Summary
Enzymes are promising catalysts with high selectivity and activity under mild reaction conditions. However, their practical application has largely been hindered by their high cost and poor stability. Metal-organic frameworks (MOFs) as host materials show potential in protecting proteins against denaturing conditions, but a systematic study investigating the stabilizing mechanism is still lacking. In this study, we stabilized enzyme cytochrome c (cyt c) by encapsulating it in a hierarchical mesoporous zirconium-based MOF, NU-1000 against denaturing organic solvents. Cyt c@NU-1000 showed a significantly enhanced activity compared to the native enzyme, and the composite retained this enhanced activity after treatment with five denaturing organic solvents. Moreover, the composite was recyclable without activity loss for at least three cycles. Our cyt c@NU-1000 model system demonstrates that enzyme@MOF composites prepared via post-synthetic encapsulation offer a promising route to overcome the challenges of enzyme stability and recyclability that impede the widespread adoption of biocatalysis.
Subject areas: Chemistry, Green chemistry, Engineering, Biocatalysis, Materials chemistry
Graphical abstract

Highlights
-
•
Catalytic activity of cytochrome c was protected against denaturing organic solvents
-
•
The mesoporous MOF stabilized cytochrome c by preventing enzyme aggregation
-
•
The enzyme@MOF composite was recyclable for at least three cycles
-
•
This enzyme@MOF system is promising for developing robust biocatalyst
Chemistry; Green chemistry; Engineering; Biocatalysis; Materials chemistry;
Introduction
Catalysis is the backbone of many industrial processes, generating 30% of global gross domestic product (GDP) with an annual sale of nearly 1.5 trillion USD (Ma and Zaera, 2011). Biocatalysis, which employs enzymes as catalysts, is an emerging sector of the catalysis industry (Sheldon and Woodley, 2018; Sanchez and Demain, 2011). The global enzyme market recorded a value of 9.9 billion USD in 2019 and it will continue to grow at a rate of 7.1% through late 2020s for analyst projects (Chapman et al., 2018). Given their green, high yielding, and selective nature, enzymes are desirable for performing chemical transformations in the syntheses of fine chemicals, such as those in cosmetics (Khan and Rathod, 2015) and pharmaceuticals (DiRocco et al., 2017; Huffman et al., 2019; Savile et al., 2010; Bornscheuer et al., 2012). Enzyme-catalyzed chemical reactions can avoid harsh energy-intensive reaction conditions (Vicente and Dean, 2017), eliminate the use of heavy and precious metal catalysts (Sheldon et al., 2020; Savile et al., 2010), and potentially reduce the number of reaction steps required during traditional synthetic routes (McLaughlin et al., 2017; Huffman et al., 2019).
Despite these desirable qualities, to date, the number and diversity of biocatalysts employed in industry remains limited due to their high price and poor operational stability (Schoemaker et al., 2003; Hauer, 2020). For example, organic solvents are essential for many organic transformations owing to their good substrate solubility, ease of removal, and a range of polarity and protic or aprotic properties that prohibit side reactions (Dordick, 1989). Unfortunately, the presence of organic solvents often disrupts the hydrophobic effect, which leads to enzyme unfolding and aggregation, rendering them catalytically inactivate (Herskovits and Jaillet, 1969). Fractions of organic solvents are often used to enhance substrate solubility and reaction rates (Dai and Klibanov, 2000), but many enzymes are unable to withstand high fractions of organic solvents in the reaction mixture. Furthermore, the presence of water tends to favor side reactions such as hydrolysis and hampers product isolation. As a result, developing new strategies to enhance enzyme stability in organic solvents would greatly propel their application in the industry.
Researchers have pursued numerous strategies to enhance the stability of enzymes for biocatalysis. For example, protein engineering methods such as directed evolution can increase enzyme durability in organic solvents (Chen and Arnold, 1993; Stimple et al., 2020). However, separating homogeneous enzymes from the product streams for subsequent reuse remains a pressing problem. Immobilizing an enzyme in or on a solid-phase support can enhance enzyme stability and heterogenize the homogeneous biocatalyst, which enables facile separation and recycling of the catalyst (Vázquez-González et al., 2020). To date, conjugation, adsorption, and physical entrapment have allowed for the immobilization of enzymes in and on an assortment of solid supports (Tran and Balkus, 2011; Homaei et al., 2013) ranging from organic polymers (Ortiz et al., 2019; Kim et al., 2020) to silica (Hou et al., 2019; Deere et al., 2003; Takahashi et al., 2000). Nevertheless, because amorphous supports lack well-defined structures, characterizing the resultant enzyme@support composites poses a challenge, making it difficult to understand and improve existing immobilization systems.
Metal-organic frameworks (MOFs) are porous coordination networks comprising metal nodes and organic linkers assembled into periodic lattices (Li et al., 1999; Farha and Hupp, 2010; Zhou et al., 2012). The inherent porosity and structural diversity of this class of materials has shown great promise for several applications including but not limited to gas storage (Chen et al., 2020c; Ma and Zhou, 2010), separation (Addicoat et al., 2017; Idrees et al., 2020), catalysis (Syed et al., 2020; Goetjen et al., 2020; Chen et al., 2020b; Bavykina et al., 2020; Konnerth et al., 2020), and drug delivery (Wang et al., 2019, 2020a; Doonan et al., 2017; Li et al., 2020b; Luzuriaga et al., 2019; Chen et al., 2018). MOFs are also attractive host materials for encapsulating enzymes as their porosity enables high enzyme loading, their crystallinity facilitates detailed bulk characterization, and their modularity allows for the design of frameworks tailored toward specific proteins (Xia et al., 2020). The two primary strategies encapsulating enzymes in MOFs are (1) de novo encapsulation where MOFs are assembled around the enzymes (He et al., 2016) and (2) post-synthetic encapsulation (PSE) during which enzymes diffuse into a premade MOF (Drout et al., 2019; Majewski et al., 2017; Huang et al., 2020; Lian et al., 2017; Liang et al., 2021; Wang et al., 2020b). With de novo encapsulation processes, the size and shape of the enzyme do not limit the portfolio of acceptable MOF supports. Several reports have demonstrated that encapsulation using the de novo method can enhance an enzyme's stability against elevated temperatures and some organic solvents (Wang et al., 2020b; Hu et al., 2018). However, controlling the spatial distribution of enzyme molecules throughout the lattice and ensuring their accessibility to substrates is challenging using the de novo technique. On the other hand, using the PSE method, proteins diffuse into pores or channels of highly porous frameworks with nanometer-scale diameters that are either inherent to the MOF structure (Lykourinou et al., 2011; Li et al., 2018; Feng et al., 2015; Deng et al., 2012) or created as defects (Li et al., 2020a; Wu et al., 2017). Without limitations on synthetic conditions, a large library of inherently mesoporous MOFs is accessible. Compared to defect-based mesopores, the inherently mesoporous MOFs allow high throughput encapsulation and activity screenings with modularity that expedite the rational design for accommodating desired enzymes with various properties (Li et al., 2018). Furthermore, with the PSE method, when the dimension of the channel or pore of the MOFs matches the size of the protein molecule, proteins are only possible to be individually hosted either along the channel or inside the pore. This single-molecule encapsulation through PSE increases proteins' accessibility and eliminates their pathway to aggregation. However, among existing reports of enzyme encapsulation in hierarchical, mesoporous MOFs (Lykourinou et al., 2011; Feng et al., 2015; Deng et al., 2012) or its organic counterpart covalent-organic frameworks (Sun et al., 2018) through the PSE method, only a few have investigated the mechanism for stabilization in details, which are important for improving future enzyme@MOF system. Herein, we undertook a systematic investigation to evaluate the stability imparted to an enzyme after immobilization in a mesoporous MOF, namely, cytochrome c in NU-1000 (cyt c@NU-1000) (Scheme 1). We optimized the encapsulation conditions and investigated the stability and recyclability of cyt c@NU-1000 after treatment with five denaturing organic solvents by quantifying the composite's catalytic activity toward pyrogallol oxidation.
