Abstract
The risk of fracture is increased in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). However, in contrast to the former, patients with T2DM usually possess higher bone mineral density. Thus, there is a considerable difference in the pathophysiological basis of poor bone health between the two types of diabetes. Impaired bone strength due to poor bone microarchitecture and low bone turnover along with increased risk of fall are among the major factors behind elevated fracture risk. Moreover, some antidiabetic medications further enhance the fragility of the bone. On the other hand, antiosteoporosis medications can affect the glucose homeostasis in these patients. It is also difficult to predict the fracture risk in these patients because conventional tools such as bone mineral density and Fracture Risk Assessment Tool score assessment can underestimate the risk. Evidence-based recommendations for risk evaluation and management of poor bone health in diabetes are sparse in the literature. With the advancement in imaging technology, newer modalities are available to evaluate the bone quality and risk assessment in patients with diabetes. The purpose of this review is to explore the pathophysiology behind poor bone health in diabetic patients. Approach to the fracture risk evaluation in both T1DM and T2DM as well as the pragmatic use and efficacy of the available treatment options have been discussed in depth.
Keywords: Diabetes, Fracture risk, Bone mineral density, Microarchitecture, Antidiabetic drugs, Antiosteoporosis therapy
Core Tip: Diabetes mellitus, either type 1 or type 2, has adverse effects on bone that translate into an elevated fracture risk. Different pathophysiological mechanisms contribute to poor bone health in patients with diabetes. Diagnosis of bone fragility in diabetic patients is challenging as traditional fracture predictors underestimate fracture risk in this population, contributing to the concept that diabetes affects bone quality. While waiting for further evidence, the prevention and management of bone fragility in diabetes should include identification of patients at risk, correction of modifiable risk factors, appropriate choice of antidiabetic medications and use of antiosteoporosis drugs with proven efficacy.
INTRODUCTION
The prevalence of diabetes mellitus (DM) is increasing worldwide, along with diabetes-related renal and cardiovascular complications, in particular, resulting in an enormous burden on healthcare systems[1].DM adversely affects the skeleton as well, and the increased risk of fragility fractures is an important complication in diabetics[2]. Given the different pathogenic mechanisms of type 1 DM (T1DM) and type 2 DM (T2DM), they exhibit a unique relationship with bones. Recent evidence shows that both T1DM and T2DM are associated with an increased risk of fracture[3]. However, the relative contribution of low bone strength and increased incidence of falls behind the higher fracture risk in diabetic patients remains unknown. Fracture risk increases with duration of disease, poor glycemic control and the presence of vascular complications[4]. From a clinical standpoint, there are several challenges in the management too. Both bone mineral density (BMD) T-score and Fracture Risk Assessment Tool (FRAX) underestimate fracture prediction in patients with diabetes, particularly T2DM[5]. Moreover, antidiabetic medications have differential effects on bone homeostasis and fracture risk. The coexistence of DM and osteoporosis runs the risk of significant associated morbidity and mortality; it may also lead to significant debilitation. Thus, understanding their complex interaction is integral to providing optimal care for these patients. The purpose of this manuscript is to review the current knowledge of the factors and interconnected mechanisms that negatively affect several determinants of bone strength in patients with DM. In addition, keeping future perspectives in mind, the considerations regarding management in this population from the glycemic and the skeletal point of view are discussed.
EFFECT OF INSULIN AND INSULIN RESISTANCE OVER BONE
Insulin is anabolic for bones. Animal studies have clarified the complex mechanism through which insulin regulates bone turnover. Insulin exerts direct anabolic actions by activation of its cognate receptor. This insulin-like growth factor 1 (IGF-1) receptor has a crucial role in the execution of anabolic effects of insulin on osteoblasts[6]. As a result, insulin-deficient conditions like T1DM are typically associated with low levels and/or action of IGF-1. The role of amylin (cosecreted with insulin from pancreatic β-cell, thus low in T1DM) is unclear to date. Few studies have shown high serum levels of this factor to correlate with high bone mass[7]. However, further studies are needed to conclude its role in bone health in T1DM.
On the other hand, insulin resistance (IR), the most important feature of T2DM, affects bone quality directly in two ways. First, high blood glucose in circulation induces osteoblast resistance to the actions of IGF-1[8]. Second, high concentrations of advanced glycated end products (AGEs) impair the stimulatory actions of IGF-1 on osteoblasts[9]. Additionally, IR and adipose tissue dysregulation contribute to chronic low-grade inflammation, which can promote bone loss. In this population, loss of Dock 7 protein and silencing of Thy-1 expression induce higher bone resorption and increased adipogenesis, which leads to the impaired bone formation which in turn contributes to low bone mass[10]. Obesity-induced hypogonadism has also been implicated in the pathogenesis of low bone mass in IR state[11].
Mechanical loading is crucial for bone health as it stimulates the mechanosensitivity of osteoblasts through the Wnt-β-catenin pathway. It also increases the expression of Runt-related transcription factor 2 and consequently promotes osteogenesis. It inhibits dickkopf-related protein 1 and sclerostin secretion, resulting in attenuated bone resorption[12]. Some suggest skeletal loading may be compromised in consequence of a decrease in muscle strength due to decreased glucose uptake by muscles; however, this postulation is yet to be confirmed. Animal data show high-fat diet-induced obesity, achieved after 14-24 wk of high-fat diet consumption, leads to higher bone resorption, lower bone formation, poor quality of bone architecture and loss of bone strength[13]. Thus, both insulin deficiency and IR is associated with low bone mass. Nevertheless, hyperinsulinemia secondary to IR might contribute to high BMD in T2DM.
BONE HEALTH IN T1DM
Fracture risk in T1DM
The risk for fractures in patients with T1DM is three-fold higher than in the general population[14,15]. According to a large meta-analysis, the pooled relative risk in patients with T1DM compared to controls was 3.78 (95% confidence interval (CI): 2.05-6.98; P< 0.001) for hip fractures and 2.88 (95%CI: 1.71-4.82; P< 0.001) for vertebral fractures[15]. Studies suggest a stronger association of hip and vertebral fractures with T1DM (relative risk 6.3 and 6.94, respectively) compared to T2DM (relative risk 1.7 and 1.38, respectively) in both men and women[16]. The increased risk of fractures in patients with T1DM extends through the entire life span and starts 10 to 15 years earlier than nondiabetic populations[17]. The fracture risk is related to the duration of diabetes, with some studies revealing a near-linear relationship[18], and some suggest a bimodal relationship, with the highest incidence occurring within the first 2.5 years of diagnosing DM and a second peak occurring after 5 years[19]. Most studies fail to establish any association with glycemic control. However, the risk is higher in T1DM with microvascular complications.
Mechanisms of increased bone fragility in T1DM
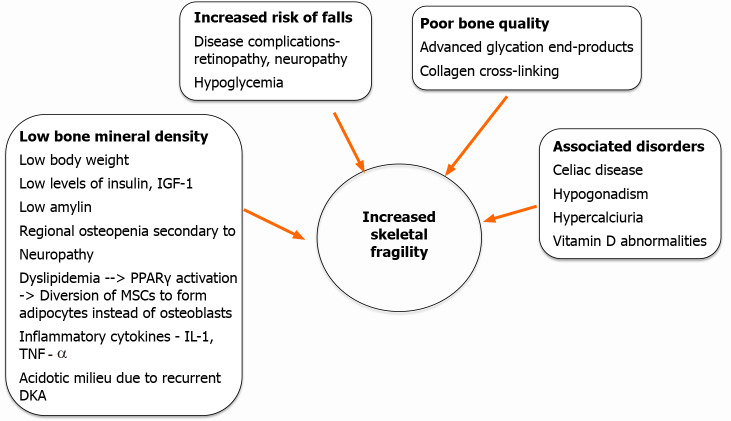
The increased fracture risk in T1DM is not solely explained by changes in BMD. Other mechanisms involving alterations in bone quality, microarchitecture and bone turnover have been suggested. Figure 1 summarizes the pathophysiologic events leading to increased risk of fractures in T1DM.
Figure 1.
Mechanisms of increased bone fragility in type 1 diabetes mellitus. DKA: Diabetic ketoacidosis; IGF-1: Insulin-like growth factor; PPAR: Peroxisome proliferator-activated receptor; MSC: Mesenchymal stem cell;IL-1: Interleukin 1; TNF: Tumor necrosis factor.
BMD in T1DM: Studies on BMD in subjects with T1DM have reported a decrease in BMD ranging from 8% to 67% with the hip being the worst affected (approximately 37%) followed by vertebrae (approximately 22%)[16,20]. A high proportion of 25(OH)D deficiency and low IGF-1 in children and adolescents with T1DM has been found, which might contribute to low axial BMD[21]. Data regarding the age of onset of osteopenia in patients with T1DM are conflicting, as studies show low Z-scores in children and young adults, but no differences in adults with T1DM in comparison to nondiabetics. A recent meta-analysis showed a significant reduction of BMD in children with T1DM[22]. Normalization of BMD or bone size over time in patients with T1DM is seen in longitudinal studies. Poor glycemic status can adversely affect BMD during childhood and adolescence, even though dual-energy X-ray absorptiometry (DXA) may not identify osteoporotic range for BMD[23]. An inverse correlation between BMD scores with glycated hemoglobin/hemoglobin A1c (HbA1c) and the duration of diabetes has been noticed in many but not all studies, yet the association with microvascular complications have been more consistent[24-26]. Results of the trabecular bone score (TBS) in T1DM have been inconsistent. Diabetics with microvascular disease have been seen to have lower total, cortical and trabecular volumetric BMD on high-resolution peripheral quantitative computed tomography (HRpQCT) of the radius[27].
Bone turnover in T1DM: Low levels of bone formation markers like osteocalcin (OC) have been seen in patients with T1DM. OC is of particular interest in T1DM because its effects on both insulin production and insulin sensitivity have been demonstrated. Markers of bone resorption including procollagen type1 N-terminal propeptide (P1NP) and C-terminal cross-link of collagen (CTX) are either low or unaltered in T1DM. These together hint towards T1DM as an overall, low bone turnover state [28,29].
Data on bone histomorphometry, the gold standard for the study of bone turnover is scarce in T1DM. No differences were seen in bone formation or resorption markers in a cohort of T1DM compared to controls. However, among those with a history of fractures, reduced activation frequency and increased degree of mineralization and nonenzymatic collagen crosslinks, were observed that suggest a low turnover state[30].
Bone geometry-size and structure: T1DM predominantly affects the cortical bone structure, whereas changes to trabecular bone are less pronounced. Lower cortical thickness but with the increased cross-sectional area has been demonstrated in long bones. The overall bone size is not smaller, but they have a larger endosteal circumference likely due to an enlarged trabecular bone compartment[31]. Though there are no differences in trabecular microarchitecture from controls, patients with T1DM and concomitant microvascular disease have thinner trabeculae, a lesser number of trabeculae per unit volume with increased spacing in between as observed on HRpQCT and magnetic resonance imaging[32]. A recent study using HRpQCT in adolescents shows detrimental changes in tibial and radial microarchitecture and bone strength, even before changes in BMD occur. Thus, the reduction in bone strength must have been related to poor glycemic control earlier in life. This study highlights that changes in bone microarchitecture and strength in early life in those with T1DM, rather than bone density, can predict the increased risk of fracture observed in adults[33].
Alteration in bone tissue quality: Although much is unknown, some analogy can be drawn with changes in T2DM, including accumulation of AGEs, increased collagen cross-linking, altered expression of noncollagenous protein expression and occlusion of vascular channels with microvascular disease, all of which can stiffen the organic matrix and increase fragility.
Reduced bone turnover in T1DM leads to the accumulation of aging bone material, and shifts to a more carbonated bone mineral matrix, which can detrimentally influence bone tissue strength[34,35].
