Abstract
Background
The new coronavirus disease (COVID-19) has caused more than 1.8 million deaths, with a fatality rate of 2.5% in more than 200 countries as of January 4, 2021. Analysis of COVID-19 clinical features can help predict disease severity and risk of mortality, early identification of high-risk patients, and provide knowledge to inform clinical interventions.
Objective
The purpose of this study is to investigate the clinical characteristics and possible predictors associated with mortality in patients with COVID-19 admitted to King Fahad (KFH), Ohood, and Miqat hospitals in Madina, Saudi Arabia.
Methods
This retrospective observational study to investigate the clinical characteristic and possible predictors associated with mortality for those 119 mild, moderate, or critically ill patients confirmed by laboratory results to have COVID-19 who were admitted to three hospitals in Madina, Saudi Arabia, from March 25, 2020, to July 30, 2020. Data were collected from December 1, 2020, to December 14, 2020.
Results
Of the 119 patients included in the study, the mean age was 54.2 (±15.7) years, with 78.2% survivors and 21.8% non-survivors. The demographic analysis indicated that the likelihood of mortality for patients in the older age group (i.e., ≥65 years) was five times higher than those in the younger age group (OR = 5.34, 95% CI 1.71–16.68, p = 0.004). The results also indicated those patients who admitted to the intensive care unit (ICU) was approximately seven times higher odds of mortality compare with those who were not admitted (OR = 6.48, 95% CI 2.52–16.63, p < 0.001). In addition, six laboratory parameters were positively associated with the odds of mortality: white blood cell count (OR = 1.11, 95% CI 1.02–1.21, p = 0.018), neutrophil (OR = 1.11, 95% CI 1.02–1.22, p = 0.020), creatine kinase myocardial band (OR = 1.02, 95% CI 1.00–1.03, p = 0.030), C-reactive protein (OR = 1.01, 95% CI 1.00–1.01, p = 0.002), urea (OR = 1.06, 95% CI 1.01–1.11, p = 0.026), and lactate dehydrogenase (OR = 1.00, 95% CI 1.00–1.01, p = 0.020).
Conclusions
In this cohort, COVID-19 patients within the older age group (≥65 years) admitted to the ICU with increased C-reactive protein levels in particular, were associated with increased odds of mortality. Further clinical observations are warranted to support these findings and enhance the mapping and control of this pandemic.
Abbreviations: COVID-19, coronavirus disease; WHO, World Health Organization; ICU, intensive care unit
Keywords: COVID-19, Coronavirus, Data analysis, Clinical characteristics, Saudi Arabia
Introduction
The new coronavirus disease (COVID-19), which is caused by the SARS-CoV-2 virus and originated in Wuhan, China in late December 2019, has spread worldwide since then and has resulted in more than 85 million confirmed cases and more than 1.8 million deaths in 200 countries, as updated on January 4, 2021 [1,2]. Although there are a high number of COVID-19 confirmed cases, the fatality rate of COVID-19 (2.5%) is lower than that of other outbreaks, such as the Middle East Respiratory Syndrome (MERS) CoV in 2012 (34.4%) and SARS-CoV in 2003 (9.5%) [1,3,4]. In Saudi Arabia, the first confirmed case of COVID-19 was reported on March 2, 2020 [5]. Since then, the number of cases has exceeded 363,061, with more than 6246 reported as of January 4, 2021 [2].
The initial manifestations reported in patients with COVID-19 vary from mild to severe symptoms [1,6]. Fever, fatigue, dyspnea, cough, anorexia, and sore throat are among the mild symptoms [7,8]. An earlier retrospective study of 1099 COVID-19 confirmed cases collected from 552 hospitals and 30 provinces in China found that the highest percentage of patients had fever (87.9%) or a cough (67.7%), and the lowest rate of patients had diarrhea and vomiting (less than 5%) [9]. In some patients, the virus may lead to severe complications such as pneumonia, sepsis, acute respiratory distress syndrome (ARDS), hyperinflammation, neurological symptoms, or multi-system organ failure [5,10].
