17 (12.2) | 0.435 | 84 (16.2) | < 0.001 | 84 (16.6) | < 0.001 |
| Admission to neonatology ward, n (%) | 3146 | 219 (11.0) | 15 (10.7) | 0.904 | 59 (11.4) | 0.813 | 103 (20.4) | < 0.001 |
| Admission to NICU, n (%) | 3146 | 75 (3.8) | 4 (2.9) | 0.576 | 22 (4.3) | 0.620 | 28 (5.5) | 0.077 |
| Shoulder dystocia, n (%) | 3173 | 26 (1.3) | 6 (4.3) | 0.015 | 7 (1.3) | 0.935 | 18 (3.5) | 0.001 |
| Apgar score 5 min < 7, n (%) | 3155 | 39 (2.0) | 4 (2.9) | 0.525 | 20 (3.9) | 0.010 | 24 (4.7) | < 0.001 |
Continuous variables are presented as mean ± SD or median (interquartile range). Percentages may not add up to 100% due to rounding.
Women in this group received treatment for gestational diabetes mellitus.
P value reported compared to group 1: non-gestational diabetes mellitus.
GDM: Gestational diabetes mellitus; NICU: Neonatal intensive care unit; WHO: World Health Organization.
In group 2, labor was more often induced (28.8% vs 17.4%, P = 0.001) compared to the non-GDM group, as this is recommended in the Dutch guidelines for GDM. Rates of birth weight > 4000 g, LGA and SGA were comparable between group 2 (15.0%, 12.2% and 4.3%) and group 1 (all P > 0.05). Other neonatal outcomes were also similar, except for shoulder dystocia, which occurred in 4.3% compared to 1.3% in group 1 (P = 0.015).
Women in group 4 were more likely to have a highest recorded diastolic blood pressure ≥ 90 mmHg, labor was induced more often and planned caesarean section rates were higher compared to the non-GDM group. Assisted vaginal deliveries were carried out less frequently in this group (3.6% vs 6.4%, P = 0.018). Rates of both spontaneous and indicated preterm birth were also significantly higher (6.9% vs 4.1%, P = 0.008 and 1.5% vs 0.3%, P = 0.006 respectively) compared to group 1. Birth weight > 4000 g and SGA did not differ between group 4 and the reference group 1. However, more infants were LGA (16.6% vs 10.2%, P < 0.001). Also, neonates were more likely to be admitted to the neonatology ward, and shoulder dystocia and a 5-min Apgar score < 7 occurred more often.
Positive and negative likelihood ratios for adverse pregnancy outcomes with corresponding 95% confidence intervals per group are presented in Table 3. Compared to the non-GDM group, positive likelihood ratios (LR+) for adverse outcomes were generally higher in women with a positive OGTT according to either the WHO 1999, WHO 2013 or both criteria, except for assisted vaginal delivery and SGA. The LR+ for adverse outcome, excluding assisted vaginal delivery and SGA, ranged from 0.76 to 2.95 for all groups. An OGTT indicative for GDM according to both criteria (group 4) had the highest LR+ for most adverse outcomes. A positive OGTT by the WHO 2013 criteria only (group 3) had higher LR+ for adverse outcomes compared to a positive OGTT by WHO 1999 criteria only (group 2). Negative likelihood ratios showed a similar but inverse pattern that were mostly close to 1.00.
Table 3.
