Abstract
To facilitate analyses of purinergic signaling in peripheral nerve glia, we review recent literature and catalog purinergic receptor mRNA expression in cultured mouse Schwann cells (SCs). Purinergic signaling can decrease developmental SC proliferation, and promote SC differentiation. The purinergic receptors P2RY2 and P2RX7 are implicated in nerve development and in the ratio of Remak SCs to myelinating SCs in differentiated peripheral nerve. P2RY2, P2RX7, and other receptors are also implicated in peripheral neuropathies and SC tumors. In SC tumors lacking the tumor suppressor NF1, the SC pathway that suppresses SC growth through P2RY2‐driven β‐arrestin‐mediated AKT signaling is aberrant. SC‐released purinergic agonists acting through SC and/or neuronal purinergic receptors activate pain responses. In all these settings, purinergic receptor activation can result in calcium‐independent and calcium‐dependent release of SC ATP and UDP, growth factors, and cytokines that may contribute to disease and nerve repair. Thus, current research suggests that purinergic agonists and/or antagonists might have the potential to modulate peripheral glia function in development and in disease.
Keywords: arrestin, ATP, glia, NF1, pain, PNS, purinergic signaling, Schwann cells
MAIN POINTS
SC purinergic receptors are implicated in suppressing SC proliferation, increasing SC differentiation, and elevating intracellular Ca2+ during nerve development in response to injury and/or in disease.
P1R, P2XR, and P2YR receptors expressed in SCs and adjacent neurons need further study.

1. INTRODUCTION
Throughout the body, purinergic signaling is ubiquitous. The term “purinergic signaling”, coined by Burnstock (Burnstock, 1972; Burnstock, Campbell, Satchell, & Smythe, 1970) refers to purines, notably adenosine triphosphate (ATP) and its breakdown products adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine, that act outside cells. The extracellular pyrimidines, which also signal through purinergic receptors, include uridine triphosphate (UTP), uridine diphosphate (UDP), and UDP‐conjugated sugars, and also act outside cells. There are several excellent reviews of nucleotide release from cells in vivo and in vitro (Burnstock, 2014; Di Virgilio & Adinolfi, 2017; Giuliani, Sarti, & Di Virgilio, 2019; Zimmermann, 2011). When released from cells, each of these ligands binds to cell surface receptors. The identification and characterization of families of purinergic receptors in the 1990s identified three major families of receptors, described below, which, upon activation by these ligands, initiate intracellular signaling responses.
Extracellular ATP which is not rapidly bound to a cell surface receptor is broken down by cell‐surface enzymes called ectonucleotidases. Degradation of ATP produces mainly ADP and adenosine, each of which activates specific purinergic receptors. The extracellular pyrimidine UTP is also released by cells and is rapidly hydrolyzed into UDP. UDP‐sugars are more stable, and more slowly degraded by ectonucleotide pyrophosphatases. This suggests that UDP‐sugars may accumulate extracellularly affecting cell function for prolonged periods (Lazarowski & Harden, 2015). The contributions of specific purinergic signaling to tissue homeostasis and dysregulation of purinergic signaling in disease is under intense investigation.
To appreciate the following discussion of purinergic signaling in the nerve, it is important to understand that during development, beginning in mid‐gestation, neural crest‐derived SC precursors (SCP) and boundary cap cells progressively differentiate into diverse peripheral nervous system glial cell types: satellite glial cells (SGC) surrounding neuronal cell bodies in peripheral and cranial ganglia; SCs associated with peripheral and cranial nerve axons; and perisynaptic SC present at neuromuscular junctions (Sanes & Lichtman, 2001). In the perinatal period, immature nerve SCs that associate with large diameter axons differentiate into myelinating SCs. In contrast, SCs that associate with a group of up to 20 small‐caliber axons differentiate into non‐myelinating SCs (Monk, Feltri, & Taveggia, 2015). Purinergic signaling is of special relevance in the peripheral nervous system (PNS), as ATP, UTP, and UDP‐sugars are released from synaptic vesicles in a Ca2+ dependent manner and along axons by non‐vesicular mechanisms: via maxi‐anion channels, pannexins, and/or connexins (Burnstock, 1972; Burnstock et al., 1970; Fields & Ni, 2010; Jung, Jo, Kwon, & Jeong, 2014). Therefore, purinergic signaling is being studied in epochs of SC development, after injury, and in disease settings.
Upon electrical stimulation of peripheral nerve axons, ATP is released along the length of axons, via maxi‐anion channels. ATP and its breakdown products activate receptors on nearby SCs (Fields & Ni, 2010; Jung et al., 2014)(Figure 4a). It is clear that SCs actively participate in purinergic signaling by responding to extracellular purines and pyrimidines and modulating cell growth and differentiation, and by the release of cytokines and growth factors, and release of additional purinergic agonists (Liu, Werry, & Bennett, 2005; Nualart‐Marti et al., 2013).
FIGURE 4.

Effects of purinergic agonists on peripheral glial cells. (a) Upon electrical stimulation, neurons release ATP which activates purinergic receptors in SCs. Activation of purinergic receptors by ATP or UTP, UDP, ADP and/or adenosine regulates SC proliferation, myelination, growth, migration and cytokine release. (b) In the tumor microenvironment, ATP, adenosine and UTP are present and regulate many aspects of tumor development. (c) Purinergic receptors are also involved in nerve damage and pain. After nerve damage, injury, or disease, ATP is released from dying cells. Purinergic signaling is involved in many aspects of pain pathology including mechanical and thermal hyperalgesia and allodynia; there may be additional purinergic receptors involved in pain, which are not shown here
The roles of extracellular purines in glial homeostasis in the brain are the subject of excellent reviews (Burnstock, 2014; Fields, 2015; Zuchero & Barres, 2015). In contrast, the purinergic receptors expressed in specific types of SCs and the pathways through which individual purinergic receptors signal in peripheral glial cells remain poorly studied and it remains unclear whether the same spectrum of purinergic ligands and receptors are present or functional in each species. Here we focus on the PNS, to organize what is known in this area, and to focus on confounding variables that limit interpretation of some studies. A common theme is that purinergic stimulation results in calcium entry into cells (through P2X receptors) and causes stimulated cells to mobilize calcium from intracellular stores (through P2X and P2Y receptors); this results in the release of a variety of factors including purines, cytokines, and growth factors that affect nerve glial cell development and function.
1.1. Purinergic receptors overview
There are two major families of purinergic receptors: P1 and P2 receptors (Burnstock, 2014; Burnstock & Verkhratsky, 2012) (Figure 1). P2X receptors are ionotropic, while P1 and P2Y include a diverse set of G‐protein coupled receptors (GPCR). To summarize purinergic receptor expression in SCs, we performed qPCR. Cultured mouse SCs expressed detectable mRNA encoding 3 of 4 P1R, and 6 of 7 P2RX, and 6 of 7 P2RY (Figure 2). Given this diversity of purinergic receptors expressed in SCs, it will be important to understand each receptor's overlapping and unique functions.
FIGURE 1.

Receptor‐mediated purinergic signaling. (a) P1 receptors (A1, A3, A2A, A2B) are activated by adenosine and signal through Gi, Gs, Gq, leading to downstream activation of diverse signaling cascades. Subsequently, β‐arrestins both diminish this signaling and activate alternative signaling. (b) P2X receptors (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7) are activated by ATP and, upon stimulation, cause an inward flux of Na+ and Ca+2 ions, and an outward flow of cellular K+. (c) P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) are activated by subsets of ADP, UTP, UDP, ATP, and UDP‐glucose and signal through Gi, Gs, and Gq, and use β‐arrestins to both diminish this signaling and activate alternative signaling cascades
FIGURE 2.
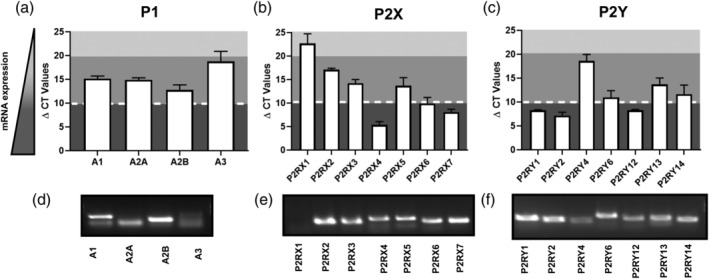
Relative levels of purinergic receptor mRNAs in mouse SC. mRNA expression levels in wildtype (WT) embryonic day (E12.5)‐derived cultured mouse SCs are expressed as ΔCT ± SD. Cells were plated as neuron/Schwann cell co‐cultures in serum‐free N2 medium with nerve growth factor, then removed from neurons and cultured in DMEM +10% FBS + 10 ng/ml β‐heregulin +2uM forskolin and used within 2 passages for RNA isolation (Kim, Rosenbaum, Marchionni, Ratner & DeClue, 1995). Color gradient: Dark gray: high mRNA levels of expression; medium gray: moderate mRNA expression detected; light gray: undetectable. (a,d) P1 receptor expression in WT mouse SCs. Receptors A1, A2A, and A2B are moderately expressed in WT SCs, while A3 expression is low. (b,e) P2X receptor expression in WT mouse SCs. Receptors P2RX4, P2RX6, and P2RX7 are highly expressed; P2RX2, P2RX3, and P2RX5 are expressed at moderate levels, and P2RX1 is undetectable. (c,f) P2Y receptor expression in WT SCs. P2RY1, P2RY2, and P2RY12 are highly expressed in SCs; P2RY6, P2RY13, and P2RY14 are expressed at moderate levels in SCs
2. P2X RECEPTOR EXPRESSION AND FUNCTION
There are seven P2X receptors: P2RX1, P2RX2, P2RX3, P2RX4, P2RX5, P2RX6, and P2RX7, all of which are ligand (ATP)‐gated ion channels (Table 1). ATP‐stimulated P2RX activation causes an inward flux of Na+ and Ca2+ ions, and an outward flow of cellular K+ (Figure 1b). Elevation of intracellular Ca2+ after P2XR activation regulates intracellular signaling, including neurotransmitter release (Burnstock & Verkhratsky, 2012; Faroni et al., 2013). P2RX receptors can also affect cell migration (Giannuzzo, 2015; Gong, 2019; Park, 2019) and importantly, cytokine release (Burnstock, 2016; Gabel, 2007). The release of cytokines downstream of ATP‐stimulated P2RX activation and resulting calcium entry into cells is important in SCs, which synthesize and release numerous growth factors, trophic factors, and cytokines that regulate nerve homeostasis. At very high levels of ATP, activation of P2XR can also cause SC death (Faroni et al., 2013).
TABLE 1.
