Abstract
Objective:
This study aims to assess the product-related, relationship-related, and sex-related factors that act as facilitators and barriers to the acceptability of a vaginal ring (VR) for HIV prevention among adolescent girls.
Design:
Qualitative study
Methods:
Ninety-six 15 to 17-year-old girls from six urban U.S. sites were enrolled in MTN-023/IPM 030, a 24-week randomized controlled trial assessing the safety and acceptability of a dapivirine VR for HIV prevention. At week 24, 21 girls were randomly selected to participate in in-depth-interviews. Interviews were transcribed verbatim, and data analyzed using a thematic analysis approach. Facilitators and barriers to VR acceptability related to participants’ relationships, sexual activity, and characteristics of the VR product were identified.
Results:
Factors related to relationships rarely appeared to act as barriers to VR acceptability; most participants disclosed VR use to sexual partners, and positive reactions from sexual partners, which were common, appeared to facilitate VR acceptability. Emotional and/or physical discomfort surrounding VR use during sex was mentioned occasionally as a barrier to VR acceptability. Product characteristics were most frequently mentioned as barriers to VR acceptability. Many participants reported concerns about the large size of the VR upon first impression. While most found the VR comfortable, some reported pain with VR insertion. Several participants were concerned about VR cleanliness, particularly during menstruation.
Conclusion:
Product considerations, specifically size and use during menstruation, were the most commonly reported barriers to VR acceptability in this study. Adolescent girls may require additional counseling to assuage product concerns regarding a VR for HIV prevention.
Keywords: Adolescent, vaginal ring, HIV, pre-exposure prophylaxis
Background
Globally, adolescent girls and young women face a disproportionate burden of HIV as compared to their male counterparts [1,2]. Each week in 2019, an estimated 5,500 adolescent girls and young women became infected with HIV [3]. In sub-Saharan Africa, five in six new HIV infections reported among adolescents aged 15 to 19 in 2019 were among girls [3]. Adolescent girls predominantly acquire HIV through heterosexual sex [4], due to a multitude of biological, structural, and behavioral factors [5–11]. New prevention methods that specifically target adolescent girls are critical to prevent HIV acquisition among this population, and to curtail spread of HIV via heterosexual transmission.
Oral pre-exposure prophylaxis (PrEP) and vaginal microbicide gels have been investigated to reduce the incidence of HIV among girls and women [12–14]. These self-initiated interventions may face fewer barriers to adherence than condoms, which require full partner cooperation, and may confer better HIV protection. While antiretroviral-based pills and gels have the potential to be efficacious among adolescent girls and women, the extent to which they protect against HIV is highly dependent on proper usage. Adherence to daily oral PrEP and a microbicide gel in some previous efficacy trials – notably in Microbicide Trials Network (MTN)-003 (VOICE) and FEM-PrEP – was demonstrated to be too low to effectively protect against HIV [12, 13]. In CAPRISA 004, an additional study assessing the effectiveness of a microbicide gel for HIV prevention, women who adhered to the gel less than 80% of the time did not experience significantly reduced HIV incidence, compared to women who received a placebo gel [14]. Additionally, in the VOICE trial, younger women were significantly less likely than older women to adhere to product use, and significantly more likely to acquire HIV [12]; no significant differences in adherence rates or HIV incidence rates by participant age were reported in FEM-PrEP or CAPRISA 004 [13–15].
A monthly vaginal ring (VR) is a longer-term HIV prevention method that may mitigate some of the behavioral barriers to daily or on-demand PrEP adherence. Safety of, and adherence to a monthly dapivirine/maraviroc VR for HIV prevention was assessed in a phase one trial among 48 HIV-negative U.S. women aged 18–40 years in MTN-013 [16]. The study found that the VR was safe and well-tolerated, with no difference in adverse events between treatment and placebo arms [16]. Additionally, relatively high adherence to the monthly VR was demonstrated via assessments by computer-assisted self-interviews, face-to-face interviews, and residual drug levels in VRs [16]. MTN-020/ASPIRE was a Phase 3 randomized placebo-controlled trial assessing the efficacy, safety, acceptability, and adherence of the dapivirine VR among 2,629 HIV-negative women ages 18–45 in Malawi, South Africa, Uganda, and Zimbabwe [17]. The ASPIRE trial found that the dapivirine VR reduced the risk of HIV infection by 27% in the study population overall, but did not reduce HIV risk among women aged 18–21 years [18]. This was in part due to lower adherence to the VR among younger women, which was assessed by drug presence in plasma and residual drug level in the ring [18]. Similarly, IPM 027 enrolled 1,959 women ages 18–45 across six sites in South Africa and one site in Uganda, and found that the dapivirine VR reduced HIV risk by about 31% in the study overall, but did not have a significant benefit over the placebo VR among women under 21 years [19].
