Highlights
-
•
Management of patients with cancer proved challenging during the COVID-19 pandemic.
-
•
Delivering standard bone care to cancer patients has often not been possible.
-
•
Alternative options, with reduced hospital attendance, have been sought.
-
•
A questionnaire explored solutions for continued provision of bone support.
-
•
This paper provides guidance for optimisation of bone health during COVID-19.
Keywords: COVID-19, Bone metastasis, Bisphosphonates, Zoledronic acid, Denosumab, Bone health
Abstract
Optimum management of patients with cancer during the COVID-19 pandemic has proved extremely challenging. Patients, clinicians and hospital authorities have had to balance the risks to patients of attending hospital, many of whom are especially vulnerable, with the risks of delaying or modifying cancer treatment. Those whose care has been significantly impacted include patients suffering from the effects of cancer on bone, where delivering the usual standard of care for bone support has often not been possible and clinicians have been forced to seek alternative options for adequate management.
At a virtual meeting of the Cancer and Bone Society in July 2020, an expert group shared experiences and solutions to this challenge, following which a questionnaire was sent internationally to the symposium’s participants, to explore the issues faced and solutions offered. 70 respondents, from 9 countries (majority USA, 39%, followed by UK, 19%) included 50 clinicians, spread across a diverse range of specialties (but with a high proportion, 64%, of medical oncologists) and 20 who classified themselves as non-clinical (solely lab-based). Spread of clinician specialty across tumour types was breast (65%), prostate (27%), followed by renal, myeloma and melanoma.
Analysis showed that management of metastatic bone disease in all solid tumour types and myeloma, adjuvant bisphosphonate breast cancer therapy and cancer treatment induced bone loss, was substantially impacted. Respondents reported delays to routine CT scans (58%), standard bone scans (48%) and MRI scans (46%), though emergency scans were less affected. Delays in palliative radiotherapy for bone pain were reported by 31% of respondents with treatments often involving only a single dose without fractionation. Delays to, or cancellation of, prophylactic surgery for bone pain were reported by 35% of respondents. Access to treatments with intravenous bisphosphonates and subcutaneous denosumab was a major problem, mitigated by provision of drug administration at home or in a local clinic, reduced frequency of administration or switching to oral bisphosphonates taken at home. The questionnaire also revealed damaging delays or complete stopping of both clinical and laboratory research.
In addition to an analysis of the questionnaire, this paper presents a rationale and recommendations for adaptation of the normal guidelines for protection of bone health during the pandemic.
1. Introduction
Patients suffering from cancer are especially vulnerable to COVID-19, often by virtue of their age, as well as from their disease or its treatment [1]. Furthermore, because of the reluctance of some patients to access care during the pandemic, we are now observing a rise in late cancer diagnosis. The need to protect such patients from exposure to the virus (for example by limiting their attendance at hospitals) has had a huge impact worldwide on the patterns of delivery of cancer care since March 2020. Key changes have included a major increase in the use of remote consultations (eg telephone or video rather than face-to face), changes to oral systemic therapies taken at home whenever possible, rather than agents administered by iv or subcutaneous injection (requiring a hospital, clinic or general practitioner (GP) visit), the need for social distancing and/or wearing of personal protective equipment when patients do attend hospital and restrictions preventing carer/relative support for patients attending hospital.
Those whose care has been significantly impacted by COVID-19 include cancer patients suffering from the effects of cancer on bone. Bone metastases are very common, occurring in 65–75% of patients with advanced breast cancers as well as approximately 80% of patients with advanced prostate cancer and 17–64% of patients with advanced lung cancer [2]. These cancers are very common, hence large numbers of patients are potentially impacted. Multiple myeloma which originates in the bone marrow causes extensive bone destruction and, while distinct from metastatic bone disease [3], there are parallels in the management of these conditions, for example in the treatment of resulting skeletal complications. Often termed skeletal related events (SREs), these include significant bone pain requiring radiotherapy, bone fractures often requiring surgery, hypercalcaemia, and spinal cord compression often requiring emergency need for radiotherapy and/or surgery [4], [5].
Recent advances in anti-cancer systemic therapies, particularly in advanced breast, prostate and lung cancers, have resulted in patients living longer, with a resultant requirement for continued and longer management of their metastatic bone disease to optimise quality of life. It is in this field where bone targeted therapies have revolutionized the treatment of metastatic bone disease, in particular the bone resorption inhibitors including bisphosphonates [6] and denosumab [7]. In the metastatic setting, although orally administered agents are available, most bisphosphonates, dosed for bone metastases, require iv administration with the frequency of dosing dependent upon the individual bisphosphonate used. In the case of denosumab, for bone metastases this is typically administered as a subcutaneous injection of 120 mg once every 4 weeks. Although COVID-19 has accentuated consideration of less frequent treatments involving hospital attendance across medicine, even before the pandemic, there has been a range of studies relating to less frequent administration of bisphosphonates and a few on denosumab. [7], [8], [9], [10], [11], [12] For metastatic breast cancer, metastatic prostate cancer, and multiple myeloma there are data to support dosing zoledronic acid every 3 months at presentation of bone metastases [10], [11] and the optimal dosing for denosumab is evolving [9]. The key study endpoints have been skeletal related events, or symptomatic skeletal related events, but the potential reduced risk of osteonecrosis of the jaw, as well as other toxicities and financial aspects, have been of great interest with less frequent dosing [13], [14].
There is strong evidence that when bisphosphonates are absorbed by bone, they reside in bone for many months or even years and remain active in suppressing resorption [15]. However, denosumab suppression of resorption falls off much more rapidly on drug cessation and this has led to concerns about the rebound effect in bone resorption and increased vertebral fracture risk. The risk of denosumab associated rebound bone resorption raises concerns when considering alternative dosing intervals in the setting of COVID19, since clinicians cannot be certain about access to future dosing. However, other measures can be taken, eg a switch to bisphosphonates [16].
As well as their use in the palliation of metastatic bone disease, for postmenopausal women with early breast cancer, the value of bisphosphonates in the adjuvant setting is now recognised with many countries using this approach to reduce the occurrence of bone metastasis [17].
It has long been recognised that, otherwise successful cancer treatments can have a negative impact on bone composition and density, resulting in bone loss, potentially leading to osteoporosis and fragility fractures [18]. This cancer treatment induced bone loss (CTIBL) is especially associated with hormonal treatments such as GnRH analogue, aromatase inhibitors in breast cancer and androgen deprivation therapy in prostate cancer [19], [20]. International guidelines in both breast cancer [21] and prostate cancer [22], are in place for the use of bone targeted agents, principally bisphosphonates and denosumab, to prevent bone loss and subsequent osteoporotic fracture in this setting and there was a clear potential for these treatments to also be negatively impacted during the pandemic.