Likelihood ratios for adverse pregnancy outcomes for group 2-4 compared to group 1
| Outcome | n (%) | LR+ | 95%CI | LR- | 95%CI |
| Highest diastolic BP ≥ 90 mmHg | |||||
| Non-GDM | 273 (13.9) | ||||
| WHO 1999 criteria only | 21 (15.9) | 1.16 | 0.74-1.82 | 0.99 | 0.96-1.02 |
| WHO 2013 criteria only | 98 (18.6) | 1.31 | 1.08-1.58 | 0.92 | 0.86-0.98 |
| Both criteria | 101 (21.0) | 1.47 | 1.22-1.78 | 0.89 | 0.84-0.95 |
| Planned caesarean | |||||
| Non-GDM | 213 (11.1) | ||||
| WHO 1999 criteria only | 17 (12.9) | 1.18 | 0.72-1.92 | 0.99 | 0.95-1.03 |
| WHO 2013 criteria only | 79 (15.5) | 1.35 | 1.10-1.66 | 0.91 | 0.85-0.98 |
| Both criteria | 81 (17.1) | 1.47 | 1.20-1.81 | 0.89 | 0.83-0.96 |
| Assisted vaginal delivery | |||||
| Non-GDM | 124 (6.4) | ||||
| WHO 1999 criteria only | 9 (6.8) | 1.06 | 0.55-2.05 | 1.00 | 0.95-1.04 |
| WHO 2013 criteria only | 29 (5.6) | 0.90 | 0.64-1.26 | 1.03 | 0.95-1.11 |
| Both criteria | 17 (3.6) | 0.59 | 0.38-0.93 | 1.10 | 1.03-1.18 |
| Emergency caesarean | |||||
| Non-GDM | 223 (11.5) | ||||
| WHO 1999 criteria only | 12 (9.1) | 0.78 | 0.44-1.39 | 1.02 | 0.98-1.05 |
| WHO 2013 criteria only | 65 (12.6) | 1.09 | 0.86-1.37 | 0.98 | 0.91-1.04 |
| Both criteria | 65 (13.6) | 1.16 | 0.92-1.46 | 0.96 | 0.90-1.03 |
| Preterm birth | |||||
| Non-GDM | 87 (4.5) | ||||
| WHO 1999 criteria only | 8 (6.1) | 1.35 | 0.68-2.67 | 0.98 | 0.92-1.04 |
| WHO 2013 criteria only | 32 (6.2) | 1.30 | 0.96-1.77 | 0.92 | 0.82-1.03 |
| Both criteria | 41 (8.6) | 1.68 | 1.29-2.19 | 0.84 | 0.74-0.95 |
| SGA | |||||
| Non-GDM | 160 (8.0) | ||||
| WHO 1999 criteria only | 6 (4.3) | 0.54 | 0.24-1.20 | 1.03 | 1.00-1.07 |
| WHO 2013 criteria only | 28 (5.4) | 0.71 | 0.50-1.00 | 1.08 | 1.01-1.15 |
| Both criteria | 34 (6.7) | 0.86 | 0.63-1.18 | 1.04 | 0.97-1.11 |
| Birth weight > 4000 g | |||||
| Non-GDM | 325 (16.2) | ||||
| WHO 1999 criteria only | 21 (15.0) | 0.92 | 0.59-1.44 | 1.01 | 0.98-1.04 |
| WHO 2013 criteria only | 117 (22.3) | 1.36 | 1.14-1.63 | 0.91 | 0.86-0.97 |
| Both criteria | 81 (15.9) | 0.98 | 0.80-1.22 | 1.00 | 0.95-1.06 |
| LGA | |||||
| Non-GDM | 203 (10.2) | ||||
| WHO 1999 criteria only | 17 (12.2) | 1.22 | 0.75-1.98 | 0.99 | 0.95-1.03 |
| WHO 2013 criteria only | 84 (16.2) | 1.50 | 1.23-1.83 | 0.88 | 0.81-0.95 |
| Both criteria | 84 (16.6) | 1.54 | 1.26-1.87 | 0.87 | 0.81-0.94 |
| Admission to NICU | |||||
| Non-GDM | 75 (3.8) | ||||
| WHO 1999 criteria only | 4 (2.9) | 0.76 | 0.29-2.00 | 1.02 | 0.97-1.07 |
| WHO 2013 criteria only | 22 (4.3) | 1.10 | 0.76-1.60 | 0.97 | 0.87-1.09 |
| Both criteria | 28 (5.5) | 1.36 | 0.98-1.88 | 0.91 | 0.81-1.03 |
| Shoulder dystocia | |||||
| Non-GDM | 26 (1.3) | ||||
| WHO 1999 criteria only | 6 (4.3) | 2.95 | 1.41-6.19 | 0.87 | 0.73-1.03 |
| WHO 2013 criteria only | 7 (1.3) | 1.03 | 0.53-1.99 | 0.99 | 0.83-1.19 |
| Both criteria | 18 (3.5) | 2.06 | 1.43-2.96 | 0.74 | 0.58-0.94 |
| 5-min Apgar score < 7 | |||||
| Non-GDM | 39 (2.0) | ||||
| WHO 1999 criteria only | 4 (2.9) | 1.43 | 0.55-3.68 | 0.97 | 0.88-1.07 |
| WHO 2013 criteria only | 20 (3.9) | 1.67 | 1.16-2.41 | 0.83 | 0.69-1.00 |
| Both criteria | 24 (4.7) | 1.92 | 1.39-2.66 | 0.77 | 0.64-0.94 |
LR+: Positive likelihood ratio; 95%CI: 95% confidence interval; LR-: Negative likelihood ratio; GDM: Gestational diabetes mellitus; BP: Blood pressure; SGA: Small-for-gestational-age; LGA: Large-for-gestational-age; WHO: World Health Organization; NICU: Neonatal intensive care unit.
