Abstract
Guidelines recommend adrenal venous sampling (AVS) to determine disease laterality in primary aldosteronism. Adrenocorticotropic hormone (ACTH) stimulation clearly improves the likelihood of successful adrenal vein catheterization, but may lead to a decrease in lateralization rates. To examine the impact of ACTH on lateralization, we performed a retrospective analysis of 340 patients with confirmed primary aldosteronism who underwent AVS with a single interventional radiology team using a protocol of sampling both before and after an ACTH bolus. In addition to this original research, we conducted a review of similar studies from the last five years to develop a consensus on the impact of ACTH on lateralization for primary aldosteronism. In the original research analysis, following a bolus of ACTH, 58% of patients had a decline in lateralization index (LI) which led to discordance between the pre- and post-ACTH classifications of lateralization in up to 26% of cases. The majority of discordant cases were due to reclassification from unilateral disease pre-ACTH to bilateral disease post-ACTH. In patients who already lateralized with unstimulated sampling, the response to ACTH did not have any impact on surgical outcomes. In a review of contemporary studies, we identified 11 similar studies in the last 5 years, of which 10 reported either no change or a decrease in LI following ACTH, resulting in approximately 25% discordance between unstimulated and stimulated lateralization rates. We conclude that ACTH-stimulation during AVS can underestimate surgically remediable primary aldosteronism and recommend that the role of ACTH be limited primarily to enhancing selectivity.
Keywords: Primary Aldosteronism, Adrenal Venous Sampling, Aldosterone, ACTH
Summary:
ACTH-stimulated adrenal venous sampling for primary aldosteronism can decrease lateralization rates and may lead to fewer patients being referred for potentially curative surgery.
Introduction:
Primary aldosteronism is a common cause of hypertension and may arise from a unilateral aldosterone-producing adrenal adenoma or bilateral hyperplasia.1–4 For most patients with primary aldosteronism in whom surgery is a consideration, current guidelines recommend adrenal venous sampling (AVS) to determine the laterality of disease; if a patient lateralizes after AVS, surgical adrenalectomy is recommended.5 When there is no lateralization on AVS, mineralocorticoid receptor antagonists are advised, which although effective, may be associated with inferior outcomes compared to surgical intervention.6–13
AVS is a highly technical and operator-dependent procedure with protocols that vary from institution to institution.14 For example, sampling may be obtained sequentially from one adrenal vein at a time or simultaneously from both adrenal veins. Additionally, samples can be drawn just once from each adrenal vein or repeated samples from each vein can be taken.15 One major difference between institutional protocols is the use of stimulated sampling with adrenocorticotropic hormone (ACTH). Administered either as a single bolus or continuous infusion, ACTH is employed by many centers to enhance the selectivity index and help confirm successful adrenal vein cannulation. Further, many centers use ACTH-stimulated aldosterone and cortisol values to calculate the lateralization index to determine whether a patient has bilateral or unilateral disease. There is no debate that ACTH increases the selectivity index and therefore greatly increases the likelihood of successful adrenal venous sampling.14,16,17 However, the effect of ACTH on the lateralization index is less clear, with several studies reporting either no change or a decrease in lateralization rates after ACTH.18–27
A potential decrease in the lateralization index following ACTH is clinically important as this may lead to under-classification of unilateral, and hence surgically curable, disease. Yet many centers continue to use ACTH-stimulated sampling to determine lateralization, as this is recommended by some expert-based guidelines.28 We investigated the effect of ACTH-stimulation on lateralization rates in our large cohort of patients with primary aldosteronism referred to our single center for AVS which we perform using bilateral simultaneous sampling both before and after a supraphysiologic bolus of ACTH. We also conducted a contemporary literature review of similar studies in the past 5 years to develop a consensus on the influence of ACTH-stimulation on lateralization indices.
Methods:
The data that support the findings of this study can be made available by contacting the corresponding author with a reasonable request, hypothesis and analytic plan, and institutional research and ethics board approval.
Study design and participants
This was a retrospective study of patients with primary aldosteronism, diagnosed on the basis of Endocrine Society guidelines with either positive dynamic confirmatory testing or an elevated aldosterone (≥ 20 ng/dL) and suppressed renin in the context of hypertension and hypokalemia,5 who underwent AVS at our high-volume center between 2005 and 2019. No specific diagnostic aldosterone-to-renin ratio (ARR) was required, but the overwhelming majority had values ≥ 30 ng/dL per ng/mL/hour. Participants were included in the study if they had successful catheterization of both adrenal veins during AVS, defined as having a stimulated selectivity index of ≥ 3 in both adrenal veins (mean adrenal vein cortisol following a bolus of 250 mcg of ACTH divided by the inferior vena cava cortisol following ACTH). A total of 340 patients out of 348 who underwent AVS met the study criteria; no patients were on a mineralocorticoid receptor antagonist for at least 4 weeks prior to AVS. All study participants gave informed consent and all study procedures were conducted in accordance with institutional guidelines (protocol 2013P000564).
AVS protocol and lateralization determination
The AVS protocol used in this study has been recently described in detail.15 Briefly, after administration of fentanyl and midazolam for conscious sedation followed by initial sampling of aldosterone and cortisol from the inferior vena cava (IVC), patients underwent bilateral simultaneous catheterization of both adrenal veins with measurements of cortisol and aldosterone obtained five minutes apart for three total measurements. A bolus of 250 mcg of ACTH was then administered, followed by three simultaneous measurements of stimulated aldosterone and cortisol from the bilateral adrenal veins at five-minute intervals. A single stimulated aldosterone and cortisol measurement was obtained from the IVC at the end of the procedure. Proper placement of the catheters was visually confirmed using adrenal venography with or without cone-beam computed tomography as necessary. The same interventional radiologist conducted all procedures.
Aldosterone-to-cortisol ratios were calculated for each paired measurement from both adrenal veins in all participants in order to calculate the lateralization index. The lateralization index was defined as the mean of the three aldosterone-to-cortisol ratios from the dominant adrenal vein divided by the mean of the three aldosterone-to-cortisol ratios from the contralateral adrenal vein. Analyses were conducted using an unstimulated (pre-ACTH) lateralization index of ≥ 2 and ≥ 4 to define laterality, as international practices differ as to which threshold to use.13,14,29 We used a stimulated (post-ACTH) lateralization index of ≥ 4 to define laterality.
