Abstract
Background
COVID-19 is usually less severe and has lower case fatality in children than in adults. We aimed to characterise the clinical features of children and adolescents hospitalised with laboratory-confirmed SARS-CoV-2 infection and to evaluate the risk factors for COVID-19-related death in this population.
Methods
We did an analysis of all patients younger than 20 years who had quantitative RT-PCR-confirmed COVID-19 and were registered in the Influenza Epidemiological Surveillance Information System (SIVEP-Gripe, a nationwide surveillance database of patients admitted to hospital with severe acute respiratory disease in Brazil), between Feb 16, 2020, and Jan 9, 2021. The primary outcome was time to recovery (discharge) or in-hospital death, evaluated by competing risks analysis using the cumulative incidence function.
Findings
Of the 82 055 patients younger than 20 years reported to SIVEP-Gripe during the study period, 11 613 (14·2%) had available data showing laboratory-confirmed SARS-CoV-2 infection and were included in the sample. Among these patients, 886 (7·6%) died in hospital (at a median 6 days [IQR 3–15] after hospital admission), 10 041 (86·5%) patients were discharged from the hospital, 369 (3·2%) were in hospital at the time of analysis, and 317 (2·7%) were missing information on outcome. The estimated probability of death was 4·8% during the first 10 days after hospital admission, 6·7% during the first 20 days, and 8·1% at the end of follow-up. Probability of discharge was 54·1% during the first 10 days, 78·4% during the first 20 days, and 92·0% at the end of follow-up. Our competing risks multivariate survival analysis showed that risk of death was increased in infants younger than 2 years (hazard ratio 2·36 [95% CI 1·94–2·88]) or adolescents aged 12–19 years (2·23 [1·84–2·71]) relative to children aged 2–11 years; those of Indigenous ethnicity (3·36 [2·15–5·24]) relative to those of White ethnicity; those living in the Northeast region (2·06 [1·68–2·52]) or North region (1·55 [1·22–1·98]) relative to those in the Southeast region; and those with one (2·96 [2·52–3·47]), two (4·96 [3·80–6·48]), or three or more (7·28 [4·56–11·6]) pre-existing medical conditions relative to those with none.
Interpretation
Death from COVID-19 was associated with age, Indigenous ethnicity, poor geopolitical region, and pre-existing medical conditions. Disparities in health care, poverty, and comorbidities can contribute to magnifying the burden of COVID-19 in more vulnerable and socioeconomically disadvantaged children and adolescents in Brazil.
Funding
National Council for Scientific and Technological Development, Research Support Foundation of Minas Gerais.
Introduction
The emergence of COVID-19 was first recognised in January, 2020, and the disease rapidly spread worldwide, being declared a global pandemic by WHO in March, 2020.1 Brazil has been one of the countries most severely affected by COVID-19, with more than 16 million cases and 450 000 deaths reported by May, 2021. A new surge in cases and deaths began in February, 2021, mainly related to a failure to mitigate the spread of a novel SARS-CoV-2 variant of concern, P.1, first recognised through genomic surveillance in the city of Manaus in January, 2021.2
Although COVID-19 occurs in all age groups, epidemiological evidence has consistently shown that children typically have less severe disease than adults.3, 4, 5 Nevertheless, with the progression of the pandemic worldwide, severe and life-threatening manifestations of the disease in paediatric patients have emerged, including multisystem inflammatory syndrome in children (MIS-C).6, 7 In addition, a systematic review has highlighted a greater impact of paediatric COVID-19 fatality in low-income and middle-income countries compared with high-income countries.8 To date, however, few paediatric cohort studies have directly investigated the risk factors associated with severe COVID-19.9 Further understanding these issues in children could provide important insights and guide the development of therapeutic and vaccination strategies for target groups.
Research in context.
Evidence before this study
Preliminary evidence, mostly from high-income countries, suggests that the clinical outcomes of COVID-19 are more favourable in children than in adults. Several studies in adults have shown that the compounding issues of racial and health-related disparities are strongly associated with the incidence and outcomes of COVID-19. We searched PubMed, Google Scholar, medRxiv, and bioRxiv on Feb 20 2021, for studies published in English since Feb 1, 2020, that described the outcomes of children with COVID-19. We used the search terms “COVID-19”, “children”, “race”, “heath disparity”, and related synonyms. Scarce data are available on the effects of ethnicity, comorbidities, social inequalities, and health disparities on outcomes in children infected with SARS-CoV-2 from middle-income or low-income countries.
Added value of this study
We describe the clinical outcomes and risk factors for death in 11 613 hospitalised children and adolescents with laboratory-confirmed SARS-CoV-2 infection in Brazil. To our knowledge, this is the largest clinical study of COVID-19 in the paediatric population to date. The data provide evidence of the effects of regional and geographical inequalities, health disparities, poverty, and ethnicity on outcomes of hospitalised children and adolescents with COVID-19 in a middle-income country.
Implications of all the available evidence
Hospitalised children and adolescents at the more severe end of the spectrum of COVID-19 in a middle-income country appear to have worse clinical outcomes than those in higher-income countries. Social and biological factors might act synergistically to increase the burden of the disease and its outcomes for this vulnerable population. The specific needs of more susceptible subgroups of paediatric patients should be considered in the context of future directions for preventive and therapeutic strategies in these populations.
