Abstract
BACKGROUND
Lactulose is approved for the symptomatic treatment of constipation, a gastrointestinal (GI) complication common in individuals with diabetes. Lactulose products contain carbohydrate impurities (e.g., lactose, fructose, galactose), which occur during the lactulose manufacturing process. These impurities may affect the blood glucose levels of individuals with type 2 diabetes mellitus (T2DM) using lactulose for the treatment of mild constipation. A previous study in healthy subjects revealed no increase in blood glucose levels after oral lactulose intake. However, it is still unclear whether the intake of lactulose increases blood glucose levels in individuals with diabetes.
AIM
To evaluate the blood glucose profile after oral lactulose intake in mildly constipated, non-insulin-dependent subjects with T2DM in an outpatient setting.
METHODS
This prospective, double-blind, randomized, controlled, single-center trial was conducted at the Clinical Research Center at the Medical University of Graz, Austria, in 24 adult Caucasian mildly constipated, non-insulin-dependent subjects with T2DM. Eligible subjects were randomized and assigned to one of six treatment sequences, each consisting of four treatments stratified by sex using an incomplete block design. Subjects received a single dose of 20 g or 30 g lactulose (crystal and liquid formulation), water as negative control or 30 g glucose as positive control. Capillary blood glucose concentrations were measured over a period of 180 min post dose. The primary endpoint was the baseline-corrected area under the curve of blood glucose concentrations over the complete assessment period [AUCbaseline_c (0-180 min)]. Quantitative comparisons were performed for both lactulose doses and formulations vs water for the equal lactulose dose vs glucose, as well as for liquid lactulose vs crystal lactulose. Safety parameters included GI tolerability, which was assessed at 180 min and 24 h post dose, and adverse events occurring up to 24 h post dose.
RESULTS
In 24 randomized and analyzed subjects blood glucose concentration-time curves after intake of 20 g and 30 g lactulose were almost identical to those after water intake for both lactulose formulations despite the different amounts of carbohydrate impurities (≤ 3.0% for crystals and approx. 30% for liquid). The primary endpoint [AUCbaseline_c (0-180 min)] was not significantly different between lactulose and water regardless of lactulose dose and formulation. Also with regard to all secondary endpoints lactulose formulations showed comparable results to water with one exception concerning maximum glucose level. A minor increase in maximum blood glucose was observed after the 30 g dose, liquid lactulose, in comparison to water with a mean treatment difference of 0.63 mmol/L (95% confidence intervals: 0.19, 1.07). Intake of 30 g glucose significantly increased all blood glucose endpoints vs 30 g liquid and crystal lactulose, respectively (all P < 0.0001). No differences in blood glucose response were observed between the different lactulose formulations. As expected, lactulose increased the number of bowel movements and was generally well tolerated. Subjects experienced only mild to moderate GI symptoms due to the laxative action of lactulose.
CONCLUSION
Blood glucose AUCbaseline_c (0-180 min) levels in mildly constipated, non-insulin dependent subjects with T2DM are not affected by the carbohydrate impurities contained in 20 g and 30 g crystal or liquid lactulose formulations.
Keywords: Lactulose, Constipation, Blood glucose, Type 2 diabetes mellitus, Laxative, Sugar substitute
Core Tip: Individuals with diabetes are at risk of developing constipation, which can be symptomatically treated with lactulose. The question arose whether carbohydrate impurities in crystal and liquid lactulose formulations would increase blood glucose levels in individuals with diabetes. This study demonstrates that, at the recommended maintenance dosage of 20 g and at a higher dosage of 30 g lactulose, the blood glucose baseline-corrected area under the curve from 0 to 180 min levels in mildly constipated, non-insulin dependent subjects with type 2 diabetes mellitus are not affected.
INTRODUCTION
Disorders of the gastrointestinal (GI) tract are common in individuals with diabetes mellitus[1,2]. It is well known that persistent hyperglycemia negatively impacts enteric motor and sensory functions[3-6]. Diabetes-related dysmotility can be attributed to a loss of enteric neurons in the colon due to increased oxidative stress and apoptosis[7], leading to a variety of GI symptoms, including constipation, that severely reduce quality of life in individuals with diabetes[8].
Evidence-based treatment options for managing diabetes-related chronic constipation include lifestyle changes and furthermore, the use of laxatives such as the stimulants bisacodyl and senna glycoside and the osmotic agents polyethylene glycol (PEG), lactitol, and lactulose[9]. Lactulose is approved as a drug for the symptomatic treatment of constipation at a dose of 10 to 30 g and portal systemic encephalopathy at doses up to 100 g[10] and restores bowel movements by facilitating intestinal motility and secretion[11].
Lactulose is a disaccharide composed of galactose and fructose. It is neither absorbed in the small intestine nor digested by enzymes of the mammalian digestive tract. As an osmotic laxative, lactulose creates an osmotic gradient that increases the retention of water in the stool and subsequently enhances stool frequency, volume, and weight[12]. In addition, lactulose is completely metabolized by saccharolytic intestinal bacteria in the colon, thereby producing metabolites, e.g., lactic acid, formic acid, and acetic acid, with osmotic abilities and peristalsis-stimulating effects[9,13]. Lactulose is known to enhance colonic transit time[14,15], which is reflected in the European Food Safety Authority-approved health claim that “lactulose contributes to an acceleration of intestinal transit”[16]. Furthermore, lactulose stimulates the growth or activity of a number of colonic bacteria referred to as bifidogenic effects[17] and is used as a prebiotic functional food ingredient.
Lactulose is produced by isomerization of the natural milk sugar lactose (galactose-glucose). During this process, carbohydrate impurities may arise and traces of the lactose may still be present in the final solution. Partial hydrolysis of lactulose can result in the formation of fructose and galactose. Tagatose can be formed by isomerization of galactose and epilactose by C2 epimerization of lactose. 3-Deoxyglyceropentuloses A and B may arise as by-products of the reaction. All these substances are listed in the European Pharmacopeia under ‘related substances’ and are denoted as “impurities” in the following)[18]. The amount and pattern of these impurities vary depending on the manufacturing process conditions. They can account for up to 3% carbohydrates in crystal lactulose and approx. 30% carbohydrates in liquid lactulose. After lactulose intake, these impurities may be absorbed in the digestive tract and thereby increase blood glucose levels. Theoretically, this may impact glycemic control in individuals with type 2 diabetes mellitus (T2DM). A previous study in healthy subjects showed no substantial increase in blood glucose after oral intake of 10 g and 20 g lactulose (crystals and liquid)[19]. These findings need to be confirmed in subjects with T2DM.
The aim of the present study was to investigate the potential impact of a single dose of 20 g or 30 g lactulose in currently marketed formulations (crystals and liquid) on blood glucose responses in mildly constipated, non-insulin-dependent subjects with T2DM in an outpatient setting.
MATERIALS AND METHODS
Study design
This was a prospective, double-blind, randomized, controlled, single-center trial with a four-period crossover and incomplete block design in subjects with mild functional constipation and T2DM. The study was conducted in accordance with the Declaration of Helsinki, the principles of Good Clinical Practice and Austrian drug law and was approved by the Independent Ethics Committee of the Medical University of Graz, Austria. All subjects gave written informed consent before any study-related activities were started. The study was registered in the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT No. 2018-002359-14).