Measurements
Characteristics of the laboratory assays are previously described, with the coefficient of variation of the LC-MS/MS aldosterone assay used throughout the entire study period ranging from 2.7%−4.4% and the coefficient of variation of the cortisol immunoassay used throughout the study period ranging from 1.5%−5.4%.15
Study objectives and statistical analysis
The primary objective of the study was to compare unstimulated (pre-ACTH) and stimulated (post-ACTH) lateralization indices and the clinical discordance between the two, with the hypothesis that ACTH-stimulation could lower lateralization rates in many patients, and at minimum, would result in non-homogeneous and unpredictable changes in lateralization indices that could influence localization and subsequent clinical decision making. We also analyzed the demographic, clinical, biochemical, radiologic, and treatment outcome differences between the group that lateralized regardless of ACTH (the ‘Concordant group’) and the group that lateralized pre-ACTH but did not lateralize post-ACTH (the ‘Discordant group’ or the ‘Unilateral-to-Bilateral group’). Finally, we examined the patients who had already lateralized on the basis of unstimulated sampling and determined if the response to ACTH was associated with any improvement in treatment outcomes; the same analysis was also performed on patients who did not lateralize on unstimulated sampling.
The Wilcoxon matched-pairs signed-ranks test was used to assess differences between inter-individual lateralization indices. The median lateralization index was used for comparison instead of the mean due to a strongly positive skew from a few very high lateralization index values after ACTH. For normally-distributed continuous variables, the t-test was used to compare differences between two groups; for non-normally distributed continuous variables, as defined by an obvious skew when represented graphically, the non-parametric Wilcoxon rank-sum test was used to compare data between two groups. The chi-square test was used to compare categorical data between groups. Analyses were performed using STATA version 15 (College Station, TX).
Contemporary literature review
In addition to this original research analysis, we performed a review of contemporary studies which also examined the effect of ACTH-stimulated AVS on lateralization indices in patients with primary aldosteronism. The primary outcome of interest was lateralization rates of unstimulated sampling versus stimulated sampling; studies were excluded if participants did not undergo both stimulated and unstimulated AVS. We searched PubMed for “adrenal venous sampling and ACTH” and “adrenal venous sampling and cosyntropin” to identify relevant studies from the last 5 years to supplement the published studies already known to the authors. The references for each of the identified publications were then individually reviewed to identify other potentially relevant studies. Each study was evaluated for the number of patients, study design, and mode of ACTH stimulation (bolus, infusion, or both), and a summary of each study’s findings were organized in tabular format.
Results:
Characteristics of the study population are displayed in Table 1. The patients were predominantly male, middle-aged, white, and obese. Patients were hypertensive despite taking an average of 3 antihypertensive medications; those patients on 3 or more antihypertensive medications were more likely to be older, male, have a higher potassium, and derive more clinical benefit from unilateral intervention than patients on 2 or fewer antihypertensives. The median aldosterone-to-renin ratio in the entire cohort was significantly elevated and the average potassium at diagnosis was in the hypokalemic range; however, since many patients were given potassium supplementation after diagnosis, the average potassium on the day of AVS was 3.9 mEq/L. Eighty-two percent of patients had an unstimulated lateralization index (LI) of 2 or greater, 66% had an unstimulated LI of 4 or greater, and 61% had a stimulated LI of 4 or greater. Sixty-nine percent of patients underwent unilateral intervention with either surgical adrenalectomy or radiofrequency ablation; 83% of these patients achieved either complete or partial clinical success and 93% of these patients achieved either complete or partial biochemical success per the PASO study criteria.30 As expected, the selectivity index (SI) increased substantially with ACTH, with the median SI increasing from 2.4 to 61.8 in the left adrenal vein and from 3.2 to 66.0 in the right adrenal vein (p<0.001 for both comparisons)(Figure S1). Regardless of the threshold, stimulated sampling led to far higher rates of successful AVS than unstimulated sampling (Table S1).