Major signaling mechanisms of purinergic receptors and their respective agonists and antagonists
| Receptor | Endogenous agonist | Receptor type | Agonists | Antagonist |
|---|---|---|---|---|
| A 1 | Adenosine | GPCR | CCPA; CPA; (S)‐ENBA; NECA; Methylthioadenosine; N6‐Cyclohexyladenosine; MLN4924; SDZ WAG 994; 2’‐MeCCPA; CVT‐510; GR79236; Ino‐8875; MRS 5474. | WRC‐0571; PSB‐36; DPCPX; 8‐Cyclopentyl‐1,3‐dimethylxanthine; N‐0840; MRS1754; SLV320; PQ‐69 |
| A 2A | CGS 21680; CV‐1808; UK‐432097; NECA; PBS 0777 ammonium salt; Regadenoson; Apadenoson | ZM 241385; SCH442416; ANR 94; 8‐(3‐Chlorostyryl) caffeine; Istradefylline; Scheme 58261; TC‐G1004 | ||
| A 2B | BAY 60‐6583; CV‐1808; NECA; LUF 5834 | MRS1754; GS 6201; PSB 0788; PSB 1115; PSB 603; MRE‐2029‐F20; Alloxazine | ||
| A 3 | CI‐IB‐MECA; MRS5698; PHPENECA; NECA; DBXRM; HEMADO | MRS1523; MRS1191; MRS1220; VUF8504; VUF5574; MRS1334; PSB 10 hydrochloride; OT 7999; MRS1292; MRS3777 hemioxalate; (3)H]MRE 3008F20; LJ‐1888 | ||
| P2X1 | ATP | Ligand‐gated ion channel | a‐bMeATP; 2MesATP; BzATP; L‐βγ‐meATP | Sumarin; TNP‐ATP; IP5I; NF023; NF449; PPADS; iso‐PPADS, NF864; Ro 0437626; NF 279; MRS 2159; PPNDS; NF157; |
| P2X2 | a‐bMeATP; BzATP; 2‐meSATP | Sumarin; PPADS; iso‐PPADS; TC‐P 262; TNP‐ATP; NF279; NF770; NF778; PSB‐1011 | ||
| P2X3 | a‐βMeATP; 2MesATP; BzATP; β,γ‐meATP; AP4A; AP5A; AP6A |
Sumarin; PPADS; iso‐PPADS; TC‐P 262 Spinorphin; TNP‐ATP; A317491; RO‐3; AF‐353 = RO‐4; Gefapixant = AF‐219; RO‐85; MK‐3901; NF110; A317491; RO51; NF279 |
||
| P2X4 | a‐βMeATP; ATPgS; BzATP; 2MesATP; AP4A; AP5A; AP6A (partial agonists); CTP | Suramin; PPADS; 5‐BDBD; BX‐430; PSB‐12054; TNP‐ATP; NF279; paroxetine; CORM2 | ||
| P2X5 | a‐βMeATP; 2MesATP; AP4A; BzATP; AP4A; AP5A; AP6A (partial agonists) | Suramin; PPADS; TNP‐ATP; BBG | ||
| P2X6 | a‐bMeATP | Suramin; PPADS; TNP‐ATP | ||
| P2X7 | BzATP; 2MesATP | PPADS; A‐438079; A740003; TNP‐ATP; NF279; KN62; AZ11645373; A839977; A804598; AZ10606120 dihydrochloride; JNJ47965567; GSK314181A; AZD‐9056; EVT‐401; CE‐224535; AZD‐9056; EVT‐401 | ||
| P2Y1 | ADP; ATP | GPCR | 2MesADP; 2MesATP; ADPβS, ATP; MRS2365; BzATP (partial agonist) | Suramin; iso‐PPADS; MRS2179; MRS2500; BPTU; MRS 2279; PIT; A3P5P |
| P2Y2 | ATP; UTP | INS37217; Ap4A; ATPgS; PSB‐1114; MRS2768; 2‐ThioUTP tetrasodium salt; UTPgS; MRS2698; INS365; Up4‐phenyl ester | AR‐C118925; Suramin; AR‐C126313; PSB‐716; MRS 2576; PSB‐0402 | |
| P2Y4 | ATP; UTP | UTPgS; MRS4062; Ap4A; 2′Azid‐dUTP | Suramin; MRS2577; PPADS | |
| P2Y6 | UDP; UTP | 5‐OMe‐UDP trisodium salt; PSB 0474; MRS 2693; MRS2957; UDPβS; | Suramin; MRS2578; PPADS; MRS2567; MRS2575 (human only) | |
| P2Y11 | ATP, UTP | ATPgS; 2MesATP; BzATP; dATP; NF 546; Ap2A (isomersP18 and p24); ARC67085; NAD+; NAADP+; Sp‐2‐propylthioATP‐α‐β (human) | Suramin; NF340; NF157 | |
| P2Y12 | ADP | 2MeSADP; 2MeSATP; ADP‐β‐S | Suramin; PSB‐0739; ticagrelor; AR‐C 66096; AZD 1283; Plavix; AR‐C 69931; ticlopidine hydrochloride; Elinogrel; Prasugrel; MRS2395; INS50589; CT50547; PSB 0413; ACT‐246475 | |
| P2Y13 | ADP; ATP | 2MeSADP; 2MeSATP; ADPbS | Suramin; MRS2211; AR‐C67085 | |
| P2Y14 | UDP; UDP‐glucose; UDP‐galactose; UDP glucuronic acid; UDP N‐acetyl‐glucosamine | MRS2905; MRS2690 | Suramin; PPTN hydrochloride; UDP disodium salt |
Abbreviations: (3)H]MRE 3008F20, [(3)H]5N‐(4‐methoxyphenylcarbamoyl)amino‐8‐propyl‐2‐(2‐furyl)pyrazolo [4,3‐e]‐1,2,4‐triazolo[1,5‐c]pyrimidine; (S)‐ENBA, N‐bicyclo[2.2.1]hept‐2‐yl‐5′‐chloro‐5′‐deoxyadenosine; 2′‐MeCCPA, 2‐chloro‐N‐cyclopentyl‐2′‐methyladenosine; 2MesADP, 2‐(methylthio)adenosine 5′‐diphosphate; 2MesATP, 2‐methylthioadenosine 5′‐triphosphate; 5‐BDBD, 5‐(3‐bromophenyl)‐1,3‐dihydro‐2H‐benzofuro[3,2‐e]‐1,4‐diazepin‐2‐one; 5‐OMe‐UDP trisodium salt, 5‐methoxyuridine 5′‐diphosphate trisodium salt; A317491, (5‐[[[(3‐phenoxyphenyl)methyl][(1S)‐1,2,3,4‐tetrahydro‐1‐naphthalenyl]amino]carbonyl]‐1,2,4‐benzenetricarboxylic acid; A317491, 5‐[[[(3‐phenoxyphenyl)methyl][(1S)‐1,2,3,4‐tetrahydro‐1‐naphthalenyl]amino]carbonyl]‐1,2,4‐benzenetricarboxylic acid disodium salt; A3P5P, adenosine‐3′‐phosphate‐5′‐phosphate; A‐740003, N‐(1‐{[(cyanoimino)(5‐quinolinylamino)methyl]amino}‐2,2‐dimethylpropyl)‐2‐(3,4‐dimethoxyphenyl)acetamide; A804598, N‐cyano‐N″‐[(1S)‐1‐phenylethyl]‐N′‐5‐quinolinyl‐guanidine; A839977, 1‐(2,3‐dichlorophenyl)‐N‐[[2‐(2‐pyridinyloxy)phenyl]methyl]‐1H‐tetrazol‐5‐amine; a‐bMeATP, α,β‐methyleneadenosine 5′‐triphosphate trisodium salt; ANR 94, 8‐Ethoxy‐9‐ethyl‐9H‐purin‐6‐amine; Ap2A (isomersP18 and p24), diadenosine polyphosphate; ARC 118925, 5‐[[5‐(2,8‐dimethyl‐5H‐dibenzo[a,d]cyclohepten‐5‐yl)‐3,4‐dihydro‐2‐oxo‐4‐thioxo‐1(2H)‐pyrimidinyl]methyl]‐N‐2H‐tetrazol‐5‐yl‐2‐furancarboxamide; AR‐C 66096, 2‐(propylthio)adenosine‐5’‐O‐(β,γ‐difluoromethylene)triphosphate tetrasodium salt; ARC67085: 2‐propylthio‐β,γ‐dichloromethylene‐d‐ATP; ARC‐C 69931, N‐[2‐(methylthio)ethyl]‐2‐[(3,3,3‐trifluoropropyl)thio]adenosine‐5’‐O‐(β,γ‐dichloromethylene)triphosphate tetrasodium salt; AZ 10606120, N‐[2‐[[2‐[(2‐Hydroxyethyl)amino]ethyl]amino]‐5‐quinolinyl]‐2‐tricyclo[3.3.1.13,7]dec‐1‐ylacetamide dihydrochloride; AZ11645373, 3‐[1‐[[(3′‐nitro[1,1′‐biphenyl]‐4‐yl)oxy]methyl]‐3‐(4‐pyridinyl)propyl]‐2,4‐thiazolidinedione; AZD 1283, ethyl 5‐cyano‐2‐methyl‐6‐[4‐[[[(phenylmethyl)sulfonyl]amino]carbonyl]‐1‐piperidinyl]‐3‐pyridinecarboxylate; BAY 60–6,583, 2‐[[6‐Amino‐3,5‐dicyano‐4‐[4‐(cyclopropylmethoxy)phenyl]‐2‐pyridinyl]thio]‐acetamide; BBG: brilliant blue G dye; BPTU, N‐[2‐[2‐(1,1‐dimethylethyl)phenoxy]‐3‐pyridinyl]‐N'‐[4‐(trifluoromethoxy)phenyl]urea; BX 430, N‐[2,6‐dibromo‐4‐(1‐methylethyl)phenyl]‐N'‐(3‐pyridinyl)urea; BzATP, [(2R,3S,4R,5R)‐5‐(6‐aminopurin‐9‐yl)‐4‐hydroxy‐2‐[[hydroxy‐(hydroxy‐phosphonooxyphosphoryl)oxyphosphoryl]oxymethyl]oxolan‐3‐yl] 4‐(benzoyl)benzoate;CCPA, 2‐Chloro‐N6‐cyclopentyladenosine; CGS 21680, 2‐p‐(2‐Carboxyethyl)phenethylamino‐5’‐N‐ethylcarboxamidoadenosine hydrochloride; CIB‐MECA, 2‐chloro‐N6‐(3‐iodobenzyl)‐adenosine‐5’‐N‐methyluronamide; CORM2, carbon monoxide donor; CPA, N6‐cyclopentyladenosine; CTP, cytidine triphosphate; CV‐1808, 2‐Phenylaminoadenosine; CVT‐510, N‐(3 [R]‐tetrahydrofuranyl)‐6‐aminopurine riboside; DBXRM, 1,3‐dibutylxanthine 7‐riboside 5’‐N‐methylcarboxa‐mide; DPCPX, 8‐Cyclopentyl‐1,3‐dipropylxanthine; GR79236, N‐[(1S,2S)‐2‐Hydroxycyclopentyl]adenosine; GS 6201, 3‐Ethyl‐3,9‐dihydro‐1‐propyl‐8‐[1‐[[3‐(trifluoromethyl)phenyl]methyl]‐1H‐pyrazol‐4‐yl]‐1H‐purine‐2,6‐dione; HEMADO, 2‐hexyn‐1‐yl‐N 6‐methyladenosine; INS37217, [P(1)‐(uridine 5′)‐P(4)‐(2′‐deoxycytidine 5′)tetraphosphate, tetrasodium salt]; INS48823, {[(3aR,4R,6R,6aR)‐2‐benzyl‐6‐(2,4‐dioxo‐1,2,3,4‐tetrahydropyrimidin‐1‐yl)‐tetrahydro‐2H‐furo[3,4‐d][1,3]dioxol‐4‐yl]methoxy}({[({[(2S,3R,4S,5S)‐5‐(2,4‐dioxo‐1,2,3,4‐tetrahydropyrimidin‐1‐yl)‐3,4‐dihydroxyoxolan‐2‐yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphinic acid; IP5I: diinosine‐5′,5″‐pentaphosphate; ISAM 140, 4‐(2‐Furanyl)‐4,10‐dihydro‐2‐methylpyrimido[1,2‐a]benzimidazole‐3‐carboxylic acid‐1‐methylethyl ester; iso‐PPADS, pyridoxalphosphate‐6‐azophenyl‐2’,5’‐disulfonic acid tetrasodium salt; Istradefylline, 8‐[(E)‐2‐(3,4‐dimethoxyphenyl)vinyl]‐1,3‐diethyl‐7‐methyl‐3,7‐dihydro‐1H‐purine‐2,6‐dione; JNJ47965567, 2‐(phenylthio)‐N‐[[tetrahydro‐4‐(4‐phenyl‐1‐piperazinyl)‐2H‐pyran‐4‐yl]methyl‐3‐pyridinecarboxamide; KF17837, (E)‐8‐(3,4‐dimethoxystyryl)‐1,3‐dipropyl‐7‐methylxanthine; KN‐62, 4‐[(2S)‐2‐[(5‐isoquinolinylsulfonyl)methylamino]‐3‐oxo‐3‐(4‐phenyl‐1‐piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester; LJ‐1888, [(2R,3R,4S)‐2‐[2‐chloro‐6‐(3‐iodobenzylamino)‐9H‐purine‐9‐yl]‐tetrahydrothiophene‐3,4‐diol]; LUF 5834, 2L‐Amino‐4‐(4‐hydroxyphenyl)‐6‐[(1H‐imidazol‐2‐ylmethyl)thio]‐3,5‐pyridinecarbonitrile; L‐βγ‐meATP, βγ‐methylene‐adenosine 