Abstract
Background:
This study examined whether recommended viral load (VL) classifications by the Department of Health and Human Services map onto changes in brain integrity observed in people living with HIV (PLWH).
Methods:
349 PLWH on combination antiretroviral therapy (cART) meeting criteria for virologic suppression (VS) (VL ≤ 20 copies/mL; n=206), “low-level viremia” (LL; 20–200 copies/mL; n=63) or virologic failure (VF) (> 200 copies/mL; n=80), and 195 demographically similar HIV- controls were compared on cognition and brain volumes from 10 regions of interest that are sensitive to HIV. Changes in cognition and brain volumes were examined in a subset of PLWH (n=132) who completed a follow-up evaluation (mean interval=28 months) and had no change in treatment regimen.
Results:
Significant differences in cognition and brain volumes were observed between the HIV- control and VS compared to the VF groups, with few differences observed between the three PLWH subgroups. Longitudinally, PLWH who continued to have VF exhibited a greater decline in cognition and brain volumes compared to PLWH who remained VS. Observed longitudinal changes in cognition correlated with brain volume changes.
Conclusion:
PLWH with continued VF (consecutive VL measurements of >200 copies/mL) represent a cause for clinical concern, and may benefit from change in treatment in addition to consideration of other potential etiologies of VF to reduce loss of brain integrity.
Keywords: Viral load, cognition, neuroimaging
Since the development of combination antiretroviral therapy (cART), human immunodeficiency virus (HIV) is described as a manageable chronic condition.1 An undetectable viral load (VL) is often used as the primary indicator of success of a particular cART regimen. However, altered brain integrity and cognitive impairment remain prominent symptoms among PLWH receiving cART.2 Previous studies have indicated persistent HIV reservoirs and chronic central nervous system inflammation may contribute to continued brain atrophy and cognitive impairment due to HIV, even among PLWH who have achieved viral suppression.3–7
Neuroimaging and tests of neuropsychological performance (NP) are sensitive to changes in the brain due to HIV.8–11 Volumetric changes are seen in subcortical and cortical gray matter regions of interest on structural magnetic resonance imaging (MRI) and are observed soon after seroconversion, with neuronal injury reduced when cART is initiated.10,12–20 Viral suppression may also mitigate the progression of cognitive decline, as cognition remains stable over time in virally suppressed PLWH.20–21 However, many individuals are not virologically suppressed on cART with questions remaining as to whether their brain integrity is at increased risk.
According to the U.S. Department of Health and Human Services (DHHS), PLWH can be categorized as experiencing virologic suppression (VS; VL ≤ 20 copies/mL), “low-level viremia” (LL; 20 copies/mL < VL ≤ 200) or “virologic failure” (VF; VL > 200 copies/mL).22 These cutoffs differ from the World Health Organization’s (WHO) threshold of >1000 copies/mL to define VF, particularly for international studies without access to more sensitive assays.23 Even when utilizing this higher threshold, reports indicate that only 53% of all PLWH worldwide achieve viral suppression.24 These findings indicate that even in the era of cART, identifying potential risks to the brain from higher viral loads remains an important clinical concern. It is unclear if differences in brain integrity are present when classifying PLWH who are on cART according to the DHHS VL guidelines.
In the present study, brain volumetrics and NP were compared in HIV- controls to PLWH who were receiving cART. PLWH were further subdivided by VL level into one of three categories: VS (VL ≤ 20 copies/mL), LL (20 copies/mL < VL ≤ 200 copies/mL) and VF (> 200 copies/mL). A subset of participants completed a follow-up scan (mean study visit interval = 24 months) to identify relationships between VL and changes in brain integrity.
METHODS
Participants
Chronically-infected (>1 year) PLWH on stable cART (>6 months) were recruited from the Washington University School of Medicine (WUSM) Infectious Disease clinic and AIDS Clinical Trial Unit (Barnes-Jewish Hospital; Saint Louis, MO) between 2012 to 2018. HIV- individuals were recruited from the Research Participant Registry at WUSM and leaflets distributed throughout the St. Louis community. 349 PLWH and 195 HIV- controls completed initial (cross-sectional) structural neuroimaging, laboratory evaluations, and NP testing. From this cohort, a subset of PLWH (n= 168) who did not have a change in cART completed a second study visit (longitudinal) between 12–48 months after their initial visit (mean=28 months).
Participants were excluded if they were <18 years old, had <8 years of education, a history of confounding neurologic disorders (including cerebrovascular events), current or past opportunistic central nervous system infections, traumatic brain injury (loss of consciousness >30 minutes), major psychiatric disorders, unable to obtain a MRI, or active substance use (other than cannabis) at a study visit as assessed by a urine drug screen. Study protocols were approved by the WUSM Human Research Protections Office and the Human Subjects Institutional Review Board. Participants provided written informed consent for participation in this study.