Table 1. Characteristics of the study population.
Mean (SD) reported for parametric data. Median [IQR] reported for non-parametric data.
| Characteristic | Mean (SD) or Median [IQR] |
|---|---|
| Age – yrs | 53 (11) |
| Male sex - % | 64% |
| Race/Ethnicity | |
| White | 50% |
| Black | 19% |
| Asian | 6% |
| Other/Unknown | 25% |
| BMI – kg/m2 | 31 (6) |
| Systolic blood pressure – mmHg | 149 (21) |
| Diastolic blood pressure – mmHg | 87 (13) |
| Number of antihypertensive medications | 3 (1) |
| Potassium at diagnosis – mEq/L | 3.4 (0.5) |
| Potassium < 3.5 mEq/L at diagnosis | 58% |
| Potassium at AVS – mEq/L | 3.9 (0.7) |
| Plasma aldosterone concentration – ng/dL | 22.8 [16.7–34.0] |
| Plasma renin activity – ng/mL/h | 0.5 [0.2–0.6] |
| Aldosterone to renin ratio – (ng/dL)/(ng/mL/h) | 70 [35–160] |
| Cross-sectional imaging findings | |
| Normal adrenal glands | 16% |
| Unilateral adrenal nodule | 76% |
| Bilateral adrenal nodules | 8% |
| Unstimulated LI ≥ 2 - % | 82% |
| Unstimulated LI ≥ 4 - % | 66% |
| Stimulated LI ≥ 4 - % | 61% |
| Unilateral intervention - % | 69% |
| Complete or partial clinical success after unilateral intervention | 83% (64% partial, 19% complete) |
| Complete or partial biochemical success after unilateral intervention | 93% (6% partial, 87% complete) |
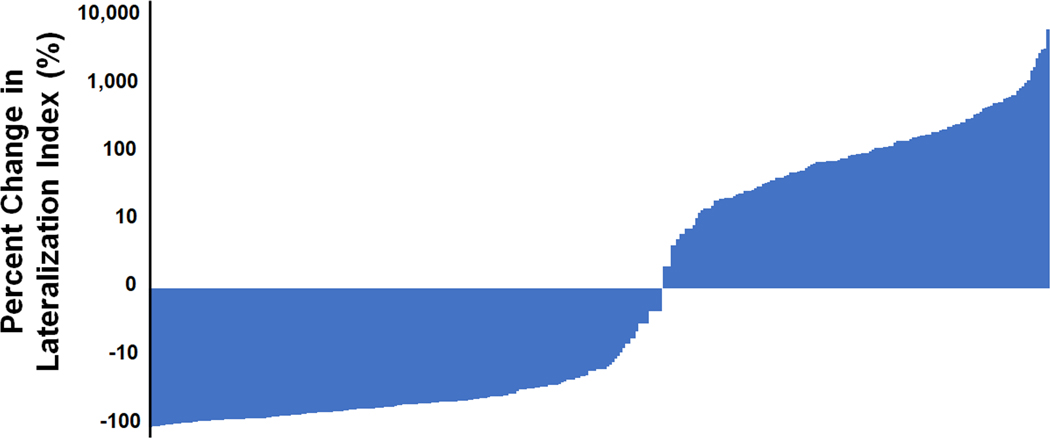
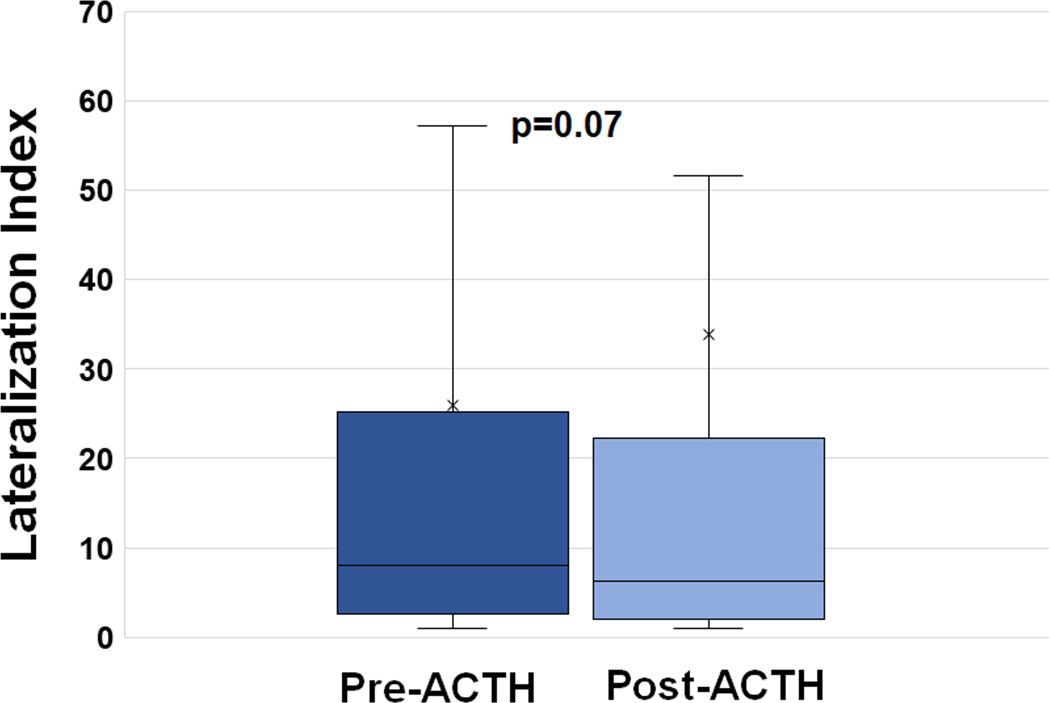
Fifty-eight percent (196/340) of patients had a decline in lateralization index following ACTH; the magnitude of this decrease ranged from 1% to 98% (Figure 1). In contrast, 144 patients (42%) had an increase in LI following ACTH; the magnitude of this increase ranged from 2% to 5585% (Figure 1). The median LI prior to ACTH was 8.0 compared to a median LI of 6.3 after ACTH (p=0.07) (Figure 2). The median intra-individual change in the lateralization index from baseline to stimulated sampling was an absolute decrease of 0.45 or a 14% percent decrease. The group with an increase in the lateralization index was more likely to be male and to have a higher BMI compared to the group with a decrease in lateralization index (Table S2). The group with an increase in LI following ACTH was also significantly less likely to lateralize on the basis of unstimulated sampling regardless of the threshold used (72% vs 89% for LI cut-off of 2; 57% vs 72% for LI cut-off of 4), but was significantly more likely to lateralize on the basis of stimulated sampling (73% vs 52%). Outcomes of surgical adrenalectomy and radiofrequency ablation were no different between these groups (Table S2).
Figure 1. Waterfall plot demonstrating the percent change in lateralization index following ACTH stimulation.
Each individual patient’s change in lateralization index following ACTH stimulation is represented on the x-axis in order of the magnitude of the change. The y-axis represents the log-scale of the percent change in the lateralization index following ACTH stimulation.
Figure 2. Baseline (pre-ACTH) vs Stimulated (post-ACTH) Lateralization Indices.
Box-and-whisker plot of the average pre-ACTH LI across all 340 patients compared to the average post-ACTH LI across all patients. Medians used for comparison given strongly positive skew, particularly with post-ACTH values.
When comparing a pre-ACTH lateralization index cut-off of ≥ 2 and a post-ACTH LI cut-off of ≥ 4, there was discordant lateralization in 26% of cases (Table 2A). Nearly all of this discordance (91%) was explained by unilateral disease per the unstimulated criteria that was reclassified as bilateral disease per the ACTH-stimulated criteria. Using a stricter unstimulated LI cut-off of ≥ 4 compared to the same ACTH-stimulated cut-off of ≥ 4, the discordance rate was 19%, with the majority of the discordance (64%) again explained by unilateral disease that was reinterpreted as bilateral disease after ACTH (Table 2B). We observed similar discordance rates when using other lateralization index thresholds (Table S3).
Table 2A.