In a previous study based on a nationwide database, Ranzani and colleagues10 retrospectively analysed the first 250 000 hospital admissions for COVID-19 among patients aged 20 years or older in Brazil. Using the same database, we investigated paediatric cases of COVID-19, with the aim of characterising the features of hospitalised children and adolescents (aged <20 years) with laboratory-confirmed SARS-CoV-2 infection and evaluating the risk factors for COVID-19-related death in this population.
Methods
Study design and participants
We analysed all COVID-19 cases in hospitalised children and adolescents (aged <20 years) recorded in the Influenza Epidemiological Surveillance Information System, SIVEP-Gripe (Sistema de Informação de Vigilância Epidemiológica da Gripe), a nationwide database established by the Brazilian Ministry of Health in 2009 for the surveillance of severe acute respiratory infections.10, 11 SIVEP-Gripe has been the primary source of information on COVID-19-related hospital admissions and deaths in Brazil. COVID-19 notification is compulsory in Brazil, and SIVEP-Gripe receives notifications of patients admitted to both public and private hospitals. For all patients enrolled in the system, data regarding demographic and clinical features are systematically registered. Our period of analysis was from Feb 16, 2020, to Jan 9, 2021 (epidemiological week 8, 2020, to epidemiological week 1, 2021). We included all patients consecutively registered in SIVEP-Gripe who were younger than 20 years, had a positive quantitative RT-PCR (RT-qPCR) test result for SARS-CoV-2, and had been admitted to hospital.
To enter the SIVEP-Gripe database, cases must present with flu-like symptoms and at least one of the following: dyspnoea, respiratory distress, oxygen saturation less than 95% in room air, cyanosis, or symptoms specific to children (intercostal retractions, nasal flaring, dehydration, or inappetence). Additionally, they must have been admitted to hospital or died without hospital admission. Detailed information about SIVEP-Gripe is available in the appendix (p 1). Through a preliminary analysis of SIVEP-Gripe data, we found that only around 60% of the selected sample met these strict criteria for database entry; therefore, we created a subset comprising only cases that fulfilled the criteria. In this subset, we did a sensitivity analysis to ascertain the robustness of our analysis (described in detail in the appendix [p 2]).
Data in SIVEP-Gripe are deidentified and publicly available. Following ethically agreed principles on open data, this analysis did not require ethical approval in Brazil.
Covariables and definitions
Demographic patient data included age, sex, ethnicity, and geopolitical macroregion (North, Northeast, Central-West, Southeast, and South, between which there are historical differences in social, economic, and health system capacity and coverage). Ethnicity was self-identified and based on the five classifications defined by the Brazilian Institute of Geography and Statistics: Branco (White), Preto (Black), Pardo (Brown), Amarelo (Asian), or Indígena (Indigenous).12 Clinical data included the date of symptom onset (defined as the day when the first symptom or sign occurred) and the date of admission to hospital. Signs and symptoms at presentation (fever, cough, respiratory distress, gastrointestinal symptoms, and reduced oxygen saturation) and presence of pre-existing comorbidities (heart disease, pulmonary disease, asthma, kidney disease, neurological disorder [including developmental delay], haematological disease, diabetes, obesity, immune deficiency, malignancy, post-transplant status, and chromosomal abnormality or syndromes) were also recorded. For analysis, the presence of comorbidities was dichotomised (yes or no) and categorised into four levels (none, one, two, and three or more pre-existing medical conditions). The presence of other risk factors, including pregnancy and puerperal period in adolescents, was also described. Clinical course was reported in terms of respiratory support (none, non-invasive oxygen support, and invasive ventilation), admission to an intensive care unit (ICU), recovery, death, and ongoing clinical situation. Date of death or discharge was also registered.
Information regarding clinical and demographic data available in SIVEP-Gripe and the recodification process are available in the appendix (p 1).
We had no access to hospital record data to allow the inclusion of information regarding pharmacotherapies, laboratory results, or the detailed clinical course of the patient. To minimise some of these limitations, one author (EAO) carefully reviewed the registry forms of all cases included in the study, including a thorough evaluation of the observations text field, which provided invaluable information about comorbidities and other clinical issues.
Outcomes
The primary outcome was time to in-hospital death. Survival time was defined from the day of admission until death or discharge. We also evaluated admission to ICU or provision of respiratory support (none, non-invasive, or invasive).
Statistical analysis
The sample included all patients younger than 20 years who had COVID-19 and were registered in the database between epidemiological week 8, 2020, and week 1, 2021. Complete data were not available for all variables, especially ethnicity and symptoms at presentation, so denominators differ between analyses. We did not impute missing data for these analyses. For those with missing data on a given comorbidity, we assumed that clinical condition to be absent.
We used medians and IQRs or means and SDs to summarise continuous variables, and calculated frequencies and proportions for categorical variables. We examined the spatial and temporal development of the COVID-19 epidemic (total cases and deaths) throughout the country by dividing our sample into quartiles, corresponding to four intervals of epidemiological weeks: 8–23, 24–30, 31–40, and 41–52 (including epidemiological week 1 of 2021).
Death was evaluated by competing risks analysis using the cumulative incidence function.13 Discharge was analysed as a competing event by competing risks analysis. The proportional sub-distribution hazards model by Fine and Gray was fitted to estimate the effect of covariates on mortality.14, 15 Covariates used in multivariate analyses were chosen on the basis of having a p value of less than 0·10 in the univariate analysis. Variables in the final model with a p value of less than 0·05 were considered statistically significant. The results are expressed as adjusted hazard ratios (HRs) and 95% CIs.
Role of the funding source
The funder of the study had no role in study design or conduct; data collection, management, analysis, or interpretation; or writing of the report.
