Abstract
Background:
The 2013 Pooled Cohort Equations (PCEs) have underestimated cardiovascular disease (CVD) events among people with HIV (PWH). We evaluate whether the addition of frailty improves PCE’s ability to estimate CVD risk among aging PWH.
Setting:
Multicenter study
Methods:
We assessed baseline frailty and 5-year atherosclerotic CVD risk using PCEs for participants in the AIDS Clinical Trials Group (ACTG) A5322 observational study. The primary outcome was incident CVD. We fit Cox proportional hazards regression models for incident CVD with: (a) PCEs alone and; (b) PCEs and frailty together (which included separate models for frailty score, frailty status, slow gait speed, and weak grip strength). We evaluated discrimination ability for the models with and without frailty by comparing their areas under the curve (AUC) and Uno’s C-statistics, as well as by calculating the net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Results:
The analysis included 944 A5322 participants (759 men, 185 women, median age 50 years, 47% white non-Hispanic). Thirty-nine experienced incident CVD during the study period. PCEs predicted 5-year CVD risk in all models. With frailty score, frailty status, slow gait speed, or weak grip strength added, the AUC and C-statistics were relatively unchanged, and the NRI and IDI indicated little improvement in model discrimination. However, frailty score independently predicted CVD risk (frailty score: hazard ratio [HR]=1.30, 95% CI=1.00–1.70, p=0.05).
Conclusions:
Frailty did not improve the predictive ability of PCEs. Baseline PCEs and frailty score independently predicted CVD. Incorporation of frailty assessment into clinical practice may provide corroborative and independent CVD risk estimation.
Keywords: Cardiovascular disease risk, frailty, HIV and aging
Introduction
Compared to the general population, persons with HIV (PWH) have elevated incidence and prevalence of cardiovascular disease (CVD) which also occurs at younger ages (1–4). A reliable CVD risk estimation tool is therefore necessary for PWH to achieve accurate risk stratification, particularly since they have higher burden of disease. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Equations (PCEs) consistently underestimates CVD risk when applied to PWH (5–11). Reasons for underperformance of the PCEs among PWH are unclear. Unique physiologic effects of HIV (such as chronic inflammation, immune dysregulation, and vascular endothelial dysfunction), demographic characteristics of PWH that include competing risks for CVD (such as increased prevalence of smoking), and a history of exposure to certain antiretroviral therapy (ART) medications may predispose to CVD (12–16).
Frailty, a syndrome of aging marked by physiologic dysregulation and functional decline, is associated with multiple adverse health consequences, such as neurocognitive impairment, falls, and disability, and excess burden of mortality and morbidity among PWH (17–20). In a recent analysis of participants in the AIDS Clinical Trials Group (ACTG) A5322 cohort, frailty was significantly associated with incident CVD (21). Furthermore, systemic levels of markers of inflammation and immune dysregulation have been associated with both frailty and CVD development among PWH, suggesting a shared antecedent (22–26). However, identifying whether consideration of frailty could improve upon CVD risk prediction models in older adults with HIV is unknown. The current lack of a validated CVD predictive tool for PWH and the recently observed association between frailty and CVD in our cohort warrant further investigation regarding whether the magnitude and components of frailty predict CVD outcomes in this population. In this analysis, we evaluated whether the consideration of frailty modifies PCEs’ ability to estimate CVD risk among aging PWH in A5322.
Methods
Study population:
ACTG A5322 (HAILO: The HIV Infection, Aging, Immune Function Long-Term Observational Study) is an ongoing, observational study of 1035 older PWH (age ≥40 years at enrollment) that longitudinally evaluates associations between ART use, aging and inflammation with incidence of non-AIDS clinical events, mortality and functional status. All HAILO participants initiated ART through an ACTG randomized clinical trial and were subsequently followed in the observational study ACTG A5001, which enrolled participants between 2000 and 2007 (27). Between November 2013 and July 2014, a subset of A5001 participants ≥ 40 years old were enrolled in HAILO for continued long-term follow-up.
We included 944 participants with baseline frailty assessments, no known history of CVD, and sufficient available data to calculate baseline PCEs. Study visits for HAILO participants in this analysis occurred semiannually during the study period (changed to annual visits in November 2019) and include medication review, chart abstractions, and falls interview. Frailty assessments, body measurements, and blood collection are performed annually. The respective institutional review boards at each study site approved the study, and written informed consent was obtained from each participant.
