Abstract
Kidney-targeted nanoparticles have become of recent interest due to their potential to deliver drugs directly to diseased tissue, decrease off-target adverse effects, and increase overall tolerability to patients with chronic kidney disease that require lifelong drug exposure. Given the physicochemical properties of nanoparticles can drastically affect their ability to extravasate past cellular and biological barriers and access the kidneys, we surveyed the literature from the past decade and analyzed how nanoparticle size, charge, shape, and material density affects passage and interaction with the kidneys. Specifically, we found that nanoparticle size impacted the mechanism of nanoparticle entry into the kidneys such as glomerular filtration or tubular secretion. In addition, we found charge, aspect ratio, and material density influences nanoparticle renal retention and provide insights for designing nanoparticles for passive kidney targeting. Finally, we conclude by highlighting active targeting strategies that bolster kidney retention and discuss the clinical status of nanomedicine for kidney diseases.
Keywords: Nanoparticle, Renal Clearance, Targeted Drug Delivery, Chronic Kidney Disease, Acute Kidney Disease
Graphical abstract
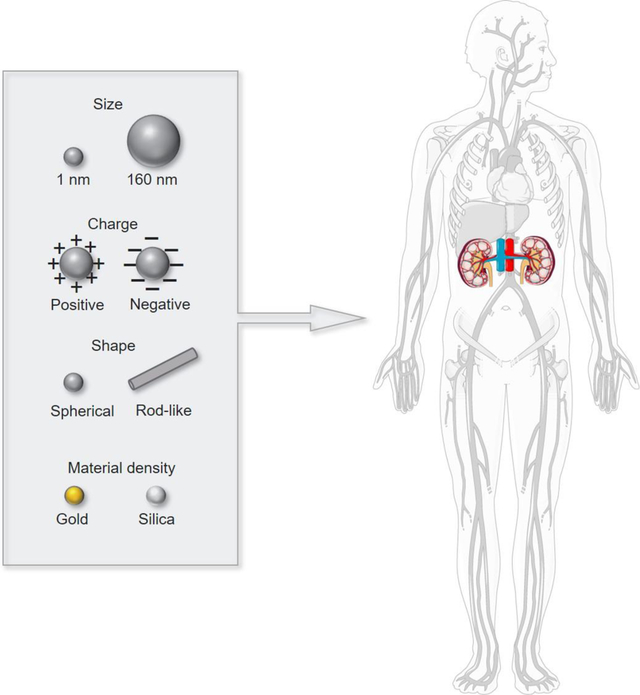
Adapted and reprint from Smart Servier Medical Art
1. Introduction
1.1. Kidney physiology
The kidney is composed of approximately one million individual functional units called nephrons, which collectively filter blood and remove waste products from the body. Each nephron contains one renal corpuscle, along with its associated renal tubules. The renal corpuscle contains the Bowman’s capsule and a network of blood capillaries called the glomerulus (Figure 1A and 1B). Upon entering the glomerulus, blood will first interact with the mesangium, which consists of mesangial cells and mesangial matrix and is located outside the glomerular capillary and directly connected to the glomerular endothelium (Figure 1B) [1]. The mesangium plays an important role in maintaining the glomerular function by producing growth factors and providing structural support for the glomerulus [2–4].
Figure 1.

Schematic of (A) the nephron, (B) the glomerulus, (C) the GFB, and (D) renal excretion. Adapted and reprinted from ref. [5–7].
The glomerular filtration barrier (GFB), which is located within the glomerulus and sandwiched between the glomerular capillary and Bowman’s space, plays a critical role as a nanoscale sieve. It is comprised of three layers: the endothelium of glomerular capillaries, glomerular basement membrane (GBM; consisting of type IV collagen, laminin, and proteoglycans), and podocyte foot processes that are located outside the GFB facing the Bowman’s space (Figure 1C) [8]. Each layer consists of varying pore sizes: the fenestrations of the endothelium measure 70–100 nm, pores in the mesh network within the GBM are 8–10 nm, and gaps between podocytes have pore sizes ranging from 4–30 nm [9–13]. The GFB, taken in its entirety, is reported to have a size cutoff of ~10 nm [13]. The endothelial cells and podocytes of the GFB both express a gel-like surface structure of proteoglycans called glycocalyx, which is negatively charged due to the presence of anionic heparan sulfate-the major component of glycocalyx [14]. The anionic proteoglycans (e.g., heparan‐sulfate and chondroitin‐sulfate glycosaminoglycan), which are the major components of the GBM, also contribute to the negative charge of the GBM [15].
When blood enters the kidney, renal excretion of wastes involves three steps: filtration, reabsorption, and secretion (Figure 1D) [16]. In addition to filtering wastes by the GFB, the kidney also helps maintain the homeostasis of ions and body fluid via reabsorption and removal of large waste in the blood via secretion. When blood enters the glomerulus from the afferent arteriole, the hydrostatic pressure in the glomerulus will force the metabolic waste and excess fluid in the blood to pass through the GFB and allow for glomerular filtration. Filtered blood including nonfilterable components like albumin will return back into systemic circulation via the efferent arteriole (Figure 1B and 1C) [17]. In the tubules, fluid, as well as ions such as potassium and hydrogen, can also be reabsorbed from proximal and distal convoluted tubules into circulation via the peritubular capillaries [16]. During secretion, unwanted ions and molecules, such as endogenous metabolites, xenobiotics, and drugs, are removed from the peritubular capillaries and secreted into the filtrate via ATPase pumps or passive diffusion [16, 18].
1.2. Kidney diseases and the use of nanoparticle-mediated drug delivery
Damage to these filtration, reabsorption, and secretion processes could lead to serious renal diseases and kidney failure. Common kidney diseases include acute kidney injury (AKI) and chronic kidney disease (CKD). Each year, approximately 15% of American adults, or 37 million people, are expected to have CKD, and 1.7 million deaths are caused by AKI worldwide [19, 20]. Many small molecule drugs prescribed for patients with kidney diseases often require high drug concentration, which can lead to non-specific uptake and undesired side effects. For instance, furosemide, a diuretic used in CKD treatment, can cause ototoxicity, aciduria, and unwanted vasoconstriction [21–23]. Similarly, tolvaptan, approved for autosomal dominant polycystic kidney disease (ADPKD), is reported to cause several adverse effects including excess thirst, polyuria, and drug-induced liver damage [24–27]. Therefore, the development of kidney-targeted nanoparticles has garnered immense interest as a therapeutic strategy for kidney diseases.
Nanoparticles are submicron (< 1 μm) particles made of polymers, lipids, or metals [28–33]. Key advantages of delivering drugs through nanoparticles are its ability to enhance bioavailability and circulation half-life and achieve targeted drug delivery through the conjugation of specific ligands to the nanoparticle surface [34]. There are several FDA-approved nanoparticle-based therapies and diagnostics already used in the clinic, such as liposomal doxorubicin for ovarian cancer treatment, iron dextran colloid for liver lesion imaging, and mRNA delivery for COVID-19 vaccination [35–37]. However, engineered nanoparticles can vary in size, charge, shape, materials, and targeting ligands, and these differences can influence the nanoparticle interaction with the kidney.
In order to understand the design rules of nanoparticles for renal applications, in this review, we summarize the nanoparticle biodistribution and kidney targeting results 24 hours post-intravenous (i.v.) injection from 54 papers published during 2007 to 2020, when increasing interest is seen for designing nanoparticles for in vivo application for kidney diseases. Before 2007, there are no articles quantitatively studying the biodistribution of kidney-associated nanoparticles. We reviewed the main text and supplementary files of all articles and collected quantitative biodistribution results derived from fluorescence imaging or MRI. Specifically, we analyzed how the size, charge, shape, and material of nanoparticles affected their biodistribution and interaction with the kidneys via specific routes of entering the kidneys and specific renal cell types. In addition, we highlight active targeting strategies broadly used by nanoparticles for renal application. Finally, we end with a discussion on clinical trials of engineered nanoparticles for kidney diseases.