Results
Between Feb 16, 2020, and Jan 21, 2021, 1 134 873 cases of severe acute respiratory illness were reported in SIVEP-Gripe. Among them, 10 184 (0·9%) cases were caused by other laboratory-confirmed viruses. 82 055 (7·3%) of the remaining 1 124 689 patients were younger than 20 years at presentation. Of these patients, we excluded 70 442 (85·8%) on the basis of SARS-CoV-2 RT-qPCR test data (49 417 negative for SARS-CoV-2, 9995 still awaiting results, 4307 not tested, 402 with inconclusive results, and 6321 with test information missing from the database). The final sample comprised 11 613 patients younger than 20 years who were hospitalised and had a confirmed positive RT-qPCR test result for SARS-CoV-2 (appendix p 3).
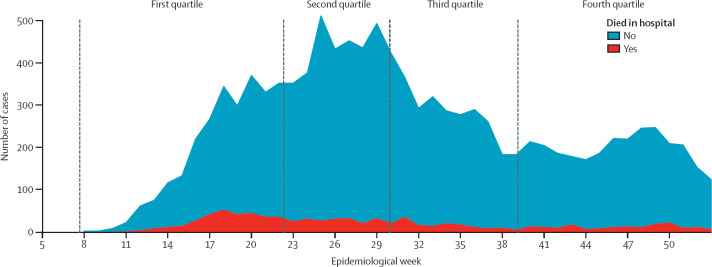
Cases of laboratory-confirmed SARS-CoV-2 infection and deaths among hospitalised people younger than 20 years over time are shown in figure 1 . It took around 16 weeks (epidemiological weeks 8–23) to reach the first quartile of cases (n=3006), 6 weeks (epidemiological weeks 24–30) to reach the second quartile (n=3172), 9 weeks (epidemiological weeks 31–40) to reach the third quartile (n=2730), and 12 weeks (epidemiological weeks 41–52) to reach the fourth quartile (n=2705). A significantly higher proportion of patients died in hospital (12·1%) in the first quartile (epidemiological weeks 8–23) than in the subsequent quartiles (6·7% in the second quartile, 5·9% in the third quartile, and 6·5% in the fourth quartile; p<0·0001).
Figure 1.
Incident cases of COVID-19 and deaths in hospitalised children and adolescents through 46 epidemiological weeks in Brazil
The median age at admission was 5·1 years (IQR 1·0–14·3; table 1 ). Patients were roughly evenly distributed among the three age groups (<2 years, 2–11 years, and 12–19 years]). There was a slight predominance of boys (6032 [52·0%] of 11 600 with available data), and Black or Brown patients accounted for about half of the sample (5784 [63·5%] of 9109). The most frequent signs and symptoms were fever, cough, respiratory distress, and dyspnoea. Respiratory distress was strongly associated with low oxygen saturation (p<0·0001). Among 11 613 cases, 8352 (71·9%) individuals had no pre-existing medical conditions, 2780 (23·9%) cases had one comorbidity, and 481 (4·1%) individuals had two or more pre-existing medical conditions. The most common conditions were asthma, neurological disorders, malignancies, and heart disease (table 1). Clinical and demographic characteristics by geopolitical macroregion are shown in the appendix (p 4). Among the general population of young people in each region, COVID-19 cases were most prevalent in the North region (330·4 cases per million) and Central-West region (330·0 cases per million). Among the study sample of hospitalised young people with COVID-19, the proportion of patients of Black or Brown ethnicity was highest in the North region (1501 [85·3%] of 1759 patients) and Northeast region (2204 [85·4%] of 2581), and the proportion of those of Indigenous ancestry was highest in the North region (90 [5·1%] of 1759).
Table 1.
Demographic and clinical characteristics of children with quantitative RT-PCR-confirmed SARS-CoV-2 infection
| Patients (n=11 613) | |
|---|---|
| Age, years | |
| Median (IQR) | 5·1 (1·0–14·3) |
| Mean (SD) | 7·5 (6·9) |
| <2 | 4051 (34·9%) |
| 2–11 | 3973 (34·2%) |
| 12–19 | 3589 (30·9%) |
| Sex | |
| Male | 6032/11 600 (52·0%) |
| Female | 5568/11 600 (48·0%) |
| Macroregion | |
| Southeast | 4065/11 613 (35·0%) |
| South | 963/11 613 (8·3%) |
| Central-West | 1356/11 613 (11·7%) |
| Northeast | 3388/11 613 (29·2%) |
| North | 1841/11 613 (15·9%) |
| Ethnicity | |
| White | 3191/9181 (34·8%) |
| Black or Brown | 5784/9181 (63·0%) |
| Asian | 79/9181 (0·9%) |
| Indigenous | 127/9181 (1·4%) |
| Signs and symptoms at presentation | |
| Fever | 7604/10 212 (74·5%) |
| Cough | 6673/9905 (67·4%) |
| Respiratory distress | 5056/9296 (54·4%) |
| Oxygen saturation <95% | 3685/8967 (41·1%) |
| Dyspnoea | 5527/9601 (57·6%) |
| Odynophagia | 2038/8512 (23·9%) |
| Anosmia | 336/4601 (7·3%) |
| Ageusia | 316/4600 (6·9%) |
| Diarrhoea | 1800/8619 (20·9%) |
| Vomiting | 1983/8651 (22·9%) |
| Abdominal pain | 668/4767 (14·0%) |
| Number of comorbidities | |
| 0 | 8352/11 613 (71·9%) |
| 1 | 2780/11 613 (23·9%) |
| 2 | 403/11 613 (3·5%) |
| ≥3 | 78/11 613 (0·7%) |
| Type of comorbidity | |
| Asthma | 867/11 613 (7·5%) |
| Pulmonary disease | 240/11 613 (2·1%) |
| Neurological disorder | 706/11 613 (6·1%) |
| Malignancy | 581/11 613 (5·0%) |
| Heart disease | 379/11 613 (3·3%) |
| Haematological disease | 269/11 613 (2·3%) |
| Kidney disease | 173/11 613 (1·5%) |
| Diabetes | 210/11 613 (1·8%) |
| Obesity | 162/11 613 (1·4%) |
| Syndrome or chromosomal aberration | 219/11 613 (1·9%) |
Data are n/N (%) of those with available data. For comorbidities, we assumed missing data to indicate the absence of the given clinical condition.