DISCUSSION
In this study, we estimated the impact of adopting new criteria for GDM in a population where adherence to the traditional criteria is the current policy, with the aim to provide insight in the potential impact of modification of disease definitions for GDM. We showed that applying the WHO 2013 criteria would result in an absolute increase of GDM diagnoses in women at risk of 13.1% or a 1.7-fold increase in comparison to the current WHO 1999 criteria. The women newly identified by the WHO 2013 criteria showed less favorable patient characteristics, i.e. higher maternal age and BMI. Newly identified women also had an increased chance of adverse pregnancy outcomes, including higher blood pressure and higher rates of indicated preterm birth, LGA and 5-min Apgar score < 7, compared to the women with a normal glucose tolerance after screening. Women only diagnosed by the 1999 criteria had similar patient characteristics and obstetric outcomes compared to those with a normal OGTT, although the latter may be because they underwent GDM treatment.
An increase in the number of GDM diagnoses, as we found in our study, is in line with the original Hyperglycemia and Adverse Pregnancy Outcome findings, which were the trigger to expand the GDM definition[10]. However, effects of implementation of the WHO 2013 criteria show regional variation, depending on the population, screening approaches or the diagnostic criteria, which are in use prior to implementation[25-28,33,34]. Studies performed in Asian populations have reported both a decrease and increase in the number of GDM diagnoses[35-37]. The 1.7-fold increase in our cohort is similar to estimations from three previous European studies with similar risk-based screening strategies[16,19,38]. Others have reported an even greater increase after actual implementation of the WHO 2013 criteria, such as a 3.5-fold increase in a study from Spain and a 4-fold increase in a Swiss cohort[39,40]. In the United Arab Emirates, introduction of the new criteria would result in almost half of the pregnant population to be labelled as GDM[14].
In 2017 a multidisciplinary working group, which included members from the Guidelines International Network, Grading of Recommendations Assessment, Development and Evaluation working group and the WHO, proposed a checklist with issues that should be considered prior to introducing modified disease definitions[41]. Although the checklist has not been formally applied to the GDM definition expansion proposed by the WHO (2013), several points on the checklist have yet to be met. For example, evaluation of incremental benefits for patients classified by the new and the previous definition is needed. Our study complements a previous cohort study from the Netherlands, which similarly found that the women newly identified by the WHO 2013 criteria are at increased risk for adverse pregnancy outcomes compared to pregnant women with normal glucose tolerance[19]. Our analysis furthermore allowed us to directly compare the newly diagnosed group to those women in which the WHO 1999 criteria and WHO 2013 criteria agree, e.g., expected ‘severe’ cases, and found that risks were generally lower in the newly added group. This is further strengthened by our analysis of likelihood ratios for adverse outcome for the different criteria. Positive likelihood ratios for adverse outcomes were lower in women with an abnormal OGTT by the WHO 2013 criteria compared to those positive by both criteria. However, an abnormal OGTT by any set of criteria showed only limited discriminative value in predicting adverse outcome, with the highest LR+ of 2.95 for shoulder dystocia and negative likelihood ratio mostly around 1.00.
Because the risk for adverse outcomes in the newly diagnosed women seems to be lower in comparison to the concordant group, it is this group of women facing possible overdiagnosis as a result of widening the diagnostic criteria for GDM. Multiple studies, all before-after studies, have shown positive effects on clinical outcomes, such as hypertensive disorders, LGA and caesarean section rates after implementation of the WHO 2013 criteria[39,42-44]. However, given the concurrent increase in the number of women diagnosed, it is unclear whether these results are attributable to effective treatment or due to inclusion of milder GDM cases. In one study that adjusted for maternal characteristics, only limited reductions in adverse outcomes were observed[42]. A randomized controlled trial found that treatment of milder GDM reduced LGA rates, shoulder dystocia, caesarean deliveries and hypertensive disorders[5]. The inclusion criteria for this trial do not fully correspond to the patients newly diagnosed by the WHO 2013 criteria, limiting extrapolation of these treatment results. To this date, no data are available for women with discordant diagnosis for GDM between the WHO 1999 and 2013 criteria and with that the treatment effect in this group specifically remains unknown. However, clinical trials on this matter are currently being undertaken[6,45,46].
