Abstract
Peripheral arterial disease (PAD) refers to partial or complete occlusion of the peripheral vessels of the upper and lower limbs. It usually occurs as part of systemic atherosclerosis in the coronary and cerebral arteries. The prevalence of PAD is expected to continue to increase in the foreseeable future owing to the rise in the occurrence of its major risk factors. Nonhealing ulcers, limb amputation and physical disability are some of its major complications. Diabetes mellitus (DM) remains a major risk for PAD, with DM patients having more than two-fold increased prevalence of PAD compared with the general population. The clinical presentation in people with DM also differs slightly from that in the general population. In addition, PAD in DM may lead to diabetic foot ulcers (DFUs), which precipitate hyperglycaemic emergencies and result in increased hospital admissions, reduced quality of life, and mortality. Despite the epidemiological and clinical importance of PAD, it remains largely under diagnosed and hence undertreated, possibly because it is largely asymptomatic. Emphasis has been placed on neuropathy as a cause of DFUs, however PAD is equally important. This review examines the epidemiology, pathophysiology and diagnosis of lower limb PAD in people with diabetes and relates these to the general population. It also highlights recent innovations in the management of PAD.
Keywords: Diabetes, Peripheral arterial disease, Diabetic foot ulcers, Lower limb complications
Core Tip: Peripheral arterial disease (PAD) is a major cause of nonhealing ulcers, lower limb amputation and mortality, especially in people with diabetes. The ominous association between PAD and diabetic foot disease is largely under-reported. Hence, it is under diagnosed and undertreated. This article reviews the impact of PAD in diabetes, its traditional and non-traditional risk factors, and pathophysiology, and examines some recent innovations in its management.
INTRODUCTION
Diabetes mellitus (DM) continues to assume pandemic proportions, affecting people across various socioeconomic groups in developed and developing nations. Globally, close to a half billion people are living with diabetes and it is expected to increase by more than 50% in the next 25 years[1]. The myriad of chronic complications attributable to the disease results in enormous physical, mental, and economic burdens. The complications are mainly vascular and lead to diabetes-specific microvascular sequelae in the retina, nerves and the glomerulus. Others are atherosclerotic macrovascular pathology in the brain, heart and lower limbs[2].
Lower extremity complications are common, showing a rising trend in many regions of the world and affecting about 131 million people worldwide, with an estimated global prevalence of 1.8%[3]. They significantly impact morbidity and mortality in people with DM, sometimes leading to leg ulcers and amputations, which are generally characterized by physical disability, reduced productivity and emotional disturbances. Although much emphasis has been laid on neuropathy as a cause, an equally important contributor to the occurrence of leg ulcers and amputations is peripheral arterial disease (PAD)[2,4-6]. Consequently, PAD is under-diagnosed and hence, may be undertreated.
PAD denotes a complete or partial occlusion of one or more of the noncardiac, non-intracranial, peripheral arteries of the upper and lower limbs, which may lead to reduced blood flow or tissue loss[7]. It usually results from atherosclerosis of the vessel wall, but may also arise as a result of embolism, thrombosis, fibromuscular dysplasia, or vasculitis[7]. Atherosclerotic PAD may be a pointer to systemic atherosclerosis in non-peripheral intra-cerebral and coronary arteries. In DM, the arteries of the lower limbs are the ones that are mostly involved; and most often the distal arteries, especially the dorsalis pedis artery[8]. This review discusses the pathophysiology of atherosclerotic PAD of the lower limbs, its epidemiology in DM, and its treatment. It also highlights recent advances in its management.
EPIDEMIOLOGY OF PAD IN DIABETES
The prevalence of PAD depends on the diagnostic measurement employed, cut-off values of the test, the limb assessed and the population studied[9]. It has been assessed using the presence of intermittent claudication (IC), palpation of the vessels of the lower limbs, and measurement of the ankle-brachial index (ABI). Prevalence generally increases with advancing age, irrespective of the measurement utilized. IC, the main symptom attributable to PAD, occurred in about 1.5% of the cohorts in the Framingham Heart Study. In all age groups, the rate in men was double that in women[10]. Also, in the Rotterdam study involving the elderly population, IC was reported by 1.6% of the participants, but the prevalence of PAD defined by an ABI < 0.9 in either leg was 19.1% in the same cohort[11]. The prevalence in men was higher in both studies. The rates of PAD using IC is generally lower compared with those obtained using ABI[11-13].