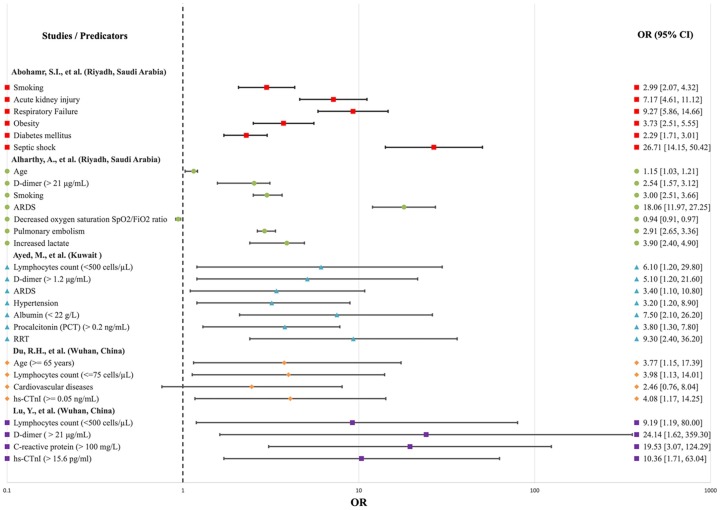
Several meta-analyses have been conducted to identify any significant risk factors associated with severe COVID-19. The first meta-analysis study was conducted by Figliozzi et al., 2020 [11] to evaluate several risk factors associated with COVID-19 using 49 published studies. Their study revealed that advanced age, male sex, cardiovascular disease, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, acute organ injury, increased levels of C-reactive protein (CRP) and D-dimer, and lymphocytopenia are associated with the highest odds of mortality [11]. Another study used 19 published papers involving a total of 1934 mild cases and 1644 severe cases of COVID-19 [2]. This study identified more than 30 potential risk factors associated with severe COVID-19. In addition to the risk factors identified by Figliozzi et al., 2020 [11], the second meta-analysis study found that severe COVID-19 cases are associated with lower levels of hemoglobin, elevated levels of leukocytes, aspartate aminotransferase, alanine aminotransferase, blood creatinine, blood urea nitrogen, high-sensitivity troponin, creatine kinase, interleukin 6, ferritin, lactate dehydrogenase, procalcitonin, and a higher erythrocyte sedimentation rate [12]. Additionally, factors affecting morbidity and mortality in patients with COVID-19 have been studied and analyzed by several research teams in different countries [5,[13], [14], [15], [16], [17], [18], [19]]. Old age, the presence of comorbidities such as hypertension, obesity, chronic pulmonary disease, diabetes mellitus, chronic kidney disease, and malignancies were factors correlated with severe cases [5]. For example, in a large study in England involving 23,698 individuals with COVID-19 who died in hospital, 7434 (31.4%) deaths occurred in patients with Type 2 diabetes (T2D) [20]. The earliest study in Saudi Arabia conducted on 1519 confirmed cases of COVID-19 found that hypertension (8.8%) was the most common comorbidity, followed by diabetes mellitus (7.6%). [21]. Additionally, the severity of this disease may be affected by factors such as distinct demographic characteristics, comorbidities, and immune system responses among diverse populations. Based on the number of multivariate studies conducted in different countries [5,[13], [14], [15], [16], [17], [18], [19]], it is evident that the significant predictors of mortality in patients with COVID-19 are age, lymphocytes, D-dimer, C-reactive protein, smoking, ARDS, acute kidney injury, estimated glomerular filtration rate (eGFR), and cardiovascular diseases (Table 1 ), and odd ratios (OR) of these significant factors of mortality in COVID-19 patients in these countries were compared using forest plot as shown in Fig. 1 .
Table 1.
Predictors based on multivariate analysis of mortality in patients with COVID-19 in different countries.
| Predictors | Study site | Sample size |
|---|---|---|
| Lymphocytes | "Mater Domini" Teaching Hospital, Catanzaro, Italy [17] | 50 |
| Cardiovascular diseases | ||
| Interleukin-6 blood level | ||
| Blood sodium level | ||
| Age | St Mary’s Hospital, London, UK [16] | 229 |
| eGFR | ||
| Renal dysfunction | ||
| Frailty | ||
| Raised troponin T | ||
| Increased creatinine | ||
| Lymphocytes | Tongji Hospital, Wuhan, China [18] | 77 |
| D-dimer | ||
| C-reactive protein | ||
| Hypersensitive cardiac troponin I | ||
| Smoking | King Saud Medical City, Riyadh, Saudi Arabia [13] | 768 |
| ARDS | ||
| Acute kidney injury | ||
| Respiratory failure | ||
| Obesity | ||
| Diabetes mellitus | ||
| Septic shock | ||
| Lymphocytes | Jaber Al-Ahmad Al Sabah Hospital, Kuwait [28] | 103 |
| D-dimer | ||
| ARDS | ||
| Hypertension | ||
| Albumin | ||
| Procalcitonin | ||
| Renal replacement therapy | ||
| Age | King Saud Medical City, Riyadh, Saudi Arabia [29] | 352 |
| D-dimer | ||
| Smoking | ||
| Decreased oxygen saturation SpO2/FiO2 ratio | ||
| Pulmonary embolism | ||
| Increased lactate | ||
| Age | Wuhan Pulmonary Hospital, Wuhan, China [15] | 179 |
| Lymphocytes | ||
| [15]Cardiovascular diseases | ||
| hs-CTnI | ||
| Age | Nationwide cohort in hospitals, Spain [19] | 4035 |
| Cardiovascular diseases | ||
| C-reactive protein | ||
| eGFR | ||
| Low age adjusted oxygen saturation | ||
| Liver cirrhosis |
*Text in bold indicates common predictors among some of the listed countries.