Interpretations of AVS comparing an unstimulated lateralization index of ≥ 2 and a stimulated lateralization index of ≥ 4
| Laterality based on stimulated lateralization index of ≥ 4 | |||||
|---|---|---|---|---|---|
| Bilateral | Left | Right | Total | ||
| Laterality based on unstimulated lateralization index of ≥ 2 | Bilateral | 54 (15.9%) | 4 (1.2%) | 4 (1.2%) | 62 |
| Left | 31 (9.1%) | 93 (27.4%) | 0 | 124 | |
| Right | 49 (14.4%) | 0 | 105 (30.9%) | 154 | |
| Total | 134 | 97 | 109 | 340 | |
Table 2B.
Interpretations of AVS comparing an unstimulated lateralization index of ≥ 4 and a stimulated lateralization index of ≥ 4
| Laterality based on stimulated lateralization index of ≥ 4 | |||||
|---|---|---|---|---|---|
| Bilateral | Left | Right | Total | ||
| Laterality based on unstimulated lateralization index of ≥ 4 | Bilateral | 93 (27.4%) | 13 (3.8%) | 10 (2.9%) | 116 |
| Left | 18 (5.3%) | 84 (24.7%) | 0 | 102 | |
| Right | 23 (6.8%) | 0 | 99 (29.1%) | 122 | |
| Total | 134 | 97 | 109 | 340 | |
Table 2A compares lateralization as defined using an unstimulated (pre-ACTH) lateralization index cut-off of ≥ 2 (rows) with lateralization based on a stimulated (post-ACTH) lateralization index cut-off of ≥ 4 (columns). Table 2B presents similar data, but uses a pre-ACTH lateralization index cut-off of ≥ 4. Bolded and italicized cells represent discordant lateralization.
We then divided patients into two groups based on the pattern of their lateralization indices: Unilateral-to-Bilateral group (unstimulated LI ≥ 2 but a stimulated LI < 4) and Concordant group (unstimulated LI ≥ 2 and stimulated LI ≥ 4). There was a third group of only 8 patients with an unstimulated LI < 2 but a stimulated LI ≥ 4 (Bilateral-to-Unilateral group) who were not analyzed in this comparison due to small sample size. The Concordant group had a lower potassium level at diagnosis, higher ARR at diagnosis, and a trend toward a higher diastolic blood pressure compared to the Unilateral-to-Bilateral group (Table 3). The Concordant group was also significantly more likely to have a unilateral adenoma on imaging, undergo unilateral intervention, and achieve complete or partial biochemical cure from the unilateral intervention than the Unilateral-to-Bilateral group (Table 3). Similar trends were observed when comparing these two groups using an unstimulated LI cut-off of ≥ 4 (Table S4).
Table 3.
Comparison of characteristics in the Discordant or Unilateral-to-Bilateral group and Concordant groups.
| Characteristic | Discordant or Unilateral-to-Bilateral group (LI ≥ 2 pre-ACTH and LI < 4 post-ACTH) (n=80) | Concordant group (LI ≥ 2 pre-ACTH and LI ≥ 4 post- ACTH) (n=198) | P value |
|---|---|---|---|
| Age – years | 54 (11) | 53 (11) | 0.64 |
| Male sex – % | 56% | 69% | 0.05 |
| Race – % | 0.86 | ||
| White | 50% | 51% | |
| Black | 19% | 16% | |
| Asian | 5% | 8% | |
| Unknown/other | 26% | 26% | |
| BMI – kg/m2 | 31 (5) | 31 (7) | 0.84 |
| SBP at diagnosis – mmHg | 150 (28) | 150 (19) | 0.87 |
| DBP at diagnosis – mmHg | 84 (17) | 88 (12) | 0.06 |
| # of BP meds at diagnosis | 3 (1) | 3 (1) | 0.71 |
| K at diagnosis – mEq/L | 3.6 (0.6) | 3.4 (0.5) | 0.006 |
| Median aldo – ng/dL | 21.3 [14.3–30.5] | 23.9 [17.0–41.0] | 0.11 |
| Median PRA – ng/mL/h | 0.5 [0.2–0.6] | 0.4 [0.1–0.6] | 0.31 |
| Median ARR – [(ng/dL)/(ng/mL/h)] | 39.2 [26.7–115] | 85.3 [42.7–183] | 0.006 |
| Imaging findings – % | <0.001 | ||
| Normal adrenal glands | 32% | 4% | |
| Unilateral nodule | 58% | 90% | |
| Bilateral nodules | 10% | 6% | |
| Unstimulated LI ≥ 4 – % | 51% | 92% | <0.001 |
| Unilateral intervention -% | 49% | 90% | <0.001 |
| Complete or partial clinical success of unilateral intervention - % | 68% (68% partial, 0% complete) | 85% (61% partial, 24% complete) | 0.08 |
| Complete or partial biochemical success of unilateral intervention - % | 83% (8% partial, 75% complete) | 98% (6% partial, 92% complete) | 0.04 |
In our final original analysis, we analyzed patients who had already lateralized on the basis of unstimulated sampling and determined if the response to ACTH was associated with any improvement in treatment outcomes. In the groups who lateralized based on unstimulated LI thresholds of both 2 and 4, an increase in LI with ACTH was significantly correlated with higher rates of intervention, but there was no difference in the number of patients who achieved complete or partial clinical or biochemical success from the unilateral intervention (Tables S5A and S5B) nor were there any other notable differences in the biochemical or clinical severity of disease at diagnosis. Further, in the groups who had bilateral disease based on unstimulated LI thresholds below either 2 or 4, the administration of ACTH did not impact the type of intervention or outcome thereof, although there were few patients who underwent unilateral intervention if they did not lateralize based on unstimulated criteria (Tables S6A and S6B). Other demographic and diagnostic characteristics were also no different between groups.