Nonosseous factors contributing to bone fragility: Recurrent hypoglycemic episodes, low body weight, microvascular complications especially peripheral neuropathy, autonomic neuropathy and retinopathy can all contribute to the increased risk of falls in patients with T1DM. Additional factors like concomitant uncorrected hypothyroidism, celiac disease, hypogonadism and low IGF-1 levels can also contribute to poor muscle strength. However, data regarding the relative contribution of these factors and susceptibility to fracture risk are not yet available.
BONE HEALTH IN T2DM
Epidemiology of fracture risk in T2DM
Patients with T2DM carry less fracture risk than the T1DM category but are subject to an increased risk of overall fractures (5%-24%)[16,36-38]. The meta-analyses that evaluated the fracture risk in T2DM patients are summarized in Table 1. Among the skeletal sites, increased risk of hip fracture (8%-70%) has been reported consistently in most of the meta-analyses[14,39-42]. Young age, prolonged duration of diabetes, use of insulin[39] and Asian ethnicity[40] are the factors that have been associated with a higher risk of hip fracture in diabetic patients. However, the risk of fracture is not comparable at all the skeletal sites. Increased risk of new (incident) vertebral fracture has been reported by Koromani et al[43], but the same meta-analysis also reports a lesser rate of prevalent vertebral fracture in diabetic patients in comparison to controls[43]. Among the other nonvertebral fractures, significantly increased risk of the ankle[37,44,45], wrist[16,45] and arm fractures[37,44] have been reported in some but not in all the meta-analyses.
Table 1.
Summary of meta-analyses evaluating risk of fracture in patients with type 2 diabetes mellitus
|
Ref.
|
Fracture site
|
Risk effect (95%CI)
|
P
value
|
Risk factors (site)
|
| Vilaca et al[39], 2020 | Hip | RR 1.33 (1.19-1.49) | S | Younger age, female gender, insulin use, longer duration of diabetes (hip) |
| Nonvertebral | RR 1.19 (1.11-1.28) | S | ||
| Koromani et al[43], 2020 | Vertebral (incident) | OR 1.35(1.27-1.44) | S | |
| Vertebral (prevalent) | OR 0.84 (0.74-0.95) | S | ||
| Wang et al[36], 2019 | All | RR 1.22 (1.13-1.31) | S | |
| Hip | RR 1.27 (1.16-1.39) | S | ||
| Distal forearm | RR 0.97 (0.66-1.09) | NS | ||
| Upper arm | RR 1.54 (1.19-1.99) | S | ||
| Ankle | RR 1.15 (1.01-1.31) | S | ||
| Vertebrae | RR 1.74 (0.96-3.16) | NS | ||
| Liu et al[44], 2018 | Limb | RR 1.18 (1.02-1.35) | S | Female gender (leg/ankle) |
| Leg/Ankle | RR 1.80 (1.13-2.87) | S | ||
| Humerus | RR 1.27 (0.60-2.68) | NS | ||
| Wrist/hand/foot | RR 1.26 (0.94-1.71) | NS | ||
| Forearm | RR 0.98 (0.78-1.23) | NS | ||
| Vilaca et al[45], 20191 | Ankle | RR 1.30 (1.15-1.48) | S | |
| Wrist | RR 0.85 (0.77-0.95) | S | ||
| Moayeri et al[37], 2017 | All | RR 1.05 (1.04-1.06) | S | Older age, male gender, duration of diabetes. Insulin use, Corticosteroid use (overall) |
| Hip | RR 1.20 (1.17-1.23) | S | ||
| Vertebral | RR 1.16 (1.05-1.28) | S | ||
| Foot | RR 1.37 (1.21-1.54) | S | ||
| Wrist | RR 0.98 (0.88-1.07) | NS | ||
| Proximal humerus | RR 1.09 (0.86-1.31) | NS | ||
| Ankle | RR 1.13 (0.95-1.32) | NS | ||
| Jia et al[38], 20172 | All | IRR 1.23 (1.12-1.35) | S | |
| Hip | IRR 1.08 (1.02-1.15) | S | ||
| Vertebrae | IRR 1.21 (0.98-1.48) | NS | ||
| Ni and Fan[42], 2017 | All LBMF | RR 1.24 (1.09-1.41) | S | Female gender |
| Dytfeld and Michalak[40], 20173 | Hip | OR 1.30 (1.07-1.57) | S | Cohort studies, Studies conducted in Asia (hip) |
| Vertebral | OR 1.13 (0.94-1.37) | NS | ||
| Fan et al[41], 2016 | Hip | RR 1.34 (1.19-1.51) | S | |
| Vestergaard[16], 2007 | Hip | RR 1.38 (1.25-1.53) | S | |
| Wrist | RR 1.19 (1.01-1.41) | S | ||
| Vertebrae | RR 0.93 (0.63-1.37) | NS | ||
| All | RR 0.96 (0.57-1.61) | NS | ||
| Janghorbani et al[14], 2007 | Hip | RR 1.7 (1.3-2.2) | S |
Study included both type 1 and type 2 diabetes.
Low energy fractures.
Low energy fractures in postmenopausal women. CI: Confidence interval; IRR: Incidence rate ratio; LBMF: Low bone mass-related fractures; NS: Not significant; OR: Odds ratio; RR: Relative risk; S: Statistically significant.
BMD in T2DM patients
In a meta-analysis[46] of 15 observational studies, BMD at different sites was compared between 3437 T2DM patients and 19139 controls. The pooled analyses showed a significant increase in BMD at hip (0.06 g/cm2), femoral neck (0.04 g/cm2) and spine (0.06 g/cm2). The meta-regression analysis showed higher HbA1c, body mass index, young age and male gender to be associated with high BMD in T2DM patients[46]. In another meta-analysis[16], where pooled analysis of BMD was evaluated in both T1DM and T2DM patients, BMD was found to be significantly increased in the latter but decreased in T1DM patients. In this meta-analysis also, body mass index was a significant predictor of BMD in T2DM patients. Obesity and hyperinsulinemia could be the major reasons behind the higher BMD in type 2 diabetic patients. Even in prediabetic male patients, BMD was found to be higher at the femoral neck in a study from South Korea[47]. However, BMD was significantly less at the femoral neck among obese T2DM children at the time of diagnosis of diabetes[48]. In a study from India, no significant difference in BMD was found between T2DM and controls[49]. Though different studies show inconsistent results, most have reported higher BMD in adult T2DM patients than in controls. The risk of increased bone fragility in T2DM patients with relatively higher BMD suggests a paradoxical phenomenon, contrary to the findings in the general population.
Structural bone quality in T2DM
Alteration in bone microarchitecture leading to poor bone quality can be one of the major reasons behind the increased fracture risk in T2DM patients. TBS can act as a surrogate for bone microarchitecture. A meta-analysis that included 40508 individuals from 12 studies found significantly lower TBS (standardized mean difference: -0.31, 95%CI: -0.45 to -0.16) in patients with T2DM than controls[50]. Even TBS in patients with prediabetes was also significantly lower (standardized mean difference: -0.13, 95%CI: -0.23 to -0.04) than those with normal blood glucose[50]. Higher accretion of pentasodine, an AGE, had been correlated with lower TBS in patients with diabetes [51].
HRpQCT is a noninvasive technique, apart from TBS, that has been used to evaluate bone architecture. In a study of elderly female diabetic patients, cortical porosity was higher at the radius (P<0.05) and tibia leading to a decrease in compressive biomechanical properties[52]. In another study, postmenopausal diabetic patients with fragility fracture had higher endocortical bone surface, intracortical pore volume and greater relative porosity at the distal tibia and ultra-distal radius than those without fracture [53].
Diabetic patients with microvascular disease had inferior cortical bone quality than those without microvascular disease[54]. Diabetes-related vascular changes (cortical microangiopathy) had been postulated as the reason behind the poor cortical bone quality in diabetic patients with a fracture[55]. Moreover, cortical porosity was significantly higher in T2DM patients with peripheral vascular disease in comparison to controls, and cortical porosity was inversely correlated with transcutaneous oxygen tension[56]. On the other hand, in the Maastricht Study[57], T2DM patients with HbA1c <7% have superior cortical bone quality than those with poor glycemic control, but no significant relation was found with the microvascular disease.
To summarize, change in bone microarchitecture as evidenced by poor cortical bone quality in T2DM patients can explain to some extent the paradox of increased bone fragility despite preserved BMD in these patients. Bone material strength index (BMSi) as calculated by in vivo bone microindentation acts as a surrogate marker of bone strength. Reduced BMSi has been reported in T2DM patients in comparison to nondiabetic controls[58,59]. Adiposity is related to decreased BMSi and increased cortical porosity in T2DM patients[60].
Bone turnover in T2DM
The studies that evaluated bone turnover markers (BTMs) in diabetic patients mostly identified diabetes as a low turnover disease. A meta-analysis that included 22 studies comprising of both T1DM and T2DM patients reported lower levels of both bone resorption (urinary N-terminal cross-linked telopeptide of type-I collagen) and formation (OC) markers[61]. However, subgroup analysis of T2DM patients showed only a trend towards lower OC levels in comparison to nondiabetic controls. In another recent meta-analysis[62], a pooled analysis showed significantly lower resorption markers (CTX and tartrate-resistant acid phosphatase) as well as formation markers (OC and P1NP) in T2DM patients. Moreover, sclerostin was found to be significantly higher in T2DM patients[62]. The elevated sclerostin level can be the link between hyperglycemia, low bone turnover and increased fracture risk in T2DM[63,64]. In a bone histomorphometry study, reduced mineralization surface, mineral apposition rate, bone formation rate and adjusted apposition rate along with a significant increase in mineralization lag time had been reported in eight (six type 2 and two type 1) diabetic patients in comparison to control[65]. In another study, significantly reduced mineralization surface, osteoblast surface and bone formation rate had been found in T2DM patients in comparison to nondiabetic control[66].
Pathophysiology of bone disease in T2DM
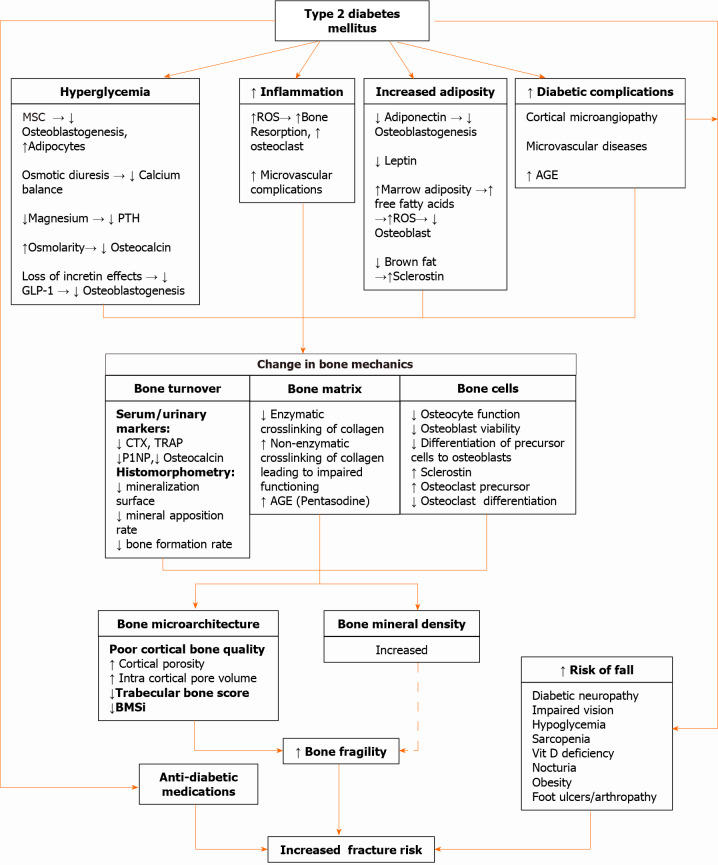
The increased fracture risk in T2DM is due to increased bone fragility and a greater risk of falls in these patients. Diminished vision, peripheral neuropathy, poor balance, diabetic arthropathy and hypoglycemic episodes can all increase the risk of falls in these patients. Moreover, antidiabetic medications can also increase fracture risk (discussed in next section). A brief outline of the pathophysiology of bone disease in T2DM has been illustrated in Figure 2. The detailed discussion of various pathophysiological mechanisms is beyond the scope of this review. The readers can find more detailed discussions on this topic elsewhere[2,67].