Fig. 1.
Forest plot showing the different predicators of mortality in patients with COVID-19 in different countries.
Despite extensive research currently being conducted on this subject, the clinical characteristics and outcomes of COVID-19 patients as a population have yet to be extensively studied in the Gulf Cooperation Council (GCC) region and Saudi Arabia in particular. Thus, filling this gap by understanding the clinical features of COVID-19 can help to map the disease, identify high-risk patients, and guide future healthcare management. In this retrospective observational study, our primary focus was to investigate the clinical characteristics of COVID-19 and identify possible predictors correlated with mortality to enhance mapping and control of this pandemic.
Method
Data collection
Data were included 119 patients diagnosed with COVID-19 who were admitted to one of three referral hospitals in Medina, Saudi Arabia, from March 25, 2020, to July 30, 2020. Data were collected from December 1, 2020, to December 14, 2020. This study was approved by the Institutional Review Board at King Fahad Medical City (IRB Log No. 20-160).
Study design and patients
This retrospective observational study to investigate the clinical characteristic and possible predictors associated with mortality for those 119 patients with confirmed COVID-19. The symptoms of those patients were mild, moderate, or critical symptoms when they admitted to King Fahad Hospital (KFH), Ohood Hospital, or Miqat hospitals in Medina, Saudi Arabia between March 25, 2020 and July 30, 2020. Son et al., 2020 published a guideline on how to classify COVID-19 cases based on disease severity and clinical outcomes based on four categories: mild (asymptomatic), moderate, severe, and critical cases [22,23]. The sample technique of this retrospective observational study was the convenience sample, and it was included only those 119 patients having complete laboratory results, as determined from patient records. Any patient who did not have a complete laboratory results in their file record were excluded. Data on the following variables were collected: age, sex, nationality, number of days in the hospital, admission to ICU, kidney disease, diabetes, and laboratory results (white blood cell count, red blood cell count, neutrophils, lymphocytes, d-dimers, prothrombin time, partial thromboplastin time, creatine kinase, creatine kinase myocardial band, hemoglobin, C-reactive protein, aspartate aminotransferase, glucose, alanine aminotransferase, urea, lactate dehydrogenase, albumin, total protein, platelets, and international normalized ratio (INR). The goal of this study was to investigate any clinical characteristics and laboratory parameters that may be predictors associated with mortality among admitted COVID-19 patients.
Statistical analysis
Categorical variables were described using frequencies and percentages. They were compared using chi-square tests. Continuous variables are presented as mean values (standard deviation (SD)) or median values based on the interquartile range (IQR). Comparisons were made using the t-test or Mann–Whitney U test for continuous variables, as appropriate. Univariate and multivariate logistic regression were used to investigate the association of clinical characteristics and laboratory parameters with the odds of death. The multivariate logistic model was determined using a stepwise selection method. This method consists of a mixture of forward and backward selection techniques. We started with the forward selection technique, which added significant predictors from the univariate logistic model one by one to the model. After adding a predictor in each step, we checked all current predictors in the model, and any predictor that was not statistically significant (p-value >0.05) was dropped from the model. The final multivariate model included only statistically significant predictors (p-value <0.05). The advantage of using the stepwise selection method is that it allows examination of various models using different combinations of predictors. The Kaplan–Meier estimator of survival probability was visually presented, while the log-rank test was used to test for any significant difference in survival probability between two different age groups of COVID-19 patients. All data were analyzed using STATA 16.0 (College Station, TX, USA).
Results
A total of 119 COVID-19 patients were included in the analysis with a mean age of 54.2 ± 15.7 years; 80 (67.2%) were men. The median length of a hospital stay was ten days (IQR: 0–19 days). Of the 119 patients, 26 (21.8%) died. The main characteristics and laboratory parameters of the 119 COVID-19 patients are presented in Table 2 , which indicates that non-survivor patients were older than those in the survivor group (60.7 ± 18.9 years vs. 52.5 ± 14.4 years, p = 0.015). Furthermore, non-survivors were more frequently Saudi (50.0% vs. 24.7%, p = 0.013), were more frequently admitted to the ICU (65.4% vs. 22.6%, p < 0.001), and experienced a longer hospital stay (15 days vs. 8 days, p = 0.003).