The study was conducted at the Clinical Research Center at the Medical University of Graz, Austria, and consisted of a screening visit and four individual study visits separated by a washout period of 7 d (allowed range 4 to 14 d) to avoid carryover effects.
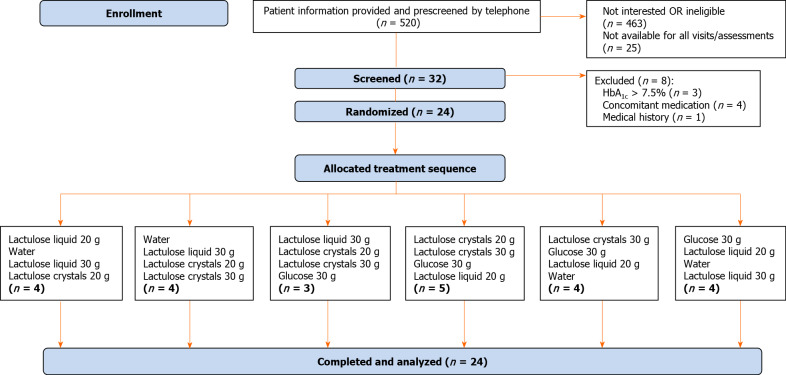
Randomization was performed by M.A.R.C.O. GmbH and Co. KG, Düsseldorf, Germany, in three blocks of six treatment sequences separately for each group stratified by sex, assigning random numbers 001 to 018 for female subjects and 021 to 038 for male subjects to treatment sequences. The randomization schedule was generated by a SAS® computer program based on the SAS® RANUNI function, which returns a random value from a uniform distribution. Subjects were assigned to random numbers in chronological order after enrollment to receive one of the six treatment sequences (Figure 1).
Figure 1.
Flow chart of trial subjects. The main reasons for pre-screening failure included: Not interested (n = 85), missing signs of functional constipation (n = 220), insufficient blood glucose control (n = 24), not allowed concomitant medication, e.g., insulin (n = 58), body mass index ≥ 35 kg/m2 (n = 31) gastrointestinal or other relevant comorbidity (cancer, lactose intolerance etc.) (n = 39), smoking habit (n = 7). HbA1c: Glycosylated hemoglobin.
On the evening before each study visit, subjects were advised to eat a standardized dinner consisting of farmhouse bread with cream cheese and cucumber. Subjects were not allowed to consume food or drink other than water for at least 10 h before study product administration. On the morning of the study visits, subjects were instructed to drink one to two glasses of water (minimum 200 mL total) upon waking. Consumption of alcohol and intensive exercise were not allowed within 24 h before each study visit. Furthermore, the use of laxatives within 48 h before each study visit was prohibited. At each study visit, the administration of any antidiabetic agents was postponed to the end of the 180-min observation period to avoid interference with the blood glucose profile.
Study population
Eligible subjects were Caucasian men or women with non-insulin-dependent T2DM under stable antidiabetic treatment 3 mo prior to screening, treated with diet and oral antidiabetic agents (e.g., metformin, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 inhibitors, sulfonylurea, sodium-dependent glucose transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists), and aged ≥ 18 and ≤ 75 years, who had glycosylated hemoglobin (HbA1c) ≤ 7.5% and mild functional constipation according to the modified Rome IV criteria (approximately three to five bowel movements per week, of which one to two usually caused discomfort) during the previous 3 mo with symptom onset at least 6 mo before study start.
The main exclusion criteria were fasting capillary blood glucose levels < 4.4 mmol/L or > 10 mmol/L; body mass index (BMI) < 18.5 or ≥ 35 kg/m2; change in body weight ≥ 10% within the last 3 mo; smoking habit; severe hepatic, renal, or cardiac disease; acute inflammatory bowel disease; GI obstruction or subocclusive syndrome; GI perforation or risk of GI perforation; abdominal pain of undetermined cause; major hospitalization or surgical event within the previous 3 mo; acute GI diseases including diarrhea and/or vomiting within the previous 2 wk; presence of disease or administration of medications/supplements other than antidiabetic treatment influencing digestion and absorption of carbohydrates or bowel habits; hereditary galactose or fructose intolerance; lactase deficiency or glucose-galactose malabsorption; intake of pre- or probiotics or medications known to affect glucose tolerance (e.g., steroids, protease inhibitors, antipsychotics); and chronic administration of substances affecting blood coagulation which, in the investigator’s opinion, would impact subject safety.
Sample size
For sample size estimation, a minimum blood glucose concentration difference of 0.6 mmol/L (corresponding to 30% of the theoretical maximum increase in blood glucose level after administration) between lactulose and water was considered. An effect size of 1 was defined for this trial. The power for detecting effect sizes of at least 1 was set to 90% at significance level α = 2.5% (one-sided). Based on this approach, 15 evaluable subjects would have been required for a complete crossover design assuming a correlation of 0.4. To obtain a balanced design, 16 subjects would have to be randomized. However, due to the incomplete block design with four periods for six treatments, a loss of efficiency of one-third was assumed. Therefore, 24 subjects with mild functional constipation and T2DM were planned to be randomized in the study.
Study products
Lactulose crystals (Laevolac® 10 g powder for oral solution) and lactulose liquid (Laevolac® 10 g/15 mL oral solution) were produced by Fresenius Kabi Austria GmbH, Linz, Austria. The maximum carbohydrate impurities of both lactulose formulations according to the European Pharmacopoeia monographs were previously published[19]. The total impurities of the study products were within the threshold value of ≤ 3.0% in the crystal and approx. 30% in the liquid lactulose formulation. The effect on the blood glucose response was assessed at doses of 20 g and 30 g lactulose for both formulations and compared to still (non sparkling) water as a negative control and 30 g glucose (33 g glucose monohydrate powder, Roquette Frères, Lestrem, France) as a positive control. The study products were prepared and blinded on site by authorized unblinded study staff according to the randomization plan. Subjects as well as the investigator were blinded to the dosage of study products and the lactulose formulation. Lactulose and glucose were dissolved in 250 mL of still water and were provided as a single oral dose under the supervision of the study staff. The single dose had to be ingested within 5 min.
Data collection
Blood glucose concentration was measured in capillary whole blood obtained by finger stick according to ISO 26642 and analyzed using a HemoCue Glucose 201 RT Analyzer (HemoCue AB, Ängelholm, Sweden). Glucose was determined photometrically using a modified glucose dehydrogenase method. Blood glucose concentrations were assessed over a period of 180 min at defined time points (0, 15, 30, 45, 60, 90, 120, 150, and 180 min post-dose).
Data were transferred on a paper case report form to M.A.R.C.O. GmbH and Co. KG, Düsseldorf, Germany, for data management and statistical analysis.