In community studies, the prevalence of PAD using ABI differs with the population, cut-off value, ankle vessel and the leg used, with values ranging from 4.3% to 9.0% in the general population[14,15]. In a systemic review assessing community-based studies of the global prevalence of PAD (using ABI ≤ 0.9) and its risk factors, prevalence differed based on the region studied and sex. It was higher among men in high-income countries, and in women in low- and middle-income countries[16]. Certain factors affect the accurate assessment of PAD in people with diabetes. PAD is often asymptomatic; the presence of peripheral neuropathy, which is a common complication of DM. may distort pain perception, and the presence of IC and absence of peripheral pulses are inadequate diagnostic indicators[8].
In hospital-based studies, PAD is two- to seven-fold more prevalent in people with diabetes than it is in those without it, with rates between 9% and 55% in people with diabetes[5,17-19]. In a national survey involving about 3000 adult Americans 40 years of age and above, PAD was two times more prevalent in people with diabetes compared with the general population[20]. Also, a systematic review of studies comparing PAD in diabetics and nondiabetics reported that PAD ranged between 20% and 50% in those with diabetes, compared with 10% and 26% in those without diabetes[21]. Also, as seen in the general population, the prevalence of PAD differed depending on the diagnostic method used (IC, palpation of vessels or ABI)[19].
Lower limb amputation resulting from foot ulcers is a major cause of disability, especially in diabetic patients. Patients with foot ulcers are more likely to present with PAD than those without ulcers, with the attendant increased mortality and lower limb amputations in those patient cohorts[22-25].
RISK FACTORS FOR PAD
The major risk factors for PAD such as DM, hypertension, smoking and hyperlipidaemia also contribute to coronary heart disease (CHD) and cerebrovascular disease (CVD). However, the influence exerted by those risks on vascular diseases is different[26-28]. In a recent systemic review that assessed community-based studies for global prevalence and risk factors of PAD, DM ranked next to smoking among the major risks and hypertension and hypercholesterolaemia followed[16,29]. In the National Health and Nutrition Examination Survey, cigarette smoking and DM were also the most significant risk factors for PAD, with a odds ratios of 4.5 and 2.7, respectively[14].
In other community-based studies, diabetes also ranked high as a risk factor for the occurrence and progression of PAD along with other traditional risks such as age, smoking, hypertension, hypercholesterolaemia and low kidney function)[11-13,30,31]. It hiked the rates of lower extremity amputation, hospital stay, and mortality[21,22,26]. While the major risk factors for PAD in people without DM remain significant even with it, other associations have also been identified in DM. They include longer duration of DM, high glycated haemoglobin (HBA1c) level, abdominal obesity, male sex and neuropathy[18,19,22,32].
The traditional risk factors do not fully explain the development of atherosclerosis in the peripheral or other vascular beds. Inflammation, abnormalities in haemostasis and blood viscosity are known to contribute to the evolution and propagation of atherosclerosis, and their markers have been studied[33-35]. High-sensitivity C-reactive protein, hyperuricaemia, and hyperhomocysteinaemia are some of the non-traditional risk factors associated with PAD in the general population and in people with DM[18,19,36-38].
PATHOPHYSIOLOGY
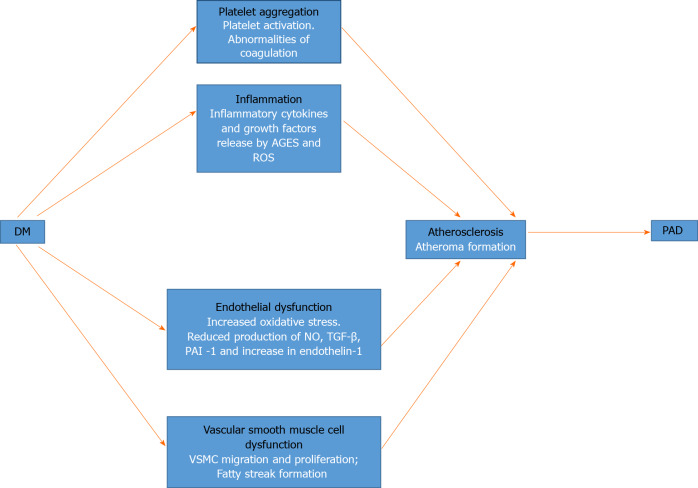
The central pathophysiological theme of PAD in DM is the process of atherosclerosis. It begins with atherogenesis, and progresses to the eventual obstruction and reduction of blood flow. In what is known as subclinical atherosclerosis, the pathological changes may predate the diagnosis of impaired fasting glucose and DM[39]. The changes are the same as those observed in other vascular beds in patients with DM. Several pathogenetic mechanisms have been identified in the initiation of atherosclerosis, including endothelial dysfunction, inflammation, platelet aggregation and vascular smooth muscle cell (VSMC) dysfunction[40]. Figure 1 shows a schematic representation of these factors and how they lead to PAD.