The safety, acceptability, and effectiveness of a dapivirine VR must also be assessed in adolescent girls ages 15 to 17 years. Given ethical and regulatory difficulties, however, few PrEP and microbicide trials have been conducted among this age group. MTN-023/IPM 030, a two-arm, randomized, double-blind, placebo-controlled Phase 2a trial, is one such study. MTN-023/IPM 030 enrolled 96 HIV-uninfected adolescent girls aged 15 to 17 years across six sites in the United States from 2014–2016 [20]. The primary aim was to assess the safety of the dapivirine VR inserted every 4 weeks during a 24-week study period [20]. Participants were randomized 3:1 to a dapivirine (25mg) VR or a placebo VR and followed for 24 weeks.
MTN-023/IPM 030 found no difference in safety outcomes between girls in the treatment vs. placebo arms of the study and indicated that adherence to the dapivirine VR was relatively high; 87% of participants demonstrated short-term adherence based on plasma dapivirine concentrations of >95 pg/mL, and 95% of participants demonstrated monthly adherence based on residual ring dapivirine levels of <23.5mg [20]. Additionally, the girls reported that they liked the VR at 93% of clinic visits, and that they experienced no discomfort at 87% of visits [20]. The current study is nested within MTN-023/IPM 30, and aims to identify VR product characteristics, relationship-related factors, and sex-related factors that may influence the acceptability of and adherence to a VR for HIV prevention among adolescent girls ages 15 to 17 years.
Methods
At week 24 of MTN-023/IPM 030, 21 of the 96 girls (21.9%) from five of the six sites (participants from St. Jude Children’s Hospital in Memphis were not included due to site limitations) were randomly selected for in-depth-interviews (IDIs) via Skype that lasted from 30 minutes to one hour, to explore the facilitators and barriers to VR acceptability and adherence throughout the study period. Participant assent and parent/legal guardian informed consent to participate in IDIs were obtained at the time of enrollment into MTN-023/IPM 030. All participants selected for IDIs completed IDIs. Three female researchers, aged mid-20s to late-30s, who were trained in IDI administration conducted the interviews. Information on partner reactions and other partner-related topics were reported by the adolescent girls who participated in IDIs – partners were not interviewed. IDIs were audio recorded and transcribed verbatim.
A thematic analysis approach was employed to identify themes pertaining to product, relationship, and sex factors that appeared to facilitate or hinder VR adherence and acceptability. The analysis of IDIs was guided by a modified version of the “Comprehensive and Flexible Conceptual Framework,” which was originally designed to investigate the acceptability of and adherence to microbicides in HIV clinical trials [21, 22]. The version of the framework adapted for this study focuses on three aspects of the use experience – product-related factors, sex-related factors, and relationship-related factors – that affect the acceptability and adherence to a VR for HIV prevention among adolescent girls (Figure 1).
Figure 1.