5′‐triphosphate; MK‐3901, N‐[1(R)‐(5‐fluoropyridin‐2‐yl)ethyl]‐3‐(5‐methylpyridin‐2‐yl)‐5‐[5(S)‐(2‐pyridyl)‐4,5‐dihydroisoxazol‐3‐yl]benzamide; MLN4924, Paeoniflorin N‐[(1S,2S)‐2‐Hydroxycyclopentyl]adenosine; MRE‐2029F20, [3H]‐N‐benzo[1,3]dioxol‐5‐yl‐2‐[5‐(2,6‐dioxo‐1,3‐dipropyl‐2,3,6,7‐tetra hydro‐1H‐purin‐8‐yl)‐1‐methyl‐1H‐pyrazol‐3‐yloxy]‐acetamide; MRS 1220, (N‐[9‐chloro‐2‐[2‐ furanyl][1,2,4]triazolo[1,5‐c]quinazolin‐5‐yl]benzeneacetamide); MRS 1523, 3‐propyl‐6‐ethyl‐5[(ethylthio)carbonyl]‐2‐phenyl‐4‐propyl‐3‐pyridine‐carboxylate; MRS 2179, 2′‐deoxy‐N 6‐methyladenosine 3′,5′‐bisphosphate ammonium salt; MRS 2395, 2,2‐dimethyl‐propionic acid 3‐(2‐chloro‐6‐methylaminopurin‐9‐yl)‐2‐(2,2‐dimethyl‐propionyloxymethyl)‐propyl ester; MRS 2500, (1R*,2S*)‐4‐[2‐Iodo‐6‐(methylamino)‐9H‐purin‐9‐yl]‐2‐(phosphonooxy)bicyclo[3.1.0]hexane‐1‐methanol dihydrogen phosphate ester tetraammonium salt; MRS 2693, 5‐Iodouridine‐5′‐O‐diphosphate trisodium salt; MRS 2768, uridine‐5′‐tetraphosphate δ‐phenyl ester tetrasodium salt; MRS 5698, (1S,2R,3S,4R,5S)‐4‐[6‐[[(3‐Chlorophenyl)methyl]amino]‐2‐[2‐(3,4‐difluorophenyl)ethynyl]‐9H‐purin‐9‐yl]‐2,3‐dihydroxy‐N‐methylbicyclo[3.1.0]hexane‐1‐carboxamide; MRS1191, 3‐ethyl 5‐benzyl 2‐methyl‐6‐phenyl‐4‐ phenylethynyl‐1,4‐(±)‐dihydropyridine‐3,5‐dicarboxylate; MRS1292, (2R,3R,4S,5S)‐2‐[N6‐3‐iodobenzyl)adenos‐9′ ‐yl]‐7‐aza‐1‐oxa‐6‐ oxospiro[4.4]‐nonan‐4,5‐diol); MRS1334, 1,4‐dihydro‐2‐methyl‐6‐phenyl‐4‐(phenylethynyl)‐3,5‐pyridinedicarboxylic acid 3‐ethyl‐5‐[(3‐nitrophenyl)methyl] ester; MRS1754, N‐(4‐Cyanophenyl)‐2‐[4‐(2,3,6,7‐tetrahydro‐2,6‐dioxo‐1,3‐dipropyl‐1H‐purin‐8‐yl)phenoxy]‐acetamide; MRS2211, 2‐[(2‐chloro‐5‐nitrophenyl)azo]‐5‐hydroxy‐6‐methyl‐3‐[(phosphonooxy)methyl]‐4‐pyridinecarboxaldehyde disodium salt; MRS2279, (1R*,2S*)‐4‐[2‐chloro‐6‐(methylamino)‐9H‐purin‐9‐yl]‐2‐(phosphonooxy)bicyclo[3.1.0]hexane‐1‐methanol dihydrogen phosphate ester diammonium salt; MRS2365, [[(1R,2R,3S,4R,5S)‐4‐[6‐Amino‐2‐(methylthio)‐9H‐purin‐9‐yl]‐2,3‐dihydroxybicyclo[3.1.0]hex‐1‐yl]methyl] diphosphoric acid mono ester trisodium salt; MRS2567, 1,2‐diphenylethane; MRS2575, 1,4‐phenylendiisothiocyanate; MRS2578, N,N″‐1,4‐butanediylbis[N'‐(3‐isothiocyanatophenyl)thiourea; MRS2690, diphosphoric acid 1‐α‐D‐glucopyranosyl ester 2‐[(4′‐methylthio)uridin‐5″‐yl] ester disodium salt; MRS2698, 2′‐amino‐2′‐deoxy‐2‐thio‐ UTP; MRS2905, 2‐thiouridine‐5′‐O‐(α, β‐methylene)diphosphate trisodium salt; MRS2957, P1‐[5′(N4‐methoxycytidyl)]‐P3‐(5′‐uridyl)‐triphosphate tri(triethylammonium) salt; MRS3777 hemioxalate, 2‐Phenoxy‐6‐(cyclohexylamino)purine hemioxalate; MRS4062, N4‐phenylpropoxycytidine‐5′‐O‐triphosphate tetra(triethylammonium) salt; MRS5474, (1R,2R,3S,4R,5S)‐4‐{2‐chloro‐6‐[(dicyclopropylmethyl)amino]‐9H‐purin‐9‐yl}bicyclo[3.1.0]hexane‐2,3‐diol; N‐0840, 5’‐N‐ethylcarboxamidoadenosine; NECA, 5’‐N‐Ethylcarboxamidoadenosine; NF 279, 8,8′‐[carbonylbis(imino‐4,1‐phenylenecarbonylimino‐4,1‐phenylenecarbonylimino)]bis‐1,3,5‐naphthalenetrisulfonic acid hexasodium salt; NF023, 1,3,5‐trisodium 8‐{[3‐({[3‐({4,6,8‐tris[(sodiooxy)sulfonyl]naphthalen1yl}carbamoyl)phenyl]carbamoyl}amino)benzene]amido}naphthalene‐1,3,5‐trisulfonate; NF110, 4,4′,4″,4‴‐[carbonylbis[imino‐5,1,3‐benzenetriylbis(carbonylimino)]]tetrakisbenzenesulfonic acid tetrasodium salt; NF157, 8,8′‐[carbonylbis[imino‐3,1‐phenylenecarbonylimino(4‐fluoro‐3,1‐phenylene)carbonylimino]]bis‐1,3,5‐naphthalenetrisulfonic acid hexasodium salt; NF340, 4,4′‐(carbonylbis(imino‐3,1‐[4‐methyl‐phenylene]carbonylimino))bis(naphthalene‐2,6‐disulfonic acid) tetrasodium salt; NF449, 1,3‐disodium 4‐{[3‐({[3,5‐bis({2,4‐bis[(sodiooxy)sulfonyl]phenyl}carbamoyl)phenyl]carbamoyl}amino)‐5‐({2,4‐bis[(sodiooxy)sulfonyl]phenyl}carbamoyl)benzene]amido}benzene‐1,3‐disulfonate; NF546: 4,4′‐(carbonylbis(imino‐3,1‐phenylene‐carbonylimino‐3,1‐[4‐methyl‐phenylene]carbonylimino))‐bis(1,3‐xylene‐alpha,alpha’‐diphosphonic acid tetrasodium salt; NF770, 7,7′‐(carbonylbis(imino‐3,1‐phenylenecarbonylimino‐3,1‐[4‐methyl‐phenylene]carbonylimino))bis(1‐methoxy‐naphthalene‐3,6‐disulfonic acid; NF778, 6,6′‐(carbonylbis(imino‐3,1‐phenylenecarbonylimino‐3,1‐[4‐methyl‐phenylene]carbonylimino))bis(1‐methoxy‐naphthalene‐3,5‐disulfonic acid; NF864, (8,8′,8″,8‴‐(carbonylbis(imino‐5,1,3‐benzenetriyl‐bis[carbonylimino]))tetrakis‐naphthalene‐1,3,5‐trisulfonic acid‐dodecasodium salt); OT‐7999, 5‐n‐butyl‐8‐(4‐ trifluoromethylphenyl)‐3H‐[1,2,4]triazolo‐[5,1‐i]purine; PBS 0777 ammonium salt, 4‐[2‐[(6‐Amino‐9‐b‐D‐ribofuranosyl‐9H‐purin‐2‐yl)thio]ethyl]benzenesulfonic acid ammonium salt; PHPNECA, 2‐(3‐hydroxy‐3‐phenyl)propyn‐1‐yl‐NECA; PIT, 2,2′‐pyridylisatogen tosylate; PPADS, pyridoxalphosphate‐6‐azophenyl‐2’,4’‐disulfonic acid tetrasodium salt; PPNDS, pyridoxal‐5′‐phosphate‐6‐(2′‐naphthylazo‐6′‐nitro‐4′,8′‐disulfonate) tetrasodium salt; PPTN hydrochloride, 4‐[4‐(4‐piperidinyl)phenyl]‐7‐[4‐(trifluoromethyl)phenyl]‐2‐naphthalenecarboxylic acid hydrochloride; PQ‐69, 4‐(Butylamino)‐2‐(3‐fluorophenyl)‐1,2‐dihydro‐3H‐pyrazolo[4,3‐c]quinolin‐3‐one; PSB 0474, 3‐(2‐oxo‐2‐phenylethyl)‐uridine‐5′‐diphosphate disodium salt; PSB 0788, 8‐[4‐[4‐(4‐Chlorobenzyl)piperazide‐1‐sulfonyl)phenyl]]‐1‐propylxanthine; PSB 10 hydrochloride, (8R)‐8‐Ethyl‐1,4,7,8‐tetrahydro‐4‐methyl‐2‐(2,3,5‐trichlorophenyl)‐5H‐imidazo[2,1‐i]purin‐5‐one monohydrochloride; PSB 1114, 4‐thiouridine‐5’‐O‐(β,γ‐difluoromethylene)triphosphate tetrasodium salt; PSB 1115, 4‐(2,3,6,7‐tetrahydro‐2,6‐dioxo‐1‐propyl‐1H‐purin‐8‐yl)‐benzenesulfonic acid; PSB 12054, N‐(p‐methylphenylsulfonyl)phenoxazine; PSB 603, 8‐[4‐[4‐(4‐chlorophenzyl)piperazide‐1‐sulfonyl)phenyl]]‐1‐propylxanthine; PSB0739, 1‐amino‐9,10‐dihydro‐9,10‐dioxo‐4‐[[4‐(phenylamino)‐3‐sulfophenyl]amino]‐2‐anthracenesulfonic acid sodium salt; PSB‐1011, disodium 1‐amino‐4‐[3‐(4,6‐dichloro[1,3,5]triazine‐2‐ylamino)‐4‐sulfophenylamino]‐9,10‐dioxo‐9,10‐dihydroanthracene‐2‐sulfonate; PSB‐1011, disodium 1‐amino‐4‐[3‐(4,6‐dichloro[1,3,5]triazine‐2‐ylamino)‐4‐sulfophenylamino]‐9,10‐dioxo‐9,10‐dihydroanthracene‐2‐sulfonate; PSB‐36, 1‐Butyl‐8‐(hexahydro‐2,5‐methanopentalen‐3a[1H]‐yl)‐3,7‐dihydro‐3‐(3‐hydroxypropyl)‐1H‐purine‐2,6‐dione; PSB‐716, 1‐amino‐4‐(2‐methoxyphenyl)‐2‐sulfoanthraquinone; Ro 0437626, N‐[(1R)‐2‐[[(1S,2R,3S)‐1‐(cyclohexylmethyl)‐3‐cyclopropyl‐2,3‐dihydroxypropyl]amino]‐2‐oxo‐1‐(4‐thiazolylmethyl)ethyl]‐1H‐benzimidazole‐2‐carboxamide; RO‐3, 5‐(2‐isopropyl‐4,5‐dimethoxy‐benzyl)‐pyrimidine‐2,4‐diamine; RO‐4, 5‐(5‐iodo‐2‐isopropyl‐4‐methoxy‐phenoxy)‐pyrimidine‐2,4‐diamine; RO‐51, 2‐[[4‐amino‐5‐[5‐iodo‐4‐methoxy‐2‐(1‐methylethyl)phenoxy]‐2,4‐pyrimidinyl]amino]‐1,3‐propanediol; SCH442416, 2‐(2‐Furanyl)‐7‐[3‐(4‐methoxyphenyl)propyl]‐7H‐pyrazolo [4,3‐e][1,2,4]triazolo[1,5‐c]pyrimidin‐5‐amine 5‐amino‐7‐(3‐(4‐methoxyphenyl)propyl)‐2‐(2 furyl)pyrazolo[4,3‐e]‐1,2,4‐triazolo[1,5‐c]pyrimidine 5‐Amino‐7‐[3‐(4‐methoxy)phenylpropyl]‐2‐(2‐furyl)‐pyrazolo[4,3‐e]‐1,2,4‐triazolo[1,5‐c]pyrimidine; Scheme 58261, 7‐(2‐phenylethyl)‐5‐amino‐2‐(2‐furyl)‐pyrazolo‐[4,3‐e]‐1,2,4‐triazolo[1,5‐c]pyrimidine; SDZ WAG 994, N‐Cyclohexyl‐2’‐O‐methyladenosine; TC‐G1004, N‐[2‐(3,5‐Dimethyl‐1H‐pyrazol‐1‐yl)‐6‐[6‐(4‐methoxy‐1‐piperidinyl)‐2‐pyridinyl]‐4‐pyrimidinyl]acetamide; TC‐P 262, 5‐[5‐methyl‐2‐(1‐methylethyl)phenoxy]‐2,4‐pyrimidinediamine; TNP‐ATP, 2′,3′‐O‐(2,4,6‐trinitrophenyl)‐ATP; UDPβS, 59‐O‐thiodiphosphate; UK‐432097, 6‐(2,2‐Diphenylethylamino)‐9‐((2R,3R,4S,5S)‐5‐(ethylcarbamoyl)‐3,4‐dihydroxytetrahydrofuran‐2‐yl)‐N‐(2‐(3‐(1‐[pyridin‐2‐yl]piperidin‐4‐yl)ureido)ethyl)‐9H‐purine2‐carboxamide; UTPgS, uridine‐5’‐(γ‐thio)‐triphosphate trisodium salt; VUF 8504, (4‐methoxy‐N‐(3‐[2‐pyridinyl]‐1‐ isoquinoli nyl)benzamide, 52); VUF5574, (N‐(2‐methoxyphenyl)‐N′ ‐(2‐[3‐ pyridyl]quina zolin‐4‐yl)urea, 53); WRC‐0571, 5‐[[9‐methyl‐8‐[methyl(propan‐2‐yl)amino]purin‐6‐yl]amino]bicyclo[2.2.1]heptan‐2‐ol; ZM 241385, 4‐(2‐[7‐Amino‐2‐[2‐furyl][1,2,4]triazolo[2,3‐a][1,3,5]triazin‐5‐ylamino]ethyl)phenol.