Figure 1.
Schematic representation of the pathophysiology of peripheral arterial disease in diabetes mellitus. Pathogenetic processes and their mechanisms are shown in black and white type, respectively. AGEs: Advanced glycation end products; DM: Diabetes mellitus; NO: Nitric oxide; PAD: Peripheral arterial disease; PAI-1: Plasminogen activator inhibitor-1; ROS: Reactive oxygen species; TGF-β: Transforming growth factor-beta; VSMC: Vascular smooth muscle cell.
Dysfunction of the vascular endothelium is the hallmark of atherosclerosis in DM, and it arises from a variety of inter-related pathogenetic factors. First, chronic hyperglycaemia activates the dormant polyol pathway. That results in increased oxidative stress from reactive oxygen species, caused by the consumption of cofactor nicotinamide adenine dinucleotide phosphate and reduced glutathione[41,42]. Chronic hyperglycaemia also causes the production of advanced glycation end products, which results in the elaboration of inflammatory cytokines and growth factors that cause vascular injury[42]. In addition, hyperglycaemia induces the activation of protein kinase C, which has various effects on gene expression. Protein kinase C is responsible for the activation of the nuclear factor κB, a transcription factor that activates a variety of proinflammatory genes[42,43]. The resultant effect is a reduction in the production of nitric oxide (NO), which is a potent vasodilator; transforming growth factor (TGF)-β and plasminogen activator inhibitor (PAI)-1. The production of the vasoconstrictor endothelin-1 is increased. NO reduces inflammation by modulating leucocyte-vascular wall interaction and inhibiting VSMC migration and platelet activation[40]. Those abnormalities in the absence of NO, result in increased endothelial permeability, leucocyte chemotaxis, adhesion and migration into the intima, thus causing inflammation. There is also low-density lipoprotein (LDL) migration into the intima where it is oxidized within monocytes to form foam cells, which are the earliest precursor of atheroma formation.
Endothelial injury and hyperglycaemia are activators of platelet adhesion, activation and aggregation. With hyperglycaemia, glucose uptake by platelets is left unchecked, resulting in platelet activation and increased oxidative stress through the release of reactive oxygen species[40]. Also, hyperglycaemia is associated with abnormalities of coagulation such as the decreased concentration of antithrombin and protein C, impaired fibrinolytic function and excess production of PAI-1[40]. Platelet activation and aggregation are therefore important elements in the development of atherosclerosis.
Hyperglycaemia is also associated with VSMC dysfunction through the effects of endothelial injury and intima inflammation. Proinflammatory mediators such as platelet-derived growth factors (PDGFs), vascular endothelial growth factors, and cytokines released in the inflammatory milieu of the intima result in VSMC migration and proliferation. The combination of VSMC and endothelial foam cells subsequently results in the development of fatty streaks that become remodelled into an atheromatous plaque. The plaque is the result of collagen production and an extracellular matrix by VSMC through the mediating effects of PDGF and TGF-β[40,44]. The increasing size of the atheromatous plaque, which causes obstruction and reduction of blood flow, is the hallmark of atherosclerosis as seen in PAD and other vascular beds in DM patients.
DIAGNOSTIC EVALUATION
History and physical examination
History taking in all DM patients should entail asking for risk factors for PAD, such as hypertension, dyslipidaemia, cigarette smoking, obesity and the duration of DM. Patients who have been diabetic for more than 10 years are more prone to the risk of PAD[19,31,45]. Similarly, longer duration of, and exposure to higher levels of the other factors (hypertension, dyslipidaemia, smoking, obesity) potentiates the risk of PAD[31]. History taking should also focus on the presence of other macrovascular complications such as CVD and CHD because they are equivalents. Symptoms of PAD include IC in about 10% of patients; pain at rest, which is indicative of critical limb ischaemia, and about 50% of patients will be asymptomatic[46]. Examiners should search for differentials of PAD such as pseudo-claudication in spinal stenosis, peripheral neuropathy, nerve root compression, deep venous thrombosis, vasculitis and musculoskeletal causes such as arthritis[46]. Examination may reveal features of ischaemia such as dependent rubor, elevated pallor, and shiny and hairless skin. Also, peripheral pulses such as the femoral, popliteal, posterior tibial and dorsalis pedis arteries may be reduced. Some patients may present with trophic skin changes and gangrene.