2. The effect of nanoparticle size on renal accumulation and clearance
2.1. Nanoparticles measuring 1–10 nm
The size of a nanoparticle is a significant factor that can drastically affect the nanoparticle-kidney interaction due to the size selectivity of the GFB [38]. The overall cut-off for nanoparticles to pass through the GFB is approximately 8–10 nm or 30–50 kDa, and most nanoparticles measuring below this threshold are reported to cross the GFB and enter the tubule system leading to renal clearance [39–41]. Renal clearance of nanoparticles is the concentration of nanoparticles in urine and represents nanoparticles that are generally cleared via glomerular filtration. One third of the total 54 studies evaluated the biodistribution of nanoparticles with diameters that ranged from 1–10 nm [42–49], and they all demonstrated a renal clearance of greater than 40% injected dose (ID) 24 hours post-injection (Figure 2A), which is not suitable to design nanoparticle for kidney targeting. For example, Singh et al. found that silicon nanoparticles with a size of 2.4 ± 0.5 nm are ~100% cleared into the urine after 24 hours, and Zhou et al. showed that copper sulfide nanodots measuring 5.6 nm had a clearance rate of 95% [42, 43].
Figure 2.

(A) Renal clearance of nanoparticles of varying size derived from urine measurements results after 24 hours post-i.v. administration [42, 45, 46, 48–50, 54, 55, 57]. Renal accumulation of nanoparticles of varying size quantified by (B) % ID [39, 46, 50, 52, 54] and (C) % ID/g [45, 47, 48, 51, 53, 55, 56].
High clearance of these small nanoparticles usually coincides with lower kidney retention. Most nanoparticles in the size range of 1–10 nm had kidney accumulation at 24 hours of less than 5% ID as the majority of nanoparticles had been cleared into urine (Figure 2B) [45, 50–53]. For instance, Hirn et al. found that 1.4 nm gold nanoparticles had kidney accumulation of ~1.9% ID at 24 hours post-injection [52]. Similarly, Yang et al. found that glutathione coated copper nanoparticles (hydrodynamic diameter (HD) = 2.2 nm) only showed renal accumulation of 0.6% ID 24 hours after injection [54]. Interestingly, the circulation and biodistribution of ultrasmall nanoclusters (~1 nm) were found to be affected by the endothelial glycocalyx of the GFB [53]. In their study, Du et al. showed through electron microscopy images that gold nanocluster of approximately 1 nm in size were trapped in the glycocalyx of the GFB in vivo after 10 minutes i.v. injection in wildtype mice [53]. Through this entrapment mechanism within the glycocalyx, nanoclusters achieved longer retention in the kidney and had a slower clearance rate than larger nanoparticles that were 2.5–6 nm. These results emphasize the significance of the glycocalyx in the interaction of ultrasmall nanoparticles with the kidney.
Instead of ID, which measures the nanoparticle quantity in each organ, % injected dose per gram of tissue (% ID/g) was reported as the unit for biodistribution results of nanoparticles of varying sizes. In another 7 articles, % ID/g was used as the unit for biodistribution results instead of ID. Renal accumulation of nanoparticles varied from 2% ID/g to 15% ID/g. Nanoparticles with sizes smaller than the GFB pore size cutoff achieved the highest renal accumulation of around 15% ID/g, while nanoparticles measuring larger than the GFB cutoff accumulated in the kidneys less than 5% ID/g (Figure 2C). For instance, quantum dots (HD = 5.6 nm) synthesized by Liang et al. and gold nanoparticles (HD = 3.1 nm) designed by Ning et al. both showed renal accumulation of around 15% ID/g 24 hours after i.v. injection, while carbon nanotubes (HD = 25 nm) synthesized by Lacerda et al. only showed renal accumulation of 0.6% ID/g 24 hours post-injection [51, 55, 56]. Hence, nanoparticles measuring smaller than the GFB cutoff can achieve the highest renal accumulation of around 15% ID/g.
2.2. Nanoparticles measuring 10–20 nm
Interestingly, nanoparticles with sizes ranging between 10–20 nm, which is slightly higher than the GFB threshold, have still been reported to traverse the GFB [56, 58–61]. Nanoparticles made with soft organic polymer-based materials also have the potential to “squeeze” through the pores of the GFB, as suggested by Du et al. [9]. For example, albumin-based nanoparticles (HD = 10 nm) designed for targeted delivery to podocytes were found to accumulate in the kidneys at 24 hours post-i.v. injection [59]. Additionally, Wang et al. found that both PEGylated kidney-targeting peptide amphiphile micelles (HD = 15 nm) and non-targeting control micelles (HD = 12 nm) showed accumulation of ~35% and 26% of total fluorescence, respectively, in the kidneys 24 hours post-i.v. injection [58].
2.3. Nanoparticles measuring 20–100 nm
For larger nanoparticles with sizes of 20–100 nm, although they are too large to pass through the GFB in its entirety, they have been reported to access the kidney through their dissociation into smaller particles and passage through the GFB. According to Zuckerman et al., cationic cyclodextrin-containing polymer (CDP)-based siRNA nanoparticles (HD = 60–100 nm, ζ = 10.6 mV) remain intact in blood circulation but become disassembled upon interaction with negatively charged proteoglycans of the GBM, allowing entry into the kidneys [13]. Moreover, nanoparticles composed of ferric iron, tannic acid, and PLG-g-mPEG (FeTNPs) (HD = 75 nm) were also reported to be disassembled by deferoxamine mesylate (DFO) via the formation of ferrioxamine with iron [62]. DFO, the iron chelation agent, was i.v. injected into the mice 24 hours after FeTNPs i.v. administration in order to trigger the dynamic disassembling of FeTNPs, and the dissembled FeTNPs, which were smaller than 6 nm in diameter, were found to be excreted into urine by renal clearance [62]. Hence, disassembly of larger particles into smaller components can be used as a strategy for nanoparticles entering the kidneys.
In addition to disassembly as a mechanism of kidney entry, nanoparticles of 20–100 nm diameter have been reported to accumulate in the kidney specifically through interaction with the mesangium. Choi et al., i.v. injected PEGylated gold nanoparticles ranging from 26 nm to 167 nm and found that after 24 hours, 79 nm nanoparticles exhibit the highest renal accumulation of ~4.5% ID. When transmission electron micrographs (TEM) of kidney sections were assessed, nanoparticles were specifically found to associate with the mesangial cells [39]. In addition, Li et al. found that 90 nm PEG-PLGA nanoparticles loaded with dexamethasone acetate had the highest kidney accumulation compared to those measuring 70 nm and 110 nm [1]. The mesangium is directly connected to the fenestrated endothelium outside the glomerulus capillaries, and nanoparticles (>15 nm) that cannot pass through the GFB will leave the glomerulus capillaries via the pores of the endothelium and can diffuse and accumulate in the mesangium [39]. Hence, the size selectivity of the mesangium should be further studied for the potential application of using therapeutic mesangium-targeted nanoparticles to treat mesangium-related kidney diseases such as mesangial cell disorders and mesangioproliferative glomerulonephritis [63–68].
2.4. Nanoparticles measuring larger than 100 nm
Although nanoparticles with diameters larger than 100 nm are too large to pass through the GFB, these particles have still been reported to enter the renal tubule system and excrete into urine [69–72]. During tubular secretion, endogenous metabolites, xenobiotics, and drugs are secreted from the peritubular capillaries to the proximal tubule, and this mechanism may also be used by nanoparticles greater than 100 nm for kidney access [73]. For example, Wyss et al. found through TEM images of kidney sections that spherical PLGA polymeric nanoparticles with diameters of 130–160 nm localize in the proximal convoluted tubules and tubule lumen [70]. Similarly, Deng et al. found that PLGA-b-mPEG mesoscale nanoparticles with a diameter of ~400 nm localize to the proximal tubule cells and exhibit the highest fluorescence intensity in the kidney among all other organs as verified by immunofluorescence imaging [72]. Naumenko et al. also found PEG-modified magnetite cubes and clusters (HD = 139 nm) translocate from the peritubular capillaries into tubule cells through in vivo intravital imaging in mice (Figure 3A) [71]. After i.v. injection of the clusters, the fluorescence signal of the clusters peaked at approximately 5 minutes and started to decrease due to the excretion of the nanoparticles into urine (Figure 3B). These studies confirmed larger nanoparticles (>100 nm) can also be directed into the kidneys through secretion by tubule epithelial cells from the peritubular capillaries. Specifically, after leaving the glomerulus via efferent arterial and entering the peritubular capillaries, larger nanoparticles > 100 nm enter the renal tubule system through exocytosis from peritubular capillary epithelial cells and endocytosis into proximal tubular epithelial cells [72].
Figure 3.

Intravital images of magnetite clusters accumulation in the renal tubules. (A) Time- lapse imaging of renal cortex upon magnetite clusters-Cy5. (red, MNP-Cy5; blue, neutrophils (arrows); green, platelets (arrowheads)). (B) Dynamics of mean fluorescence intensity in capillaries (pink) and tubules (yellow); results are shown as means ± SEM; Reprinted and adapted with permission from Journal of Controlled Release [71].