Data analysis and statistics
For the primary endpoint, capillary blood glucose levels as baseline-corrected area under the curve from 0 to 180 min [AUCbaseline_c (0-180 min)] were determined, and quantitative comparisons after oral intake of lactulose products with water or glucose were performed. Secondary endpoints related to blood glucose concentrations were maximum blood glucose concentration (Cmax), time to reach maximum concentration (Tmax), maximum blood glucose concentration minus baseline value (Max_increase), AUC(0-180 min), and incremental AUC(0-180 min) [iAUC(0-180 min)] (i.e., area above the baseline blood glucose concentration after oral intake of lactulose formulations or control products). An increase in blood glucose concentration ≥ 2.2 mmol/L is considered clinically relevant and is usually caused by an additional administration of 10 g carbohydrates, which might contribute to an increase in HbA1c by 0.1% in the long term[20,21]. The following comparisons were made for both primary and secondary endpoints: (1) Both lactulose doses and formulations (20 g/30 g crystals/liquid) vs water as a negative control; (2) 30 g lactulose (crystals/liquid) vs 30 g glucose as a positive control; and (3) Crystal lactulose vs liquid lactulose (for each dose 20 g/30 g). 95% two-sided confidence intervals (CIs) were calculated for all comparisons, which correspond to exploratory two-sided tests at 5% significance levels. In particular, if CIs did not include the threshold of clinical relevance, it could be concluded that lactulose has no clinically relevant impact on blood glucose levels.
GI tolerability was assessed at each study visit during the initial 180-min period and 24 h post dose using a 4-point Likert scale (none, mild, moderate or severe) to describe symptoms. The number of bowel movements was counted at each study visit until 24 h post dose for the different treatment groups. Consistency of stool was graded based on the Bristol Stool Form Scale (BSFS)[22] (type 1 = separate hard lumps, like nuts (hard to pass); type 2 = sausage-shaped but lumpy; type 3 = like a sausage with cracks on its surface; type 4 = like a sausage or snake, smooth and soft; type 5 = soft blobs with clear-cut edges, passed easily; type 6 = fluffy pieces with ragged edges, a mushy stool; type 7 = liquid, no solid pieces) with types 1 and 2 reflecting severe and mild constipation, types 3 to 5 showing normal stool, and types 6 and 7 reflecting mild and severe diarrhea.
Adverse events (AEs) were recorded in diaries over the entire study period after written informed consent was obtained. AEs were coded according to the latest Medical Dictionary for Regulatory Activities (version 22.0). The Common Terminology Criteria for Adverse Events (version 5.0) was used to assess the intensity of AEs. At each study visit, AEs were reviewed by the investigator and recorded in the case report form.
All parameters were listed and summarized with descriptive statistics (n = number of no-missing values, arithmetic mean, standard deviation, minimum, median, maximum, first and third quartiles) or frequency tables by treatment, as appropriate.
The primary endpoint, untransformed AUCbaseline_c (0-180 min), was analyzed using a mixed analysis-of-variance model with sex (as between-subject fixed effect), treatment (six levels), period (four levels) and baseline blood glucose level to adjust for potential inter- and intraindividual differences in baseline blood glucose according to the study period as fixed effect and subject as random effect. No further covariates were considered. Least square (LS) means including 95%CIs were calculated for all treatments and treatment differences. Secondary endpoints were evaluated analogously to the primary endpoint. Raw data listings, summary tables and inferential analyses were carried out using SAS® software (version 9.3).
Data are presented for the intention-to-treat population, which was identical to the per-protocol population in this study. Exploration of possible carryover effects was not obligatory due to the 7-d (allowed range 4 to 14 d) washout period.
RESULTS
A total of 32 subjects were screened, and 24 subjects were enrolled from November 2018 to March 2019. Demographic and baseline data of randomized subjects are summarized in Table 1. Overall, 16 subjects (66.7%) were male, and eight subjects (33.3%) were female. The treatment sequence groups were comparable to the subject’s baseline age, height, weight, and BMI. The mean baseline values of fasting blood glucose ranged from 6.9 mmol/L to 7.3 mmol/L. Before randomization, 75% of the subjects reported bowel symptoms, and 67% had constipation for more than one year. The average number of bowel movements per week was three to five movements, and almost all subjects (n = 22) had an average of one to two bowel movements with discomfort per week prior to randomization. Only two patients had three to five bowel movements with discomfort per week. Only two subjects used laxatives to encourage defecation before randomization. However, these subjects abstained from using laxatives two days before and up to 24 h after the respective study visits.
Table 1.
Demographic and baseline data of subjects (n = 24)
|
Variable
|
mean
|
SD
|
Min
|
Median
|
Max
|
| Age (yr) | 62.2 | 7.61 | 45 | 62.5 | 73 |
| BMI (kg/m2) | 30.0 | 3.0 | 23.3 | 30.1 | 34.7 |
| Systolic BP (mmHg) | 142.8 | 19.1 | 114.0 | 141.5 | 196.0 |
| Diastolic BP (mmHg) | 88.1 | 9.1 | 73.0 | 90.0 | 105.0 |
| HbA1c (%) | 6.6 | 2.6 | 5.5 | 6.6 | 7.5 |
BMI: Body mass index; BP: Blood pressure; HbA1c: Glycosylated hemoglobin; Max: Maximum; Min: Minimum, SD: Standard deviation.
Four sequence groups achieved the anticipated size of four subjects, whereas one more subject was allocated to one sequence group (n = 5) and one less to another sequence group (n = 3) (Figure 1). This slight imbalance did not constitute a protocol deviation. All 24 subjects were treated according to the randomization schedule and successfully completed the study without any major protocol deviations.
Lactulose vs negative control (water)
The primary endpoint AUCbaseline_c (0-180 min) did not significantly differ between lactulose and water intake, regardless of lactulose dose and formulation. The estimated LS means for AUCbaseline_c (0-180 min) of all lactulose doses and formulations ranged from -30.70 to -54.40 min/mmol/L. The mean AUCbaseline_c (0-180 min) after water intake was -53.21 min/mmol/L (95%CI: -99.14, -7.28). This implies a net decrease in blood glucose concentration over time after lactulose intake compared to the respective baseline blood glucose level. The average net decrease over the assessment period, calculated as AUCbaseline_c (0-180 min)/180 min, was approx. -0.3 mmol/L.
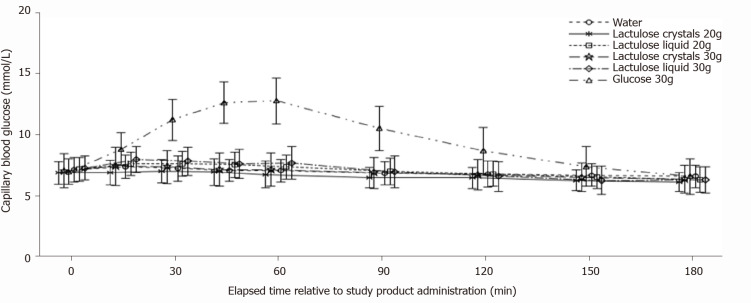
Mean blood glucose concentration-time curves after intake of 20 g (Table 2) or 30 g (Table 3) crystal lactulose did not differ from the mean blood glucose concentration-time curve after intake of water (Figure 2). The mean blood glucose concentration-time curve for 20 g liquid lactulose was also comparable to that of water (Table 4). The maximum blood glucose concentrations appeared slightly higher after intake of the 30 g liquid lactulose compared to water, showing mean maximum increases of 1.00 mmol/L and 0.37 mmol/L after intake of 30 g liquid lactulose and water, respectively (Table 5). Thus, the mean maximum increase after 30 g liquid lactulose was 0.63 mmol/L (95%CI: 0.19, 1.07) (P = 0.0059) higher than that after water. The median (range) Tmax was 30 min (0 to 60 min) after 30 g liquid lactulose intake and 22.5 min (0 to 150 min) after the intake of water.