Another point raised in the checklist is evaluation of potential incremental harm to patients. We found in our cohort that the proportion of women with an OGTT discordant between WHO 1999 and WHO 2013 was larger than the proportion of women in which both criteria agree. Implementation of the WHO 2013 criteria would subsequently result in unevaluated treatment of more than half the GDM population. This treatment could result in unnecessary exposure to interventions with possible harmful side effects, including induction of labor and caesarean sections[8]. Also, GDM diagnosis and subsequent medicalization can have profound negative effects on a patient’s quality of life[47-51]. Similarly, underdiagnosis could occur in the group of women that are currently receiving treatment but would be excluded with the new criteria. Although this was a relatively small group in our cohort, evaluation is necessary to establish whether these women can be safely left untreated.
Other forms of incremental harm include increased costs and use of health care resources because of implementation of the new criteria[52]. With estimated costs for GDM treatment in excess of standard antenatal care of €6843 per individual[53], implementation of the new criteria would result in a direct increase of medical costs of over 3 million euros for the study period in our center alone. In the Netherlands, approximately 5% of the pregnant population (approximately 8500 women) is diagnosed with GDM with the current criteria. A conservative 2% increase would result in 11900 diagnoses on a yearly basis, amounting up to over 23 million euros in additional health care costs. Data on cost-effectiveness are conflicting and are particularly influenced by the discriminative power of the new criteria to detect longer-term maternal, neonatal and infant outcome, which are currently largely unknown[39,54-56].
The strength of this study is the large sample size and availability of OGTT results, allowing for the reclassification in the four groups as presented in this study. This classification provides more insight on the impact of the WHO 2013 criteria by analyzing the women with discordant results separately, as proposed by the checklist on modifying disease definitions[41]. However, because the WHO 1999 criteria were used to diagnose GDM in our cohort, a treatment effect is present in the women meeting these criteria. Furthermore, the reference group in this study consisted of women with normal OGTT results. Because women were either screened because of the presence of risk factors or clinical signs suggestive for GDM, this group is a selection and therefore not fully representative of the general obstetric population in the Netherlands. However, comparison of pregnancy outcomes with a reference group including women without any risk factors for GDM would probably only further strengthen the associations we found. Another limitation is that there were no 1-h post load OGTT measurements available in this cohort, which are used in the WHO 2013 criteria. This may have resulted in an underestimation of the proportion of women diagnosed with GDM upon implementation of the WHO 2013 criteria.
CONCLUSION
The results from this retrospective study indicate a marked increase in the number of women diagnosed with GDM with the adoption of the WHO 2013 criteria as compared to WHO 1999 criteria. We have shown that the new criteria identify a new group of women at risk for adverse pregnancy outcomes but also result in exclusion of a number of women that currently receive treatment. Randomized trials are urgently needed to establish whether treatment of women with mild hyperglycemia, formerly not labelled as GDM, indeed leads to improvement of perinatal outcome. Treatment effects should be assessed both on the short-term and on the long-term of both mother and child to establish benefits, harms and cost-effectiveness of adopting the new criteria prior to implementation.
ARTICLE HIGHLIGHTS
Research background
Gestational diabetes mellitus (GDM) is the most common metabolic disorder of pregnancy. It is associated with both short- and long-term fetal, neonatal and maternal complications. Treatment of GDM has been shown to improve pregnancy outcomes.
Research motivation
Worldwide different diagnostic criteria to diagnose GDM are being used. Recently the Hyperglycemia and Adverse Pregnancy Outcome study has shown that maternal glucose levels below the most used thresholds increase the risk of adverse outcomes. As a result, new diagnostic criteria have been proposed by the World Health Organization (WHO) in 2013. These new, more stringent criteria have been shown to greatly affect the number of women diagnosed with GDM, which in turn can have great consequences for health care costs and effectiveness of current treatment strategies. However, the effects vary in different populations and are influenced by patient characteristics such as ethnicity and maternal body mass index.
Research objectives
We aimed to estimate the impact of the WHO 2013 criteria, compared with the WHO 1999 criteria, on the incidence of gestational diabetes mellitus as well as to determine the diagnostic accuracy for detecting adverse pregnancy outcomes. We sought to evaluate the patient characteristics and pregnancy outcomes of women with a discordant diagnosis specifically, as these are of importance for the treatment effects that may be expected. Currently, the treatment effects in these women are unknown.
Research methods
For this study we evaluated a cohort of 3338 women that were tested for GDM using a 75 g oral glucose tolerance test in the University Medical Center Utrecht. We applied both the current WHO 1999 criteria and the newly proposed WHO 2013 criteria for GDM. We determined the change in the number of GDM diagnoses. Also, we separately reported on patient characteristics and pregnancy outcomes of women with discordant diagnoses and compared these to the non-GDM women. Lastly, we determined the likelihood ratios for adverse outcomes for the different groups.