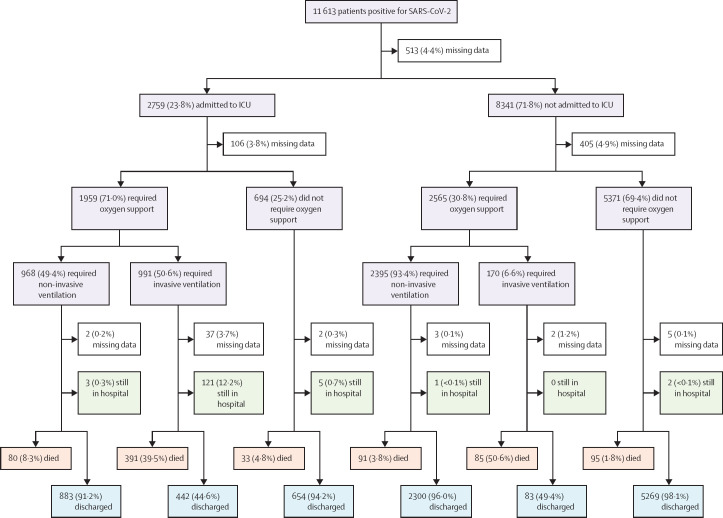
The median time from symptom onset to hospitalisation was 3 days (IQR 1–6). 2759 (23·8%) individuals were admitted to ICU, for a median duration of 5 days (2–12). Among all 11 613 patients, 6203 (53·4%) did not require respiratory support at any stage, 3399 (29·3%) needed non-invasive oxygen support, 1167 (10·0%) required invasive ventilation, and for 844 (7·3%) this information was not available. Figure 2 shows clinical outcomes according to ICU admission and requirement for oxygen support. 10 410 patients (89.6%) were alive at the time of analysis (including 10 041 [86·5%] who had been discharged and 369 [3·2%] who were still in hospital when the database was analysed), and 317 (2·7%) were missing information on outcome. 886 (7·6%) patients died in hospital, at a median 6 days (3–15) after hospital admission.
Figure 2.
Clinical outcomes at the time of database download (Jan 10, 2021), according to ICU admission and oxygen support
Of 513 children with missing data regarding ICU admission, 111 (21·6%) died in hospital. ICU=intensive care unit.
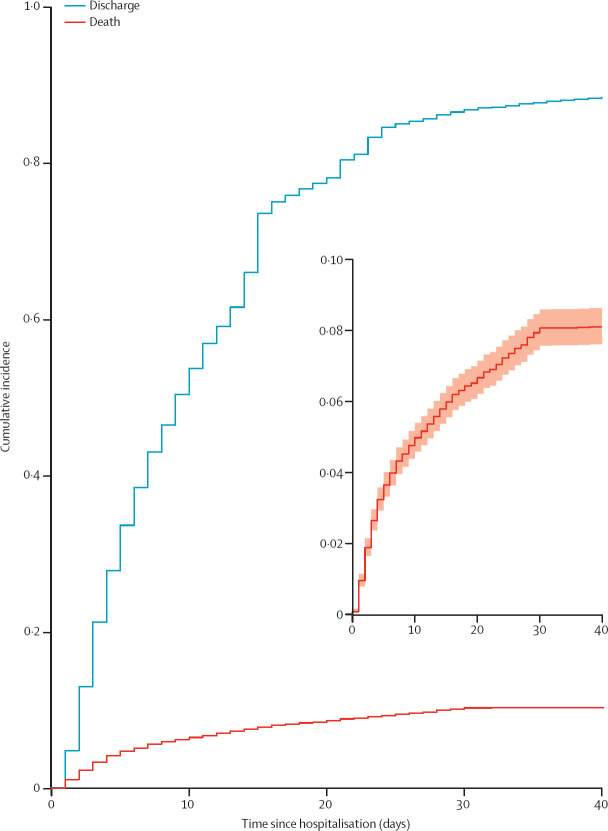
The cumulative incidence functions for death and discharge are shown in figure 3 . The estimated probability of death was 4·8% during the first 10 days after hospital admission, 6·7% during the first 20 days, and 8·1% at the end of follow-up. Probability of discharge was 54·1% during the first 10 days, 78·4% during the first 20 days, and 92·0% at the end of follow-up. The univariate competing risks analysis for death with respect to demographic characteristics and clinical features in the study sample are shown in the appendix (p 5). Using the Fine and Gray model, risk of death was increased in infants younger than 2 years (HR 1·88 [95% CI 1·58–2·24]) and adolescents aged 12–19 years (1·98 [1·65–2·36]) relative to children aged 2–11 years, and in patients from the Northeast region (2·03 [1·72–2·38])and North region (1·53 [1·25–1·87]) relative to those from the Southeast region. Compared with White patients, children of Black or Brown ethnicity (1·30 [1·11–1·53]) and Indigenous ethnicity (3·27 [2·17–4·93]) were also at increased risk of death (appendix p 5). With respect to signs and symptoms at presentation, the presence of respiratory distress (2·78 [2·35–3·29]) and oxygen saturation below 95% (3·66 [3·11–4·30]) were strongly associated with death, whereas fever, cough, and gastrointestinal symptoms were not associated with death. The presence of any comorbidity and the number of comorbidities (one, two, or three or more vs none) increased the risk of death in the univariable analysis, as did all the main specific pre-existing medical conditions analysed, with the exception of asthma, which decreased the risk of death (0·60 [0·42–0·85]).