ABI
The ABI is a sensitive and specific screening tool for PAD. It has a sensitivity of 90% and specificity of 98% in detecting PAD[47]. The European Society of Cardiology (ESC), and American Heart Association recommend the use of ABI to screen for PAD in all diabetics older than 50 years of age. Others include diabetics younger than 50 years of age with a DM duration of more than 10 years or with other risk factors for PAD such as smoking, hypertension, dyslipidaemia and PAD equivalents[45,48]. An ABI of < 0.9 is indicative of PAD, and is associated with a 2- to 4 -fold increase in mortality[45]. An ABI of > 1.3 is indicative of poorly compressible vessels resulting from vascular calcification, which is also associated with an increased risk of mortality and amputation (Table 1)[40].
Table 1.
Interpretation of ankle-brachial index
|
Ankle-brachial index
|
Interpretation
|
| < 0.4 | Severe obstruction |
| 0.4-0.69 | Moderate obstruction |
| 0.7-0.90 | Mild obstruction |
| 0.91-1.30 | Normal |
| > 1.30 | Poorly compressible |
Duplex ultrasound
Duplex ultrasound is a combination of conventional and doppler ultrasonography. It is indicated as a first-line imaging method to detect the site and extent of severity of vascular lesions[45].
Computed tomography and magnetic resonance angiography
Angiography is indicated in patients with planned revascularization to guide optimal revascularization strategies. Computed tomography angiography is non-invasive, widely available, and has a high resolution. The disadvantages include exposure to irradiation, use of iodinated contrast agents and contrast nephrotoxicity, particularly in patients with chronic kidney disease (CKD)[45]. Magnetic resonance angiography has the advantage of being acceptable in mild to moderate CKD, with higher soft-tissue resolution. It is limited by frequent motion artefacts, claustrophobia, severe CKD, and in patients with magnetic resonance imaging noncompliant pacemakers or implantable cardioverter defibrillators[45].
TREATMENT
The management of PAD in DM includes symptomatic control and reduction of the risk of cardiovascular (CV) events. Management includes CV risk factor treatment and lifestyle modifications such as regular physical exercise, promotion of a healthy diet, weight reduction and smoking cessation. If medical management fails because of disabling symptoms or in the presence of chronic life-threatening ischaemia, then revascularization is indicated.
Exercise
Regular physical activity improves claudication distance in PAD. It also improves quality of life and reduces the risk of CV disease, which often accompanies PAD[49,50]. Home-based walking exercise is recommended for a minimum of 30 min, at least 3 d of the week[49]. Randomized controlled trials of 493 patients with PAD showed that home-based walking exercise improved walking ability in patients and also improved 6-min walk more than supervised treadmill exercise[51].
Statins
High-intensity statin therapy is recommended for all patients with PAD[52,53]. Observational and randomized clinical studies have shown that statin therapy reduced all-cause mortality and CV events in patients with PAD. The goal is to reduce LDL cholesterol (LDL-C) to < 1.8 mmol/L (70 mg/dL) or to reduce it by ≥ 50% if baseline values are 1.8-3.5 mmol/L (70-135 mg/dL)[45]. In statin-benefit groups such as PAD, ezetimibe is a reasonable and beneficial addition if LDL-C remains > 1.8 mmol/L (70 mg/dL) with maximally tolerated statin therapy[45,54]. If LDL-C remains > 1.8 mmol/L (70 mg/dL) on statins and ezetimibe, the addition of evolocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 is reasonable and beneficial to reduce CV events[45,55].