Scheme 1.
Schematic illustration of cytochrome c encapsulated in NU-1000, and the oxidation reaction of pyrogallol
Results and discussion
Cytochrome c is a relatively small enzyme involved in electron transport in mitochondria. Its structure (Mirkin et al., 2008) and activity (Prasad et al., 2002; Vazquez-Duhalt, 1999; Maiti et al., 2012; Childs and Bardsley, 1975; Feng et al., 2015) are well documented, and its engineered derivatives have shown unique enzymatic reactivity such as C–Si bond formation (Kan et al., 2016). The activity of cytochrome c can be quantified by monitoring its catalytic oxidation of UV-active substrates such as pyrogallol (Prasad et al., 2002; Vazquez-Duhalt, 1999; Maiti et al., 2012; Childs and Bardsley, 1975; Feng et al., 2015), making it a model candidate for our study. Based on the dimensions of cytochrome c (2.6 × 3.2 × 3.3 nm, PDB: 2b4z) (Mirkin et al., 2008), we chose NU-1000 as its host material, which has a mesopore with a diameter of 3.2 nm, matching the size of cytochrome c. NU-1000 consists of Zr6 clusters and tetracarboxylate pyrene-based linkers (Li et al., 2018). This MOF maintains excellent crystallinity and porosity when exposed to organic and aqueous media over a wide range of pH (Howarth et al., 2016) owing to its strong Zr–O bonds between the nodes and linkers. In addition to the framework’s mesoporous channel, it also features a 1.3 nm microporous channel capable of facilitating substrate diffusion (Li et al., 2016, 2018). In this study, cytochrome c was encapsulated into the mesopore of NU-1000 through the PSE technique. We were able to control the enzyme loading of the cyt c@NU-1000 composite, identify the cause of cytochrome c denaturation in organic solvents, circumvent this denaturation process through NU-1000 encapsulation, and recover the enzyme composite from reaction mixtures for repeated cycles of catalysis. Specifically, we assessed the enzymatic activity of free cytochrome c and cyt c@NU-1000 by the turnover number (TON) of pyrogallol oxidation product, purpurogallin, quantified by ultraviolet-visible (UV-Vis) spectrophotometry over 10 min at room temperature.
Enzyme encapsulation optimization
Very early during our study, water has shown to be an ineffective media for cytochrome c diffusion into NU-1000. Since previous reports have shown that buffer concentration influences enzyme loading in a different system through the PSE technique (Phipps et al., 2020), we first explored encapsulation conditions for obtaining the cyt c@NU-1000 composite using Tris buffer at four different concentrations (0.00 M, 0.25 M, 0.50M, and 0.90 M). Prior to assessing the composites’ activities, we examined the structural integrity of the resultant materials. The composite particles from all four encapsulation conditions retained the NU-1000 morphology (Figure S1) and crystallinity (Figure S2) as confirmed by scanning electron microscopy (SEM) images and powder X-ray diffraction (PXRD), respectively. More importantly, SEM energy-dispersive X-ray spectroscopy (EDS) line scans for Zr and S indicate a uniform distribution of cytochrome c throughout the NU-1000 particles under all conditions (Figure S3). We used two complementary techniques to quantify the amount of cytochrome c immobilized within the framework. We calculated the mass percent of cytochrome c by measuring the amount of iron and sulfur coming from cytochrome c and zirconium from NU-1000 node in the composite through elemental analysis, namely inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectroscopy (ICP-OES). We also calculated the enzyme loading by quantifying the residual amount of cytochrome c in the supernatant using UV-Vis (more details on these methods can be found in the STAR Methods). The enzyme loadings calculated from these different methods roughly agree with each other, and we observed that a higher Tris buffer concentration leads to higher cytochrome c loadings in NU-1000, up to 13% in 0.90 M Tris (Figure 1 and Table S1). To explore the driving forces leading to these differences, we measured the surface charge of cytochrome c and NU-1000 at the four different encapsulation conditions (0.00 M, 0.25 M, 0.50M, and 0.90 M). Zeta potentials of cytochrome c and NU-1000 particle carry the same charge in Millipore water (both positive) and Tris buffer (both negative) (Figure S4); thus, we excluded surface electrostatic interactions as an explanation for our observation. This result demonstrates that the ionic strength of the solution affects enzyme-MOF interactions and poses an interesting topic for future enzyme@MOF encapsulation investigation examining the kinetics and thermodynamics of immobilization.
Figure 1.
Mass percent of cytochrome c encapsulated in NU-1000 under different buffer conditions
Amount of cytochrome c encapsulated in NU-1000 determined by UV-Vis and ICP-OES (S) and ICP-MS (Fe) in 0.00 M, 0.25 M, 0.50 M, and 0.90 M Tris buffer. Error bars indicated standard deviations calculated from three independent runs.
