Abstract
This study examines whether neuroticism is differentially associated with risk of incident Alzheimer’s disease (AD), vascular dementia (VD), and frontotemporal dementia (FTD) using a prospective study design. Participants from the UK Biobank (N = 401,422) completed a self-report neuroticism scale in 2006–2010 and incident all-cause dementia, AD, VD, and FTD were ascertained using electronic health records or death records up to 2018. During an average follow-up of 8.8 years (3,566,123 person-years), there were 1,798 incident of all-cause dementia, 675 AD, 376 VD, and 81 FTD. Accounting for age and sex, compared to individuals in the low quartile, individuals in the top quartile of neuroticism had higher risk of all-cause dementia (HR = 1.70; 95% CI: 1.49–1.93), AD (HR = 1.42; 1.15–1.75), VD (HR = 1.73; 1.30–2.29), but not FTD (HR = 0.89; 0.49–1.63). The associations with AD and VD were attenuated but remained significant after further accounting for education, household income, deprivation index, diabetes, hypertension, stroke, heart attack, ever smoker, physical activity, obesity, hemoglobin A1c, C-reactive protein, and low-density lipoprotein. The associations were not moderated by socioeconomic status. The findings were consistent in analyses that excluded cases that occurred within the first 5 years of follow-up. In conclusion, neuroticism is a robust predictor of incident AD and VD, but not FTD. This pattern suggests that the affective symptoms that distinguish dementia types may partly reflect premorbid differences in trait neuroticism.
Keywords: Personality, Alzheimer’s disease, Pick’s disease, Emotional distress, Neurodegenerative disease
Introduction
Higher neuroticism (i.e., the tendency to experience negative emotions and vulnerability to stress) has been consistently associated with worse performance on cognitive tasks, steeper cognitive decline, higher likelihood of subjective cognitive complaints, and risk of pre-dementia syndromes (Caselli et al., 2016; Hock et al., 2014; Könen and Karbach, 2020; Munoz et al., 2020; Stephan et al., 2020). Furthermore, multiple prospective cohort studies have found that high neuroticism is a risk factor for Alzheimer’s disease (AD) and related dementias (ADRD)(Duchek et al., 2019; Johansson et al., 2014; Wilson et al., 2005). A recent meta-analysis of 12 studies (n = 33,054) found a pooled hazard ratio (HR) = 1.24 (95% Confidence Interval (CI) = 1.17, 1.31) with modest evidence of heterogeneity across studies and no significant differences between studies that examined AD compared to the broader diagnosis of dementia (Aschwanden et al., 2021). While current evidence indicates that neuroticism is related to cognitive health (Segerstrom, 2020; Terracciano and Sutin, 2019), it is unclear whether neuroticism is differentially associated with specific types of dementia, besides AD. The primary objective of this study was to test whether the association between neuroticism and risk of dementia varies across AD, vascular dementia (VD), and frontotemporal dementia (FTD; also known as Pick’s disease). This question is of scientific and clinical relevance because the emotional vulnerabilities assessed by neuroticism could be differentially related to the underlying neuropathology that characterize the different types of dementia and have pathoplastic effects on the clinical course of the disease.
This work builds on a previous UK Biobank study that included neuroticism as a covariate to predict dementia risk (Calvin et al., 2019). We extend the previous study (Calvin et al., 2019) by considering a longer follow-up period (up to 12 years compared to 8 years), more than five-fold higher number of incident cases, and the full range of neuroticism scores (the previous study used half the sample by contrasting the top and bottom quartile of neuroticism scores). Most importantly, we focus on the differential associations between neuroticism and specific types of dementia, including the less common FTD. To our knowledge, no study to date has examined whether neuroticism is prospectively associated with risk of incident FTD. We expected neuroticism to be more strongly related to VD than AD (Barnes et al., 2012; Calvin et al., 2019), but we had no hypothesis for FTD. We also considered the extent to which socioeconomic and vascular risk factors attenuated the association between neuroticism and dementia risk, which may suggest potential pathways between personality and health outcomes. Finally, we tested whether there was an interaction between neuroticism and socioeconomic status on dementia risk, as suggested by a previous large study (Chapman et al., 2020).
Methods
Participants and study design
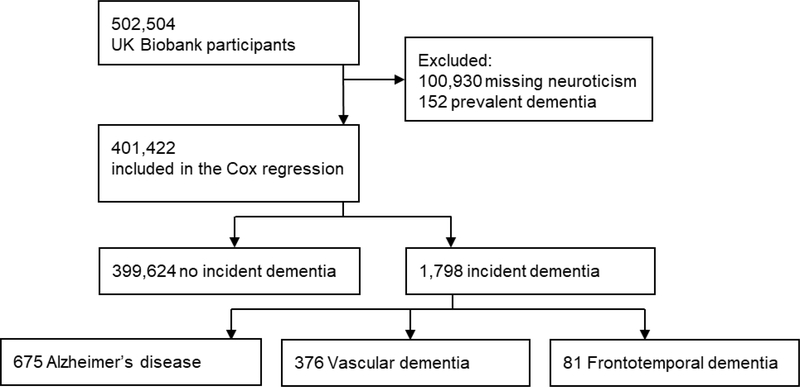
The participants are part of the UK Biobank (http://www.ukbiobank.ac.uk), an ongoing prospective cohort study that aims to improve the prevention, diagnosis, and treatment of common diseases. A sample of more than 500,000 individuals registered with the UK National Health Service (NHS) was recruited from across the UK. The first assessment was conducted at 22 assessment centers between 2006 and 2010, when neuroticism and the covariates included in the present study were assessed. Incident dementia was ascertained through linked health records from the NHS. See Figure 1 for the study sample flow chart.