Common ways that have been utilized to adjust the size of nanoparticles to study nanoparticle-kidney interaction include modifying the PEG/PLGA length of PEGylated/PLGA nanoparticles, applying gold nanoparticle core of different diameters, changing the amino acid length of decorated peptide on the nanoparticle surface [7, 39, 74]. For example, Choi et al. changed both the diameter of gold nanoparticle core and PEG length to adjust the size of nanoparticles and study the optimum size for mesangium accumulation [39]. Similarly, Yu et al. modified nanoparticles with different PLGA length and obtained nanoparticles with sizes of 100 nm, 200 nm, and 300 nm to study the size-dependent targeting of nanoparticle for AKI [74].
To sum up, nanoparticles that range from 1 nm to 160 nm in diameter can achieve renal accumulation using multiple mechanisms to target various kidney components. Nanoparticles that are ~1 nm can be leveraged to accumulate in the glycocalyx of the GFB while those that are ~80–90 nm can be harnessed for mesangium targeting. Larger nanoparticles (> 100 nm) can also access the kidney via the secretion from the peritubular capillaries to the proximal tubule. As such, nanoparticles of different sizes can be tailored for targeting various kidney cell types, which brings flexibility and possibilities for kidney nanomedicine.
3. The effect of nanoparticle charge on renal accumulation and clearance
The GFB behaves like a charge-selective barrier due to the presence of negatively charged heparan sulfate in the glycocalyx and the anionic proteoglycans on the GBM, and hence, the charge of nanoparticles’ can also significantly affect their interaction with the kidney [75, 76]. To evaluate how charge affects nanoparticles targeting to the kidneys and clearance, in this section, we summarized the biodistribution and renal clearance findings from 7 papers that used nanoparticles of varying charges but similar sizeS ranging 1.4–5.7 nm. Even though all these nanoparticles are small enough to pass through the GFB, the charge-selectivity of the GFB was found to affect the interaction with the nanoparticles of different charges. It has been observed that positively charged nanoparticles tend to pass through the GFB more easily than negatively charged nanoparticles due to electrostatic repulsion (Table 1) [51]. For instance, as found by Liang et al., kidney accumulation of cationic polyethylenimine-conjugated quantum dots (ζ = 23.4 mV, HD = 5.67 nm) was ~15% ID/g 24 hours after injection, which is higher than anionic mercaptosuccinic acid-capped quantum dots (ζ = −52 mV, HD = 3.7 nm) at ~3% ID/g [51]. Similarly, our group has found that positively charged peptide amphiphile micelles (ζ = 14 mV, HD = 15.0 nm) have significantly higher kidney accumulation than negatively charged micelles (ζ = −41 mV, HD = 10.6 nm) [7]. In addition to nanoparticles, polymeric macromolecules, including N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers, have been reported as a vehicle to achieve renal accumulation of up to 40% ID. Specifically, Borgman et al. synthesized HPMA copolymers with varying charge by altering the content of the side group, trans-(S,S)- cyclohexane-1,2-diamine-N,N,N’,N”,N”-pentaacetic acid (CHX-A″-DTPA) [77]. The authors found that more negatively charged HPMA copolymers with higher content ratio of CHX-A″-DTPA showed higher renal retention than HPMA copolymers with less CHX-A″-DTPA after 24 hours in vivo [77, 78]. The mechanism of how HPMA copolymers enter the kidney, however, was not reported and will need additional studies.
Table 1.
Kidney accumulation and renal clearance of nanoparticles of varying charge after 24 hours post-i.v. injection
| Nanoparticle | Charge [mV] | Size [nm] | Renal accumulation | Renal clearance | Reference |
|---|---|---|---|---|---|
| Quantum dot | 23.4 | 5.7 | 15% ID/g | - | [51] |
| Quantum dot | −52.0 | 3.7 | 3% ID/g | - | [51] |
| Gold nanoparticle | −30.0 | 2.9 | 9% ID | - | [45] |
| Gold nanoparticle | −20.0 | 1.4 | 5% ID | - | [52] |
| Nanodot | 2.9 | 5.6 | - | 95% ID | [43] |
| Silicon nanoparticle | 5.4 | 2.4 | - | 100% ID | [72] |
| Gold nanoparticle | −27.0 | 3.1 | - | 42% ID | [45] |
| Gold nanoparticle | −30.0 | 2.9 | - | 45% ID | [46] |
| Gold nanoparticle | −50.0 | 2.4 | - | 53% ID | [55] |
In terms of renal clearance, positively charged nanoparticles have been reported to be cleared faster by the kidney than negatively charged nanoparticles because positively charged nanoparticles can pass through the GFB more readily and enter the tubule system (Table 1) [43, 72]. For example, copper sulfide nanodot (ζ = 2.9 mV, HD = 5.6 nm) and silicon nanoparticles (ζ = 5.4 mV, HD = 2.4 nm) were 95% ID and 100% ID cleared after 24 hours post-injection, respectively [43, 72]. In contrast, the renal clearance of negatively charged nanoparticles of similar size is found to be lower 24 hours after injection. For instance, gold nanoparticles with ζ of −27 mV and HD of 3.1 nm showed renal clearance of 42% ID, and 45% ID for gold nanoparticles with ζ of −30 mV and HD of 2.9 nm, and 52.5% ID for gold nanoparticles with ζ of −50 mV and HD of 2.4 nm [45, 46, 55]. Since positively charged nanoparticles are generally cleared faster than negatively charged nanoparticles, the overall charge of nanoparticles should be tuned into negative charge in order to achieve longer retention in the body. Although these nanoparticles were synthesized from different materials, charge influenced their renal clearance properties, and positively charged nanoparticles were generally cleared faster than negatively charged nanoparticles. A few methods have been commonly utilized to adjust the charge of nanoparticles including conjugating charged ligands like peptide and applying surface coating of amine or carboxyl/silanol groups [7, 79]. For example, Hu et al. synthesized fluorescent carbon dots with surface functionalized of amine-rich coatings and carboxyl or silanol groups to obtain nanoparticles with charges varying from 36.6 mV to −52.3 mV [79].
Neutral and zwitterionic coatings on the nanoparticle surface have also been reported to influence the access of nanoparticles to the kidney [50, 76, 80]. Such coatings can prevent opsonin adsorption and liver uptake by the MPS system, and suppress the formation of protein corona [50, 76, 80, 81]. In contrast, nanoparticles with a high net charge are more likely to get opsonized and can become larger due to protein adsorption and corona formation, which can prevent nanoparticles into the kidney through the GFB [82]. Zwitterionic nanoparticles have been studied to show stability during in vitro experiments. For example, Debayle et al. reported that sulfobetaine-coated, zwitterionic quantum dots are stable for up to 4 days in 1000 μM BSA or 100% human serum, as measured by DLS [81]. In addition, Estephan et al. found that organosiloxane-coated, zwitterionic silica nanoparticles are much more stable than non-coated silica nanoparticles which showed aggregation at 24 hours in 50% fetal bovine serum [83]. In addition to in vitro studies, zwitterionic coatings have been found to assist nanoparticles to enter the kidney more readily using in vivo systems as well. For example, our group has reported that peptide amphiphile micelles with more zwitterionic peptide ligands conjugated on the surface show higher kidney accumulation 24 hours after injection [7]. Specifically, we found that micelles with additional sequence repeats of the zwitterionic peptides, KKEEE and EEKKK, generally achieved higher renal accumulation, and enhanced zwitterionic properties provide resistance to protein corona formation [7]. In addition, Kang et al. found that 800-β-cyclodextrins-conjugated ε-polylysine nanoparticles (ZW800-CDPL±, zwitterionic) had faster blood concentration decay and greater plasma clearance than the positively charged ZW800-CDPL+ and negatively charged ZW800-CDPL− 4 hours after administration [80]. Therefore, neutral and zwitterionic coatings can be used as a strategy to assist nanoparticles to pass the GFB and enter the kidneys more readily, and upon passage, targeting ligands can be applied for cell- specific targeting and kidney retention.