Clinical Characterization of Participants
For cross-sectional analyses, VL and CD4 T-cell count were obtained for all PLWH from medical records within one month of neuropsychological assessment and neuroimaging. Of the 349 PLWH, a majority (n=206; 59%) were VS (≤ 20 copies/mL), 18% were LL (n=63) (20 copies/mL < VL ≤ 200 copies/mL) and 23% had VF (VL > 200 copies/mL) (n=80). In the VF group, VL copies ranged from 208–555,495 copies/mL (interquartile range = 1,821–55,800 copies mL).
For the longitudinal analyses, additional VLs were obtained from participant medical records at all clinical visits that occurred between study visits. Only participants who met either VS or VF criteria at both study visits and at all clinical visits in the study interval were included in further analyses.
Within this cohort, participants included in these analyses either remained VS at all clinical and study visits (VS-VS; n=93), remained VF at all clinical and study visits (VF-VF; n=21), or VF at the baseline visit and changed to VS by the time of the second study visit (VF-VS; n=18). Only one participant converted from VS at the first visit to VF at the second visit and this participant was not included in further analyses.
All HIV- controls were confirmed to be negative using a rapid oral HIV test at WUSM.
Neuropsychological Performance Evaluations
Participants completed a comprehensive neuropsychological battery designed to assess cognitive impairment in PLWH.25 Fifteen standard tests were administered that represented five cognitive domains: learning, retention, executive functioning, motor/psychomotor speed, and language. Raw scores were transformed into Z-scores adjusted for appropriate demographics (age, sex, race, education).26 Individual test Z-scores for tests within a single cognitive domain were averaged into domain Z-scores.26 Domain Z-scores were then further averaged to create a global Z-score.
A classification of cognitive impairment was defined based on a Z-score <−1 in two or more cognitive domains, or a Z-score <−2 in at least one cognitive domain. This classification system has previously been used to examine cognitive impairment in PLWH.26
MRI Acquisition and Processing
All participants had neuroimaging performed on the same 3T Siemens Tim Trio MR scanner (Siemens AG, Erlangen Germany) with a 12-channel head coil. A high-resolution, 3-dimensional, sagittal, magnetization-prepared rapid gradient echo scan (MPRAGE) T1 scan was acquired (repetition time [TR] = 2400ms, echo time (TE) = 3.16 ms, flip angle = 8°, inversion time = 1000 ms, voxel size = 1×1×1 mm3 voxels, 256×256×256 acquisition matrix, 162 slices). FreeSurfer v.5.3.0 (Martinos Center, Harvard University, Boston, MA, USA) was used to reconstruct the cortical surface for volumetric segmentation.
Structural volumes from brain regions were generated by FreeSurfer version 5.3. Visual inspection of the automated segmentation results was performed for quality assurance purposes, and manual corrections were made when necessary. Volumetric data for the FreeSurfer brain regions were extracted and normalized with respect to intracranial volume using a linear regression to account for possible variations in head size.27 Regional volumes were averaged between hemispheres and separated into subcortical and cortical brain areas. Volumes were additionally corrected for significant (p < 0.05) covariates: age, sex, and race, then converted to z-scores. The z-scores for regions within a lobe (frontal, occipital, parietal, and temporal) were averaged to create a z-score for each lobe. 28
Statistical Analysis
Demographic variables were compared between HIV- controls, PLWH with VS, LL, or VF using t-tests for continuous variables and chi-square tests for categorical variables. Group differences in NP, cortical (frontal, parietal, temporal, and occipital lobes; total cortical volume) and subcortical (thalamus, caudate, putamen, pallidum, hippocampus, amygdala and total subcortical volume) brain volumes were assessed using linear models with maximum likelihood selection. Post-hoc Tukey’s tests evaluated specific group differences. A chi-square analysis also examined differences in group rates of cognitive impairment. False discovery rate (FDR) was applied to account for multiple comparisons. Pearson’s correlations were calculated on a group-by-group basis to identify relationships between total cortical and total subcortical brain volumes and NP.
For longitudinal analyses, rate of change values were calculated for each NP and brain volume outcome variable ((follow-up value – initial value)/number of months between study visits). Generalized liner models compared rate of change for outcome variables amongst the three PLWH groups (VS-VS, VF-VF, VF-VS), with post-hoc Tukey’s tests conducted to evaluate group differences. FDR was applied to correct for multiple comparisons.