Cyt c@NU-1000 stability against denaturing organic solvents
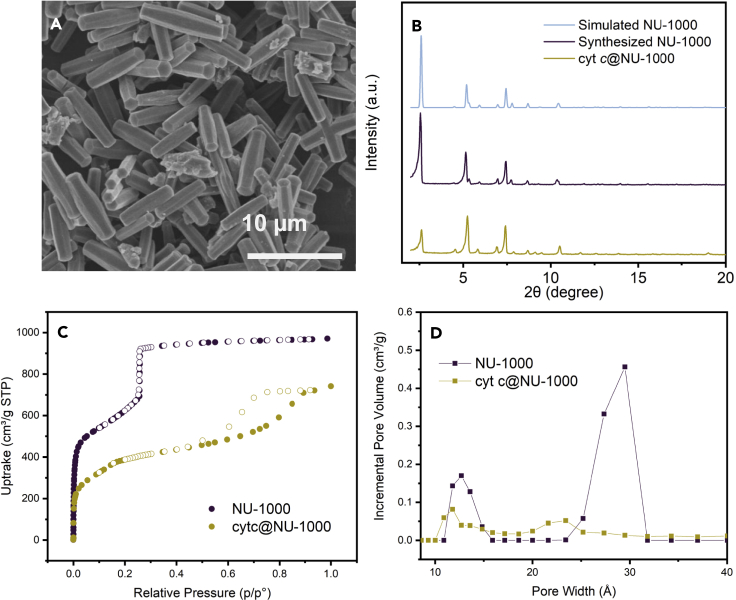
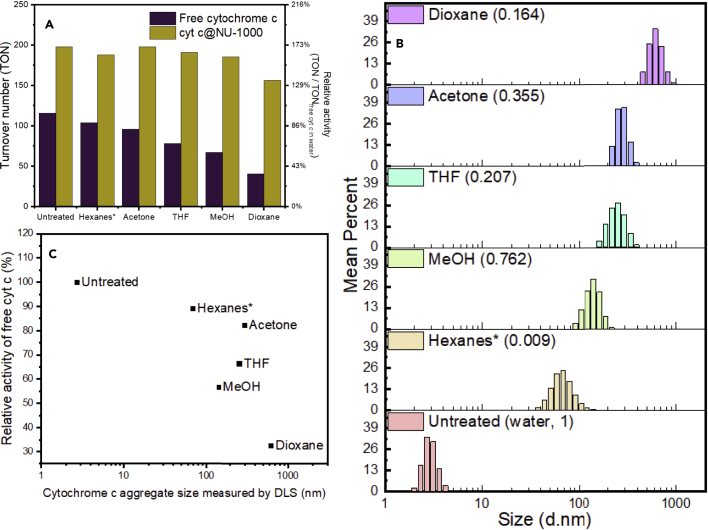
Based on our encapsulation results, we chose 0.50 M as our optimal condition for remainder of our study, given that this composite displays relatively high enzyme loading and its morphology and PXRD pattern closely match with those of the host NU-1000 material (Figures 2A and 2B). Decreases in N2 uptake (Figure 2C), mesopore volume (Figure 2D), and the intensity of the first peak in the PXRD pattern (Figure 2B) all indicate that cytochrome c occupies the 3 nm hexagonal pore. The N2 isotherm also indicates a 20% decrease in pore volume upon encapsulation, which roughly agrees with the cytochrome c loading mass ratio around 10% determined by UV-Vis and ICP. Enzymatic activity of cytochrome c and cyt c@NU-1000 was quantified by the TON of purpurogallin from pyrogallol oxidation over 10 min under identical enzyme concentration, and relative activity in percentage was calculated against untreated free cytochrome c. Untreated cytochrome c encapsulated in NU-1000 demonstrated an enhanced enzymatic activity compared to untreated free cytochrome c in accordance with our previous findings (Chen et al., 2020a). We next examined the composite's activity after exposure to 5 commonly used laboratory organic solvents: hexanes, acetone, tetrahydrofuran (THF), methanol, and dioxane (Figure 3A). After being incubated in organic solvents for 24 hr, all composites were isolated, and their enzymatic activity was assessed under the same condition (0.1 M Tris buffer, pH 7.5). After treating with organic media, free cytochrome c’s activity decreases with a maximum reduction of over 60% in dioxane. In contrast, maximum activity loss of cyt c@NU-1000 is only at 20% compared to the activity of the untreated composite, which is still approximately 30% higher than untreated native cytochrome c. Cyt c@NU-1000 treated with solvents other than dioxane largely retained its activity with decreases of less than 6%, demonstrating the importance of the NU-1000 framework in preserving cytochrome c activity (Figure 3A and Table S2). Initial rates of each reaction were calculated from the first 50s of the reaction (Table S3), showing all encapsulated enzymes with a higher rate than free enzyme. Post-catalysis cyt c@NU-1000 retained the morphology, crystallinity, and enzyme loading of the pre-catalysis materials (Figure S6).
Figure 2.
Characterization of cyt c@NU-1000 encapsulated in 0.50 M Tris buffer
(A) SEM image of cytochrome c@NU-1000 demonstrates the retention of the NU-1000 morphology. Scale bar indicates 10 µm.
(B) PXRD patterns of NU-1000 simulated (top trace in light blue), synthesized (activated, middle trace in maroon), and with cytochrome c encapsulated (activated, bottom trace in yellow) demonstrate the retention of crystallinity.
(C) N2 isotherms at 77k of NU-1000 (maroon) and cyt c@NU-1000 (yellow) show a porosity reduction because of enzyme encapsulation.
(D) DFT-calculated pore size distributions of NU-1000 (maroon) and cyt c@NU-1000 (yellow) suggest that the encapsulated enzyme occupies the framework's mesopore.
Figure 3.
Catalytic activities of free cytochrome c and cyt c@NU-1000 after treated with organic solvents
(A) Activity comparison between free and encapsulated cytochrome c for pyrogallol oxidation recovered from various denaturing solvents.
(B) Size distribution of free cytochrome c recovered from various solvents determined by dynamic light scattering (DLS) measurements. Relative polarity of the solvent is shown in parenthesis.
(C) Relative activity of free cytochrome c plotted against aggregate size after solvent treatment.
∗Note that among all solvents listed, hexanes is the only water-immiscible solvent.