4. The effect of nanoparticle shape on renal accumulation and clearance
In addition to size and charge, a correlation between nanoparticle shape and physiological response has been reported in nanomedicine literature [75, 84]. This was broadly demonstrated as early as the 1990’s by Lippmann et al., where shape-dependent toxicity was observed in studies showing asbestos fibers causing different pathologies due to differences in aspect ratios. [85]. Such non-spherical nanoparticles have a wide variety of nomenclatures in the literature. In general, nanorods, nanowires, and nanofibers are defined as having one long, non-nanoscale dimension. Alternatively, nanomaterials can have two lateral dimensions that are much larger than the third, such as the case for nanosheets, nanodisks, and nanochips [86]. More recently, nanoparticle shape has been elucidated to affect particle biodistribution, which has been harnessed for developing useful nanomaterials such as tumor targeting iron oxide nanochains [87], RNA polygons [88], and filamentous micelles for long-circulating imaging agents (Table 2) [89].
Table 2.
Kidney accumulation and renal clearance of nanoparticles of varying shape.
| Nanoparticle | Shape | Size (nm) | Advantage Over Control Particle | Reference |
|---|---|---|---|---|
| Liposome-encapsulated actin-hemoglobin (LEAcHb) | Disk-like | 140.8 | Increase in half-life of 52 hours | [90] |
| Filomicelles | Rod-like | 20 nm × 500 nm | Ten times longer half-life | [91] |
| Single walled carbon nanotubes (SWCNTs) | Rod-like | 1.2 nm × 100–1000 nm | 65% ID increase in renal clearance | [92] [93] |
| PAMAM | Rod-like | 18 nm core, 20 kD PEG chains | ~80% ID/g increase in renal accumulation | [94] |
| Malleable poly(glycidyl methacrylate) (L-PGMA) | Rod-like | 43 nm | 4.2 ID/g increase in renal accumulation | [95] |
| Mesoporous silca nanoparticles, long rod (NLR) | Rod-like | 159 nm | 16% renal increase in renal accumulation | [96] |
| RNA nanosquare | Square | 10 nm | 66% increase in renal accumulation | [97] |
| Gold nanostar | Star | 55 nm | ~40% increase in renal accumulation. | [98] |
Compared to spherical nanoparticles, disk or rod-like nanoparticles display longer circulation half-lives. For example, spherical liposomes consisting of DMPC, cholesterol and DMPG showed a circulation half-life of 19 hours in rat models, but this increased to greater than 72 hours when the liposomes are modified to become disk-like [90]. Similarly for rods, i.v. injected filamentous micelles with a diameter of 20 nm and a length of 500 nm possess a circulation half-life ten times longer than spherical micelle controls [91].
One proposed mechanism for such a difference in vivo is the dynamic flow conditions in circulation. Upon interaction of one end of a high aspect ratio nanoparticle with macrophages, the free end is subject to higher fluid shear stresses, and the whole particle can be swept away from the cell surface before endocytosis and cell uptake [91, 99]. Therefore, they are less prone to be cleared by macrophages and remain in circulation longer than spherical counterparts. A second mechanism to explain this phenomenon relates to membrane curvature. Successful endocytosis depends on local membrane curvature caused by nanoparticle contact with a circulating phagocytic cell [100]. Rods, sheets, and fibers are unlikely to induce curvature when the long dimension contacts the cell in circulation, while spherical nanoparticles can promote this at every interaction [101]. Again, cylindrical nanoparticles are able to avoid phagocytic uptake, and remain in free circulation for extended periods of time.
This benefit of longer circulation half-lives can be useful for designing nanoparticles that provide enhanced kidney accumulation [102]. To access the renal tubule epithelial cells, the nanoparticle must squeeze through the aforementioned pores in the GFB [75]. Spherical nanoparticles with a diameter smaller than the ~10 nm GFB size cutoff can be filtered into the Bowman’s space [94, 103]. Cylindrical or rod-like nanoparticles can readily take advantage of this phenomenon, despite having a total molecular weight larger than the cutoff for renal clearable proteins (30–50 kDa) by aligning the long axis of the particle towards the filtration slits. As long as nanoparticle width is below the ~10 nm size cutoff, lengths may be orders of magnitude higher without compromising kidney filtration. For example, single walled carbon nanotubes (SWCNTs) with a molecular weight of ~350–500 kDa, length of 100–1000 nm, and diameter of 1.2 nm were found to be cleared via the renal route when administered to mice. ~15% of the injected dose was found to accumulate in the cell nuclei of proximal tubule cells, while ~65% was renally cleared, after 20 minutes post-i.v. injection [92]. This is in contrast to studies that used spherical carbon nanoparticles of various forms (diamond, graphite, and graphene oxide) between 3–8 nm in diameter that showed negligible kidney accumulation and aggregation [93]. As long as nanoparticle width is below the ~10 nm size cutoff, lengths are allowed to be orders of magnitude higher while preserving kidney filtration.
Additionally, this difference in organ accumulation due to geometry has been verified using dendrimer nanoparticles, which are traditionally spherical nanoparticles. 18 nm diameter PAMAM dendrimers with an outer layer of 2 kD molecular weight PEGylation did not show renal excretion (<10% ID/g), and instead accumulated in higher amounts in the lung, heart, and liver compared to the kidney [104]. However, when two 20 kD PEG chains were conjugated to the base dendrimer, imparting a long cylindrical aspect ratio while preserving the 18 nm central core, high kidney excretion was observed (~80% ID/g) (Figure 4) [94]. Despite the 20 kD molecules being substantially larger size than the unmodified PAMAM dendrimers, the shape effect was a significant driver for renal clearance. It is hypothesized that these cylindrical “arms” allowed initial entrapment of the particle in filtration slits, allowing a longer interaction time of the soft, organic 18nm PAMAM core to “squeeze” through the GFB.
Figure 4.

PAMAM dendrimers conjugated with short (2 kD) PEG, which are roughly spherical in shape, show negligible renal accumulation (left). However, when two long, linear PEG (20 kD) are conjugated, imparting a cylindrical shape, enhanced renal accumulation is observed (right). Reprinted and adapted with permission from American Chemical Society [94].
When investigating geometry in such studies, it is important that all other properties of the nanoparticle be kept consistent. One study performed by Zhengkui et al. specifically addresses this issue on previous observations of nanoparticle shape effects. Using a malleable poly(glycidyl methacrylate) (L-PGMA) material, cylindrical polymer brushes (CPBs) and spherical polymer nanoparticles (SPNPs) were synthesized with the same volume, surface chemistry, and polydispersity, with an average diameter of 43 nm. In the kidney, at 5 minutes post-i.v. injection, the biodistribution to the kidney of CPBs was 9.9% ID/g, while SPNPs remained at 5.7% ID/g [95]. These results corroborate that the shape effect alone can change a nanoparticle to favor renal accumulation and excretion, while other parameters are held constant. However, it is important to note nanoparticle biodistribution and kidney accumulation are time dependent, and results may differ depending on the timepoint of analysis. Zhao et al. utilized mesoporous silica nanoparticles with an average diameter of 159 nm, but with varying aspect ratios of 4, 2, and 1, for long rod (NLR), short rod (NSR), and spherical nanoparticles (NS), respectively [96]. After 2 and 24 hours post-oral administration, the short rod and spherical rod nanoparticles exhibited greater kidney accumulation compared to long rods. Interestingly, this trend is reversed 7 days post-oral administration, with long rods being retained in the kidney to a larger extent than short rod and spherical nanoparticles [96] (Figure 5). The authors present a plausible explanation that the NLR nanoparticles were able to have a longer circulation in the blood than NSR and NS nanoparticles and avoid RES uptake, therefore accumulating in the kidney at a much later timepoint of 7 days.
Figure 5.

In vivo biodistribution of RNA nanoparticles of varying shape. Time-course fluorescence images of triangle, square, and pentagon nanoparticles. H, heart; K, kidneys; Li, liver; Lu, lung; S, spleen; T, tumors. Adapted and reprinted with permission from Springer Nature [97].