Figure 1.
Study sample flow chart.
UK Biobank obtained ethical approval from the North West Multicenter Research Ethics Committee, and all participants gave informed consent. The study used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies (see supplementary material for the checklist).
Measures
Neuroticism.
Neuroticism was assessed as part of a touchscreen questionnaire using the 12-item Eysenck Personality Questionnaire-Revised (EPQ-R) Short Form (Eysenck et al., 1985). Items were short sentences (e.g., “Are you a worrier?”) and response options were “Yes”, “No”, “Do not know”, and “Prefer not to answer”. Respondents who did not answer “Yes” or “No” to all 12 items were excluded from the analyses. The neuroticism score was the sum of “Yes” answers across the 12 items. Raw scores were transformed into z-scores (Mean=0, SD=1) to facilitate comparison across studies. We also compared individuals in the bottom quartile (scores 0 or 1; 26.9% of the sample) with the top quartile (scores 7 to 12; 24.2% of the sample) of neuroticism. The internal consistency in this sample was α = 0.84.
Dementia.
All-cause dementia, AD, VD, and FTD were ascertained using NHS hospital inpatient records or death records. We used dementia adjudication and dates generated by the UK Biobank Outcome Adjudication Group (http://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_dementia.pdf). AD, VD, and FTD were also classified as all-cause dementia, but not every all-cause dementia case had a cause-specific ascertainment. Incident cases were the earliest known hospitalization with relevant International Classification of Diseases (ICD) code. Dementia cases were also ascertained from death register records. The source of dementia report was 93.4% from hospital admission and 6.6% from death records. Our analyses of incident cases excluded individuals with a relevant ICD code prior to recruitment or self-reported diagnosis at recruitment (as part of the enrollment interview with a nurse, participants were asked if they had a history of “Dementia or Alzheimer’s or Cognitive Impairment”, to which they answered “Yes” or “No”). A validation study compared the UK Biobank dementia case ascertainment with clinical expert adjudication based on full medical records and found a high positive predictive value for the ascertainment of all-cause dementia, but lower for AD and VD (Wilkinson et al., 2019).
Demographic, socioeconomic, and vascular risk covariates
Demographic.
Age (in years) and sex (0 = male, 1 = female) were assessed at baseline and included in all analyses.
Socioeconomic status.
Education was coded as a college/university degree or equivalent (yes/no). The Townsend deprivation index was used as a proxy for socioeconomic status and assigned to participants based on their postcodes. The Townsend deprivation index is based on unemployment, non-home ownership, non-car ownership, and household overcrowding in a geographic area. Total household income was assessed with the question “What is the average total income before tax received by your HOUSEHOLD?” with the following response options: “<18,000”, “18,000 to 30,999”, “31,000 to 51,999”, “52,000 to 100,000”, “>100,000”, “Prefer not to answer”, and “Do not know”. The last two options were set to missing and income was included in the analyses on a scale ranging from 1 (<18,000) to 5 (>100,000).
Vascular/behavioral risk factors.
As part of a touchscreen questionnaire, diabetes was assessed with the question “Has a doctor ever told you that you have diabetes?”. The question “Has a doctor ever told you that you have had any of the following conditions? (You can select more than one answer)” was used to assess hypertension, stroke, and heart attack. Never smokers were compared to current and former smokers based on questions about current (“Do you smoke tobacco now?”) and past (“In the past, how often have you smoked tobacco?”) tobacco smoking. Physical activity was measured as the sum of minutes performing walking, moderate and vigorous activity based on the International Physical Activity Questionnaire. Obesity (body mass index ≥ 30) was calculated from standing height and weight measured during the initial assessment center visit. At the same visit, trained staff members obtained non-fasting venous blood samples (~ 50 ml) to assay high sensitivity C-reactive protein (CRP), low-density lipoprotein (LDL), and hemoglobin A1c (HbA1c). CRP is a marker of inflammation, LDL is a marker of hyperlipidemia, HbA1c is an additional measure of diabetes, and all three were part of the vascular risk factor covariates. For assay information see https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf.