Results
Demographics
Table 1 summarizes group demographics and clinical information, including age, sex, race, and education level for HIV- controls and PLWH on cART within the various subgroups, along with recent and nadir CD4 T-cell count for PLWH. The four groups significantly differed in age and sex. The HIV- controls and the PLWH with VF were significantly younger compared to the PLWH with VS or LL groups (p<.001). In addition, the HIV- control group had a significantly higher proportion of female participants compared to the three PLWH groups. Although NP and neuroimaging variables were corrected for age and sex, these variables were added as covariates in subsequent analyses in order to minimize their potential influence on results. Recent CD4 and duration of infection also significantly differed between PLWH viral load groups (p <.001), with CD4 T-cell counts and duration of infection dropping with increasing viral load.
Table 1.
Demographic and clinical characteristics of participants at initial and follow-up study visit.
| Initial study visit | Follow-up study visit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV− (n=195) | VS (n=206) | LL (n=63) | VF (n=80) | P-value | VS-VS (n=93) | VF-VS (n=18) | VF-VF(n=21) | P-value | |
| Age in years; mean (SD) | 38.1 (17.7) | 49.4(13.9)^* | 47.9(16.3)^* | 35.8 (15.3) | <.001 | 50.1(12.0) | 36.1(16.1)+ | 35.8 (14.7)+ | <.001 |
| Sex (% Male) | 48% | 75%^ | 75%^ | 84%^ | <.001 | 69% | 67% | 86% | .20 |
| Race (% AAa) | 57% | 66% | 63%^ | 79% | .07 | 66% | 89% | 81% | .17 |
| Education in years; mean (SD) | 13.7 (2.0) | 13.2 (3.2) | 13.5 (2.7) | 13.0 (1.8) | .08 | 13.2 (2.8) | 12.7 (2.2) | 13.1 (2.2) | .92 |
| Recent CD4 T-cell count; median (IQR) | - | 638 (471, 816)* | 522 (360, 685)* | 297 (172, 437) | <.001 | 606 (401, 818) | 563 (318, 888) | 365 (198, 413) | <.001 |
| Nadir CD4 T-cell count; median (IQR) | - | 213 (36, 343) | 111 (55, 251) | 256 (90, 387) | .05 | 158 (20, 282) | 170 (33, 293) | 293 (170, 423) | .06 |
| Duration of infection in months; mean (SD) | - | 184 (109)* | 147 (113) | 111 (102) | .001 | 197 (94) | 131 (113) | 114 (107)+ | .004 |
| Length of follow-up; mean (SD) | 26.6 (8.9) | 32.5 (9.2) | 31.2 (6.3) | .17 | |||||
AA=African American
significant difference from HIV− group (p<.05)
significant difference from VF group (p<.05)
significant difference from VS-VS group (p<.05)
(VS= virologically suppressed; LL= low-level viremia; VF= virologic failure
At the follow-up visit (Table 1), PLWH groups significantly differed in age and recent CD4 T-cell counts. VS-VS participants were significantly older and had higher recent CD4 T-cell counts compared to those in the VF-VS or VF-VF groups (p<.001). Additionally, participants in the VF-VF group had significantly shorter duration of infection compared to individuals within the VS-VS group. These variables were included as covariates in appropriate analyses.
Demographic and clinical characteristics did not significantly differ between PLWH who did or did not have a follow-up visit (all p-values >.05).