Antiplatelet therapy
Single antiplatelet therapy is indicated in all patients with symptomatic PAD and in those who have had revascularization[45]. Antiplatelet agents are effective in preventing limb-related and CV events[56]. A post hoc analysis of the CAPRIE (Clopidogrel vs Aspirin in Patients at Risk of Ischaemic Events) trial in 6452 patients with clinical lower extremity artery disease (LEAD) showed that at 3 years, clopidogrel was superior with significant reductions in CV mortality [hazard ratio (HR) 0.76 (95%CI: 0.64-0.91)] and major adverse cardiovascular events HR 0.78 (95%CI: 0.65-0.93)][57]. A similar benefit was seen in the subgroup of LEAD patients with DM[57]. In the randomized EUCLID (Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease) trial, ticagrelor did not show any difference compared to clopidogrel[58]. Clopidogrel is therefore the recommended antiplatelet drug in symptomatic PAD.
Vasodilators
Cilostazol, an oral phosphodiesterase type III inhibitor is useful in managing IC. It inhibits platelet aggregation and causes vasodilation. Randomized controlled trials have shown improved walking distance and quality of life with the use of cilostazol[59]. However, it has been suggested that improvement in walking distance is mild to moderate, with great variability.
Glycaemic control
There are no randomized controlled trials with arms comparing intensive or standard arm glucose lowering in those with DM and PAD. However, there is evidence that glucose control is associated with a reduction in microvascular and macrovascular complications. In type 1 DM, the DCCT (Diabetes Control and Complications Trial) showed a reduction in CV events in the intensive arm compared with the standard arm, both with long-term follow-up[60]. With long-term follow-up, intensive control showed a 57% reduction in nonfatal myocardial infarction (MI), stroke, CV death, as well as some reduction in all-cause mortality[61]. However, in type 2 DM, evidence of the benefit of intensive lowering of glycaemia was not as compelling. There is a need, therefore, for a general goal of a glycated haemoglobin level of < 7%, while individualizing the goal of treatment for each patient’s characteristics[62].
In the UKPDS (United Kingdom Prospective Diabetic Study), short-term follow-up did not show a significant benefit in the reduction of CV events of combined fatal and nonfatal MI, sudden death (P = 0.052) and stroke[63]. However, after 10 years of follow-up, patients in the intensive glycaemia control arm showed significant reductions in MI and all-cause mortality[63]. The short-term and long-term results of the UKPDS are contradicted by those of ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial and the VADT (Veterans Affairs Diabetic Trial[64]. In those trials, short-term follow-up of 3.5 to 5.6 years did not show any reduction in CV events in those in the intensive arm. Long-term follow-up in the ADVANCE trial showed no evidence of CV benefit or harm[65]. In the ACCORD trial, the glycaemic control comparison was stopped early because of increased mortality in the intensive (1.41% per year) compared with the standard (1.14% per year) treatment arms [HR 1.22 (95%CI: 1.01-1.46)]. But the long-term follow-up at 10 years in the VADT showed a reduction in CV events in the intensive arm[66,67]. In those three trials, the patients had high CV risks, longer DM duration, and were relatively older than patients in UKPDS. Also, severe hypoglycaemia was more likely in the intensive arms, hence the importance of individualizing control for those groups of patients to reduce the high risk of CV events and mortality because of hypoglycaemia.
Blood pressure control
The ESC/European Society of Hypertension recommends that systolic blood pressure (SBP) be targeted to < 130 mmHg and that the diastolic blood pressure should < 80 mmHg and that the SBP should not be < 120 mmHg in patients with DM[68]. This is supported by evidence from the ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) and ACCORD trials showing an overall reduction in CV events with intensive SBP lowering to < 130 mmHg[69,70]. Diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and beta-blockers can all be used in PAD. However, the HOPE (Heart Outcomes Prevention) and ONTARGET studies have shown that ACEIs and ARBs reduce CV events in PAD and should therefore be considered in treating patients with PAD and hypertension with or without DM[69,71]. Care should be taken with the use of beta-blockers in patients with chronic limb-threatening ischaemia[68].
Revascularization
Revascularization is indicated if claudication impairs quality of life after the failure of exercise therapy and pharmacotherapy in patients whose general condition allows invasive treatment. Strategies include endovascular therapy, open surgery, or a combination of the two. Endovascular therapy is generally recommended for short (< 25 cm) occlusive lesions, and in those with high surgical risk. It includes balloon dilation (angioplasty), stents, and atherectomy[72]. Open surgery is recommended for patients with long (≥ 25 cm) lesions who are young and fit[45].