Baseline assessment:
We assessed frailty among all participants using the Fried frailty phenotype (28). As previously described, the phenotype includes the five components of weak grip, slow gait speed on a 4-meter walk, and self-reported weight loss, exhaustion, and limitations in ability to undertake vigorous physical activity (29). We evaluated frailty at baseline (time of HAILO entry visit) and categorized participants as frail if they met 3–5 criteria, pre-frail if they met 1–2 criteria, or non-frail if they did not meet any criteria. In addition to frailty status, we evaluated frailty score (from 0–5), as well as the individual frailty components of slow gait speed and weak grip strength.
We calculated 5-year atherosclerotic cardiovascular disease (ASCVD) risk at baseline for all participants using the 2013 American College of Cardiology/American Heart Association (ACC/AHA) PCEs. As previously described, PCEs estimate risk of myocardial infarction or equivalent using age, sex, race (White, Black, or other), systolic blood pressure, total and HDL cholesterol concentrations, history of diabetes (hemoglobin A1c ≥6.5% or a medical diagnosis of diabetes), current smoking status, and hypertension treatment status (30).
Clinical CVD outcomes:
We included clinical CVD events that occurred after the baseline frailty and ASCVD risk assessment. Clinical CVD outcomes were defined as acute coronary syndrome, cerebrovascular accident, peripheral arterial disease, unstable angina, and/or need for revascularization procedure. We excluded persons with prevalent CVD (any history of a diagnosis prior to baseline).
Demographic, behavioral and clinical factors of the study population:
We summarized several additional factors for the cohort overall and by incident CVD status. Race/ethnicity was categorized as black non-Hispanic, white non-Hispanic, Hispanic/other, age as 40–49, 50–59, and ≥60 years, sex as male or female. Self-reported smoking was categorized as “never smoker”, “former smoker”, or “current smoker”. Body mass index (BMI) was categorized as underweight (BMI: <18.5 kg/m2), normal (BMI: 18.5 – <25 kg/m2), overweight (BMI: 25–30 kg/m2), and obese (BMI: >30 kg/m2). HIV-related factors included CD4 T-lymphocyte cell count/mm3 (CD4) at time of ART initiation and at baseline, plasma HIV-RNA level at time of ART initiation and baseline, and proportion of time with HIV RNA level <200 copies/mL between ART initiation and HAILO study entry.
Statistical Analysis:
Basic summary statistics for PCEs and frailty components and other demographic and clinical characteristics at HAILO baseline were calculated by incident CVD status. Because the mean follow-up duration of participants at the time of the analysis was 5 years, PCEs 5-year risk scores were used in this analysis, and follow-up time was censored at year 5. Follow-up was defined as time from baseline to date of the most recent visit, last clinic date for off-study participants or date of clinical CVD outcome, whichever occurred first (with the restriction specified above). We calculated the predicted CVD risk at 5 years using the baseline survival function at 5 years (S0[t=5]) in the PCE model (31).
To estimate the predictive ability of PCEs risk score with and without the incorporation of frailty, we first fit a univariate Cox proportional hazards regression model for incident CVD with PCEs risk score (Model 0). Then both PCEs risk score and frailty score at baseline were included in a separate Cox regression model (Model 1). Similarly, PCEs risk score and frailty status (Model 2), PCEs risk score and slow gait speed (Model 3), and PCEs risk score and weak grip strength (Model 4) (all at baseline) were included in separate Cox regression models. The integrated area under receiver operating characteristic curve (AUC) (the average of all available AUC statistics over time) and the Uno’s C-statistic from these models with and without frailty variables (frailty score, frailty status, slow gait speed, and weak grip strength) were compared to assess the difference in ability of the models to discriminate between persons with and without cardiovascular disease. We also calculated the continuous net reclassification improvement (NRI), category-based NRI (with CVD risk categories <5%, 5–20%, and >20%), and the integrated discrimination improvement (IDI) as additional measures of the incremental value of adding frailty to the PCEs-only model. The bootstrap method was used to calculate 95% confidence intervals for the NRI and IDI.
Results:
Among 759 men and 185 women, 47% were white non-Hispanic and 46% between 40–49 years of age. Information on gender was not explicitly available. The majority (94%) were virally suppressed (HIV RNA <200 copies/mL) at baseline with a median baseline CD4 of 618 cells/μL. Participant demographic and clinical characteristics are shown in Table 1. At baseline, 57 participants (6%) were frail. Thirty-nine participants experienced a clinical CVD outcome (as defined in Methods); median (Q1, Q3) time from enrollment to CVD outcome was 25.1 (11.9, 48.8) months. Among persons experiencing a CVD event, 4 (10%) were frail at baseline and 15% at the visit closest to the CVD event.