Analysis of the expression and function of P2X receptors in peripheral glia is ongoing. Among P2X receptor mRNAs, P2RX4, P2RX6, P2RX7 mRNAs are expressed at the highest levels in cultured mouse SC (Figure 2b,e). Early immunocytochemical evidence suggested P2RX1 and P2RX3 expression in mouse SCs in vivo. However, some tissue staining was likely artefactual as the authors failed to identify P2RX1 mRNA in the SCs (consistent with Figure 2b,e) (Barden, Cottee, & Bennett, 1999). P2RX2 and P2RX3 mRNAs are robustly expressed in mouse SCs, and rat SCs (Zhang et al., 2018). P2RX4 is expressed in the lysosomes of rat SCs (Su et al., 2018). Although P2RX5 and P2RX6 mRNAs are expressed in mouse SCs (Figure 2b,e), there has been no analysis of P2RX5 or P2RX6 expression in vivo. P2RX6 is expressed on endothelial cells in the human endoneurium (Dong & Uboqu, 2018).
Of the P2X receptors expressed in SCs, the best studied is P2RX7. Upon activation, P2RX7 can change conformation and allow membrane flux of molecules/complexes of up to 900kD in size (Albuquerque, Pereira, Alkondon, & Rogers, 2009). P2RX7 mRNA is abundant in cultured mouse SCs (Figure 2b,e). In vivo, P2RX7 localizes to the SC cytoplasm and the abaxonal SC membrane during myelination in the mouse, and it is present in some Remak SCs (Colomar et al., 2003; Faroni et al., 2014). Cultured SC differentiated from adipocyte stem cells also have upregulated P2RX7 (and P2RX4) expression (Faroni et al., 2013). Using Ca2+ imaging techniques, Faroni et al. (2013) showed that P2RX7 is functional in SC; ATP stimulation increased intracellular Ca2+ and led to cell death, which could be prevented by the P2RX7 antagonist AZ10606120. Importantly, whole body genetic inactivation of P2RX7 reduced numbers of myelinated fibers while increasing numbers of Remak bundles (Faroni et al., 2014). Whether loss of P2RX7 in neurons causes reduced axon diameter (reducing axon diameter causes SCs to form ensheathing rather than myelinating SCs), and/or loss of P2RX7 in SCs causes the effect (by regulating the release of factor[s] from SCs), is not known. The roles and localization of other P2RXs in the peripheral nerve glia remain poorly studied in vitro and uncharacterized in vivo.
3. P1 AND P2Y OVERVIEW
As noted above P1 and P2Y receptors are a diverse set of GPCR.
4. GPCR SIGNALING
When agonists bind P1 and P2Y receptors, these GPCR receptors bind to intracellular heterotrimeric complexes of small heterotrimeric G‐proteins, containing one Gα, one Gβ, and one Gγ subunit. These small G‐proteins signal rapidly upon agonist binding. Gα proteins are encoded by four families of GNA genes. GNA proteins of the Gs family activate adenylyl cyclase; Gi inactivate adenylyl cyclase, Gq/11 activate PLCβ, and G12/13 which signal predominantly through the small G‐protein Rho. GPCR including the P2RY2 receptor can also couple to Go in an integrin‐dependent manner, activating Rac1 (Bagchi et al., 2005). This Go/Rac1 pathway is linked to cell proliferation in neural crest cells and their maturing progeny, including SC (Fuchs et al., 2009). Gβγ subunits, in contrast, signal independently to activate additional downstream targets (Senarath et al., 2018).
Multiple mechanisms contribute to the termination of P1 and P2Y GPCR signaling. G‐protein coupled receptor kinases (GRKs) phosphorylate active receptors to terminate receptor signaling. Inactivation of Gα proteins are carried out by Regulators of G protein signaling (RGS), GTPase activating protein (GAPs) that accelerate the hydrolysis of GTP on small G‐proteins. In addition, the β‐arrestin proteins, β‐arrestin1, and β‐arrestin2, terminate GPCR signaling through small G‐proteins, at least in part by stimulating endocytosis.
β‐arrestins also act as scaffolding proteins that bring together some GPCRs with diverse signaling complexes (DeWire, Ahn, Lefkowitz, & Shenoy, 2007; Lefkowitz, Rajagopal, & Whalen, 2006). This β‐arrestin scaffolding results in a second, late phase of GPCR‐initiated signaling that is receptor and cell‐type dependent. For example, β‐arrestin scaffolds associated with specific receptors shuttle them to lysosomes. Alternatively, β‐arrestin scaffolds form complexes with sets of cytoplasmic signaling molecules including the MAPK pathway components, Raf/MEK/ERK. Other β‐arrestin scaffolds include AKT and PP2A phosphatase, or NF‐kB components. It is important to emphasize that during cell differentiation, patterns of purinergic receptor expression change, which is predicted to modulate response to specific β‐arrestin recruitment by various GPCRs. The role(s) that β‐arrestins play in SC signaling and development are little studied, and most research to date has focused on ligand‐mediated GPCR signaling through Gαβγ. Figure 1a–c provide a general overview of the three classes of purinergic receptors and their known downstream signaling pathways.
5. P1 RECEPTOR EXPRESSION AND FUNCTION IN PNS GLIA
The four P1R are the adenosine receptors A1R, A2AR, A2BR, and A3R (gene names: ADORA1, ADORA2A, ADORA2B, ADORA3) (Table 1). A1R is coupled to Gi/o, which inhibits adenylate cyclase (Figure 1a). In frog, A1R are present in perisynaptic SCs that ensheath the axon terminals of the neuromuscular junction (Robitaille, 1995). To date, there is no analysis of A1 in mammalian SCs in vivo. A1 mRNA was present in mouse primary SCs (Figure 2a,d). When tissue‐specific A1A knockout mice become available, it will be of interest to analyze them for effects on the peripheral nervous system. A recent report shows ADORA1 upregulation in cancer, in hypoxia‐activated SCs of the human pancreas (Demir et al., 2016).
A2AR is Gs coupled and, therefore, its activation results in cAMP generation. Adenosine‐stimulated cultured SCs increase levels of intracellular cAMP, and genetic loss of A2AR in mouse SCs decreases this cAMP accumulation stimulated by adenosine, confirming A2AR expression and function (Stevens, Ishibashi, Chen, & Fields, 2004). Our qPCR analysis of mouse SC is consistent with A2AR mRNA expression (Figure 2a,d). It is known that increasing cAMP in SCs in vitro elevates proliferation stimulated by several growth factors (FGF2, TGFβ, or neuregulin) (Monje, Athauda, & Wood, 2008). However, adenosine added to SCs in the presence of PDGF blunts, rather than stimulates mitogen‐stimulated proliferation (Stevens et al., 2004). In addition, when SC attach to axons in culture and proliferate in response to axonal neuregulin, proliferation is unaffected by the added adenosine (Stevens & Fields, 2000). Why these responses differ is not clear. Activation of different downstream effects of A2AR stimulation versus direct stimulation by cAMP elevation, and/or the duration or levels of effector signaling may contribute to these different responses.
The biological effects of A2AR activation on nerves in vivo are unknown, and, there is no evidence that increasing cAMP affects developmental SC proliferation. Prolonged exposure (days) of cultured SC to higher levels of cAMP than those needed to stimulate SC proliferation in vitro, increases expression of myelin genes. In vivo, effects of cAMP on myelination appear to be largely mediated through the adhesion GPCR126 (Monk et al., 2015; Petersen et al., 2015).
A2BR can be Gs coupled, increasing cAMP upregulation and/or Gq coupled, increasing intracellular calcium (Sheth, Brito, Mukherjea, Rybak, & Ramkumar, 2014). A2BR mRNA transcripts are detected in mouse SCs in culture (Figure 2a,d), but A2BR is not yet studied in the nerve in vivo. A3R are Gi coupled receptors that inhibit adenylate cyclase and decrease intracellular cAMP (Figure 1a). A3R are present in the mouse nerve terminal at the neuromuscular junction and in sensory neurons (Garcia et al., 2014), but have not been studied in peripheral nerve SCs. A3R mRNA expression was low in mouse SC cultures (Figure 2a,d).
6. P2Y RECEPTOR EXPRESSION AND FUNCTION IN PNS GLIA
The metabotropic P2Y receptors include eight GPCRs: P2RY1, P2RY2, P2RY4, P2RY6, P2RY11, P2RY12, P2RY13, and P2RY14. Each of these receptors is activated by subsets of the agonists ATP, ADP, UTP, UDP and UDP‐glucose, and UDP‐GlcNac (Rafehi & Muller, 2018) (Table 1). The P2RY1, P2RY2, P2RY4, and P2RY6 receptors are expressed in primary cultured rat SCs (Chen et al., 2015; Del Puerto, Wandosell, & Garrido, 2013; Lamarca et al., 2014). The P2RY2 receptor is also expressed in a rat immortalized SC line (Berti‐Mattera, Wilkins, Madhun, & Suchovsky, 1996). P2RY1, P2RY2, P2RY6, P2RY12, P2RY13, and P2RY14 mRNA are expressed in mouse SCs (Figure 2c,f).
UTP, UDP‐glucose, and UDP‐GlcNac are extracellular signaling molecule agonists of several P2RYs, which play key roles in the regulation of programmed cell death, energy generation, and cellular signaling (Manhaes et al., 2016). Because they are difficult to measure in the extracellular milieu, their precise physiological concentrations are unknown (Lazarowski & Harden, 2015). Application of UTP to cultured SCs results in Ca2+ dependent release of ATP via suramin‐sensitive P2RY(s) (Liu et al., 2005). Given the lack of specificity of suramin, the responsible UTP receptor(s) are unknown since P2RY2, P2RY4, P2RY6, and P2RY14 can all use UTP as a ligand.