Table 2.
Main characteristics and laboratory parameters of COVID-19 patients who survived (n = 93) or did not survive (n = 26) in our study population.
| Total patients (n = 119) | Non-survivors (n = 26, 21.8%) | Survivors (n = 93, 78.2%) | p-Value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 54.2 15.7 | 60.7 18.9 | 52.5 14.4 | 0.015* |
| Sex (male: n, %) | 80 (67.2) | 14 (53.9) | 66 (71.0) | 0.100 |
| Nationality (non-Saudi: n, %) | 83 (69.8) | 13 (50.0) | 70 (75.3) | 0.013* |
| Hospital length of stay (days, IQR) | 10 (0−19) | 15 (9−21) | 8 (0−16) | 0.003* |
| Admission to ICU (yes: n, %) | 38 (31.9) | 17 (65.4) | 21 (22.6) | <0.001* |
| Kidney disease (yes: n, %) | 16 (13.5) | 3 (11.5) | 13 (14.0) | 0.747 |
| Diabetes (yes: n, %) | 35 (29.4) | 4 (15.4) | 31 (33.3) | 0.076 |
| Laboratory parameters | ||||
| White blood cell counts (, normal: 4–10) | 10.1 5.3 | 12.6 6.2 | 9.4 4.9 | 0.003* |
| Red blood cell counts (, normal: 4.5−5.5) | 4.1 0.8 | 3.6 1.0 | 4.2 0.8 | 0.001* |
| Neutrophil (l, normal: 2–7) | 7.4 5.4 | 10.0 6.9 | 6.7 4.7 | 0.014* |
| Lymphocytes (l, normal: 1.1–3.2) | 1.6 1.4 | 1.8 2.7 | 1.5 0.7 | 0.069 |
| D-dimers (mcg/mL, normal: 2–5) | 121551.3 | 33.5 48.2 | 145.9 621.6 | 0.358 |
| Prothrombin time (seconds, normal: 11–16) | 13.9 2.2 | 14.2 3.1 | 13.8 1.9 | 0.914 |
| Partial thromboplastin time (seconds, normal: 26–36) | 35.8 12.0 | 37.5 17.2 | 35.3 10.2 | 0.907 |
| Creatine kinase (, normal: 21–232) | 330.8 568.2 | 444.6 534.8 | 298.9 575.9 | 0.135 |
| Creatine kinase myocardial band (mg/l, normal: 7–25) | 29.8 28.1 | 42.3 44.7 | 26.3 20.3 | 0.023* |
| Hemoglobin (g/dl, normal: 13–17) | 11.2 2.4 | 9.9 2.4 | 11.5 2.2 | <0.001* |
| C-reactive protein (mg/l, normal: 1–10) | 78.8 80.4 | 125.8 76.8 | 65.6 76.7 | <0.001* |
| Aspartate aminotransferase (μ/l, normal range 15–37) | 70.5 156.4 | 132.2 308.3 | 53.3 64.6 | 0.184 |
| Glucose (mmol/l, normal: 3.9–6.1) | 10.3 6.6 | 10.7 8.8 | 10.3 5.9 | 0.320 |
| Alanine aminotransferase (μ/l, normal: 30–65) | 62.1 184.5 | 136.3 385.0 | 41.4 37.5 | 0.361 |
| Urea (mmol/l, normal: 2.5–8.3) | 10.0 8.0 | 13.3 8.4 | 9.1 7.7 | 0.006* |
| Creatinine (mg/dl, normal: 44–115) | 188.0 239.8 | 257.7 274.7 | 168.5 226.9 | 0.058 |
| Lactate dehydrogenase, (u/l, normal: 100–190) | 375.8 415.6 | 612.2 828.5 | 309.7 121.8 | 0.072 |
| Albumin (g/l, normal: 30–50) | 28.1 6.8 | 25.7 8.2 | 28.8 6.2 | 0.068 |
| Total protein (g/l, normal: 60–85) | 66.2 8.7 | 63.0 8.2 | 67.1 8.7 | 0.054 |
| Platelets (ml, normal: 150–410) | 292.1 127.6 | 257.9 120.6 | 301.7 128.5 | 0.129 |
| International normalized ratio (normal: 0.9–1.4) | 1.1 0.2 | 1.1 0.3 | 1.1 0.2 | 0.935 |
p < 0.05, results were statistically significant.