Group differences in neuropsychological performance
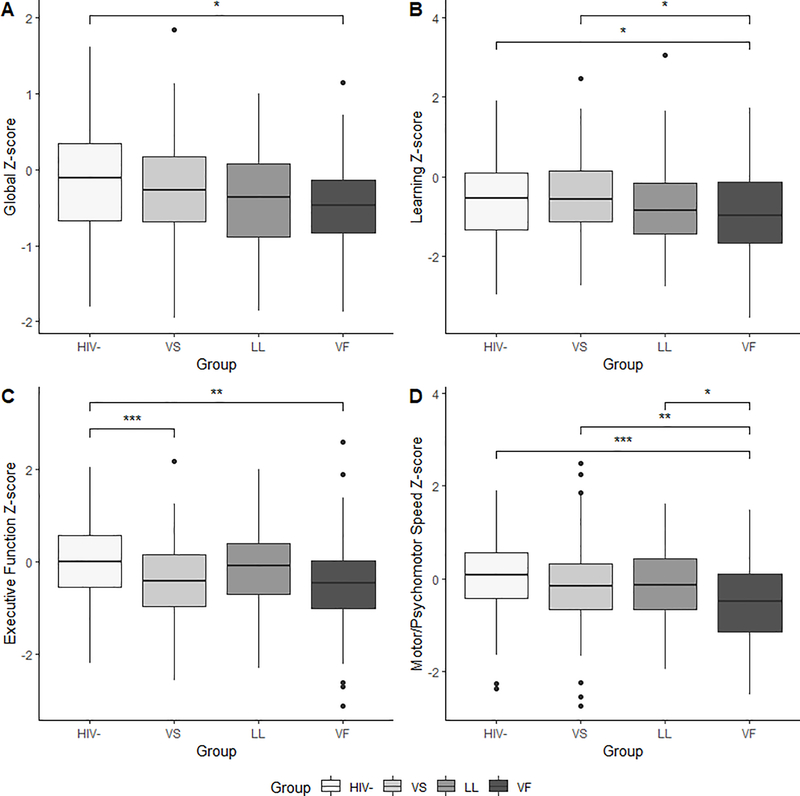
Analyses revealed significant group differences for executive function (p<.001), motor/psychomotor (p<.001), language (p=.001), and learning (p=.03) (Figure 1) on the initial visit. Within the executive function domain, the HIV- control group performed significantly better compared to PLWH in the VS (p <.001) and VF groups (p=.001). In the motor/psychomotor domain, individuals in the VF group performed significantly worse than HIV- controls, and PLWH in either the VS and LL groups (p-values <.05). Within the learning domain, PLWH in the VF group performed significantly worse compared to HIV- controls and PLWH in the VS group (p-values <.05). Finally, HIV- controls performed significantly better on tests of language compared to PLWH in either the LL and VF groups (p-values <.05). The omnibus test for the global Z-score was also significant (p=.007). HIV- controls had significantly higher global Z-scores compared to PLWH in the VF group (p=.02). However, there were no significant differences amongst the PLWH subgroups with regards to the global Z- score.
Figure 1.
Group differences in neuropsychological performance Z-scores: (A) global cognition, (B) learning domain, (C) executive function domain, and (D) motor/psychomotor speed domain. (*p<.05; **p<.01; ***p<.001) (VS= virologically suppressed; LL= low-level viremia; VF= virologic failure)
When examining rate of cognitive impairment, there was a significant group difference (p=.01). PLWH in the VF group demonstrated a significantly higher rate of cognitive impairment (36%) compared to HIV- (18%) and individuals in the VS group (22%). HIV- controls and VS did not significantly differ from each other, and the LL group did not significantly differ from any other group (p-values >.05).
Group differences in cortical volumes
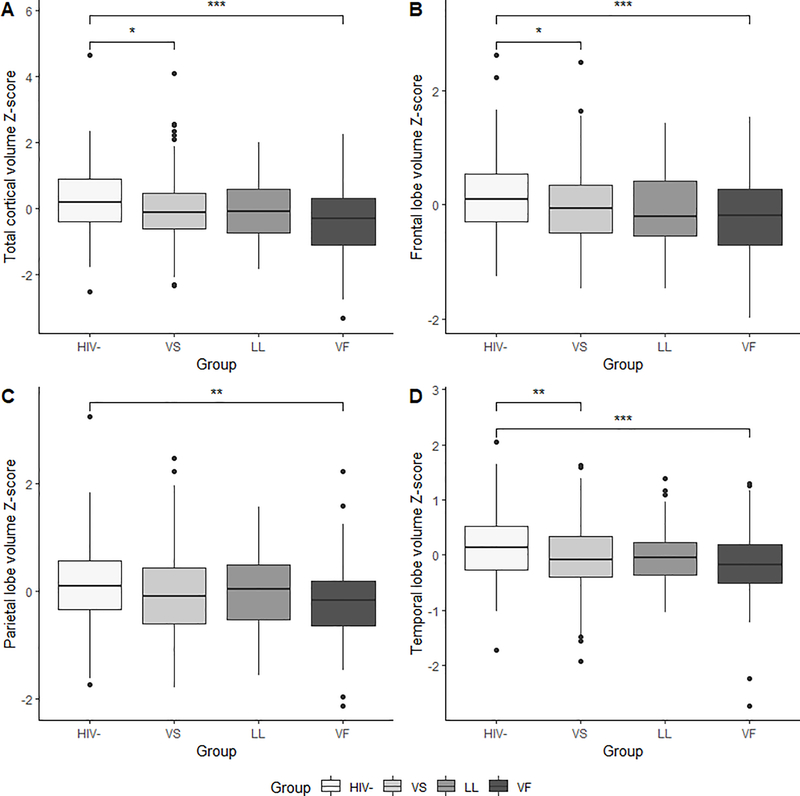
Total cortical volume, and volumes from the four lobes (frontal, parietal, temporal, occipital) were compared amongst the groups at the initial visit. Group differences in frontal lobe, parietal lobe, temporal lobe, and total cortical volume were significant (p-values <.01) (Figure 2). The HIV- control group exhibited significantly larger frontal, parietal, temporal, and total cortical volumes compared to PLWH in the VF group (p-values <.05). Additionally, the HIV- control group exhibited significantly larger frontal, temporal and total cortical volumes compared to PLWH in the VS group (p-values <.01). There were no significant differences in cortical volumes amongst the PLWH subgroups.