P2RY1 mRNA was first identified in cultured mouse SCs by Stevens et al. (2004), who initially ascribed ATP effects on inhibition growth factor‐induced SC proliferation to this receptor. Purines from the nerve terminal, released via calcium‐mediated release of synaptic vesicles, activate P2RY1 in terminal SCs (Darabid, St‐Pierre‐See, & Robitaille, 2018; Heredia, Feng, Hennig, Renden, & Gould, 2018). As reagents allowed discrimination among P2YR receptor effects, several lines of evidence now support the idea that ATP‐mediated inhibition of growth factor‐induced SC is mediated through P2YR2 (Coover et al., 2018). In an attempt to clarify the mechanisms involved in the ATP‐mediated block of axonal neuregulin‐stimulated SC proliferation in vitro, we studied this effect in human and mouse SCs (Coover et al., 2018). Using antagonists and shRNAs, we confirmed that this inhibitory pathway requires P2RY2 receptors. As mentioned above, β‐arrestins can terminate GPCR signaling and modulate the formation of signaling scaffolds. Surprisingly, the inhibitory effect of ATP, required β‐arrestin mediated PP2A and AKT complexes rather than heterotrimeric G‐protein signaling. Specifically, inhibition of PP2A or β‐arrestins, using pharmacological inhibitors or shRNA, were each sufficient to rescue the growth inhibitory effects of ATP. Whether this P2RY2 β‐arrestin‐mediated pathway regulates developmental myelination remains to be tested.
In vivo, inhibiting P2RY2 during developmental myelination (with short‐hairpin P2RY2 [shP2RY2]) or suppressing IP3 signaling with IP3‐5 phosphatase (5ppase), suppressed ATP‐induced mitochondrial Ca2+ responses and caused hypomyelination in rats (Ino et al., 2015). This purinergic signaling enhances SC mitochondrial bioenergetics, and blocking a mitochondrial transporter increased mitochondrial but not cytosolic Ca2+, implying a primary effect on mitochondrial Ca2+. This study strongly suggests a key role for ATP via P2RY2 and calcium, in vivo. However, verification of roles of P2RY2 on SC proliferation and/or myelination in mice in vivo awaits detailed analysis of mice with genetic loss of P2RY2.
P2RY4 is expressed in rat dorsal root and trigeminal ganglion neurons (Ruan & Burnstock, 2003), but functional analysis has not yet been reported in SCs. P2Y11 is expressed in human cells but has no mouse homolog. P2RY12 is expressed in rat SGCs (Kobayashi et al., 2006; Yi et al., 2018), and P2RY13 and P2RY14 mRNA are expressed in mouse SCs (Figure 2c,f). P2RY14 is also expressed in the cultured rat “SCGs” (these were not satellite glia, but rather dedifferentiated cultured SCs) where it mediated IL‐1β and CCL2 release (Lin et al., 2019). P2RY14 receptor activation also increases ERK1/2 and p38/JNK phosphorylation in these cells, but has not been studied in vivo (Lin et al., 2019).
7. POTENTIAL SOURCES OF PURINERGIC RECEPTOR LIGANDS IN THE PNS
Neuronal activity, through ATP release, can modulate SC differentiation. In a seminal paper, Stevens and Fields (2000) showed that when neurons in tissue culture fire action potentials, they release ATP, which activates SC purinergic receptors. This, in turn, reduces axonal neuregulin‐stimulated SC proliferation along cultured axons and correlates with upregulated expression of the differentiation (early myelination) marker O4 and ultimately of MBP+ myelin sheaths (Fields & Ni, 2010). Electrically active neurons in vivo release ATP through stretch‐activated maxi‐ion channels (Fields & Ni, 2010). Ino et al. (2015) confirmed that PNS axons release ATP via nonvesicular maxi‐ion channels in vivo and showed, using microdialysis, that extracellular levels of ATP (and its breakdown products) are elevated 10‐fold by stimulation of rat sciatic nerve. How the stimulation paradigms used correspond to endogenous firing patterns, and thus levels of extracellular ATP, remains unclear.
During perinatal myelination in vivo, electrical stimulation and neuronal ATP release increases Ca2+ signaling (mitochondrial and cytosolic) in surrounding SCs (Ino et al., 2015). This results in release of ATP from SCs that requires elevation of intracellular calcium. Vesicular ATP was released by Ca2+ dependent exocytosis of lysosomal vesicles by cultured SCs (Lin et al., 2019). Additional mechanisms of ATP release by SC have been proposed, including passage through connexin 32 hemi‐channels (Nualart‐Marti et al., 2013). Another potential source of elevated extracellular ATP, UTP, and their derivatives are dying cells caused by nerve damage. These dying cells release signals that stimulate purinergic receptors in peripheral immune cells to aid in nerve repair (Oberlander & Berghoff, 2010; Shen et al., 2018; Wang et al., 2006).
As noted above, extracellular ATP is rapidly converted to ADP, AMP, and then adenosine by extracellular ectonucleotidases. ATP/ADP to AMP degradation is carried out by ectonucleoside triphosphate diphosphohydrolase (ENTPD), also known as CD39, which is the rate‐limiting step for adenosine production. ADP to adenosine degradation is carried out by 5′‐nucleotidase (NT5E), also known as CD73. The expression of each of these ectonucleotidases is robust and dynamic in dorsal root ganglia in SCs and possibly also in SGCs (Street et al., 2013). Expression changes in either purinergic agonists and/or endonucleases are predicted to have a significant impact on amounts of purinergic agonists present in the cellular environment at specific times. It is likely that glial cells are exposed to variable levels of purinergic agonists under specific conditions, which is predicted to modulate cell‐to‐cell communication.
8. PURINERGIC SIGNALING IN SCHWANN CELL TUMORS
In many tissues, after injury, high levels of adenosine are present. Adenosine is also elevated in the tumor microenvironment, where it supports cell growth (A1, A2A), regulates hypoxia (A2A, A2B), and/or stimulates angiogenesis (A2B) (Burnstock, 2014; Di Virgilio & Adinolfi, 2017), and can modulate the tumor immune and stromal environment. Very little is known about purinergic signaling in nerve tumors.
We recently studied purinergic signaling in the context of tumor suppressor gene loss of function in SCs. Tumors of the peripheral nervous system are largely due to loss of NF1 or NF2 tumor suppressor gene function. Individuals with neurofibromatosis type 1 (NF1) have a loss of function of the NF1 tumor suppressor gene, which encodes off‐signal for Ras proteins (a RAS‐GAP), so that loss of function in the NF1 gene results in increased Ras‐GTP after SC stimulation, and formation of benign tumors in peripheral nerves (Ratner & Miller, 2015). In contrast to wild type SCs (Figure 3a), Nf1 deficient SCs can show growth suppression in response to ATP, on exposure to higher levels of extracellular ATP than required to suppress the growth of normal cells. These Nf1−/− SCs are resistant to P2RY2‐β‐arrestin‐mediated PP2A dependent dephosphorylation of AKT (Figure 3b) (Coover et al., 2018). Supporting the relevance of purinergic signaling to tumor growth in NF1, administration of ATP to neurofibroma‐bearing mice significantly reduced tumor cell proliferation and reduced tumor size (Coover et al., 2018). Defining purinergic ligands and receptors that mediate these effects, and testing therapeutic implications of these findings requires further investigation. For example, it has been shown that UTP, through an unidentified receptor, induces expression of the neural cell adhesion molecule (N‐cadherin) in ErbB2‐transformed “schwannoma” cell lines in vitro (transformed SCs), but the mechanism underlying this effect is unknown, as is its potential in vivo relevance (Martiañez, Lamarca, Casals, & Gella, 2013). Figure 4b provides an overview on how, in the tumor microenvironment, ATP, adenosine, and UTP are present and regulate many aspects of tumor development.
FIGURE 3.

P2RY2 signaling in WT and Nf1−/− SCs. (a) In WT SCs, ATP binds to P2Y2 causing β‐arrestin activation (by phosphorylation), which in turn activates PP2A complex resulting in AKT dephosphorylation and suppression of SC growth. (b) In Nf1−/− SCs, ATP suppresses SC growth only on exposure to elevated levels of ATP; normal heterotrimeric G‐protein signaling is initiated upon activation of P2RY2 by ATP, resulting in Gq signaling, and increasing release of Ca+2 from intracellular stores. However, β‐arrestin signaling is reduced in Nf1−/− SCs (shown by the dashed line/arrow), and Nf1−/− SCs do not show the subsequent, characteristic, β‐arrestin‐mediated transient calcium decrease (modified from Coover et al., 2018)
9. PURINERGIC RECEPTORS AND PERIPHERAL NERVE DAMAGE
Damage to the peripheral nerve reduces nerve conduction velocity. Nerve damage can also lead to sensory and motor nerve dysfunction, and pain. Rapidly, after nerve injury or in peripheral neuropathies like Charcot–Marie‐Tooth (CMT) disease and Autoimmune Neuritis (EAN), T cells and inflammatory macrophages invade the injured nerve. If an injury persists, permanent loss of function and disability occurs (Zuchero & Barres, 2015). In all tissues, the presence of elevated extracellular ATP, UTP, and their derivatives are signs of tissue damage. These tissue damage signals are released from dying cells and stimulate purinergic signaling in immune cells, which promotes injury responses and contributes to tissue repair (Oberlander & Berghoff, 2010; Shen et al., 2018; Wang et al., 2006). Acutely after nerve injury, ATP is released from dying cells. Subsequently, when the axons are no longer conducting electrical impulses, axon‐stimulated ATP levels in nerves are anticipated to decrease. Notably, resupply of ATP prevents the myelin degradation that follows nerve damage (Shin, Chung, Park, Jung, & Jeong, 2014) but, the mechanisms responsible are unknown.
Purinergic ligands such as ATP that increase locally after nerve injury are broken down by local ectonucleotidases to adenosine. Adenosine signals through receptors on immune cells that invade injured tissues (Hasko et al., 2000; Hasko, Linden, Crostein, & Pacher, 2008; Kreckler, Wan, Ge, & Auchampach, 2006; Lappas, Rieger, & Linden, 2005; Naganuma et al., 2006; Sevingny et al., 2007). Adenosine signaling may also affect Schwann cells acutely after nerve injury. Purinergic signaling (through an unknown P2 receptor) is required for rapid glial cell line‐derived neurotrophic factor (GDNF) dependent transcription and translation in myelinating and in Remak SCs after nerve injury (Xu et al., 2013). GDNF contributes to neuron survival and repair, so this pathway may contribute to purinergic signaling actions in nerve repair. How during injury, elevated adenosine levels via adenosine receptors on SC, might affect nerve disease and nerve repair is unknown.
Some ATP receptors are upregulated after nerve injury and in certain pathological conditions. For example, duplication of the PMP22 gene in the peripheral neuropathy CMT1A upregulates P2RX7 receptor causing the formation of aberrant myelin and demyelination which are CMT1A disease features (Nobbio et al., 2009; Nobbio et al., 2014). ATP‐mediated P2RX7 signaling increased Ca2+ and decreased cAMP in SCs cultured from a rat model of CMT1A, affecting SC migration and neurotrophic factor release (Nobbio et al., 2009). Downregulation of P2RX7 rescued these features and improved in vitro myelination (Nobbio et al., 2009).
In terms of P2RX4, Su et al. (2018) overexpressed P2RX4 in mouse SCs. This promoted motor and sensory functional recovery and accelerated remyelination after nerve injury. In a model of polyneuropathy induced by HIV‐derived GP120, P2RY12 expression is up‐regulated in GFAP+ SGCs, concurrent with increases in P‐P38, Ca2+, and release of the inflammatory proteins TNFα and IL1β (Yi et al., 2018). In vitro, shRNA targeting P2RY12 reduced these effects, suggesting a role for P2RY12 (as for P2RX7) in modulating cytokine release from SGC in diseased nerve.
SC cytokine release is also stimulated by pyrimidine receptor stimulation. The P2RY14 ligand UDP‐glucose increases GFAP in satellite cells, correlating with increases in P‐ERK, P‐P38, and P‐JNK, and release of IL1β and CCL2 (Lin et al., 2019). UTP/UDP‐sugars activate P2RY14, which can itself trigger ATP release. Therefore, it is possible that P2RY14‐mediated ATP release indirectly mediates the effect. It was suggested that SC stimulation by UTP causes ATP release from SC through LAMP1+ vesicle exocytosis, which may be relevant during Wallerian degeneration (Jung et al., 2014).