Statistical analyses
We calculated descriptive statistics for the study variables at baseline as means and standard deviations (SD) or proportions in the full sample and by dementia outcome. We computed Cox proportional hazard regression models with neuroticism (z-score) as the main predictor of incident dementia. Time was coded in years from the date of the personality assessment (2006–2010) up to the date of the first dementia report, death, or censoring date (8 February 2018). We used the date of the first report of dementia for the analyses of all-cause and cause-specific dementia. These specific diagnoses may have occurred in the health record at a later date, but we used the date of the first dementia code. The proportional hazard assumption was met. We report HRs and their 95% confidence interval for 1 SD difference in neuroticism. In line with past research, we also examined the risk for individuals in the top quartiles of neuroticism as compared to the bottom quartile. Model 1 included age and sex as covariates. Using the covariates of Model 1, four sensitivity analyses were performed: Model 1.1 excluded individuals with more than one cause of dementia; Model 1.2 excluded incident dementia cases within the first five years of follow-up to reduce the likelihood that participants already had dementia at baseline; Model 1.3 excluded dementia cases ascertained from death records; Model 1.4 excluded participants younger than 50 years old at baseline because they were less likely to develop dementia in the time span considered. Model 2 added education and the Townsend deprivation index as covariates, in addition to age and sex. Model 2.1 added total household income as an additional covariate. Because a previous study indicated that the association between neuroticism-related traits and dementia risk may vary by socioeconomic status (Chapman et al., 2020), we tested the interaction between (a) neuroticism and the Townsend deprivation index and (b) neuroticism and total household income on dementia risk. Model 3 included the covariates in Model 2 and the vascular risk factors (self-reported diabetes, hypertension, stroke, heart attack, ever smoker, BMI > 30). Because of missing data, we added the HbA1c, CRP, LDL, and sum of minutes performing walking, moderate and vigorous activity to Model 3.1.
Results
Baseline characteristics for the entire sample and by the outcome groups are reported in Table 1. The neuroticism scale was completed by 401,422 participants who had no record of dementia at baseline. At baseline, participants were aged 39 to 73 years (average = 56.41 years, SD = 8.07). During up to 12 years of follow-up (average = 8.88 years, SD = 1.23) and 3,566,123 person-years, there were 1,798 incident all-cause dementia, 675 AD, 376 VD, and 81 FTD. Note that the number of cause-specific dementia cases do not add up to the number of all-cause dementia cases because some individuals had no cause-specific diagnostic codes.
Table 1.
Baseline descriptive statistics for the full sample and by dementia outcome at follow-up.
| Total | Not impaired | All-cause dementia | Alzheimer’s disease | Vascular dementia | Frontotemporal dementia | |
|---|---|---|---|---|---|---|
| N | 401,422 | 399,624 | 1,798 | 675 | 376 | 81 |
| Neuroticism | 4.12 (3.27) | 4.12 (3.27) | 4.31 (3.41) | 4.11 (3.37) | 4.26 (3.42) | 3.70 (3.45) |
| Age, years | 56.41 (8.07) | 56.38 (8.06) | 64.04 (5.09) | 64.53 (4.38) | 64.59 (4.36) | 61.99 (5.63) |
| Follow-up, years | 8.88 (1.23) | 8.90 (1.21) | 5.95 (2.10) | 6.19 (1.89) | 5.78 (2.14) | 5.49 (2.12) |
| Sex (Women) | 215,518 (53.7%) | 214,733 (53.7%) | 785 (43.7%) | 318 (47.1%) | 138 (36.7%) | 36 (44.4%) |
| College | 133,363 (33.2%) | 133,013 (33.3%) | 350 (19.5%) | 131 (19.4%) | 58 (15.4%) | 27 (33.3%) |
| Deprivation | −1.39 (3.04) | −1.39 (3.03) | −0.86 (3.37) | −1.14 (3.23) | −0.55 (3.47) | −1.53 (3.16) |
| Income | 2.66 (1.20) | 2.66 (1.20) | 1.87 (1.00) | 1.88 (0.96) | 1.72 (0.96) | 2.08 (1.06) |
| Diabetes | 20,418 (5.1%) | 20,154 (5.0%) | 264 (14.7%) | 83 (12.3%) | 94 (25.0%) | 9 (11.1%) |
| Hypertension | 96,621 (24.1%) | 96,071 (24.0%) | 550 (30.6%) | 198 (29.3%) | 127 (33.8%) | 22 (27.2%) |
| Stroke | 4,937 (1.2%) | 4,854 (1.2%) | 83 (4.6%) | 18 (2.7%) | 34 (9.0%) | 2 (2.5%) |
| Heart attack | 9,934 (2.3%) | 9,174 (2.3%) | 160 (8.9%) | 55 (8.1%) | 58 (15.4%) | 7 (8.6%) |
| Obesity | 97,319 (24.2%) | 96,834 (24.2%) | 485 (27.0%) | 163 (24.1%) | 134 (35.6%) | 21 (25.9%) |
| HbA1c, mmol/mol | 36.03 (6.69) | 36.02 (6.67) | 38.88 (9.78) | 38.48 (9.95) | 41.41 (11.16) | 37.18 (9.42) |
| CRP, mg/L | 2.57 (4.32) | 2.57 (4.32) | 3.02 (5.24) | 2.6825 (4.90) | 3.3631 (5.11) | 3.1368 (4.53) |
| LDL, mmol/L | 3.56 (0.87) | 3.56 (0.87) | 3.37 (0.96) | 3.3902 (0.95) | 3.2138 (1.01) | 3.4556 (0.80) |
| Ever smoker | 181,675 (45.3%) | 180,686 (45.2%) | 989 (55.0%) | 346 (51.3%) | 231 (61.4%) | 44 (54.3%) |
| Physical activity | 127.80 (101.73) | 127.83 (101.72) | 120.36 (104.71) | 129.42 (106.83) | 111.80 (103.92) | 116.77 (100.55) |
Notes. Sample size as indicated in the Table except for Income (n = 348,523), physical activity (n = 335,202), HbA1c (n = 373,318), CRP (n = 375,036), and LDL (n = 375,112). Income is the household average total income before tax on a scale from 1 to 5. Physical activity is the sum of minutes performing walking, moderate and vigorous activity.