Table 2.
Blood glucose endpoints: 20 g crystal lactulose vs water
|
20 g crystal lactulose
|
Water
|
Treatment
|
P
value
|
|||
|
Variable
|
n
|
LS mean (95%CI)
|
n
|
LS mean (95%CI)
|
Difference (95%CI)
|
|
| AUCbaseline_c (0-180 min) (min/mmol/L) | 16 | -54.40 (-100.23, -8.57) | 16 | -53.21 (-99.14, -7.28) | -1.20 (-60.78, 58.39) | 0.9682 |
| AUC(0-180 min) (min/mmol/L) | 16 | 1218.33 (1172.50, 1264.16) | 16 | 1219.53 (1173.60, 1265.46) | -1.20 (-60.78, 58.39) | 0.9682 |
| iAUC(0-180 min) (min/mmol/L) | 16 | 17.00 (-14.43, 48.43) | 16 | 18.44 (-13.04, 49.92) | -1.44 (-45.16, 42.29) | 0.9478 |
| Cmax (mmol/L) | 16 | 7.50 (7.17, 7.82) | 16 | 7.44 (7.11, 7.77) | 0.06 (-0.38, 0.50) | 0.7999 |
| Max_increase (mmol/L) | 16 | 0.43 (0.10, 0.75) | 16 | 0.37 (0.04, 0.70) | 0.06 (-0.38, 0.50) | 0.7999 |
AUCbaseline_c (0-180 min): Baseline-corrected area under the curve from 0 to 180 min; AUC(0-180 min): Area under the curve from 0 to 180 min; iAUC(0-180 min): Incremental AUC(0-180 min), i.e., area above baseline levels of blood glucose concentration; Cmax: Maximum blood glucose concentration; Max_increase: Maximum blood glucose concentration minus baseline value; LS mean: Adjusted least square mean obtained from analysis of covariance model.
Table 3.
Blood glucose endpoints: 30 g crystal lactulose vs water
|
30 g crystal lactulose
|
Water
|
Treatment
|
P
value
|
|||
|
Variable
|
n
|
LS mean (95%CI)
|
n
|
LS mean (95%CI)
|
Difference (95%CI)
|
|
| AUCbaseline_c (0-180 min) (min/mmol/L) | 16 | -30.70 (-76.75, 15.35) | 16 | -53.21 (-99.14, -7.28) | 22.51 (-37.05, 82.06) | 0.4529 |
| AUC(0-180 min) (min/mmol/L) | 16 | 1242.03 (1195.98, 1288.08) | 16 | 1219.53 (1173.60, 1265.46) | 22.51 (-37.05, 82.06) | 0.4529 |
| iAUC(0-180 min) (min/mmol/L) | 16 | 27.65 (-3.91, 59.21) | 16 | 18.44 (-13.04, 49.92) | 9.21 (-34.39, 52.81) | 0.6744 |
| Cmax (mmol/L) | 16 | 7.77 (7.44, 8.09) | 16 | 7.44 (7.11, 7.77) | 0.33 (-0.11, 0.76) | 0.1426 |
| Max_increase (mmol/L) | 16 | 0.70 (0.37, 1.02) | 16 | 0.37 (0.04, 0.70) | 0.33 (-0.11, 0.76) | 0.1426 |
AUCbaseline_c (0-180 min): Baseline-corrected area under the curve from 0 to 180 min; AUC(0-180 min): Area under the curve from 0 to 180 min; iAUC(0-180 min): Incremental AUC(0-180 min), i.e., area above baseline levels of blood glucose concentration; Cmax: Maximum blood glucose concentration; Max_increase: Maximum blood glucose concentration minus baseline value; LS mean: Adjusted least square mean obtained from analysis of covariance model.
Figure 2.
Blood glucose concentration-time curves. Each line represents mean ± SD. n = 16 each for water, 20 g crystal lactulose, 30 g crystal lactulose, and 30 g glucose, n = 17 for 20 g liquid lactulose, n = 15 for 30 g liquid lactulose.
Table 4.
Blood glucose endpoints: 20 g liquid lactulose vs water
|
20 g liquid lactulose
|
Water
|
Treatment
|
P
value
|
|||
|
Variable
|
n
|
LS mean (95%CI)
|
n
|
LS mean (95%CI)
|
Difference (95%CI)
|
|
| AUCbaseline_c (0-180 min) (min/mmol/L) | 17 | -30.72 (-75.26, 13.83) | 16 | -53.21 (-99.14, -7.28) | 22.49 (-35.18, 80.16) | 0.4387 |
| AUC(0-180 min) (min/mmol/L) | 17 | 1242.02 (1197.47, 1286.56) | 16 | 1219.53 (1173.60, 1265.46) | 22.49 (-35.18, 80.16) | 0.4387 |
| iAUC(0-180 min) (min/mmol/L) | 17 | 34.42 (3.98, 64.86) | 16 | 18.44 (-13.04, 49.92) | 15.98 (26.78, 58.75) | 0.4578 |
| Cmax (mmol/L) | 17 | 7.747 (7.43, 8.06) | 16 | 7.44 (7.11, 7.77) | 0.31 (-0.12, 0.73) | 0.1560 |
| Max_increase (mmol/L) | 17 | 0.68 (0.36, 0.99) | 16 | 0.37 (0.043, 0.70) | 0.31 (-0.12, 0.73) | 0.1560 |
AUCbaseline_c (0-180 min): Baseline-corrected area under the curve from 0 to 180 min; AUC(0-180 min): Area under the curve from 0 to 180 min; iAUC(0-180 min): Incremental AUC(0-180 min), i.e., area above baseline levels of blood glucose concentration; Cmax: Maximum blood glucose concentration; Max_increase: Maximum blood glucose concentration minus baseline value; LS mean: Adjusted least square mean obtained from analysis of covariance model
Table 5.