10. PURINERGIC RECEPTORS IN PAIN
The role of SC purinergic receptors in pain is not well studied. Most studies to date do not distinguish direct effects on SCs, versus effects of receptors directly on neuronal cell bodies or indirect effects of purinergic signaling in SCs that subsequently modulate adjacent axons. However, given that activation of P2X and P2Y receptors stimulate SC to release inflammatory cytokines, this suggests that these known mediators of pain could provide a link between tissue injury and pain. Here, we summarize recent data on the effects caused by the release of purines from SCs (and/or other sources) by ATP, working through sensory neuron purinergic receptors.
It has been hypothesized that ATP release from SCs might be important for the activation of peripheral pain pathways. In the rat vagus nerve, ATP increases unmyelinated nociceptive C‐fiber excitability, which may be attributed to SC and/or neuronal effects (Irnich, Burgstahler, Bostock, & Grafe, 2001). The tactile allodynia that occurs after nerve injury in rats was reversed by administration of the non‐selective P2 antagonist PPADS, which blocks P2RX1, P2RX2, P2RX3, P2RX5, P2RY2, and P2RY4 (Inoue, 2006). Barclay et al. (2002) downregulated the ATP receptor P2RX3 using antisense oligonucleotides in models of neuropathic and inflammatory pain, and inhibited hyperalgesia. More recently, Zhang et al. (2018) showed microencapsulated SC transplantation reduces neuropathic pain through P2RX2/3 receptors. ATP stimulation of keratinocyte P2RX4 also modulates cutaneous hypersensitivity (Moehring et al., 2018).
As noted above, GDNF is produced by SCs after nerve injury in response to purinergic signaling (Xu et al., 2013). Queme, Weyler, Cohen, Hudgins, and Jankowski (2020) showed that P2RX5 is downstream of GDNF signaling in muscle nociceptors, indicating that several purinergic receptors could be involved in the pain response. P2RX6 receptor expression is increased in visceral pain in rat models (Chen et al., 2016). Mice that lack P2RX7 show no chronic inflammatory pain in response to mechanical or thermal stimuli (Chessell et al., 2005). Chronic constriction injury of a peripheral nerve increases levels of nNOS, iNOS, and pro‐algesic factors such as IL‐1β and IL‐6 (Martucci et al., 2008). In this mouse model, treatment of injured mice with PPADS reversed nociceptive hypersensitivity, halted nNOS overproduction, and decreased IL‐1β and IL‐6 levels (Martucci et al., 2008). SC may produce and release these factors (Murwani, Hodgkinson, & Armati, 1996), although the majority of the literature suggests that they are mainly produced by nerve‐infiltrating immune cells. More research is needed to understand if and/or how P2X receptors in SCs contribute to the regulation of pain in the PNS.
In general, Gs and Gi coupled P2Y receptors have been proposed to have opposing effects in pain (Malin & Molliver, 2010). In cutaneous sensory neurons, Molliver, Rau, McIlwrath, Jankowski, and Koerber (2011) suggested that P2Y1 is important for the normal heat sensitivity of polymodal nociceptors, and P2Y1 knockdown during cutaneous inflammation reduced afferent sensitization to heat stimuli (Jankowski et al., 2012; Lu et al., 2017). Queme, Ross, Lu, Hudgins, and Jankowski (2016) suggested a role for P2Y1 in muscle afferent sensitization after ischemia/reperfusion injury. There is also evidence showing that P2RY2 could be involved in thermal hypersensitivity (Moriyama et al., 2003). Stucky, Medler, and Molliver (2004) showed that receptors P2YR2 and P2RY4 are involved in pain by promoting sustained action potential firing of C‐fibers in mice, and P2Y2 can regulate both mechanical and thermal sensitivity in cutaneous sensory neurons (Malin et al., 2008; Molliver et al., 2016). The contribution of glial cells to these findings is still unknown.
UTP and UDP can also have analgesic effects. For example, UTP or UDP administration to rats increases the mechanical nociceptive threshold and thermal nociceptive latency (Okada, Nakagawa, Minami, & Satoh, 2002). Trigeminal neurons carrying proprioceptive, tactile, and nociceptive information upregulate P2RY receptors on SGCs, suggesting the use of P2RY receptor antagonists in the treatment of trigeminal pain (Magni, Merli, Verderio, Abbracchio, & Ceruti, 2015). However, more research is needed to clarify the roles of P2YRs in SCs in relation to pain, and peripheral versus central nervous system contributions to pain.
It is well documented that humans experience pain upon administration of adenosine (Bleehen & Keele, 1977; Sylven, Jonzon, Fredholm, & Kaijser, 1988), and administration of an A2R agonist enhances pain responses in mice (Karlsten, Gordh, & Post, 1992), as does an A3R agonist in rats (Sawynok, Zarrindast, Reid, & Doak, 1997). However, adenosine can also reduce pain. Administration of an A1 receptor agonist to the hind paw of a rat produced anti‐nociception (Gong et al., 2010; Taiwo & Levine, 1990) and adenosine infusion reduced the area of dynamic tactile allodynia in patients suffering from neuropathic pain (Sjolund et al., 2001). A2B receptor agonists have shown spinal anti‐nociceptive effects in preclinical neuropathic pain models (Sawynok, 2016). A3R agonists have recently been suggested to alleviate persistent pain states. A3A receptor activation showed analgesic effects and reduced the excitability of spinal neurons by inhibiting nociception through the activation of serotonergic and noradrenergic neural circuits in the CNS (Little et al., 2014). Whether these receptors might have roles in SCs is not known. Taken together, while SCs have a clear role in pain development (Wei, Ying, Wenfeng, & Gang, 2019) purinergic receptor signaling within SCs may play a significant and underappreciated role in pain processing which requires further investigation. An overview of receptors implicated in response to nerve damage and pain is shown in (Figure 4c).
11. THERAPEUTIC POTENTIAL OF TARGETING PURINERGIC RECEPTORS
Currently, purinergic receptors are being targeted for their potential to ameliorate central nervous system neurodegenerative disorders, autoimmune disease, inflammation, and cancer (Di Virgilio, 2015; Di Virgilio & Adinolfi, 2017; Rafehi & Muller, 2018), and purinergic signaling may be targetable in peripheral nerve disorders (Faroni et al., 2014; Nobbio et al., 2014; Rodella et al., 2017). Given that several P2X and P2Y receptors are implicated in mediating the release of cytokines and modulating SC development, targeting one or several receptors may show efficacy in PNS‐specific contexts. While the lack of conditional alleles to study specific receptors in individual cell types has limited progress, the availability of agonists and antagonists selective for specific purinergic receptors, and/or inhibitors of ectonucleotidases are anticipated to enable defining the effects of individual agonists and receptors. These reagents have been summarized in recent reviews (Rafehi & Muller, 2018). Unfortunately, many P2RY analogs and antagonists have poor oral bioavailability, are rapidly degraded by ectonucleotidases, and/or show insufficient selectivity, so that testing effects of P2RY awaits next‐generation delivery strategies. However, in the CNS, P2RX7 antagonists prevented demyelination in autoimmune encephalomyelitis in rodent models (Matute et al., 2007) and prevented secondary neurological injury after spinal cord injury (Peng et al., 2009); these agents are yet to be tested in the PNS. In conclusion, there is strong evidence supporting the idea that targeting purinergic receptors has the potential for treating PNS disease. However, more research is needed to define the roles of purinergic receptors and how certain pathologies affect individual receptors and signaling cascades.
ACKNOWLEDGMENTS
This work was supported by grants NIH‐R01‐NS28840, NIH‐R01‐NS105715, and NIH‐R37‐NS083580 and the Department of Defense Program on Neurofibromatosis W81XWH‐17‐1‐010289 to N. R. J. P‐C was supported by NIH‐T32‐NS007453 and a Children's Tumor Foundation Young Investigator Award. R. A. C. was a Pelotonia Postdoctoral Fellow of The Ohio State University Research Alliance.
Patritti‐Cram J, Coover RA, Jankowski MP, Ratner N. Purinergic signaling in peripheral nervous system glial cells. Glia. 2021;69:1837–1851. 10.1002/glia.23969
Funding information Children's Tumor Foundation Young Investigator Award, Grant/Award Number: CTF‐2020‐01‐006; Department of Defense Program on Neurofibromatosis, Grant/Award Number: W81XWH‐17‐1‐010289; National Institutes of Health, Grant/Award Numbers: NIH‐R01‐NS105715, NIH‐R01‐NS28840, NIH‐R37‐NS083580, NIH‐T32‐NS007453; The Ohio State University Research Alliance., Grant/Award Number: Pelotonia Postdoctoral Fellow
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Albuquerque, E. , Pereira, E. , Alkondon, M. , & Rogers, S. (2009). Mammalian nicotinic acetylcholine receptors: From structure to function. Physiological Reviews, 89, 73–120. 10.1152/physrev.00015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi, S. , Liao, Z. , Gonzalez, A. , Chorna, N. E. , Seye, C. I. , Weisman, G. A. , & Erb, L. (2005). The P2Y2 nucleotide receptor interacts with αv Integrins to activate go and induce cell migration. Journal of Biological Chemistry, 280(47), 39050–39057. 10.1074/jbc.M504819200 [DOI] [PubMed] [Google Scholar]
- Barclay, J. , Patel, S. , Dorn, G. , Wotherspoon, G. , Moffatt, S. , Eunson, L. , … Ganju, P. (2002). Functional downregulation of P2X3receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. The Journal of Neuroscience, 22(18), 8139–8147. 10.1523/JNEUROSCI.22-18-08139.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden, J. , Cottee, L. , & Bennett, M. (1999). Vesicle‐associated proteins and P2X receptor clusters at single sympathetic varicosities in mouse vas deferens. Journal of Neurocytology, 28, 469–480. [DOI] [PubMed] [Google Scholar]
- Berti‐Mattera, L. , Wilkins, P. , Madhun, Z. , & Suchovsky, D. (1996). P2‐purigenic receptors regulate phospholipase C and adenylate cyclase activities in immortalized Schwann cells. Biochemical Journal, 314(2), 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen, T. , & Keele, C. (1977). Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain, 3(4), 367–377. [DOI] [PubMed] [Google Scholar]
- Burnstock, G. (1972). Purinergic nerves. Pharmacological Reviews, 24(3), 509–581. [PubMed] [Google Scholar]
- Burnstock, G. (2014). Purinergic signalling: From discovery to current developments. Experimental Physiology, 99(1), 16–34. 10.1113/expphysiol.2013.071951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. (2016). P2X ion channel receptors and inflammation. Purinergic Signalling, 12, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. , Campbell, G. , Satchell, D. , & Smythe, A. (1970). Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non‐adrenergic inhibitory nerves in the gut. British Journal of Pharmacology, 40(4), 668–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. , & Verkhratsky, A. (2012). Purinergic signalling in the central nervous system. SpringerLink. 433‐581. 10.1007/978-3-642-28863-0_8. [DOI]
- Chen, L. , Liu, Y. , Yue, K. , Ru, Q. , Xiong, Q. , Ma, B. , … Li, C. (2016). Differential expression of ATP‐gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signal, 12, 79–87. 10.1007/s11302-015-9481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Xia, S. , Sun, Y. , Li, M. , Song, X. , Li, G. , … Chen, D. (2015). Expression of purinergic receptor P2Y4 in Schwann cell following nerve regeneration. International Journal of Clinical and Experimental Medicine, 8(8), 13203–13210. [PMC free article] [PubMed] [Google Scholar]