Figure 2.
Mechanisms underlying bone fragility in type 2 diabetes mellitus. AGE: Advanced glycated end product; BMSi: Bone material strength index; CTX: C-terminal cross-linked telopeptide; GLP-1: Glucagon-like peptide-1; MSC: Mesenchymal stem cells; P1NP: Procollagen type 1 N-terminal propeptide; PTH: Parathyroid hormone; ROS: Reactive oxygen species; TRAP: Tartrate-resistant acid phosphatase.
IMPACT OF DIABETES TREATMENTS ON BONE METABOLISM: CURRENT EVIDENCE
The complexity of bone changes in diabetes is made more complicated by the plethora of effects that pharmacotherapy induces. Therapeutic agents for diabetes affect bone quality and fracture risk by different mechanisms. First, they can affect bone formation and resorption at the molecular level. Second, some agents induce weight loss that can independently be associated with a reduction of bone mass. Third, agents that increase the chance of fall especially in the elderly can increase the chance of fracture, irrespective of bone quality. The effects of individual agents are discussed in Table 2[68-87].
Table 2.
The effect of diabetes therapies on skeletal parameters and fracture risk
|
Agents
|
Effect on bone metabolism
|
Additional effects on fracture risk
|
Effect on bone markers and BMD
|
Effect on fracture
|
Overall effect
|
| Insulin | Anabolic | Increases fall risk[68] | No negative effect | Hip, peripheral and osteoporotic fracture risk is magnified[69]. A propensity matched cohort analysis demonstrated adjusted sub hazard ratio of 1.38 (95%CI: 1.06-1.80) for major fractures with insulin use as compared with nonusers[70]. Females are more prone. No increased risk with glargine use[71] | Effect on bone +ve. Fracture risk ↑ |
| Metformin | Anabolic (via AMPK). Skew the mesenchymal stem cells from the adipogenic to the osteogenic arm[72] and inhibit osteoclast differentiation[73] | Reductions in oxidative stress and cell apoptosis | In a meta-analysis the use of metformin was associated with a reduced risk of fracture (RR 0.86, 95%CI: 0.75-0.99). It was mostly prescribed in the early stages of T2DM, and there was less hypoglycemia that might explain fewer fractures with metformin[74] | Effect on bone +ve. Fracture risk ↓ | |
| Sulfonylurea | Negligible effect | Increases fall risk due to hypoglycemia | Negligible effect | A recent meta-analysis including 11 studies involving 255644 individuals showed 14% increase in the risk of developing fracture[75]. Most of the fractures were attributable to increased fall due to hypoglycemia[76] | Effect on bone-neutral. Fracture risk ↔/↑ |
| Pioglitazone | Proadipogenic. Inhibits osteoblast differentiation. Inhibits osteoclast differentiation[77] | None | The bone resorption marker(CTX) was elevated, while indicators of bone formation were reduced[78]. It was also associated with significant reduction in BMD among women at the lumbar spine as well in femoral neck. | An updated meta-analysis including 24544 participants from 22 RCTS showed significantly increased incidence of fracture was found in women (OR=1.94; 95%CI: 1.60-2.35; P<0.001), but not in men (OR=1.02; 95%CI: 0.83-1.27; P=0.83). The fracture risk was independent of age, and there was no clear association with duration of TZD exposure[79] | Effect on bone -ve. Fracture risk ↑ |
| DPP-4 inhibitors | Preclinical studies demonstrated antiresorptive evidence[80] | None | None | The overall risk of fracture did not differ between patients exposed to DPP-4 inhibitors and controls (RR, 0.95; 95%CI: 0.83-1.10; P = 0.50) in a meta-analysis including 62 RCTs[81] | Effect on bone- neutral. Fracture risk ↔ |
| GLP-1 Analogues | Pro-osteoblast. Suppress sclerostin and increase osteocalcin[82] | By virtue of weight loss, they are supposed to cause a decrease in BMD | BMD did not significantly change after exenatide-induced weight loss (-3.5 ± 0.9 kg); suggesting that exenatide treatment attenuated BMD decrements after weight loss[83] | The Bayesian network meta-analysis suggested that GLP-1 RAs had a decreased bone fracture risk compared to other antihyperglycemic drugs, and exenatide is the safest agent with regard to the risk of fracture[84] | Effect on bone +ve. Fracture risk ↔ |
| SGLT-2 inhibitors | Preclinical data are conflicting | Weight loss causes BMD loss. Increased PTH due to phosphate reabsorption | A randomized controlled study (104 wk) found that canagliflozin induced reductions in hip BMD (−1.2% relative to placebo)[85] | A recent meta-analysis including 30 RCTs demonstrated that the incidence of bone fractures was not significantly different between patients taking SGLT2 inhibitors and placebo[86] | Effect on bone ↔. Fracture risk ↔ |
| Metabolic surgery | No direct effect. Mechanical unloading, nutritional deficiencies and hormonal changes are catabolic to bone | Massive weight loss causes a reduction of BMD. The severity of bone outcomes seems to be related to the degree of malabsorption varies depending on different procedures | Patients undergoing gastric bypass surgery, BMD was 5%-7% lower at the spine and 6%–10% lower at the hip compared with nonsurgical controls, as assessed by QCT and dual-energy X-ray absorptiometry[87] | In a large database from the United Kingdom. RYGB is associated with a 43% increased risk of nonvertebral fracture compared with AGB, with risk increasing >2 yr after surgery. The risk was highest after 5 yr of surgery (HR 3.91)[87] | Effect on bone -ve. Fracture risk ↑ |
AGB: Adjustable gastric banding; AMPK: AMP-activated protein kinas; BMD: Bone mineral density; CI: Confidence interval; CTX: C-terminal cross-linked telopeptide; DPP-4: Dipeptidyl-peptidase 4; GLP-1: Glucagon -like peptide-1; HR: Hazard ratio; OR: Odds ratio; PTH: Parathormone; QCT: Quantitative computed tomography; RCT: Randomized controlled trial; RYGB: Roux-en-Y gastric bypass; RR: Relative risk; SGLT-2: Sodium-glucose cotransporter 2; T2DM: Type 2 diabetes mellitus; TZD: Thiazolidinedione; ↑: Increase; ↓: Decrease; ↔: Unchanged; +ve: Positive; -ve: Negative.
Overall, insulin, metformin and glucagon-like peptide 1 analogs have a beneficial effect, and pioglitazone and bariatric surgery have a negative effect on bone morphology. Agents like sulfonylureas, dipeptidyl peptidase-4 inhibitors and sodium-glucose cotransporter-2 inhibitors do not have any direct beneficial or detrimental effects on bone morphology. But the fracture outcome data with all these agents depend on concomitant weight loss and risk for hypoglycemia. To date, only pioglitazone, insulin and bariatric surgery have demonstrated an increased risk for fracture in a real-world setting. However, whether insulin actually increases the fracture risk is controversial. Insulin-treated patients on average have longer disease duration and a higher prevalence of micro-and macrovascular complications. Thus, insulin use may just be a surrogate for severity or duration of T2DM, risk of hypoglycemia, presence of complications or increased risk of fall, which may explain the increased fracture risk in patients with T2DM. However, given there is a paucity of evidence of fracture outcome data from randomized controlled trials (RCTs) as a primary outcome, the conclusions reached herein are subject to change with additional future evidence.
PREDICTION OF FRACTURE RISK IN INDIVIDUALS WITH DIABETES: AN EMERGING CHALLENGE
Given the increasing number of patients with diabetes and consequently increasing the population-attributable risk of fracture, it is imperative to find out predictors of fracture. Even with increased fracture risk in diabetes, risk stratification of patients with diabetes is still lacking.
Clinical and radiologic assessment
The association between standard clinical risk factors (CRFs) for osteoporotic fractures as well as incident fractures is comparable in individuals with and without diabetes[88]. Nevertheless, other factors specific to the diabetic population need to be considered. For the duration of diabetes, studies have shown positive associations with fracture risk[89]. Poor glycemic control may impact differently on fracture risk depending on the type of diabetes. In some studies, a higher risk of fracture was observed in the presence of chronic complications of diabetes[16,90]. However, the impact of diabetic complications on fracture risk is debatable. In patients with diabetes, a history of fall is of particular importance.T2DM patients with fractures have more frequent episodes of fall and are more likely to be affected by peripheral neuropathy and reduced physical performance[91]. Vitamin D deficiency is also more common in patients with diabetes, and it is generally accepted that vitamin D-deficient subjects are at greater risk of fractures, but specific data on vitamin D-deficient patients with diabetes are not available[92].
In day-to-day practice, fracture risk is usually determined by measuring BMD (at the lumbar spine and the proximal femur) and by CRF assessment. These well-established RFs are part of a questionnaire-based FRAX released in 2008[93]. In general, BMD measured by DXA is regarded as the gold standard for bone health assessment in clinical practice. However, the estimated fracture probabilities by the BMD T-score and FRAX significantly underestimate fracture risk in patients with T1DM and T2DM[3,5]. This situation poses considerable challenges for the primary prevention of fragility fractures in these patients.
Risk assessment modalities
The bone status and fracture risk in diabetic patients may be evaluated by different approaches: BMD, CRFs, fracture probability, bone microarchitecture and bone strength.
BMD, CRFs, fracture probability: Studies have consistently demonstrated lower BMD in patients with T1DM compared to subjects without diabetes[16] and higher BMD in patients with T2DM. Importantly, for patients with both T1DM and T2DM, the BMD T-score underestimates the fracture risk[5,16]. Schwartz et al[5] showed that a T-score in a diabetic woman that is associated with risk of hip fracture corresponds to a T-score of approximately 0.5 units lower in a nondiabetic woman[5]. Though BMD underestimates the risk of fracture, it stratifies the risk in elderly patients with T2DM[94].
The FRAX algorithm allows for calculations of the 10year probability of fracture. The assessment is based on CRFs and the hip BMD T-score and permits for the incorporation of secondary osteoporosis for example in T1DM but not in T2DM. One prospective study found that the FRAX algorithm underestimated fracture risk in patients with T2DM[5], and a retrospective cohort study showed that FRAX underestimated the risk of hip fracture and major osteoporotic fracture in a group of combined T1DM and T2DM patients[3].
Overall, neither BMD T-score nor the FRAX tool provides a satisfactory fracture risk evaluation for patients with diabetes, and additional considerations on this topic are described in the next section.
Bone microarchitecture and bone quality: HRpQCT can be used to image and quantify volumetric BMD and bone microarchitecture including cortical porosity at a low radiation dose. Further, the estimated bone strength and failure load can be calculated. An association between high cortical porosity and T2DM was first described by Burghardt and others[52,53,95]. As determined by finite element analysis, pathologic cortical microarchitecture translated into major deficits in stiffness, failure load and cortical load fraction[52]. Recently, the Framingham Study found that T2DM patients had lesser cortical volumetric BMD, higher cortical porosity and smaller tibial cross-sectional area, independent of age, sex, weight and height[96]. Although the HRpQCT data is promising and could be a better fracture risk predictor than DXA, this research technique is unlikely to become widely available for routine clinical use.