The laboratory parameter results indicated that patients in the non-survivor group had a higher statistically significant mean with respect to white blood cell (12.6 vs. 9.4, p = 0.003), neutrophil (10.0 vs. 6.7, p = 0.014), creatine kinase myocardial band (42.3 vs. 26.3, p = 0.023), C-reactive protein (12.8 vs. 65.6, p < 0.001), and urea levels (compared to patients in the survivor group. However, the patients in the non-survivor group had a lower statistically significant mean for red blood cell count (3.6 vs. 4.2, p = 0.001) and hemoglobin (9.9 vs. 11.5, p < 0.001) compared to those in the survivor group (Table 2).
Table 3 shows the mortality odds ratios (ORs) for risk predictors based on univariate logistic regression among patients with COVID-19. Patients in the older age group (i.e., ≥65 years) had five times higher odds of mortality compared to those in the younger age group (i.e., ≤49 years) (OR = 5.34, 95% CI 1.71–16.68, p = 0.004). Furthermore, non-Saudi patients had 77% lower odds of mortality compared to Saudi patients (OR = 0.33, 95% CI 0.13–0.81, p = 0.016). Additionally, the results indicated that those patients who were admitted to the intensive care unit (ICU) had approximately seven times higher odds of mortality compared to those who were not admitted (OR = 6.48, 95% CI 2.52–16.63, p < 0.001). Laboratory parameters were positively associated with the odd of mortality were white blood cell count (OR = 1.11, 95% CI 1.02−1.21, p = 0.018), neutrophil (OR = 1.11, 95% CI 1.02–1.22, p = 0.020), creatine kinase myocardial band (OR = 1.02, 95% CI 1.00–1.03, p = 0.030), C-reactive protein (OR = 1.01, 95% CI 1.00–1.01, p = 0.002), urea (OR = 1.06, 95% CI 1.01–1.11, p = 0.026), and lactate dehydrogenase (OR = 1.00, 95% CI 1.00–1.01, p = 0.020) (Table 3). However, laboratory parameters were negatively associated with the odds of mortality were red blood cell (OR = 0.41, 95% CI 0.23–0.76, p = 0.004), elevated hemoglobin (OR = 0.71, 95% CI 0.57–0.89, p = 0.003), albumin (OR = 0.93, 95% CI 0.87–0.99, p = 0.043), and total protein levels (OR = 0.94, 95% CI 0.89–0.99, p = 0.033) (Table 4 ).
Table 3.
Univariate logistic regression model mortality in patients with COVID-19.
| OR (95% CI) | p-Value | |
|---|---|---|
| Characteristics | ||
| Age, years | 1.04 (1.01−1.07) | 0.021* |
| Age group | ||
| 0−49 years | 1(reference) | |
| 50−64 years | 1.05 (0.32−3.41) | 0.932 |
| ≥65 years | 5.34 (1.71−16.68) | 0.004* |
| Sex (male vs. female) | 0.48 (0.20−1.16) | 0.104 |
| Nationality (non-Saudi vs. Saudi) | 0.33 (0.13−0.81) | 0.016* |
| Hospital length of stay | 1.02 (1.00−1.05) | 0.066 |
| Admission to ICU (yes vs. no) | 6.48 (2.52−16.63) | <0.001* |
| Kidney (yes vs. no) | 0.80 (0.21−3.06) | 0.748 |
| Diabetics (yes vs. no) | 0.36 (0.12−1.15) | 0.084 |
| Laboratory parameters | ||
| White blood cell counts (, normal: 4–10) | 1.11 (1.02−1.21) | 0.018* |
| Red blood cell counts (, normal: 4.5–5.5) | 0.41 (0.23−0.76) | 0.004* |
| Neutrophil (l, normal: 2–7) | 1.11 (1.02−1.22) | 0.020* |
| Lymphocytes (l, normal: 1.1–3.2) | 1.14 (0.86−1.50) | 0.368 |
| D-dimers (mcg/mL, normal: 2–5) | 1.00 (0.99−1.00) | 0.463 |
| Prothrombin time (second, normal: 11–16) | 1.08 (0.90−1.31) | 0.391 |
| Partial thromboplastin time (second, normal: 26–36) | 1.01 (0.98−1.05) | 0.422 |
| Creatine kinase (, normal: 21–232) | 1.00 (0.99−1.00) | 0.266 |
| Creatine kinase myocardial band (mg /l, normal: 7–25) | 1.02 (1.00−1.03) | 0.030* |
| Hemoglobin (g/dl, normal: 13–17) | 0.71 (0.57−0.89) | 0.003* |
| C-reactive protein (mg/l, normal: 1–10) | 1.01 (1.00−1.01) | 0.002* |
| Aspartate aminotransferase (μ/l, normal range 15–37) | 1.00 (0.99−1.01) | 0.103 |
| Glucose (mmol/l, normal: 3.9–6.1) | 1.01 (0.95−1.08) | 0.766 |
| Alanine aminotransferase (μ/l, normal: 30–65) | 1.00 (1.00−1.01) | 0.117 |
| Urea (mmol/l, normal: 2.5–8.3) | 1.06 (1.01−1.11) | 0.026* |
| Creatinine (mg/dl, normal: 44–115) | 1.00 (0.99−1.00) | 0.106 |
| Lactate dehydrogenase, (u/l, normal: 100–190) | 1.00 (1.00−1.01) | 0.020* |
| Albumin (g/l, normal: 30–50) | 0.93 (0.87−0.99) | 0.043* |
| Total protein (g/l, normal: 60–85) | 0.94 (0.89−0.99) | 0.033* |
| Platelets (ml, normal: 150–410) | 1.00 (0.99−1.00) | 0.124 |
| International normalized ratio (normal: 0.9–1.4) | 1.76 (0.23−13.31) | 0.583 |
p < 0.05, results were statistically significant.