- Chessell, I. , Hatcher, J. , Bountra, C. , Michel, A. , Hughes, J. , Green, P. , … Buell, G. (2005). Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain, 114(3), 386–396. 10.1016/j.pain.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Colomar, A. , Marty, V. , Medina, C. , Combe, C. , Parnet, P. , & Amedee, T. (2003). Maturation and release of interleukin‐1β by lipopolysaccharide‐primed mouse Schwann cells require the stimulation of P2X7Receptors. Journal of Biological Chemistry, 278, 30732–30740. 10.1074/jbc.M304534200 [DOI] [PubMed] [Google Scholar]
- Coover, R. , Healy, T. , Guo, L. , Chaney, K. , Hennigan, R. , Thomson, C. , … Ratner, N. (2018). Tonic ATP‐mediated growth suppression in peripheral nerve glia requires arrestin‐PP2 and is evaded in NF1. Acta Neuropathologica Communications, 6(1), 127. 10.1186/s40478-018-0635-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabid, H. , St‐Pierre‐See, A. , & Robitaille, R. (2018). Purinergic‐dependent glial regulation of synaptic plasticity of competing terminals and synapse elimination at the neuromuscular junction. Cell Reports, 25, 2070–2082. [DOI] [PubMed] [Google Scholar]
- Del Puerto, A. , Wandosell, F. , & Garrido, J. (2013). Neuronal and glial purinergic receptors functions in neuron development and brain disease. Frontiers in Cellular Neuroscience, 7, 1–15. 10.3389/fncel.2013.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir, I. , Tieftrunk, E. , Schorn, S. , Saricaoglu, Ö. , Pfitzinger, P. , Teller, S. , … Ceyhan, G. (2016). Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut, 65, 1001–1014. [DOI] [PubMed] [Google Scholar]
- DeWire, S. , Ahn, S. , Lefkowitz, R. , & Shenoy, S. (2007). Beta‐arrestins and cell signaling. Annual Review of Physiology, 69, 483–510. 10.1146/annurev.physiol.69.022405.154749 [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F. (2015). P2RX7: A receptor with a split personality in inflammation and cancer. Molecular & Cellular Oncology, 3(2), e1010937. 10.1080/23723556.2015.1010937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio, F. , & Adinolfi, E. (2017). Extracellular purines, purinergic receptors and tumor growth. Oncogene, 36(3), 293–303. 10.1038/onc.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C. , & Uboqu, E. (2018). GDNF enhances human blood‐nerve barrier function in vitro via MAPK signaling pathways. Tissue Barriers, 6(4), 1–22. 10.1080/21688370.2018.1546537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroni, A. , Rothwell, S. , Grolla, A. , Terenghi, G. , Magnaghi, V. , & Verkhratsky, A. (2013). Differentiation of adipose‐derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death & Disease, 4(7), e743. 10.1038/cddis.2013.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroni, A. , Smith, R. , Procacci, P. , Castelnovo, L. , Puccianti, E. , Reid, A. , … Verkhrastsky, A. (2014). Purinergic signaling mediated by P2X7 receptors controls myelination. Journal of Neuroscience Research, 92, 1259–1269. 10.1002/jnr.23417 [DOI] [PubMed] [Google Scholar]
- Fields, R. (2015). A new mechanism of nervous system plasticity: Activity‐dependent myelination. Nature Review Neuroscience, 16(12), 756–767. 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R. , & Ni, Y. (2010). Nonsynaptic communication through ATP release from volume‐activated anion channels in axons. Science Signaling, 3(142), ra73. 10.1126/scisignal.2001128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, S. , Herzog, D. , Sumara, G. , Buchmann‐Moller, S. , Civenni, G. , Wu, X. , … Sommer, L. (2009). Stage‐specific control of neural crest stem cell proliferation by the small rho GTPases Cdc42 and Rac1. Cell Press, 4(3), 236–247. 10.1016/j.stem.2009.01.017 [DOI] [PubMed] [Google Scholar]
- Gabel, C. (2007). P2 purinergic receptor modulation of cytokine production. Purinergic Signalling, 3, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, N. , Priego, M. , Hurtado, E. , Obis, T. , Santafe, M. , Tomas, M. , … Tomas, J. (2014). Adenosine A2B and A3 receptor location at the mouse neuromuscular junction. Journal of Anatomy, 225(1), 109–117. 10.1111/joa.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannuzzo, A. P. (2015). The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Molecular Cancer, 14, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani, A. , Sarti, A. , & Di Virgilio, F. (2019). Extracellular nucleotides and nucleosides as signalling molecules. Immunology Letters, 205, 16–24. [DOI] [PubMed] [Google Scholar]
- Gong, D. Z. (2019). The m6A‐suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP‐induced Ca2+ influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. Journal of Experimental & Clinical Cancer Research, 38, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Q. , Li, Y. , Xin, W. , Wei, X. , Cui, Y. , Wang, J. , … Liu, X. (2010). Differential effects of adenosine A1 receptor on pain‐related behavior in normal and nerve‐injured rats. Brain Research, 1361, 23–30. 10.1016/j.brainres.2010.09.034 [DOI] [PubMed] [Google Scholar]
- Hasko, G. , Kuhel, D. , Chen, J. , Schwarzschild, M. , Deitch, E. , Mabley, J. , … Szabo, C. (2000). Adenosine inhibits IL‐12 and TNF‐α production via adenosine A2a receptor‐dependent. The FASEB Journal, 14, 2065–2074. 10.1096/fj.99-0508com [DOI] [PubMed] [Google Scholar]
- Hasko, G. , Linden, J. , Crostein, B. , & Pacher, P. (2008). Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nature Reviews Drug Discovery, 7(9), 759–770. 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia, D. , Feng, C. , Hennig, G. , Renden, R. , & Gould, T. (2018). Ctivity‐induced Ca2+ signaling in perisynaptic Schwann cells of the early postnatal mouse is mediated by P2Y1 receptors and regulates muscle fatigue. eLife, 7, 30839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino, D. , Sagara, H. , Suzuki, J. , Kanemaru, K. , Okubo, Y. , & Iino, M. (2015). Neuronal regulation of Schwann cell mitochondrial Ca2+ signaling during myelination. Cell Press, 12(12), 1951–1959. 10.1016/j.celrep.2015.08.039 [DOI] [PubMed] [Google Scholar]
- Inoue, K. (2006). The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacology & Therapeutics, 109(1–2), 210–226. 10.1016/j.pharmthera.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Irnich, D. , Burgstahler, R. , Bostock, H. , & Grafe, P. (2001). ATP affects both axons and Schwann cells of unmyelinated C fibres. Pain, 92(3), 343–350. 10.1016/s0304-3959(01)00277-9 [DOI] [PubMed] [Google Scholar]
- Jankowski, M. , Rau, K. , Soneji, D. , Ekmann, K. , Anderson, C. , Molliver, D. , & Koerber, H. (2012). Purinergic receptor P2Y1 regulates polymodal C‐fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain, 153, 410–419. 10.1016/j.pain.2011.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J. , Jo, H. , Kwon, H. , & Jeong, N. (2014). ATP release through lysosomal exocytosis from peripheral nerves: The effect of lysosomal exocytosis on peripheral nerve degeneration and regeneration after nerve injury. BioMed Research International, 2014, 1–6. 10.1155/2014/936891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsten, R. , Gordh, T. , & Post, C. (1992). Local Antinociceptive and hyper algesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacology & Toxicology, 70(6), 434–438. [DOI] [PubMed] [Google Scholar]
- Kim, H. , Rosenbaum, T. , Marchionni, M. , Ratner, N. , & DeClue, J. (1995). Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene, 11(2), 325–335. [PubMed] [Google Scholar]
- Kobayashi, K. , Fukuoka, T. , Yamanaka, H. , Dai, Y. , Obata, K. , Tokunaga, A. , & Noguchi, K. (2006). Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. The Journal of Comparative Neurology, 498, 443–454. 10.1002/cne.21066 [DOI] [PubMed] [Google Scholar]
- Kreckler, L. , Wan, T. , Ge, Z. , & Auchampach, J. (2006). Adenosine inhibits tumor necrosis factor‐α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. The Journal of Pharmacology and Experimental Therapeutics, 317, 172–180. 10.1124/jpet.105.096016 [DOI] [PubMed] [Google Scholar]
- Lamarca, A. , Gella, A. , Martiañez, T. , Segura, M. , Figueiro‐Silva, J. , Grijota‐Martinez, C. , … Casals, N. (2014). Uridine 59‐triphosphate promotes in vitro Schwannoma cell migration through matrix Metalloproteinase‐2 activation. PLoS One, 9(6), e98998. 10.1371/journal.pone.0098998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas, C. M. , Rieger, J. , & Linden, J. (2005). A2A adenosine receptor induction inhibits IFN‐γ production in immune CD4+ T cells. The Journal of Immunology, 174, 1073–1080. 10.4049/jimmunol.174.2.1073 [DOI] [PubMed] [Google Scholar]
- Lazarowski, E. , & Harden, T. (2015). UDP‐sugars as extracellular signaling molecules: Cellular and physiologic consequences of P2Y14 receptor activation. Molecular Pharmacology, 88, 151–160. 10.1124/mol.115.098756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz, R. , Rajagopal, K. , & Whalen, E. (2006). New roles for β‐arrestins in cell signaling: Not just for seven‐transmembrane receptors. Molecular Cell, 24(5), 643–652. 10.1016/j.molcel.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Lin, J. , Liu, F. , Zhang, Y. , Song, N. , Liu, M. , Fang, X. , … Shen, J. (2019). P2Y14 receptor is functionally expressed in satellite glial cells and mediates interleukin‐1β and chemokine CCL2 secretion. Journal of Cellular Physiology, 234, 21199–21210. 10.1002/jcp.28726 [DOI] [PubMed] [Google Scholar]
- Little, J. , Ford, A. , Symons‐Liquori, A. , Chen, Z. , Janes, K. , Doyle, T. , … Salvemini, D. (2014). Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain, 138(1), 28–35. 10.1093/brain/awu330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. , Werry, E. , & Bennett, m. (2005). Secretion of ATP from Schwann cells in response to uridine triphosphate. The European Journal of Neuroscience, 21(1), 151–160. 10.1111/j.1460-9568.2004.03831.x [DOI] [PubMed] [Google Scholar]
- Lu, P. , Hudgins, R. , Liu, X. , Ford, Z. , Hofmann, M. , Queme, L. , & Jankowski, M. (2017). Upregulation of P2Y1 in neonatal nociceptors regulates heat and mechanical sensitization during cutaneous inflammation. Molecular Pain, 13, 174480691773025. 10.1177/1744806917730255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni, G. , Merli, D. , Verderio, C. , Abbracchio, M. , & Ceruti, S. (2015). P2Y2 receptor antagonists as anti‐allodynic agents in acute and sub‐chronic trigeminal sensitization: Role of satellite glial cells. Glia, 63(7), 1256–1269. 10.1002/glia.22819 [DOI] [PubMed] [Google Scholar]
- Malin, S. , Davis, B. , Koerber, H. , Reynolds, I. , Albers, K. , & Molliver, D. (2008). Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain, 138, 484–496. 10.1016/j.pain.2008.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin, S. A. , & Molliver, D. C. (2010). Gi‐ and Gq‐coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Molecular Pain, 6, 1744‐8069‐6‐21. 10.1186/1744-8069-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhaes, M. , Cesar, M. , Justo, R. , Geller, M. , Suchmacher, M. , & Cisne, R. (2016). The role of nucleotides in glial cells during peripheral nerve trauma and compressive disorders. IntechOpen. DOI: 10.5772/68068. [DOI]