In order to better understand the impact of the coronavirus on such patients, on the professional healthcare workers who care for them and on the many linked research programmes, and how these issues are approached in different parts of the world, the Cancer and Bone Society (CABS) held a special webinar symposium as part of (and in association with) a virtual meeting of the American Society for Bone and Mineral Research (ASBMR) in July 2020 entitled ‘COVID-19 and managing bone in patients with all stages of cancer’. Recognising that the clinical management of patients with cancer has been dramatically affected by the COVID-19 pandemic, the CABS/ASBMR Webinar presenters outlined challenges and solutions for bone care in the setting of cancer and COVID-19. The session, which was attended by an international audience, included presentations covering management of bone in early and advanced cancer, as well as a question-and-answer session for interactive dialogue with the Webinar attendees. Following the Webinar, a questionnaire was sent out to all attendees to better evaluate how the pandemic has impacted their clinical and research programmes. This paper presents the questionnaire results as well as recommendations for adaptations of standard treatment guidance during the pandemic.
2. Methods
2.1. Questionnaire design and distribution:
The questionnaire was constructed within ‘Google Forms’, which allows on-line completion and use of the integrated analysis tools. The questions within the survey were initially created by a subset of the authors of this publication, and circulated, via a shared folder on Google Drive, to the members of the CABS Board who span a range of countries of residence and are internationally respected authorities in the field of cancer and bone. These reviewers of the survey in the construction phase include a wide range of perspectives in the field of cancer and the skeleton including clinical management of patients, clinically-based research and pre-clinical, lab-based research in bone oncology. Each member of the CABS Board had the opportunity to comment on each question at each iteration. Suggestions were incorporated into the questionnaire form and, following a total of three successive iterations of this process, the completed survey was tested/piloted by two clinicians, not part of the original design team, to ensure that it was clear and well understood. The questionnaire was designed so that it would take a maximum of 20–30 min to complete.
The final questionnaire contained 45 questions of which 40 were addressed towards staff working clinically and 5 were directed towards both clinical and non-clinical staff members. Where respondents stated that they were non-clinical, they were directed towards a separate section at the end of the questionnaire. The form encompassed a range of question types from multiple choice questions through to yes / no questions and strongly agree / strongly disagree answers. In cases where the respondent replied with “other” a box was provided for text response of answers. Some questions were mandatory.
The questions, which were intended for professionals in cancer and bone and not patients, inquired into the recipient’s area of work, the sector they were involved in, the effect of COVID-19 upon the treatment and bone-imaging choices made, the effects of the COVID-19 pandemic upon how patients are consulted within clinics and the effects of the COVID-19 pandemic upon staff recruitment and training within the institutions as well as the effects of COVID-19 on basic, translational and clinical research.
The questionnaire was uploaded to Google Drive on 19th July 2020 and the link was distributed by email immediately after the above CABS symposium and a reminder was sent out after two weeks. Responses to the survey accumulated within the period 19th July – 8th October 2020 at which point the responses were analysed. As responses were received from global specialists, the responses reveal the effects of COVID-19 upon cancer treatment within countries at different phases of the first wave of virus infection.
The data analysis tools within Google forms were used to provide an overview of the responses in terms of large-scale distribution of replies (both as pie charts and as histograms). The analyses and initial drafting of the report was carried out by an author not involved with clinical management. Where questions asked for more individual or in-depth responses (or provided the possibility of providing “other” answers within a drop-down menu with a subsequent additional answer box), these replies were interpreted manually for inclusion within this publication.
The survey questionnaire is available within the supplementary data section of the publication.
2.2. Setting
The link to the survey was distributed internationally via the CABS organisation by e-mail and also to all who attended the webinar symposium (including members of ASBMR). There was no restriction on who could return a completed questionnaire.
2.3. Ethical considerations
The survey respondents were assured that their responses would be anonymised and that no data would be shared which could be used to identify the individual responders.
3. Results
3.1. Respondents and demographics
In total, there were 70 respondents, including 50 clinicians and 20 who classified themselves as non-clinical (solely lab-based). These were spread across a diverse range of specialisations within the field of bone oncology. Two clinicians dropped out after the first two questions, one in orthopaedic surgery and the other in radiology, probably because many of the following questions related to physicians giving systemic therapy. Although not mandatory, 54/70 (77%) respondents provided their email address. There would be no reason to expect duplication and, since a high proportion (77%) were known to be not duplicated, the potential of an effect due to multiple entries is very small. Of the clinicians who completed the survey, 87.5% were senior doctors and 12.5% were trainees. Since no limit had been placed upon the distribution of the questionnaire (indeed respondents were encouraged to involve other colleagues) and since not all respondents provided email addresses, it is not possible to define the response rate as the denominator is not known.
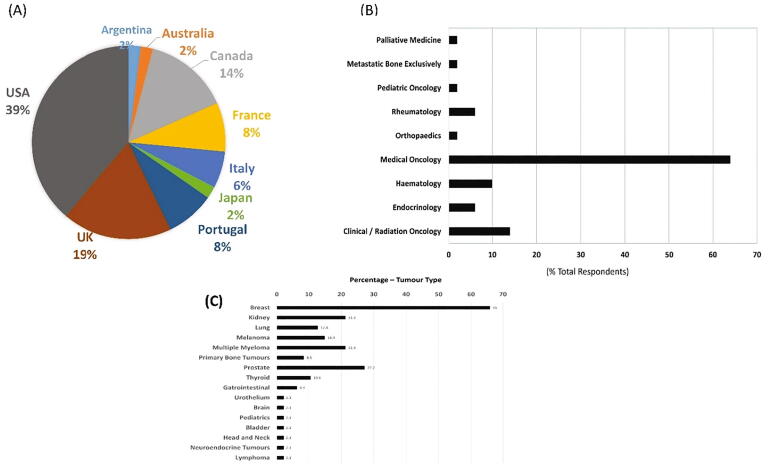
Respondents who provided information on the country in which they practice revealed coverage within Europe, USA/Canada and Asia with the USA providing the most responses – (see Fig. 1a). In terms of the respondent’s area of work, the predominant field was listed as “Medical Oncology” (responsible for 64% of replies) and “clinical / radiation oncology” was the second most prevalent response (see Fig. 1b). The majority of respondents (90%) worked within academic institutions with 10% of respondents working within non-academic healthcare institutions.
Fig. 1.
Demographic data Area of work and global distribution of questionnaire respondents. (A) Country of practice of the respondents to the survey – 49 responses. Global distribution of respondents – encompassing Europe, Asia and America / Canada. (B) Area of work of the respondents – 50 responses, (C) Cancer types treated by the clinician respondents – 47 responses.
Clinicians responding to this questionnaire worked in a wide range of tumour types with breast cancer being the most prominent, followed by prostate cancer, renal cancer, myeloma, melanoma and lung cancer (see Fig. 1c).