Table 4.
Multivariate logistic regression model mortality in patients with COVID-19.
| aOR (95% CI) | p-Value | |
|---|---|---|
| Characteristics | ||
| Age group (≥65 years vs. <65 years) | 5.20 (1.74–15.49) | 0.003 |
| Admission to ICU (yes vs. no) | 5.68 (1.99–16.19) | 0.001 |
| Laboratory parameters | ||
| C-reactive protein (mg/l, normal: 1–10) | 1.01 (1.00–1.02) | 0.004 |
Table 4 shows the adjusted odds ratios (aORs) for risk predictors based on multivariate logistic regression for mortality among patients with COVID-19. Patients in the older age group (≥65 years), admitted to the ICU, and exhibiting increased C-reactive protein levels were positively associated with the odds of mortality (aOR = 5.20, 95% CI 1.74–15.49, p = 0.003, aOR = 5.68, 95% CI 1.99–16.19, p = 0.001 and aOR = 1.01, 95% CI 1.00–1.02, p = 0.004, respectively).
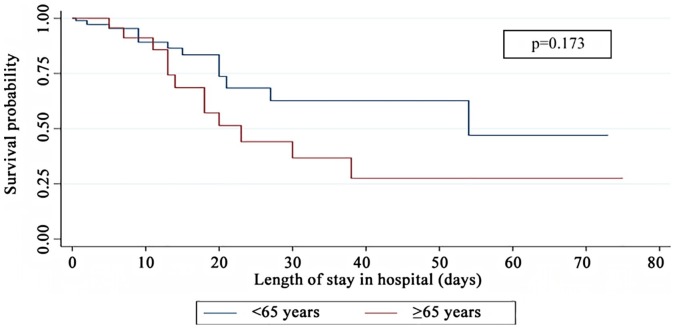
Kaplan–Meier curves for the length of stay in the hospital that was observed in the two age groups of this cohort of COVID-19 patients are illustrated in Fig. 2 . The mean hospital stay was shorter for patients aged 65 years or older compared to those who were younger than 65 years (39 days vs. 49 days); however, this difference was not statistically significant (log-rank test, p = 0.173).
Fig. 2.
Kaplan–Meier survival curves for the two age groups (<65 years, ≥65 years) based on the length of stay in the hospital for COVID-19 patients.
Discussion
This retrospective observational study aimed to investigate and identify the dominant predictors and associated clinical characteristics that increase the odds of mortality in COVID-19 patients. Identifying the mortality predictors may help to provide appropriate medical interventions and levels of care for COVID-19 patients [16]. The main findings of our study are that the high associations between various predictors, which are being older than 65 years, being admitted to the ICU, and having high C-reactive protein levels, increased the odds of mortality in the non-survivor group compared to other clinical characteristics based on multivariate logistic regression analyses.
Several studies conducted in various regions have found different sets of factors that increased the odds of mortality among COVID-19 patients [5,8,13,[15], [16], [17], [18], [19],24]. Age was frequently considered a significant predictor of mortality in COVID-19 patients in the majority of these studies [5,13,15,18]. Univariate and multivariate logistic regression analyses conducted on all patients in our study indicated that age (≥65 years) is a strong predictor of mortality in COVID-19 patients. This finding is consistent with many studies conducted in different countries [5,13,15,18]. An earlier study involving 179 COVID-19 patients in Wuhan, China found that 17 (81%) of the deceased patients were older than 65, with an associated OR of 3.765 [14]. In another study conducted in London on 229 patients, the mean age was 78 years among the deceased group (75 patients) [16]. More extensive research was conducted in Spain, where 1131 of 4035 patients died, and 85.6% of deaths occurred in patients older than 65 [19]. A separate recent study conducted in Saudi Arabia found that the time in ICU is a predictor of death with an OR of 1.06 based on univariate analysis, which is close to the OR of our study for any age (without subdividing age groups (OR = 1.04)) [5]. However, our study's ORs for patients older than 65 years were 5.34 and 5.20 based on univariate and multivariate analysis, respectively.