Figure 3.
Cumulative incidence functions for death and discharge
Day 0 represents the day of hospital admission. The inset panel shows the cumulative incidence function for death (the primary outcome) in detail, with the shaded region showing 95% CI.
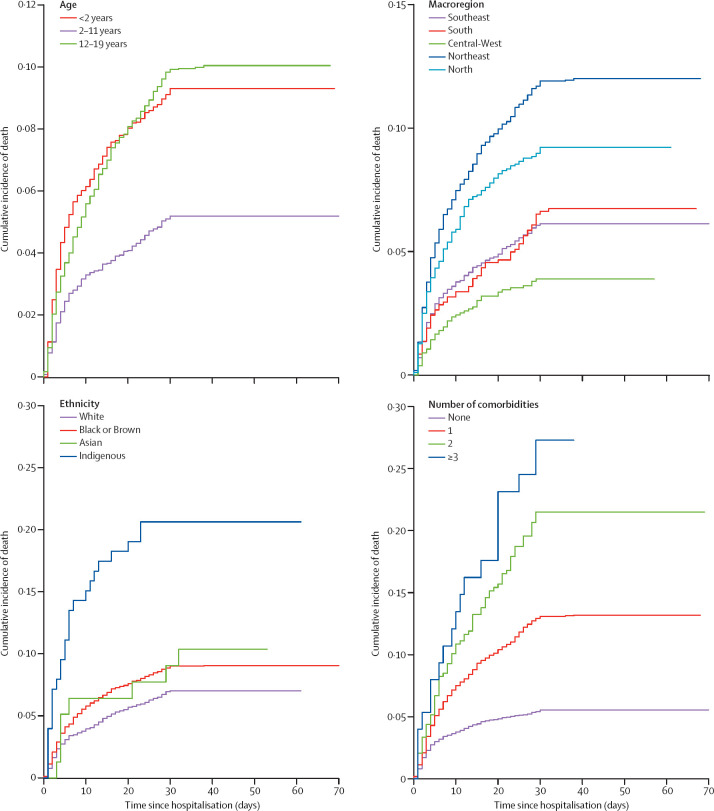
After adjustment for the competing risk of discharge through multivariate regression analysis, factors associated with death were age younger than 2 years (HR 2·36 [95% CI 1·94–2·88], p<0·0001) or 12–19 years (2·23 [1·84–2·71], p<0·0001), living in the Northeast region (2·06 [1·68–2·52], p<0·0001) or North region (1·55 [1·22–1·98], p<0·0001), Indigenous ethnicity (3·36 [2·15–5·24], p<0·0001), and presence of one (2·96 [2·52–3·47], p<0·0001), two (4·96 [3·80–6·48], p<0·0001), or three or more (7·28 [4·56–11·60, p<0·0001) pre-existing comorbidities (table 2 ). Figure 4 shows the cumulative incidence functions for death in the presence of these risk factors.
Table 2.
Multivariate competing risks analysis of discharge and in-hospital death
|
Discharge |
In-hospital death |
||||||
|---|---|---|---|---|---|---|---|
| Events, n (%) | HR (95% CI) | p value | Events, n (%) | HR (95% CI) | p value | ||
| Overall* | 10 041 (91·9%) | .. | .. | 886 (8·1%) | .. | .. | |
| Age, years | |||||||
| <2 | 3558/10 041 (35·4%) | 0·82 (0·78–0·86) | <0·0001 | 194/886 (21·9%) | 2·36 (1·94–2·88) | <0·0001 | |
| 2–11 | 3453/10 041 (34·4%) | 1 (ref) | .. | 354/886 (40·0%) | 1 (ref) | .. | |
| 12–19 | 3030/10 041 (30·2%) | 0·73 (0·70–0·77) | <0·0001 | 338/886 (38·1%) | 2·23 (1·84–2·71) | <0·0001 | |
| Macroregion | |||||||
| Southeast | 3648/10 041 (36·3%) | 1 (ref) | .. | 236/886 (26·6%) | 1 (ref) | .. | |
| South | 881/10 041 (8·8%) | 1·03 (0·95–1·13) | 0·41 | 63/886 (7·1%) | 1·16 (0·87–1·55) | 0·227 | |
| Central-West | 1183/10 041 (11·8%) | 0·69 (0·65–0·74) | <0·0001 | 49/886 (5·5%) | 0·80 (0·56–1·13) | 0·202 | |
| Northeast | 2751/10 041 (27·4%) | 0·66 (0·63–0·71) | <0·0001 | 377/886 (42·6%) | 2·06 (1·68–2·52) | <0·0001 | |
| North | 1578/10 041 (15·7%) | 0·82 (0·76–0·87) | <0·0001 | 161/886 (18·2%) | 1·55 (1·22–1·98) | <0·0001 | |
| Ethnicity† | |||||||
| White | 2854/7964 (35·8%) | 1 (ref) | .. | 214/741 (28·9%) | 1 (ref) | .. | |
| Black or Brown | 4941/7964 (62·0%) | 0·95 (0·90–1·00) | 0·072 | 493/741 (66·5%) | 1·12 (0·92–1·35) | 0·213 | |
| Asian | 69/7964 (0·9%) | 0·90 (0·71–1·14) | 0·42 | 8/741 (1·1%) | 1·45 (0·71–2·99) | 0·312 | |
| Indigenous | 100/7964 (1·3%) | 0·70 (0·56–0·88) | 0·0026 | 26/741 (3·5%) | 3·36 (2·15–5·24) | <0·0001 | |
| Number of comorbidities | |||||||
| 0 | 7399/10 041 (73·7%) | 1 (ref) | .. | 438/886 (49·4%) | 1 (ref) | .. | |
| 1 | 2291/10 041 (22·8%) | 0·72 (0·68–0·76) | <0·0001 | 347/886 (39·2%) | 2·96 (2·52–3·47) | <0·0001 | |
| 2 | 298/10 041 (3·0%) | 0·49 (0·43–0·55) | <0·0001 | 81/886 (9·1%) | 4·96 (3·80–6·48) | <0·0001 | |
| ≥3 | 53/10 041 (0·5%) | 0·37 (0·28–0·49) | <0·0001 | 20/886 (2·3%) | 7·28 (4·56–11·60) | <0·0001 | |
369 cases were still hospitalised (censored in the analysis).