3.2. Effects upon patient care
3.2.1. Effects of COVID-19 upon tumour imaging
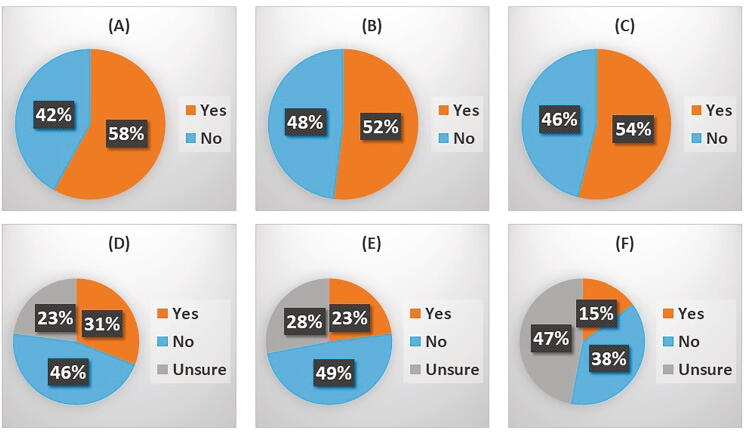
Imaging is critical to management of patients with established or suspected bone metastases for which patients need to attend a medical setting. Across all respondents, 58% reported delays to routine CT scans (Fig. 2a), 47.9% reported a delay in carrying out standard bone scans (Fig. 2b) and 45.8% reported delays in MRI scans due to COVID-19 (Fig. 2c). Delays appeared to be in respect of routine care and not for emergency needs, such as spinal cord compression.
Fig. 2.
Effect of COVID-19 upon tumour imaging and radiotherapeutic treatments of bone cancers: The effects of COVID19 upon tumour imaging and use of radiotherapies for bone cancers was assessed using a series of questions. (A) Have there been delays to routine bone imaging such as CT? – 48 responses, (B) Have there been delays in getting bone scans due to COVID19? – 48 responses (C) Have there been delays in getting MRI scans due to COVID19? – 48 responses, (D) Has there been an impact on palliative external beam radiotherapy at your centre due to COVID19? – 48 responses, (E) Has there been an impact upon access to stereotactic radiotherapy at your centre due to COVID19? – 47 responses and (F) Has there been an impact upon access to iMRT for small volume bone metastases at your centre due to COVID19? – 47 responses.
Reported delays in bone scans ranged from 1 to 2 weeks in about 50% of responses, to up to 2–3 months for the remainder of the respondents. The average delays to MRI scans followed a similar pattern with approximately 50% of respondents reporting delays of 1–3 weeks and the remaining 50% reporting delays of up to 2–3 months. The majority of the respondents indicated that these delays were not uniform across sites within their area of practice.
It should be noted that delays could be due either to a delay in scanning and reporting following scan request to radiology or a delayed scan because clinicians had been asked to change normal practice and request these less frequently in the pandemic. Text responses in the questionnaire suggested that both sources of delay were often applicable. Overall, during the first 4 months of the pandemic, 50% or more of the respondents experienced delays in obtaining imaging studies for their patients with bone metastases.
3.2.2. Effects of COVID-19 on the treatment of patients with metastatic bone disease and multiple myeloma
Treatment of bone metastases and multiple myeloma involves radiotherapeutic and surgical approaches as well as the use of bone-targeted agents (including oral and i.v. bisphosphonates, denosumab and, in the case of prostate cancer, 223Radium treatment). With the exception of oral bisphosphonates, all of these treatments normally require clinic or hospital attendance. Depending upon the therapeutic agent in question, dosing may range from every 4 weeks to less frequently out to every 3 – 6 months.
3.2.2.1. Radiotherapy and delays due to COVID-19
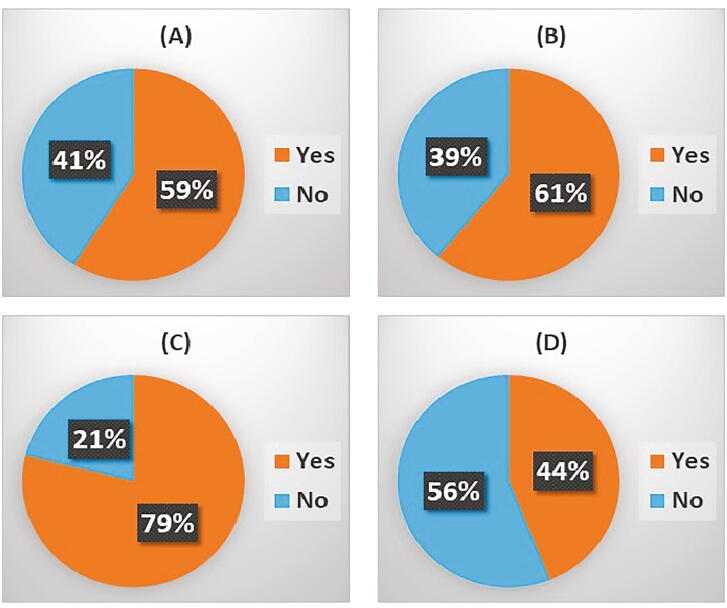
Effects of COVID-19 upon radiotherapy (palliative external beam radiotherapy for bone pain – Fig. 2d, stereotactic radiotherapy – Fig. 2e, and iMRT for small volume bone metastases – Fig. 2f) was assessed, with 14.3–31.3% of respondents reporting an impact upon the provision of this radiotherapy (Table 1). Reported delays to palliative external beam radiotherapy ranged from 1 to 2 weeks to a month and when respondents were asked about how treatments were impacted, delay of treatment was commonly cited. The number of fractions which would normally be given for palliation were reduced in some institutions, eg a single fraction for bone pain, rather than multiple fractions. For stereotactic treatment, whilst some respondents said there was no delay, this may have been partly because adaptations were made to the sequence of treatments. For IMRT, the high level of ‘don’t knows’ is probably because most respondents were not radiation oncologists. Delays in radiation therapy to bone metastases were reported in up to one third of respondents with reports of decreases in number of radiation fractions occurring to minimise clinic visits.
Table 1.
Effects of COVID-19 upon bone-targeted treatments within metastatic disease:
| Treatment Modality | Impacted | Not-Impacted | Unsure | |
|---|---|---|---|---|
| Denosumab Administration | 43.8% | Increased −21.2% Decreased −78.8% |
41.7% | 14.6% |
| I.V. Bisphosphonate Administration | 68.8% | Increased − 5.4% Decreased − 94.6% |
20.8% | 10.4% |
| Oral Bisphosphonates | 14.9% | Increased − 76.5% Decreased − 23.5% |
59.6% | 25.5% |
| 223Radium Administration | 18.2% | Increased – 40% Decreased – 60% |
13.6% | 68.2% recipients don’t treat prostate cancer |
3.2.2.2. Prophylactic surgery
Prophylactic surgery is frequently employed for patients with metastatic bone disease at risk of developing bone fractures. Alterations to the use of prophylactic surgery for bone pain were reported by 34.8% of the 46 questionnaire respondents, with delays frequently cited as well as cancellation of prophylactic surgery during the initial intense phase of COVID-19 infections. Some respondents reported increased thresholds being employed for performing prophylactic surgery as well as cancellation of non-essential operations during the peak of the pandemic. Comments included ‘surgery delayed unless absolutely necessary’, ‘prophylactic surgery was stopped for 4 months, now resumed’, ‘All non-emergency surgeries were postponed’. The impact of a possible increased future incidence of pathological fracture remains to be determined and is of concern.