This result prompted us to investigate the source of the enhanced stability of cyt c@NU-1000. Dynamic light scattering (DLS) measurements revealed that native cytochrome c aggregates within 24 h of exposure to organic solvents, forming aggregates up to 600 nm in diameter (Figure 3B). The extent of aggregation roughly follows the relative polarity values of the solvents (Reichardt and Welton, 2011) (Figure 3B), and the activity loss of free cytochrome c trends with the size of native cytochrome c aggregates (Figure 3C). Previous studies have reported that cytochrome c undergoes conformational changes and denaturation in many solvents, including alcohols and acetonitrile (Amin et al., 2016; Herskovits et al., 1970), and nonfunctional unfolded conformations of the enzyme aggregate more readily than folded states (Lin et al., 2016; Furkan et al., 2016). Native and solvent-treated cytochrome c shares similar diffuse-reflective UV-Vis solid-state spectra (Figure S5), showing low-spin ferricytochrome c as the predominate active center without major changes from the native state (Collinson and Bowden, 1992). Given that hexagonal pore can only accommodate single enzyme molecules along its channel, the retained activity was therefore largely attributed to the prevention of cytochrome c aggregation in the MOF matrix. Hexanes are an exception to the trend as it did not cause extensive aggregation and activity loss in native cytochrome c despite having the lowest relative polarity. This can be explained by the low solubility of cytochrome c in nonpolar solvents and hexanes’ immiscibility with water. For this experiment, native cytochrome c which is highly soluble in water, was first dissolved in 20 μL water and then diluted with 500 μL of solvent to circumvent solubility issues in dioxane and THF. In the case of hexanes, free cytochrome c mostly remained in the small aqueous phase even after sonication, which likely prevented hexanes from disrupting the enzyme’s structure as extensively as the water-miscible dioxane.
Cyt c@NU-1000 recyclability
Lastly, one of the primary benefits of employing heterogeneous catalysts is their facile separation from reaction mixtures and their subsequent reuse. Through a set of recycling experiments, we found that the composite material demonstrates excellent recyclability for up to 3 cycles even without accounting for physical material loss during the separation and recycling processes (Figure 4). Each reaction was carried out with the same amount of encapsulated cytochrome c, and each experiment was performed in triplicates. Catalytic activity of cyt c@NU-1000 was retained throughout the three cycles within one standard deviation. This demonstrates that despite the ability of solvent to permeate the MOF framework, the enzyme is largely protected against denaturation.
Figure 4.
Recyclability of cyt c@NU-1000 recovered from various denaturing solvents
Error bars indicated standard deviations calculated from three independent runs.
Conclusion
In this study, we encapsulated cytochrome c inside the 3.2 nm mesopore of NU-1000 using the post-synthetic encapsulation (PSE) technique. We were able to control the enzyme loading in the composite cyt c@NU-1000 system, achieving enzyme loadings exceeding 10% by mass. Cyt c@NU-1000 showed a significant increase in activity compared to native cytochrome c, and this enhanced activity was retained after treatment with five common denaturing organic solvents. The cyt c@NU-1000 composite recovered after solvent treatment was also recycled up to three times without major activity losses. Our comprehensive investigation of enzyme encapsulation poses a few interesting questions for future explorations in the field, specifically regarding the thermodynamic forces driving enzyme encapsulation and how properties such as ionic strength of the encapsulation media influence the encapsulation process. The excellent stability and recyclability of cyt c@NU-1000 after treatment with organic solvents demonstrated that our composite provides an excellent biocatalyst system with great potential to improve existing biocatalytic processes.
Limitations of the study
This work focuses on the cytochrome c and NU-1000 system, but given the large library of MOFs and enzymes with their distinct properties, different enzyme-MOF systems may exhibit varying trends for encapsulation and pathways for deactivation. Furthermore, the underlying mechanism that causes the difference in encapsulation under different buffer concentrations is still to be explored. Lastly, although we did not observe diffusion limiting the catalysis of cytc c@NU-1000, substrate-product diffusion in other enzyme@MOF systems should be considered.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Cytochrome c from bovine heart | Sigma | CAS: 9007-43-6 |
| UltraPureTM 1M Tris-HCl pH 7.5 | Invitrogen | CAS: 15567-027 |
| Water used in all experiments | Millipore Milli-Q water purification system | N/A |
| Zirconyl chloride octahydrate (ZrOCl2·8H2O) | Sigma-Aldrich | CAS: 13520-92-8 |
| Benzoic Acid (C6H5COOH) | Sigma-Aldrich | CAS: 65-85-0 |
| Dimethylformamide | Sigma-Aldrich | CAS: 68-12-2 |
| Trifluoroacetic acid (CF3COOH) | Sigma-Aldrich | CAS: 76-05-1 |
| Hydrochloric acid (HCl) | Sigma-Aldrich | CAS: 7647-01-0 |
| Acetone (CH3COCH3) | Sigma-Aldrich | CAS: 67-64-1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Omar K. Farha (o-farha@northwestern.edu).
Materials availability
NU-1000 was synthesized and characterized according to a reported method (Islamoglu et al., 2018). A more detailed procedure can be found under method details.
Data and code availability
This study did not generate any unique datasets or code.
Method details
General
Crystallinity of each NU-1000 materials was verified with powder X-ray diffraction (PXRD) on a STOE STADI P with CuKα1 radiation and compared to a simulated NU-1000 pattern. Porosity of NU-1000 and cyt c@NU-1000 were measured using isothermal N2 adsorption at 77 K on Micromeritics Tristar II 3020. Pore size distributions (PSDs) were calculated by N2 DFT model with slit geometry. Prior to N2 adsorption measurements, cyt c@NU-1000 was activated with supercritical CO2 and at 80°C under vacuum overnight on Micromeritics Smart VacPrep instrument. UV-vis measurements were performed on Shimadzu UV-2600 in a quartz cuvette. SEM images and SEM-EDS line scans were taken on Hitachi SU8030 equipped with Oxford AZtec software after 9 nm of Os plasma coating. ICP-OES was performed on Thermo iCap7600, and ICP-MS was performed on Thermo iCapQ instrument. DLS and zeta potential were measured by Zetasizer (Malvern Instruments Ltd, NanoZS).