Blood glucose endpoints: 30 g liquid lactulose vs water
|
30 g liquid lactulose
|
Water
|
Treatment
|
P
value
|
|||
|
Variable
|
n
|
LS mean (95%CI)
|
n
|
LS mean (95%CI)
|
Difference (95%CI)
|
|
| AUCbaseline_c (0-180 min) (min/mmol/L) | 15 | -41.01 (-88.72, 6.70) | 16 | -53.21 (-99.14, -7.28) | 12.20 (-47.48, 71.88) | 0.6843 |
| AUC(0-180 min) (min/mmol/L) | 15 | 1231.73 (1184.02, 1279.43) | 16 | 1219.53 (1173.60, 1265.46) | 12.20 (-47.48, 71.88) | 0.6843 |
| iAUC(0-180 min) (min/mmol/L) | 15 | 42.05 (9.51, 74.60) | 16 | 18.44 (-13.04, 49.92) | 23.61 (-20.52, 67.75) | 0.2890 |
| Cmax (mmol/L) | 15 | 8.07 (7.73, 8.41) | 16 | 7.44 (7.11, 7.77) | 0.63 (0.19, 1.07) | 0.0059 |
| Max_increase (mmol/L) | 15 | 1.00 (0.66, 1.34) | 16 | 0.37 (0.043, 0.70) | 0.63 (0.19, 1.07) | 0.0059 |
AUCbaseline_c (0-180 min): Baseline-corrected area under the curve from 0 to 180 min; AUC(0-180 min): Area under the curve from 0 to 180 min; iAUC(0-180 min): Incremental AUC(0-180 min), i.e., area above baseline levels of blood glucose concentration; Cmax: Maximum blood glucose concentration; Max_increase: Maximum blood glucose concentration minus baseline value; LS mean: Adjusted least square mean obtained from analysis of covariance model
Lactulose vs positive control (30 g glucose)
A glucose dose of 30 g was expected to induce higher blood glucose concentrations than 30 g of lactulose. Indeed, significant differences (P < 0.0001) in all study endpoints were observed between glucose and both lactulose formulations (Table 6). The mean AUCbaseline_c (0-180 min) of 460 min/mmol/L for glucose and especially the means of -41 min/mmol/L and -31 min/mmol/L for 30 g lactulose formulations (i.e., even slightly lowered glucose levels) demonstrated no effect of lactulose intake on blood glucose levels.
Table 6.
Blood glucose endpoints: 30 g glucose vs 30 g liquid and crystal lactulose
|
|
Glucose 30 g
|
Liquid and crystal lactulose 30 g
|
Treatment
|
P
value
|
|||
|
Variable
|
n
|
LS mean (95%CI)
|
Formu-lation
|
n
|
LS mean (95%CI)
|
Difference (95%CI)
|
|
| AUCbaseline_c (0-180 min) (min/mmol/L) | 16 | 459.83 (413.74, 505.92) | Liquid | 15 | -41.01 (-88.72, 6.70) | 500.84 (439.43, 562.26) | < 0.0001 |
| Crystals | 16 | -30.70 (-76.75, 15.35) | 490.54 (431.89, 549.18) | < 0.0001 | |||
| AUC(0-180 min) (min/mmol/L) | 16 | 1732.57 (1686.48, 1778.66) | Liquid | 15 | 1231.73 (1184.02, 1279.43) | 500.84 (439.43, 562.26) | < 0.0001 |
| Crystals | 16 | 1242.03 (1195.98, 1288.08) | 490.54 (431.89, 549.18) | < 0.0001 | |||
| iAUC(0-180 min) (min/mmol/L) | 16 | 481.14 (449.56, 512.72) | Liquid | 15 | 42.05 (9.51, 74.60) | 439.08 (394.55, 483.62) | < 0.0001 |
| Crystals | 16 | 27.65 (-3.91, 59.21) | 453.49 (409.96, 497.02) | < 0.0001 | |||
| Cmax (mmol/L) | 16 | 13.22 (12.90, 13.55) | Liquid | 15 | 8.07 (7.73, 8.41) | 5.16 (4.71, 5.61) | < 0.0001 |
| Crystals | 16 | 7.77 (7.44, 8.09) | 5.46 (5.02, 5.89) | < 0.0001 | |||
| Max_increase (mmol/L) | 16 | 6.15 (5.83, 6.48) | Liquid | 15 | 1.00 (0.66, 1.34) | 5.16 (4.71, 5.61) | < 0.0001 |
| Crystals | 16 | 0.70 (0.37, 1.02) | 5.46 (5.02, 5.89) | < 0.0001 | |||
AUCbaseline_c (0-180 min): Baseline-corrected area under the curve from 0 to 180 min; AUC(0-180 min): Area under the curve from 0 to 180 min; iAUC(0-180 min): Incremental AUC(0-180 min), i.e., area above baseline levels of blood glucose concentration; Cmax: Maximum blood glucose concentration; Max_increase: Maximum blood glucose concentration minus baseline value; LS mean: Adjusted least square mean obtained from analysis of covariance model.
Likewise, iAUC(0-180 min), AUC(0-180 min), Cmax and maximum increase were significantly lower for 30 g of both lactulose formulations compared to 30 g glucose.
As expected for subjects with T2DM, a pronounced increase in blood glucose concentration to 13.2 mmol/L with a median Tmax of 60 min was observed after glucose administration. Blood glucose returned to nearly baseline levels after 180 min without any use of antidiabetic agents.
Crystal vs liquid lactulose
No noticeable differences in blood glucose response were observed between the different lactulose formulations. After 20 g lactulose intake, the LS mean AUCbaseline_c (0-180 min) was -54.40 min/mmol/L (95%CI: -100.23, -8.57) and -30.72 min/mmol/L (95%CI: -75.26, 13.83) for the crystal and liquid formulations, respectively (P = 0.4218). After 30 g lactulose intake, the LS mean AUCbaseline_c (0-180 min) values were -30.70 min/mmol/L (95%CI: -76.75, 15.35) and -41.01 min/mmol/L (95% CI: -88.72, 6.70) for the crystal and liquid formulations, respectively, (P = 0.7379). The AUC(0-180 min) and iAUC(0-180 min) results were similar for both formulations (Tables 2-6). For the different lactulose formulations, the mean Cmax was 7.50 and 7.77 mmol/L for 20 g and 30 g crystal lactulose, respectively, and 7.75 and 8.07 mmol/L for 20 g and 30 g liquid lactulose, respectively. The median Tmax was similar for all lactulose formulations and doses (Tmax data not shown).
Bowel movements and consistency
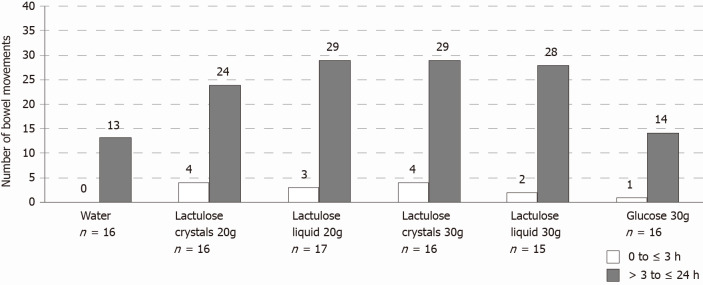
Overall, the total number of bowel movements was greater after lactulose intake regardless of dose, formulation, or time period (from 0 to ≤ 3 h or from > 3 to ≤ 24 h) compared to the control groups and occurred more frequently between > 3 to ≤ 24 h after study product administration (Figure 3). As expected, 88% (20 g crystal lactulose), 100% (30 g crystal lactulose), 94% (20 g liquid lactulose), and 93% (30 g liquid lactulose) of subjects had at least 1 bowel movement during the first 24 h after lactulose intake compared to 63% and 69% of subjects after intake of water and 30 g glucose, respectively.
Figure 3.
Cumulative numbers of bowel movements per treatment and time after oral intake of water, glucose, or lactulose for all subjects.
On average, the number of bowel movements was higher in the lactulose groups [20 g crystal lactulose: 1.75 ± 1.39 (mean ± SD), range 0-5; 30 g crystal lactulose: 2.06 ± 1.00, range 1-4; 20 g liquid lactulose: 1.88 ± 1.05, range 0-4; 30 g liquid lactulose: 2.00 ± 1.25, range 0-4] compared to water (0.81 ± 0.83, range 0-3) and 30 g glucose (0.94 ± 0.77, range 0-2).