3.2.2.3. Palliative care
In terms of the management of bone pain, access to palliative care for bone pain was judged to be negatively impacted in 30% of the clinicians questioned. Of those, some reported clinic locations were closed during the height of the outbreak and there were reports of very poor access to community palliative care services in many areas (but not all). Clinician access was mainly via
telephone, with a significant decline in home and face-to-face visits and it was felt that the quality of assessments may have been affected as palliative care patients need to have face-to-face assessments. There remains concern over access to palliative care services during the pandemic.
3.2.2.4. Bisphosphonates and denosumab
The survey and the webinar have highlighted the variety of practice across countries in the delivery of bone-targeted agents: home or office-based delivery of bisphosphonates or denosumab vs hospital administration. Nevertheless, it is clear that COVID-19 has had a significant global impact upon the treatment of metastatic bone disease using bisphosphonates or denosumab. Decreased administration of i.v.bisphosphonates and denosumab was reported by 94.6% and 78.8% of respondents, respectively (see Table 1). The reduced administration of i.v. bisphosphonates in this study was accompanied by an increased use of home-administered oral bisphosphonates (as reported by 76.5% of respondents). Thirty three respondents answered the question of whether denosumab use had increased (7 respondents) or decreased (26 respondents) during the pandemic. From the textual responses, decreased use of denosumab appears to be related to the logistics of use of chemotherapy suites which were at reduced capacity with other treatments receiving priority (including curative chemotherapy). There was also clear concern as to the possible rebound effect after stopping denosumab which needs to be avoided, possibly by replacing with a bisphosphonate. Those that suggested an increase in denosumab use may have been in countries where IV bisphosphonates were routinely used and where denosumab could be administered without use of chemotherapy infusion suites.
These alterations to the administration of oral and i.v. bisphosphonates and subcutaneous denosumab were accompanied by increased use of speciality clinics (16–18%) to ensure rapid patient throughput and increase in home administration (up to 15%) with one report of a “drive through” administration.
Treatment of bone metastases arising from prostate cancer has increasingly involved the use of 223Radium (in those countries where it is available), a short range alpha-emitting radioactive isotope of radium which is taken up into bones in the place of calcium, and which therefore specifically targets bone-resident cancer cells. Of the 44 respondents to the question on the use of 223Radium during the pandemic, 30 indicated that they did not treat prostate cancer and of the remaining 14 respondents who treated prostate cancer, 4 reported no change, with 6 reporting decreased use and 4 reporting increased use of 223Radium.
Treatment of bone metastases and myeloma bone disease with bisphosphonates and denosumab has been significantly impacted, but alterations to use and scheduling of iv and oral bisphosphonates and denosumab have been implemented to optimise patient care (see Table 3)
Table 3.
Cancer and bone: Recommendations for consideration if current management guidance is disrupted during the COVID-19 pandemic (ASCO guidelines are included for breast cancer, prostate cancer and myeloma and ESMO guidelines for lung cancer and other solid tumours, though it should be emphasised that, as expected, there is a high level of commonality among guidelines. Therefore, recommended adaptations for disruption caused by the pandemic are intended to guide decisions whichever guidelines are normally used).
| GUIDANCE COMMON TO ALL SOLID TUMOUR TYPES AND MYELOMA | ||
|---|---|---|
| CURRENT GUIDANCE (ASCO, ESMO) | RECOMMENDATIONS FOR CONSIDERATION IF ADHERENCE TO GUIDANCE IS DISRUPTED DURING COVID-19 PANDEMIC | |
| Radiotherapy is one of the main therapeutic approaches to palliate pain in patients with bone metastasis. | Continue radiotherapy as needed, but consider giving as a single (not fractionated) dose. | |
| Calcium and vitamin D supplementation (eg, calcium 500 mg and vitamin D 400 IU daily) has been prescribed or strongly recommended within clinical trials of zoledronic acid or denosumab and is recommended within the package inserts of both drugs. | Continue with calcium and vitamin D supplementation. | |
| Orthopaedic surgery (high risk of fracture, metastatic long bone fracture, spinal cord compression) | These situations represent a medical emergency and surgery should not be delayed. | |
| Dental evaluation prior to start of zoledronic acid or denosumab is recommended as invasive dental procedures or ill-fitting dental appliances during therapy are a common predisposing factor in cases of ONJ. | Maintain dental evaluation if at all possible, or at least patient/physician visual inspection when dentistry appointments are not available. | |
| BREAST CANCER | ||
| INDICATION | CURRENT GUIDANCE (ASCO) | RECOMMENDATIONS FOR CONSIDERATION IF ADHERENCE TO GUIDANCE IS DISRUPTED DURING COVID-19 PANDEMIC |
| Advanced Breast Cancer, bone Metastases [24] | Patients with breast cancer who have evidence of bone metastases should be treated with a bone modifying agent. Options include
|
Patients with breast cancer who have evidence of bone metastases should be treated with a bone modifying agent. Options include
|
| Early Breast Cancer, adjuvant bisphosphonates [25] | It is recommended that, if available, zoledronic acid (4 mg intravenously every 6 months) or clodronate (1,600 mg/d orally) be considered as adjuvant therapy for postmenopausal patients with breast cancer who are deemed candidates for adjuvant systemic therapy | It is recommended that, if available, zoledronic acid (4 mg intravenously every 6 months) or clodronate (1,600 mg/d orally) be considered as adjuvant therapy for postmenopausal patients with breast cancer who are deemed candidates for adjuvant systemic therapyWhen infusion therapy is limited, consider zoledronic acid 5 mg once a year |
| Early Breast Cancer, prevention of bone loss [21] | Patients with osteoporosis or who are at increased risk of osteoporotic fractures based on clinical assessment or risk assessment tools, bone-modifying agents, such as oral bisphosphonates, intravenous (IV) bisphosphonates or subcutaneous denosumab at the osteoporosis-indicated dosage, may be offered to reduce the risk of fracture. Hormonal therapies for osteoporosis management (eg, estrogens) are generally avoided in patients with hormonal-responsive cancers Additionally, specific populations may be considered appropriate candidate for bone-modifying agents:
|