RECENT INNOVATIONS IN THE MANAGEMENT OF PAD
Endovascular therapy has continued to evolve with the modification and development of new technologies, including drug-eluting stents, self-expanding stents, cutting balloons (CBs), and cryoplasty balloons. Other interventions are focal pressure balloons and drug-coated balloons (DCBs). These reduce post-treatment cell proliferation or restenosis, thereby improving patency, and new atherectomy systems, especially for calcified lesions[72-74]. CBs and cryoplasty balloons are modifications of standard percutaneous transluminal angioplasty (PTA). PTA is done by placing a wire within the artery beyond the target lesion and then expanding the inserted balloon with appropriate pressure. That leads to fracture of the lesion and stretching of the arterial wall[73]. Cryoplasty balloons induce an inflammatory response and dilate plaques by utilizing a combination of hypothermia and pressure. DCBs inhibit hyperplasia by including medication (usually paclitaxel) after performing a standard PTA[73].
Lithoplasty (Shockwave Medical) is an atherectomy device that combines a balloon angioplasty catheter with sound waves that break up calcifications that otherwise would not be broken with the use of DCBs and stents[74]. The Pantheris Lumivascular Atherectomy System (Avinger, Inc.) is a directional atherectomy system that includes optical coherence tomography. It utilizes light to provide three-dimensional visual guidance rather than two-dimensional X-ray images with fluoroscopy. It aids better navigation for removal of plaque, reduces damage to the artery and may reduce exposure to radiation from fluoroscopic imaging procedures[74].
CONCLUSION
DM is a major risk for PAD, resulting in increased morbidity and mortality. Morbidity is characterized by an increased risk of other cardiovascular complications, increased hospital admissions, disability from leg ulcers and amputation, reduced productivity and reduced quality of life.
Early detection of PAD in diabetic patients at risk is imperative to reduce morbidity and mortality. At-risk diabetics include older patients, those with a DM duration longer than 10 years, high HBA1c, obesity and neuropathy. The ABI is a highly sensitive and specific simple tool to screen for PAD in DM. It is also valuable as a follow-up tool, and also for stratifying CV risk[45].
Prevention of CV events and symptom control in symptomatic patients are the paramount pillars of the treatment of PAD in DM. They should include treatment of CV risk factors, and treatment of PAD, including pharmacological and nonpharmacological interventions and revascularization if medical treatment fails.
Open surgery used to be the mainstay of revascularization, endovascular therapy has however evolved recently to improve outcomes, with the development of new innovations, such as the DES, CBs, self-expanding stents, and cryoplasty balloons. More studies are needed to evaluate quality of life and wound healing using these newer endovascular modalities and to compare surgical and endovascular revascularization in symptomatic patients[45].
Footnotes
Conflict-of-interest statement: The authors have no competing interests to declare.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association of Clinical Endocrinologists; Society for Endocrinology United Kingdom; and Endocrine and Metabolism Society of Nigeria.
Peer-review started: February 13, 2021
First decision: March 16, 2021
Article in press: May 7, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yuan B S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
David Olubukunmi Soyoye, Department of Medicine, Obafemi Awolowo University, Ile-Ife 220282, Osun State, Nigeria; Department of Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife 220282, Osun State, Nigeria. bksoyoye@yahoo.com.
Olugbenga Olusola Abiodun, Department of Medicine, Federal Medical Centre, Jabi 900211, Abuja, Nigeria.
Rosemary Temidayo Ikem, Department of Medicine, Obafemi Awolowo University, Ile-Ife 220282, Osun State, Nigeria; Department of Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife 220282, Osun State, Nigeria.
Babatope Ayodeji Kolawole, Department of Medicine, Obafemi Awolowo University, Ile-Ife 220282, Osun State, Nigeria; Department of Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife 220282, Osun State, Nigeria.