A newer approach for the assessment of bone quality is bone indentation. Some studies using tibial outer cortex microindentation have shown that the estimated BMSi is reduced in T2DM compared to controls[59,97]. Moreover, AGE accumulation is negatively related to BMSi[97]. Nevertheless, its wide use as a clinical tool is restricted because of the invasive procedure. Taken together, available data points towards deficits in the cortical compartment and lesser resistance to the indentation in patients with diabetes.
The TBS is a parameter that reveals bone microarchitecture through analysis of DXA image pixel gray-level variations. Leslie et al[98] evaluated 2356 diabetic women (both T1DM and T2DM) and 27051 women without diabetes and revealed lower TBS in diabetic patients in comparison to controls in spite of higher lumbar spine and hip BMD in patients with diabetes. Current studies suggest the potential of TBS in fracture risk prediction for diabetic patients[99-101]. Clinical studies directly comparing differences in TBS between T1DM and T2DM are scarce. To summarize, because TBS is DXA based, it can be accessed without the need of new equipment, and TBS is more helpful for predicting fracture risk when combined with BMD. However, there is a lack of evidence demonstrating how post-treatment TBS improvement can decrease fracture risk[102].
Histomorphometry and BTMs: Studies in rodent models have found a reduced rate of bone turnover, worse microstructure, and lower strength in T1DM and T2DM. However, as the bone biopsy is an invasive test, only a small number of clinical studies have investigated the bone quality of patients using bone histomorphometry[102]. Moreover, results are inconsistent among different studies. A recent paper has shown that premenopausal women with T2DM have low bone turnover rates compared to healthy controls, and histomorphometry parameters are influenced by disease control and the presence of chronic complications[103]. Additional high-quality studies are necessary to determine the histologic changes of diabetic bone.
BTMs have been extensively investigated in patients with DM. A recent meta-analysis on levels of circulating BTMs in children and adolescents withT1DM reported reduced levels of OC compared to subjects without diabetes, while data were not conclusive for CTX and P1NP[104]. Another meta-analysis evaluating BTMs in both T1DM and T2DM subjects showed increased levels of alkaline phosphate in diabetic patients and decreased OC, CTX, and 25 (OH) vitamin D levels compared to controls[61]. Neither P1NP, N-terminal propeptide type1 collagen, deoxypyridinoline, bone-specific alkaline phosphatase nor parathyroid hormone (PTH) differed significantly from controls. This meta-analysis also reported considerable heterogeneity between the studies. Newer evidence suggested BTMs are decreased as CTX, OC, P1NP, u-N-terminal propeptide type 1 collagen and PTH were lower in T2DM than controls[105-107]. The association between BTMs and fracture has been evaluated in cross-sectional trials. CTX and sclerostin may potentially predict fractures, but longitudinal trials are required[108,109]. In general, BTMs are poorly related to fracture risk in patients with diabetes as bone marker levels differ from study to study. It should also be pointed out that nephropathy may alter bone turnover and modify fracture risk in diabetes.
BTMs seem to be lower in patients with diabetes, whereas bone-specific alkaline phosphatase is normal to higher, suggesting that the matrix becomes hypermineralized in diabetic patients[110]. This may clarify, in part, the paradox of low bone strength and increased BMD.
Evaluation of bone health in T1DM
There are no specific recommendations on BMD screening for T1DM patients. Following pediatric guidelines in children and adolescents with T1DM, osteoporosis can only be diagnosed in the presence of vertebral compression fractures or clinically significant long bone fractures (≥2 long-bone fractures up to the age of 10 years and ≥ 3 long-bone fractures up to age 19 years) with a BMD Z-score of 2.0 or lower[111]. The preferred sites for bone mineral content and areal BMD measurements in children include spine and total body less head but not the hip. However, there is a lack of normative data in children, and areal BMD requires adjustments for differences in height and bone size. The effects of height and bone size on BMD can be offset by an automated radiogrammetric measurement of cortical BMD of the second to fourth metacarpal bones using BoneXpert, expressed as a bone health index, and one study has reported significantly decreased cortical bone density using this technique in children and adolescents with T1DM[112].
The FRAX algorithm is used to estimate an individual’s 10year probability of major osteoporotic fracture and hip fracture in subjects greater than 40 years of age. However, T1DM is considered as a cause of secondary osteoporosis, and therefore it increases fracture probability only if BMD is not included in the calculations[20]. A low TBS value can increase the predicted fracture probability in T1DM[113].
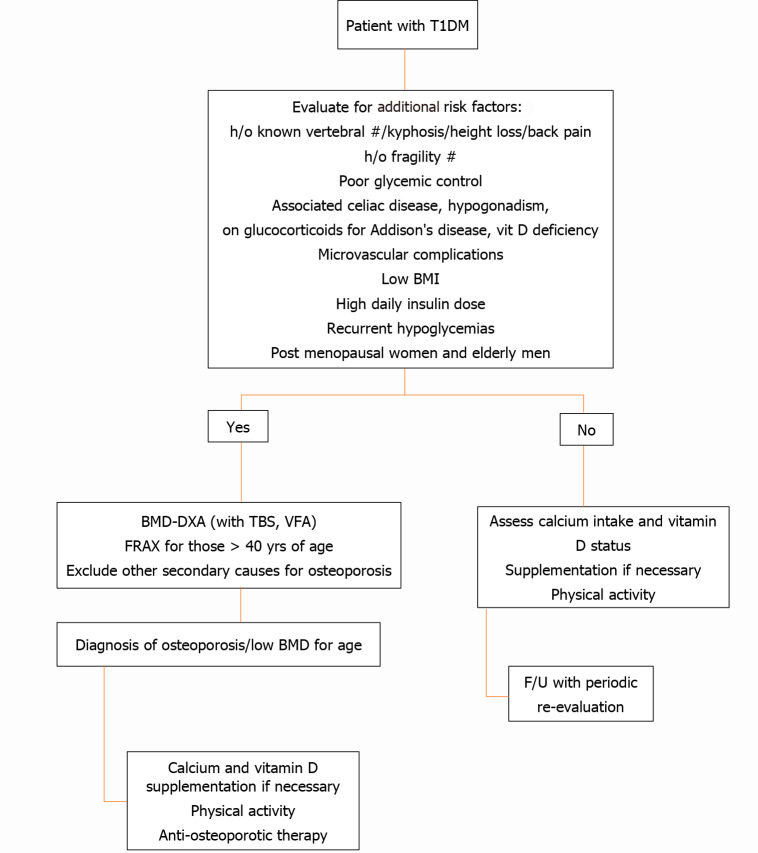
To date, it is not clear who should undergo a BMD assessment among T1DM patients. One single study suggested a number of risk factors for fractures in patients with T1DM, the presence of which should dictate the need for DXA scanning and further evaluation. Figure 3 provides an approach for investigating osteoporosis in T1DM.
Figure 3.
Algorithm for evaluation of bone health in type 1 diabetes mellitus. BMI: Body mass index; BMD-DXA: Bone mineral density by dual energy X-ray absorptiometry; F/U: Follow up; FRAX: Fracture Risk Assessment Tool; H/o: History of; T1DM: Type 1 diabetes mellitus; TBS: Trabecular bone score; VFA: Vertebral fracture assessment.
Evaluation of fracture risk in patients with T2DM-an algorithm
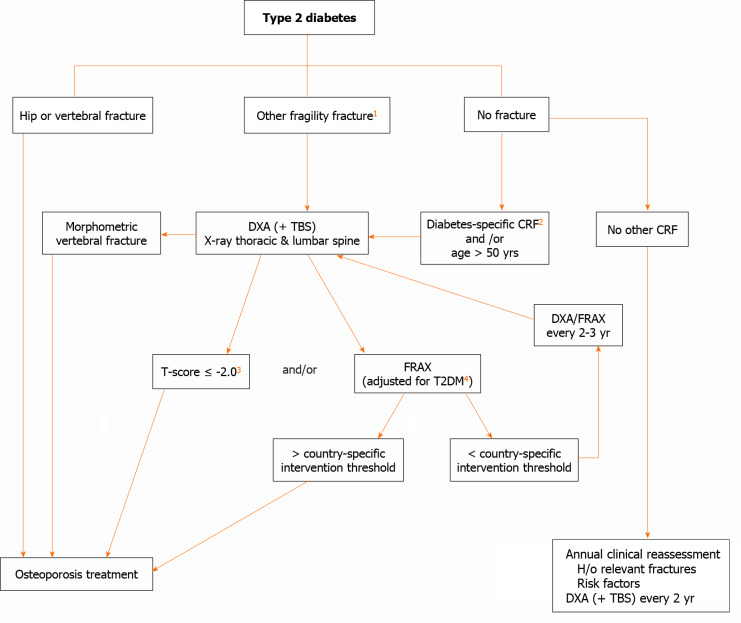
The identification of fracture risk in patients with T2DM remains challenging and the optimal approach has not yet been established. An algorithm for the evaluation of fracture risk in diabetic patients is proposed in Figure 4. This approach may change over time as additional evidence accumulates.
Figure 4.
Evaluation of fracture risk in patients with type 2 diabetes mellitus. 1: ≥ 1 nonvertebral nonhip fragility fracture might be required to initiate therapy; 2: Diabetes-specific clinical risk factors (diabetes duration, antidiabetic medications,, hemoglobin A1c and microvascular complications); 3: In diabetes, fracture risk at T-score < -2 equivalent for nondiabetes at T-score < -2.5; 4: See text. CRF: Clinical risk factor; TBS: Trabecular bone score; DXA: Dual energy X-ray absorptiometry; T2DM: Type 2 diabetes mellitus; FRAX: Fracture Risk Assessment Tool; H/o: History of. Modified from Ferrari et al[123]: Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R, El-Hajj Fuleihan G, Josse R, Kendler DL, Kraenzlin M, Suzuki A, Pierroz DD, Schwartz AV, Leslie WD; Bone and Diabetes Working Group of IOF. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 2018; 29:2585-2596.Copyright ©The Author(s) 2018. Published by Springer Nature.
COMPREHENSIVE MANAGEMENT OF FRACTURE RISK IN PATIENTS WITH DIABETES
Nonpharmacologic management
Patients with diabetes should modify their lifestyle with optimal exercise and a balanced diet. Exercise is beneficial to improve bone strength and bone biomechanical properties[114]. Nevertheless, weight loss-associated muscle and bone loss may enhance the risk of sarcopenia and skeletal fragility. Sarcopenia and sarcopenic obesity should be prevented by sufficient protein intake and weight-bearing exercise to reduce the risk of falls and frailty[115]. Calcium and vitamin D are important in the maintenance of bone health and are included in the treatment of osteoporosis. Even though the skeletal benefits of vitamin D supplementation in diabetes have not been shown, in correspondence to the nondiabetic population, daily intake of 800 IU vitamin D may be advocated. Nevertheless, it may not be enough to attain optimal serum levels (30 ng/mL) in T2DM. Sufficient calcium intake (1000 mg/d) is also recommended (preferably from food sources). For children and adolescents with T1DM, calcium and vitamin D supplementation is particularly important. Screening for celiac disease and early introduction of gluten-free diet is very important in T1DM subjects. Other nonpharmacological measures like avoidance of smoking, decrease in sodium intake and limitation in alcohol consumption (< 3 units/d) remain vital.
Optimal management of diabetes in patients with concomitant osteoporosis
Glycemic control and diabetic chronic complications: Although strict glycemic control does not necessarily lessen the fracture risk[116], numerous studies have suggested that poor glycemic control enhances the fracture risk compared to control in T1DM[117,118] and T2DM[119,120]. Therefore, for diabetic patients, a smooth reduction in blood glucose level is required to avert hypoglycemia and its consequences, including fracture[121]. A strong association has been documented between diabetic complications and risk of fracture[119,122]. Peripheral neuropathy, retinopathy and any impairment of vision, predisposition to hypoglycemia, hypotension and recent history of fall should be taken into account and corrected where possible.