Timely access to DEXA scans to assess BMD may not be possible, but fracture risk assessment, such as the WHO FRAX score (https://www.sheffield.ac.uk/FRAX/tool.aspx) should be considered to assess those at high risk of osteoporotic fracture. Patients with osteoporosis or who are at increased risk of osteoporotic fractures, bone-modifying agents, such as oral bisphosphonates, intravenous bisphosphonates are favoured agents to avoid the potential interruption of denosumab dosing and associated rebound fracture risk. In patients presently receiving denosumab or zoledronic acid consider off site, drive-through or home administration if feasible. In patients presently on denosumab (60 mg every 6 months) consider oral bisphosphonate via telemedicine immediately in patients at high risk of fracture until they can resume original therapy. |
| PROSTATE CANCER | ||
| INDICATION | CURRENT GUIDANCE (ASCO) | RECOMMENDATIONS FOR CONSIDERATION IF ADHERENCE TO GUIDANCE IS DISRUPTED DURING COVID-19 PANDEMIC |
| Advanced Prostate Cancer – Bone Metastases [26] | In men with metastatic CRPC (mCRPC), either zoledronic acid (minimally symptomatic or asymptomatic disease) or denosumab (disease independent of symptoms) (both at bone metastasis-indicated dosages: zoledronic acid, 4 mg iv, 3–4 weekly; denosumab, 120 mg sc, 4 weekly) is recommended for preventing or delaying skeletal-related events (SREs). | Patients with prostate cancer who have evidence of bone metastases should be treated with a bone modifying agent. Options include • denosumab, 120 mg subcutaneously
|
| In men with symptomatic mCRPC, Ra-223 is recommended to extend overall survival. In men with symptomatic mCRPC and bone pain, radium-223 (Ra-223) should be considered for reducing symptomatic skeletal events and improving health-related QoL. Systemic therapies for the treatment of mCRPC such as abiraterone/prednisone, enzalutamide, docetaxel and cabazitaxel have been shown to reduce SREs, improve bone pain and health-related QoL, and/or improve overall survival in mCRPC. Mitoxantrone has also been shown to improve pain and health-related QoL. The optimal sequencing or combination of these therapies with bone-targeted agents is unclear, and recommendations to patients should be done in consultation with a clinician. |
If Ra-223 not available, consider external beam or sterotactic radiotherapy for bone pain and bisphosphonates or denosumab for reducing SREs and improving health-related QoL (please see above for recommend options). Consider using oral anticancer therapies, that have demonstrated a decrease in SREs such as abiraterone or enzalutamide. |
|
| There is evidence to suggest harm in the form of increased fracture risk with the combination of Ra-223 when administered with abiraterone and prednisone initiation; that combination should be avoided. Current guidelines do not support concurrent use of Ra-223 with other secondary therapies known to prolong survival for mCRPC. | Avoid combination of Ra-223 with other therapies. | |
| Bone loss due to ADT or other treatments affecting hormone levels [26] | For men with non-metastatic prostate cancer at high risk of fracture receiving ADT, denosumab at the osteoporosis-indicated dosage should be considered to reduce the risk of fracture. In situations or jurisdictions where denosumab is contraindicated or not available, a bisphosphonate is a reasonable option. Baseline bone mineral density (BMD) testing with conventional dual x-ray absorptiometry is encouraged for men before starting ADT to help determine fracture risk and to identify those individuals who would probably benefit from pharmacological intervention. | Timely access to DEXA scans to assess BMD may not be possible, but fracture risk assessment, such as the WHO FRAX score (https://www.sheffield.ac.uk/FRAX/tool.aspx) should be considered to assess those at high risk of osteoporotic fracture. If denosumab administration is not possible, consider annual infusion of zoledronic acid at osteoporosis dose. If this is not possible, consider oral bisphosphonates at osteoporosis doses |
| MULTIPLE MYELOMA | ||
| INDICATION | CURRENT GUIDANCE(ASCO) | RECOMMENDATIONS FOR CONSIDERATION IF ADHERENCE TO GUIDANCE IS DISRUPTED DURING COVID-19 PANDEMIC |
| MGUS, asymptomatic myeloma (SMM) [27] | Watchful waiting for standard risk SMM. Clinical trial may be considered for high risk SMM. |
Watchful waiting. Scheduled visits of patients with stable disease can be delayed with safety. Alternatively, blood examination in local laboratories and consultation via telemedicine is encouraged. |
| Symptomatic myeloma [27] | Intravenous administration of pamidronate 90 mg or zoledronic acid 4 mg every 3 to 4 weeks, or denosumab 120 mg every 4 weeks If patients have problems of renal impairment, denosumab is preferred compared to iv aminobisphosphonates Treatment with the bone-modifying agents is recommended to continue for up to 2 years Continuous use of the agents depends on discretion of physicians |
|
| Relapsed and/or refractory myeloma [27] | Treatment of biochemically relapsed should be individualized. All clinically relapsed patients with symptoms due to myeloma should be treated immediately. | Watchful waiting may be considered for patients with biochemical relapses, especially for patients with a slow and gradual increase in the paraprotein level. New onset of end-organ damage features (CRAB) and a history of aggressive relapse with rapid deterioration of the clinical presentation should receive next-line treatment without delay. Regarding the selection of treatment regimen, orally administered agents (ixazomib, lenalidomide, pomalidomide, and panobinostat) should be considered. |
| LUNG and OTHER SOLID TUMOUR SITES (except breast and prostate cancer) | ||
| INDICATION | CURRENT GUIDANCE (ESMO) | RECOMMENDATIONS FOR CONSIDERATION IF ADHERENCE TO GUIDANCE IS DISRUPTED DURING COVID PANDEMIC |
| Advanced cancer, bone metastases [28] | Bone targeted agents:
|
Bone targeted agents:
• denosumab, 120 mg subcutaneously
|
3.2.3. Impact upon use of adjuvant bisphosphonates:
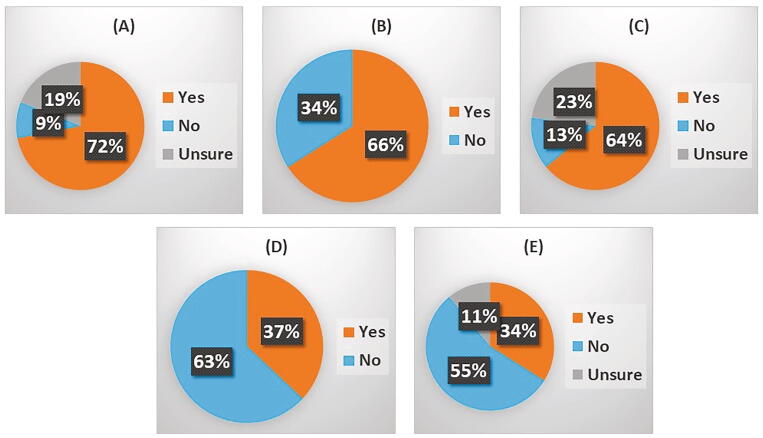
Adjuvant bisphosphonates are increasingly used for the reduction/prevention of bone metastases in breast cancer. Overall 72% of 47 clinicians responding reported that adjuvant bisphosphonates were normally used within their patient populations. Of these clinicians, 66% (23 respondents) reported that COVID-19 had impacted upon adjuvant bisphosphonate use (Fig. 3). These alterations to normal use included delays to treatment, delayed or missed appointments (in some cases due to patients declining to come in for appointments), reduced clinical availability owing to the demands of caring for COVID-19 patients and the switch from i.v. to an oral route of administration. There appeared to be a number of assessments that postponing adjuvant treatment represented a lower risk than exposing patients to the higher risks of hospital attendance which was necessary for iv zoledronic acid infusion. In some cases, adjuvant bisphosphonates had been restarted after 4 months. Revised guidelines in the UK imposed by COVID-19 suggested not giving adjuvant bisphosphonates during the pandemic.