Constipation, expressed by BSFS types 1 and 2, was observed more often in subjects receiving 30 g glucose (25%) compared to 20 g crystal lactulose (13%), 30 g crystal lactulose (6%), 20 g liquid lactulose (18%), 30 g liquid lactulose (20%), and water (0%). Constipation did not lead to a discontinuation of participation in the study.
Normal stool, reflected by BSFS types 3-5, was present more often in subjects receiving lactulose (20 g crystal lactulose: 81%; 30 g crystal lactulose: 88%; 20 g liquid lactulose: 88%; 30 g liquid lactulose: 67%) compared to water (69%) and 30 g glucose (56%).
Diarrhea, expressed by BSFS types 6 and 7, was observed more often in subjects receiving lactulose (20 g crystal lactulose: 44%; 30 g crystal lactulose: 56%; 20 g liquid lactulose: 59%; 30 g liquid lactulose: 80%) compared to water (13%) and 30 g glucose (13%). Diarrhea did not lead to a discontinuation of participation in the study.
AEs and GI tolerability
All 24 subjects experienced at least one AE: 12 (75.0%) subjects after intake of 20 g crystal lactulose, 14 (87.5%) subjects after intake of 30 g crystal lactulose, 15 (88.2%) subjects after intake of 20 g liquid lactulose, 15 (100%) subjects after intake of 30 g liquid lactulose, seven (43.8%) subjects after intake of water, and nine (56.3%) subjects after intake of 30 g glucose. Of note, the reported AEs mainly affected the digestive system with mild to moderate abdominal distension, diarrhea, flatulence, and abnormal GI sounds. Moderate AEs, such as rumbling or abdominal pain, were reported more frequently after intake of the 30 g liquid lactulose. Overall, none of the AEs were serious, and no AE led to study discontinuation or modification of the study product dosage.
Distension and flatulence of mild to severe intensity occurred in 36% and 52% of subjects after lactulose intake (> 3 to ≤ 24 h post-dose), respectively. For comparison, distension was reported in 19% of subjects in the water group and 25% of subjects in the glucose group, and flatulence was reported in 31% of subjects in the water group and 25% of subjects in the glucose group.
Overall, all study products were well tolerated. Tolerability 3 h after intake was assessed as “very good” (100% of subjects in the 20 g crystal lactulose, water, and 30 g glucose, 81% in the 30 g crystal lactulose, 94% in the 20 g liquid lactulose, and 93% in the 30 g liquid lactulose group) and “good” (19% in the 30 g crystal lactulose, 6% in the 20 g liquid lactulose, and 7% in the 30 g liquid lactulose group). Similar tolerability was reported up to 24 h post-dose (“very good” in 47%-94% of subjects, “good” in 6%-53% of subjects). Only in the 30 g lactulose groups did some subjects also report “moderate” GI tolerability (6% and 13% of subjects in the 30 g crystal and liquid lactulose groups, respectively). No subjects reported “poor” GI tolerability through 24 h after intake of study products.
DISCUSSION
The present study tested the hypothesis that single oral doses of 20 g and 30 g of crystal and liquid lactulose have no clinically relevant impact on blood glucose levels in mildly constipated, non-insulin-dependent subjects with T2DM. The study was designed as a prospective, double-blind, randomized trial with a four-period crossover and incomplete block design. Compared to a previous study in healthy subjects that used doses of 10 g and 20 g[19], a higher lactulose dose (30 g) was used to address potential safety concerns regarding “impurity load” in subjects with T2DM. However, with respect to the amount of carbohydrate impurities (up to 3% for crystal lactulose and approx. 30% for liquid lactulose), only minor effects on blood glucose concentrations were expected. Another study objective was to compare the two lactulose formulations in terms of blood glucose concentration-time responses.
According to the prescribing information, the recommended maintenance dosage range of lactulose in adults with chronic constipation is 10-20 g per day, both for crystal and liquid formulations. The higher dose of 30 g per day can be indicated as a starter dose, to achieve an immediate laxative effect. Crystal and liquid lactulose at doses of 20 g as well as crystal lactulose at a dose of 30 g did not affect any measures of blood glucose response in mildly constipated, non-insulin dependent subjects with T2DM when compared to water, whether expressed as AUCbaseline_c (0-180 min), Cmax or maximum increase. Merely after the intake of 30 g liquid lactulose, a small significant increase in calculated blood glucose parameters Cmax and maximum increase compared to water (negative control) was observed. However, in the interpretation of this result, it should be taken into account that maximum increase is a secondary endpoint in our study and is solely based on a single sampling point and calculation. Furthermore, individual glucose profiles showed a rather heterogenic pattern with maximum values occurring at different times ranging between baseline and 180 min (as a second peak) after administration. Thus, this observation presumably appeared due to random variability and is unlikely to be induced by 30 g liquid lactulose. The observed result is clinically not relevant, since the upper limit of the CI is clearly below the 2.2 mmol/L threshold of clinical relevance and the AUCbaseline_c (0-180 min) was comparable to that of water.
These findings are in agreement with previous studies demonstrating that a 25 g lactulose dose did not affect blood glucose levels in female lactose digesters and maldigesters[23] or in individuals with diabetes[24]. There is only one case report referring to higher blood glucose levels after changing the lactulose syrup brand in a subject with diet-controlled diabetes and cirrhosis[25]. It is notable that the carbohydrate impurity amount and pattern in lactulose products vary depending on the manufacturing process conditions. A different brand may, therefore, have a higher content of impurities, which may have been the reason for the increase in blood glucose levels described in this case report.
The oral intake of unabsorbable disaccharides may affect carbohydrate metabolism by reducing transit time and, possibly, glucose absorption[25,26]. The intake of both the 20 g and 30 g lactulose doses, regardless of the formulation, resulted in a slight net decrease in blood glucose concentrations of approx. -0.3 mmol/L from baseline as assessed by an overall negative AUCbaseline_c (0-180 min). This decrease, however, was within the normal physiological range of fasting blood glucose and comparable to what was observed after intake of water. Lactulose-induced impairment of intestinal carbohydrate uptake and carbohydrate metabolism was not observed under fasting conditions. The blood glucose concentrations remained largely stable despite a continuous fasting period for 3 h after oral intake of lactulose. Therefore, there is no risk for hypoglycemia after oral lactulose intake in individuals with T2DM.
With regard to safety and tolerability, the GI symptoms experienced by the participating subjects after single oral lactulose intake are well known. The reported AEs included diarrhea, flatulence, and abdominal discomfort that, as expected, were reported more frequently after intake of the higher lactulose dose. Usually, GI symptoms disappear after some days of lactulose treatment. Most treatment-emergent AEs were mild to moderate in severity, considered to be related to the study treatment, and resolved by the end of the 24 h posttreatment observation period. Overall, lactulose was well tolerated, and no unexpected safety issues were identified.
Both lactulose formulations and doses showed the desired laxative action by increasing bowel movements compared to the control treatments predominantly between > 3 to ≤ 24 h after intake.