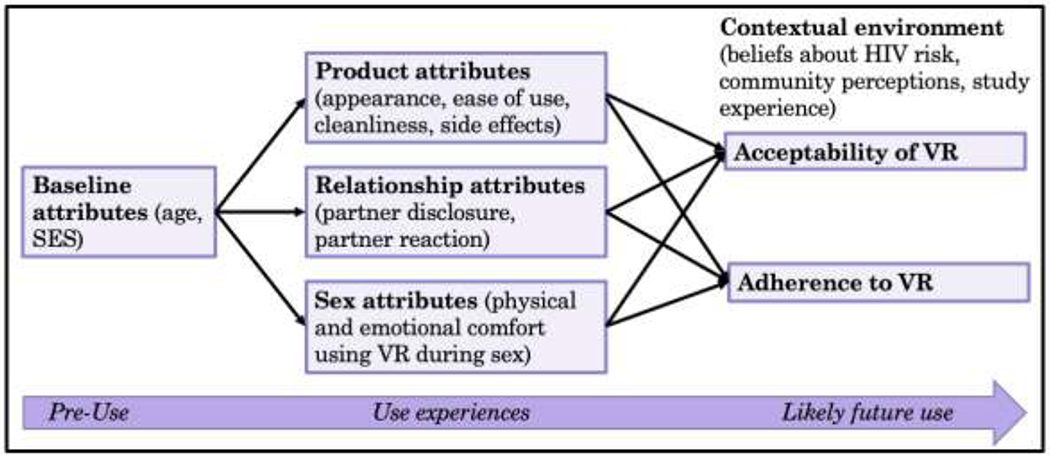
Conceptual model for VR use experiences predicting barriers/facilitators to VR acceptability and adherence, based on results from IDIs among adolescent girls in MTN-023/IPM 030; adapted from the Comprehensive and Flexible Conceptual Framework
Transcribed data were analyzed qualitatively using Atlas.Ti v1.5.0. A detailed codebook was created, defining the relevant product, sex, and relationship portions of the IDIs. Codes were grouped according to categories specified in the conceptual model (Appendix I). Once coded, data were analyzed to decipher recurrent themes. Fourteen percent of IDIs were double-coded for inter-rater reliability (IRR), yielding an IRR of 82% and indicating a relatively high level of coder agreement. All discrepancies were resolved by discussing and obtaining consensus within the research team.
Results
Sociodemographics
The 21 participants who completed IDIs had a mean age of 16.3 years (Table 1). Eleven (52%) of the girls identified as African-American, seven (33%) identified as White, and three (14%) identified with another racial group (Table 1). Four participants (19%) identified as Hispanic (Table 1). Participants had the option to select as many racial/ethnic groups with which they identified. Participants who completed IDIs were similar to study participants who were not selected for IDIs with regards to age, race/ethnicity, and sexual history (Table 1). Among those selected for IDIs, seventeen girls (81%) had been randomly allocated to the treatment VR, while four (19%) had been assigned to the placebo VR. Four (19%) participants were from the Boston site, four (19%) were from the Bronx, six (29%) were from Colorado, four (19%) were from Pittsburgh, and three (14%) were from the University of Alabama.
Table 1.
Demographics and Sexual History of Participants in MTN-023/IPM 030, Separated by those who Completed IDIs, and those not Selected to Complete IDIs
| Variable | Statistic | Total | IDI | Non-IDI |
|---|---|---|---|---|
| n | 96 | 21 | 75 | |
| Age | mean (sd) (min, max) |
16.3 (0.8) (15, 17) |
16.3 (0.9) (15, 17) |
16.2 (0.8) (15, 17) |
| Race | ||||
| African-American | n (%) | 57 (59%) | 11 (52%) | 46 (61%) |
| White | n (%) | 24 (25%) | 7 (33%) | 17 (23%) |
| Other | n (%) | 15 (16%) | 3 (14%) | 12 (16%) |
| Ethnicity | ||||
| Latina or Hispanic | n (%) | 20 (21%) | 4 (19%) | 16 (21%) |
| Not Latina or Hispanic | n (%) | 76 (79%) | 17 (81%) | 59 (79%) |
| Sexual History | ||||
| Lifetime sex partners | median (min, max) |
3 (0, 23) |
2 (1, 15) |
3 (0, 23) |
| Sex partners in past 30 days | median (min, max) |
1 (0, 7) |
1 (0,2) |
1 (0,7) |
| No condom use at last sex act | n (%) | 30 (49%) | 6 (40%) | 24 (52%) |
Relationship factors
“[I told] my boyfriend… The way [he] reacted was generally positive, [he] just wanted to make sure that I was safe… but [he was] totally fine with me doing what I wanted to do.”
- 15-year-old girl from Colorado
Relationship factors, including disclosure of VR use to partners (i.e. whether girls told their partner that they were using a VR as part of a clinical trial), and partners’ reaction to the girls’ VR use, were most frequently mentioned as facilitators to VR acceptability and adherence (Table 2). The majority of girls (n=18; 86%) felt comfortable telling their main sex partners, most commonly referred to as “boyfriends,” about their VR use. A few participants (n=3; 14%), however, decided not to disclose VR use to their partners, due to feeling uncomfortable and embarrassed about approaching this conversation, and/or due to concerns that their partner would have a negative reaction to the VR.