Fig. 3.
COVID-19 and Bone Health: The effects of COVID19 upon bone health was assessed via the following questions: (A) Are adjuvant bisphosphonates used for patients with breast cancer at your centre? – 47 responses. Among respondents who answered “yes” the following questions were then asked: (B) If yes, has this been impacted by COVID19? – 47 responses. (C) Has bone health monitoring e.g. bone density DEXA scans, been impacted by COVID19 at your centre? – 47 responses. (D) Has your treatment of cancer treatment induced bone loss been changed due to COVID19? – 46 responses, and (E) Have you changed your antiresorptive therapy regimens for cancer treatment induced bone loss due to the COVID19 pandemic? – 47 responses.
Adjuvant bisphosphonate therapy has been significantly disrupted because postponing was thought to be lower risk than potential exposure to COVID-19.
3.2.4. Impact on bone mass and osteoporosis (CTIBL).
DEXA scans are employed to monitor bone health within both cancer patients and patients with benign conditions such as osteoporosis. These are done relatively infrequently (eg annually or every 2 years). COVID-19 appears to have substantially impacted upon the use of DEXA scans with 63.8% of respondents reporting a reduced frequency. Many clinicians reported the reduced availability of DEXA scanning, as well as delays, in many cases of a 3–4 month duration (though this may be equivalent to saying that scans had not been done at all up until the questionnaire was completed). Long term bone health was probably regarded as low priority compared with many other needs and, when treatment was given, this was increasingly without repeating the DEXA scan.
Common alterations reported included a move to the use of oral bisphosphonates (eg ibandronate) and away from i.v. infusion and reduced frequency of infusion as well as cancellation of treatments owing to COVID-19. Within CTIBL, up to 44% of clinicians reported a change to the anti-resorptive therapies they administer during the COVID-19 pandemic.
The effects of COVID-19 upon treatment of osteoporotic fractures produced a similarly mixed response albeit with slightly less concern about the long-term impact of COVID-19. These responses may reflect the timescale over which the respective medical conditions require treatment and the hopes that a treatment (such as a COVID-19 vaccine) might return a degree of normality to treatment in future and these patients could subsequently be treated when more routine services were available.
Long-term bone mass and risk of osteoporosis was regarded as not highest priority as there could be a return to treatment post-pandemic.
3.2.5. Patient follow-up and access to pharmacies:
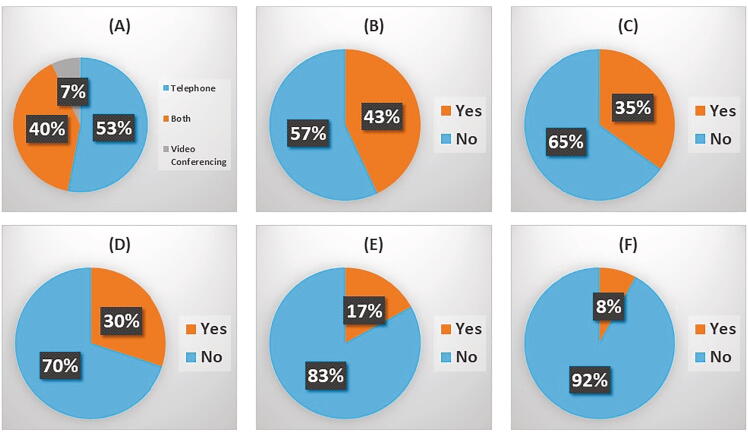
The adoption of remote follow-up of patients varied widely between respondents and when this question was further pursued in terms of timing, many centres adopted remote follow-up of all patients at the height of the first wave of COVID-19 and then moved more towards face-to-face assessments later, once the peak of infection had receded. For many countries, the first wave had already subsided when the questionnaire was completed. Non-face-to-face methods of follow up were commonly adopted within 85.1% of the respondents (Fig. 4), with 53.3% reporting the use of telephone follow-up only, 6.7% video conferencing only and 40% reporting use of both methods. In addition, just over half of respondents (57.4%) reported the increased use of more local care providers, to decrease outpatient attendance either at the hospital or large oncology centre.
Fig. 4.
Follow-up and management of patients with bone metastases: Within the questionnaire 85% of respondents indicated that their patients did have non face to face consultations. Further questions were asked relating to patient care and treatments including (A) For non-face to face meetings have you been using video conferencing, telephone or both? – 45 responses, (B) Has there been increased use of local provider(s) for local care? – 47 responses, (c) Has the use of prophylactic surgery for patients at risk of fracture been affected at your centre? – 46 responses, (D) Has access to palliative care for pain control been affected in your centre due to COVID19? – 47 responses, (E) Has access to outpatient pharmacies and associated medications been impacted by COVID19 for your patients with bone metastases? – 47 responses, and (F) Have you noticed a change in your opiate for bone pain prescribing pattern during COVID19? – 48 responses.
Only 17% of questionnaire respondents felt that there was a negative impact of COVID-19 on access to medication via outpatient pharmacies, the main issue being the closure of hospital pharmacies with redirection of patients towards local pharmacies. Alterations to the prescription of opiates occurred in only 8.3% of respondents and this appears to have mainly involved the increased dispensation of opiate medications by GPs and palliative care teams and less by hospital pharmacies.
3.2.6. Other concerns
For the question relating to reimbursement, 60% of respondents said this was not relevant, which probably means they had national health services such as the NHS in the UK. Of the remaining 40% of the respondents who were Yes/No (as to whether billing was an issue), only 21% replied “yes” but it is noteworthy that a significant proportion of these respondents were from within the USA.
Questions relating to the effect upon SRE incidence in patients with bone metastases revealed a disparity in answers, with roughly a third of respondents stating it would probably have no significant long-term effect upon SRE incidence. However, this was at a time when the first wave had subsided and care was returning to something like normal. It is likely that views will be very different with greater concerns during and after second and subsequent waves.
3.2.7. Effects on cancer and bone research and trainees:
COVID-19 has clearly also had a major impact upon bone metastasis and bone health research. In our study, 59.3% of 59 respondents reported a negative impact of COVID-19 upon the progress of bone-related clinical trials (see Fig. 5). Indeed, this interruption to trials evoked the strongest reaction of all the questions. Many respondents reported reduced recruitment of patients to clinical trials, and the suspension of all non-COVID-related clinical trials was also frequently mentioned as a major concern. Issues with patient sampling and data capture were also highlighted. In many cases, trial suspension seems to have been a temporary measure as a significant number of the respondents reported (at the time of the questionnaire) the resumption of patient recruitment following the peak of the first wave of the pandemic. However, the impact of the second wave on non-COVID-19 clinical research, is of growing concern.
Fig. 5.