In a contemporary literature review, the PubMed search of “Adrenal Venous Sampling and ACTH” yielded 86 studies from 2015-present and the search “Adrenal Venous Sampling and cosyntropin” yielded 37 studies in that time frame, many of them duplicates. Ten studies with titles and abstracts fitting our inclusion criteria were reviewed in detail and one additional study (El Ghorayeb et al)19 was added after in-depth review of the references for these 10 papers. In Table 4, we present a summary of these 11 studies from the past 5 years, plus the current study, which compared lateralization indices before and after ACTH. Note that AVS procedures were highly heterogeneous between studies, with some using simultaneous sampling whereas others used sequential sampling, and some using an ACTH bolus whereas others used an ACTH infusion preceded by a bolus or an ACTH infusion alone. Despite this study heterogeneity, a consistent finding that emerged was that on average, lateralization rates either did not change following ACTH or they decreased. Only one study showed that lateralization rates increased following ACTH, but this was only observed using an LI cut-off of 2 for both unstimulated and stimulated AVS and was not seen when using a cut-off of 4.31 Whereas one study found that post-ACTH LI correlated better with postoperative clinical outcomes,21 other studies, including our own, reported that post-adrenalectomy outcomes were not improved by ACTH.22,26
Table 4.
Summary of recent AVS studies comparing stimulated and unstimulated lateralization indices (LI).
| Patient characteristics | Type of study | AVS procedure | Finding | |
|---|---|---|---|---|
| Yozamp et al, 2021 | 340 patients | Retrospective cohort | - Simultaneous sampling - ACTH bolus |
- LI decreased in 58% after ACTH - Most discordance driven by Unilateral-to-Bilateral group after ACTH - Concordant unilateral group had more biochemically severe disease - Response to ACTH not associated with improved outcomes |
| Sung et al, 202031 | 76 patients, all of whom had adrenalectomy | Retrospective cohort | - Simultaneous sampling - ACTH bolus followed by infusion |
- Mean LI increased with ACTH - 9.2% in Unilateral-to-Bilateral group after ACTH (using LI cut-off of 4 for both) |
| Chee et al, 202018 | 201 patients | Retrospective | - Sequential sampling - ACTH bolus followed by infusion |
- Significant decrease in LI after ACTH - Lateralization rates decreased with ACTH (70% vs 54% using LI cut-offs of 3 pre- and 4 post-ACTH) |
| Yatabe et al, 202021 | 185 patients, 81 of whom had adrenalectomy for unilateral disease | Retrospective | - Sequential sampling - ACTH bolus only |
- Significant decrease in LI after ACTH - Lateralization rates decreased after ACTH (72% pre vs 36% post using LI cut-offs of 2 and 2.6 respectively) - Post-ACTH LI correlated better with postop blood pressure |
| Kobayashi et al, 202036 | 1854 patients, 314 of whom underwent adrenalectomy | Retrospective cohort | - Sequential sampling - ACTH bolus only, bolus + infusion, or infusion only |
- Lateralization discordance rates of 40% in total cohort and 22% in adrenalectomy cohort, driven by Unilateral-to-Bilateral (U to B) group after ACTH - U to B group had poorer outcomes compared to consistent unilateral group |
| Rossitto et al, 202022 | 1625 individual AVS studies across 19 centers; 402 patients had both stimulated and unstimulated AVS | Retrospective | - Roughly half of centers with sequential sampling, half with simultaneous - ACTH infusion |
- ACTH reduced LI and lateralization rates - Post-adrenalectomy outcomes not improved by ACTH |
| Violari et al, 201942 | 32 patients, all of whom had adrenalectomy | Retrospective | - ACTH bolus followed by infusion | - 22% lateralized pre-ACTH but not post-ACTH (using LI cut-offs of 4 for both) |
| Wannachalee et al, 201920 | 222 patients | Retrospective | - Simultaneous - ACTH bolus only |
- LI increased in 27%, decreased in 33%, and remained stable in 40% after ACTH - Discordant lateralization in 24% of cases, driven by U to B group and those with milder disease - KCNJ5 mutations associated with decreasing LI after ACTH - ATP1A1 and ATP2B3 mutations associated with increasing LI |
| Takeda et al, 201926 | 1425 patients with successful AVS from 28 sites | Retrospective | - Varied | - Decrease in lateralization rates (62% pre vs 28% post, using cut-offs of 2 and 4) - ACTH did not contribute to better outcomes |
| Laurent et al, 201816 | 14 studies | Meta-analysis | - Varied | - AVS with ACTH did not change lateralization |
| El Ghorayeb et al, 201619 | 171 successful AVS procedures | Retrospective cohort | - Simultaneous - ACTH bolus only |
- Discordant lateralization in 28%, mostly U to B group after ACTH - LI decreased in 53%; median LI 7 pre-ACTH vs 6.7 post-ACTH |
| Wolley et al, 201635 | 47 AVS procedures | Retrospective | - Sequential - ACTH bolus only |
- 9% discordance rate pre- and post-ACTH |
Discussion:
The use of ACTH-stimulation during AVS is a well-established maneuver to increase the selectivity index and confirm cannulation of the adrenal veins; however, there is a lack of consensus on the use of ACTH-stimulation to assess for lateralization.5,14,17,28 In this large retrospective cohort study of patients with primary aldosteronism who underwent AVS both before and after a single bolus of ACTH, 58% of patients had a decrease in the post-ACTH lateralization index that resulted in discordant lateralization in 19–26% of cases depending on the unstimulated lateralization index cut-off. The vast majority of these discordant cases were due to reclassification from unilateral disease pre-ACTH to bilateral disease post-ACTH. Although patients with concordant lateralization pre- and post-ACTH had evidence of more biochemically severe primary aldosteronism and responded better to unilateral intervention than those with discordant lateralization, the response to ACTH (i.e., increase or decrease) was not associated with better outcomes when compared to lateralization or non-lateralization based on unstimulated sampling alone. These findings underscore results from other studies in recent years that have overwhelmingly found lower or unchanged lateralization rates with ACTH-stimulated AVS.