It has been demonstrated that individuals with diabetes experience a decrease in gut microbial diversity with an increase in opportunistic pathogens[9]. In contrast to other laxatives, lactulose is metabolized by gut bacteria, thereby contributing to the maintenance or development of a healthy colonic microbiota. Lactulose is used as an energy source by bifidobacteria and lactobacilli in the colon, allowing lactulose to be regarded as a prebiotic agent[9]. In a prospective, randomized, controlled trial in 65 chronically constipated nondiabetic adults who received PEG-4000 or lactulose over 4 wk, fecal bifidobacterial counts were higher in the lactulose group than in the PEG group (P = 0.04)[17]. Other types of laxatives (e.g., bulk-forming laxatives such as psyllium and methylcellulose, other osmotic laxatives such as sorbitol and PEG, or stimulant agents such as senna glycoside and bisacodyl) may have further disadvantages. Specifically, bulk-forming laxatives may interfere with the absorption of medications commonly prescribed for use by older subjects (e.g., warfarin, aspirin, iron, calcium)[27-29], while stimulant laxatives such as bisacodyl indicated only for short-term use may induce dehydration and loss of electrolytes[28]. PEG may cause anaphylaxis[9] with a contraindication of use in severe inflammatory conditions of the intestinal tract (e.g., ulcerative colitis, Crohn’s disease, toxic megacolon) or intestinal perforation[9].
Our study confirms that based on the AUCbaseline_c (0-180 min) the recommended maintenance doses of 20 g and the higher dose of 30 g lactulose (crystals or liquid) can be used in mildly constipated, non-insulin dependent subjects with T2DM. These individuals may particularly benefit from the prebiotic effect of this laxative without experiencing an impact on blood glucose levels and glycemic management.
The present study has several strengths and limitations. First, an obvious strength is that the study was conducted in a relatively short time period, with high reliability and power. Second, the intention-to-treat population was identical to the per-protocol population in this study.
One limitation of the current study is that subjects may have distinguished between water and the other study products due to the slightly sweet taste of lactulose and glucose. Although subjects were blinded to both the dose and formulation of lactulose, as well as both control products, it was not feasible to ensure an identical taste of all study products. However, placebo effects on blood glucose concentration were not identified in a previous study in healthy subjects[19]. Therefore, a potential impact of this confounding factor on the blood glucose response is not expected. Adherence of subjects to the pre-visit restrictions was verified using diaries and questionnaires that were checked by the investigator at the start of each study visit. In case of noncompliance, the study visit was to be postponed. Thus, the potential bias is considered negligible. All lactulose doses and formulations were only tested in a single oral dose. During the study, 16 participants received three different lactulose doses, while 8 participants received two different lactulose doses. We assume that repeated daily doses will unlikely impact blood glucose levels if single doses do not increase blood glucose levels.
Eventually, applying the listed inclusion and exclusion criteria, the study population consisted exclusively of outpatients with T2DM and mild constipation without any endocrine or GI comorbidities. Since our aim was to specifically investigate the effect of lactulose on blood sugar response, we defined these criteria to ensure that any confounders masking the potential effects of lactulose, such as medications or comorbidities, can be ruled out. In fact, it is common practice to define strict inclusion/exclusion criteria for clinical studies to minimize the influence of potential confounders and achieve a certain degree of homogeneity. We consider the study population to be representative for the patient group who may benefit from lactulose administration.
CONCLUSION
In conclusion, the present study demonstrates that, at the recommended maintenance dose of 20 g and at the higher dose of 30 g lactulose, the blood glucose AUCbaseline_c (0-180 min) levels in mildly constipated, non-insulin dependent subjects with T2DM are not affected by the carbohydrate impurities contained in crystal or liquid lactulose formulations. Lactulose increased the number of bowel movements with only mild to moderate known GI side effects.
ARTICLE HIGHLIGHTS
Research background
Lactulose is approved for the symptomatic treatment of constipation, a gastrointestinal (GI) complication common in individuals with diabetes. Lactulose products contain carbohydrate impurities that occur during the lactulose manufacturing process. These impurities may affect the blood glucose levels of individuals with type 2 diabetes mellitus (T2DM) using lactulose for the treatment of mild constipation.
Research motivation
Currently, there is no information on whether lactulose in marketed formulations (crystals and liquid) has an impact on the blood glucose profile in mildly constipated, non-insulin-dependent subjects with T2DM.
Research objectives
The main objective was to assess possible changes in blood glucose levels after oral intake of lactulose in mildly constipated, non-insulin-dependent subjects with T2DM in an outpatient setting.
Research methods
The study was performed as a prospective, double-blind, randomized, controlled, single-center trial with a four-period crossover and incomplete block design in a total of 24 mildly constipated non-insulin-dependent subjects with T2DM. Capillary blood glucose concentrations were assessed over a period of 180 min after a single oral dose of 20 g or 30 g lactulose (crystal and liquid formulation). Water and 30 g glucose served as a negative and positive control, respectively.
Research results
Lactulose when administered at the recommended maintenance dose of 20 g and at a higher dose of 30 g (crystal or liquid formulation) had no impact on blood glucose baseline-corrected area under the curve of blood glucose concentrations over the complete assessment period [AUCbaseline_c (0-180 min)]. The early, small, self-limited increase in maximal blood glucose increase of 0.63 mmol/L (maximum blood glucose concentration, P = 0.0059 vs water) compared to water is not clinically relevant. As expected for subjects with T2DM, the dose of 30 g glucose (positive control) resulted in a pronounced increase in blood glucose concentration. No differences in blood glucose response were observed between the different lactulose formulations. Lactulose increased the number of bowel movements and was generally well tolerated with only mild to moderate GI symptoms due to the laxative action of lactulose.
Research conclusions
As expressed by the AUCbaseline_c (0-180 min) carbohydrate impurities in oral lactulose products administered at the recommended doses of 20 g/d and 30 g/d do not have to be considered for the blood glucose management of mildly constipated, non-insulin-dependent individuals with T2DM taking lactulose as a laxative.
Research perspectives
Future research could focus on the impact of oral lactulose supplementation at different doses over a longer period of time on blood glucose profile and gut microbiota.
ACKNOWLEDGEMENTS
The authors thank all subjects who took part in this clinical trial. Furthermore, the authors acknowledge Marlene Czarny (TechMedWriting Services, LLC, Jacksonville, Florida) and Christina Gatschelhofer (Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Austria) for reviewing and editing the manuscript.
Footnotes
Institutional review board statement: This study protocol was reviewed and approved by the Independent Ethics Committee of the Medical University of Graz, Austria.
Clinical trial registration statement: This study was registered in the European Union Drug Regulating Authorities Clinical Trials Database, No. 2018-002359-14.
Informed consent statement: All study participants provided written informed consent prior to enrollment.
Conflict-of-interest statement: The study was sponsored by Fresenius Kabi Deutschland GmbH, Germany. The sponsor’s representatives contributed to the study design and the selection of outcome measures before study start but had no role in study execution or data collection, or data analyses. All other authors declare no potential conflicts of interest related to this paper.
CONSORT 2010 statement: The authors read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to that statement.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Parenteral and Enteral Nutrition (ASPEN), No. 7658540.