Research results
Retrospectively applying the WHO 2013 criteria increased the cohort incidence by 13.1%, from 19.3 to 32.4%. Discordant diagnoses occurred in 21.3%; 4.1% would no longer be labelled as GDM, and 17.2% were newly diagnosed. Compared to the non-GDM group, women newly diagnosed were older, had higher rates of obesity, higher diastolic blood pressure and higher rates of caesarean deliveries. Their infants were more often delivered preterm, large-for-gestational-age and were at higher risk of a 5-min Apgar score < 7. Women excluded from GDM were older and had similar pregnancy outcomes compared to the non-GDM group, except for higher rates of shoulder dystocia (4.3% vs 1.3%, P = 0.015). Positive likelihood ratios for adverse outcomes in all groups were generally low, ranging from 0.54 to 2.95.
Research conclusions
The number of women diagnosed with GDM increases substantially with the WHO 2013 compared to the WHO 1999 criteria. Women additionally diagnosed are at increased risk for adverse pregnancy outcomes. However, they seem to be at lower risk than women who would be diagnosed with GDM by both the old and new criteria. Also, likelihood ratios for adverse outcomes comparing both diagnostic criteria are generally low. Treatment effects may therefore be lower in newly diagnosed women, which may result in overtreatment of women newly diagnosed with GDM according to the WHO 2013 criteria.
Research perspectives
Adopting the WHO 2013 criteria results in an increased number of women diagnosed with GDM and translates to an excess risk of adverse pregnancy outcomes, supporting the need for intervention studies to estimate the treatment benefit and cost-effectiveness to improve clinically relevant outcomes for these previously untreated pregnant women.
Footnotes
Institutional review board statement: This study was exempt from approval of the Medical Research Ethics Committee of the University Medical Center Utrecht (reference number 16-711/C), which granted a waiver after reviewing the protocol because the Dutch Medical Research Involving Human Subjects Act (WMO) did not apply to this study.
Informed consent statement: With this document we would like to inform you that for the manuscript regarding our study entitled ’Estimated impact of introduction of new diagnostic criteria for gestational diabetes mellitus’ no individual informed consent forms have been signed by participants as approved by our Institutional Review Board (MREC of the University Medical Center Utrecht). See the attached Institutional Review Board Approval Form.
Conflict-of-interest statement: The authors declare that there is no duality of interest associated with this manuscript.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Invited manuscript
Peer-review started: February 2, 2021
First decision: February 25, 2021
Article in press: April 25, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ling ZX S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Leon de Wit, Department of Obstetrics and Gynaecology, University Medical Center Utrecht, Utrecht 3584 EA, Netherlands.
Anna B Zijlmans, Department of Obstetrics and Gynaecology, Gelderse Vallei Hospital, Ede 6716 RP, Netherlands.
Doortje Rademaker, Department of Obstetrics and Gynaecology, Amsterdam University Medical Centers- Location AMC, Amsterdam 1105 AZ, Netherlands.
Christiana A Naaktgeboren, Department of Obstetrics and Gynaecology, Amsterdam University Medical Centers–Location AMC, Amsterdam 1105 AZ, Netherlands.
J Hans DeVries, Department of Endocrinology, Amsterdam University Medical Centers–Location AMC, Amsterdam 1105 AZ, Netherlands.
Arie Franx, Department of Obstetrics and Fetal Medicine, Erasmus MC Sophia Children Hospital, Rotterdam 3015 GD, Netherlands.
Rebecca C Painter, Department of Obstetrics and Gynaecology, Amsterdam University Medical Centers–Location AMC, Amsterdam 1105 AZ, Netherlands.
Bas B van Rijn, Department of Obstetrics and Fetal Medicine, Erasmus MC Sophia Children Hospital, Rotterdam 3015 GD, Netherlands. b.vanrijn@erasmusmc.nl.
Data sharing statement
No additional data are available.