For years, AVS has been the procedure of choice to guide surgical candidacy for primary aldosteronism. Current guidelines recommend AVS in all patients with primary aldosteronism in whom surgery is being considered, with the possible exception of patients younger than age 35 with severe primary aldosteronism and a clear unilateral adrenal adenoma on imaging.5 Yet, the procedure itself is technically challenging and is not standardized, even across high-volume centers of expertise. For instance, in the AVIS trial published in 2012, Rossi and colleagues reported that among twenty major hypertension referral centers worldwide, 13 out of 20 used sequential catheterization of the adrenal veins while 7 used bilaterally simultaneous catheterization and 11 centers used ACTH stimulation.29 In centers that use ACTH stimulation, some use a bolus only, others use a continuous infusion, and still others use a bolus followed by an infusion.17 Although some centers (Table 4) obtain cortisol and aldosterone values before and after ACTH administration, others obtain values only after administration of ACTH.32 Further, the cut-offs used to determine adrenal vein selectivity and lateralization are heterogeneous and differ depending on whether they are calculated with or without ACTH stimulation.14,29 These limitations of AVS have prompted investigation of other modalities, such as CT alone or the response of peripheral aldosterone levels to ACTH stimulation, to ascertain laterality in primary aldosteronism, but thus far outcomes have been inferior compared to AVS.33,34
Although the ability of ACTH to enhance successful adrenal vein catheterization (selectivity) is well-established and also confirmed in our study,18,19,21–27,31,35 the role of ACTH in determining lateralization is more controversial. Compared to unstimulated sampling, several studies have reported an overall decrease in lateralization index values following ACTH,18,21,22,24–27 others have reported no net difference,19,20,23 while one has reported net increases in the lateralization index.31 In the many studies reporting an overall decrease in LI following ACTH, this corresponded to a significant decrease in actual lateralization rates compared to unstimulated values,18,21,22,25–27 such that there can be as little as 9% and up to 40% discordance between unstimulated and stimulated lateralization rates.18–21,26,35,36 This decrease in lateralization rates with ACTH is seen regardless of the mode of ACTH administration. Data regarding the effect of ACTH on clinical outcomes are mixed, with some studies reporting no improvement of post-adrenalectomy outcomes with ACTH22,26 while one found that the post-ACTH lateralization index correlated better with positive postoperative clinical outcomes than the unstimulated LI.21
There are several proposed mechanisms that may account for this variability in the lateralization index following ACTH. Even in patients with primary aldosteronism, high doses of ACTH stimulate production of aldosterone from normal adrenocortical cells, which may falsely lower the stimulated lateralization index in a patient with mild primary aldosteronism.37 Conversely, some patients with an aldosterone-producing adenoma may demonstrate a robust increase in aldosterone secretion and hence lateralization index following ACTH, which may be attributed to differential expression of melanocortin type 2 receptors (MC2R).37,38 The response of cortisol itself to ACTH may also alter the lateralization index as many patients with primary aldosteronism demonstrate co-secretion of cortisol and other glucocorticoid metabolites.39 Next-generation sequencing of adrenal tissue has afforded further insights into the molecular drivers of the differential response to ACTH in primary aldosteronism. Wannachalee and others recently reported that patients with a mutation in KCNJ5 had a higher baseline lateralization index that declined with ACTH stimulation.20 On the other hand, patients with mutations in ATP1A1 and ATP2B3 tended to have an increase in lateralization index after ACTH. More study is needed to further elucidate the other mechanisms that drive differential responses of the lateralization index to ACTH.
The primary concern regarding the decrease in lateralization rates observed by numerous studies is that unilateral primary aldosteronism may be underestimated, particularly in patients with milder disease, resulting in fewer referrals for potentially curative unilateral intervention. This study and others have shown no improvement in outcomes when surgery was performed on the basis of ACTH-stimulated lateralization indices compared to unstimulated values alone.16,22 Based on unstimulated and stimulated discordance rates, upwards of 1/3 fewer patients would be eligible for unilateral intervention if relying solely on the post-ACTH lateralization index.21,36 Surgical adrenalectomy is the only potentially curative procedure for primary aldosteronism and appears to have superior cardiovascular, metabolic, renal, and quality of life outcomes compared to medical therapy.6–13,40 So as not to exclude any patients who might benefit from surgery, we underscore the recommendation that ACTH stimulation in the context of AVS be limited primarily to determination of the selectivity index, and that the lateralization index be primarily calculated on the basis of pre-ACTH or unstimulated sampling.
One limitation of this study is that ACTH was administered as a bolus, and not as an infusion, although sampling was performed within minutes and it is reassuring that other studies have reported similar results when ACTH was given as an infusion and when AVS was done sequentially instead of simultaneously from both adrenal veins. A second limitation is that not all patients in our cohort with lateralizing disease underwent unilateral intervention, nor did every patient who underwent unilateral intervention have available outcome data. However, the high number of patients who underwent unilateral intervention and did have outcome data was sufficient to draw the conclusion that postoperative clinical and biochemical outcomes per the PASO study criteria were no different when post-ACTH lateralization indices were compared to pre-ACTH lateralization indices. A third limitation is that we were not able to conduct histopathologic, molecular, or genetic analyses on surgical samples to better understand the factors that may determine ACTH responses. Finally, our results may not be generalizable to the entire primary aldosteronism population at large, given the very small percentage of patients with severe enough disease that underwent screening, confirmation of the diagnosis, and adrenal venous sampling – all of which had to be satisfied for inclusion in the study. Indeed, the percentage of patients herein who lateralized based on unstimulated criteria and underwent unilateral intervention is relatively high compared to reported prevalence rates of patients with bilateral (~60%) and unilateral (~40%) disease.41
Perspectives:
AVS with administration of an ACTH bolus resulted in 58% of patients with primary aldosteronism experiencing a decline in lateralization index. This decrease in lateralization indices created a discordance between pre-ACTH and post-ACTH lateralization in up to 26% of patients, with up to 91% of this discordance explained by unilateral disease being reclassified as bilateral after ACTH. Although patients who lateralized on the basis of both stimulated and unstimulated criteria had more severe primary aldosteronism and appeared to benefit more from surgery compared to patients who lateralized before ACTH but failed to lateralize after ACTH, the response to ACTH did not impart any diagnostic or prognostic benefit compared to lateralization or lack thereof calculated on the basis of unstimulated sampling alone. Our study adds to the growing body of literature that AVS with ACTH-stimulation decreases lateralization rates in primary aldosteronism, which may lead to fewer patients being referred for potentially curative surgery. In conclusion, we recommend that ACTH be used primarily to enhance selectivity of AVS for primary aldosteronism whereas unstimulated sampling should be used primarily to calculate laterality.
Supplementary Material
Novelty and Significance:
What is New?