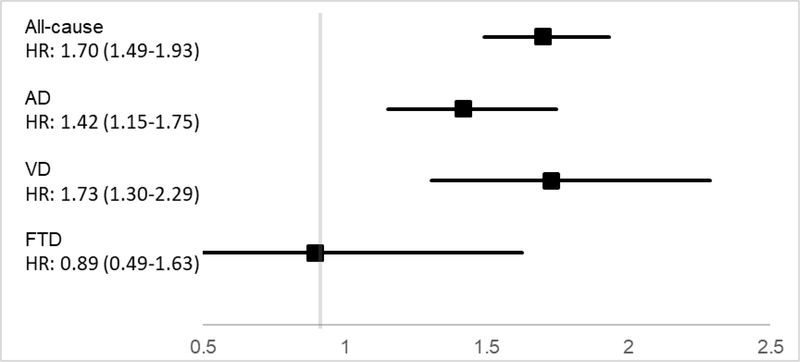
Table 2 presents the HRs and 95% CIs for all-cause and cause-specific dementia. The primary Model 1, with age and sex as covariates, indicated that one SD higher score on neuroticism was associated with ~20% higher risk of incident all-cause dementia. The same model indicated a weaker association for incident AD (HR = 1.15, 95% CI = 1.06, 1.24) and a stronger association for VD (HR = 1.24, 95% CI = 1.13, 1.38). Neuroticism was not related to risk of FTD (HR = 0.97, 95% CI = 0.77, 1.22). Figure 2 shows a similar pattern when contrasting individuals in the top and bottom quartile of neuroticism. In a first sensitivity analysis (Model 1.1), there was a slightly stronger association between neuroticism and each type of dementia when individuals with more than one cause of dementia were excluded. The second to fourth sensitivity analyses found that neuroticism remained a significant predictor of all-cause dementia, AD, and VD with the exclusion of dementia cases that occurred within 5 years of the baseline assessment (Model 1.2), with the exclusion of dementia ascertained from death records (Model 1.3), or with the exclusion of younger participants (Model 1.4).
Table 2.
Cox regression results of neuroticism predicting risk of incident of all-cause dementia, Alzheimer’s disease, vascular dementia, and frontotemporal dementia.
| All-cause dementia | Alzheimer’s disease | Vascular dementia | Frontotemporal dementia | |
|---|---|---|---|---|
| Model 1, # cases | 1,798/401,416 | 675/401,341 | 376/401,235 | 81/400,734 |
| Model 1, HR (95% CI) | 1.22 (1.17–1.28) | 1.15 (1.06–1.24) | 1.24 (1.13–1.38) | 0.97 (0.77–1.22) |
| Model 1, categories HR (95% CI) | 1.70 (1.49–1.93) | 1.42 (1.15–1.75) | 1.73 (1.30–2.29) | 0.89 (0.49–1.63) |
| Sensitivity analyses / Robustness check | ||||
| Model 1.1, # cases | - | 596/401,338 | 304/401,228 | 69/400,705 |
| Model 1.1, HR (95% CI) | - | 1.17 (1.08–1.27) | 1.28 (1.15–1.43) | 1.10 (0.87–1.40) |
| Model 1.2, # cases | 1,262/394,319 | 475/394,319 | 250/394,272 | 53/394,042 |
| Model 1.2, HR (95% CI) | 1.23 (1.16–1.30) | 1.20 (1.10–1.32) | 1.22 (1.08–1.38) | 0.99 (0.75–1.32) |
| Model 1.3, # cases | 1,680/401,298 | 649/401,220 | 357/401,110 | 74/400,588 |
| Model 1.3, HR (95% CI) | 1.23 (1.17–1.29) | 1.13 (1.05–1.23) | 1.25 (1.13–1.38) | 0.95 (0.74–1.21) |
| Model 1.4, # cases | 1,757/306,170 | 669/305,995 | 371/305,995 | 78/305,534 |
| Model 1.4, HR (95% CI) | 1.22 (1.17–1.28) | 1.15 (1.06–1.24) | 1.25 (1.13–1.38) | 0.98 (0.77–1.24) |
| Socioeconomic covariates | ||||
| Model 2, # cases | 1,796/400,925 | 674/400,850 | 376/400,744 | 80/400,245 |
| Model 2, HR (95% CI) | 1.19 (1.14–1.25) | 1.12 (1.04–1.22) | 1.19 (1.08–1.32) | 0.99 (0.79–1.25) |
| Model 2.1, # cases | 1,347/348,067 | 495/348,011 | 285/347,925 | 58/347,202 |
| Model 2.1, HR (95% CI) | 1.17 (1.11–1.24) | 1.12 (1.02–1.23) | 1.21 (1.08–1.36) | 0.96 (0.73–1.27) |
| Vascular and behavioral covariates | ||||
| Model 3, # cases | 1,796/400,925 | 674/400,847 | 376/400,737 | 80/400,216 |
| Model 3, HR (95% CI) | 1.18 (1.13–1.24) | 1.13 (1.04–1.22) | 1.16 (1.05–1.28) | 0.97 (0.77–1.22) |
| Model 3.1, # cases | 1,242/298,447 | 464/298,321 | 268/298,328 | 53/297,952 |
| Model 3.1, HR (95% CI) | 1.18 (1.12–1.25) | 1.11 (1.01–1.22) | 1.18 (1.05–1.33) | 1.01 (0.76–1.34) |
Notes. Model 1 to Model 1.4 include the covariates age and sex; Model 1.1 excludes cases with more than one type of dementia code (e.g., AD and VD); Model 1.2 excludes incident cases of dementia within 5 years of baseline assessment; Model 1.3 excludes dementia ascertained from death records; Model 1.4 excludes participants younger than age 50 at baseline; Model 2 includes the covariates of Model 1 plus education and Townsend deprivation index; Model 2.1 further adds household income; Model 3 includes the covariates of Model 2 plus vascular risk factors (self-reported diabetes, hypertension, stroke, heart attack, ever smoker, BMI > 30); Model 3.1 further add HbA1c, CRP, LDL, and the sum of minutes performing walking, moderate and vigorous activity. HR = Hazard Ratio; CI = Confidence Interval.