Choose antidiabetic drugs carefully: Medications used in the treatment of T2DM may have an impact on bone metabolism. For people with diabetes at high fracture risk, antidiabetic agents with neutral effects or even with a protective effect on bone, like metformin, dipeptidyl peptidase-4inhibitors or glucagon-like peptide-1RA, should be preferred. Thiazolidinediones should be used with caution in elderly patients with T2DM who are at risk for fracture, especially in postmenopausal women, and the concurrent use of thiazolidinediones and sulfonylureas should be avoided in particular. Caution should be exercised when using sodium-glucose cotransporter-2 inhibitors in elderly patients with cardiovascular diseases or those taking high-dose diuretics. Insulin should be used with caution and careful measures to prevent hypoglycemia.
Indications for treatment of osteoporosis in diabetic patients
In individuals with diabetes, treatment should be considered at more favorable BMD and FRAX values compared to the nondiabetic population. Recently, the Bone and Diabetes Working Group of the International Osteoporosis Foundation[123] recommended use of an intervention threshold of a BMD T-score of -2.0 at the hip or spine in patients with diabetes (Figure 4). Although possibly appropriate in Western populations, this proposed adjustment and absolute cut-off may not apply to Asian and the Middle East populations. This working group also suggested a monitoring every 2 years of BMD in diabetes. If significant BMD loss is observed upon two consecutive measurements (≥ 5% in 2 years), or the T-score reaches close to -2.0, treatment should be considered (Figure 4).
Risk assessment tools, such as FRAX, do not entirely capture the elevated risks in patients with T2DM. Therefore, for a given FRAX score, a higher risk of fracture is observed in T2DM patients than in patients without T2DM[5]. As T2DM confers an elevated fracture risk that is not dependent on standard CRFs, it has been suggested that inclusion of T2DM be considered in future FRAX versions[3]. The FRAX calculated fracture risk in diabetes is estimated to be equivalent to an addition of 10 years of age or decreasing the BMD T-score by 0.5 SD[5]. Rheumatoid arthritis input to FRAX as a proxy for the T2DM effect is one option. Clinically, such a FRAX adjustment for T2DM can be useful despite limitations[124].
Osteoporosis therapies-efficacy in diabetes and cautions
Does diabetes modify the effectiveness of medications for osteoporosis? There is very little information available from comparative studies on the efficacy of osteoporosis therapies in diabetes-induced osteoporosis in general and in T1DM specifically. This has been worsened by the fact that diabetes is frequently an exclusion criterion for enrollment in clinical trials. In addition, there are concerns that in the setting of diabetes, antiresorptive therapies that suppress bone turnover may not be as effective[125]. Regarding antiresorptive therapies in people with diabetes, the efficacy of bisphosphonates and raloxifene in diabetic individuals are discussed here. Until now, the efficacy of denosumab in diabetes has been investigated in only one study.
Post hoc analyses of RCTs comparing results in people with diabetes randomized to treatment vs placebo have provided the strongest clinical evidence concerning the efficacy of bisphosphonates in diabetic population. In any particular trial, however, the number of diabetic patients is often insufficient to evaluate the fracture-related outcome. Conducted among postmenopausal women in the United States, a post hoc analysis of the Fracture Intervention Trial, showed that the lumbar spine and hip BMDs were increased following alendronate therapy for 3 years relative to placebo in women with T2DM[126]. The size of these effects is comparable in diabetic and nondiabetic women. Risedronate efficacy in diabetic patients is established from the results of three RCTs that were conducted in Japan[127]. Risedronate has similar effects on bone resorption and formation markers and BMD at the lumbar spine in diabetic and nondiabetic patients. Similar antifracture efficacy for bisphosphonates has been reported in diabetic compared with nondiabetic subjects by observational studies[128,129]. No trials or observational studies have assessed whether the efficacy of osteoporosis therapies in the diabetic population differs by BMD T-score. Analyses of two different RCTs of raloxifene, a nonsteroidal selective estrogen receptor modulator, show similar efficacy for diabetic and nondiabetic women for the prevention of vertebral fractures[130,131]. However, raloxifene also has the limitation of lack of efficacy in nonvertebral fractures similar to results in nondiabetic women. A meta-analysis of antiosteoporosis medications in T1DM and T2DM patients indicated that the efficacy of alendronate, risedronate and raloxifene in improving BMD and decreasing fracture rate is comparable between diabetic and nondiabetic individuals [132].
Effects of denosumab in diabetic patients with osteoporosis have been investigated in the subgroup analysis of the FREEDOM study and FREEDOM extension[133]. Long-term denosumab treatment reduced the risk of vertebral fractures and increased BMD in both diabetic and nondiabetic women with osteoporosis. No reduction in nonvertebral fractures has been observed.
Anabolic agents are of special interest in diabetes, which is associated with lower bone formation, in comparison to postmenopausal osteoporosis that is characterized by increased turnover[125]. Rodent studies are available for PTH and sclerostin antibodies. For PTH 1–34 (teriparatide), post hoc analyses of the DANCE observational study show that effects on nonvertebral fracture risk and BMD gain are similar in patients with T2DM and controls. Furthermore, patients with T2DM have a larger increase in femoral neck BMD during 18 mo of treatment with teriparatide in comparison to controls[134]. At present, clinical studies on the effectiveness of anti-sclerostin monoclonal antibody (romosozumab) in patients with diabetes are not available.
Overall, both antiresorptive and anabolic therapies reduce the risk of fractures in diabetic patients. Table 3 summarizes the efficacy of osteoporosis therapies in patients with diabetes.
Table 3.
Effects of osteoporosis medications in patients with type 2 diabetes mellitus
|
Medication
|
Effect on glucose metabolism
|
BMD
|
Risk of fracture
|
| Alendronate | Reduction in the risk of diabetes | Increase | NA/unchanged |
| Risedronate | Reduction in the risk of diabetes | Increase | NA |
| Etidronate | NA | NA | Unchanged |
| Denosumab | No effect on blood glucoselevels | Increase | Decrease |
| Raloxifene | Improves insulin sensitivity | NA | Decrease/unchanged |
| Teriparatide | No effect blood glucose levels | Increase | Unchanged |
BMD: Bone mineral density; NA: Not available.
Special points when diabetic patients receive osteoporosis therapy: For osteoporosis treatment in diabetes, a vitamin D-sufficient status must be attained through supplementation, and current evidence supports the use of both antiresorptive and anabolic agents[135]. People with diabetes may develop some degree of renal impairment and gastrointestinal complications. Therefore, it is imperative to assess renal function and gastrointestinal symptoms prior to starting antiresorptive drugs. Denosumab may be a favored choice in patients with diabetes who are older and/or have a worsening kidney function. Because diabetes is characterized by poor bone quality and low bone turnover, when sequential osteoporosis treatment is considered, an anabolic agent should be administered initially, followed by an antiresorptive drug[135].
A higher frequency of atypical femur fractures and osteonecrosis of the jaw is observed with the use of bisphosphonates and denosumab[125]. There is conflicting evidence on diabetes being associated with an increased incidence of atypical femur fractures[136]. In the oncology population, diabetes is considered a risk factor for the development of osteonecrosis of the jaw. In this population, the combined effects of osteoporosis therapy and diabetes are unknown.
Antiosteoporosis medications and glucose metabolism
The presence of crosstalk between the bone and energy metabolism has been established with animal models. Thus, the possible effects of antiosteoporosis drugs on glucose metabolism should be noted. Specifically, rodent models point out that OC has favorable effects on glucose metabolism[137]. Given that bone resorption inhibitors suppress OC, the concern is there that these therapies might enhance the risk of diabetes. Post hoc analyses of randomized trials of alendronate, zoledronic acid and denosumab indicated that the risk of diabetes is not increased by the use of antiresorptive therapies[138].
Observational studies have also shown that bisphosphonate use is associated with a lower risk of incident diabetes[139,140]. These findings provide reassurance that antiresorptive therapy will not increase the risk of incident diabetes. Mouse studies have found that downregulation of receptor activator of nuclear factor kappa-Β signaling leads to improved hepatic insulin sensitivity and plasma glucose levels[141]. This appears to imply that receptor activator of nuclear factor kappa-Β ligand blocking may have a favorable effect on diabetes prevention. Clinical trials, however, did not prove any correlation between denosumab (human monoclonal antibody that inhibits receptor activator of nuclear factor kappa-Β ligand) treatment and fasting glucose, IR or diabetes risk[138]. Regarding raloxifene effects on glycemic control, a post hoc analysis found no difference in fasting glucose or HbA1c changes over 3 years between raloxifene and placebo in women with and without diabetes[142]. Using the Danish registry data, an observational study reported that raloxifene was associated with a reduced incidence of diabetes[128].
PTH 1–34 improved IR and increased serum OC in T2DM rats[143]. One short study revealed that teriparatide did not affect glucose metabolism after 6 mo of treatment[144]. However, another study showed that after 6 mo of treatment with teriparatide (20 μg/d) fasting glucose and Homeostatic Model Assessment for IR index increased in postmenopausal women[145].
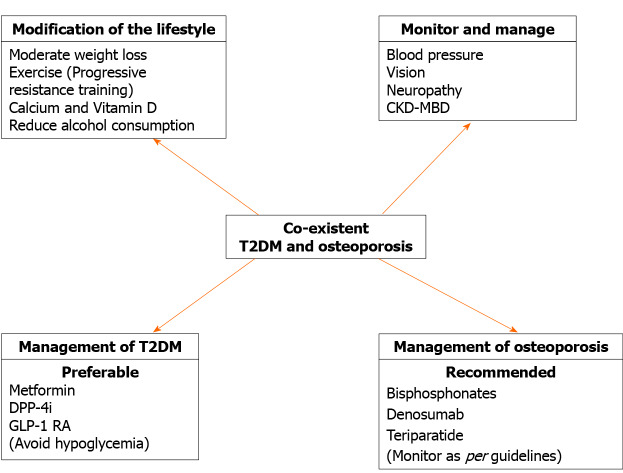
In conclusion, the data indicate that antiosteoporosis medications have minimal, if any, effects on glucose metabolism (Table 3). The findings of a reduction in the risk of developing diabetes with bisphosphonate use merit further investigation. Strategies for the best possible management of patients with T2DM and coexisting osteoporosis have been detailed elsewhere[146]. Figure 5 provides an outline of management.
Figure 5.
Strategies for treating type 2 diabetes mellitus and concurrent osteoporosis. CKD-MBD: Chronic kidney disease–mineral and bone disorder; DPP-4i: Dipeptidyl-peptidase 4 inhibitor; GLP-1: Glucagon-like peptide-1; T2DM: Type 2 diabetes mellitus.
Osteoporosis targeted pharmacotherapy in T1DM
Given that T1DM is a low bone turnover state, anabolic agents like intermittent recombinant human PTH therapy and antisclerostin agent romosozumab seem to be interesting therapeutic options, but there are no human studies in the T1DM population. Bisphosphonates have shown no difference in efficacy in T1DM compared to T2DM or nondiabetics)[126]. However, caution must be exercised while using bisphosphonates in women of reproductive age. Denosumab increases predominantly cortical BMD, which makes it another intriguing option in T1DM, but there is no data yet. A novel agent, recombinant IGF-1, has shown promising results in T1DM rodent models[147].