We compared unstimulated (pre-ACTH) and stimulated (post-ACTH) lateralization rates in patients with primary aldosteronism who underwent adrenal venous sampling (AVS) and reviewed the findings of recent similar studies since the use of ACTH to determine lateralization remains common practice
What is Relevant?
58% of patients had a decrease in the lateralization index following ACTH-stimulation
Pre- and post-ACTH lateralization rates differed by up to 26%, which was overwhelmingly driven by patients with unilateral disease pre-ACTH who failed to lateralize after ACTH
The post-ACTH lateralization index did not add meaningful diagnostic or prognostic information beyond the pre-ACTH lateralization index
10 of 11 recent similar studies also demonstrated either no change or a decrease in lateralization rates following ACTH
Acknowledgements:
We thank the interventional radiology staff that assisted with adrenal venous sampling protocols and clinical research center staff that assisted with specimen processing and storage.
Sources of funding: We thank our funding sources for supporting this work: R01 DK115392 (AV), R01 HL153004 (AV), R01 DK16618 (AV), and 2T32 HL007609-32 (NY).
Footnotes
Disclosures: AV reports consulting fees unrelated to the contents of this work from Corcept Therapeutics, Mineralys, HRA Pharma.
References
- 1.Vaidya A, Mulatero P, Baudrand R, Adler GK. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr Rev. 2018;39(6):1057–1088. doi: 10.1210/er.2018-00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–1820. doi: 10.1016/j.jacc.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 3.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J Am Coll Cardiol. 2006;48(11):2293–2300. doi: 10.1016/j.jacc.2006.07.059 [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020;173(1):10–20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 6.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic Outcomes and Mortality in Medically Treated Primary Aldosteronism: A Retrospective Cohort Study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hundemer Gregory L., Curhan Gary C., Yozamp Nicholas, Wang Molin, Vaidya Anand. Renal Outcomes in Medically and Surgically Treated Primary Aldosteronism. Hypertension. 2018;72(3):658–666. doi: 10.1161/HYPERTENSIONAHA.118.11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol. 2018;3(8):768–774. doi: 10.1001/jamacardio.2018.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indra T, Holaj R, Štrauch B, Rosa J, Petrák O, Šomlóová Z, Widimský J. Long-term effects of adrenalectomy or spironolactone on blood pressure control and regression of left ventricle hypertrophy in patients with primary aldosteronism. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2015;16(4):1109–1117. doi: 10.1177/1470320314549220 [DOI] [PubMed] [Google Scholar]
- 10.Kline GA, Pasieka JL, Harvey A, So B, Dias VC. Medical or Surgical Therapy for Primary Aldosteronism: Post-treatment Follow-up as a Surrogate Measure of Comparative Outcomes. Ann Surg Oncol. 2013;20(7):2274–2278. doi: 10.1245/s10434-013-2871-3 [DOI] [PubMed] [Google Scholar]
- 11.Meng X, Ma W-J, Jiang X-J, Lu P-P, Zhang Y, Fan P, Cai J, Zhang H-M, Song L, Wu H-Y, Zhou X-L, Lou Y. Long-term blood pressure outcomes of patients with adrenal venous sampling-proven unilateral primary aldosteronism. J Hum Hypertens. Published online September 5, 2019. doi: 10.1038/s41371-019-0241-8 [DOI] [PubMed] [Google Scholar]
- 12.Velema M, Dekkers T, Hermus A, Timmers H, Lenders J, Groenewoud H, Schultze Kool L, Langenhuijsen J, Prejbisz A, van der Wilt G-J, Deinum J. Quality of Life in Primary Aldosteronism: A Comparative Effectiveness Study of Adrenalectomy and Medical Treatment. J Clin Endocrinol Metab. 2018;103(1):16–24. doi: 10.1210/jc.2017-01442 [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, et al. Clinical Outcomes of 1625 Patients With Primary Aldosteronism Subtyped With Adrenal Vein Sampling. Hypertens Dallas Tex 1979. 2019;74(4):800–808. doi: 10.1161/HYPERTENSIONAHA.119.13463 [DOI] [PubMed] [Google Scholar]
- 14.Rossi GP, Auchus RJ, Brown M, Lenders JWM, Naruse M, Plouin PF, Satoh F, Young WF. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertens Dallas Tex 1979. 2014;63(1):151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097 [DOI] [PubMed] [Google Scholar]
- 15.Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Variability of Aldosterone Measurements During Adrenal Venous Sampling for Primary Aldosteronism. Am J Hypertens. Published online November 12, 2020. doi: 10.1093/ajh/hpaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent I, Astère M, Zheng F, Chen X, Yang J, Cheng Q, Li Q. Adrenal venous sampling with or without adrenocorticotropic hormone stimulation: A meta-analysis. J Clin Endocrinol Metab. Published online November 6, 2018. doi: 10.1210/jc.2018-01324 [DOI] [PubMed] [Google Scholar]
- 17.Turcu AF, Auchus R. Approach to the Patient with Primary Aldosteronism: Utility and Limitations of Adrenal Vein Sampling. J Clin Endocrinol Metab. Published online December 31, 2020. doi: 10.1210/clinem/dgaa952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chee NYN, Abdul-Wahab A, Libianto R, Gwini SM, Doery JCG, Choy KW, Chong W, Lau KK, Lam Q, MacIsaac RJ, Chiang C, Shen J, Young MJ, Fuller PJ, Yang J. Utility of adrenocorticotropic hormone in adrenal vein sampling despite the occurrence of discordant lateralization. Clin Endocrinol (Oxf). 2020;93(4):394–403. doi: 10.1111/cen.14220 [DOI] [PubMed] [Google Scholar]
- 19.El Ghorayeb N, Mazzuco TL, Bourdeau I, Mailhot J-P, Zhu PS, Thérasse E, Lacroix A. Basal and Post-ACTH Aldosterone and Its Ratios Are Useful During Adrenal Vein Sampling in Primary Aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826–1835. doi: 10.1210/jc.2015-3915 [DOI] [PubMed] [Google Scholar]