Additional shapes beyond spherical nanoparticles and nanorods have also been investigated in the context of kidney uptake. Using RNA folding methods, Jasinski et al. studied kidney affinity of nanoscale polygons such as nanosquares, nanotriangles, and nanopentagons with a 10-nm edge length. After 12 hours post-i.v. injection, nanosquares showed high fluorescence in the kidneys (2.0 × 108 epi-fluorescence), whereas triangular and pentagon shaped particles showed levels similar to baseline (1.2 × 108 epi-fluorescence) (Figure 5) [97]. Fluorescence was cleared out from all organs in all three particle types after 24 hours. Interestingly, the authors did not address why uptake was only seen in one kidney to a much greater extent than the other, and raises questions regarding size dependent filtration characteristics between the left and right kidneys. Nonetheless, this study demonstrated that complex 3D shape, a different shape parameter than aspect ratio, can also influence kidney biodistribution. Another study corroborating such findings by Talamini et al. demonstrated the renal affinity of gold 50 nm spheres, 60 × 30 nm rods, and 55 nm stars. Comparing the biodistribution 120 hours after i.v. injection, only 20% of the ID of rods remained in the kidney, while 60–80% of the ID of stars and spheres was detected [98]. It was predicted that since the stars and spheres had similar aspect ratios, kidney accumulation would be similar. However, higher retention of the stars was found within the liver, potentially caused by the sharp protrusions of the star geometry that were recognized by circulating macrophages. The exact mechanisms for shape-dependent accumulation of nanoparticles need deeper study. Mechanistic studies comparing the relative contribution of increased passive filtration, versus efficient evasion of circulating phagocytic cells due to geometry will need to be conducted. Nevertheless, shape effects may be used to exploit renal pathways and offer an interesting platform for kidney targeted applications.
5. Effect of material choice on renal accumulation and clearance
Nanoparticles are composed of varying materials, and the material properties have also been found to affect kidney accumulation. For instance, when the material density of ultrasmall metal nanoparticles (< 5 nm) is low, such as silica (2.32 g/cm3), kidney targeting ability is also low, while renal clearance is high (Table 3). This trend, as stated by Tang et al., can be attributed to the density-dependent circulation rate in blood flow for ultrasmall metal nanoparticles [49, 105]. Nanoparticles with higher density (> 10 g/cm3) tend to have higher buoyancy force towards the endothelium and can reach the endothelium more rapidly than nanoparticles of low-density (< 10 g/cm3) material [105]. Hence, most dense nanoparticles do not stay in the middle flow lines of the blood vessel, where fluid speed is the highest [49]. In contrast, nanoparticles of lower density circulate faster in the blood stream and are distributed more easily in the body, resulting in shorter blood retention time and faster renal clearance. For example, gold nanoparticles with material density of 19.32 g/cm3 and HD of 1.4 nm had 5% ID in the kidney 24 hours after injection [52], which is higher than CdSe/ZnS nanoparticles with a density of 4.09–5.82g/cm3 and HD of 4.36 nm at 2.6% ID [55]. Silica nanoparticles (HD = 2.4–3.3 nm) with a density of 2.32g/cm3 showed even lower kidney accumulation at 24 hours post-injection, 0.2–2% ID [42, 50].
Table 3.
Kidney accumulation and renal clearance of nanoparticles synthesized with varying materials 24 hours post-injection.
| Nanoparticle material | Material density [g/cm3] |
Size [nm] |
Renal accumulation | Renal clearance | Reference |
|---|---|---|---|---|---|
| Gold | 19.32 | 1.4 | 5.0% ID | - | [45] |
| CdSe/ZnS | 4.09–5.82 | 4.4 | 2.6% ID | - | [55] |
| Silica | 2.32 | 3.3 | 2.0% ID | - | [42] |
| Silica | 2.32 | 2.4 | 0.2% ID | - | [50] |
| Gold | 19.32 | 2.5 | - | 50% ID | [45] |
| CdSe/ZnS | 4.09–5.82 | 4.4 | - | 75% ID | [55] |
| Silica | 2.32 | 3.3 | - | 98% ID | [42] |
In terms of renal clearance, gold nanoparticles (density = 19.32 g/cm3, HD = 2.5 nm) had 50% ID cleared 24 hours after injection [26]. In contrast, QD-Cys (density = 4.09–5.82 g/cm3) showed higher renal clearance of 75% ID at 24 hours post-injection [31]. Similarly, Si NPs (density: 2.32g/cm3, HD = 3.3 nm) showed one of the highest renal clearance of 98% ID 24 hours after injection [42].
6. Active targeting strategies for kidney nanoparticles
Although the nanoparticle size, charge, shape, and materials can allow for passive accumulation in the kidneys, nanoparticles with active targeting strategies are also being investigated to enhance kidney targeting, mainly through the addition of peptides and antibodies [58, 59, 106]. The kidney targeting peptide-(KKEEE)3K was found to bind to megalin, a multiligand binding receptor in proximal tubule cells [107]. Wang et al. reported that PEGylated micelles with a size of 15 nm that were conjugated to (KKEEE)3K showed renal fluorescence level of ~35% of total fluorescence 24 hours after i.v. injection [58]. Moreover, lipid nanoparticles containing CSAVPLC, which binds to the glomerular endothelial cells, were found to increase kidney accumulation by 2.6 times than the control, non-targeting nanoparticles of similar size and charge as confirmed by ex vivo organ imaging [108–111]. In addition to these two peptides, various peptide sequences including cyclo(RGDfC), PKNGSDP, and ELRGD(R/M)AX(W/L), have shown promise binding to podocytes, endothelium, glomerular basement membrane, or tubule cells within the kidneys, but have yet to be incorporated into nanoparticles [112–114].
In addition to peptides, Tietjen et al. used anti-CD31 antibodies to target endothelial cells in animal models [115]. They confirmed the high expression of CD31 on renal cortical vasculature endothelial cells with fluorescence immunohistochemistry and synthesized anti-CD31 antibody conjugated PLA-PEG nanoparticles [115]. The nanoparticles were found to have 10-fold higher accumulation in the renal vasculature than the control nanoparticles after administration to the kidneys via ex vivo normothermic machine perfusion (NMP) [115]. In addition to endothelial cells, podocytes were also chosen as targets for active renal targeting. Wu et al. synthesized an albumin-based glucocorticoids methylprednisolone nanoparticle (BSA663- MP) in order to target the glucocorticoid receptors and the neonatal Fc receptors on renal podocytes [59]. Glucocorticoids are commonly used to treat proteinuria glomerular nephropathy, and glucocorticoid receptors were found on glomerular podocytes [116]. BSA was covalently conjugated to glucocorticoid methylprednisolone to target the neonatal Fc receptor, a natural receptor of albumin that is highly expressed on the renal podocytes. Similar nanoparticle signals were found in liver and kidney 24 hours post-i.v. injection (Figure 6) [59].
Figure 6.

Biodistribution of the BSA633-MP nanoconjugates. (A) In vivo imaging, (B) ex vivo imaging of heart, liver, lung, kidney, and spleen. (C) Quantitative analysis of fluorescence imaging values in various organs. (n=3) Reprinted and adapted with permission from International Journal of Molecular Medicine [59].
7. Current applications of nanoparticles for the kidney
With the appropriate selection of nanoparticle size, charge, shape, material, and targeting ligands, nanomaterials have been developed with drug delivery or imaging capabilities [117]. In this section, we present recent efforts in imaging, and the treatment of acute and chronic kidney conditions via nanomedicine strategies, with a focus on those undergoing clinical trials.
Surprisingly, no FDA approved materials or clinical trials utilize active or passive targeting nanoparticles to specifically increase kidney accumulation. Several have been approved for conditions relating to the kidney, which are compiled in Table 4, but the majority are indicated as iron replacement therapies to mitigate anemia in CKD patients [118]. These relatively simple nanoparticles are composed of an iron oxide core decorated with hydrophilic polymers such as dextran, which provide a slower release profile of iron following IV injection. Additionally, Rapamune, a nanoscale variant of the drug sirolimus, has been used since its approval in 2000 as an immunosuppressant to prevent immune rejection after kidney transplant [119]. While the active component of Rapamune is fairly insoluble, the nano-crystallinity provides an extended- release profile compared to unmodified drug, increasing therapeutic its window [120].
Table 4.
Current FDA approved nanoparticles with kidney related indications.
| Nanoparticle | Indication | Manufacturer | Nanoparticle Type |
|---|---|---|---|
| Venofer | Anemia in CKD | American Regent | Iron colloid |
| Ferrlecit | Anemia in CKD | Sanofi | Iron colloid |
| Feraheme | Anemia in CKD | AMAG | Iron colloid |
| Rapamune | Immunosuppressant for kidney transplant | Wyeth Pharmaceuticals | Nanocrystalline drug |
Ongoing research uses unique advantages of nanoscale materials for imaging the kidney, specifically the early detection of acute kidney damage. Kobayashi et al. investigated a generation 4 PAMAM with a gadolinium core MRI contrast agent which accumulated in proximal tubules and in the renal medullary ray due to its size (HD=7 nm, 59 kDa) [121]. Upon acute kidney damage, accumulation in the renal medullary is inhibited as filtrate flow is interrupted, and a detectable change in MRI signal can be used as a marker for glomerular injury. Importantly, the contrast agent is able to detect changes before the rise in traditional kidney injury markers such as serum creatinine and blood urea nitrogen [122]. This technology and relatively straightforward nanoparticle platform can be utilized in applications where time-sensitive reporting of kidney health is needed.