Table 1:
Baseline demographic and clinical characteristics
| Total (N = 944)a | Persons with incident CVD (N=39) | Persons without incident CVD (N=905) | P-value | |
|---|---|---|---|---|
| Age, years, Median (Q1, Q3) | 50 (46, 56) | 55 (50, 58) | 50 (45, 55) | <0.001 |
| Male sex, % | 80 | 90 | 80 | 0.1 |
| Race/Ethnicity, % | 0.8 | |||
| White, non-Hispanic | 47 | 41 | 47 | |
| Black, non-Hispanic | 30 | 31 | 30 | |
| Hispanic, other | 23 | 28 | 23 | |
| BMI, kg/m2, Median (Q1, Q3) | 27.2 (24.1, 30.9) | 28.0 (25.3, 32.0) | 27.2 (24.1, 30.9) | 0.4 |
| Smoking status, % | 0.4 | |||
| Never | 42 | 36 | 42 | |
| Former | 33 | 31 | 33 | |
| Current | 25 | 33 | 24 | |
| Fasting cholesterol, Median (Q1, Q3) | ||||
| Total cholesterol, mg/dL | 185 (162, 210) | 197 (172, 235) | 184 (162, 209) | 0.03 |
| HDL, mg/dL | 46 (39, 57) | 42 (38, 50) | 46 (39, 57) | 0.05 |
| LDL, mg/dL | 107 (87, 128) | 118 (90, 138) | 106 (87, 127) | 0.09 |
| Diabetes, % | 10 | 23 | 9 | 0.005 |
| Hypertensionb, % | 33 | 44 | 33 | 0.2 |
| 5-year ASCVD risk, %, Median (Q1, Q3) | 2.003 (1.034, 3.916) | 4.626 (2.084, 9.690) | 1.930 (1.021, 3.653) | <0.001 |
| Frailty scorec, Median (Q1, Q3) | 0 (0, 1) | 1 (0, 1) | 0 (0, 1) | 0.07 |
| Frailty status, N (%) | 0.2 | |||
| Fraild | 57 (6) | 4 (10) | 53 (6) | |
| Pre-fraile | 350 (37) | 18 (46) | 332 (37) | |
| Non-frail | 537 (57) | 17 (44) | 520 (57) | |
| Nadir CD4, cells/μL, Median (Q1, Q3) | 194 (63, 304) | 72 (26, 205) | 198 (67, 305) | 0.004 |
| Baseline CD4, cells/μL, Median (Q1, Q3) | 618 (450, 828) | 494 (372, 831) | 620 (454, 828) | 0.1 |
| ART duration, years, Median (Q1, Q3) | 7.7 (4.4, 11.9) | 10.7 (6.8, 14.3) | 7.7 (4.3, 11.9) | 0.01 |
| Pre-ART HIV RNA, copies/mL, Median (Q1, Q3) | 55,597 (19,918, 179,031) | 95,567 (33,050, 312,275) | 54,517 (19,646, 173,093) | 0.05 |
| HIV RNA suppressed to <200 copies/mL ≥75% of the time pre-enrollment, N (%) | 745 (79%) | 31 (79%) | 714 (79%) | 0.9 |
| Baseline HIV RNA <200 copies/mL, N (%) | 892 (94) | 34 (87) | 858 (94) | 0.06 |
| History of clinical AIDS, N (%) | 200 (21%) | 10 (26%) | 190 (21%) | 0.5 |
36 participants were not included in 5-year ASCVD risk and frailty assessments due to lack of data; from N=999, an additional 55 participants not included due to prior CVD events
defined by use of antihypertensive medication at baseline
frailty score determined by the 5 components of the Fried frailty phenotype: weak grip, slow gait speed on a 4-meter walk, and self-reported weight loss, exhaustion, and limitations in ability to undertake vigorous physical activity
frailty status defined by ≥3 components of the Fried frailty phenotype
pre-frailty status defined as 1–2 components of the Fried frailty phenotype
BMI=body mass index; HDL=high density lipoprotein; LDL=low-density lipoprotein; ART=antiretroviral therapy; ASCVD=atherosclerotic cardiovascular disease; ART=antiretroviral therapy; HIV=Human Immunodeficiency Virus; RNA=ribonucleic acid; AIDS=Acquired Immune Deficiency Syndrome
In the model including PCEs and the continuous variable of frailty score (Model 1), both variables separately predicted CVD (PCEs: hazard ratio [HR]=1.13, 95% confidence interval [CI]=1.09–1.17, p=<0.001; frailty score: HR per unit increase=1.30, 95% CI=1.00–1.70, p=0.05). In the model including PCEs and the categorical variable of frailty status (Model 2), PCEs predicted CVD (HR=1.13, 95% CI=1.09–1.18, p=<0.001), while being frail (vs non-frail) did not (HR=2.11; 95% CI=0.71–6.29, p=0.20). In the models including PCEs and the continuous variables of slow gait speed or weak grip (Models 3 and 4), PCEs also predicted CVD but the frailty measures did not.