317 cases were missing primary outcome data.
2222 cases were missing ethnicity data.
Figure 4.
Cumulative incidence functions for death of children and adolescents with COVID-19 according to age group, macroregion, ethnicity, and number of comorbidities
We did a sensitivity analysis in the subset of 7081 cases who fulfilled the strict criteria for entry into the SIVEP-Gripe database. Cases included in this subset had a more severe spectrum of the disease. 2047 (28·9%) individuals were admitted to ICU, 964 (13·6%) required invasive ventilation, and 714 (10·1%) patients died. When discharge was treated as a competing event, despite the increased severity of cases in this subset, the results were similar to those based on the entire set of 11 613 cases, with the same covariates associated with death in the multivariate analysis: age younger than 2 years (HR 2·31 [95% CI 1·85–2·88, p<0·0001) or 12–19 years (2·47 [1·99–3·01], p<0·0001), living in the Northeast region (2·18 [1·74–2·73], p<0·0001) or North region (1·52 [1·16–1·97], p=0·0023), Indigenous ethnicity (3·24 [2·02–5·17], p<0·0001), and presence of one (2·58 [2·15–3·09], p<0·0001), two (4·18 [3·13–5·58], p<0·0001), or three or more (6·31 [3·85–10·3], p<0·0001) pre-existing comorbidities (appendix p 8). Complete results are presented in the appendix (pp 6–13).
Discussion
We evaluated a nationwide database of hospitalised children and adolescents with symptomatic laboratory-confirmed COVID-19 during the first year of the pandemic in Brazil. To our knowledge, this is the largest clinical study of COVID-19 in a paediatric population to date. The inclusion of such a substantial number of cases was made possible because of mandatory reporting of all cases of severe acute respiratory illness in Brazil, established by the Ministry of Health in 2009 during the H1N1 influenza pandemic.10, 11 With the arrival of COVID-19, the registry form of the Brazilian Ministry of Health was updated to include information on SARS-CoV-2, allowing this study to provide one of the most detailed epidemiological datasets of COVID-19 in hospitalised children and adolescents. Patients younger than 20 years accounted for 82 022 (7·3%) of the 1 124 689 cases in the SIVEP-Gripe database overall, but represented 11 613 (1·5%) of the 781 365 laboratory-confirmed cases of COVID-19 in the database—a percentage similar to those reported in the USA (1·7%),16 the UK (0·9%),17 and China (2%).18
In our sample, 23·8% individuals were admitted to the ICU, 10·0% required invasive ventilation, and 7·5% died. The proportion of patients admitted to critical care in our sample was higher than those reported in the UK (18%),17 in a multicentre multinational European study (13%),19 and in the USA (10%).16 Mortality was also higher than that reported in other paediatric studies.20, 21 Previous reports have shown that COVID-19 in children has a less severe pattern of disease then adults, with a small proportion of patients requiring intensive care.3, 22 In Wang and colleagues' meta-analysis of COVID-19 among 11 671 children,23 only 3·3% (95% CI 2·03–4·94) of cases were severe and mortality was 0·28% (0·19–0·39). However, unlike our study, most of these series reported data from mild, non-hospitalised, or even asymptomatic cases. In the context of hospitalised paediatric patients with COVID-19, Swann and colleagues'17 prospective cohort study in the UK showed that 116 (18%) of 632 hospitalised children were admitted to the ICU, and six (1%) of 627 patients (all six of whom had comorbidities) died in hospital.