Figure 2.
Group differences in cortical brain volume Z-scores: (A) total cortical volume, (B) frontal lobe, (C) parietal lobe, and (D) temporal lobe. (*p<.05; **p<.01; ***p<.001) (VS= virologically suppressed; LL= low-level viremia; VF= virologic failure)
Group differences in subcortical volumes
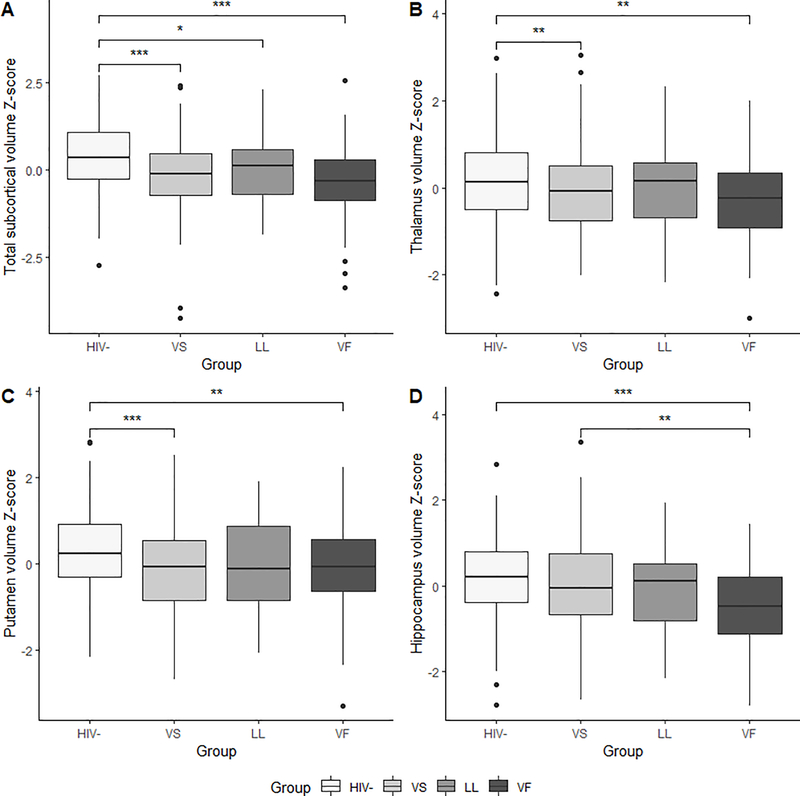
Group differences in thalamus, caudate, putamen, hippocampus, and amygdala volumes were significant at the initial visit (p-values <.01) (Figure 3). Post-hoc testing revealed larger volumes in the thalamus, caudate, putamen, and amygdala in HIV- controls compared to PLWH in the VS group (p-values <.05), and larger volumes in the thalamus, putamen, and amygdala compared to PLWH in the VF group (p-values <.01). The only significant difference amongst PLWH subgroups was identified in the hippocampus, with the VS group exhibiting larger hippocampal volumes compared to the VF group (p=.003). The group difference in total subcortical volume was also significant (p<.001). Post-hoc pairwise comparison testing revealed significantly larger total subcortical volumes in the HIV- controls compared to PLWH in the VS (p<.001), LL (p=.03), and VF (p<.001) groups. There were no significant differences in total subcortical volumes amongst the PLWH subgroups.
Figure 3.
Group differences in subcortical brain volume Z-scores: (A) total subcortical brain volume, (B) thalamus, (C) putamen, and (D) hippocampus. (*p<.05; **p<.01; ***p<.001) (VS= virologically suppressed; LL= low-level viremia; VF= virologic failure)
Relationships between neuropsychological performance and brain volumes
Correlational analyses revealed a general pattern of positive relationships between all cognitive domain scores and total cortical and subcortical volumes (r values ranged from 0.18 to 0.34) for both HIV- controls and the various PLWH subgroups. Specifically, within the HIV- control group, better performance in the learning domain and executive function domain was positively correlated with larger total subcortical (p=.02) and cortical (p=.02) volumes, respectively. Within the PLWH, larger cortical volumes were significantly correlated with better performance in the executive function domain (r=.27, p<.001) and motor/psychomotor domain (r=.020, p=.003) for the VS subgroup. Additionally, better performance in the motor/psychomotor domain significantly correlated with larger total subcortical volume (r=.26, p<.001) for the VS subgroup. No other significant relationships were seen for the PLWH subgroups after correcting for multiple comparisons.