Additionally, the results indicated that patients admitted to the ICU had approximately six to seven times higher risk of mortality based on the multivariate analysis compared with those who were not admitted. This outcome is in agreement with a recent study conducted in Wuhan, China, which found that 70% of patients admitted to the ICU died [18]. This finding has also been supported by Saudi research that revealed that ICU length of stay is among eight significant predictors of mortality in COVID-19 patients based on univariate logistic regression analysis [5]. The third significant predictor of mortality based on multivariate logistic regression analysis in our study was an increase in the C-reactive protein (CRP) level. This finding is consistent with a larger study conducted in Spain, which found that 990 of 1023 patients who died had elevated CRP levels [19].
Moreover, a recent study conducted on 77 young COVID-19 patients found that CRP levels are a significant predictor of mortality in these patients based on multivariate analysis [18]. Furthermore, it has been shown that increased CRP levels are linked with the overproduction of inflammatory cytokines that play an essential role in the severity of COVID-19 and can cause damage to lung tissue, even in patients with non-severe symptoms [25,26]. Taken together, this suggests that CRP can be used as a helpful predictor marker to identify high-risk COVID-19 patients at an early stage for early disease management. Furthermore, targeting CRP levels may reduce the inflammation status in COVID-19 patients, and may be a promising treatment for COVID-19 patients. Our results highlighted a set of significant predictors associated with high odds of mortality. This significance was based on univariate logistic regression analysis involving some parameters, such as white blood cell, neutrophils, myocardial kinase, urea, and lactate dehydrogenase counts. These parameters are included in a set of 27 predictors of COVID-19 patient mortality collected from several studies and summarized in Table 1.
Controlling the spread of COVID-19 is one of the most important challenges facing different countries. These challenges include both economic and social aspects. Therefore, addressing the economic and social impacts resulting from the spread of the disease has been a major priority in many countries [18]. Chen et al. [27] reported that 65 and older age patients who diagnosed with COVID-19 were more likely to develop comorbidities, serious symptoms and experience death compared to younger age group (<65 years). Accordingly, the Ministry of Health in Saudi Arabia has attached special importance to this group by focusing on vaccines as a primary strategy and emphasizing several recommendations such as staying home, staying away from gatherings, taking prescribed medicines regularly and using apps to order and have food and prescribed drugs delivered. The Ministry of Health has issued several directives to family members and caregivers for the elderly, including paying increased attention to the personal hygiene of the elderly and ensuring that their rooms are clean and well-ventilated [19]. In addition, while these groups are at risk of disease, other studies have investigated the association between patients with comorbidities that may pose a significant threat to their lives and COVID-19 [[20], [21], [22]]. The findings from our study and other studies highlight the need for public health intervention to minimize the risk of infection by COVID-19 among vulnerable populations, develop clinical guidelines, and improve treatment strategies including counseling, and discussion for COVID-19 patients identified as having high odds of mortality. To our knowledge, this is the first study in the Medina region that has reported the characteristics and clinical parameters of COVID-19 patients as predictors of mortality.
This study includes some potential limitations. First of the limitation, the data was recorded only at the time of the hospital admission. Further, the collection of the data was utilized on the patients’ medical record and some variables were not available in the medical record such as obesity, cardiovascular and hypertension. In addition, the study was conducted in the Medina region; therefore, other studies should be performed in other regions within Saudi Arabia to evaluate the external validity of our model so that these results can be generalized to the COVID-19 population in Saudi Arabia. Furthermore, the sample size was 119 patients, which could have led to some non-significant differences in the clinical characteristics in the subgroup analysis and also to non-significant predictors for the odds of mortality because the study was limited to a relatively small sample size.
Conclusion
Our retrospective observational study involving patients who were diagnosed with COVID-19 with mild, moderate, or critical symptoms highlighted several significant factors that can be used to predict mortality based on patient characteristics and laboratory parameters. This study concluded that the older age group (≥65 years) had five times higher odds of mortality compared to patients in the younger age group (≤49 years). Moreover, ICU patients with COVID-19 had approximately seven times higher odds of mortality compared to those who were not admitted into the ICU. Additionally, older age groups (≥65 years) who were admitted to the ICU and exhibited increased C-reactive protein levels were positively associated with the odds of mortality. Further clinical observations are warranted to support these findings and enhance the mapping and control of the COVID-19 pandemic.