The differences in unfavourable outcomes between this study and previous studies might be related to some of the features of our sample. First, the SIVEP-Gripe database captured data from children and adolescents who were managed within the hospital setting. In addition, testing capacity for SARS-CoV-2 in Brazil is lower than clinical demand and, therefore, many hospitalised children with symptoms consistent with COVID-19 are not appropriately tested24—for instance, of 82 055 patients younger than 20 years included in the database, 49 417 had a negative test, 11 163 had a positive test (our sample), but around 10 000 individuals did not yet have results available, 4000 were not tested, and 6000 had this information missing from the dataset. Taken together, these characteristics suggest that our sample tends to represent individuals at the more severe end of the disease spectrum, because patients with more severe disease were also more likely to be prioritised for testing. Second, Brazil is a middle-income country with substantial socioeconomic disparities, which might affect the quality of regional health services (including the availability of paediatric ICUs) and, thus, the likelihood of unfavourable clinical outcomes. For example, of the 838 patients who died in our study, 261 (31%) were not admitted to the ICU, probably because of the lack of these facilities, reflecting regional economic disparities even within the same region. The proportion of children who died without ICU support was about 36% in the poorest regions (Northeast and North), compared with about 23% in the richest regions (Southeast and South). Notably, in the systematic review reported by Kitano and colleagues,8 among 138 countries, Brazil had the highest rate of deaths in the young population, reaching about 23·6 per 1 000 000 children. Consequently, the high mortality observed in our sample is likely to reflect both the inclusion of hospitalised individuals at the more severe end of the spectrum and an apparent inability to provide the highest level of care to the most critically ill patients, especially in less developed regions.25
This study, to our knowledge, is the first large-scale study to assess competing risks in children and adolescents with COVID-19. In competing risk univariate and multivariate analyses, higher risk of death was significantly associated with age group (infants and adolescents), poor geopolitical macroregions (North and Northeast), ethnicity (Indigenous), and comorbidities. In the COVID-19 setting, because patients can recover, die, or still have an ongoing clinical situation, analysis of survival data is complicated by competing risks, and, therefore, commonly used survival analysis techniques (eg, the Kaplan-Meier method) are often not appropriate for such real-time situations.26 The competing risks model recognises effective and non-effective predictors of the outcome. Of note, in our analysis, a complex interaction emerged between social and clinical risk factors for fatal outcome. Among social factors, patients from the poorest regions of the country and of Indigenous ethnicity had a significantly higher risk of poor outcomes. Importantly, Black or Brown ethnicity was also associated with poor outcomes in the univariate analysis, but did not remain significantly associated with death after adjustment in the multivariate analysis. A possible explanation for this finding is that patients of Black or Brown ethnicity represented about 50% of our overall sample, but 85% of the sample from the North and Northeast regions (data not shown). Therefore, both variables are probably providing similar information related to social inequalities and low socioeconomic status. Several studies have showed that the interweaving issues of race, social inequality, and health disparity are strongly associated with the incidence and prognosis of COVID-19, whether in high-income or lower-income countries or in adult or paediatric populations.27, 28 Accordingly, Baqui and colleagues'12 preliminary analysis of the SIVEP-Gripe dataset including all Brazilian age groups showed increased mortality in the North region and in those of Black or Brown ethnicity.
Another important clinical question highlighted by our results is the negative effect of the presence of any pre-existing medical condition. In our study, the prevalence of comorbidity was around 30% in the overall sample, but around 50% in patients who died. When the number of comorbidities was categorised as none, one, two, or three or more, a step gradient effect was observed in the multivariate analysis, with risk of death increasing with number of pre-existing medical conditions. However, the presence of asthma was associated with a decreased risk of death. The presence of pre-existing medical conditions has been shown to be strongly associated with the prognosis of COVID-19 in adults,29 but the evidence in the paediatric setting is limited by the paucity of data. In a European multicentre study including 582 children and adolescents, multivariable analysis showed that the presence of pre-existing medical conditions was associated with ICU admission (odds ratio 3·27 [95% CI 1·67–6·42], p=0·0015).19 Additionally, Shekerdemian and colleagues30 reported that 40 (83%) of 48 paediatric patients admitted to US and Canadian paediatric ICUs had significant pre-existing comorbidities.
Our study is unique in terms of the large sample size of paediatric patients with laboratory-confirmed COVID-19, allowing analysis of the clinical characteristics, risk factors, and outcomes of SARS-CoV-2 infection in hospitalised children. However, it has several limitations. First, the sample comprised only hospitalised children, who are at the more severe end of the disease spectrum. Second, the likelihood of inappropriate selection of patients within the database could have biased the findings. Of the 32 638 patients younger than 20 years with clinically suspected COVID-19, 21 025 cases had no laboratory confirmation of the disease, for several reasons. We did several preliminary analyses to compare the clinical features and outcomes between patients with versus without laboratory-confirmed infection, and found significant differences between them, with a more favourable outcome for the not-confirmed group in terms of in-hospital death. Consequently, we decided to exclude patients without laboratory confirmation of SARS-CoV-2 infection, but this option might have contributed further to the selection of patients at the more severe spectrum of the disease for our study sample.
Another important limitation of our study is that mild cases in which the patient is not hospitalised are not included in the registry. Therefore, the main limitation concerns the generalisability of the results. Our sample comprised hospitalised children, mortality was limited to in-hospital death, and discharged patients were assumed to still be alive during the study period. Consequently, the reported clinical outcomes should be interpreted with consideration of this fundamental limitation. In addition, we were unable to assess some relevant information regarding the treatment and management of the patients, for which data were unavailable because of the nature of the database. We had no access to hospital record data to include laboratory results or information on the detailed clinical courses of the patients; therefore, for example, only four cases of possible MIS-C were anecdotally reported in the observations field of the record, but without relevant clinical details.