Anthony Olubunmi Akintomide, Department of Medicine, Obafemi Awolowo University, Ile-Ife 220282, Osun State, Nigeria; Department of Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife 220282, Osun State, Nigeria.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39 Suppl 1:S4–S5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care. 2020;43:964–974. doi: 10.2337/dc19-1614. [DOI] [PubMed] [Google Scholar]
- 4.Akanji AO, Adetuyidi A. The pattern of presentation of foot lesions in Nigerian diabetic patients. West Afr J Med. 1990;9:1–5. [PubMed] [Google Scholar]
- 5.Ikem R, Ikem I, Adebayo O, Soyoye D. An assessment of peripheral vascular disease in patients with diabetic foot ulcer. Foot (Edinb) 2010;20:114–117. doi: 10.1016/j.foot.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Peters EJ, Armstrong DG, Lavery LA. Risk factors for recurrent diabetic foot ulcers: site matters. Diabetes Care. 2007;30:2077–2079. doi: 10.2337/dc07-0445. [DOI] [PubMed] [Google Scholar]
- 7.Kullo IJ, Rooke TW. CLINICAL PRACTICE. Peripheral Artery Disease. N Engl J Med. 2016;374:861–871. doi: 10.1056/NEJMcp1507631. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 12.Bennett PC, Lip GY, Silverman S, Blann AD, Gill PS. The contribution of cardiovascular risk factors to peripheral arterial disease in South Asians and Blacks: a sub-study to the Ethnic-Echocardiographic Heart of England Screening (E-ECHOES) study. QJM. 2010;103:661–669. doi: 10.1093/qjmed/hcq102. [DOI] [PubMed] [Google Scholar]
- 13.Ramos R, Quesada M, Solanas P, Subirana I, Sala J, Vila J, Masiá R, Cerezo C, Elosua R, Grau M, Cordón F, Juvinyà D, Fitó M, Isabel Covas M, Clarà A, Angel Muñoz M, Marrugat J REGICOR Investigators. Prevalence of symptomatic and asymptomatic peripheral arterial disease and the value of the ankle-brachial index to stratify cardiovascular risk. Eur J Vasc Endovasc Surg. 2009;38:305–311. doi: 10.1016/j.ejvs.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 16.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 17.Arora E, Maiya AG, Devasia T, Bhat R, Kamath G. Prevalence of peripheral arterial disease among type 2 diabetes mellitus in coastal Karnataka. Diabetes Metab Syndr. 2019;13:1251–1253. doi: 10.1016/j.dsx.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Akalu Y, Birhan A. Peripheral Arterial Disease and Its Associated Factors among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia. J Diabetes Res. 2020;2020:9419413. doi: 10.1155/2020/9419413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soyoye DO, Ikem RT, Kolawole BA, Oluwadiya KS, Bolarinwa RA, Adebayo OJ. Prevalence and Correlates of Peripheral Arterial Disease in Nigerians with Type 2 Diabetes. Adv Med. 2016;2016:3529419. doi: 10.1155/2016/3529419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L 1999-2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 21.Stoberock K, Kaschwich M, Nicolay SS, Mahmoud N, Heidemann F, Rieß HC, Debus ES, Behrendt CA. The interrelationship between diabetes mellitus and peripheral arterial disease - a systematic review. Vasa. 2020:1–8. doi: 10.1024/0301-1526/a000925. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Yang BH, Wang G, Gao Y, Cao X, Zhang XF, Yan CC, Lian XT, Liu BH, Ju S. A meta-analysis of the relationship between foot local characteristics and major lower extremity amputation in diabetic foot patients. J Cell Biochem. 2019;120:9091–9096. doi: 10.1002/jcb.28183. [DOI] [PubMed] [Google Scholar]
- 23.Beach KW, Bedford GR, Bergelin RO, Martin DC, Vandenberghe N, Zaccardi M, Strandness DE Jr. Progression of lower-extremity arterial occlusive disease in type II diabetes mellitus. Diabetes Care. 1988;11:464–472. doi: 10.2337/diacare.11.6.464. [DOI] [PubMed] [Google Scholar]
- 24.Costa RHR, Cardoso NA, Procópio RJ, Navarro TP, Dardik A, de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Syndr. 2017;11 Suppl 2:S583–S587. doi: 10.1016/j.dsx.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Wang A, Sun X, Wang W, Jiang K. A study of prognostic factors in Chinese patients with diabetic foot ulcers. Diabet Foot Ankle. 2014;5 doi: 10.3402/dfa.v5.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 27.Forrest KY, Becker DJ, Kuller LH, Wolfson SK, Orchard TJ. Are predictors of coronary heart disease and lower-extremity arterial disease in type 1 diabetes the same? Atherosclerosis. 2000;148:159–169. doi: 10.1016/s0021-9150(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994;7:7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 29.Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 30.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 31.Weragoda J, Seneviratne R, Weerasinghe MC, Wijeyaratne SM. Risk factors of peripheral arterial disease: a case control study in Sri Lanka. BMC Res Notes. 2016;9:508. doi: 10.1186/s13104-016-2314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla V, Fatima J, Ali M, Garg A. A Study of Prevalence of Peripheral Arterial Disease in Type 2 Diabetes Mellitus Patients in a Teaching Hospital. J Assoc Physicians India. 2018;66:57–60. [PubMed] [Google Scholar]