Secondary analyses: Rate of change in NP and brain volumes over time
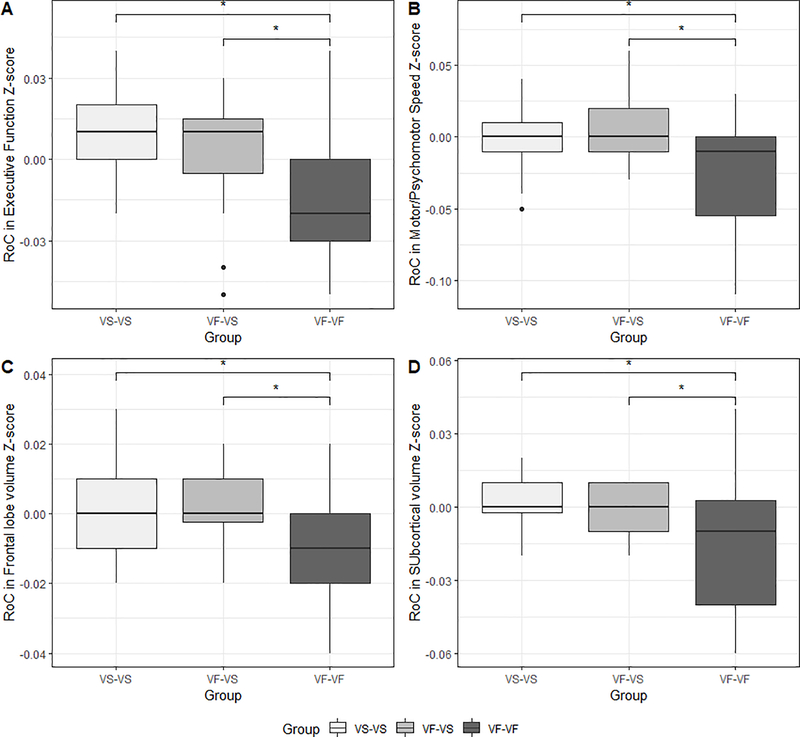
For the subgroup of PLWH who had longitudinal evaluations, significant group differences in the rate of change (per month of study interval) in the motor/psychomotor domain Z-score (p<.001) and executive function domain Z-score (p=.001) (Figure 4) were identified. Post-hoc testing revealed the VF-VF group exhibited significantly greater decreases in the motor/psychomotor and executive function domains compared to both the VS-VS (p-values<.01) and VF-VS (p-values<.05) groups. The VS-VS and VF-VS groups did not significantly differ from each other (p-values>.05).
Figure 4.
Group differences in rate of change (RoC) (per month over the study interval) in (A) executive function Z-score, (B) motor/psychomotor speed Z-score, (C) frontal lobe volume Z-score, and (D) subcortical gray matter volume Z-score. (*p<.05; **p<.01; ***p<.001) (VS= virologically suppressed; LL= low-level viremia; VF= virologic failure)
Regarding change in brain volumes, significant group differences were also identified for rate of change in frontal lobe volume (p=.009), occipital lobe volume (p=.001), total subcortical gray matter volume (p=.004), and putamen volume (p=.006) (see Figure 4). Post-hoc pairwise comparisons revealed significantly greater rate of decline in cortical (frontal and occipital lobe) volume Z-scores for the VF-VF group compared to both the VS-VS and VF-VS groups (p-values <.05). Additionally, the VF-VF group exhibited a significantly greater rate of decline in subcortical (putamen and total subcortical gray matter) volume Z-scores compared to the VF-VS group (p=.01 and p <0.05 respectively). The VS-VS and VF-VS did not significantly differ for any of the volumes (p-values >.05).