Missing data are inherent to the nature of a registry based on point-of-care case report forms. In this regard, around 20% of patients in our study sample were missing data on ethnicity; this percentage reached about 40% for infants. This finding is somewhat expected because of the difficulties in ascertaining this characteristic in small children in a self-report survey. Nevertheless, we cannot exclude the introduction of bias in the analysis of ethnicity. For instance, the missing data might account for absence of an association between Black or Brown ethnicity and the primary outcome in the multivariate analysis, as shown in adult cohorts. In addition, our multivariate analysis did not include variables related to some symptoms at presentation because of the heterogeneity in the data captured and the amount of missing information.
Finally, another important limitation was the absence of a national audit system, under the scope of the Brazilian Ministry of Health, to verify the consistency of the SIVEP-Gripe data. To try to overcome some of the limitations of missing variables, we carefully reviewed all forms for all cases in the study, including a thorough evaluation of the observations text field, which provided invaluable information about comorbidities and other clinical issues. We also did a sensitivity analysis in a subset of cases that met the strict criteria for entry into the SIVEP-gripe database. Our analyses showed similar results in both datasets.
In this analysis of a large, nationwide database of hospitalised children and adolescents with laboratory-confirmed COVID-19, our data showed that death was associated with age younger than 2 years or 12–19 years, Indigenous ethnicity, living in the poorest macroregions, and presence of pre-existing medical conditions. Therefore, health-care disparities and social inequalities, exacerbated by interweaving comorbidities, might have contributed synergistically to magnify the COVID-19 burden for more socioeconomically deprived and vulnerable individuals. Additionally, rates of poor outcomes in patients from Brazil, a middle-income country, were higher than those found in other studies from higher-income countries. These findings provide evidence for the effects of regional and geographical inequalities on outcomes. Furthermore, our analysis identified subgroups of higher-risk paediatric patients whose characteristics should inform the direction of preventive and therapeutic strategies for this population.
Data sharing
SIVEP-Gripe data are publicly available online at https://opendatasus.saude.gov.br/dataset/bd-srag-2020/resource/d89ea107-4a2b-4bd5-8b8b-fa1caaa96550. Our analysis code is available upon request to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported by the National Council for Scientific and Technological Development and the Research Support Foundation of Minas Gerais. We are grateful to all front-line health-care workers for their systematic collection of data for the SIVEP-Gripe database and for their efforts to tackle the COVID-19 pandemic in Brazil.
Contributors
EAO and MCLO conceptualised the study. EAO, MCLO, and EAC contributed to the methodology. EAO, MCLO, ACSS, DBM, HMJ, and LRS contributed to the investigation. EAO, MCLO, ACSS, DBM, HMJ, LRS, RHM, and EAC did the formal analysis. EAO, EAC, RHM, and ACSS prepared the original draft of the manuscript. EAO, MCLO, ACSS, and RHM contributed to reviewing and editing the manuscript. EAO and MCLO were responsible for data curation. EAO, MCLO, and EAC accessed and verified the data underlying the study. All authors had full access to all data in the study, participated in data interpretation, revised the manuscript, and approved the final version of the manuscript for publication. All authors contributed important intellectual content during manuscript drafting or revision and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work were appropriately investigated and resolved. EAO takes responsibility for the honest, accurate, and transparent reporting of the study.
Supplementary Material
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021 doi: 10.1126/science.abh2644. published online April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Rinaldi E, Zusi C, Beatrice G, Saccomani MD, Dalbeni A. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: a meta-analysis. Pediatr Res. 2021;89:733–737. doi: 10.1038/s41390-020-1015-2. [DOI] [PubMed] [Google Scholar]
- 5.Singh T, Heston SM, Langel SN, et al. Lessons from COVID-19 in children: key hypotheses to guide preventative and therapeutic strategies. Clin Infect Dis. 2020;71:2006–2013. doi: 10.1093/cid/ciaa547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitano T, Kitano M, Krueger C, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastos LS, Niquini RP, Lana RM, et al. COVID-19 and hospitalizations for SARI in Brazil: a comparison up to the 12th epidemiological week of 2020. Cad Saude Publica. 2020;36 doi: 10.1590/0102-311X00070120. [DOI] [PubMed] [Google Scholar]
- 12.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8:e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Zuccaro V, Celsa C, Sambo M, et al. Competing-risk analysis of coronavirus disease 2019 in-hospital mortality in a Northern Italian centre from SMAtteo COvid19 REgistry (SMACORE) Sci Rep. 2021;11 doi: 10.1038/s41598-020-80679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus disease 2019 in children—United States, Feb 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 21.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JG, Zhong ZJ, Mo YF, Wang LC, Chen R. Epidemiological features of coronavirus disease 2019 in children: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:1146–1157. doi: 10.26355/eurrev_202101_24685. [DOI] [PubMed] [Google Scholar]
- 24.Silveira MF, Barros AJD, Horta BL, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26:1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 25.Castro MC, Kim S, Barberia L, et al. Spatiotemporal pattern of COVID-19 spread in Brazil. Science. 2021 doi: 10.1126/science.abh1558. published online April 14. [DOI] [PubMed] [Google Scholar]
- 26.McCaw ZR, Tian L, Vassy JL, et al. How to quantify and interpret treatment effects in comparative clinical studies of COVID-19. Ann Intern Med. 2020;173:632–637. doi: 10.7326/M20-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SIVEP-Gripe data are publicly available online at https://opendatasus.saude.gov.br/dataset/bd-srag-2020/resource/d89ea107-4a2b-4bd5-8b8b-fa1caaa96550. Our analysis code is available upon request to the corresponding author.