- 33.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 34.Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25:153–175. doi: 10.1210/er.2002-0034. [DOI] [PubMed] [Google Scholar]
- 35.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 36.Shou Z, Zhao Y, Zhang Y, Li S. Risk factors for peripheral arterial disease in elderly patients with Type-2 diabetes mellitus: A clinical study. Pak J Med Sci. 2020;36:1344–1348. doi: 10.12669/pjms.36.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aronow WS, Ahn C. Association between plasma homocysteine and peripheral arterial disease in older persons. Coron Artery Dis. 1998;9:49–50. doi: 10.1097/00019501-199809010-00008. [DOI] [PubMed] [Google Scholar]
- 38.Bembi V, Singh S, Singh P, Aneja GK, Arya TV, Arora R. Prevalence of peripheral arterial disease in a cohort of diabetic patients. South Med J. 2006;99:564–569. doi: 10.1097/01.smj.0000221624.68378.5d. [DOI] [PubMed] [Google Scholar]
- 39.Drexel H, Aczel S, Marte T, Benzer W, Langer P, Moll W, Saely CH. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care. 2005;28:101–107. doi: 10.2337/diacare.28.1.101. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 41.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 42.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 43.Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–126. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–1380. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 45.Aboyans V, Björck M, Brodmann M, Collet JP, Czerny M, De Carlo M, Naylor AR, Roffi M, Tendera M, Vlachopoulos C, Ricco JB ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:e35–e41. doi: 10.1093/eurheartj/ehx499. [DOI] [PubMed] [Google Scholar]
- 46.Hennion DR, Siano KA. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013;88:306–310. [PubMed] [Google Scholar]
- 47.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 48.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, Golzarian J, Gornik HL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1555–1570. doi: 10.1016/j.jacc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12:CD000990. doi: 10.1002/14651858.CD000990.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDermott MM. Exercise Rehabilitation for Peripheral Artery Disease: A REVIEW. J Cardiopulm Rehabil Prev. 2018;38:63–69. doi: 10.1097/HCR.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiter-Brennan C, Osei AD, Iftekhar Uddin SM, Orimoloye OA, Obisesan OH, Mirbolouk M, Blaha MJ, Dzaye O. ACC/AHA lipid guidelines: Personalized care to prevent cardiovascular disease. Cleve Clin J Med. 2020;87:231–239. doi: 10.3949/ccjm.87a.19078. [DOI] [PubMed] [Google Scholar]
- 54.Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, Reist C, Im K, Bohula EA, Isaza D, Lopez-Sendon J, Dellborg M, Kher U, Tershakovec AM, Braunwald E. Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial. J Am Coll Cardiol. 2016;67:353–361. doi: 10.1016/j.jacc.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 55.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 56.Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301:1909–1919. doi: 10.1001/jama.2009.623. [DOI] [PubMed] [Google Scholar]
- 57.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 58.Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, Blomster J, Millegård M, Reist C, Patel MR EUCLID Trial Steering Committee and Investigators. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376:32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 59.Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2008:CD003748. doi: 10.1002/14651858.CD003748.pub3. [DOI] [PubMed] [Google Scholar]
- 60.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 61.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Professional Practice Committee: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S3. doi: 10.2337/dc20-Sppc. [DOI] [PubMed] [Google Scholar]
- 63.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 64.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 66.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 68.Williams B, Mancia G. Ten Commandments of the 2018 ESC/ESH HTN Guidelines on Hypertension in Adults. Eur Heart J. 2018;39:3007–3008. doi: 10.1093/eurheartj/ehy439. [DOI] [PubMed] [Google Scholar]
- 69.Margolis KL, O'Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, Cutler JA, Evans GW, Gerstein HC, Grimm RH Jr, Lipkin EW, Narayan KM, Riddle MC Jr, Sood A, Goff DC Jr. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721–1728. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ontarget Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 71.Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 72.Pereira K. Treatment Strategies for the Claudicant. Semin Intervent Radiol. 2018;35:435–442. doi: 10.1055/s-0038-1676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazar A, Morrissey N. Recent advances in endovascular treatment of peripheral arterial disease. F1000Res. 2020;9 doi: 10.12688/f1000research.20398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Topfer LA, Spry C. New Technologies for the Treatment of Peripheral Artery Disease. 2018 Apr 1. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016–. 172. [PubMed] [Google Scholar]