Emerging treatment options
A potential new antiosteoporosis treatment, romosozumab, is a monoclonal antibody against sclerostin that causes a loss of osteoblast inhibition along with inhibition of osteoclast activation. Romosozumab improves BMD at different skeletal sites and decreases the risk of fracture compared with placebo or other antiosteoporosis treatments[148]. Because elevated levels of sclerostin in diabetes may contribute to bone disease, it will be interesting to investigate the effect of romosozumab in diabetic patients. Further, research into the role of PTH for bone protection in patients with diabetes can provide interesting insights into its use as it is by far the best treatment for this patient population. Because AGEs play an important role in the pathogenesis of DM and osteoporosis, prevention of AGE-induced glycation of proteins connected with the maintenance of bone health can be a potential way of managing diabetes-induced osteoporosis.
CONCLUSION
Both T1DM and T2DM are associated with bone fragility although via different mechanisms. The situation seems more complex in T2DM as BMD is elevated, and the bone quality alterations are multifactorial. The contribution of antidiabetic medications, if any exists, appears limited except through the induction of hypoglycemic episodes responsible for falls. Diabetes-associated osteoporosis and fracture are important complications to consider when evaluating patients with long-standing DM. However, there is no clear consensus on how to screen for fracture risk and when to initiate treatment of osteoporosis in patients with DM. Therefore, realistic measures should be taken, with special attention to the prevention of falls. Good glycemic control is essential for reducing the risk of bone fragility, but hypoglycemia should be avoided and medications with a neutral effect on bone metabolism are preferred. Future research should continue evaluating the structural determinants of bone fragility in diabetes and improving the fracture prediction tools to facilitate timely intervention and fracture prevention. The available data, albeit small, suggest that antiosteoporosis medications are equally effective in patients with and without diabetes. Dedicated trials investigating the effects of new osteoporosis drugs, such as sclerostin antibodies, on bone strength and fracture outcomes in diabetes are needed.
Footnotes
Conflict-of-interest statement: Nothing to disclose.
Manuscript source: Invited manuscript
Peer-review started: January 27, 2021
First decision: March 30, 2021
Article in press: April 29, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pastromas S S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Rajan Palui, Department of Endocrinology, The Mission Hospital, Durgapur 713212, West Bengal, India.
Subhodip Pramanik, Department of Endocrinology, Neotia Getwel Healthcare Centre, Siliguri 734010, West Bengal, India.
Sunetra Mondal, Department of Endocrinology, Institute of Post Graduate Medical Education and Research (IPGMER), Kolkata 700020, West Bengal, India.
Sayantan Ray, Department of Endocrinology, Medica Superspeciality Hospital and Medica Clinic, Kolkata 700099, West Bengal, India. sayantan.ray30@gmail.com; Department of Endocrinology, Jagannath Gupta Institute of Medical Sciences and Hospital, Kolkata 700137, West Bengal, India.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 8th edition 2017. [cited 30 December 2020]. Available from: https://diabetesatlas.org/en/
- 2.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 3.Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27:301–308. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 4.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM Study of Osteoporotic Fractures (SOF) Research Group; Osteoporotic Fractures in Men (MrOS) Research Group; Health; Aging and Body Composition (Health ABC) Research Group. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulzele K, DiGirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem. 2007;282:25649–25658. doi: 10.1074/jbc.M700651200. [DOI] [PubMed] [Google Scholar]
- 7.Cornish J, Naot D. Amylin and adrenomedullin: novel regulators of bone growth. Curr Pharm Des. 2002;8:2009–2021. doi: 10.2174/1381612023393341. [DOI] [PubMed] [Google Scholar]
- 8.Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, Otani S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 2001;38:113–122. doi: 10.1007/s005920170007. [DOI] [PubMed] [Google Scholar]
- 10.Picke AK, Campbell GM, Blüher M, Krügel U, Schmidt FN, Tsourdi E, Winzer M, Rauner M, Vukicevic V, Busse B, Salbach-Hirsch J, Tuckermann JP, Simon JC, Anderegg U, Hofbauer LC, Saalbach A. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aao6806. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Lee JY, Sung J. Obesity and Bone Health Revisited: A Mendelian Randomization Study for Koreans. J Bone Miner Res. 2019;34:1058–1067. doi: 10.1002/jbmr.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:319–338. doi: 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–297. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 14.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 15.Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32:1134–1142. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 17.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN) Diabetes Care. 2015;38:1913–1920. doi: 10.2337/dc15-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao J, Brismar K, Nyrén O, Ugarph-Morawski A, Ye W. Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care. 2005;28:2850–2855. doi: 10.2337/diacare.28.12.2850. [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 20.Hough FS, Pierroz DD, Cooper C, Ferrari SL IOF CSA Bone and Diabetes Working Group. MECHANISMS IN ENDOCRINOLOGY: Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174:R127–R138. doi: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 21.Hamed EA, Faddan NH, Elhafeez HA, Sayed D. Parathormone--25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2011;12:536–546. doi: 10.1111/j.1399-5448.2010.00739.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Xu J, Zhou M, Lian X, Shi J. Association between type 1 diabetes mellitus and reduced bone mineral density in children: a meta-analysis. Osteoporos Int. 2021 doi: 10.1007/s00198-020-05715-3. [DOI] [PubMed] [Google Scholar]
- 23.Fuusager GB, Christesen HT, Milandt N, Schou AJ. Glycemic control and bone mineral density in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2019;20:629–636. doi: 10.1111/pedi.12861. [DOI] [PubMed] [Google Scholar]
- 24.Gunczler P, Lanes R, Paz-Martinez V, Martins R, Esaa S, Colmenares V, Weisinger JR. Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J Pediatr Endocrinol Metab. 1998;11:413–419. doi: 10.1515/jpem.1998.11.3.413. [DOI] [PubMed] [Google Scholar]
- 25.Eller-Vainicher C, Zhukouskaya VV, Tolkachev YV, Koritko SS, Cairoli E, Grossi E, Beck-Peccoz P, Chiodini I, Shepelkevich AP. Low bone mineral density and its predictors in type 1 diabetic patients evaluated by the classic statistics and artificial neural network analysis. Diabetes Care. 2011;34:2186–2191. doi: 10.2337/dc11-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen P, Feldt-Rasmussen B, Jacobsen P, Rossing K, Parving HH, Nielsen PK, Feldt-Rasmussen U, Olgaard K. Microalbuminuria as an early indicator of osteopenia in male insulin-dependent diabetic patients. Diabet Med. 1997;14:1038–1043. doi: 10.1002/(SICI)1096-9136(199712)14:12<1038::AID-DIA509>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K. Bone Geometry, Volumetric Density, Microarchitecture, and Estimated Bone Strength Assessed by HR-pQCT in Adult Patients With Type 1 Diabetes Mellitus. J Bone Miner Res. 2015;30:2188–2199. doi: 10.1002/jbmr.2573. [DOI] [PubMed] [Google Scholar]
- 28.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 29.Pater A, Sypniewska G, Pilecki O. Biochemical markers of bone cell activity in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2010;23:81–86. doi: 10.1515/jpem.2010.23.1-2.81. [DOI] [PubMed] [Google Scholar]
- 30.Armas LA, Akhter MP, Drincic A, Recker RR. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone. 2012;50:91–96. doi: 10.1016/j.bone.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verroken C, Pieters W, Beddeleem L, Goemaere S, Zmierczak HG, Shadid S, Kaufman JM, Lapauw B. Cortical Bone Size Deficit in Adult Patients With Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2017;102:2887–2895. doi: 10.1210/jc.2017-00620. [DOI] [PubMed] [Google Scholar]
- 32.Abdalrahaman N, McComb C, Foster JE, McLean J, Lindsay RS, McClure J, McMillan M, Drummond R, Gordon D, McKay GA, Shaikh MG, Perry CG, Ahmed SF. Deficits in Trabecular Bone Microarchitecture in Young Women With Type 1 Diabetes Mellitus. J Bone Miner Res. 2015;30:1386–1393. doi: 10.1002/jbmr.2465. [DOI] [PubMed] [Google Scholar]
- 33.Devaraja J, Jacques R, Paggiosi M, Clark C, Dimitri P. Impact of Type 1 Diabetes Mellitus on Skeletal Integrity and Strength in Adolescents as Assessed by HRpQCT. JBMR Plus. 2020;4:e10422. doi: 10.1002/jbm4.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah VN, Carpenter RD, Ferguson VL, Schwartz AV. Bone health in type 1 diabetes. CurrOpinEndocrinol Diabetes Obes. 2018;25:231–236. doi: 10.1097/MED.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan TS, Fraser LA. Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos. 2015;2015:174186. doi: 10.1155/2015/174186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Ba Y, Xing Q, Du JL. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9:e024067. doi: 10.1136/bmjopen-2018-024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:455–468. doi: 10.2147/TCRM.S131945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia P, Bao L, Chen H, Yuan J, Liu W, Feng F, Li J, Tang H. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017;28:3113–3121. doi: 10.1007/s00198-017-4183-0. [DOI] [PubMed] [Google Scholar]
- 39.Vilaca T, Schini M, Harnan S, Sutton A, Poku E, Allen IE, Cummings SR, Eastell R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone. 2020;137:115457. doi: 10.1016/j.bone.2020.115457. [DOI] [PubMed] [Google Scholar]
- 40.Dytfeld J, Michalak M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: meta-analysis of observational studies. Aging Clin Exp Res. 2017;29:301–309. doi: 10.1007/s40520-016-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y, Wei F, Lang Y, Liu Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int. 2016;27:219–228. doi: 10.1007/s00198-015-3279-7. [DOI] [PubMed] [Google Scholar]