References
- 1.criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 3.Martis R, Crowther CA, Shepherd E, Alsweiler J, Downie MR, Brown J. Treatments for women with gestational diabetes mellitus: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2018;8:CD012327. doi: 10.1002/14651858.CD012327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, Lange S, Siebenhofer A. Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;340:c1395. doi: 10.1136/bmj.c1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung NW, Moses RG. Gestational Diabetes Mellitus: Is It Time to Reconsider the Diagnostic Criteria? Diabetes Care. 2018;41:1337–1338. doi: 10.2337/dci18-0013. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal MM. Gestational diabetes mellitus: An update on the current international diagnostic criteria. World J Diabetes. 2015;6:782–791. doi: 10.4239/wjd.v6.i6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348:g1567. doi: 10.1136/bmj.g1567. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal MM. Consensus in Gestational Diabetes MELLITUS: Looking for the Holy Grail. J Clin Med. 2018;7 doi: 10.3390/jcm7060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HAPO Study Cooperative Research Group , Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [Google Scholar]
- 11.International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser GH, de Valk HW. Is the evidence strong enough to change the diagnostic criteria for gestational diabetes now? Am J Obstet Gynecol. 2013;208:260–264. doi: 10.1016/j.ajog.2012.10.881. [DOI] [PubMed] [Google Scholar]
- 13.Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes: differences between the current international diagnostic criteria and implications of switching to IADPSG. J Diabetes Complications. 2015;29:544–549. doi: 10.1016/j.jdiacomp.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Arora GP, Thaman RG, Prasad RB, Almgren P, Brøns C, Groop LC, Vaag AA. Prevalence and risk factors of gestational diabetes in Punjab, North India: results from a population screening program. Eur J Endocrinol. 2015;173:257–267. doi: 10.1530/EJE-14-0428. [DOI] [PubMed] [Google Scholar]
- 16.Boyadzhieva MV, Atanasova I, Zacharieva S, Tankova T, Dimitrova V. Comparative analysis of current diagnostic criteria for gestational diabetes mellitus. Obstet Med. 2012;5:71–77. doi: 10.1258/om.2011.110073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helseth R, Salvesen O, Stafne SN, Mørkved S, Salvesen KA, Carlsen SM. Gestational diabetes mellitus among Nordic Caucasian women: prevalence and risk factors according to WHO and simplified IADPSG criteria. Scand J Clin Lab Invest. 2014;74:620–628. doi: 10.3109/00365513.2014.928942. [DOI] [PubMed] [Google Scholar]
- 18.Jenum AK, Mørkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, Voldner N, Rognerud-Jensen OH, Berntsen S, Mosdøl A, Skrivarhaug T, Vårdal MH, Holme I, Yajnik CS, Birkeland KI. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol. 2012;166:317–324. doi: 10.1530/EJE-11-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koning SH, van Zanden JJ, Hoogenberg K, Lutgers HL, Klomp AW, Korteweg FJ, van Loon AJ, Wolffenbuttel BHR, van den Berg PP. New diagnostic criteria for gestational diabetes mellitus and their impact on the number of diagnoses and pregnancy outcomes. Diabetologia. 2018;61:800–809. doi: 10.1007/s00125-017-4506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kun A, Tornóczky J, Tabák AG. The prevalence and predictors of gestational diabetes mellitus in Hungary. Horm Metab Res. 2011;43:788–793. doi: 10.1055/s-0031-1287795. [DOI] [PubMed] [Google Scholar]
- 21.Olagbuji BN, Atiba AS, Olofinbiyi BA, Akintayo AA, Awoleke JO, Ade-Ojo IP, Fasubaa OB Gestational Diabetes Study Group-Nigeria. Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan African population. Eur J Obstet Gynecol Reprod Biol. 2015;189:27–32. doi: 10.1016/j.ejogrb.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Pan L, Leng J, Liu G, Zhang C, Liu H, Li M, Tan L, Tian H, Chan JC, Hu G, Yu Z, Yang X. Pregnancy outcomes of Chinese women with gestational diabetes mellitus defined by the IADPSG's but not by the 1999 WHO's criteria. Clin Endocrinol (Oxf) 2015;83:684–693. doi: 10.1111/cen.12801. [DOI] [PubMed] [Google Scholar]
- 23.Trujillo J, Vigo A, Duncan BB, Falavigna M, Wendland EM, Campos MA, Schmidt MI. Impact of the International Association of Diabetes and Pregnancy Study Groups criteria for gestational diabetes. Diabetes Res Clin Pract. 2015;108:288–295. doi: 10.1016/j.diabres.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Dahanayaka NJ, Agampodi SB, Ranasinghe OR, Jayaweera PM, Wickramasinghe WA, Adhikari AN, Chathurani HK, Dissanayaka UT. Inadequacy of the risk factor based approach to detect gestational diabetes mellitus. Ceylon Med J. 2012;57:5–9. doi: 10.4038/cmj.v57i1.4193. [DOI] [PubMed] [Google Scholar]
- 25.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, Duncan BB, Schmidt MI. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeedi M, Cao Y, Fadl H, Gustafson H, Simmons D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108642. doi: 10.1016/j.diabres.2020.108642. [DOI] [PubMed] [Google Scholar]
- 27.Ramezani Tehrani F, Naz MSG, Yarandi RB, Behboudi-Gandevani S. The Impact of Diagnostic Criteria for Gestational Diabetes Mellitus on Adverse Maternal Outcomes: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10 doi: 10.3390/jcm10040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muche AA, Olayemi OO, Gete YK. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: a systematic review and meta-analysis. Arch Public Health. 2019;77:36. doi: 10.1186/s13690-019-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NVOG Nederlandse Vereniging voor Gynaecologie & Obstetrie. Diabetes Mellitus en Zwangerschap 2.0 2010. [cited 10 January 2021]. Available from: https://www.nvog.nl/wp-content/uploads/2018/02/Diabetes-mellitus-en-zwangerschap-2.0-04-06-2010.pdf .