Nanoparticles can also be an effective tool to deliver medicines for the treatment of CKD, which are characterized by the long term, gradual loss of kidney function. CKD can be caused by diabetes, high blood pressure, inflammation such as lupus nephritis, or genetic kidney conditions. Renal carcinoma, a devastating long-term progressive disease, is a prevalent comorbidity to chronic kidney disease [123]. Only recently have nanoparticles been used in the clinic for renal carcinoma. Submicron particle docetaxel (NanoDoce) is now in Phase 1 clinical trials (NCT04260360) for renal cell carcinoma. Pre-clinical in vivo experiments showed promise due to the specific nanoscale formulation of this drug, where a proprietary synthesis method creates uniform nanoparticles approximately 900 nm in diameter [124]. Locally administered NanoDoce showed tumor reduction in xenograft models of 786-O human renal cell carcinoma, UM-UC-3 human transitional cell bladder carcinoma, and PC-3 human prostate carcinoma. In contrast, the gold standard of IV docetaxel resulted in no renal cell tumor volume reduction, demonstrating the distribution advantages of nanoscale formulations such as NanoDoce for the kidney [125]. While NanoDoce is likely too large to take advantage of efficient glomerular filtration, its potency is likely attributed to the enhanced permeability and retention (EPR) effect in the solid renal tumors. Lower dosages for efficacy, and therefore milder systemic side effects, can be obtained when glomerular filtration can also be harnessed to access renal carcinoma cells. Logical improvements to NanoDoce can be made with physiochemical design considerations mentioned in this review, such as size, shape, and charge of the nanoparticle. While there are currently less than ten total clinical trials using nanoparticles for kidney applications, the myriad of promising pre-clinical research represents an opportunity to improve the standard of care in the clinic.
8. Conclusions
Herein, we surveyed the biodistribution and renal clearance properties of nanoparticles published between 2007 to 2020 and analyzed how the size, charge, shape, material, and active targeting ligands can be used to tailor the interaction with the kidney. Ultrasmall nanoparticles (<10 nm) without kidney targeting ligands generally show high renal clearance, while large nanoparticles (>100 nm) access the kidney through the secretion from the peritubular capillaries to the proximal tubule. In addition, positively-charged nanoparticles with high aspect ratios and high material density are usually found to have higher renal accumulation. Still, some fundamental questions remain unknown and are worthy of comprehensive investigation in the future. First, the relationship between the formation of the protein corona and physicochemical properties of nanoparticles is not well understood. The formation and the specific components of the protein corona may significantly affect the circulation of nanoparticles and how they interact with the kidneys. In addition, questions remain regarding larger nanoparticles that are >100 nm but showed high accumulation in the kidney. These nanoparticles are too large to pass through the GFB, but they are able to be retained in the kidney from the peritubular capillary. It is still not clear which features of the nanoparticle lead to secretion by tubule epithelial cells from the peritubular capillary.
It is noteworthy to address the limitations of the studies included here. Broadly, we summarized the results from only 54 papers, and forthcoming, additional studies will need to be included to further validate our conclusions on how nanoparticles interact within the kidneys. Specifically, 20 out of these 54 papers did not report the sex of mice used for their nanoparticle biodistribution study. Sex differences are one of the most important factors in pharmacokinetic research, and the rate of progression for many chronic kidney diseases, including autosomal dominant polycystic kidney disease in which disease progression is faster in male vs. female patients [126–128]. Additionally, significant differences in nanoparticle renal accumulation have also been observed between male and female rats [129–132]. Hence, it will be necessary to investigate how nanoparticles target the kidney based on sex for effective therapeutic strategies.
Nonetheless, effective nanoparticle design for renal applications using passive and active targeting mechanisms is thriving and viable strategies. Optimal nanoparticle drug delivery devices should consider both the physicochemical properties to access the desired component of the kidney and utilize active targeting ligands to further enhance specificity and retention in the kidneys to achieve a desired outcome.
Highlights.
Nanoparticle size impacts the mechanism of nanoparticle entry into the kidneys.
Charge, aspect ratio, and material density influences nanoparticle renal retention.
Active targeting strategies can be applied to bolster nanoparticle kidney retention.
Opportunities for clinical application of kidney targeted nanomedicine.
Acknowledgements
The authors would like to acknowledge the financial support from the University of Southern California (USC) Alfred E. Mann Institute (AMI) fellowship awarded to JW, the National Heart, Lung, and Blood Institute (NHLBI), R00HL124279 and NIH New Innovator Award (DP2- DK121328) awarded to EJC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li S, et al. , Design and evaluation of glomerulus mesangium-targeted PEG-PLGA nanoparticles loaded with dexamethasone acetate. Acta Pharmacologica Sinica, 2019. 40(1): p. 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little MH, Kidney development, disease, repair and regeneration. 2015: Academic Press. [Google Scholar]
- 3.Wallace D and Hahn BH, Dubois’ Lupus Erythematosus and Related Syndromes E-Book: Expert Consult-Online. 2012: Elsevier Health Sciences. [Google Scholar]
- 4.Alpern RJ and Hebert SC, Seldin and Giebisch’s The Kidney: Physiology & Pathophysiology 1–2. 2007: Elsevier. [Google Scholar]
- 5.Huang JG and Gretz N, Light-Emitting Agents for Noninvasive Assessment of Kidney Function. Chemistryopen, 2017. 6(4): p. 456–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts JGJOSC, Anatomy & physiology.(2013).
- 7.Huang Y, et al. , The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles. Bioengineering & Translational Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arif E and Nihalani D, Glomerular Filtration Barrier Assembly: An insight. Postdoc J, 2013. 1(4): p. 33–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Du BJ, Yu MX, and Zheng J, Transport and interactions of nanoparticles in the kidneys. Nature Reviews Materials, 2018. 3(10): p. 358–374. [Google Scholar]
- 10.Isogai S, et al. , Initial ultrastructural changes in pore size and anionic sites of the glomerular basement membrane in Streptozotocin-induced diabetic rats and their prevention by insulin treatment. Nephron, 1999. 83(1): p. 53–58. [DOI] [PubMed] [Google Scholar]
- 11.Akilesh S, et al. , Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(3): p. 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahdenkari AT, et al. , Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol, 2004. 15(10): p. 2611–8. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerman JE, et al. , Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proceedings of the National Academy of Sciences of the United States of America, 2012. 109(8): p. 3137–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita H, Yoshimura A, and Kimata K, The role of heparan sulfate in the glomerular basement membrane. Kidney Int, 2008. 73(3): p. 247–8. [DOI] [PubMed] [Google Scholar]
- 15.Miner JH, The glomerular basement membrane. Experimental Cell Research, 2012. 318(9): p. 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felmlee MA, Dave RA, and Morris ME, Mechanistic models describing active renal reabsorption and secretion: a simulation-based study. AAPS J, 2013. 15(1): p. 278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeppen BM and Stanton BA, Renal Physiology E-Book: Mosby Physiology Monograph Series. 2012: Elsevier Health Sciences. [Google Scholar]
- 18.Katz A.J.A.J.o.P.-R.P., Renal Na-K-ATPase: its role in tubular sodium and potassium transport. 1982. 242(3): p. F207–F219. [DOI] [PubMed] [Google Scholar]
- 19.Luyckx VA, Tonelli M, and Stanifer JW, The global burden of kidney disease and the sustainable development goals. Bulletin of the World Health Organization, 2018. 96(6): p. 414.-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Control C.f.D., et al. , Chronic kidney disease in the United States, 2019. 2019.