- 42.Ni Y, Fan D. Diabetes mellitus is a risk factor for low bone mass-related fractures: A meta-analysis of cohort studies. Medicine (Baltimore) 2017;96:e8811. doi: 10.1097/MD.0000000000008811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koromani F, Oei L, Shevroja E, Trajanoska K, Schoufour J, Muka T, Franco OH, Ikram MA, Zillikens MC, Uitterlinden AG, Krestin GP, Anastassiades T, Josse R, Kaiser SM, Goltzman D, Lentle BC, Prior JC, Leslie WD, McCloskey E, Lamy O, Hans D, Oei EH, Rivadeneira F. Vertebral Fractures in Individuals With Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care. 2020;43:137–144. doi: 10.2337/dc19-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Cao L, Qian YW, Chen ZX, Guo SF, Sun WQ, He ZR. The association between risk of limb fracture and type 2 diabetes mellitus. Oncotarget. 2018;9:31302–31310. doi: 10.18632/oncotarget.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vilaca T, Walsh J, Eastell R. Discordant pattern of peripheral fractures in diabetes: a meta-analysis on the risk of wrist and ankle fractures. Osteoporos Int. 2019;30:135–143. doi: 10.1007/s00198-018-4717-0. [DOI] [PubMed] [Google Scholar]
- 46.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319–332. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang M, Kim H, Lea S, Oh S, Kim JS, Oh B. Effect of duration of diabetes on bone mineral density: a population study on East Asian males. BMC EndocrDisord. 2018;18:61. doi: 10.1186/s12902-018-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HS, Yoon JS, Park KJ, Lim JS, Hwang JS. The Relationship Between Bone Mineral Density and Type 2 Diabetes in Obese Children and Adolescents at the Time of Initial Diagnosis. HormMetab Res. 2019;51:42–46. doi: 10.1055/a-0755-2799. [DOI] [PubMed] [Google Scholar]
- 49.Asokan AG, Jaganathan J, Philip R, Soman RR, Sebastian ST, Pullishery F. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J Nat Sci Biol Med. 2017;8:94–98. doi: 10.4103/0976-9668.198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho-Pham LT, Nguyen TV. Association between trabecular bone score and type 2 diabetes: a quantitative update of evidence. Osteoporos Int. 2019;30:2079–2085. doi: 10.1007/s00198-019-05053-z. [DOI] [PubMed] [Google Scholar]
- 51.Choi YJ, Ock SY, Jin Y, Lee JS, Kim SH, Chung Y- Urinary Pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus. Osteoporos Int. 2018;29:907–915. doi: 10.1007/s00198-017-4359-7. [DOI] [PubMed] [Google Scholar]
- 52.Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:5045–5055. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28:313–324. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016;174:115–124. doi: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- 55.Heilmeier U, Cheng K, Pasco C, Parrish R, Nirody J, Patsch JM, Zhang CA, Joseph GB, Burghardt AJ, Schwartz AV, Link TM, Kazakia G. Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int. 2016;27:2791–2802. doi: 10.1007/s00198-016-3614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samakkarnthai P, Sfeir JG, Atkinson EJ, Achenbach SJ, Wennberg PW, Dyck PJ, Tweed AJ, Volkman TL, Amin S, Farr JN, Vella A, Drake MT, Khosla S. Determinants of Bone Material Strength and Cortical Porosity in Patients with Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Waard EAC, de Jong JJA, Koster A, Savelberg HHCM, van Geel TA, Houben AJHM, Schram MT, Dagnelie PC, van der Kallen CJ, Sep SJS, Stehouwer CDA, Schaper NC, Berendschot TTJM, Schouten JSAG, Geusens PPMM, van den Bergh JPW. The association between diabetes status, HbA1c, diabetes duration, microvascular disease, and bone quality of the distal radius and tibia as measured with high-resolution peripheral quantitative computed tomography-The Maastricht Study. Osteoporos Int. 2018;29:2725–2738. doi: 10.1007/s00198-018-4678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29:787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res. 2017;32:1062–1071. doi: 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- 60.Sundh D, Rudäng R, Zoulakis M, Nilsson AG, Darelid A, Lorentzon M. A High Amount of Local Adipose Tissue Is Associated With High Cortical Porosity and Low Bone Material Strength in Older Women. J Bone Miner Res. 2016;31:749–757. doi: 10.1002/jbmr.2747. [DOI] [PubMed] [Google Scholar]
- 61.Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P. Biochemical markers of bone turnover in diabetes patients--a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos Int. 2014;25:1697–1708. doi: 10.1007/s00198-014-2676-7. [DOI] [PubMed] [Google Scholar]
- 62.Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. MECHANISMS IN ENDOCRINOLOGY: Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–R157. doi: 10.1530/EJE-16-0652. [DOI] [PubMed] [Google Scholar]
- 63.Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 64.Heilmeier U, Carpenter DR, Patsch JM, Harnish R, Joseph GB, Burghardt AJ, Baum T, Schwartz AV, Lang TF, Link TM. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int. 2015;26:1283–1293. doi: 10.1007/s00198-014-2988-7. [DOI] [PubMed] [Google Scholar]
- 65.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 66.Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, Kousteni S, Rubin MR. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3240–3250. doi: 10.1210/jc.2012-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4:159–173. doi: 10.1016/S2213-8587(15)00283-1. [DOI] [PubMed] [Google Scholar]
- 68.de Waard EAC, Driessen JHM, de Jong JJA, van Geel TACM, Henry RMA, van Onzenoort HAW, Schram MT, Dagnelie PC, van der Kallen CJ, Sep SJS, Stehouwer CDA, Schaper NC, Koster A, Savelberg HHCM, Neef C, Geusens PPMM, de Vries F, van den Bergh JPW. The association between insulin use and volumetric bone mineral density, bone micro-architecture and bone strength of the distal radius in patients with type 2 diabetes - The Maastricht study. Bone. 2017;101:156–161. doi: 10.1016/j.bone.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Wallander M, Axelsson KF, Nilsson AG, Lundh D, Lorentzon M. Type 2 Diabetes and Risk of Hip Fractures and Non-Skeletal Fall Injuries in the Elderly: A Study From the Fractures and Fall Injuries in the Elderly Cohort (FRAILCO) J Bone Miner Res. 2017;32:449–460. doi: 10.1002/jbmr.3002. [DOI] [PubMed] [Google Scholar]
- 70.Losada-Grande E, Hawley S, Soldevila B, Martinez-Laguna D, Nogues X, Diez-Perez A, Puig-Domingo M, Mauricio D, Prieto-Alhambra D. Insulin use and Excess Fracture Risk in Patients with Type 2 Diabetes: A Propensity-Matched cohort analysis. Sci Rep. 2017;7:3781. doi: 10.1038/s41598-017-03748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pscherer S, Kostev K, Dippel FW, Rathmann W. Fracture risk in patients with type 2 diabetes under different antidiabetic treatment regimens: a retrospective database analysis in primary care. Diabetes MetabSyndrObes. 2016;9:17–23. doi: 10.2147/DMSO.S101370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tolosa MJ, Chuguransky SR, Sedlinsky C, Schurman L, McCarthy AD, Molinuevo MS, Cortizo AM. Insulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metformin. Diabetes Res Clin Pract. 2013;101:177–186. doi: 10.1016/j.diabres.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L, Jia CH, Wen ZH, Jin DD, Bai XC. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem. 2011;112:2902–2909. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- 74.Hidayat K, Du X, Wu MJ, Shi BM. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: Systematic review and meta-analysis of observational studies. Obes Rev. 2019;20:1494–1503. doi: 10.1111/obr.12885. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Z, Cao Y, Tao Y, E M, Tang J, Liu Y, Li F. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res Clin Pract. 2020;159:107990. doi: 10.1016/j.diabres.2019.107990. [DOI] [PubMed] [Google Scholar]
- 76.Choi HJ, Park C, Lee YK, Ha YC, Jang S, Shin CS. Risk of fractures and diabetes medications: a nationwide cohort study. Osteoporos Int. 2016;27:2709–2715. doi: 10.1007/s00198-016-3595-6. [DOI] [PubMed] [Google Scholar]
- 77.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 78.Zinman B, Haffner SM, Herman WH, Holman RR, Lachin JM, Kravitz BG, Paul G, Jones NP, Aftring RP, Viberti G, Kahn SE ADOPT Study Group. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:134–142. doi: 10.1210/jc.2009-0572. [DOI] [PubMed] [Google Scholar]
- 79.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Wang C, Xiao F, Qu X, Zhai Z, Hu G, Chen X, Zhang X. Sitagliptin, An Anti-diabetic Drug, Suppresses Estrogen Deficiency-Induced OsteoporosisIn Vivo and Inhibits RANKL-Induced Osteoclast Formation and Bone Resorption In Vitro. Front Pharmacol. 2017;8:407. doi: 10.3389/fphar.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu J, Zhu J, Hao Y, Guo C, Zhou Z. Dipeptidyl peptidase-4 inhibitors and fracture risk: an updated meta-analysis of randomized clinical trials. Sci Rep. 2016;6:29104. doi: 10.1038/srep29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nuche-Berenguer B, Moreno P, Esbrit P, Dapía S, Caeiro JR, Cancelas J, Haro-Mora JJ, Villanueva-Peñacarrillo ML. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009;84:453–461. doi: 10.1007/s00223-009-9220-3. [DOI] [PubMed] [Google Scholar]
- 83.Bunck MC, Eliasson B, Cornér A, Heine RJ, Shaginian RM, Taskinen MR, Yki-Järvinen H, Smith U, Diamant M. Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes ObesMetab. 2011;13:374–377. doi: 10.1111/j.1463-1326.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang YS, Weng WY, Xie BC, Meng Y, Hao YH, Liang YM, Zhou ZK. Glucagon-like peptide-1 receptor agonists and fracture risk: a network meta-analysis of randomized clinical trials. Osteoporos Int. 2018;29:2639–2644. doi: 10.1007/s00198-018-4649-8. [DOI] [PubMed] [Google Scholar]
- 85.Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J Clin Endocrinol Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng L, Li YY, Hu W, Bai F, Hao HR, Yu WN, Mao XM. Risk of bone fracture associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials. Diabetes Metab. 2019;45:436–445. doi: 10.1016/j.diabet.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 87.Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, Butsch WS, Finkelstein JS. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100:1452–1459. doi: 10.1210/jc.2014-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraser LA, Pritchard J, Ioannidis G, Giangegorio LM, Adachi JD, Papaioannou A, Leslie WD. Clinical risk factors for fracture in diabetes: a matched cohort analysis. J Clin Densitom. 2011;14:416–421. doi: 10.1016/j.jocd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Majumdar SR, Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, Kanis JA. Longer Duration of Diabetes Strongly Impacts Fracture Risk Assessment: The Manitoba BMD Cohort. J Clin Endocrinol Metab. 2016;101:4489–4496. doi: 10.1210/jc.2016-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee RH, Sloane R, Pieper C, Lyles KW, Adler RA, Van Houtven C, LaFleur J, Colón-Emeric C. Clinical Fractures Among Older Men With Diabetes Are Mediated by Diabetic Complications. J Clin Endocrinol Metab. 2018;103:281–287. doi: 10.1210/jc.2017-01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165:1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 92.Poiana C, Capatina C. Fracture Risk Assessment in Patients With Diabetes Mellitus. J Clin Densitom. 2017;20:432–443. doi: 10.1016/j.jocd.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 93.World Health Organization. 2008 Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK. [cited 30 December 2020]. Available from: http://www.shef.ac.uk/FRAX/index.htm .