Table 2.
Perceived facilitators to VR acceptability and adherence for HIV prevention according to IDIs among adolescent girls in MTN-023/IPM 030, separated by relationship, sex, and product factors
| Recurrent Themes | Sample Quotations |
|---|---|
| Relationship-related factors | |
| Deciding to disclose VR use to partners | “[I told] my mom and my partner.” |
| “[I told] my sex partner, and my sister.” | |
| Positive reaction to VR use from partners | “He was very supportive… like I was already on the Nuva ring, so he knew about that too, so really was not much of a difference.” |
| “[My partner] asked what it was. But I explained it to him and it didn’t seem to bother him.” | |
| Sex-related factors | |
| Emotional comfort with VR use during sex | “I liked that it didn’t make me feel uncomfortable. That any time I have sex… my partner didn’t feel it…” |
| “At first I was like ‘Is he going to feel it, like how is it going to feel?’ but then once I did have intercourse everything was okay, he didn’t even feel it.” | |
| Physical comfort with VR use during sex | “[My boyfriend] said he doesn’t feel it when we have intercourse and he doesn’t actually really know it’s there.” |
| “My boyfriend didn’t even notice it. Like he felt it, but it didn’t bother him he said.” | |
| Product-related factors | |
| Inability to feel VR/comfort of VR inside body | “What I liked most about the ring was that you can’t feel it.” |
| “… It didn’t move around at all, and then I couldn’t even tell it was really up there most of the time, so I mostly just forgot about it, so that was really the most beneficial thing that came out of it.” | |
| Ease of use | “I like how simple it is, because when you’re using like birth control you have to take a shot over a certain period of time or take a pill and remember what time to take it at, but with the ring you just put it in and you can take it out too, it’s not like a pill where you eat it and you can’t take it out again. If you want options.” |
| “It was easy to put in and take out.” | |
| Lack of interference with daily activities | “Well I can’t feel it when you’re going about your business… I thought I might be able to feel it like when I run and stuff cause I do a few races and I do a lot of like training… But I can’t feel it… I like that.” |
| “Like when I was doing certain activities I couldn’t feel it at all. Like I was, I have to do a lot of bending. I didn’t feel it, it never came out.” | |
| “I liked the fact that you didn’t like, feel it, like as you were walking around and I was cheerleading, so I didn’t even feel it with me cheerleading.” | |
Partner reactions were generally neutral or positive, with most partners (n=15; 71%) exhibiting acceptance or support of participants’ VR use (Table 2). A few partners (n=3; 14%) exhibited additional interest in the VR, wanting to know more about the product and the study. Some partners (n=3; 14%) offered additional support, helping their partner re-insert the VR if it became dislodged during sex. Despite the majority of partners reacting positively or neutrally to the VR, a couple of girls (n=2; 10%) reported that partners had negative reactions (Table 3). One girl reported that her partner did not like that he could feel the VR during sex. Infrequently, partners expressed emotional discomfort, confusion, or fears associated with their partner’s use of the VR (n=2; 10%). One partner expressed fear that using the VR could increase his partner’s susceptibility to HIV infection.
Table 3.