- 21.Ho KM and Power BM, Benefits and risks of furosemide in acute kidney injury. Anaesthesia, 2010. 65(3): p. 283–93. [DOI] [PubMed] [Google Scholar]
- 22.Dormans TP, et al. , Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol, 1996. 28(2): p. 376–82. [DOI] [PubMed] [Google Scholar]
- 23.Stason WB, et al. , Furosemide. A clinical evaluation of its diuretic action. Circulation, 1966. 34(5): p. 910–20. [DOI] [PubMed] [Google Scholar]
- 24.Wang A, et al. , Hydrochlorothiazide ameliorates polyuria caused by tolvaptan treatment of polycystic kidney disease in PCK rats. Clin Exp Nephrol, 2019. 23(4): p. 455–464. [DOI] [PubMed] [Google Scholar]
- 25.Khan MY, et al. , Tolvaptan-induced Liver Injury: Who is at Risk? A Case Report and Literature Review. Cureus, 2019. 11(6): p. e4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres VE, et al. , Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med, 2012. 367(25): p. 2407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardenas A, et al. , Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol, 2012. 56(3): p. 571–8. [DOI] [PubMed] [Google Scholar]
- 28.Khan I, Saeed K, and Khan I, Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, 2019. 12(7): p. 908–931. [Google Scholar]
- 29.Dreaden EC, et al. , The golden age: gold nanoparticles for biomedicine. Chem Soc Rev, 2012. 41(7): p. 2740–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent S, et al. , Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev, 2008. 108(6): p. 2064–110. [DOI] [PubMed] [Google Scholar]
- 31.Muller RH, Mader K, and Gohla S, Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm, 2000. 50(1): p. 161–77. [DOI] [PubMed] [Google Scholar]
- 32.Tuncel D and Demir HV, Conjugated polymer nanoparticles. Nanoscale, 2010. 2(4): p. 484–94. [DOI] [PubMed] [Google Scholar]
- 33.Rao JP and Geckeler KE, Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science, 2011. 36(7): p. 887–913. [Google Scholar]
- 34.Gelperina S, et al. , The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American Journal of Respiratory and Critical Care Medicine, 2005. 172(12): p. 1487–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anselmo AC and Mitragotri S, Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine, 2019. 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polack FP, et al. , Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh EE, et al. , Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med, 2020. 383(25): p. 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubbad L, et al. , Reduced glomerular size selectivity in late streptozotocin-induced diabetes in rats: application of a distributed two-pore model. Physiol Rep, 2015. 3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi CH, et al. , Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A, 2011. 108(16): p. 6656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longmire MR, et al. , Biologically optimized nanosized molecules and particles: more than just size. Bioconjug Chem, 2011. 22(6): p. 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longmire M, Choyke PL, and Kobayashi H, Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond), 2008. 3(5): p. 703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh G, et al. , Ultrasmall silicon nanoparticles as a promising platform for multimodal imaging. Faraday Discuss, 2020. 222(0): p. 362–383. [DOI] [PubMed] [Google Scholar]
- 43.Zhou M, et al. , CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano, 2015. 9(7): p. 7085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F, et al. , Dynamic Positron Emission Tomography Imaging of Renal Clearable Gold Nanoparticles. Small, 2016. 12(20): p. 2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu M, et al. , Interactions of Renal-Clearable Gold Nanoparticles with Tumor Microenvironments: Vasculature and Acidity Effects. Angew Chem Int Ed Engl, 2017. 56(15): p. 4314–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, et al. , Luminescent Gold Nanoparticles with Efficient Renal Clearance. Angewandte Chemie-International Edition, 2011. 50(14): p. 3168–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou C, et al. , Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Abstracts of Papers of the American Chemical Society, 2013. 245. [DOI] [PubMed] [Google Scholar]
- 48.Yang SY, et al. , Renal Clearance and Degradation of Glutathione-Coated Copper Nanoparticles. Bioconjugate Chemistry, 2015. 26(3): p. 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang S, et al. , Tailoring Renal Clearance and Tumor Targeting of Ultrasmall Metal Nanoparticles with Particle Density. Angew Chem Int Ed Engl, 2016. 55(52): p. 16039–16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns AA, et al. , Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nanomedicine. Nano Letters, 2009. 9(1): p. 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X, et al. , Short- and Long-Term Tracking of Anionic Ultrasmall Nanoparticles in Kidney. ACS Nano, 2016. 10(1): p. 387–95. [DOI] [PubMed] [Google Scholar]
- 52.Hirn S, et al. , Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. European Journal of Pharmaceutics and Biopharmaceutics, 2011. 77(3): p. 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du BJ, Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Abstracts of Papers of the American Chemical Society, 2018. 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S, et al. , Renal clearance and degradation of glutathione-coated copper nanoparticles. Bioconjug Chem, 2015. 26(3): p. 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning X, et al. , Physiological stability and renal clearance of ultrasmall zwitterionic gold nanoparticles: Ligand length matters. APL Mater, 2017. 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacerda L, et al. , Dynamic Imaging of functionalized multi-walled carbon nanotube systemic circulation and urinary excretion. Advanced Materials, 2008. 20(2): p. 225.-+. [Google Scholar]
- 57.Zhou C, et al. , Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angewandte Chemie-International Edition, 2012. 51(40): p. 10118–10122. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, et al. , Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Research, 2018. 11(10): p. 5584–5595. [Google Scholar]
- 59.Wu L, et al. , Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. International Journal of Molecular Medicine, 2017. 39(4): p. 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang DW, et al. , Efficient renal clearance of DNA tetrahedron nanoparticles enables quantitative evaluation of kidney function. Nano Research, 2019. 12(3): p. 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng QY, et al. , Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Scientific Reports, 2018. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang YB, et al. , A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials, 2019. 197: p. 284–293. [DOI] [PubMed] [Google Scholar]
- 63.Brunskill EW and Potter SS, Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. Bmc Nephrology, 2012. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Floege J, Johnson RJ, and Couser WG, Mesangial cells in the pathogenesis of progressive glomerular disease in animal models. Clin Investig, 1992. 70(9): p. 857–64. [DOI] [PubMed] [Google Scholar]
- 65.Yang XQ, Wang YG, and Gao GQ, High glucose induces rat mesangial cells proliferation and MCP-1 expression via ROS-mediated activation of NF-kappa B pathway, which is inhibited by eleutheroside E. Journal of Receptors and Signal Transduction, 2016. 36(2): p. 152–157. [DOI] [PubMed] [Google Scholar]
- 66.Zhang PY, et al. , Long non-coding RNA Rpph1 promotes inflammation and proliferation of mesangial cells in diabetic nephropathy via an interaction with Gal-3. Cell Death & Disease, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen B, et al. , circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol, 2019. 234(11): p. 21249–21259. [DOI] [PubMed] [Google Scholar]
- 68.Guo L, et al. , Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nature Communications, 2017. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams RM, et al. , Selective Nanoparticle Targeting of the Renal Tubules. Hypertension, 2018. 71(1): p. 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyss PP, et al. , Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials, 2020. 230. [DOI] [PubMed] [Google Scholar]
- 71.Naumenko V, et al. , Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. Journal of Controlled Release, 2019. 307: p. 368–378. [DOI] [PubMed] [Google Scholar]
- 72.Deng XT, et al. , Kidney-targeted triptolide-encapsulated mesoscale nanoparticles for high- efficiency treatment of kidney injury. Biomaterials Science, 2019. 7(12): p. 5312–5323. [DOI] [PubMed] [Google Scholar]
- 73.Inui K. i. and Okuda M, Cellular and molecular mechanisms of renal tubular secretion of organic anions and cations. Clinical and Experimental Nephrology, 1998. 2(2): p. 100–108. [Google Scholar]
- 74.Yu H, et al. , Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials, 2019. 219: p. 119368. [DOI] [PubMed] [Google Scholar]
- 75.Liu J, et al. , Renal clearable inorganic nanoparticles: a new frontier of bionanotechnology. Materials Today, 2013. 16(12): p. 477–486. [Google Scholar]
- 76.Choi HS, et al. , Renal clearance of quantum dots. Nat Biotechnol, 2007. 25(10): p. 1165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borgman MP, et al. , Tumor-targeted HPMA copolymer-(RGDfK)-(CHX-A”-DTPA) conjugates show increased kidney accumulation. J Control Release, 2008. 132(3): p. 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolman ME, et al. , Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv Drug Deliv Rev, 2010. 62(14): p. 1344–57. [DOI] [PubMed] [Google Scholar]