- 20.Wannachalee T, Zhao L, Nanba K, Nanba AT, Shields JJ, Rainey WE, Auchus RJ, Turcu AF. Three Discrete Patterns of Primary Aldosteronism Lateralization in Response to Cosyntropin During Adrenal Vein Sampling. J Clin Endocrinol Metab. 2019;104(12):5867–5876. doi: 10.1210/jc.2019-01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatabe M, Bokuda K, Yamashita K, Morimoto S, Yatabe J, Seki Y, Watanabe D, Morita S, Sakai S, Ichihara A. Cosyntropin stimulation in adrenal vein sampling improves the judgment of successful adrenal vein catheterization and outcome prediction for primary aldosteronism. Hypertens Res Off J Jpn Soc Hypertens. 2020;43(10):1105–1112. doi: 10.1038/s41440-020-0445-x [DOI] [PubMed] [Google Scholar]
- 22.Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Schultzekool L, et al. Subtyping of Primary Aldosteronism in the AVIS-2 Study: Assessment of Selectivity and Lateralization. J Clin Endocrinol Metab. 2020;105(6). doi: 10.1210/clinem/dgz017 [DOI] [PubMed] [Google Scholar]
- 23.Monticone S, Satoh F, Giacchetti G, Viola A, Morimoto R, Kudo M, Iwakura Y, Ono Y, Turchi F, Paci E, Veglio F, Boscaro M, Rainey W, Ito S, Mulatero P. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertens Dallas Tex 1979. 2012;59(4):840–846. doi: 10.1161/HYPERTENSIONAHA.111.189548 [DOI] [PubMed] [Google Scholar]
- 24.Rossi GP, Ganzaroli C, Miotto D, De Toni R, Palumbo G, Feltrin GP, Mantero F, Pessina AC. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens. 2006;24(2):371–379. doi: 10.1097/01.hjh.0000202818.10459.96 [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008;26(5):989–997. doi: 10.1097/HJH.0b013e3282f9e66a [DOI] [PubMed] [Google Scholar]
- 26.Takeda Y, Umakoshi H, Takeda Y, Yoneda T, Kurihara I, Katabami T, Ichijo T, Wada N, Yoshimoto T, Ogawa Y, et al. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J Hypertens. 2019;37(5):1077–1082. doi: 10.1097/HJH.0000000000001964 [DOI] [PubMed] [Google Scholar]
- 27.Seccia Teresa M, Miotto Diego, De Toni Renzo, Pitter Gisella, Mantero Franco, Pessina Achille C., Rossi Gian Paolo. Adrenocorticotropic Hormone Stimulation During Adrenal Vein Sampling for Identifying Surgically Curable Subtypes of Primary Aldosteronism. Hypertension. 2009;53(5):761–766. doi: 10.1161/HYPERTENSIONAHA.108.128553 [DOI] [PubMed] [Google Scholar]
- 28.Young WF. Diagnosis of primary aldosteronism. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA, 2021 [Google Scholar]
- 29.Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97(5):1606–1614. doi: 10.1210/jc.2011-2830 [DOI] [PubMed] [Google Scholar]
- 30.Williams TA, Lenders JW, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, et al. Outcome of adrenalectomy for unilateral primary aldosteronism: International consensus and remission rates. Lancet Diabetes Endocrinol. 2017;5(9):689–699. doi: 10.1016/S2213-8587(17)30135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung T-Y, Alobuia WM, Tyagi MV, Ghosh C, Kebebew E. Adrenal Vein Sampling to Distinguish Between Unilateral and Bilateral Primary Hyperaldosteronism: To ACTH Stimulate or Not? J Clin Med. 2020;9(5). doi: 10.3390/jcm9051447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf). 2009;70(1):14–17. doi: 10.1111/j.1365-2265.2008.03450.x [DOI] [PubMed] [Google Scholar]
- 33.Dekkers T, Prejbisz A, Kool LJS, Groenewoud HJMM, Velema M, Spiering W, Kołodziejczyk-Kruk S, Arntz M, Kądziela J, Langenhuijsen JF, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739–746. doi: 10.1016/S2213-8587(16)30100-0 [DOI] [PubMed] [Google Scholar]
- 34.St-Jean M, Bourdeau I, Therasse É, Lacroix A. Use of peripheral plasma aldosterone concentration and response to ACTH during simultaneous bilateral adrenal veins sampling to predict the source of aldosterone secretion in primary aldosteronism. Clin Endocrinol (Oxf). 2020;92(3):187–195. doi: 10.1111/cen.14137 [DOI] [PubMed] [Google Scholar]
- 35.Wolley MJ, Ahmed AH, Gordon RD, Stowasser M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin Endocrinol (Oxf). 2016;85(5):703–709. doi: 10.1111/cen.13110 [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi H, Nakamura Y, Abe M, Kurihara I, Itoh H, Ichijo T, Takeda Y, Yoneda T, Katabami T, Tsuiki M, et al. Effect of cosyntropin during adrenal venous sampling on subtype of primary aldosteronism: analysis of surgical outcome. Eur J Endocrinol. 2020;182(3):265–273. doi: 10.1530/EJE-19-0860 [DOI] [PubMed] [Google Scholar]
- 37.El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and Other Hormones in the Regulation of Aldosterone Production in Primary Aldosteronism. Front Endocrinol. 2016;7:72. doi: 10.3389/fendo.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim JS, Plaska SW, Rege J, Rainey WE, Turcu AF. Aldosterone-Regulating Receptors and Aldosterone-Driver Somatic Mutations. Front Endocrinol. 2021;12:644382. doi: 10.3389/fendo.2021.644382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8). doi: 10.1172/jci.insight.93136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hundemer GL, Vaidya A. MANAGEMENT OF ENDOCRINE DISEASE: The role of surgical adrenalectomy in primary aldosteronism. Eur J Endocrinol. 2020;183(6):R185–R196. doi: 10.1530/EJE-20-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. doi: 10.1111/j.1365-2265.2007.02775.x [DOI] [PubMed] [Google Scholar]
- 42.Violari EG, Arici M, Singh CK, Caetano CM, Georgiades CS, Grady J, Tendler BR, Shichman SJ, Malchoff CD. Adrenal vein sampling with and without cosyntropin stimulation for detection of surgically remediable aldosteronism. Endocrinol Diabetes Metab. 2019;2(2):e00066. doi: 10.1002/edm2.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.