Perceived barriers to VR acceptability and adherence for HIV prevention according to IDIs among adolescent girls in MTN-023/IPM 030, separated by relationship, sex, and product factors
| Recurrent Themes | Sample Quotations |
|---|---|
| Relationship-related factors | |
| Deciding not to disclose VR use to partner(s) | “The one [partner] I thought might have something negative to say, I just didn’t tell.” |
| “It was just like sometimes embarrassing, I would like, sneakily try and take it out… it was just like… an awkward thing to bring up because people don’t really know about it.” | |
| Negative partner reaction to VR | “My sex partner wasn’t really okay with it. He had some questions about it… He was basically worried about if I could get HIV from using it… He doesn’t really like the ring.” |
| “What I didn’t like probably was like, insecure with having relations, but then like it wasn’t an issue for me… it was just my partner… he just felt like weird.” | |
| Sex-related factors | |
| Emotional discomfort with VR use during sex | “I didn’t want to have sex. I don’t like sex with this ring.” |
| “[I dislike that] your partner is able to feel it during sex.” | |
| Physical discomfort/interference with VR use during sex | “When I got the ring there would be some weeks when we wouldn’t have [sex] cause I was feeling raw or uncomfortable down there.” |
| “During sex it’s not the best feeling, I don’t know, it’s kind of uncomfortable.” | |
| Product-related factors | |
| Concerns with size and rigidity of VR | “It was bigger than what I imagined.” |
| “I thought it was really big. I thought it wasn’t going to fit.” | |
| Concerns/ discomfort with using the VR during menstruation | “I feel like that’s kind of gross, to have blood on the ring.” |
| “I’d be having my period while I had the ring in… and… I could just assume that it was probably gross, so I just cleaned it out when I was in the shower.” | |
| Problems with ring insertion | “It’s just difficult to insert, it’s just really rigid. So it’s kind of harder to put it in.” |
| “I didn’t like how difficult it was to put in. Because it was very, very difficult.” | |
| Pain or discomfort when VR was inside body | “When I was working out, it was like pulling or pushing.” |
| “When I was at school… I don’t know it would move and then there was no place to like really fix the ring.” | |
| Unwanted expulsion of the VR | “There was one time it came out in the shower.” |
| “It came out about five times [during sexual intercourse].” | |
Sex factors
“I was anxious; I’m like what if they don’t like it, what if they feel it’s there… During sex it’s not the best feeling… it’s kind of uncomfortable.”
- 17-year-old girl from Boston
Emotional discomfort surrounding VR use during sex was expressed fairly frequently (n=6; 29%; Table 3). Some of these expressions of anticipated discomfort appeared to be related to relationship factors. For example, some girls (n=3; 14%) explained that they did not like using the VR during sex, because their partners could feel it. Most partners who felt the VR during sex, however, expressed that this did not bother them. More commonly, when partners expressed that they could feel the VR during sex, this caused the girls to dislike or feel uncomfortable using the VR. One girl expressed feelings of anxiety during sex while using the VR, because she did not feel able to disclose her VR use and did not want her partner to find out about it.
In addition to feelings of embarrassment and anticipated concerns regarding partner reactions surrounding VR interference with sex, some girls reported physical discomfort, pain, or VR expulsion during sex (n=6; 29%). Three girls (14%) reported that this physical discomfort led them to remove the VR before or during sex. Two girls (10%) stated that they had a decreased desire to have sex, and were thus having sex less frequently, which they attributed to discomfort with having sex while using the VR. Three girls (14%) reported unwanted VR expulsion during sex. Several girls (n=5; 24%) reported that having to leave the VR in while having sex was one of the things they liked least about the product.
Despite the fact that some girls felt emotional and/or physical discomfort using the VR during sex, the majority (n=14; 67%) reported that they did not experience such discomfort. Most girls reported that they felt comfortable, that their partners could not feel the VR, and that their sexual experiences did not change as a result of using the VR (Table 2).
Product factors
“I thought it was pretty big… [Insertion] is kind of painful.”
- 16-year-old girl from Alabama
“I’d be having my period while I had the ring in… and… I could just assume that it was probably gross, so I just cleaned it out when I was in the shower.”
- 15-year-old girl from Colorado
Concerns with the product itself were much more commonly mentioned than relationship- or sex-related concerns (Table 3). Upon being presented with the VR for the first time, the majority of participants (n=16; 76%) reported that the VR was much larger and/or much more rigid than they had anticipated. Four girls (19%) reported fears that the VR would not fit or would be painful. Several participants (n=9; 43%) reported that inserting the VR was difficult, uncomfortable, or painful (Table 3). However, most girls disclosed that despite the VR’s size they found insertion and use easy and painless (n=12; 57%; Table 2). Even among girls who reported problems associated with insertion or fears about the VR not fitting, most found that once the VR was in place, they could not feel it. Many girls reported that the fact that they could not feel the VR, and that the VR did not interfere with any of their daily activities, was what they liked most about the product.
Despite most girls reporting no physical concerns once the VR was in place, three girls (14%) reported that they did feel physical discomfort after insertion. While one of these girls reported that this physical discomfort lessened as she became more experienced with the VR, the other two girls who reported instances of discomfort noted that they occurred sporadically throughout the study period. This physical discomfort reportedly occurred during daily activities, at school, or while exercising. One girl expressed that she believed her discomfort was associated with the size of the VR.