In the Cox proportional hazards regression model for incident CVD with baseline PCEs, the integrated AUC and C-statistic were 0.777 and 0.719, respectively, indicating a moderate level of discrimination between participants with and without events. These indices are for all models presented in Table 2. In all instances, the AUC and C-statistics were relatively unchanged, indicating that model discrimination did not improve with addition of the frailty measures. The C-statistics, NRI, and IDI between all models are presented in Table 3. The continuous NRI for the addition of frailty score was 0.273 (95% CI=−0.042, 0.586) and for the addition of frailty status it was also 0.273 (95% CI=−0.042, 0.586), suggesting a modest but not significant improvement in discrimination, while the categorical NRI did not suggest any improvement in discrimination. The IDI with the addition of frailty score was 0.0088 (95% CI=0.0018, 0.0183) and with the addition of frailty status was 0.0082 (95% CI=0.0021, 0.0154), suggesting a very small improvement in discrimination ability. The continuous and category-based NDI did not indicate any improvement in discrimination with the addition of slow gait, while the IDI suggested a small improvement with the addition of slow gait vs the PCEs-only model (0.009 [0.0024, 0.0179]). The IDI and NRI suggested a small improvement in discrimination with the addition of weak grip. The differences in the model C-statistics also indicate no improvement in discrimination with the addition of the frailty measures.
Table 2:
Cox proportional hazards regression models for incident CVD with baseline PCEs and frailty
| Model 0: PCEs only | Model 1: PCEs + frailty score | Model 2: PCEs + frailty status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Integrated AUC | C-statistic | Hazard Ratio (95% CI) | P-value | Integrated AUC | C-statistic | Hazard Ratio (95% CI) | P-value | Integrated AUC | C-statistic | |
| PCEs scorea | 1.13 (1.09,1.17) | <0.001 | 0.777 | 0.719 | 1.13 (1.09,1.17) | <0.001 | 0.763 | 0.708 | 1.13 (1.09,1.18) | <0.001 | 0.774 | 0.711 |
| Frailty scoreb | 1.30 (1.00,1.70) | 0.05 | ||||||||||
| Frailty statusc | 2.11 (0.71,6.19) | 0.2 | ||||||||||
| Pre-frailty statusd | 1.64 (0.84, 3.2) | 0.1 | ||||||||||
| Model 3: PCEs + slow gait | Model 4: PCEs + weak grip | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Integrated AUC | C-statistic | Hazard Ratio (95% CI) | P-value | Integrated AUC | C-statistic | |
| PCEs scorea | 1.13 (1.09,1.17) | <0.001 | 0.764 | 0.710 | 1.13 (1.09,1.17) | <0.001 | 0.802 | 0.722 |
| Slow gaite | 1.57 (0.84, 2.95) | 0.2 | ||||||
| Weak gripf | 1.65 (0.85,3.21) | 0.1 | ||||||
for every one percent increase
for every one unit increase
frail (vs. non-frail)
pre-frail (vs. non-frail)
>4 seconds/4 meters (vs. ≤4 seconds)
men: ≤29 kg for body mass index (BMI) ≤24 kg/m2, ≤30 kg for BMI 24.1–28 kg/m2, ≤32 kg for BMI >28 kg/m2; women: ≤17 for BMI ≤23 kg/m2, ≤17.3 for BMI 23.1–26 kg/m2, ≤18 for BMI 26.1–29 kg/m2, ≤21 for BMI >29 kg/m2
CVD=cardiovascular disease; PCEs=Pooled Cohort Equations; CI = Confidence Interval; AUC= area under the receiver operating characteristic curve
Table 3:
C-Statistic differences, Net Reclassification Improvement, and Integrated Discrimination Improvement between all models
| Difference in C-statistics | Continuous NRI | Category-based NRI | IDI | |||||
|---|---|---|---|---|---|---|---|---|
| Modelsa | Difference | 95% CI | NRI | NRI 95% CI | NRI | NRI 95% CI | IDI | IDI 95% CI |
| Model 1 (vs Model 0) | −0.0112 | (−0.0453, 0.023) | 0.2726 | (−0.0423, 0.5863) | −0.0145 | (−0.1263, 0.1016) | 0.0088 | (0.0018, 0.0183) |
| Model 2 (vs Model 0) | −0.0077 | (−0.0527, 0.0374) | 0.2726 | (−0.0423, 0.5863) | 0.0066 | (−0.1187, 0.148) | 0.0082 | (0.0021, 0.0154) |
| Model 3 (vs Model 0) | −0.0088 | (−0.0539, 0.0362) | 0.2351 | (−0.0927, 0.5645) | −0.0335 | (−0.1496, 0.0806) | 0.009 | (0.0024, 0.0179) |