Discussion
We utilized neuroimaging and NP evaluations to investigate the relationship between VL classification, brain volumetrics, and cognitive performance cross-sectionally and longitudinally. We observed significant group differences in NP (executive function, motor/psychomotor, learning, and language), cortical volumes (frontal, parietal, and temporal lobes; total cortical volume) and subcortical volumes (thalamus, putamen, and amygdala; total subcortical volume) between HIV- controls and PLWH at an initial visit. The greatest differences were seen between the HIV- controls and VF group, with few differences between the three PLWH VL groups. Better performance in the executive functioning and motor/psychomotor cognitive domains was significantly associated with larger brain volumes, particularly for VS PLWH. Longitudinally, PLWH who continued to have VF at both study visits experienced the greatest rate of decline in NP (executive function, motor/psychomotor speed) and brain volumes (frontal lobe, occipital lobe, total subcortical gray matter, and putamen) compared to PLWH who were VS at one or both study visits.
The consistent significant group differences across NP and brain volumes between the VF and HIV- control group suggest that once an individual reaches virologic failure, there are potential significant risks to brain structure and function. These risks are even more notable longitudinally, where PLWH who had VF over a period of time (average time = ~2 years) demonstrated significantly greater rates of decline in NP and brain volumes compared to PLWH who remained VS, and PLWH who transitioned from VF to VS over the study visit interval. Consequently, while a single observation of a detectable viral load may not impart considerable risk to brain integrity, sustained high-level viremia (>200 copies/mL) remains a significant cause for clinical concern. These results complement those identifying significant relationships between VL and smaller brain volumes only among PLWH with the highest viral loads.29
Few differences in brain integrity measures were observed between the three PLWH subgroups (VS, LL, and VF). Specifically, significant differences amongst the PLWH subgroups were identified only in the motor/psychomotor and learning cognitive domains, and in hippocampal volume. These results suggest little risk attributed to detectable, yet low-level (21–200 copies/mL), VL in PLWH at a single time point. A lack of consistent significant group differences between PLWH groups in NP and neuroimaging outcomes, combined with the observation of widespread differences between the HIV- control group and one or more viral load groups supports the presence of two differing effects on brain integrity. First, there is an effect of HIV serostatus on brain integrity despite use of cART. These results complement previous studies identifying strong differences between HIV- controls and PLWH on NP and brain volumes even in PLWH who have achieved viral suppression.8–16 Next, if VL is not well-controlled (>200 copies/mL), particularly over time, there may be additional deleterious effects on brain integrity in PLWH. The lack of consistent VL effects between the three PLWH VL groups cross-sectionally could also point to the importance of other clinical factors beyond viral load contributing to brain health, such as early initiation of cART, cardiovascular health, and management of other comorbidities.20,30–32
It is important to note that our results indicating a significant relationship between high VL and worse brain integrity defined VF as >200 copies/mL, a recommendation given by the DHHS. The WHO, which provides health recommendations worldwide, has suggested that a threshold of <1000 copies/mL at two consecutive evaluations can be used as evidence of VS in international studies that do not have access to sensitive assays.23 However, our data illustrate a potential threat to brain integrity with VL starting at 200 copies/mL, suggesting that using a threshold of <1000 copies to define VS might result in a missed opportunity to implement clinical strategies capable of reducing the risk of long-term HIV disease complications after the initiation of cART. This disparity in thresholds for VS emphasizes the need for future studies to examine the associations between VL and brain integrity specifically in the interval between 200–1000 copies/mL.
Several limitations should be considered with our current analysis. First, although secondary analyses contained a longitudinal component, we did not have longitudinal data available for all participants and therefore could not take into account changes in viral load over time or history of disease severity for every participant. For example, it is possible that some PLWH in the LL and VF groups may have been experiencing a “viral blip” at the time of study, or a temporary increase in viral load to detectable levels for a short period. Additionally, the number of VF participants with longitudinal data was relatively limited. Cognitively, potential practice effects from repeated neuropsychological test administration were not accounted for in the current study. However, alternate test forms were given when possible to mitigate potential effects. Next, we recognize that the four cross-sectional groups were not well-matched in terms of age and sex. We accounted for these differences by regressing out the effects of these variables on outcome variables before performing further analyses in addition to including these variables as covariates in appropriate analyses. Furthermore, we did not have data available to examine interactions between VL and other variables of potential interest, including delay in initiating cART after diagnosis and the presence and management of comorbidities. The collection and analysis of these variables in future studies will aid in our understanding the relationships between HIV infection, VL, and brain integrity. Finally, many brain regions were aggregated to calculate volumes by lobe rather than performing analyses at a voxel-by-voxel level. Although these methods support an initial attempt to identify patterns in relationships between VL and brain volumes, future analyses can use more detailed approaches in order to identify specific regions that may be most vulnerable to elevated VL.