Figure 2.
Association of neuroticism with risk of all-cause, Alzheimer’s Disease, Vascular, and Frontotemporal Dementia.
Next, we evaluated the impact of socioeconomic variables on the association between neuroticism and risk of incident dementia. In Model 2, the associations were attenuated but remained significant when education and the Townsend deprivation index were included as covariates. Similarly, when further adding household income as a covariate (Model 2.1), the associations were attenuated but remained significant. There was no significant interaction between neuroticism and the Townsend deprivation index on risk of all-cause dementia (HR = 1.03, 95%CI: 0.99, 1.07), AD (HR = 1.05, 95%CI: 0.98, 1.13), VD (HR = 0.93, 95%CI: 0.85, 1.01), or FTD (HR = 1.18, 95%CI: 0.97, 1.46). Likewise, there was no significant interaction between neuroticism and household income on risk of all-cause dementia (HR = 0.99, 95%CI: 0.94, 1.05), AD (HR = 0.98, 95%CI: 0.89, 1.07), VD (HR = 1.00, 95%CI: 0.88, 1.13), or FTD (HR = 1.23, 95%CI: 0.97, 1.58).
Finally, the association between neuroticism and incident all-cause dementia, AD, and VD was attenuated but remained significant when self-reported diabetes, hypertension, stroke, heart attack, ever smoker, and obesity were included as covariates (Model 3). In a subsample with available blood biomarkers (HbA1c, CRP, LDL) and physical activity, the pattern of findings remained unchanged.
Discussion
In one of the largest prospective studies on neuroticism and dementia risk to date, we found that higher neuroticism was related to higher risk of incident all-cause dementia. Individuals in the top quartiles of neuroticism had about 70% higher risk of all-cause dementia compared to the individuals who scored in the lower quartile of neuroticism. The association was slightly stronger for VD (about 70% higher risk) compared to AD (about 40% higher risk). These effects were robust and remained significant after accounting for relevant demographics, education, socioeconomic status, cardiovascular conditions, blood biomarkers, and health-related behaviors that are risk factors for dementia. Neuroticism was not associated with FTD.
AD is the most common cause of dementia and the most frequently examined in studies of personality and dementia. In the current sample, the proportion of AD cases was relatively lower than in previous studies (Goodman et al., 2017), and the association between neuroticism and risk of incident AD was weaker than previously reported (Terracciano et al., 2014). One potential reason is the relatively younger age of the UK Biobank compared to previous studies. Most UK Biobank participants have not yet reached the typical age of onset for AD, which is generally older than VD and even more so than FTD (Goodman et al., 2017). Another potential reason is that previous studies that reported larger effects relied on extensive neuropsychological testing, informant/subject structured interview, and neurological examination to reach diagnosis at consensus conference (Terracciano et al., 2014). Nevertheless, the association between neuroticism and AD risk remained significant in sensitivity analyses and after accounting for other risk factors. The robust evidence from prospective studies is supported by research on potential neurobiological mechanisms underlying the relationship between neuroticism and AD. First, a growing number of studies suggest that the association between neuroticism and AD extends to amyloid, tau, and neurodegeneration (ATN) biomarkers (Jack et al., 2018). Indeed, neuroticism has been associated with higher risk of extracellular deposition of Aβ plaques, the pathological aggregation of the misfolded and abnormally phosphorylated protein tau in neurofibrillary tangles, and neuronal losses in key brain regions (Graham et al., 2020; Jackson et al., 2011; Schultz et al., 2020; Terracciano et al., 2013a). Moreover, evidence from autopsy studies indicated that even in the presence of amyloid and tau pathology, individuals who score lower on neuroticism were less likely to develop dementia (Graham et al., 2020; Terracciano et al., 2013a). Neuroticism is also related to lower levels of brain-derived neurotrophic factor (BDNF)(Lang et al., 2004; Terracciano et al., 2011). BDNF deficits are thought to contribute to neurodegenerative diseases, via the BDNF critical role in synaptic and neuronal maintenance (Giuffrida et al., 2018). The greater vulnerability to stress and maladaptive coping strategies of individuals scoring higher on neuroticism (Gunthert et al., 1999) may also result in chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis and dysregulation of the physiological stress response (O’Súilleabháin et al., 2019). Abnormal stress response may in turn lead to neuronal loss and neurodegeneration in the hippocampus and prefrontal cortex, two key regions involved in the pathophysiology of AD (Carroll et al., 2017; Dedovic et al., 2009). Ultimately, chronic cortisol secretion can increase risk of dementia (Ennis et al., 2017).