| Model 4 (vs Model 0) | 0.0029 | (−0.0347, 0.0406) | 0.2324 | (−0.0698, 0.5299) | −0.0333 | (−0.1676, 0.0977) | 0.0062 | (0.0003, 0.0141) |
Model 0: Pooled Cohort Equations (PCEs) only, Model 1: PCEs + frailty score, Model 2: PCEs + frailty status, Model 3: PCEs + slow gait, Model 4: PCEs + weak grip
NRI = Net Reclassification Improvement
IDI = Integrated Discrimination Improvement
CI = Confidence Interval
Discussion:
In this large, well-characterized, prospectively-followed cohort of virally suppressed PWH with age ≥40 years, we observed that frailty score independently predicted CVD events. Nonetheless, the various discrimination measures indicate that the addition of frailty did not improve the ability of the 2013 American College of Cardiology/American Heart Association PCEs to predict CVD events. Prior attempts to modify existing CVD risk estimation tools that were developed in the general population to optimize risk prediction for PWH have not led to substantial improvements in the models’ discrimination (10, 32). Feinstein et al. previously incorporated HIV-specific covariates (HIV viral load, CD4 lymphocyte count, ART exposure, and protease inhibitor use), factors known to influence CVD development, into PCEs; however this addition did not result in improved CVD prediction among PWH (32). HIV-specific covariates are widely variable among PWH and their individual roles as quantifiable CVD risk factors are poorly understood. Thus, it was not our intent to create a novel risk estimation tool for PWH, nor was it to evaluate the impact of adding HIV-specific parameters to existing PCEs, an undertaking previously not shown to be impactful on improving CVD prediction. Rather, since the assessment of frailty is easily accomplished in a clinical setting, and since it has also been useful for determining the risks for other clinical outcomes, incorporating frailty into the available measures of CVD risk stratification appears to be worthwhile. We know of no other studies to-date that have attempted such an analysis.
Frailty’s ability to predict multiple negative health consequences among PWH has been previously described; frailty has been observed to predict bone fracture (33), falls (18), multimorbidity (34), hospitalizations (35), and overall mortality (34–36). Prior to our analyses of this cohort, frailty had not previously been observed to predict CVD among PWH. Among HIV-uninfected persons, however, individual components of frailty have been associated with increased CVD risk; low energy, exhaustion, and slow gait speed conferred the highest risk among the different frailty components, with overall highest risk of CVD occurring with higher cumulative frailty score, which is consistent with our observations (37). In a cohort of older HIV-uninfected persons (age ≥65 years), frailty was shown to enhance the 12-month mortality predictive ability of the Global Registry of Acute Coronary Events (GRACE) model (38). Among HIV-uninfected persons with CVD, frailty was predictive of cerebrovascular accident (39), and slow gait speed associated with increased cardiovascular mortality (40). However, while well-validated CVD risk models developed in the general population exist and are widely utilized for CVD risk stratification, these same tools do not accurately estimate CVD risk and often perform inconsistently across different sexes and healthcare settings among PWH (10, 11). To date, only one CVD risk equation (from The Data Collection on Adverse Effects on Anti-HIV Drugs [D:A:D] Cohort) incorporates an HIV-specific variable (ART exposure), yet it too underestimates CVD risk among PWH (5, 41, 42). Other currently used disease risk prediction tools have not yet been adapted to include HIV-specific variables nor validated among PWH. Therefore, the clinical use of an adjunctive CVD predictor, such as frailty, is particularly relevant to PWH.