- 94.Schacter GI, Leslie WD. DXA-Based Measurements in Diabetes: Can They Predict Fracture Risk? Calcif Tissue Int. 2017;100:150–164. doi: 10.1007/s00223-016-0191-x. [DOI] [PubMed] [Google Scholar]
- 95.Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26:673–679. doi: 10.1007/s00198-014-2927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, Boyd SK, McLean RR, Broe KE, Kiel DP, Bouxsein ML. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-pQCT Study. J Bone Miner Res. 2018;33:54–62. doi: 10.1002/jbmr.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furst JR, Bandeira LC, Fan WW, Agarwal S, Nishiyama KK, McMahon DJ, Dworakowski E, Jiang H, Silverberg SJ, Rubin MR. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:2502–2510. doi: 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leslie WD, Aubry-Rozier B, Lamy O, Hans D Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 99.Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, Shin CS. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100:475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 100.Martineau P, Leslie WD, Johansson H, Oden A, McCloskey EV, Hans D, Kanis JA. Clinical Utility of Using Lumbar Spine Trabecular Bone Score to Adjust Fracture Probability: The Manitoba BMD Cohort. J Bone Miner Res. 2017;32:1568–1574. doi: 10.1002/jbmr.3124. [DOI] [PubMed] [Google Scholar]
- 101.Neumann T, Lodes S, Kästner B, Lehmann T, Hans D, Lamy O, Müller UA, Wolf G, Sämann A. Trabecular bone score in type 1 diabetes--a cross-sectional study. Osteoporos Int. 2016;27:127–133. doi: 10.1007/s00198-015-3222-y. [DOI] [PubMed] [Google Scholar]
- 102.Jiang N, Xia W. Assessment of bone quality in patients with diabetes mellitus. OsteoporosInt. 2018;29:1721–1736. doi: 10.1007/s00198-018-4532-7. [DOI] [PubMed] [Google Scholar]
- 103.Andrade VFC, Chula DC, Sabbag FP, Cavalheiro DDDS, Bavia L, Ambrósio AR, da Costa CRV, Dos Reis LM, Borba VZC, Moreira CA. Bone Histomorphometry in Young Patients With Type 2 Diabetes is Affected by Disease Control and Chronic Complications. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgz070. [DOI] [PubMed] [Google Scholar]
- 104.Madsen JOB, Jørgensen NR, Pociot F, Johannesen J. Bone turnover markers in children and adolescents with type 1 diabetes-A systematic review. Pediatr Diabetes. 2019;20:510–522. doi: 10.1111/pedi.12853. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar PD, Choudhury AB. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 2013;17:1631–1635. [PubMed] [Google Scholar]
- 106.Yamamoto M, Yamaguchi T, Nawata K, Yamauchi M, Sugimoto T. Decreased PTH levels accompanied by low bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1277–1284. doi: 10.1210/jc.2011-2537. [DOI] [PubMed] [Google Scholar]
- 107.Reyes-García R, Rozas-Moreno P, López-Gallardo G, García-Martín A, Varsavsky M, Avilés-Perez MD, Muñoz-Torres M. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013;50:47–52. doi: 10.1007/s00592-011-0347-0. [DOI] [PubMed] [Google Scholar]
- 108.Jiajue R, Jiang Y, Wang O, Li M, Xing X, Cui L, Yin J, Xu L, Xia W. Suppressed bone turnover was associated with increased osteoporotic fracture risks in non-obese postmenopausal Chinese women with type 2 diabetes mellitus. Osteoporos Int. 2014;25:1999–2005. doi: 10.1007/s00198-014-2714-5. [DOI] [PubMed] [Google Scholar]
- 109.Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013;56:355–362. doi: 10.1016/j.bone.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 110.Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - A systematic review. Bone. 2016;82:69–78. doi: 10.1016/j.bone.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 111.Gordon CM, Leonard MB, Zemel BS International Society for Clinical Densitometry. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Slavcheva-Prodanova O, Konstantinova M, Tsakova A, Savova R, Archinkova M. Bone Health Index and bone turnover in pediatric patients with type 1 diabetes mellitus and poor metabolic control. Pediatr Diabetes. 2020;21:88–97. doi: 10.1111/pedi.12930. [DOI] [PubMed] [Google Scholar]
- 113.Zhukouskaya VV, Eller-Vainicher C, Shepelkevich AP, Dydyshko Y, Cairoli E, Chiodini I. Bone health in type 1 diabetes: focus on evaluation and treatment in clinical practice. J Endocrinol Invest. 2015;38:941–950. doi: 10.1007/s40618-015-0284-9. [DOI] [PubMed] [Google Scholar]
- 114.Picke AK, Sylow L, Møller LLV, Kjøbsted R, Schmidt FN, Steejn MW, Salbach-Hirsch J, Hofbauer C, Blüher M, Saalbach A, Busse B, Rauner M, Hofbauer LC. Differential effects of high-fat diet and exercise training on bone and energy metabolism. Bone. 2018;116:120–134. doi: 10.1016/j.bone.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 115.Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V. Sarcopenic Obesity and Its Temporal Associations With Changes in Bone Mineral Density, Incident Falls, and Fractures in Older Men: The Concord Health and Ageing in Men Project. J Bone Miner Res. 2017;32:575–583. doi: 10.1002/jbmr.3016. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, Josse RG, Schnall AM, Simmons DL, Hue TF, Palermo L, Hamilton BP, Green JB, Atkinson HH, O'Connor PJ, Force RW, Bauer DC. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35:1525–1531. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vavanikunnel J, Charlier S, Becker C, Schneider C, Jick SS, Meier CR, Meier C. Association Between Glycemic Control and Risk of Fracture in Diabetic Patients: A Nested Case-Control Study. J Clin Endocrinol Metab. 2019;104:1645–1654. doi: 10.1210/jc.2018-01879. [DOI] [PubMed] [Google Scholar]
- 118.Chen SC, Shepherd S, McMillan M, McNeilly J, Foster J, Wong SC, Robertson KJ, Ahmed SF. Skeletal Fragility and Its Clinical Determinants in Children With Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:3585–3594. doi: 10.1210/jc.2019-00084. [DOI] [PubMed] [Google Scholar]
- 119.Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hofman A, van Leeuwen JP, Witteman JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li CI, Liu CS, Lin WY, Meng NH, Chen CC, Yang SY, Chen HJ, Lin CC, Li TC. Glycated Hemoglobin Level and Risk of Hip Fracture in Older People with Type 2 Diabetes: A Competing Risk Analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res. 2015;30:1338–1346. doi: 10.1002/jbmr.2462. [DOI] [PubMed] [Google Scholar]
- 121.Ntouva A, Toulis KA, Keerthy D, Adderley NJ, Hanif W, Thayakaran R, Gokhale K, Thomas GN, Khunti K, Tahrani AA, Nirantharakumar K. Hypoglycaemia is associated with increased risk of fractures in patients with type 2 diabetes mellitus: a cohort study. Eur J Endocrinol. 2019;180:51–58. doi: 10.1530/EJE-18-0458. [DOI] [PubMed] [Google Scholar]
- 122.de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 123.Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R, El-Hajj Fuleihan G, Josse R, Kendler DL, Kraenzlin M, Suzuki A, Pierroz DD, Schwartz AV, Leslie WD Bone and Diabetes Working Group of IOF. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int. 2018;29:2585–2596. doi: 10.1007/s00198-018-4650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leslie WD, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D. Comparison of Methods for Improving Fracture Risk Assessment in Diabetes: The Manitoba BMD Registry. J Bone Miner Res. 2018;33:1923–1930. doi: 10.1002/jbmr.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz AV. Efficacy of Osteoporosis Therapies in Diabetic Patients. Calcif Tissue Int. 2017;100:165–173. doi: 10.1007/s00223-016-0177-8. [DOI] [PubMed] [Google Scholar]
- 126.Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL fracture intervention trial. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27:1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 127.Inoue D, Muraoka R, Okazaki R, Nishizawa Y, Sugimoto T. Efficacy and Safety of Risedronate in Osteoporosis Subjects with Comorbid Diabetes, Hypertension, and/or Dyslipidemia: A Post Hoc Analysis of Phase III Trials Conducted in Japan. Calcif Tissue Int. 2016;98:114–122. doi: 10.1007/s00223-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88:209–214. doi: 10.1007/s00223-010-9450-4. [DOI] [PubMed] [Google Scholar]
- 129.Abrahamsen B, Rubin KH, Eiken PA, Eastell R. Characteristics of patients who suffer major osteoporotic fractures despite adhering to alendronate treatment: a National Prescription registry study. Osteoporos Int. 2013;24:321–328. doi: 10.1007/s00198-012-2184-6. [DOI] [PubMed] [Google Scholar]
- 130.Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, Zhou C, Pavo I. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res. 2004;19:764–772. doi: 10.1359/JBMR.040211. [DOI] [PubMed] [Google Scholar]
- 131.Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, Zhao Q, Agnusdei D, Cauley JA. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23:112–120. doi: 10.1359/jbmr.070904. [DOI] [PubMed] [Google Scholar]
- 132.Anagnostis P, Paschou SA, Gkekas NN, Artzouchaltzi AM, Christou K, Stogiannou D, Vryonidou A, Potoupnis M, Goulis DG. Efficacy of anti-osteoporotic medications in patients with type 1 and 2 diabetes mellitus: a systematic review. Endocrine. 2018;60:373–383. doi: 10.1007/s12020-018-1548-x. [DOI] [PubMed] [Google Scholar]
- 133.Ferrari S, Eastell R, Napoli N, Schwartz A, Hofbauer LC, Chines A, Wang A, Pannacciulli N, Cummings SR. Denosumab in postmenopausal women with osteoporosis and diabetes: Subgroup analysis of FREEDOM and FREEDOM extension. Bone. 2020;134:115268. doi: 10.1016/j.bone.2020.115268. [DOI] [PubMed] [Google Scholar]
- 134.Schwartz AV, Pavo I, Alam J, Disch DP, Schuster D, Harris JM, Krege JH. Teriparatide in patients with osteoporosis and type 2 diabetes. Bone. 2016;91:152–158. doi: 10.1016/j.bone.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 135.Liu JM, Zhu DL, Mu YM, Xia WB Chinese Society of Osteoporosis and Bone Mineral Research; the Chinese Society of Endocrinology, Chinese Diabetes Society, Chinese Medical Association Chinese Endocrinologist Association; Chinese Medical Doctor Association. Management of fracture risk in patients with diabetes-Chinese Expert Consensus. J Diabetes. 2019;11:906–919. doi: 10.1111/1753-0407.12962. [DOI] [PubMed] [Google Scholar]
- 136.Lo JC, Huang SY, Lee GA, Khandelwal S, Provus J, Ettinger B, Gonzalez JR, Hui RL, Grimsrud CD. Clinical correlates of atypical femoral fracture. Bone. 2012;51:181–184. doi: 10.1016/j.bone.2012.02.632. [DOI] [PubMed] [Google Scholar]
- 137.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schwartz AV, Schafer AL, Grey A, Vittinghoff E, Palermo L, Lui LY, Wallace RB, Cummings SR, Black DM, Bauer DC, Reid IR. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J Bone Miner Res. 2013;28:1348–1354. doi: 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- 139.Chan DC, Yang RS, Ho CH, Tsai YS, Wang JJ, Tsai KT. The use of alendronate is associated with a decreased incidence of type 2 diabetes mellitus--a population-based cohort study in Taiwan. PLoS One. 2015;10:e0123279. doi: 10.1371/journal.pone.0123279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vestergaard P. Risk of newly diagnosed type 2 diabetes is reduced in users of alendronate. Calcif Tissue Int. 2011;89:265–270. doi: 10.1007/s00223-011-9515-z. [DOI] [PubMed] [Google Scholar]
- 141.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, Moschen AR, Muscogiuri G, Sorice GP, Kireva T, Summerer M, Wirtz S, Luther J, Mielenz D, Billmeier U, Egger G, Mayr A, Oberhollenzer F, Kronenberg F, Orthofer M, Penninger JM, Meigs JB, Bonora E, Tilg H, Willeit J, Schett G. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 142.Barrett-Connor E, Ensrud KE, Harper K, Mason TM, Sashegyi A, Krueger KA, Anderson PW. Post hoc analysis of data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial on the effects of three years of raloxifene treatment on glycemic control and cardiovascular disease risk factors in women with and without type 2 diabetes. Clin Ther. 2003;25:919–930. doi: 10.1016/s0149-2918(03)80114-5. [DOI] [PubMed] [Google Scholar]
- 143.Kimura S, Sasase T, Ohta T, Sato E, Matsushita M. Parathyroid hormone (1-34) improves bone mineral density and glucose metabolism in Spontaneously Diabetic Torii-Lepr(fa) rats. J Vet Med Sci. 2012;74:103–105. doi: 10.1292/jvms.11-0328. [DOI] [PubMed] [Google Scholar]
- 144.Anastasilakis AD, Efstathiadou Z, Plevraki E, Koukoulis GN, Slavakis A, Kita M, Avramidis A. Effect of exogenous intermittent recombinant human PTH 1-34 administration and chronic endogenous parathyroid hormone excess on glucose homeostasis and insulin sensitivity. HormMetab Res. 2008;40:702–707. doi: 10.1055/s-2008-1078729. [DOI] [PubMed] [Google Scholar]
- 145.Celer O, Akalın A, Oztunali C. Effect of teriparatide treatment on endothelial function, glucose metabolism and inflammation markers in patients with postmenopausal osteoporosis. Clin Endocrinol (Oxf) 2016;85:556–560. doi: 10.1111/cen.13139. [DOI] [PubMed] [Google Scholar]
- 146.Paschou SA, Dede AD, Anagnostis PG, Vryonidou A, Morganstein D, Goulis DG. Type 2 Diabetes and Osteoporosis: A Guide to Optimal Management. J Clin Endocrinol Metab. 2017;102:3621–3634. doi: 10.1210/jc.2017-00042. [DOI] [PubMed] [Google Scholar]
- 147.Fowlkes JL, Bunn R C, Thrailkill KM. Contributions of the Insulin/Insulin-Like Growth Factor-1 Axis to Diabetic Osteopathy. J Diabetes Metab. 2011;1 doi: 10.4172/2155-6156.S1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y, Cao Y, Zhang S, Zhang W, Zhang B, Tang Q, Li Z, Wu J. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric. 2018;21:189–195. doi: 10.1080/13697137.2018.1433655. [DOI] [PubMed] [Google Scholar]