Another common product concern was related to menstruation while using the VR. According to the study protocol, the VR needed to be in place continuously even during menstruation, and only removed monthly for a new VR to be immediately reinserted. Several girls (n=9; 43%) reported emotional or physical discomfort with using the VR during menstruation (Table 3). Six girls (29%) expressed that they felt the VR was unclean, that they needed to remove the VR to wash it during menstruation, and/or that having blood on the VR was “gross”. Three girls (14%) reported that using tampons while having the VR in place was physically uncomfortable, or that they were told not to use tampons while using the VR, despite the fact that tampon use was not prohibited by the study protocol. Some girls described that having to leave the VR inside their bodies while they menstruated was one of the things they liked least about the VR.
Discussion
Relationship issues, and particularly partner disclosure of VR use, were infrequently mentioned as barriers to VR acceptability in this study. However, fears or discomfort surrounding partner reactions were mentioned by a small number of participants. Although most participants in MTN-023/IPM 030 decided to tell their partners about their use of the ring, partner disclosure may remain an important barrier to VR acceptability among some adolescent girls. Concerns regarding partner disclosure are especially pertinent among adolescent girls, as this population may face barriers in communicating sexual concerns or preferences to their partners [23]. Lack of open communication with partners has also been linked to high rates of unprotected sex among adolescent girls [23].
Several girls in MTN-023/IPM 030 indicated that they did not believe it would be possible to use the VR without telling their partners, and many partners reported feeling the VR during sex. Some girls were worried that partners would dislike being able to feel the VR during sex, even though most partners who felt the VR during sex did not find this bothersome. Similarly, a qualitative analysis of sexual experiences among women enrolled in MTN-020/ASPIRE found that women were frequently concerned about partners still having a positive sexual experience while using the VR [24]. Feeling unable to discuss the VR with partners or concerns that VR use would negatively impact sexual experiences may prevent girls from using a VR for HIV prevention.
Negative partner reactions to the VR, or partner beliefs that use of the product could increase susceptibility to HIV, which was reported by one partner, could have serious consequences regarding VR adherence. Adolescent girls may be particularly vulnerable to opinions of sex partners and may be persuaded to discontinue use of a product that their partners dislike [25, 26]. For example, adolescent girls are more likely than older women to discontinue condom use if partners indicate that they dislike condoms [25–28]. Thus, adolescent girls may be more likely than older women to discontinue use of a VR for HIV prevention if their sexual partners state that they dislike the VR.
In addition to worries and concerns about partners feeling the VR during sex, several girls reported that VR use during sex was painful or resulted in unwanted VR expulsion. Some girls reported removing the VR before sex, which could potentially have implications for product efficacy. It is possible that the VR would confer reduced protection against HIV if removed frequently for sex and/or if not reinserted after sex [20]. Considering that 58% of the 96 girls in MTN-023 reported removing the VR at least once over the study period [20], greater evaluation of VR efficacy given occasional removal is warranted.
Pain and discomfort associated with using the VR during insertion, intercourse, and sporadically through the study period were expressed by some of the girls in this study. Although most girls reported that the VR was comfortable once in place, almost all were concerned about the size and/or rigidity of the VR when it was first presented to them. However, these perceptions usually changed over time as most girls found the VR comfortable to wear. Similar initial fears about the size and rigidity of the dapivirine VR that dissipated after use were expressed among women enrolled in MTN-020/ASPIRE [29]. Fears about pain or discomfort could potentially dissuade adolescents from trying the VR for HIV prevention. To combat this, girls should receive counseling describing how the VR is placed in the vagina, and that it is not expected to cause any pain or discomfort once it has been inserted. Similarly, counseling may help to assuage concerns regarding VR cleanliness during menstruation. Informing young women that it is not necessary to clean the VR during menstruation may be particularly important; results from in-depth interviews among adult women in sub-Saharan Africa with low reported VR adherence in a 12-month open-label extension of the dapivirine VR indicate that VR removal during menses was a frequent challenge to adherence [30]. Clinic staff should counsel girls to assure them that the VR is not unhygienic to use during menstruation, that tampons can still be used, and that it is not necessary to clean or rinse the VR.