Among the different causes of dementia, neuroticism had the strongest association with risk of VD. This association was attenuated by about a third in the model that accounted for vascular risk factors, including hypertension, diabetes, obesity, and cigarette smoking. This suggests that vascular risk factors play an explanatory role in the link between neuroticism and VD. Indeed, neuroticism has been associated with a multitude of risky behaviors and clinical conditions that compromise vascular health across the lifespan (Turiano et al., 2018). For example, children who score higher on neuroticism-related traits are more likely to start smoking (Strickhouser et al., 2020). In adulthood, neuroticism is robustly linked to a higher probability of smoking, lower likelihood to quit smoking, and greater risk of smoking relapse (Hakulinen et al., 2015). Similarly, neuroticism is robustly associated with physical inactivity and sedentary behaviors in every age group (Sutin et al., 2016). In part through these health-risk behaviors, individuals who score high on neuroticism tend to have more chronic conditions (Charles et al., 2008; Sutin et al., 2013), are more likely to have metabolic syndrome (Phillips et al., 2010), and lower cardiovascular fitness (Terracciano et al., 2013b). Together, these vascular risk factors represent major pathways through which higher neuroticism increases risk of VD. The vascular pathways may also contribute to the risk of AD, although the level of attenuation for AD was smaller in this sample.
Neuroticism was not a significant predictor of FTD. This null finding could be due to limited power given the relatively small number of FTD cases (n = 81). However, as recognized in consensus diagnostic criteria (Neary et al., 1998), the hallmark clinical symptoms of FTD include emotional blunting, the lower tendency to worry, the failure of showing appropriate embarrassment, lack of empathy, and failures to display or recognize basic emotions (Bathgate et al., 2001). These clinical criteria suggest low level of neuroticism in FTD patients. By contrast, the AD clinical criteria include increased emotional distress, mood swings, anxiety, and tearfulness as key parts of the AD clinical spectrum (Islam et al., 2019; McKhann et al., 2011). Our findings add to this literature and suggest that differences in the emotional profile of patients with FTD, AD, and VD (Bathgate et al., 2001) might reflect long-term psychological characteristics (i.e., traits) that are present well before the clinical onset of dementia. Consistent with this hypothesis, two small retrospective studies found that premorbid neuroticism, as rated by a proxy, was slightly lower for individuals who later developed FTD, compared to controls (Mahoney et al., 2011) and patients with mild cognitive impairment or AD (Lykou et al., 2013). Enduring personality traits such as neuroticism moderate risk of cause-specific dementia and are likely to influence pathoplastic processes that lead to different behavioral and psychological symptoms of dementia (Rouch et al., 2019; Sutin et al., 2018).
Strengths, Limitations, and future directions
Among the strengths of this study are the large sample size and the ascertainment of AD, VD, and FTD, in addition to all-cause dementia. The study design has both strengths and weaknesses, but complements many previous studies on personality and incident dementia (Aschwanden et al., 2021). In terms of weaknesses, there is neuropathologic and clinical overlap among the different causes of dementia and it is often difficult to distinguish AD from VD in clinical contexts. The ascertainment of dementia through hospital and death records is likely to under-detect cases, lead to diagnostic delays, or misclassification. More traditional longitudinal studies with repeated in-depth neuropsychological evaluations likely have greater sensitivity and accuracy. However, the reliance on health records has the advantage that attrition is less of a concern because outcomes are ascertained without the need for participants to be evaluated by the research study. The consistency of the association between neuroticism and risk of dementia across different study designs supports the robustness and generalizability of the findings, which are not dependent on any particular method to determine dementia.
The use of a validated neuroticism scale is another strength. A limitation of the UK Biobank, however, is the lack of assessment of the other four major dimensions of personality. Conscientiousness has been consistently associated with dementia risk (Aschwanden et al., 2021), while lower agreeableness may play a stronger role in VD and FTD relative to AD. An important question for future studies is whether lower conscientiousness and lower agreeableness are significant predictors of incident FTD, as one would hypothesize on the basis of the clinical and behavioral features of FTD. Future studies should also examine associations with other causes of dementia, for example, the Lewy Body dementia.
Reverse causality is a potential explanation of the observed associations, particularly because there was not a full neuropsychological screening at baseline. However, multiple findings suggest that reverse causality is unlikely to explain the association between neuroticism and risk of incident dementia. First, in this study, the effects remained significant (and were even stronger for AD and all-cause dementia) when cases that occurred within the first five years were excluded. Second, a meta-analysis has found that the length of follow-up was unrelated to the strength of the associations, contrary to what would be expected from reverse causality (Aschwanden et al., 2021). Third, a long-term longitudinal study found that neuroticism did not increase in the preclinical phase of AD in individuals who later developed cognitive impairment or dementia (Terracciano et al., 2017). These findings indicate that neuroticism reflects an etiologic risk factor more than a dementia prodrome.