- 79.Hu P, et al. , Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano, 2020. 14(7): p. 7970–7986. [DOI] [PubMed] [Google Scholar]
- 80.Kang H, et al. , Renal Clearable Organic Nanocarriers for Bioimaging and Drug Delivery. Adv Mater, 2016. 28(37): p. 8162–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Debayle M, et al. , Zwitterionic polymer ligands: an ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials, 2019. 219: p. 119357. [DOI] [PubMed] [Google Scholar]
- 82.Nie S, Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine (Lond), 2010. 5(4): p. 523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Estephan ZG, Jaber JA, and Schlenoff JB, Zwitterion-stabilized silica nanoparticles: toward nonstick nano. Langmuir, 2010. 26(22): p. 16884–9. [DOI] [PubMed] [Google Scholar]
- 84.Li B and Lane LA, Probing the biological obstacles of nanomedicine with gold nanoparticles. WIREs Nanomedicine and Nanobiotechnology, 2019. 11(3): p. e1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippmann M, Effects of fiber characteristics on lung deposition, retention, and disease. Environmental health perspectives, 1990. 88: p. 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Gao H, and Bao G, Physical Principles of Nanoparticle Cellular Endocytosis. ACS nano, 2015. 9(9): p. 8655–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peiris PM, et al. , Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS nano, 2012. 6(5): p. 4157–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu C, et al. , Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer letters, 2018. 414: p. 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christian DA, et al. , Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol Pharm, 2009. 6(5): p. 1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li S, Nickels J, and Palmer AF, Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials, 2005. 26(17): p. 3759–69. [DOI] [PubMed] [Google Scholar]
- 91.Geng Y, et al. , Shape effects of filaments versus spherical particles in flow and drug delivery. Nature Nanotechnology, 2007. 2(4): p. 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruggiero A, et al. , Paradoxical glomerular filtration of carbon nanotubes. Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(27): p. 12369–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurantowicz N, et al. , Biodistribution of a High Dose of Diamond, Graphite, and Graphene Oxide Nanoparticles After Multiple Intraperitoneal Injections in Rats. Nanoscale Research Letters, 2015. 10(1): p. 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Longmire MR, et al. , Biologically Optimized Nanosized Molecules and Particles: More than Just Size. Bioconjugate Chemistry, 2011. 22(6): p. 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, et al. , Shape Effects of Cylindrical versus Spherical Unimolecular Polymer Nanomaterials on in Vitro and in Vivo Behaviors. Research, 2019. 2019: p. 2391486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, et al. , A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Scientific Reports, 2017. 7(1): p. 4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jasinski DL, Li H, and Guo P, The Effect of Size and Shape of RNA Nanoparticles on Biodistribution. Molecular Therapy, 2018. 26(3): p. 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Talamini L, et al. , Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano, 2017. 11(6): p. 5519–5529. [DOI] [PubMed] [Google Scholar]
- 99.Champion JA and Mitragotri S, Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(13): p. 4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jarsch IK, Daste F, and Gallop JL, Membrane curvature in cell biology: An integration of molecular mechanisms. The Journal of cell biology, 2016. 214(4): p. 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toy R, et al. , Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine (London, England), 2014. 9(1): p. 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petros RA and DeSimone JM, Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov, 2010. 9(8): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 103.Longmire M, Choyke PL, and Kobayashi H, Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (London, England), 2008. 3(5): p. 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kojima C, et al. , Influence of dendrimer generation and polyethylene glycol length on the biodistribution of PEGylated dendrimers. International Journal of Pharmaceutics, 2010. 383(1): p. 293–296. [DOI] [PubMed] [Google Scholar]
- 105.Decuzzi P, et al. , A theoretical model for the margination of particles within blood vessels. Annals of Biomedical Engineering, 2005. 33(2): p. 179–190. [DOI] [PubMed] [Google Scholar]
- 106.Tietjen GT, et al. , Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Science Translational Medicine, 2017. 9(418). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wischnjow A, et al. , Renal Targeting: Peptide-Based Drug Delivery to Proximal Tubule Cells. Bioconjug Chem, 2016. 27(4): p. 1050–7. [DOI] [PubMed] [Google Scholar]
- 108.Wang G, et al. , Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics, 2019. 9(21): p. 6191–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pasqualini R and Ruoslahti E, Organ targeting in vivo using phage display peptide libraries. Nature, 1996. 380(6572): p. 364–366. [DOI] [PubMed] [Google Scholar]
- 110.Bidwell GL, et al. , A kidney-selective biopolymer for targeted drug delivery. American Journal of Physiology-Renal Physiology, 2017. 312(1): p. F54–F64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Masehi-Lano JJ, and Chung EJ, Peptide and antibody ligands for renal targeting: nanomedicine strategies for kidney disease. Biomaterials Science, 2017. 5(8): p. 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pollinger K, et al. , Kidney Podocytes as Specific Targets for cyclo(RGDfC)-Modified Nanoparticles. Small, 2012. 8(21): p. 3368–3375. [DOI] [PubMed] [Google Scholar]
- 113.Jung E, et al. , Identification of tissue-specific targeting peptide. J Comput Aided Mol Des, 2012. 26(11): p. 1267–75. [DOI] [PubMed] [Google Scholar]
- 114.Odermatt A, et al. , Identification of receptor ligands by screening phage-display peptide libraries ex vivo on microdissected kidney tubules. J Am Soc Nephrol, 2001. 12(2): p. 308–16. [DOI] [PubMed] [Google Scholar]
- 115.Tietjen GT, et al. , Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci Transl Med, 2017. 9(418). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan K, et al. , Subcellular localization of glucocorticoid receptor protein in the human kidney glomerulus. Kidney International, 1999. 56(1): p. 65–73. [DOI] [PubMed] [Google Scholar]
- 117.Zavaleta C, Ho D, and Chung EJ, Theranostic Nanoparticles for Tracking and Monitoring Disease State. SLAS Technology: Translating Life Sciences Innovation, 2017. 23(3): p. 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nikravesh N, et al. , Factors influencing safety and efficacy of intravenous iron-carbohydrate nanomedicines: From production to clinical practice. Nanomedicine: Nanotechnology, Biology and Medicine, 2020. 26: p. 102178. [DOI] [PubMed] [Google Scholar]
- 119.Sirolimus. AY 22989, NSC 226080, NSC 606698, rapamycin, Rapamune. Drugs R D, 1999. 1(1): p. 100–7. [DOI] [PubMed] [Google Scholar]
- 120.Bobo D, et al. , Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharmaceutical Research, 2016. 33(10): p. 2373–2387. [DOI] [PubMed] [Google Scholar]
- 121.Kobayashi H, et al. , Polyamine dendrimer-based MRI contrast agents for functional kidney imaging to diagnose acute renal failure. Journal of Magnetic Resonance Imaging, 2004. 20(3): p. 512–518. [DOI] [PubMed] [Google Scholar]
- 122.Petzold K, et al. , Urinary biomarkers at early ADPKD disease stage. PloS one, 2015. 10(4): p. e0123555–e0123555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russo P, End stage and chronic kidney disease: associations with renal cancer. Frontiers in oncology, 2012. 2: p. 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maulhardt HA, Marin AM, and diZerega GS, Intratumoral submicron particle docetaxel inhibits syngeneic Renca renal cancer growth and increases CD4+, CD8+, and Treg levels in peripheral blood. Investigational new drugs, 2020. 38(5): p. 1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maulhardt HA, et al. , Local Injection of Submicron Particle Docetaxel is Associated with Tumor Eradication, Reduced Systemic Toxicity and an Immunologic Response in Uro-Oncologic Xenografts. Cancers, 2019. 11(4): p. 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishikawa I, et al. , Gender difference in the mean age at the induction of hemodialysis in patients with autosomal dominant polycystic kidney disease. American Journal of Kidney Diseases, 2000. 35(6): p. 1072–1075. [DOI] [PubMed] [Google Scholar]
- 127.Gretz N, et al. , Is Gender a Determinant for Evolution of Renal-Failure - a Study in Autosomal Dominant Polycystic Kidney-Disease. American Journal of Kidney Diseases, 1989. 14(3): p. 178–183. [DOI] [PubMed] [Google Scholar]
- 128.Cobo G, et al. , Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci (Lond), 2016. 130(14): p. 1147–63. [DOI] [PubMed] [Google Scholar]
- 129.James BD, Guerin P, and Allen JB, Let’s Talk About Sex-Biological Sex Is Underreported in Biomaterial Studies. Adv Healthc Mater, 2020: p. e2001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Z, et al. , Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats. J Appl Toxicol, 2019. 39(5): p. 807–819. [DOI] [PubMed] [Google Scholar]
- 131.Chen J, et al. , Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice. Int J Nanomedicine, 2013. 8: p. 2409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JH, et al. , Sex-specific accumulation of silver nanoparticles in rat kidneys is not ovarian hormone regulated but elimination limited. 2020. 20: p. 100255. [Google Scholar]