Funding
The authors extend their appreciation to the Deanship of Scientific Research, Majmaah University, for supporting this study under project number No. (R-2021-119).
Competing interests
None declared.
Ethical approval
Data were collected from three referral hospitals in Medina, Saudi Arabia. This study was approved by the Institutional Review Board of King Fahad Medical City (IRB Log No. 20-160). Informed consent to participate was waived or not required because only laboratory data and demographic data were used for this study.
Authors’ contributions
All authors contributed equally to this document.
Acknowledgments
The authors would like to thank the three referral hospitals in Medina for their valuable contributions to data collection. Special thanks to the Research Center at King Fahad Medical City, Riyadh, for their valuable help and support.
References
- 1.Alharbi Y., Alqahtani A., Albalawi O., Bakouri M. Epidemiological modeling of COVID-19 in Saudi Arabia: spread projection, awareness, and impact of treatment. Appl Sci. 2020;10(17):5895. [Google Scholar]
- 2.Worldometers.info. Coronavirus Cases. [04 January 2021]. Available from: https://www.worldometers.info/coronavirus/.
- 3.Alguwaihes A.M., Al-Sofiani M.E., Megdad M., Albader S.S., Alsari M.H., Alelayan A. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alharthy A., Aletreby W., Faqihi F., Balhamar A., Alaklobi F., Alanezi K. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2020;11(1):98–104. doi: 10.2991/jegh.k.200928.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Shareef K., Bakouri M. Cytokine blood filtration responses in COVID-19. Blood Purif. 2020;50(2):141–149. doi: 10.1159/000508278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani A.M., AlMalki Z.S., Alalweet R.M., Almazrou S.H., Alanazi A.S., Alanazi M.A. Assessing the severity of illness in patients with coronavirus disease in Saudi Arabia: a retrospective descriptive cross-sectional study. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.593256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figliozzi S., Masci P.G., Ahmadi N., Tondi L., Koutli E., Aimo A. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10) doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 12.Mudatsir M., Fajar J.K., Wulandari L., Soegiarto G., Ilmawan M., Purnamasari Y. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res. 2020;9(1107):1107. doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abohamr S.I., Aldossari M.A., Alaklobi F.A., Amer H.A., Alzarzour S.H., Abdelhamid S.W. Clinical characteristics and in-hospital outcome of medical staff infected with COVID-19 in Saudi Arabia. A retrospective single-center study. Saudi Med J. 2020;41(12):1336–1343. doi: 10.15537/smj.2020.12.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alkundi A., Mahmoud I., Musa A., Naveed S., Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in UK: a retrospective single centre study. Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moledina S.M., Maini A.A., Gargan A., Harland W., Jenney H., Phillips G. Clinical characteristics and predictors of mortality in patients with COVID-19 infection outside intensive care. Int J Gen Med. 2020;13:1157–1165. doi: 10.2147/IJGM.S271432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trecarichi E.M., Mazzitelli M., Serapide F., Pelle M.C., Tassone B., Arrighi E. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci Rep. 2020;10(1):20834. doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Huang Z., Wang M., Tang K., Wang S., Gao P. Clinical characteristics and predictors of mortality in young adults with severe COVID-19: a retrospective observational study. Ann Clin Microbiol Antimicrob. 2021;20(1):3. doi: 10.1186/s12941-020-00412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenguer J., Ryan P., Rodríguez-Baño J., Jarrín I., Carratalà J., Pachón J. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsofayan Y.M., Althunayyan S.M., Khan A.A., Hakawi A.M., Assiri A.M. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13(7):920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son K.B., Lee T.J., Hwang S.S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull World Health Organ. 2021;99(1):62–66. doi: 10.2471/BLT.20.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health, O . World Health Organization; Geneva: 2021. COVID-19 clinical management: living guidance, 25 January 2021. [Google Scholar]
- 24.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T., Dai Z., Mo P., Li X., Ma Z., Song S. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–1795. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayed M., Borahmah A.A., Yazdani A., Sultan A., Mossad A., Rawdhan H. Assessment of clinical characteristics and mortality-associated factors in COVID-19 critical cases in Kuwait. Med Princ Pract. 2020;30(2):185–192. doi: 10.1159/000513047. [DOI] [PMC free article] [PubMed] [Google Scholar]