NU-1000 synthesis
NU-1000 and its corresponding linker 4,4’,4’’,4’’’-(pyrene-1,3,6,8-tetrayl)tetrabenzoic acid (H4TBAPy) were synthesized following the published procedures (Islamoglu et al., 2018; Wang et al., 2016). In short, ZrOCl2·8H2O (0.97 g, 3.01 mmol) and benzoic acid (20 g, 164 mmol) were dissolved in 60 mL dimethylformamide (DMF) in a 100-mL glass bottle and sonicated for 15 min. The clear solution was incubated in an oven at 100°C for 1 h. H4TBAPy (0.4 g, 0.586 mmol) was dissolved in 20 mL DMF, sonicated for 15 min, and heated to 100°C for 1 h. After cooling down to room temperature, the H4TBAPy solution and trifluoroacetic acid (0.4 mL, 5.22 mmol) were added to the pre-made Zr node containing solution and sonicated for 15 min. The yellow suspension was placed in a pre-heated oven at 120°C for 18 h. After cooling down to room temperature, yellow powder was collected by centrifugation (7 min, 7500 rpm) and washed with DMF three times (~100 mL each, soaked ~1 h between washes). Then the yellow powder was suspended in 130 mL DMF in a 200-mL glass bottle, and 5 mL of 8 M aqueous HCl was added. This mixture was heated in an oven at 100°C for 18 h. After cooling to room temperature, the powder was isolated by centrifugation and washed with DMF three times (~100 mL each, soaked ~1 h between washes) and acetone three times (~300 mL each, soaked ~1 h between washes) and soaked in acetone for an additional 16 h. NU-1000 crystals were collected by centrifugation and dried in a vacuum oven at 120°C overnight, and then activated at Micromeritics Smart VacPrep instrument at 120°C under vacuum before characterization or experiments.
Materials preparation
For the encapsulation study, 0.2 mg of cytochrome c and 1 mg of NU-1000 were dispersed in 1 mL of 0.00 M (water), 0.25 M, 0.50 M, or 0.90 M Tris buffer in 1.5 mL Eppendorf tubes. Each sample was left on an incubator shaker at 350 rpm for 24 hr under room temperature. For activity determination, cytochrome c was encapsulated with 0.5 M Tris buffer, and the composite’s enzyme loading was determined by residual cytochrome c left in the supernatant by UV-Vis before each reaction. All NU-1000 particles used in the study are ~7.5 μm in size.
DLS measurements
Prior to dynamic light scattering measurements, 2 mg of native cytochrome c was dissolved in 20 uL Millipore water and diluted with 500 uL of solvent (hexanes, acetone, methanol, THF, dioxane). All solutions were sonicated until evenly dispersed. Solvents were dried off after 24 hours, and the dried enzyme was redissolved in 2 mL Millipore water (1 g/L). The aqueous solution was prepared by 2 mg of native cytochrome c in 2 mL 0.1 M Tris buffer.
Cytochrome c encapsulation determination in mass percent
The amount of enzyme encapsulated was determined by analyzing the supernatant with UV-Vis and the solid material by ICP. Free cytochrome c in the supernatant was quantified by the absorbance at 409 nm with absorbance normalized at 900 nm. The amount of encapsulation was determined against a 5-point calibration curve of cytochrome c in each buffer matrix (Figure S7), and each encapsulation experiment was performed in triplicate. Solid cyt c@NU-1000 materials were analyzed by ICP-OES for Zr and S, and by ICP-MS for Fe in nitric acid matrix with 6 calibration points. Each cytochrome c unit (MW: 12,327 Da) contains one iron atom from the heme active center and four sulfur atoms from two cysteine and two methionine residues, and we were able to calculate the mass percent of cytochrome c loading based on the ratios of iron and sulfur to zirconium from the NU-1000 Zr6 node (NU-1000, MW: 2184 g/mol).
Cytochrome c activity evaluation
Native and cyt c@NU-1000 (10 uL, 1.1 g/L, 90 uM) were treated with five different organic solvents, hexanes, acetone, THF, methanol (500 uL), and dioxane for 24 hours, and the enzyme was recovered by drying off the solvents under N2 flow. The activity was monitored in 0.1 M Tris buffer by oxidation of pyrogallol (Figure S9). Reactions were carried out with cytochrome c (0.9 uM), pyrogallol (5 mM), H2O2 (26.4 mM) in 0.1 M of Tris buffer in a total of 1 mL of water solution. Both the Tris buffer and water were purged by N2 for at least 10 minutes to reduce O2 content that may cause pyrogallol autoxidation.(Gao et al., 1998; Siegel and Siegel, 1958) Absorbance at 420 nm was recorded for 10 minutes immediately after the addition of pyrogallol and H2O2 solutions. Activity of cyt c@NU-1000 was determined similarly under the same cytochrome c concentration (0.9 uM) (Figure S9). Mass of cyt c@NU-1000 used in the reaction was determined based on mass percent of the encapsulated cytochrome c measured by UV-Vis. Activities were calculated by the increase of absorbance at 420 nm after 600s, normalized to the absorbance at 0 s and baseline reaction with all reagents but cytochrome c present (Figure S8). The decreasing baseline in the NU-1000 control likely results due to MOFs particles settling.
Recyclability test
Each cycle of the recycling experiments was carried in three independent trials, and the cyt c@NU-1000 material was recovered by centrifuging the reaction mixture after 600s reaction. Sample UV-Vis traces of recycling experiments on dioxane-treated material are included (Figure S10).
Leaching study
No evidence of enzyme leaching was observed in this study. Cyt c@NU-1000 encapsulated in water was dispersed and washed with ethanol, and the solid composite material was then isolated by centrifugation. Mass loading based on ICP-OES sulfur and zirconium measurement before and after the wash were both 3.0% (Table S1), indicating no leaching of cytochrome c from the composite with ethanol wash. Elemental analysis of pre- and post-catalysis material also shows same amount of enzyme loading. And lastly, cyt c@NU-1000 was recycled three times without observable loss of reactivity during recyclability test from aqueous reaction solution, which serves as indirect evidence for minimal enzyme leaching from cyt c@NU-1000 material.
Acknowledgments
The authors kindly acknowledge financial support from Defense Threat Reduction Agency (HDTRA1-19-1-0007), Merck & Co., Inc, and Northwestern University. This research made use of the X-ray diffraction capability of Northwestern University's IMSERC facilities, which receive support from the NSF (CHE-1048773 and DMR0521267), Northwestern University Quantitative Bio-element Imaging Center generously supported by NASA Ames Research Center NNA06CB93G for metal analysis, the imaging instrumentation in the EPIC facility and Zetasizer in the Keck-II facility of Northwestern University's NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633), the MRSEC program (NSF DMR-1720139), the International Institute for Nanotechnology (IIN), the Keck Foundation, and the State of Illinois through the IIN.