- Martiañez, T. , Lamarca, A. , Casals, N. , & Gella, A. (2013). N‐cadherin expression is regulated by UTP in schwannoma cells. Purinergic Signal, 9(2), 259–270. 10.1007/s11302-012-9348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci, C. , Trovato, A. , Costa, B. , Borsani, E. , Franchi, S. , Magnaghi, V. , … Colleoni, M. (2008). The purinergic antagonist PPADS reduces pain related behaviours and interleukin‐1β, interleukin‐6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain, 137(1), 81–95. 10.1016/j.pain.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Matute, C. , Torre, I. , Perez‐Cerda, F. , Perez‐Samartin, A. , Alberdi, E. , Etxebarria, E. , … Domercq, M. (2007). P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. Journal of Neuroscience, 27, 9525–9533. 10.1523/JNEUROSCI.0579-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring, F. , Cowie, A. , Menzel, A. , Weyer, A. , Grzybowski, M. , Arzua, T. , … Stucky, C. (2018). Keratinocytes mediate innocuous and noxious touch via ATP‐P2X4 signaling. eLife, 7, 31684. 10.7554/eLife.31684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver, D. , Rau, K. , Jankowski, M. , Soneji, D. , Baumbauer, K. , & Koerber, H. (2016). Deletion of the murine ATP/UTP receptor P2Y2 alters mechanical and thermal response properties in polymodal cutaneous afferents. Neuroscience, 332, 223–230. 10.1016/j.neuroscience.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver, D. , Rau, K. , McIlwrath, S. , Jankowski, M. , & Koerber, H. (2011). The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Molecular Pain, 7, 1744‐8069‐7‐13. 10.1186/1744-8069-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje, P. , Athauda, G. , & Wood, P. (2008). Protein kinase A‐mediated gating of neuregulin‐dependent ErbB2‐ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. The Journal of Biological Chemistry, 283(49), 34087–34100. 10.1074/jbc.M802318200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk, K. , Feltri, M. , & Taveggia, C. (2015). New insights on Schwann cell development. Glia, 63(8), 1376–1393. 10.1002/glia.22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama, T. , Iisa, T. , Kobayashi, K. , Higashi, T. , Fukuoka, T. , Tsumura, H. , … Tominaga, M. (2003). Possible involvement of P2Y2Metabotropic receptors in ATP‐induced transient receptor potential Vanilloid receptor 1‐mediated thermal hypersensitivity. Journal of Neuroscience, 23(14), 6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murwani, R. , Hodgkinson, S. , & Armati, P. (1996). Tumor necrosis factor alpha and interleukin‐6 mRNA expression in neonatal Lewis rat Schwann cells and a neonatal rat Schwann cell line following interferon gamma stimulation. Journal of Neuroimmunology, 71, 65–71. [DOI] [PubMed] [Google Scholar]
- Naganuma, M. , Wiznerowicz, E. B. , Lappas, C. M. , Linden, J. , Worthington, M. T. , & Ernst, P. B. (2006). Cutting edge: Critical role for A2A adenosine receptors in the T cell‐mediated. The Journal of Immunology, 177, 2765–2769. 10.4049/jimmunol.177.5.2765 [DOI] [PubMed] [Google Scholar]
- Nobbio, L. , Sturla, L. , Florese, F. , Usai, C. , Basile, G. , Moreschi, I. , … Bruzzone, S. (2009). P2X7‐mediated increased intracellular calcium causes functional derangement in Schwann cells from rats with CMT1A neuropathy. Journal of Biological Chemistry, 284, 23146–23158. 10.1074/jbc.M109.027128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobbio, L. , Visigalli, D. , Mannino, E. , Fiorese, F. , Kassack, M. , Sturla, L. , … Schenone, A. (2014). The diadenosine homodinucleotide P18 improves in vitro myelination in experimental Charcot‐Marie‐tooth type 1A. Journal of Cellular Biochemistry, 115(1), 161–167. 10.1002/jcb.24644 [DOI] [PubMed] [Google Scholar]
- Nualart‐Marti, A. , del Molino, E. , Grandes, X. , Bahima, L. , Martin‐Satue, M. , Puchal, R. , … Solsona, C. (2013). Role of connexin 32 hemichannels in the release of ATP from peripheral nerves. Glia, 61(12), 1976–1986. 10.1002/glia.22568 [DOI] [PubMed] [Google Scholar]
- Oberlander, M. , & Berghoff, M. (2010). Effects of the CC chemokine receptor 2 in mice deficient for the myelin protein zero (P0). Molecular and Cellular Neurosciences, 45, 59–65. 10.1016/j.mcn.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Okada, M. , Nakagawa, T. , Minami, M. , & Satoh, M. (2002). Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. Journal of Pharmacology and Experimental Therapeutics, 303(1), 66–73. 10.1124/jpet.102.036079 [DOI] [PubMed] [Google Scholar]
- Park, M. K. (2019). Involvement of the P2X7 receptor in the migration and metastasis of tamoxifen‐resistant breast cancer: Effects on small extracellular vesicles production. Scientific Reports, 9, 11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, W. , Cotrina, M. , Han, X. , Yu, H. , Bekar, L. , Blum, L. , … Nedergaard, M. (2009). Systemic administration of an antagonist of the ATP‐sensitive receptor P2X7 improves recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 106, 12489–12493. 10.1073/pnas.0902531106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, S. , Luo, R. , Liebescher, I. , Giera, S. , Jeong, S. , Mogha, A. , … Monk, K. (2015). The adhesion GPCR GPR126 has distinct, domain‐dependent functions in Schwann cell development mediated by interaction with laminin‐211. Neuron, 85(4), 755–757. 10.1016/j.neuron.2014.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queme, L. , Ross, J. , Lu, P. , Hudgins, R. , & Jankowski, M. (2016). Dual modulation of nociception and cardiovascular reflexes during peripheral ischemia through P2Y1 receptor‐dependent sensitization of muscle afferents. Journal of Neuroscience, 36, 19–30. 10.1523/JNEUROSCI.2856-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queme, L. , Weyler, A. , Cohen, E. , Hudgins, R. , & Jankowski, M. (2020). A dual role for peripheral GDNF signaling in nociception and cardiovascular reflexes in the mouse. Proceedings of the National Academy of Sciences of the United States of America, 117, 698–707. 10.1073/pnas.1910905116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafehi, M. , & Muller, C. (2018). Tools and drugs for uracil nucleotide‐activated P2Y receptors. Pharmacology & Therapeutics, 190, 24–80. 10.1016/j.pharmthera.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Ratner, N. , & Miller, S. (2015). A RASopathy gene commonly mutated in cancer: The neurofibromatosis type 1 tumour suppressor. Nature Reviews. Cancer, 15, 290–301. 10.1038/nrc3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille, R. (1995). Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. The Journal of Neuroscience, 15(11), 7121–7131. 10.1523/jneurosci.15-11-07121.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodella, U. , Negro, S. , Scorzeto, M. , Bergamin, E. , Jalink, K. , Montecucco, C. , … Rigoni, M. (2017). Schwann cells are activated by ATP released from neurons in an invitro cellular model of Miller fisher syndrome. Disease Models & Mechanisms, 10(5), 597–560. 10.1242/dmm.027870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, H. , & Burnstock, G. (2003). Localisation of P2Y 1 and P2Y 4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochemistry and Cell Biology, 120(5), 415–426. 10.1007/s00418-003-0579-3 [DOI] [PubMed] [Google Scholar]
- Sanes, J. , & Lichtman, J. I. (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Reviews. Neuroscience, 2, 791–805. [DOI] [PubMed] [Google Scholar]
- Sawynok, J. (2016). Adenosine receptors target for pain. Neuroscience, 338, 1–18. 10.1016/j.neuroscience.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Sawynok, J. , Zarrindast, M. , Reid, A. , & Doak, G. (1997). Adenosine A3 receptor activation produces nociceptive behaviour and edema by release of histamine and 5‐hydroxytryptamine. European Journal of Pharmacology, 333(1), 1–7. 10.1016/s0014-2999(97)01110-2 [DOI] [PubMed] [Google Scholar]
- Senarath, K. , Kankanamqe, D. , Samaradivakara, S. , Ratnayake, K. , Tennakoon, M. , & Karunarathne, A. (2018). Regulation of G protein βγ signaling. International Review of Cell and Molecular Biology, 339, 133–191. 10.1016/bs.ircmb.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Sevingny, C. , Li, L. , Awad, A. , Huang, L. , McDuffie, M. , … Okusa, M. (2007). Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. Journal of Immunology, 178, 4240–4249. 10.4049/jimmunol.178.7.4240 [DOI] [PubMed] [Google Scholar]
- Shen, D. , Chu, F. , Lang, Y. , Geng, Y. , Zheng, X. , Zhu, J. , & Liu, K. (2018). Beneficial or harmful role of macrophages in Guillain‐Barré syndrome and experimental autoimmune neuritis. Mediators of Inflammation, 2018, 1–10. 10.1155/2018/4286364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, S. , Brito, R. , Mukherjea, D. , Rybak, L. , & Ramkumar, V. (2014). Adenosine receptors: Expression, function and regulation. International Journal of Molecular Sciences, 15(2), 2024–2052. 10.3390/ijms15022024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y. , Chung, H. , Park, C. , Jung, J. , & Jeong, N. (2014). Adenosine 5′‐triphosphate (ATP) inhibits schwann cell demyelination during Wallerian degeneration. Cellular and Molecular Neurobiology, 34(3), 361–368. 10.1007/s10571-013-0020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolund, K. , Belfrage, M. , Karlsten, R. , Segerdahl, M. , Arner, S. , Gordh, T. , & Solevi, A. (2001). Systemic adenosine infusion reduces the area of tactile allodynia in neuropathic pain following peripheral nerve injury: A multi‐centre, placebo‐controlled study. European Journal of Pain, 5, 199–209. 10.1053/eujp.2001.0237 [DOI] [PubMed] [Google Scholar]
- Stevens, B. , & Fields, R. (2000). Response of Schwann cells to action potentials in development. Science, 287(5461), 2267–2271. [DOI] [PubMed] [Google Scholar]
- Stevens, B. , Ishibashi, T. , Chen, J. , & Fields, R. (2004). Adenosine: An activity‐dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biology, 1(01), 23–24. 10.1017/s1740925x04000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street, S. E. , Kramer, N. J. , Walsh, P. L. , Taylor‐Blake, B. , Yadav, M. C. , King, I. F. , … Zylka, M. J. (2013). Tissue‐nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsa. Journal of Neuroscience, 33, 11314–11322. 10.1523/JNEUROSCI.0133-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky, C. , Medler, K. , & Molliver, D. (2004). The P2Y agonist UTP activates cutaneous afferent fibers. Pain, 109(1), 36–44. 10.1016/j.pain.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Su, W. , Wu, F. , Jin, Z. , Gu, Y. , Chen, Y. , Fei, Y. , … Chen, G. (2018). Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia, 67(1), 78–90. 10.1002/glia.23527 [DOI] [PubMed] [Google Scholar]
- Sylven, C. , Jonzon, B. , Fredholm, B. , & Kaijser, L. (1988). Adenosine injection into the brachial artery produces ischaemia like pain or discomfort in the forearm. Cardiovascular Research, 22(9), 674–678. [DOI] [PubMed] [Google Scholar]
- Taiwo, Y. , & Levine, J. (1990). Direct cutaneous hyperalgesia induced by adenosine. Neuroscience, 38(3), 757–762. [DOI] [PubMed] [Google Scholar]
- Wang, I. C. , Kroner, A. , Fischer, S. , Berghoff, M. , Kobsar, I. , Maurer, M. , & Martini, R. (2006). Role of immune cells in animal models for inherited peripheral neuropathies. Neuromolecular Medicine, 8(1–2), 175–190. 10.1385/nmm:8:1-2:175 [DOI] [PubMed] [Google Scholar]
- Wei, Z. , Ying, F. , Wenfeng, S. , & Gang, C. (2019). Emerging role of Schwann cells in neuropathic pain: Receptors, glial mediators and myelination. Frontiers in Cellular Neuroscience, 13, 116–123. 10.3389/fncel.2019.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P. , Rosen, K. , Hedstrom, K. , Rey, O. , Guha, S. , Hart, C. , & Corfas, G. (2013). Nerve injury induces glial cell line‐derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC‐PKD pathway. Glia, 61, 1029–1040. 10.1002/glia.22491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, Z. , Xie, L. , Zhou, C. , Yuan, H. , Ouyang, S. , Fang, Z. , … Liang, S. (2018). P2Y12 receptor upregulation in satellite glial cells is involved in neuropathic pain induced by HIV glycoprotein 120 and 2′,3′‐dideoxycytidine. Purinergic Signal, 14(1), 47–58. 10.1007/s11302-017-9594-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Liu, Y. , Chen, D. , Yang, B. , Liu, T. , Li, J. , … Liu, Z. (2018). Microencapsulated Schwann cell transplantation inhibits P2X2/3 receptors overexpression in a sciatic nerve injury rat model with neuropathic pain. Neuroscience Letters, 676, 51–57. 10.1016/j.neulet.2018.03.063 [DOI] [PubMed] [Google Scholar]
- Zimmermann, H. (2011). Purinergic signaling in neural development. Seminars in Cell & Developmental Biology, 22, 194–204. [DOI] [PubMed] [Google Scholar]
- Zuchero, J. , & Barres, B. (2015). Glia in mammalian development and disease. Development, 142(22), 3805–3809. 10.1242/dev.129304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