While traditional risk factors clearly play a role in the development in CVD among PWH, higher levels of chronic systemic inflammation and immune activation that are prevalent among PWH compared to the general population have been associated with CVD risk as well (13, 43, 44), and perhaps account for “missing” CVD risk factors more common among PWH that are not included in current risk prediction tools (10). As suggested by Triant et al., improving the discrimination of CVD risk prediction among PWH should involve consideration of factor(s) related to HIV-associated inflammation and immune activation (10); frailty is one such factor. Associations between heightened levels of inflammation and the development of frailty in PWH have similarly been observed (22, 24). In fact, greater levels of specific inflammatory biomarkers have been independently associated with both frailty and CVD among PWH, and include interleukin-6 (IL-6), high sensitivity C-reactive protein (hsCRP), sCD14 and sCD163 (22, 24, 26, 44–46). Frailty may therefore represent, at least in part, a cumulative consequence of excess chronic systemic inflammation from HIV infection, years of ART exposure, and excesses in the prevalence of competing CVD risks, consonant with our current observations that frail PWH are particularly vulnerable to CVD, which is a known sequela of chronic inflammation. The multiple associations we describe between HIV, frailty, and CVD are depicted in Figure 1. (It should be noted that there is a difference between identifying an association between two clinical entities [frailty and CVD] and confirming causality. For example, inflammation may lead to both CVD and frailty but that does not mean frailty causes CVD or vice versa.) Without an accurate CVD risk estimator “customized” for use among PWH, use of other available risk prediction strategies may improve the sensitivity of CVD event risk assessment in this vulnerable population. We believe that clinical frailty assessment can be considered a viable adjunctive CVD risk prediction tool for PWH; higher baseline frailty scores predicted CVD risk in our cohort.
Figure 1:
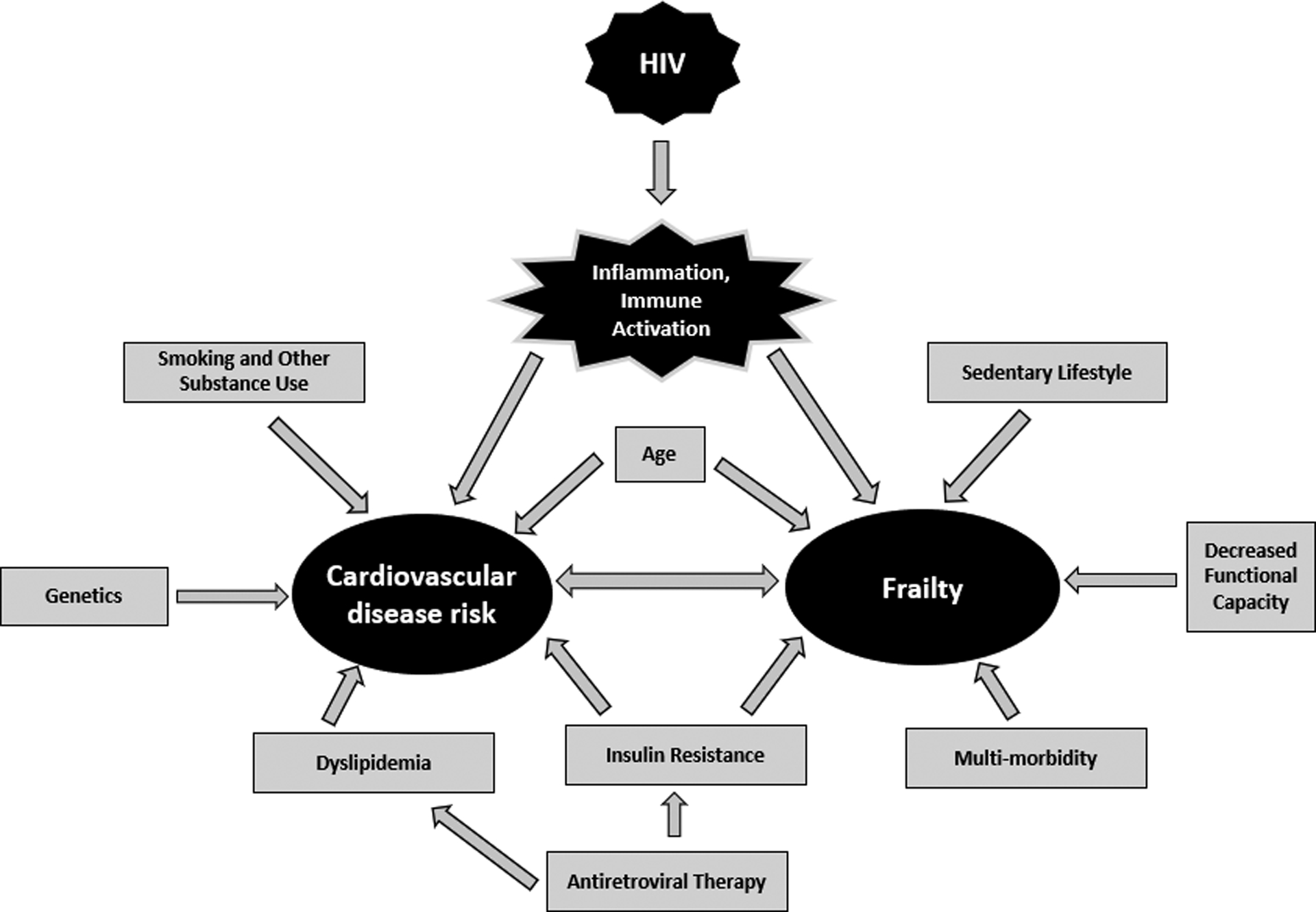
Associations between HIV, frailty, and cardiovascular disease risk