Author contributions
F.S. designed and performed the experiments and prepared the manuscript draft. K.B.I. synthesized and provided the linker. Y.C., X.Z., and O.K.F. provided conceptual advice for the project. O.K.F. and X.Z. supervised the project. O.K.F. acquired funding for the project. All authors reviewed and edited the manuscript.
Declaration of interests
O.K.F. has financial interest in NuMat Technologies, a startup company that is seeking to commercialize MOFs.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102641.
Contributor Information
Xuan Zhang, Email: xuan.zhang@northwestern.edu.
Omar K. Farha, Email: o-farha@northwestern.edu.
Supplemental information
References
- Addicoat M., Bennett T., Chapman K., Denysenko D., Dincă M., Doan H., Easun T., Eddaoudi M., Farha O., Gagliardi L. New directions in gas sorption and separation with MOFs: general discussion. Faraday Discuss. 2017;201:175–194. doi: 10.1039/c7fd90044a. [DOI] [PubMed] [Google Scholar]
- Amin M.A., Halder R., Ghosh C., Jana B., Bhattacharyya K. Effect of alcohol on the structure of cytochrome C: FCS and molecular dynamics simulations. J. Chem. Phys. 2016;145:235102. doi: 10.1063/1.4972065. [DOI] [PubMed] [Google Scholar]
- Bavykina A., Kolobov N., Khan I.S., Bau J.A., Ramirez A., Gascon J. Metal–organic frameworks in heterogeneous catalysis: recent progress, new trends, and future perspectives. Chem. Rev. 2020;120:8468–8535. doi: 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- Bornscheuer U.T., Huisman G.W., Kazlauskas R.J., Lutz S., Moore J.C., Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- Chapman J., Ismail A.E., Dinu C.Z. Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts. 2018;8:238. [Google Scholar]
- Chen K., Arnold F.H. Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proc. Natl. Acad. Sci. U S A. 1993;90:5618–5622. doi: 10.1073/pnas.90.12.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li P., Modica J.A., Drout R.J., Farha O.K. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018;140:5678–5681. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiménez-Ángeles F., Qiao B., Krzyaniak M.D., Sha F., Kato S., Gong X., Buru C.T., Chen Z., Zhang X. Insights into the enhanced catalytic activity of cytochrome c when encapsulated in a metal–organic framework. J. Am. Chem. Soc. 2020;142:18576–18582. doi: 10.1021/jacs.0c07870. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li P., Zhou J., Buru C.T., Đorđević L., Li P., Zhang X., Cetin M.M., Stoddart J.F., Stupp S.I. Integration of enzymes and photosensitizers in a hierarchical mesoporous metal–organic framework for light-driven CO2 reduction. J. Am. Chem. Soc. 2020;142:1768–1773. doi: 10.1021/jacs.9b12828. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li P., Anderson R., Wang X., Zhang X., Robison L., Redfern L.R., Moribe S., Islamoglu T., Gómez-Gualdrón D.A. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science. 2020;368:297–303. doi: 10.1126/science.aaz8881. [DOI] [PubMed] [Google Scholar]
- Childs R.E., Bardsley W.G. The steady-state kinetics of peroxidase with 2,2'-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson M., Bowden E.F. UV-visible spectroscopy of adsorbed cytochrome c on tin oxide electrodes. Anal. Chem. 1992;64:1470–1476. [Google Scholar]
- Dai L., Klibanov A.M. Peroxidase-catalyzed asymmetric sulfoxidation in organic solvents versus in water. Biotechnol. Bioeng. 2000;70:353–357. doi: 10.1002/1097-0290(20001105)70:3<353::aid-bit13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Deere J., Magner E., Wall J.G., Hodnett B.K. Oxidation of ABTS by silicate-immobilized cytochrome c in nonaqueous solutions. Biotechnol. Progr. 2003;19:1238–1243. doi: 10.1021/bp0340537. [DOI] [PubMed] [Google Scholar]
- Deng H., Grunder S., Cordova K.E., Valente C., Furukawa H., Hmadeh M., Gándara F., Whalley A.C., Liu Z., Asahina S. Large-pore apertures in a series of metal-organic frameworks. Science. 2012;336:1018. doi: 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- DiRocco D.A., Ji Y., Sherer E.C., Klapars A., Reibarkh M., Dropinski J., Mathew R., Maligres P., Hyde A.M., Limanto J. A multifunctional catalyst that stereoselectively assembles prodrugs. Science. 2017;356:426–430. doi: 10.1126/science.aam7936. [DOI] [PubMed] [Google Scholar]
- Doonan C., Riccò R., Liang K., Bradshaw D., Falcaro P. Metal–organic frameworks at the biointerface: synthetic strategies and Applications. Acc. Chem. Res. 2017;50:1423–1432. doi: 10.1021/acs.accounts.7b00090. [DOI] [PubMed] [Google Scholar]
- Dordick J.S. Enzymatic catalysis in monophasic organic solvents. Enzyme Microb. Technol. 1989;11:194–211. [Google Scholar]
- Drout R.J., Robison L., Farha O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2019;381:151–160. [Google Scholar]
- Farha O.K., Hupp J.T. Rational design, synthesis, purification, and activation of metal−organic framework materials. Acc. Chem. Res. 2010;43:1166–1175. doi: 10.1021/ar1000617. [DOI] [PubMed] [Google Scholar]
- Feng D., Liu T.-F., Su J., Bosch M., Wei Z., Wan W., Yuan D., Chen Y.-P., Wang X., Wang K. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015;6:5979. doi: 10.1038/ncomms6979. [DOI] [PubMed] [Google Scholar]
- Furkan M., Fazili N.A., Afsar M., Naeem A. Analysing cytochrome c aggregation and fibrillation upon interaction with acetonitrile: an in vitro study. J. Fluoresc. 2016;26:1959–1966. doi: 10.1007/s10895-016-1889-x. [DOI] [PubMed] [Google Scholar]
- Gao R., Yuan Z., Zhao Z., Gao X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998;45:41–45. [Google Scholar]
- Goetjen T.A., Liu J., Wu Y., Sui J., Zhang X., Hupp J.T., Farha O.K. Metal–organic framework (MOF) materials as polymerization catalysts: a review and recent advances. Chem. Commun. 2020;56:10409–10418. doi: 10.1039/d0cc03790g. [DOI] [PubMed] [Google Scholar]