COVID-19 and Bone Research: Effects of COVID19 upon bone research was addressed by asking the following questions: (A) Have bone directed clinical trials been affected at your institution during COVID19? – 59 responses, (B) Have bone oriented or cancer oriented labs been affected at your institution during COVID19? – 66 responses, (c) Have the trainees in the cancer and bone field experienced significant changes to their learning opportunities during COVID19? – 66 responses, and (D) Have there been financial constraints to cancer and bone research funding during COVID19? – 61 responses.
Cancer-orientated laboratory research appears to have been hugely impacted by the COVID-19 pandemic with 60.7% of respondents reporting major inhibition, often with research having to be scaled back to only ‘essential’ work. The impacts reported commonly included the closure of laboratory spaces for all but COVID19-related research. The reduction in research activity was reported as easing slightly, however a frequent approach within research departments has been to re-open gradually with less than full capacity staff members and many respondents reported departmental re-opening at 10%-50% capacity. The delays to bone-related research have been particularly felt within longer timescale experiments, especially work involving animals, where delays have been substantial and many more sophisticated planned experiments have been impossible to pursue.
Effects upon trainees within the cancer and bone field have been particularly acute with 78.8% of respondents reporting decreased access to training as well as reduced training hours, increased reliance upon online training, reduced lab- and campus-access and greatly reduced face-to-face interactions with peers, with less supervision and mentorship. This has been especially problematical for researchers trying to complete PhD and postdoctoral research. The funding of bone research was raised as a concern by 44.3% of respondents with notable effects of COVID-19 upon the level of funding for non-COVID based research, fewer openings for submitting grant applications and the consumption of grant funding via less productive routes owing to the effects of COVID-19. Many respondents expressed concern for the long-term implications of COVID-19 upon bone research.
4. Discussion:
Of those clinicians and scientists who responded to the questionnaire, analysis of the number of responses to each question showed that there was strong engagement through to the end of the questionnaire (see Table 2). There was also a good response to the opportunities for provision of textual information which, although anecdotal, painted an informative picture of the impacts of the pandemic on patients and care systems involving cancer and bone.
Table 2.
Most common questions with comments.
| Q8. Have there been any delays in getting routine bone imaging such as CT due to COVID19? What is the average length of the delay?
|
| Q11. Have there been any delays in getting bone scans due to COVID19? what is the average length of the delay?
|
| Q12. Have there been any delays in getting MRI scans due to COVID19? what is the average length of the delay?
|
| Q13. Has there been an impact on palliative external beam radiotherapy at your centre due to COVID19? What is an average length of the delay?
|
| Q14. Has there been an impact upon access to stereotactic radiotherapy at your centre due to COVID19? What is an average length of the delay?
|
Q16. What percentage of your patients with bone metastases have been having remote follow-up?
|
Q41. Have bone directed clinical trials been affected at your institution during COVID19?
|
Q42. Have bone oriented or cancer-oriented labs been affected at your institution during COVID19?
|
Q43. Have the trainees in the cancer and bone field experienced significant changes to their learning opportunities during COVID19?
|
Q44. Have there been financial constraints to cancer and bone research funding during COVID19?
|
The COVID-19 pandemic has been, and still is, one of the greatest challenges ever faced by the global healthcare and medical research community. The requirement to reduce face-to-face contact in order to reduce viral transmission rates has placed strain on the healthcare systems of every country worldwide. The present questionnaire results reveal the effect of COVID-19 upon the clinical management of patients with metastatic bone disease and CTIBL, as well as the wider impacts upon the field of bone research.
In terms of the clinical management of such patients, all aspects of treatment have been impacted from bone scans, treatment for metastasis and the use of therapeutic agents in the adjuvant setting to prevent the recurrence of bone metastasis. Bone scans and radiotherapies have been administered less frequently with serious delays to normal standards of care. There has been a clear shift towards oral administration of bisphosphonates and away from i.v. infusion, enabling a reduction in outpatient visits for patients. The use of telephone and video conferencing has enabled the follow-up of patients to continue without the need for face-to-face contact. However, as the length of the pandemic has now exceeded 1 year, patients may be suffering from lack of face-to-face contact with their clinical team, with psychological effects.
Other aspects of coping during the pandemic were raised by responses to the questionnaire. Clearly there was a delay in standard treatment and some delays became built into modified standard treatments. Greater flexibility was shown in the order in which treatments were carried out, where this could assist in solving logistical problems. It is also clear that administrative and logistical issues arose because normal procedures were continually needing to be changed and staff were having to adapt to the new ‘norm’.
There seems no doubt that despite major efforts, optimal treatment of patients with bone metastases has been difficult to achieve during the pandemic. For many such patients the likely timescale of restrictions due to the pandemic is of the same order of magnitude as their survival time following bone metastasis diagnosis, typically 2–3 years. It is possible that a cohort of patients may not be able to achieve optimum quality of their remaining life because of the pandemic. However, the unexpected ‘experiment’ in care delivery imposed by the COVID-19 pandemic may uncover the possibility that our “optimal” regimens may have been over treating some patients. In the absence of prospectively designed studies, it will be challenging to evaluate the impact of COVID-19 on bone specific endpoints. Long-term effects on patients susceptible to CTIBL may also become more significant.
Some care in interpretation of the questionnaire needs to be emphasised. It represents a particular snapshot in time from a workforce with special interests in cancer and the skeleton, not representative of the wider body of practicing oncologists. However, this also has benefits in that the resulting recommendations, including those in Table 3, are available to all oncologists, including non-bone specialists. Our purpose is to highlight major effects of the pandemic and how these can best be mitigated, as perceived by a group of experts from a range of countries involved in day-to-day care of patients with cancer and its impact on bone. The respondents were heavily weighted towards oncologists and mainly medical oncologists. The perceptions of the respondents may therefore not be representative of those of radiation oncologists, surgical oncologists, haematologists or clinicians managing benign bone disease.
The above evidence has clearly demonstrated that clinicians have responded to the necessity, caused by COVID-19, of adapting normal practice in the treatment of metastatic bone disease and other scenarios involving cancer and bone. We believe it is useful to bring together recommendations on how national and international guidelines may be adapted to provide best continuing care, given the continuing pandemic. These recommendations are contained in Table 3 for breast cancer, prostate cancer, other solid tumours and multiple myeloma.
In the vital area of research into cancer and bone, there has been a marked decrease in activity and this seems likely to continue for many months. This is well documented in a recent Lancet Oncology article, which demonstrates a 60% decrease in new clinical trials for cancer drugs and biological therapies during the pandemic [23]. New funding opportunities available for work outside of the field of COVID-19-related research have been restricted. The recruitment and training of laboratory staff has also been very severely impacted with reduced face-to-face supervision of students, increased online training and extension of deadlines necessary for submission of both grant applications and outputs of work such as doctoral theses. The overall impact on research into cancer and bone is likely to be substantial and may be long-lasting.