Our findings may not be generalizable to PWH who experience intermittent virologic non-suppression or disengagement in care, as the majority of HAILO participants are durably virally suppressed and compliant with healthcare and research participation. The numbers of frail participants at baseline (57, 6%) and CVD events observed (39, 4%) were low, and may not accurately reflect the magnitude of CVD risk for all frail PWH. Additionally, the mean follow-up of the HAILO cohort was only 5 years at the time of this analysis and we therefore calculated 5-year rather than 10-year CVD risk. While we did observe an association between frailty and CVD risk after 5 years of follow-up, frailty’s effect on the performance of PCEs may be evident with longer follow-up. Therefore, longer follow-up of our cohort is needed. Our findings should also be further evaluated in larger cohorts that include participants with and without HIV, and with variable prevalances of frailty and CVD risk factors in order to evaluate how the relationship between frailty and CVD risk may vary. Exercise interventions targeting reversible components of frailty and thereby their potential effects on mitigating CVD risk should also be explored. Performance of alternative measures of physical function (such as the 400-meter walk) may provide additional information regarding CVD risk as well.
In summary, we found that PCEs and baseline frailty score are independent risk factors for CVD events. While the addition of frailty did not substantially modify the performance of PCEs in our cohort, since no CVD risk estimator has yet been validated among PWH, frailty may provide additional or adjunctive CVD risk estimation value for aging PWH with marginal or indeterminate CVD risk based on PCEs. Our observations support the incorporation of annual frailty assessment into the routine care of older PWH, enhancing CVD risk stratification.
Acknowledgements:
All authors contributed to study design, data interpretation, manuscript revision, and approved of the final draft. KW and KT performed the data analysis. KW and KT had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SGK, KW, KT, KME, SLK, and FLP prepared the initial manuscript. We wish to thank all study volunteers of A5322 (HAILO), the ACTG clinical units, and the ACTG.
Conflicts of Interest and Source of Funding:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. KME has received grant support from Gilead Sciences and has served on advisory panels for ViiV and Theratechnologies. SLK has received grant support from Gilead Sciences. FJP has received personal fees from Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and ViiV. All other authors report no potential conflicts of interest. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636, UM1 AI106701, and UM1 AI069494. This research is also supported in part by NIA AG054366.