Another limitation was the relatively short follow-up (an average of almost 9 years), but studies with longer follow-up have reported similar results (Johansson et al., 2014; Terracciano et al., 2014). Finally, despite the large and heterogeneous sample, the UK Biobank may underrepresent individuals with poor health and lower socioeconomic status. Nevertheless, we found that a deprivation index and household income did not significantly moderate the associations. This null finding also raises doubts that neuroticism interacts with socioeconomic indicators in predicting cognitive outcomes (Chapman et al., 2020; Luchetti et al., in press).
Conclusions
To summarize, this study advances knowledge on the role of personality in modulating the risk of dementia in one of the largest studies to date. The highlight is that neuroticism is associated with risk of incident AD and VD, but not FTD. The findings indicate that differences in the clinical symptoms among types of dementia (Bathgate et al., 2001) may partly reflect lifelong differences in trait neuroticism.
Supplementary Material
Acknowledgments
This research has been conducted using the UK Biobank Resource (Application Reference Number 57672). The study was not pre-registered and the authors do not have permission to distribute the data, but data is available upon application from http://www.ukbiobank.ac.uk.
Funding.
This work was supported by the National Institute on Aging of the National Institutes of Health (grant numbers R01AG068093, R01AG053297). Luca Passamonti is funded by the Medical Research Council (MRC) (MR/P01271X/1) at the University of Cambridge, UK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding Institutes.
Footnotes
Conflicts of Interest: None to declare.
References.
- Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, Terracciano A, 2021. Is personality associated with dementia risk? A meta-analytic investigation. Ageing Res Rev 67, 101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA, 2012. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Archives of general psychiatry 69(5), 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D, 2001. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta neurologica Scandinavica 103(6), 367–378. [DOI] [PubMed] [Google Scholar]
- Calvin CM, Wilkinson T, Starr JM, Sudlow C, Hagenaars SP, Harris SE, Schnier C, Davies G, Fawns-Ritchie C, Gale CR, Gallacher J, Deary IJ, 2019. Predicting incident dementia 3–8 years after brief cognitive tests in the UK Biobank prospective study of 500,000 people. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 15(12), 1546–1557. [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR, 2017. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience & Biobehavioral Reviews 77, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Henslin BR, Johnson TA, Woodruff BK, Hoffman-Snyder C, Geda YE, 2016. Impact of Personality on Cognitive Aging: A Prospective Cohort Study. Journal of the International Neuropsychological Society : JINS 22(7), 765–776. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Huang A, Peters K, Horner E, Manly J, Bennett DA, Lapham S, 2020. Association Between High School Personality Phenotype and Dementia 54 Years Later in Results From a National US Sample. JAMA psychiatry 77(2), 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Gatz M, Kato K, Pedersen NL, 2008. Physical health 25 years later: the predictive ability of neuroticism. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 27(3), 369–378. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC, 2009. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 47(3), 864–871. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Aschenbrenner AJ, Fagan AM, Benzinger TL, Morris JC, Balota DA, 2019. The Relation Between Personality and Biomarkers in Sensitivity and Conversion to Alzheimer-Type Dementia. Journal of the International Neuropsychological Society, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis GE, An Y, Resnick SM, Ferrucci L, O’brien RJ, Moffat SD, 2017. Long-term cortisol measures predict Alzheimer disease risk. Neurology 88(4), 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ, Barrett P, 1985. A revised version of the psychoticism scale. Pers Indiv Differ 6(1), 21–29. [Google Scholar]
- Giuffrida ML, Copani A, Rizzarelli E, 2018. A promising connection between BDNF and Alzheimer’s disease. Aging 10(8), 1791–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM, 2017. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 13(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, James BD, Jackson KL, Willroth EC, Boyle P, Wilson R, Bennett DA, Mroczek DK, 2020. Associations Between Personality Traits and Cognitive Resilience in Older Adults. The Journals of Gerontology: Series B, 10.1093/geronb/gbaa1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S, 1999. The role of neuroticism in daily stress and coping. Journal of personality and social psychology 77(5), 1087–1100. [DOI] [PubMed] [Google Scholar]
- Hakulinen C, Hintsanen M, Munafò MR, Virtanen M, Kivimäki M, Batty GD, Jokela M, 2015. Personality and smoking: Individual-participant meta-analysis of nine cohort studies. Addiction 110(11), 1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock RS, Lee HB, Bienvenu OJ, Nestadt G, Samuels JF, Parisi JM, Costa PT Jr., Spira AP, 2014. Personality and cognitive decline in the Baltimore Epidemiologic Catchment Area follow-up study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 22(9), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, Mazumder M, Schwabe-Warf D, Stephan Y, Sutin AR, Terracciano A, 2019. Personality Changes With Dementia From the Informant Perspective: New Data and Meta-Analysis. Journal of the American Medical Directors Association 20(2), 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D, 2011. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of aging 32(12), 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Duberstein PR, Hallstrom T, Waern M, Ostling S, Skoog I, 2014. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology 83(17), 1538–1544. [DOI] [PubMed] [Google Scholar]