Despite the promise of effective vaccines, it has become clear that COVID-19 will remain a serious healthcare challenge well into 2021 and beyond. Providing the best care for patients with cancer and bone involvement, as well as improving the outlook for such patients via continued scientific and medical research will be key challenges going forwards during and following the COVID-19 pandemic. Whilst there is evidence that some normality returned to this field following the first wave, the severity of the second and subsequent waves and the effects of more easily transmitted variants, suggest that the issues restricting optimum treatment of patients with metastatic bone disease or CTIBL will continue for many months. At that time it will be important to consider, through new programmes of research, the possible long term effects of the restrictions caused by the pandemic on patients suffering from the effects of cancer on the skeleton.
CRediT authorship contribution statement
J.E. Brown: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Project administration. S.L. Wood: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. C. Confavreux: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. M. Abe: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. K. Weilbaecher: Conceptualization, Investigation, Writing - review & editing. P. Hadji: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. R.W. Johnson: Conceptualization, Investigation, Writing - review & editing. J.A. Rhoades: Conceptualization, Investigation, Writing - review & editing. C.M. Edwards: Conceptualization, Investigation, Writing - review & editing. P.I. Croucher: Conceptualization, Investigation, Writing - review & editing. P. Juarez: Conceptualization, Investigation, Writing - review & editing. S. El Badri: Conceptualization, Investigation, Writing - review & editing. G. Ariaspinilla: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. S. D’Oronzo: Conceptualization, Investigation, Writing - review & editing. T.A. Guise: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. C. Van Poznak: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Funding:
No funding was required for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100375.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Almaghlouth N.K., Davis M.G., Davis M.A. Risk factors for mortality among patients with SARS-CoV-2 infection: A longitudinal observational study. J Med Virol. 2020 doi: 10.1002/jmv.26560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman R.E., Croucher P.I., Padhani A.R. Bone metastases. Nat Rev Dis Primers. 2020;6(1):83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 3.Barwick B.G., Gupta V.A., Vertino P.M., Boise L.H. Cell of Origin and Genetic Alterations in the Pathogenesis of Multiple Myeloma. Front Immunol. 2019;10:1121. doi: 10.3389/fimmu.2019.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravalos C., Rodriguez C., Sabino A. SEOM Clinical Guideline for bone metastases from solid tumours. Clin Transl Oncol. 2016;18(12):1243–1253. doi: 10.1007/s12094-016-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kan C., Vargas G., Pape F.L., Clezardin P. Cancer Cell Colonisation in the Bone Microenvironment. Int J Mol Sci. 2016;17(10) doi: 10.3390/ijms17101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roelofs A.J., Thompson K., Ebetino F.H. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des. 2010;16(27):2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 7.Sousa S., Clezardin P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcif Tissue Int. 2018;102(2):227–250. doi: 10.1007/s00223-017-0353-5. [DOI] [PubMed] [Google Scholar]
- 8.Yang M., Yu X. Management of bone metastasis with intravenous bisphosphonates in breast cancer: a systematic review and meta-analysis of dosing frequency. Support Care Cancer. 2020;28(6):2533–2540. doi: 10.1007/s00520-020-05355-7. [DOI] [PubMed] [Google Scholar]
- 9.Clemons M., Ong M., Stober C. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur J Cancer. 2021;142:132–140. doi: 10.1016/j.ejca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himelstein A.L., Foster J.C., Khatcheressian J.L. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hortobagyi G.N., Van Poznak C., Harker W.G. Continued Treatment Effect of Zoledronic Acid Dosing Every 12 vs 4 Weeks in Women With Breast Cancer Metastatic to Bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA Oncol. 2017;3(7):906–912. doi: 10.1001/jamaoncol.2016.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amadori D., Aglietta M., Alessi B. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 13.Ng T.L., Tu M.M., Ibrahim M.F.K. Long-term impact of bone-modifying agents for the treatment of bone metastases: a systematic review. Support Care Cancer. 2021;29(2):925–943. doi: 10.1007/s00520-020-05556-0. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro C.L., Moriarty J.P., Dusetzina S. Cost-Effectiveness Analysis of Monthly Zoledronic Acid, Zoledronic Acid Every 3 Months, and Monthly Denosumab in Women With Breast Cancer and Skeletal Metastases: CALGB 70604 (Alliance) J Clin Oncol. 2017;35(35):3949–3955. doi: 10.1200/JCO.2017.73.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J.E., Ellis S.P., Lester J.E., S Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res. 2007;13(18 Pt 1):5406–5410. doi: 10.1158/1078-0432.CCR-07-0247. [DOI] [PubMed] [Google Scholar]
- 16.Anastasilakis A.D., Polyzos S.A., Makras P., B Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J Bone Miner Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 17.Coleman R. Bisphosphonates and breast cancer - From cautious palliation to saving lives. Bone. 2020;140 doi: 10.1016/j.bone.2020.115570. [DOI] [PubMed] [Google Scholar]
- 18.Russell N., Grossmann M. Management of bone and metabolic effects of androgen deprivation therapy. Urol Oncol. 2018 doi: 10.1016/j.urolonc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Song Y., Xu Y.L., Lin Y. Fractures due to Aromatase Inhibitor Therapy for Breast Cancer: A Real-World Analysis of FAERS Data in the Past 15 Years. Oncol Res Treat. 2020;43(3):96–102. doi: 10.1159/000505376. [DOI] [PubMed] [Google Scholar]
- 20.Skolarus T.A., Caram M.V., Shahinian V.B. Androgen-deprivation-associated bone disease. Curr Opin Urol. 2014;24(6):601–607. doi: 10.1097/MOU.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro C.L., Van Poznak C., Lacchetti C. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J Clin Oncol. 2019 Nov 1;37(31):2916–2946. doi: 10.1200/JCO.19.01696. Epub 2019 Sep 18PMID: 31532726. [DOI] [PubMed] [Google Scholar]
- 22.Brown J.E., Handforth C., Compston J.E. Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group, J Bone Oncol. 2020;25 doi: 10.1016/j.jbo.2020.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson E. Dramatic drop in new cancer drug trials during the COVID-19 pandemic. Lancet Oncol. 2021 doi: 10.1016/S1470-2045(21)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Poznak C., Somerfield M.R., Barlow W.E. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol. 2017 Dec 10;35(35):3978–3986. doi: 10.1200/JCO.2017.75.4614. [DOI] [PubMed] [Google Scholar]
- 25.Dhesy-Thind S., Fletcher G.G., Blanchette P.S. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017 Jun 20;35(18):2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 26.Saylor P.J., Rumble R.B., Tagawa S. Bone Health and Bone-Targeted Therapies for Prostate Cancer: ASCO Endorsement of a Cancer Care Ontario Guideline. J Clin Oncol. 2020;38:1736–1743. doi: 10.1200/JCO.19.03148. [DOI] [PubMed] [Google Scholar]
- 27.Anderson K., Ismaila N., Kyle R.A. Role of Bone-Modifying Agents in Multiple Myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Practice. 2018;14:266–269. doi: 10.1200/JOP.17.00013. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R, Hadji P, Body J-J, et al., Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 202; 31: 165-1663. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.