- Hauer B. Embracing nature’s catalysts: a viewpoint on the future of biocatalysis. ACS Catal. 2020;10:8418–8427. [Google Scholar]
- He H., Han H., Shi H., Tian Y., Sun F., Song Y., Li Q., Zhu G. Construction of thermophilic lipase-embedded metal–organic frameworks via biomimetic mineralization: a biocatalyst for ester hydrolysis and kinetic resolution. ACS Appl. Mater. Interfaces. 2016;8:24517–24524. doi: 10.1021/acsami.6b05538. [DOI] [PubMed] [Google Scholar]
- Herskovits T.T., Gadegbeku B., Jaillet H. On the structural stability and solvent denaturation of proteins: I. denatureation by the alcohols and glycols. J. Biol. Chem. 1970;245:2588–2598. [PubMed] [Google Scholar]
- Herskovits T.T., Jaillet H. Structural stability and solvent denaturation of myoglobin. Science. 1969;163:282–285. doi: 10.1126/science.163.3864.282. [DOI] [PubMed] [Google Scholar]
- Homaei A.A., Sariri R., Vianello F., Stevanato R. Enzyme immobilization: an update. J. Chem. Biol. 2013;6:185–205. doi: 10.1007/s12154-013-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Ghéczy N., Messmer D., Szymańska K., Adamcik J., Mezzenga R., Jarzębski A.B., Walde P. Stable immobilization of enzymes in a macro- and mesoporous silica monolith. ACS Omega. 2019;4:7795–7806. doi: 10.1021/acsomega.9b00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth A.J., Liu Y., Li P., Li Z., Wang T.C., Hupp J.T., Farha O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016;1:15018. [Google Scholar]
- Hu Y., Dai L., Liu D., Du W., Wang Y. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs) Renew. Sustain. Energy Rev. 2018;91:793–801. [Google Scholar]
- Huang S., Kou X., Shen J., Chen G., Ouyang G. Armor-plating" enzymes with metal-organic frameworks (MOFs) Angew. Chem. Int. Ed. Engl. 2020;59:8786–8798. doi: 10.1002/anie.201916474. [DOI] [PubMed] [Google Scholar]
- Huffman M.A., Fryszkowska A., Alvizo O., Borra-Garske M., Campos K.R., Canada K.A., Devine P.N., Duan D., Forstater J.H., Grosser S.T. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science. 2019;366:1255–1259. doi: 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- Idrees K.B., Chen Z., Zhang X., Mian M.R., Drout R.J., Islamoglu T., Farha O.K. Tailoring pore aperture and structural defects in zirconium-based metal–organic frameworks for krypton/xenon separation. Chem. Mater. 2020;32:3776–3782. [Google Scholar]
- Islamoglu T., Otake K.-I., Li P., Buru C.T., Peters A.W., Akpinar I., Garibay S.J., Farha O.K. Revisiting the structural homogeneity of NU-1000, a Zr-based metal–organic framework. CrystEngComm. 2018;20:5913–5918. [Google Scholar]
- Kan S.B.J., Lewis R.D., Chen K., Arnold F.H. Directed evolution of cytochrome c for carbon–silicon bond formation: bringing silicon to life. Science. 2016;354:1048–1051. doi: 10.1126/science.aah6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.R., Rathod V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process. Biochem. 2015;50:1793–1806. [Google Scholar]
- Kim S., Kwon K., Cha J., Yoo S., Han M.S., Tae G., Kwon I. Pluronic-based nanocarrier platform encapsulating two enzymes for cascade reactions. ACS Appl. Bio Mater. 2020;3:5126–5135. doi: 10.1021/acsabm.0c00591. [DOI] [PubMed] [Google Scholar]
- Konnerth H., Matsagar B.M., Chen S.S., Prechtl M.H.G., Shieh F.-K., Wu K.C.W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020;416:213319. [Google Scholar]
- Li H., Eddaoudi M., O'keeffe M., Yaghi O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature. 1999;402:276–279. [Google Scholar]
- Li P., Chen Q., Wang T.C., Vermeulen N.A., Mehdi B.L., Dohnalkova A., Browning N.D., Shen D., Anderson R., Gómez-Gualdrón D.A. Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chemistry. 2018;4:1022–1034. [Google Scholar]
- Li P., Modica J.A., Howarth A.J., Vargas l E., Moghadam P.Z., Snurr R.Q., Mrksich M., Hupp J.T., Farha O.K. Toward design rules for enzyme immobilization in hierarchical mesoporous metal-organic frameworks. Chemistry. 2016;1:154–169. [Google Scholar]
- Li S.-F., Zhai X.-J., Zhang C., Mo H., Zang S.-Q. Enzyme immobilization in highly ordered macro–microporous metal–organic frameworks for rapid biodegradation of hazardous dyes. Inorg. Chem. Front. 2020;7:3146–3153. [Google Scholar]
- Li X., Wang X., Ito A., Tsuji N.M. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nat. Commun. 2020;11:3858. doi: 10.1038/s41467-020-17637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X., Fang Y., Joseph E., Wang Q., Li J., Banerjee S., Lollar C., Wang X., Zhou H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017;46:3386–3401. doi: 10.1039/c7cs00058h. [DOI] [PubMed] [Google Scholar]
- Liang W., Wied P., Carraro F., Sumby C.J., Nidetzky B., Tsung C.-K., Falcaro P., Doonan C.J. Metal–organic framework-based enzyme biocomposites. Chem. Rev. 2021;121:1077–1129. doi: 10.1021/acs.chemrev.0c01029. [DOI] [PubMed] [Google Scholar]
- Lin Y., Kardos J., Imai M., Ikenoue T., Kinoshita M., Sugiki T., Ishimori K., Goto Y., Lee Y.-H. Amorphous aggregation of cytochrome c with inherently low amyloidogenicity Is characterized by the metastability of supersaturation and the phase diagram. Langmuir. 2016;32:2010–2022. doi: 10.1021/acs.langmuir.5b03810. [DOI] [PubMed] [Google Scholar]
- Luzuriaga M.A., Welch R.P., Dharmarwardana M., Benjamin C.E., Li S., Shahrivarkevishahi A., Popal S., Tuong L.H., Creswell C.T., Gassensmith J.J. Enhanced stability and controlled delivery of MOF-encapsulated vaccines and their immunogenic response in vivo. ACS Appl. Mater. Interfaces. 2019;11:9740–9746. doi: 10.1021/acsami.8b20504. [DOI] [PubMed] [Google Scholar]
- Lykourinou V., Chen Y., Wang X.-S., Meng L., Hoang T., Ming L.-J., Musselman R.L., Ma S. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011;133:10382–10385. doi: 10.1021/ja2038003. [DOI] [PubMed] [Google Scholar]