- Könen T, Karbach J, 2020. Self-Reported Cognitive Failures in Everyday Life: A Closer Look at Their Relation to Personality and Cognitive Performance. Assessment 27(5), 982–995. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Gallinat J, 2004. BDNF serum concentrations in healthy volunteers are associated with depression-related personality traits. Neuropsychopharmacology 29, 795–798. [DOI] [PubMed] [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, Aschwanden D, Sutin A, in press. Personality traits and memory: A multilevel analysis across 27 countries from the Survey of Health, Ageing and Retirement in Europe. Psychological science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykou E, Rankin KP, Chatziantoniou L, Boulas C, Papatriantafyllou O, Tsaousis I, Neuhaus J, Karageorgiou C, Miller BL, Papatriantafyllou JD, 2013. Big 5 personality changes in Greek bvFTD, AD, and MCI patients. Alzheimer disease and associated disorders 27(3), 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Rohrer JD, Omar R, Rossor MN, Warren JD, 2011. Neuroanatomical profiles of personality change in frontotemporal lobar degeneration. The British journal of psychiatry : the journal of mental science 198(5), 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz E, Stawski RS, Sliwinski MJ, Smyth JM, MacDonald SWS, 2020. The Ups and Downs of Cognitive Function: Neuroticism and Negative Affect Drive Performance Inconsistency. The journals of gerontology. Series B, Psychological sciences and social sciences 75(2), 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF, 1998. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51(6), 1546–1554. [DOI] [PubMed] [Google Scholar]
- O’Súilleabháin PS, Hughes BM, Oommen AM, Joshi L, Cunningham S, 2019. Vulnerability to stress: Personality facet of vulnerability is associated with cardiovascular adaptation to recurring stress. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 144, 34–39. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Weiss A, Deary I, Gale CR, Thomas GN, Carroll D, 2010. Neuroticism, cognitive ability, and the metabolic syndrome: The Vietnam Experience Study. Journal of psychosomatic research 69(2), 193–201. [DOI] [PubMed] [Google Scholar]
- Rouch I, Dorey JM, Padovan C, Trombert-Paviot B, Benoit M, Laurent B, Boublay N, Krolak-Salmon P, 2019. Does Personality Predict Behavioral and Psychological Symptoms of Dementia? Results from PACO Prospective Study. Journal of Alzheimer’s disease : JAD 69(4), 1099–1108. [DOI] [PubMed] [Google Scholar]
- Schultz SA, Gordon BA, Mishra S, Su Y, Morris JC, Ances BM, Duchek JM, Balota DA, Benzinger TLS, 2020. Association between personality and tau-PET binding in cognitively normal older adults. Brain imaging and behavior 14, 2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, 2020. Personality and Incident Alzheimer’s Disease: Theory, Evidence, and Future Directions. The journals of gerontology. Series B, Psychological sciences and social sciences 75(3), 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Canada B, Terracciano A, 2020. Personality and Motoric Cognitive Risk Syndrome. Journal of the American Geriatrics Society 68(4), 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickhouser JE, Terracciano A, Sutin AR, 2020. Parent-reported childhood temperament and adolescent self-reported substance use initiation. Addictive behaviors 110, 106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Luchetti M, Artese A, Oshio A, Terracciano A, 2016. The five-factor model of personality and physical inactivity: A meta-analysis of 16 samples. J Res Pers 63, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Luchetti M, Terracciano A, 2018. Self-reported personality traits are prospectively associated with proxy-reported behavioral and psychological symptoms of dementia at the end of life. International journal of geriatric psychiatry 33(3), 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Zonderman AB, Ferrucci L, Terracciano A, 2013. Personality Traits and Chronic Disease: Implications for Adult Personality Development. The journals of gerontology. Series B, Psychological sciences and social sciences 68, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, An Y, Sutin AR, Thambisetty M, Resnick SM, 2017. Personality Change in the Preclinical Phase of Alzheimer Disease. JAMA psychiatry 74(12), 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Iacono D, O’Brien RJ, Troncoso JC, An Y, Sutin AR, Ferrucci L, Zonderman AB, Resnick SM, 2013a. Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiology of aging 34, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, Sutin AR, Zonderman AB, Uda M, Crisponi L, Schlessinger D, 2011. Neuroticism, depressive symptoms, and serum BDNF. Psychosomatic medicine 73(8), 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Schrack JA, Sutin AR, Chan W, Simonsick EM, Ferrucci L, 2013b. Personality, metabolic rate and aerobic capacity. PloS one 8(1), e54746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin A, 2019. Personality and Alzheimer’s disease: An integrative review. Personality Disorders: Theory, Research, and Treatment 10(1), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, Resnick SM, 2014. Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 10, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Hill P, Graham EK, Mroczek DK, 2018. Associations Between Personality and Health Behaviors Across the Life Span. The Oxford Handbook of Integrative Health Science, 305. [Google Scholar]
- Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall DE, Lerpiniere C, Allen NE, Flaig R, Russ TC, Bathgate D, Pal S, O’Brien JT, Sudlow CLM, 2019. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. European journal of epidemiology 34(6), 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA, 2005. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology 64(2), 380–382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.