Peer-review started: December 3, 2020
First decision: March 14, 2021
Article in press: April 22, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Austria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dahiya K, Firneisz G, García-Compeán D S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
Contributor Information
Thomas R Pieber, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Graz 8036, Austria.
Eva Svehlikova, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Graz 8036, Austria.
Ines Mursic, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Graz 8036, Austria.
Tamara Esterl, Division of Endocrinology and Diabetology, Department of Internal Medicine, Medical University of Graz, Graz 8036, Austria.
Manfred Wargenau, Department of Statistic, M.A.R.C.O. GmbH & Co. KG, Institute for Clinical Research and Statistics, Düsseldorf 40211, Germany.
Tina Sartorius, Department of Nutritional CRO, BioTeSys GmbH, Esslingen 73728, Germany.
Lioba Pauly, Department of Medical & Clinical Affairs, Market Access & Education Business Unit Enteral Nutrition, Bad Homburg 61352, Germany.
Susann Schwejda-Guettes, Department of Medical & Clinical Affairs, Market Access & Education Business Unit Enteral Nutrition, Bad Homburg 61352, Germany.
Annalena Neumann, Department of Medical, Clinical & Regulatory Affairs, Business Unit Parenteral Nutrition, Keto-Analogues and Standard I.V. Fluids, Fresenius Kabi Deutschland GmbH, Bad Homburg 61352, Germany.
Valentin Faerber, Department of Medical, Clinical & Regulatory Affairs, Business Unit Parenteral Nutrition, Keto-Analogues and Standard I.V. Fluids, Fresenius Kabi Deutschland GmbH, Bad Homburg 61352, Germany. valentin.faerber@fresenius-kabi.com.
John Friedrich Stover, Department of Consultancy, Stover-Solutions, Zurich 8006, Switzerland.
Barbara Gaigg, Market Unit Lactulose, Fresenius Kabi Austria GmbH, Linz 4020, Austria.
Angelika Kuchinka-Koch, Market Unit Lactulose, Fresenius Kabi Austria GmbH, Linz 4020, Austria.
Data sharing statement
No additional data are available.
References
- 1.Zhao J, Frøkjaer JB, Drewes AM, Ejskjaer N. Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World J Gastroenterol. 2006;12:2846–2857. doi: 10.3748/wjg.v12.i18.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao M, Liao D, Zhao J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World J Diabetes. 2017;8:249–269. doi: 10.4239/wjd.v8.i6.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 4.el-Salhy M. The possible role of the gut neuroendocrine system in diabetes gastroenteropathy. Histol Histopathol. 2002;17:1153–1161. doi: 10.14670/HH-17.1153. [DOI] [PubMed] [Google Scholar]
- 5.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 6.Ricci JA, Siddique R, Stewart WF, Sandler RS, Sloan S, Farup CE. Upper gastrointestinal symptoms in a U.S. national sample of adults with diabetes. Scand J Gastroenterol. 2000;35:152–159. doi: 10.1080/003655200750024317. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–138, e26. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper MS, Saad RJ. Diabetes Mellitus and the Colon. Curr Treat Options Gastroenterol. 2017;15:460–474. doi: 10.1007/s11938-017-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad VG, Abraham P. Management of chronic constipation in patients with diabetes mellitus. Indian J Gastroenterol. 2017;36:11–22. doi: 10.1007/s12664-016-0724-2. [DOI] [PubMed] [Google Scholar]
- 10.Cash BD, Lacy BE. Systematic Review: FDA-Approved Prescription Medications for Adults With Constipation. Gastroenterol Hepatol (N Y) 2006;2:736–749. [PMC free article] [PubMed] [Google Scholar]
- 11.Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25:12–19. doi: 10.1055/s-0032-1301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697–1702. [PubMed] [Google Scholar]
- 13.DiPalma JA. Current treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4 Suppl 2:S34–S42. [PubMed] [Google Scholar]
- 14.Fritz E, Hammer HF, Lipp RW, Högenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther. 2005;21:259–268. doi: 10.1111/j.1365-2036.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 15.Pontes FA, Silva AT, Cruz AC. Colonic transit times and the effect of lactulose or lactitol in hospitalized patients. Eur J Gastroenterol Hepatol. 1995;7:441–446. [PubMed] [Google Scholar]
- 16.Panel on Dietetic Products N, Allergies. Scientific Opinion on the substantiation of health claims related to lactulose and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 806) and reduction in intestinal transit time (ID 807) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA. 2010;8:1806. [Google Scholar]
- 17.Bouhnik Y, Neut C, Raskine L, Michel C, Riottot M, Andrieux C, Guillemot F, Dyard F, Flourié B. Prospective, randomized, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation. Aliment Pharmacol Ther. 2004;19:889–899. doi: 10.1111/j.1365-2036.2004.01918.x. [DOI] [PubMed] [Google Scholar]
- 18.Panesar PS, Kumari S. Lactulose: production, purification and potential applications. Biotechnol Adv. 2011;29:940–948. doi: 10.1016/j.biotechadv.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Steudle J, Schön C, Wargenau M, Pauly L, Schwejda-Güttes S, Gaigg B, Kuchinka-Koch A, Stover JF. Blood glucose response after oral intake of lactulose in healthy volunteers: A randomized, controlled, cross-over study. World J Gastrointest Pharmacol Ther. 2018;9:22–30. doi: 10.4292/wjgpt.v9.i3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, Kowalski AJ, Madden P, McAuliffe-Fogarty AH, McElwee-Malloy M, Peters A, Raman S, Reifschneider K, Rubin K, Weinzimer SA. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40:1622–1630. doi: 10.2337/dc17-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–447. doi: 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 22.Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teuri U, Vapaatalo H, Korpela R. Fructooligosaccharides and lactulose cause more symptoms in lactose maldigesters and subjects with pseudohypolactasia than in control lactose digesters. Am J Clin Nutr. 1999;69:973–979. doi: 10.1093/ajcn/69.5.973. [DOI] [PubMed] [Google Scholar]
- 24.Conn HO, Bircher J. Hepatic encephalopathy: management with lactulose and related carbohydrates. Medi-Ed Press. 1989 [Google Scholar]
- 25.Bianchi G, Ronchi M, Marchesini G. Effect of lactulose on carbohydrate metabolism and diabetes mellitus. Scand J Gastroenterol Suppl. 1997;222:62–64. doi: 10.1080/00365521.1997.11720721. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins DJ, Goff DV, Leeds AR, Alberti KG, Wolever TM, Gassull MA, Hockaday TD. Unabsorbable carbohydrates and diabetes: Decreased post-prandial hyperglycaemia. Lancet. 1976;2:172–174. doi: 10.1016/s0140-6736(76)92346-1. [DOI] [PubMed] [Google Scholar]
- 27.Day M, Wills T, Coffey A. Constipation and the pros and cons of laxatives for older adults. Nurs Res Care. 2014;16:2–4. [Google Scholar]
- 28.Waterfield J. Laxatives: choice, mode of action and prescribing issues. Nurse Prescri. 2007;5:456–461. [Google Scholar]
- 29.Yakabowich M. Prescribe with care. The role of laxatives in the treatment of constipation. J Gerontol Nurs. 1990;16:4–11. doi: 10.3928/0098-9134-19900701-04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.