Limitations
This study faced a few notable limitations. Firstly, participant enrollment was challenging. Parental permission was required for study participation, which may have caused some girls to decline participation due to concerns that their participation would necessitate disclosure of their sexual experiences to their parent. This was identified as a major barrier to study recruitment. Additionally, adolescent girls who chose to participate in the study were likely more comfortable communicating about sex and with using a VR than the average sexually active adolescent. Girls who participated in this study may have had fewer barriers to VR acceptability and adherence than girls who would choose not to participate in such a study. Additionally, the findings of this study among U.S. adolescent girls may not be generalizable to adolescent girls in other regions of the world.
Multiple interviewers were also involved in conducting the IDIs, potentially contributing to lack of standardization in interview style. A highly detailed standardized interview script was utilized, however, and transcriptions suggest that the same or very similar questions were asked across the sample. IDIs were also conducted via Skype, as opposed to in person, necessitated by the fact that the girls in the study were spread geographically throughout the U.S. This may have impacted interviewer-subject rapport, and affected girls’ willingness to answer sensitive questions. Additionally, Skye-based interviewing may restrict interviewers from recognizing interviewee’s non-verbal cues such as body language, which may have prevented interviewers from fully deciphering interviewees’ responses. Skype-based interviewing has, however, been successfully used previously in microbicide research with young women [31]. Additionally, while interviewers were comprised of graduate students and younger staff members, there was an age difference between interviewers and subjects, which could have also impacted rapport.
It is possible that girls in this study may have been hesitant to discuss questions that were sensitive in nature, such as questions about sex. Interviewers attempted to alleviate this issue by ensuring girls of the confidentiality of their responses, and probing interviewees with relevant questions. Additionally, due to social desirability bias, it is possible that girls may have over-reported adherence to the VR during IDIs. To overcome this barrier, VR adherence in MTN-023/IPM 030 was assessed by plasma measurements and residual drug levels in rings, which was reported in the primary paper [20].
Conclusion
Overall, there was a relatively high level of acceptability of a VR for HIV prevention among participants in the qualitative component of MTN-023/IPM 030. Most girls were comfortable disclosing VR use to their sexual partners and received positive partner reactions to their VR use. Although most girls reported no issues with the VR during sex, some experienced emotional distress as they worried their partners would dislike feeling the VR, and others experienced physical discomfort and/or VR expulsion during sex. More universally, barriers to VR acceptability in this U.S. adolescent population related to concerns with the product’s size, rigidity, and required use during menstruation. Concerns relating to VR size and rigidity usually alleviated over time as most girls found the VR comfortable to use. Several girls disliked using the VR during menstruation, as they felt the VR was unclean. If VRs are approved for use, the issues described in this study must be addressed by clinic counselors. Health care providers will need to discuss with potential users the concerns reported by the adolescent girls in MTN-023/IPM 030 to ensure that girls and young women are well-informed about the VR prior to use.
Supplementary Material
Acknowledgements
We would like to acknowledge the MTN-023/IPM 030 Protocol Team and study participants for their valuable contributions to this study. The MTN-023 study was designed and implemented by the Microbicide Trials Network (MTN) funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funder: US National Institutes of Health
References
- 1.Celum C, Delany-Moretlwe S, McConnell M, van Rooyen H, Bekker L, Kurth A et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc. 2015;18(4 Suppl 3):20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh J Why vulnerable young women at risk of HIV should be prioritized for access to preexposure prophylaxis. AIDS. 2013;27(12):1998–1999. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. UNAIDS Data 2020. Geneva: 2016. https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf [Google Scholar]
- 4.Case K, Ghys P, Gouws E, Eaton J, Borquez A, Stover J et al. Understanding the modes of transmission model of new HIV infection and its use in prevention planning. World Health Organization. 2012; 90(11):831–838A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellar R, Dlamini S, Karim Q. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc. 2015;18(2 Suppl 1):19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomerantz R, de la Monte S, Donegan S, Rota T, Vogt M, Craven D et al. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108(3):321–327. [DOI] [PubMed] [Google Scholar]
- 7.Nagelkerke N, Moses S, de Vlas S, Bailey R. Modelling the public health impact of male circumcision for HIV prevention in high prevalence areas in Africa. BMC Infect Dis. 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal S, Cohen S, Stanberry L. Topical microbicides. Current status and research considerations for adolescent girls. Sex Transm Dis. 1998;25(7):368–377. [DOI] [PubMed] [Google Scholar]
- 9.Shannon K, Leiter K, Phaladze N, Hlanze Z, Tsai A, Heisler M et al. Gender inequity norms are associated with increased male-perpetrated rape and sexual risks for HIV infection in Botswana and Swaziland. PLoS One. 2012;7(1):e28739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66 Suppl 2:S144–153. [DOI] [PubMed] [Google Scholar]
- 11.Maughan-Brown B, Kenyon C, Lurie M. Partner age differences and concurrency in South Africa: Implications for HIV-infection risk among young women. AIDS Behav. 2014;18(12):2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Eng J Med. 2015; 372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Eng J Med. 2012; 367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2011; 329(5996):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, et al. FEM-PrEP: Adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2014; 66(3):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: A double-blind randomized trial. J Aacquir Immune Defic Syndr 2015; 70(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten J, Palanee-Phillips T, Brown E, Schwartz K, Soto-Torres L, Govender V et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375(22):2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mensch B, Richardson B, Husnik M, Brown E, Matovu Kiweewa F, Mayo A et al. Vaginal Ring Use in a Phase 3 Microbicide Trial: A Comparison of Objective Measures and Self-reports of Non-adherence in ASPIRE. AIDS Behav. 2019;23(2):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel A, van Niekerk N, Kapiga S, Bekker L, Gama C, Gill K et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. NEJM. 2016; 375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 20.Bunge K, Levy L, Szydlo D, Zhang J, Gaur A, Reirden D et al. Brief Report: Phase IIa Safety Study of a Vaginal Ring Containing Dapivirine in Adolescent Young Women. J Acquir Immune Defic Syndr. 2020. 83(2):135–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin-Hodoglugil N, Montgomery E, Kacanek D, Morar N, Mtetwa S, Nkala B et al. User experiences and acceptability attributes of the diaphragm and lubricant gel in an HIV prevention trial in southern Africa. AIDS Care. 2011;23(8):1026–1034. [DOI] [PubMed] [Google Scholar]
- 22.Severy L, Tolley E, Woodsong C, Guest G. A framework for examining the sustained acceptability of microbicides. AIDS Behav. 2005;9(1):121–131. [DOI] [PubMed] [Google Scholar]
- 23.Crosby R, DiClemente R, Wingood G, Sionean C, Cobb B, Harrington K. Correlates of unprotected vaginal sex among African American female adolescents: importance of relationship dynamics. Arch Pediatr Adolesc Med. 2000;154(9):893–899. [DOI] [PubMed] [Google Scholar]
- 24.Laborde N, Pleasants E, Reddy K, Atujuna M, Nakyanzi T, Chitukuta M, et al. Impact of the dapivirine vaginal ring on sexual experiences and intimate partnerships of women in an HIV prevention clinical trial: Managing ring detection and hot sex. AIDS Behav. 2018:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacPhail C, Campbell C. 'I think condoms are good but, aai, I hate those things': condom use among adolescents and young people in a Southern African township. Soc Sci Med. 2001;52(11):1613–1627. [DOI] [PubMed] [Google Scholar]
- 26.Sex Poulin M., money, and premarital partnerships in southern Malawi. Soc Sci Med. 2007;65(11):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gage AJ. Sexual activity and contraceptive use: the components of the decisionmaking process. Stud Fam Plann. 1998;29(2):154–166. [PubMed] [Google Scholar]
- 28.Selikow T, Ahmed N, Flisher A, Mathews C, Mukoma W. I am not “umqwayito”: a qualitative study of peer pressure and sexual risk behaviour among young adolescents in Cape Town, South Africa. Scand J Public Health. 2009;37 Suppl 2:107–112. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery E, van der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, et al. Acceptability and use of a dapiviring vaginal ring in a phase III trial. AIDS 2017; 31(8):1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naidoo K, Mansoor LE, Katz AW, Garcia M, Kemigisha D, Morar NS, et al. Qualitative perceptions of dapivirine vaginal ring adherence and drug level feedback following an open-label extension trial. J Acquir Immune Defic Syndr. 2020. Dec 3. doi: 10.1097/QAI.0000000000002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carballo-Dieguez A, Giguere R, Dolezal C, Chen B, Kahn J, Zimet G et al. "Tell Juliana": Acceptability of the candidate microbicide VivaGel(R) and two placebo gels among ethnically diverse, sexually active young women participating in a phase 1 microbicide study. AIDS Behav. 2012;16(7):1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