- Ma Z., Zaera F. Heterogeneous catalysis by metals. In: Scott R.A., editor. Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons, Ltd; 2011. [Google Scholar]
- Ma S., Zhou H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010;46:44–53. doi: 10.1039/b916295j. [DOI] [PubMed] [Google Scholar]
- Maiti S., Das K., Dutta S., Das P.K. Striking improvement in peroxidase activity of cytochromec by modulating hydrophobicity of surface-functionalized gold nanoparticles within cationic reverse micelles. Chemistry. 2012;18:15021–15030. doi: 10.1002/chem.201202398. [DOI] [PubMed] [Google Scholar]
- Majewski M.B., Howarth A.J., Li P., Wasielewski M.R., Hupp J.T., Farha O.K. Enzyme encapsulation in metal–organic frameworks for applications in catalysis. CrystEngComm. 2017;19:4082–4091. [Google Scholar]
- McLaughlin M., Kong J., Belyk K.M., Chen B., Gibson A.W., Keen S.P., Lieberman D.R., Milczek E.M., Moore J.C., Murray D. Enantioselective synthesis of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) via enzymatic desymmetrization. Org. Lett. 2017;19:926–929. doi: 10.1021/acs.orglett.7b00091. [DOI] [PubMed] [Google Scholar]
- Mirkin N., Jaconcic J., Stojanoff V., Moreno A. High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins. 2008;70:83–92. doi: 10.1002/prot.21452. [DOI] [PubMed] [Google Scholar]
- Ortiz C., Ferreira M.L., Barbosa O., Dos Santos J.C.S., Rodrigues R.C., Berenguer-Murcia Á., Briand L.E., Fernandez-Lafuente R. Novozym 435: the “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019;9:2380–2420. [Google Scholar]
- Phipps J., Chen H., Donovan C., Dominguez D., Morgan S., Weidman B., Fan C., Beyzavi M.H. Catalytic activity, stability, and loading trends of alcohol dehydrogenase enzyme encapsulated in a metal-organic framework. ACS Appl. Mater. Inter. 2020;12:26084–26094. doi: 10.1021/acsami.0c06964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Maiti N.C., Mazumdar S., Mitra S. Reaction of hydrogen peroxide and peroxidase activity in carboxymethylated cytochrome c: spectroscopic and kinetic studies. Biochim. Biophys. Acta. 2002;1596:63–75. doi: 10.1016/s0167-4838(02)00205-4. [DOI] [PubMed] [Google Scholar]
- Reichardt C., Welton T. Solvents and Solvent Effects in Organic Chemistry. 4th. Wiley-VCH; Weinheim, Germany: 2011. Empirical parameters of solvent polarity; pp. 389–469. [Google Scholar]
- Sanchez S., Demain A.L. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org. Process. Res. Dev. 2011;15:224–230. [Google Scholar]
- Savile C.K., Janey J.M., Mundorff E.C., Moore J.C., Tam S., Jarvis W.R., Colbeck J.C., Krebber A., Fleitz F.J., Brands J. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- Schoemaker H.E., Mink D., Wubbolts M.G. Dispelling the myths—biocatalysis in industrial synthesis. Science. 2003;299:1694–1697. doi: 10.1126/science.1079237. [DOI] [PubMed] [Google Scholar]
- Sheldon R.A., Brady D., Bode M.L. The Hitchhiker's guide to biocatalysis: recent advances in the use of enzymes in organic synthesis. Chem. Sci. 2020;11:2587–2605. doi: 10.1039/c9sc05746c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R.A., Woodley J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018;118:801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- Siegel S.M., Siegel B.Z. Autoxidation of pyrogallol : general characteristics and inhibition by catalase. Nature. 1958;181:1153–1154. doi: 10.1038/1811153a0. [DOI] [PubMed] [Google Scholar]
- Stimple S.D., Smith M.D., Tessier P.M. Directed evolution methods for overcoming trade-offs between protein activity and stability. Alche J. 2020;66:e16814. doi: 10.1002/aic.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Fu C.-W., Aguila B., Perman J., Wang S., Huang H.-Y., Xiao F.-S., Ma S. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 2018;140:984–992. doi: 10.1021/jacs.7b10642. [DOI] [PubMed] [Google Scholar]
- Syed Z.H., Sha F., Zhang X., Kaphan D.M., Delferro M., Farha O.K. Metal–organic framework nodes as a supporting platform for tailoring the activity of metal catalysts. ACS Catal. 2020;10:11556–11566. [Google Scholar]
- Takahashi H., Li B., Sasaki T., Miyazaki C., Kajino T., Inagaki S. Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem. Mater. 2000;12:3301–3305. [Google Scholar]
- Tran D.N., Balkus K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011;1:956–968. [Google Scholar]
- Vazquez-Duhalt R. Cytochrome c as a biocatalyst. J. Mol. Catal. B Enzym. 1999;7:241–249. [Google Scholar]
- Vázquez-González M., Wang C., Willner I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat. Catal. 2020;3:256–273. [Google Scholar]
- Vicente E.J., Dean D.R. Keeping the nitrogen-fixation dream alive. Proc. Natl. Acad. Sci. U S A. 2017;114:3009–3011. doi: 10.1073/pnas.1701560114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Chen Y., Wang S., Li P., Mirkin C.A., Farha O.K. DNA-functionalized metal–organic framework nanoparticles for intracellular delivery of proteins. J. Am. Chem. Soc. 2019;141:2215–2219. doi: 10.1021/jacs.8b12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.C., Vermeulen N.A., Kim I.S., Martinson A.B.F., Stoddart J.F., Hupp J.T., Farha O.K. Scalable synthesis and post-modification of a mesoporous metal-organic framework called NU-1000. Nat. Protoc. 2016;11:149–162. doi: 10.1038/nprot.2016.001. [DOI] [PubMed] [Google Scholar]
- Wang D., Jana D., Zhao Y. Metal-organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 2020;53:1389–1400. doi: 10.1021/acs.accounts.0c00268. [DOI] [PubMed] [Google Scholar]
- Wang X., Lan P.C., Ma S. Metal–organic frameworks for enzyme immobilization: beyond host matrix materials. ACS Cent. Sci. 2020;6:1497–1506. doi: 10.1021/acscentsci.0c00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Yang C., