Abstract
Background:
Excessive weight gain affects some HIV-positive individuals prescribed dolutegravir- containing regimens. Mechanisms underlying such weight gain are unknown.
Setting:
Data and DNA from antiretroviral therapy-naïve participants who were randomized to initiate dolutegravir with emtricitabine plus either tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF) in the ADVANCE study (NCT03122262) were used to characterize associations between human genetic polymorphisms and magnitude of weight gain.
Methods:
Associations with percent weight gain from baseline to week 48 were assessed using multivariable linear regression models. Primary analyses a priori considered 59 polymorphisms and 10 genes of potential relevance to dolutegravir, TAF or TDF pharmacokinetics. We also explored genome-wide associations.
Results:
Among the 314 (92%) of 340 dolutegravir recipients who were successfully genotyped, 160 (47%) and 154 (45%) were randomized to TAF/emtricitabine and TDF/emtricitabine, respectively. In target gene analyses, the lowest P-values for the dolutegravir and tenofovir groups were ABCG2 rs4148149 (P = 7.0×10−4) and ABCC10 rs67861980 (P = 1.0×10−2), respectively, which were not significant after correction for multiple testing. In genome-wide analyses the lowest P-values for dolutegravir, was rs7590091 in TMEM163 (P = 3.7×10−8), rs17137701 in LOC105379130 (P = 6.4×10− 8) for TAF, and rs76771105 in LOC105371716 (P = 9.7×10−8) for TDF.
Conclusion:
Among South African participants in a randomized clinical trial of dolutegravir plus either TAF/emtricitabine or TDF/emtricitabine, we identified several potential genetic associations with weight gain. Only TMEM163 rs7590091 withstood correction for multiple testing. These associations warrant replication in other cohorts.
Keywords: Weight gain, dolutegravir, tenofovir, pharmacogenetics
Précis
Greater weight gain has been reported with dolutegravir and tenofovir alafenamide. We characterized genetic associations with weight gain among participants in a randomized clinical trial in South Africa. A polymorphism in TMEM163 achieved genome-wide significance.
Introduction
The HIV-1 integrase strand transfer inhibitor (INSTI) dolutegravir effectively controls viral replication, is generally well tolerated, and has out-performed several other drugs.1–6 Dolutegravir and other INSTIs are preferred components of first-line antiretroviral therapy (ART) in the United States, Europe, by WHO, and increasingly worldwide.7,8 In Africa, dolutegravir is replacing non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as efavirenz in first-line ART, and HIV-1 protease inhibitors in second-line ART regimens.9,10 However, recent reports have associated INSTIs, especially the second generation INSTIs dolutegravir and bictegravir, with greater weight gain than other agents either following ART initiation or switch to INSTI-containing ART.11–17 An early report was an observational cohort study of 495 patients with sustained virologic suppression, 136 of whom switched from efavirenz-containing regimens to INSTI-containing regimens. At 18 months, patients who switched to INSTI-containing regimens gained significantly more weight, especially those who switched to dolutegravir, than those who remained on efavirenz.11 Similar findings of greater weight gain with INSTI-containing regimens as compared to NNRTI or protease inhibitor-containing regimens were reported in a pooled analysis of eight randomized controlled trials among treatment-naïve individuals initiating ART,16 and in cohort studies of patients with viral suppression on protease inhibitor- or NNRTI-containing ART who then switched to INSTIs.18,19
Specific concomitant NRTIs have also been associated with magnitude of weight gain. In randomized controlled trials among HIV-positive patients, the tenofovir pro-drug, tenofovir alafenamide fumarate (TAF), has been associated with greater weight gain than tenofovir disoproxil fumarate (TDF),16 and in a study of pre-exposure prophylaxis in HIV-negative individuals.20 TAF has become the most widely prescribed NRTI in high-income countries because it causes less kidney and bone toxicity than TDF. There is considerable interest in using TAF rather than TDF in low- and middle-income countries based on its more favorable safety profile, and likely lower cost for generic manufacture given its much lower dose.10
Additional factors that have been associated with greater weight gain in individuals who initiate ART, and in people with viral suppression on ART who switch to INSTIs, have included Black race and female sex.16,18 Two randomized controlled trials of dolutegravir in Africa have reported 48-week findings including changes in weight. In the NAMSAL study, which randomized ART-naïve participants in Cameroon to dolutegravir or efavirenz, both with TDF and lamivudine, there was greater median weight gain and more treatment-emergent obesity at 48 weeks in the dolutegravir arm.15 In the ADVANCE study, a three-arm (1) dolutegravir, TAF, and emtricitabine; 2) dolutegravir, TDF, and emtricitabine; or 3) efavirenz, TDF, and emtricitabine randomized controlled trial of ART-naïve participants in South Africa; there was greater mean weight gain and treatment-emergent obesity at 48 weeks in the dolutegravir arms, with the greatest weight increases in the dolutegravir and TAF arm.17 This may be explained, in part, by lesser weight gain among participants receiving efavirenz with TDF, particularly with CYP2B6 slow metabolizer genotypes.21,22
Genomic studies could help elucidate mechanisms underlying greater weight gain with dolutegravir and TAF, which are currently unknown. We conducted analyses to characterize associations between human genetic polymorphisms and magnitude of weight gain among ART- naïve individuals who were randomized to initiate dolutegravir-containing regimens in the ADVANCE study.
Methods
Study population
The ADVANCE study in South Africa is a phase 3 non-inferiority clinical trial in which 1053 HIV-positive, ART-naïve participants were randomly assigned to one of three treatment arms:17 1) dolutegravir, TAF and emtricitabine; 2) dolutegravir, TDF and emtricitabine; or 3) efavirenz, TDF and emtricitabine. DNA samples were collected from 340 (48%) of 702 treatment arm 1 and 2 participants who consented to genetic testing. While some individuals opted to not participate in the genetic study, many individuals were not offered the opportunity to participate. Ethics approval was granted by the University of Cape Town and Wits University Human Research Ethics Committees.
Genetic Polymorphisms
Whole blood was collected from consenting participants, and DNA extracted using the salting out method as described elsewhere.23 Samples were labeled with coded identifiers. Stored DNA was genotyped using the Illumina Infinium Multi-Ethnic Global BeadChip (MEGAEX) at Vanderbilt Technologies for Advanced Genomics (VANTAGE). Post-genotype quality control was performed by Vanderbilt Technologies for Advanced Genomics Analysis and Research Design (VANGARD). All quality control steps were performed using PLINK version 1.9.24 Genotyping efficiency per participant was >95% for all samples. Markers with genotyping efficiency <95% were censored, as were those with minor allele frequency (MAF) <5%. We excluded 21 samples with overall genotyping call rates <95%. After quality control, data were imputed to 1000 Genomes after transforming to genome build 37 using liftOver and stratification by chromosome to parallelize the imputation process.25,26 For each chromosome in each phase, 100% concordance with genotyped data was assessed. Imputed polymorphisms with imputation scores less <0.7, genotyping call rates <95%, or MAF <0.05 were excluded. To address population stratification, we performed data-reduction using multidimensional scaling (MDS) implemented in PLINK, which produces a k-dimensional representation of structure. Associations between percentage weight gain from baseline to week 48 and genes relevant to dolutegravir, TAF, and/or TDF metabolism were characterized.
We focused on genes that encode proteins relevant to dolutegravir and tenofovir disposition. Dolutegravir is primarily metabolized by uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and less so by cytochrome P450 (CYP) 3A4.27 Dolutegravir is also a substrate for ATP-binding cassette transporters B1 (encoded by ABCB1) and G2 (encoded by ABCG2).28 In some studies, polymorphisms in SCL22A6, ABCC2 and ABCC4 were associated with higher tenofovir concentrations29–32 although a subsequent study did not replicate associations between tenofovir concentrations and ABCC family polymorphisms.33 Both TAF and TDF are substrates of P- glycoprotein and human breast cancer resistance protein (BCRP).34 TDF is a substrate of the OAT1 and OAT3 organic anion transporters,35 and is minimally metabolized by CYP3A4.36 We also considered genome-wide associations.
Association analyses
Multivariable linear regression models were used to characterize associations between polymorphisms relevant to dolutegravir, TAF, and TDF with percentage weight gain from study baseline to week 48. Covariates included baseline age, self-reported sex, and concomitant NRTI, as well as the first three MDS coordinates to adjust for population stratification.37 We report the regression coefficient (β) for additive associations with polymorphisms, where positive β values indicate an association with greater weight gain. From the NHGRI-EBI GWAS Catalog (accessed 26 November 2020),38 we identified 86 polymorphisms previously associated with obesity trait in the general population at P < 5.0 ×10−8 in at least one published study. Of these, 77 polymorphisms were represented in our genotype data. The Bonferroni method was used to determine significance threshold, with P = 5.0×10−8 for genome-wide analyses, and 0.05 divided by number of polymorphisms tested in targeted polymorphism and gene analyses.
Results
Characteristics of participants
Among the 340 (92%) participants who consented for genetic analyses, 314 were successfully genotyped and had genome-wide genotype data. Participant disposition is presented in Figure 1. Baseline characteristics of study participants are shown in Table 1. All participants were black South Africans, and most were females. Baseline characteristics were similar between those who consented and those who did not consent for genotyping (Table 1).
Figure 1: Disposition of study participants.
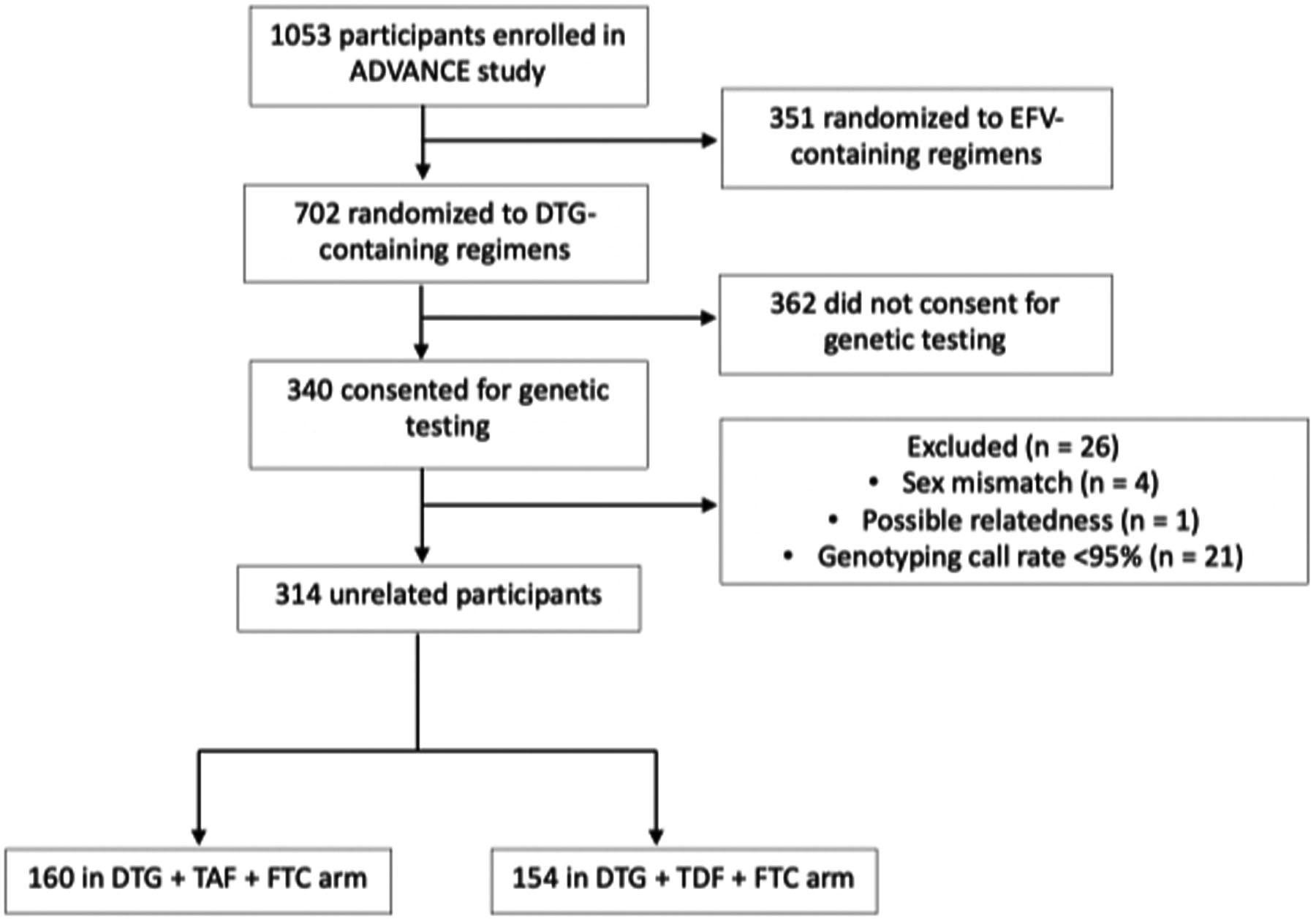
Of 1053 participants enrolled in the ADVANCE study, 314 who had been randomized to dolutegravir-containing regimens were evaluable for genetic associations.
Table 1:
Baseline characteristics in all dolutegravir recipients
| Participants with genetic consent | Participants without genetic consent | ||
|---|---|---|---|
| DTG, TAF and FTC | DTG, TDF and FTC | DTG, TAF or TDF and FTC | |
| n=154 | n=166 | n= 362 | |
| Age in years, (IQR) | 32 (27, 38) | 32 (27, 37) | 32 (26, 37) |
| Sex | |||
| Male, n (%) | 65 (37.4%) | 61 (36.8%) | 154 (42.5%) |
| Female, n (%) | 109 (62.6%) | 105 (63.3%) | 208 (57.5%) |
| Baseline BMI, kg/m2(IQR) | 23.6 (20.6, 26.5) | 23.0 (20.0, 27.1) | 23.1 (20.1, 27.0) |
| Weight gain at week 48, kg (IQR) | +5.8 (+2.4, +9.4) | +2.5 (0.0 +5.6) | +2.9 (+0.2, +7.6) |
| Baseline CD4 count (IQR) | 326 (170, 502) | 272 (159, 424) | 295 (173, 451) |
| Baseline viral load (IQR) | 28 968 (6 180, 75 314) | 25 703 (6 408, 75 619) | 22 064 (5 973, 73 796) |
| Viral load suppression (< 50 c/μL) at week 48, n (%) | 164 (95.9%) | 157 (96.3%) | 262 (92.6%) |
IQR =interquartile range, kg= kilograms, DTG=dolutegravir, TAF= tenofovir alafenamide, FTC= emtricitabine, TDF= tenofovir disoproxil fumarate
Associations with dolutegravir-relevant polymorphisms and genes
We first focused on dolutegravir, characterizing associations in polymorphisms that have previously been associated with dolutegravir metabolism or transport (selected a priori for this analysis) and percentage weight gain from baseline to week 48 among all dolutegravir recipients, by multivariable linear regression analysis. Results for all 314 dolutegravir recipients are presented in Table 2. The lowest P-value for association was ABCG2 rs13137622 (β = −1.24, P = 0.03), which was not significant after correcting for multiple testing (cut-off P<1.0×10−3). We next assessed 1729 polymorphisms in ABCB1, ABCG2, CYP3A4 and UGT1A1(± 50kB for each gene) for association with percentage weight gain among all dolutegravir recipients, by multivariable linear regression analysis. The two lowest P-values in each gene are presented in Table S1, Supplemental Digital Content. The lowest P-value overall was ABCG2 rs4148149 (β = −1.96, P = 7.0×10−4), which did not achieve the Bonferroni cut-off of P<2.9×10−5.
Table 2:
Association among 314 dolutegravir recipients between percentage weight gain at week 48 and selected polymorphisms relevant to dolutegravir
| SNPa | Gene | MAFc | Beta | P-valued |
|---|---|---|---|---|
| rs13137622 | ABCG2 | 0.46 | −1.24 | 0.03 |
| rs2725252 | ABCG2 | 0.19 | −1.29 | 0.06 |
| rs1042640 | UGT1A1 | 0.18 | 1.34 | 0.07 |
| rs10929302 | UGT1A1 | 0.29 | 1.01 | 0.09 |
| rs28401781 | ABCB1 | 0.25 | −1.12 | 0.09 |
| rs2246709 | CYP3A4 | 0.31 | −0.94 | 0.12 |
| rs1976391 | UGT1A1 | 0.41 | 0.84 | 0.13 |
| rs887829b | UGT1A1 | 0.41 | 0.81 | 0.15 |
| rs2231137 | ABCG2 | 0.06 | 1.73 | 0.16 |
| rs10011796 | ABCG2 | 0.25 | −0.92 | 0.16 |
| rs12505410 | ABCG2 | 0.1 | −1.1 | 0.24 |
| rs4148738 | ABCB1 | 0.14 | −0.89 | 0.25 |
| rs4124874 | UGT1A1 | 0.37 | 0.92 | 0.26 |
| rs3789243 | ABCB1 | 0.38 | 0.66 | 0.27 |
| rs1922242 | ABCB1 | 0.45 | 0.59 | 0.28 |
| rs10276036 | ABCB1 | 0.15 | −0.81 | 0.28 |
| rs1045642 | ABCB1 | 0.89 | 0.93 | 0.35 |
| rs3842 | ABCB1 | 0.28 | 0.55 | 0.36 |
| rs2235015 | ABCB1 | 0.36 | 0.47 | 0.41 |
| rs3213619 | ABCB1 | 0.17 | 0.55 | 0.47 |
| rs10248420 | ABCB1 | 0.35 | 0.4 | 0.5 |
| rs2235067 | ABCB1 | 0.15 | −0.42 | 0.6 |
| rs10267099 | ABCB1 | 0.1 | 0.49 | 0.6 |
| rs1128503 | ABCB1 | 0.06 | 0.59 | 0.61 |
| rs2740574 | CYP3A4 | 0.25 | −0.3 | 0.62 |
| rs11983225 | ABCB1 | 0.21 | −0.26 | 0.72 |
| rs3735451 | CYP3A4 | 0.17 | 0.24 | 0.76 |
| rs4646440 | CYP3A4 | 0.08 | −0.28 | 0.78 |
| rs4728709 | ABCB1 | 0.39 | 0.15 | 0.79 |
| rs4148740 | ABCB1 | 0.19 | −0.17 | 0.81 |
| rs2242480 | CYP3A4 | 0.14 | 0.19 | 0.82 |
| rs7699188 | ABCG2 | 0.44 | −0.12 | 0.83 |
| rs3114020 | ABCG2 | 0.15 | −0.17 | 0.83 |
| rs8330 | UGT1A1 | 0.4 | 0.12 | 0.84 |
| rs10280101 | ABCB1 | 0.2 | −0.11 | 0.88 |
| rs4646437 | CYP3A4 | 0.13 | 0.09 | 0.92 |
| rs2235047 | ABCB1 | 0.23 | −0.05 | 0.94 |
| rs10929303 | UGT1A1 | 0.37 | −0.03 | 0.95 |
| rs7787082 | ABCB1 | 0.27 | −0.02 | 0.97 |
| rs2235040 | ABCB1 | 0.17 | −0.02 | 0.98 |
SNP: Single nucleotide polymorphism.
UGT1A1 rs887829 T allele is known to be in strong linkage disequilibrium (LD) with the Gilbert trait decrease expression allele, UGT1A1*28.
MAF: Minor allele frequency.
Significance threshold was 1.0×10−3 for the subset of 40 polymorphisms.
Associations with tenofovir-relevant polymorphisms and genes
We next focused on tenofovir, characterizing associations in polymorphisms that have previously been associated with tenofovir disposition (selected a priori for this analysis) and percentage weight gain from baseline to week 48 among all tenofovir recipients (i.e., TDF and TAF), by multivariable linear regression analysis. Results for all 314 tenofovir recipients are presented in Table S2, Supplemental Digital Content. The lowest P-value for association was ABCC4 rs3742106 (β = 1.59, P = 0.04), which did not achieve the Bonferroni cut-off of P<3.0×10−3. We next assessed all 3945 polymorphisms in ABCC2, ABCC4, ABCC10, SLC22A2, SLC22A6 and SLC22A11 (± 50kB for each gene) for association with percentage weight gain among all tenofovir recipients, by multivariable linear regression analysis. The two lowest P-values in each gene are presented in Table S3, Supplemental Digital Content. The lowest P-value for association overall was ABCC10 rs67861980 (β = 1.82, P = 1.0×10−2), which did not achieve the Bonferroni cut-off of P<1.3×10−5.
Genome-wide associations with percent weight gain at 48 weeks
Associations between genetic polymorphisms and percentage weight gain from baseline to week 48 were explored by multivariable linear regression analysis in all participants, followed by separate regression models for those randomized to TDF and TAF. Among all 314 dolutegravir recipients, the lowest P-value for association was TMEM163 rs7590091 (P = 3.7×10−8), presented in Figure 2A. In analyses limited to the 160 TAF recipients, the two lowest P-values for association were LOC105379130 rs17137701 (P = 6.4×10−8) and rs76794506 in an intergenic region on chromosome 10 (P = 8.8×10−8), presented in Figure 2B. In analyses limited to the 154 TDF recipients, the lowest P-values for association was LOC105371716 rs76771105 (P = 9.7×10−8), presented in Figure 2C. Only TMEM163 rs7590091 was genome-wide significant after correcting for multiple testing. There was no evidence of genomic inflation of each analysis, based on QQ-plots.
Figure 2. Genome-wide associations with weight gain from baseline to week 48 in ADVANCE study participants.
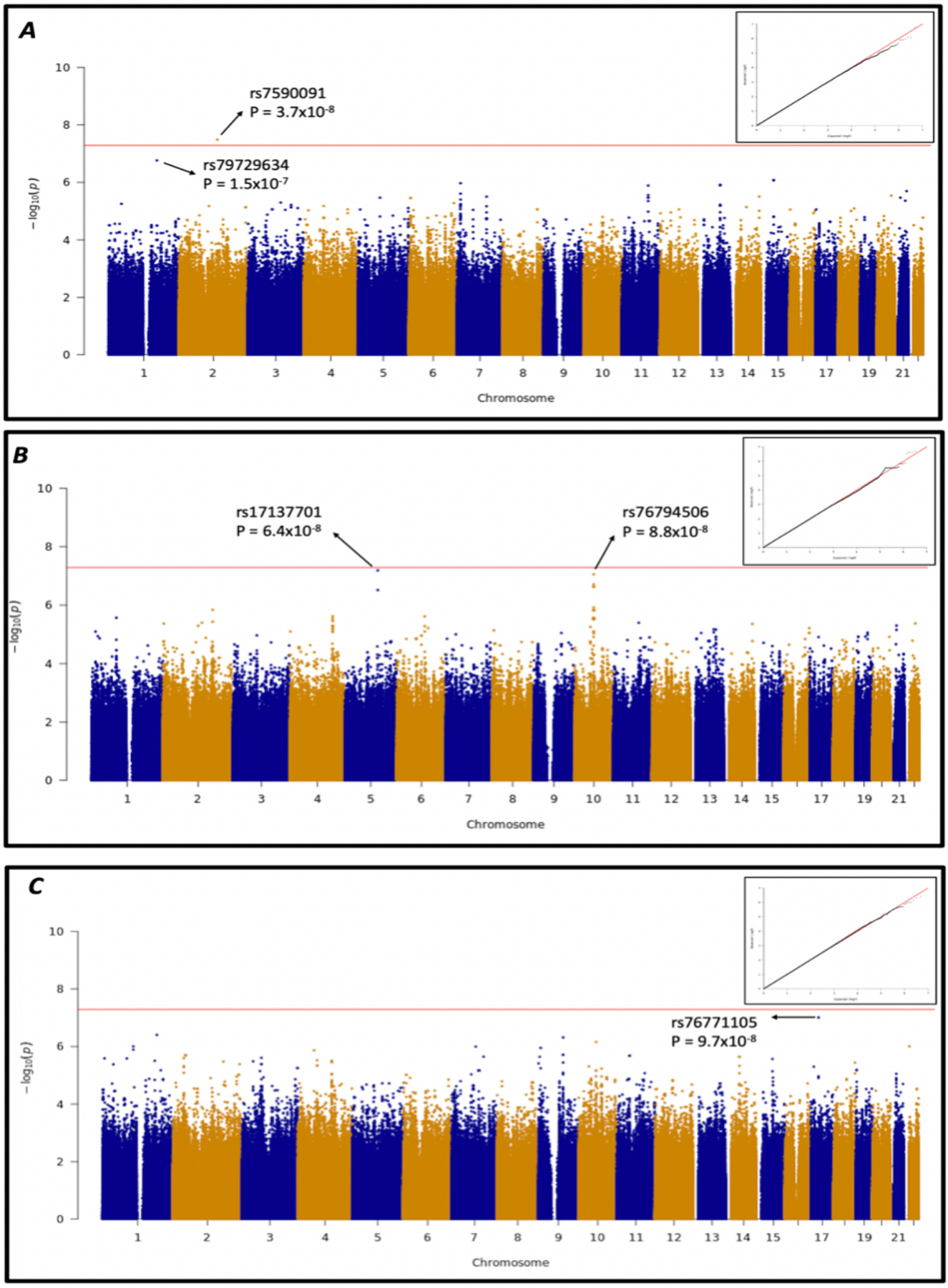
Manhattan plots and QQ-plot for genome-wide associations with percentage weight gain from baseline to week 48. Analyses controlled for age, sex, first three MDS coordinates, and, in the dolutegravir analysis, concomitant NRTI. Red lines indicate a genome-wide significance threshold of P<5.0×10−8. Panel A: Associations among 314 participants in the dolutegravir arm. A polymorphism in TMEM163 (rs7590091) achieved genome-wide significance (P = 3.7×10−8). Panel B: Associations among 160 participants in the tenofovir alafenamide fumarate (TAF) arm. Two polymorphisms, LOC105379130 rs17137701, and rs76794506 in an intergenic region on chromosome 10, had P- vales approaching statistical significance (P = 6.4×10−8 and P = 8.8×10−8, respectively). Panel C: Associations among 154 participants in the tenofovir disoproxil fumarate arm. The polymorphism LOC105371716 rs76771105 had the lowest p-value (P = 9.7×10–8).
Of the 86 polymorphisms identified in GWAS Catalog, 77 were represented in our genotype data. These are presented in Table S4, Supplemental Digital Content. The lowest P-value in our analysis was for ETV5 rs1516725 (β = −1.58, P = 0.01). None of the 77 polymorphisms were significant after correcting for multiple testing.
Discussion
In our analysis for association with percentage weight gain, and 59 selected polymorphisms relevant to dolutegravir and tenofovir disposition, ABCG2 rs13137622 and ABCC4 rs3742106 had the lowest P-values, respectively, but were not significant after Bonferroni correction. In targeted analysis involving 1729 polymorphisms in genes suggested to affect dolutegravir disposition, ABCG2 rs4148149 had the lowest P-value, while in analysis of 3945 polymorphisms in genes suggested to affect tenofovir disposition, ABCC10 rs67861980 had the lowest P-value. In genome-wide association analysis for each study drug (dolutegravir, TAF, and TDF), we found no associations between polymorphisms relevant to dolutegravir and tenofovir disposition and weight gain. For dolutegravir, only TMEM163 rs7590091 achieved genome-wide significance (β = 3.3, P = 3.7×10−8). For TAF and TDF, no polymorphism achieved genome-wide significance
This is the first study to explore associations between dolutegravir or tenofovir-associated weight gain and polymorphisms in genes relevant to their disposition, including ABCG2 and ABCC4. In studies of ABCG2-null mice, ABCG2 deficiency did not affect dolutegravir metabolism or excretion.39 In addition, polymorphisms in the ABCC family have previously been associated with proximal kidney tubule cell dysfunction, including ABCC2 rs717620, ABCC4 rs3742106, ABCC10 rs2125739 and ABCC10 rs9349256,40–42 with possibly greater risk of renal dysfunction among HIV- positive patients of African descent than of European descent.43 In our study ABCC4 rs3742106 (P = 0.05) and ABCC10 rs2125739 (P = 0.77) were not significantly associated with weight gain. The lack of association between ABCG2 or ABCC family polymorphisms and weight gain suggests a relationship that may not be driven by plasma drug concentrations.
In genome-wide analyses, TMEM163 rs7590091 was significantly associated with weight gain. TMEM163 encodes transmembrane protein 163,44 a zinc-binding protein that has been reported to interact with the ion channel transient receptor potential mucolipin-1 (TRPML1), which may help maintain zinc homeostasis in a cell-type specific manner.45 A previous study observed a reduction in TMEM163 mRNA and protein expression levels in mucolipidosis type IV (MLIV) fibroblast cells which correlated with increased lysosomal zinc levels.45 It has been reported that variants of zinc transported proteins on pancreatic β-cells are associated with type 2 diabetes mellitus.46,47 Two other TMEM163 polymorphisms, rs6723108 and rs998451, have been associated with reduced fasting plasma insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR), suggesting that TMEM163 might modulate susceptibility to type 2 diabetes mellitus by affecting insulin secretion.48 The TMEM163 rs6723108 polymorphism has also been associated with waist circumference.48 However, a relationship between TMEM163 and dolutegravir has not been previously reported. This possible association warrants replication. In analyses focused on polymorphisms previously associated with obesity trait in the general population, the lowest P-value was with ETV5 rs1516725 (β = −1.58, P = 0.01). The gene ETV5 encodes a transcription factor. Polymorphisms in ETV5 have been associated with body mass index and bipolar disorder,49 and inhibition of ETV5 homolog in Drosophila melanogaster induced bipolar disorder and obesity-related phenotypes.50
Mechanisms underlying greater weight gain with dolutegravir and with TAF are unknown. One hypothesis for the greater weight gain observed with TAF compared with TDF is that this reflects TDF-induced depletion of mitochondrial DNA in adipocytes, or some other off-target metabolic effect. In addition, TDF may cause loss of appetite. In a clinical trial of patients in the United States randomized to initiate efavirenz-containing ART, lesser weight gain occurred among CYP2B6 slow metabolizers who were also randomized to receive concomitant TDF, but not those randomized to receive concomitant abacavir.21
Our study had limitations. We only considered individual polymorphisms. It is possible that other approaches, such as polygenetic risk score, may identify associations not apparent in our analyses. We did not consider mitochondrial genetics or epigenetics. Although associations with pharmacogenetic variants can often be identified with small sample sizes, because of large effect sizes, a larger study would have greater power to detect associations, especially in the TDF and TAF subgroup analyses. Fifty two percent of participants from the main ADVANCE study did not consent to genotyping, resulting in a smaller sample size and reduced power to detect associations. We are encouraged that baseline characteristics were similar between the two groups, suggesting that our results are likely generalizable to the larger ADVANCE study population.
In summary, we identified potential associations between human genetic polymorphisms and percentage weight gain during the first 48 weeks of ART initiation with dolutegravir plus emtricitabine and either TAF or TDF. No polymorphisms in genes relevant to dolutegravir, TAF or TDF were significantly associated with weight gain after correcting for multiple testing. In a genome-wide association analysis among dolutegravir recipients, a polymorphism in TMEM163 achieved genome-wide significance. This association needs to be replicated in other cohorts before a causal link can be made with weight gain.
Supplementary Material
Acknowledgements:
We thank the Fogarty International Center of the National Institutes of Health for the support. In addition, the authors are grateful to the study participants.
Conflicts of Interests and Source of Funding:
The authors declare no potential conflicts of interest related to this work. Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health (NIH) under Award Number D43 TW010559. Furthermore, this work was supported by National Research Foundation through the Thuthuka Grant [113983] and Black Academic Advancement Program grant [120647], the South African Medical Research Council (SAMRC) through self-initiated research grant (PS), the Wellcome Trust (WT) through an investigator award [212265/Z/18/Z], the National Research Foundation (NRF) of South Africa (Grant Number 119078), and core funding for the Wellcome Centre for Infectious Diseases Research in Africa [203135/Z/16/Z] (GM). Grant support included TW010559, AI110527, AI077505, TR000445 and AI110527 (DWH). Gilead Sciences and ViiV Healthcare donated study drugs for the conduct of the parent study. FV reports grants from USAID, Unitaid, SAMRC, and ViiV; personal fees and non-financial support from ViiV Healthcare and Gilead Sciences, during the conduct of the study; and personal fees from Mylan, Merck, Adcock-Ingram, Aspen, Abbott, Roche, and Johnson and Johnson, outside the submitted work. SS reports grants from USAID, Unitaid, SAMRC, and ViiV Healthcare during the conduct of the study. NC reports grants from USAID, Unitaid, SAMRC and ViiV Healthcare during the conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, WT, NRF, SAMRC or other funders.
Footnotes
Part of these data were presented at the International Workshop on Clinical Pharmacology of HIV, Hepatitis & Other Antiviral Drugs. A virtual program that took place on 28–30 September 2020.
References
- 1.Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380–1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–2072. [DOI] [PubMed] [Google Scholar]
- 2.Sax PE, DeJesus E, Crofoot G, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–e160. [DOI] [PubMed] [Google Scholar]
- 3.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–264. [DOI] [PubMed] [Google Scholar]
- 4.Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, Phase IIIb study. Antivir Ther. 2017;22(4):295–305. [DOI] [PubMed] [Google Scholar]
- 5.Clotet B, Feinberg J, Van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. [DOI] [PubMed] [Google Scholar]
- 6.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. [DOI] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services, December 18, 2019. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf [Google Scholar]
- 8.World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland: World Health Organization; 2019. Available from: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/ [Google Scholar]
- 9.World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post- exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018: Available from: https://www.who.int/hiv/pub/guidelines/ARV2018update/en/ [Google Scholar]
- 10.Vitoria M, Hill A, Ford N, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: What are the issues? AIDS. 2018;32(12):1551–1561. [DOI] [PubMed] [Google Scholar]
- 11.Norwood J, Turner M, Bofill C, et al. Weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakal D, Coelho L, Luz PM, et al. Obesity Following Antiretroviral Therapy (ART) Initiation is Common and Influenced by Both Traditional and HIV-/ART-Specific Risk Factors. Open Forum Infect Dis. 2017;4(suppl_1):S37–S38. [Google Scholar]
- 13.Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain. AIDS. 2017;31(10):1499–1500. [DOI] [PubMed] [Google Scholar]
- 14.Kerchberger AM, Sheth AN, Angert CD, et al. Weight Gain Associated With Integrase Stand Transfer Inhibitor Use in Women. Clin Infect Dis. 2020;71(3):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouanfack C, Mpoudi-Etame M, Bassega PO, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. [DOI] [PubMed] [Google Scholar]
- 16.Sax PE, Erlandson KM, Lake JE, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020; 71(6): 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. [DOI] [PubMed] [Google Scholar]
- 18.Lake JE, Wu K, Erlandson KM, et al. Risk factors for excess weight gain following switch to integrase inhibitor–based art. Presented at: Conference on Retroviruses and Opportunistic Infections; March 6, 2019; Seattle, Washington. [Google Scholar]
- 19.Gomez M, Seybold U, Roider J, Härter G, Bogner JR. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection. 2019;47(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hare CB, Coll J, Ruane P, et al. The phase 3 DISCOVER study: Daily F/TAF or F/TDF for HIV preexposure prophylaxis. Presented at: Conference on Retroviruses and Opportunistic Infections; March 6, 2019; Seattle, Washington. [Google Scholar]
- 21.Leonard MA, Cindi Z, Bradford Y, et al. Efavirenz Pharmacogenetics and Weight Gain following Switch to Integrase Inhibitor-containing Regimens. Clin Infect Dis. August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griesel R, Maartens G, Chirehwa M, et al. CYP2B6 Genotype and Weight Gain Differences Between Dolutegravir and Efavirenz. Clin Infect Dis. September 2020; [online ahead of print DOI: 10.1093/cid/ciaa1073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genomes Project C, Altshuler DL, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lift Genome Annotations. Accessed February 17, 2020. Available from: https://genome.ucsc.edu/cgi-bin/hgLiftOver
- 27.Hirano A, Tomishima K, Togami H, et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infect Dis. 2017;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya K, Hayashida T, Hamada A, Oki S, Oka S, Gatanaga H. High plasma concentrations of dolutegravir in patients with ABCG2 genetic variants. Pharmacogenet Genomics. 2017;27(11):416–419. [DOI] [PubMed] [Google Scholar]
- 29.Manosuthi W, Sukasem C, Thongyen S, Nilkamhang S, Sungkanuparph S. ABCC2*1C and plasma tenofovir concentration are correlated to decreased glomerular filtration rate in patients receiving a tenofovir-containing antiretroviral regimen. J Antimicrob Chemother. 2014;69(8):2195–2201. [DOI] [PubMed] [Google Scholar]
- 30.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):298–303. [DOI] [PubMed] [Google Scholar]
- 31.Rungtivasuwan K, Avihingsanon A, Thammajaruk N, et al. Influence of ABCC2 and ABCC4 polymorphisms on tenofovir plasma concentrations in Thai HIV-infected patients. Antimicrob Agents Chemother. 2015;59(6):3240–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J Pharmacol Exp Ther. 2005;314(2):923–931. [DOI] [PubMed] [Google Scholar]
- 33.Wanga V, Venuto C, Morse GD, et al. Genomewide association study of tenofovir pharmacokinetics and creatinine clearance in AIDS Clinical Trials Group protocol A5202. Pharmacogenet Genomics. 2015;25(9):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atta MG, De Seigneux S, Lucas GM. Clinical pharmacology in HIV therapy. Clin J Am Soc Nephrol. 2019;14(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687–692. [DOI] [PubMed] [Google Scholar]
- 36.Cerrone M, Alfarisi O, Neary M, et al. Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J Antimicrob Chemother. 2019;74(6):1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purcell S, Neale B, Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buniello A, Macarthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Tian X, Shehu A, et al. ABCG2 Deficiency Does Not Alter Dolutegravir Metabolism and Pharmacokinetics. J Pharmacol Exp Ther. 2020. [online ahead of print DOI: 10.1124/jpet.119.264424] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushpakom SP, Liptrott NJ, Rodríguez-Nóvoa S, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011;204(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacomet V, Cattaneo D, Viganò A, et al. Tenofovir-induced Renal Tubular Dysfunction in Vertically HIV-infected Patients Associated with Polymorphisms in ABCC2, ABCC4 and ABCC10 Genes. Pediatr Infect Dis J. 2013;32(10):e403–e405. [DOI] [PubMed] [Google Scholar]
- 42.Danjuma MI, Egan D, Abubeker IY, Post F, Khoo S. Polymorphisms of tenofovir disoproxil fumarate transporters and risk of kidney tubular dysfunction in HIV-positive patients: genetics of tenofovir transporters. Int J STD AIDS. 2018;29(14):1384–1389. [DOI] [PubMed] [Google Scholar]
- 43.Neary M, Owen A. Pharmacogenetic considerations for HIV treatment in different ethnicities: an update. Expert Opin Drug Metab Toxicol. 2017;13(11):1169–1181. [DOI] [PubMed] [Google Scholar]
- 44.Burré J, Zimmermann H, Volknandt W. Identification and characterization of SV31, a novel synaptic vesicle membrane protein and potential transporter. J Neurochem. 2007;103(1):276–287. [DOI] [PubMed] [Google Scholar]
- 45.Cuajungco MP, Basilio LC, Silva J, et al. Cellular Zinc Levels Are Modulated by TRPML1-TMEM163 Interaction. Traffic. 2014;15(11):1247–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58(9):2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemaire K, Chimienti F, Schuit F. Zinc transporters and their role in the pancreatic β-cell. J Diabetes Investig. 2012;3(3):202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabassum R, Chauhan G, Dwivedi OP, et al. Genome-wide association study for type 2 diabetes in indians identifies a new susceptibility locus at 2q21. Diabetes. 2013;62(3):977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pisanu C, Williams MJ, Ciuculete DM, et al. Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI. Transl Psychiatry. 2019;9(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams MJ, Klockars A, Eriksson A, et al. The Drosophila ETV5 Homologue Ets96B: Molecular Link between Obesity and Bipolar Disorder. PLoS Genet. 2016;12(6):e1006104–e1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