- 30.Visser GH, Eilers PH, Elferink-Stinkens PM, Merkus HM, Wit JM. New Dutch reference curves for birthweight by gestational age. Early Hum Dev. 2009;85:737–744. doi: 10.1016/j.earlhumdev.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eusebi P. Diagnostic accuracy measures. Cerebrovasc Dis. 2013;36:267–272. doi: 10.1159/000353863. [DOI] [PubMed] [Google Scholar]
- 33.Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:11. doi: 10.1186/s13098-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, Kim MY, Jang HC. Pregnancy Outcomes of Women Additionally Diagnosed as Gestational Diabetes by the International Association of the Diabetes and Pregnancy Study Groups Criteria. Diabetes Metab J. 2019;43:766–775. doi: 10.4093/dmj.2018.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi C, Loy SL, Chan SY, Choong C, Cai S, Soh SE, Tan KH, Yap F, Gluckman PD, Godfrey KM, Shek LP, Chan JKY, Kramer MS, Chong YS. Impact of adopting the 2013 World Health Organization criteria for diagnosis of gestational diabetes in a multi-ethnic Asian cohort: a prospective study. BMC Pregnancy Childbirth. 2018;18:69. doi: 10.1186/s12884-018-1707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilder ME, Zin TW, Wai NS, Ner M, Say PS, Htoo M, Say S, Htay WW, Simpson JA, Pukrittayakamee S, Nosten F, McGready R. Gestational diabetes mellitus prevalence in Maela refugee camp on the Thai-Myanmar border: a clinical report. Glob Health Action. 2014;7:23887. doi: 10.3402/gha.v7.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yew TW, Khoo CM, Thai AC, Kale AS, Yong EL, Tai ES. The Prevalence of Gestational Diabetes Mellitus Among Asian Females is Lower Using the New 2013 World Health Organization Diagnostic Criteria. Endocr Pract. 2014;20:1064–1069. doi: 10.4158/EP14028.OR. [DOI] [PubMed] [Google Scholar]
- 38.Saleh L, Shareef M, Meiracker AHVD, Visser W. The impact of implementing the WHO-2013 criteria for gestational diabetes mellitus on its prevalence and pregnancy outcomes: A comparison of the WHO-1999 and WHO-2013 diagnostic thresholds. Eur J Obstet Gynecol Reprod Biol. 2020;249:107. doi: 10.1016/j.ejogrb.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Duran A, Sáenz S, Torrejón MJ, Bordiú E, Del Valle L, Galindo M, Perez N, Herraiz MA, Izquierdo N, Rubio MA, Runkle I, Pérez-Ferre N, Cusihuallpa I, Jiménez S, García de la Torre N, Fernández MD, Montañez C, Familiar C, Calle-Pascual AL. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care. 2014;37:2442–2450. doi: 10.2337/dc14-0179. [DOI] [PubMed] [Google Scholar]
- 40.Huhn EA, Massaro N, Streckeisen S, Manegold-Brauer G, Schoetzau A, Schulzke SM, Winzeler B, Hoesli I, Lapaire O. Fourfold increase in prevalence of gestational diabetes mellitus after adoption of the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. J Perinat Med. 2017;45:359–366. doi: 10.1515/jpm-2016-0099. [DOI] [PubMed] [Google Scholar]
- 41.Doust J, Vandvik PO, Qaseem A, Mustafa RA, Horvath AR, Frances A, Al-Ansary L, Bossuyt P, Ward RL, Kopp I, Gollogly L, Schunemann H, Glasziou P Guidelines International Network (G-I-N) Preventing Overdiagnosis Working Group. Guidance for Modifying the Definition of Diseases: A Checklist. JAMA Intern Med. 2017;177:1020–1025. doi: 10.1001/jamainternmed.2017.1302. [DOI] [PubMed] [Google Scholar]
- 42.Wu ET, Nien FJ, Kuo CH, Chen SC, Chen KY, Chuang LM, Li HY, Lee CN. Diagnosis of more gestational diabetes lead to better pregnancy outcomes: Comparing the International Association of the Diabetes and Pregnancy Study Group criteria, and the Carpenter and Coustan criteria. J Diabetes Investig. 2016;7:121–126. doi: 10.1111/jdi.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djaković I, Sabolović Rudman S, Gall V, Košec A, Markuš Sandrić M, Košec V. Do Changing Diagnostic Criteria for Gestational Diabetes Influence Pregnancy Outcome? Acta Clin Croat. 2016;55:422–427. doi: 10.20471/acc.2016.55.03.11. [DOI] [PubMed] [Google Scholar]
- 44.Hung TH, Hsieh TT. The effects of implementing the International Association of Diabetes and Pregnancy Study Groups criteria for diagnosing gestational diabetes on maternal and neonatal outcomes. PLoS One. 2015;10:e0122261. doi: 10.1371/journal.pone.0122261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amsterdam UMC Leading the Change the Netherlands. TANGO-DM Study. [cited 10 January 2021]. Available from: https://zorgevaluatienederland.nl/tango-dm/study-information .
- 46.Crowther CA, McCowan LME, Rowan JA, Edlin R, McKinlay CJD GEMS Study Group. Lower vs higher diagnostic criteria for the detection of gestational diabetes for reducing maternal and perinatal morbidity: study protocol for the GEMS randomised trial. BMC Pregnancy Childbirth. 2020;20:547. doi: 10.1186/s12884-020-03252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchetti D, Carrozzino D, Fraticelli F, Fulcheri M, Vitacolonna E. Quality of Life in Women with Gestational Diabetes Mellitus: A Systematic Review. J Diabetes Res. 2017;2017:7058082. doi: 10.1155/2017/7058082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter SM, Rogers W, Heath I, Degeling C, Doust J, Barratt A. The challenge of overdiagnosis begins with its definition. BMJ. 2015;350:h869. doi: 10.1136/bmj.h869. [DOI] [PubMed] [Google Scholar]
- 49.Parsons J, Sparrow K, Ismail K, Hunt K, Rogers H, Forbes A. Experiences of gestational diabetes and gestational diabetes care: a focus group and interview study. BMC Pregnancy Childbirth. 2018;18:25. doi: 10.1186/s12884-018-1657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalra B, Gupta Y, Baruah MP. Renaming gestational diabetes mellitus: A psychosocial argument. Indian J Endocrinol Metab. 2013;17:S593–S595. doi: 10.4103/2230-8210.123539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence JM. Women with diabetes in pregnancy: different perceptions and expectations. Best Pract Res Clin Obstet Gynaecol. 2011;25:15–24. doi: 10.1016/j.bpobgyn.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Ng E, Neff M, Sztal-Mazer S. Insights uncovered from experiencing a rise in the incidence of gestational diabetes at a Melbourne hospital. Diabetologia. 2018;61:1881–1883. doi: 10.1007/s00125-018-4631-1. [DOI] [PubMed] [Google Scholar]
- 53.Lenoir-Wijnkoop I, van der Beek EM, Garssen J, Nuijten MJ, Uauy RD. Health economic modeling to assess short-term costs of maternal overweight, gestational diabetes, and related macrosomia - a pilot evaluation. Front Pharmacol. 2015;6:103. doi: 10.3389/fphar.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, Fairley L, Golder S, Tuffnell D, Bland M, Dunne F, Whitelaw D, Wright J, Sheldon TA. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess. 2016;20:1–348. doi: 10.3310/hta20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cade TJ, Polyakov A, Brennecke SP. Implications of the introduction of new criteria for the diagnosis of gestational diabetes: a health outcome and cost of care analysis. BMJ Open. 2019;9:e023293. doi: 10.1136/bmjopen-2018-023293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacklin PB, Maresh MJ, Patterson CC, Stanley KP, Dornhorst A, Burman-Roy S, Bilous RW. A cost-effectiveness comparison of the NICE 2015 and WHO 2013 diagnostic criteria for women with gestational diabetes with and without risk factors. BMJ Open. 2017;7:e016621. doi: 10.1136/bmjopen-2017-016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.