Measurement of VL has remained an important marker of HIV disease severity, and the success of a particular cART regimen is often determined by the achievement of an undetectable VL. Results from our study indicate that detectable, but low-level (≤ 200 copies/mL), viremia at a single time-point may not be a significant marker of reduced brain integrity in PLWH. However, results from the current study suggest that a VL >200 copies/mL may be a cause for concern, particularly if this level is sustained over time. Comprehensive longitudinal analyses are needed to determine interactions between VL and other factors on NP and neuroimaging in PLWH.
Acknowledgements
Conflicts of Interest and Sources of Funding
No conflicts of interest were reported.
This work was supported by grants from the National Institute for Nursing Research [R01NR014449, R01NR015738] and the National Institute of Mental Health [R01MH118031]. Research was conducted and supported by the Washington University Institute of Clinical and Translational Sciences [UL-TR000448 from the National Center for Advancing Translational Sciences]. The authors report no conflicts of interest.
References
- 1.Sabin CA Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med, 2013; 11: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford DB, Ances BM HIV-associated neurocognitive disorder. Lancet Infect Dis, 2013; 13(11): 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewey J, Hana G, Russell T, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. NeuroImage 2010; 51(4): 1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallianpur KJ, Jahanshad N, Sailasuta N, et al. Regional brain volumetric changes despite 2 years of treatment initiated during actue HIV infection. AIDS, 2020; 34(3): 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant I, Franklin DR Jr, Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology, 2014; 82(23): 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS, 2011; 25(5): 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 2010; 75(23): 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ances Beau M, Ortega M, Vaida F, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndromes, 2012; 59(5): 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elovaara I, Poutiainen E, Raininko R, et al. Mild brain atrophy in early HIV infection: the lack of association with cognitive deficits and HIV-specific intrathecal immune response. Journal of the Neurological Sciences, 1990; 99(2): 121–136. [DOI] [PubMed] [Google Scholar]
- 10.Holt JL, Kraft-Terry SD, & Chang L Neuroimaging studies of the aging HIV-1-infected brain. Journal of Neurovirology, 2012; 18(4): 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jernigan TL, Archibald S, Hesselink JR, et al. Magnetic Resonance Imaging Morphometric Analysis of Cerebral Volume Loss in Human Immunodeficiency Virus Infection. JAMA Neurology, 1993; 50(3): 250–255. [DOI] [PubMed] [Google Scholar]
- 12.Guha A, Brier MR, Ortega M, et al. Topographies of Cortical and Subcortical Volume Loss in HIV and Aging in the cART Era. Journal of Acquired Immune Deficiency Syndromes, 2016; 73(4): 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson PM, Dutton RA, Hayashi KM, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America, 2005; 102(43): 15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JT, Maruca V, Kingsley LA, et al. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology, 2012; 54(2): 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology, 2013; 80(19): 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young GS, Geschwind MD, Fischbein NJ, et al. (2005). Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol, 2005; 26(6): 1551–62 [PMC free article] [PubMed] [Google Scholar]
- 17.Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology, 2009; 73(9): 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ances Beau M., & Ellis RJ Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in Neurology, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Ragin AB, Du H, Ochs R, et al. Structural brain alterations can be detected early in HIV infection. Neurology, 2012; 79(24): 2328–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanford R, Fellows LK, Ances BM, et al. Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurology, 2018; 75(1): 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhanya V, Jacobs GB, Glashoff RH, et al. Clinical Relevance of Total HIV DNA in Peripheral Blood Mononuclear Cell Compartments as a Biomarker of HIV-Associated Neurocognitive Disorders (HAND). Viruses, 2017; 9(11): 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/virologic-failure [Google Scholar]
- 23.Bennet DE, Bertagnolio S, Sutherland D, Gilks CF The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther, 2008, 13(Suppl 2): 1–13. [PubMed] [Google Scholar]
- 24.Marsh K, Eaton JW, Mahy M et al. Global, regional and country-level 90–90-90 estimates for 2018. AIDS, 2019; 33: S213–S226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul RH, Cooley SA, Garcia-Egan PM, et al. Cognitive performance and frailty in older HIV-positive adults. JAIDS, 2018; 79(3): 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul RH, Rhee G, Baker LM, et al. Effort and neuropsychological performance in HIV-infected individuals on stable combination antiretroviral therapy. Journal of Neurovirology, 2017; 23: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage, 2004; 23(2): 724–738. [DOI] [PubMed] [Google Scholar]
- 28.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 2006; 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 29.Cohen RA, Harezlak J, Schifitto, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of Neurovirol, 2010; 16: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology, 2009; 73(16): 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley J, Ettenhofer M, Wright MJ et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol, 2010; 24(2): 265–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright EJ, Grund B, Robertson K et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology, 2010; 75(10): 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]