Footnotes
Preliminary data were presented at AIDS 2020: Virtual, July 9, 2020
References:
- 1.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23(8):625–30. [DOI] [PubMed] [Google Scholar]
- 4.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. Aids. 2010;24(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson-Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr., Skarbinski J, Chmiel JS, et al. Cardiovascular Disease Risk Prediction in the HIV Outpatient Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(11):1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monroe AK, Haberlen SA, Post WS, Palella FJ Jr., Kinsgley LA, Witt MD, et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. Aids. 2016;30(13):2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanni MV, Fitch KV, Feldpausch M, Han A, Lee H, Lu MT, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS (London, England). 2014;28(14):2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto Neto L, Dias FR, Bressan FF, Santos CRO. Comparison of the ACC/AHA and Framingham algorithms to assess cardiovascular risk in HIV-infected patients. Braz J Infect Dis. 2017;21(6):577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosepele M, Regan S, Massaro J, Meigs JB, Zanni MV, D’Agostino RB Sr., et al. Impact of the American College of Cardiology/American Heart Association Cholesterol Guidelines on Statin Eligibility Among Human Immunodeficiency Virus-Infected Individuals. Open Forum Infect Dis. 2018;5(12):ofy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triant VA, Lyass A, Hurley LB, Borowsky LH, Ehrbar RQ, Silverberg MJ, et al. , editors. Cardiovascular Risk Estimation is Sub-optimal Across Two HIV Cohort. AIDS 2020; 2020. July 6–10; San Francisco. [Google Scholar]
- 12.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211(8):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–96. [DOI] [PubMed] [Google Scholar]
- 14.Lopez M, San Roman J, Estrada V, Vispo E, Blanco F, Soriano V. Endothelial dysfunction in HIV infection--the role of circulating endothelial cells, microparticles, endothelial progenitor cells and macrophages. AIDS Rev. 2012;14(4):223–30. [PubMed] [Google Scholar]
- 15.Castronuovo D, Cacopardo B, Pinzone MR, Di Rosa M, Martellotta F, Schioppa O, et al. Bone disease in the setting of HIV infection: update and review of the literature. Eur Rev Med Pharmacol Sci. 2013;17(18):2413–9. [PubMed] [Google Scholar]
- 16.Kelesidis T, Currier JS, Yang OO, Brown TT. Role of RANKL-RANK/osteoprotegerin pathway in cardiovascular and bone disease associated with HIV infection. AIDS Rev. 2014;16(3):123–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Erlandson KM, Perez J, Abdo M, Robertson K, Ellis RJ, Koletar SL, et al. Frailty, Neurocognitive Impairment, or Both in Predicting Poor Health Outcomes Among Adults Living With Human Immunodeficiency Virus. Clin Infect Dis. 2019;68(1):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassiopoulos K, Abdo M, Wu K, Koletar SL, Palella FJ Jr., Kalayjian R, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS (London, England). 2017;31(16):2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–49. [DOI] [PubMed] [Google Scholar]
- 21.Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty Is an Independent Risk Factor for Mortality, Cardiovascular Disease, Bone Disease, and Diabetes Among Aging Adults With Human Immunodeficiency Virus. Clin Infect Dis. 2019;69(8):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2015;70(12):1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ Jr., et al. Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men With or at Risk for HIV Infection. The Journal of infectious diseases. 2017;215(2):228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and Circulating Markers of Inflammation in HIV+ and HIV- Men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2017;74(4):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeoh HL, Cheng AC, Cherry CL, Weir JM, Meikle PJ, Hoy JF, et al. Immunometabolic and Lipidomic Markers Associated With the Frailty Index and Quality of Life in Aging HIV+ Men on Antiretroviral Therapy. EBioMedicine. 2017;22:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 29.Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, et al. Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy. J Infect Dis. 2017;215(6):933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muntner P, Colantonio LD, Cushman M, Goff DC Jr., Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. Jama. 2014;311(14):1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Shi Q, Hoover DR, Tien PC, Plankey MW, Cohen MH, et al. Frailty predicts fractures among women with and at-risk for HIV. Aids. 2019;33(3):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guaraldi G, Brothers TD, Zona S, Stentarelli C, Carli F, Malagoli A, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS (London, England). 2015;29(13):1633–41. [DOI] [PubMed] [Google Scholar]
- 35.Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. Journal of acquired immune deficiency syndromes (1999). 2014;67(4):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlandson KM, Perez J, Abdo M, Robertson K, Ellis RJ, Koletar SL, et al. Frailty, Neurocognitive Impairment, or Both in Predicting Poor Health Outcomes Among Adults Living with HIV. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. Journal of the American College of Cardiology. 2015;65(10):976–83. [DOI] [PubMed] [Google Scholar]
- 38.Anand A, Cudmore S, Robertson S, Stephen J, Haga K, Weir CJ, et al. Frailty assessment and risk prediction by GRACE score in older patients with acute myocardial infarction. BMC Geriatr. 2020;20(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campo G, Maietti E, Tonet E, Biscaglia S, Ariza-Solè A, Pavasini R, et al. The Assessment of Scales of Frailty and Physical Performance Improves Prediction of Major Adverse Cardiac Events in Older Adults with Acute Coronary Syndrome. J Gerontol A Biol Sci Med Sci. 2020;75(6):1113–9. [DOI] [PubMed] [Google Scholar]
- 40.Dumurgier J, Elbaz A, Ducimetière P, Tavernier B, Alpérovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. Bmj. 2009;339:b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. [DOI] [PubMed] [Google Scholar]
- 42.Nery MW, Martelli CM, Silveira EA, de Sousa CA, Falco Mde O, de Castro Ade C, et al. Cardiovascular risk assessment: a comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. ScientificWorldJournal. 2013;2013:969281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3(3):e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. The Journal of infectious diseases. 2013;208(11):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206(10):1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


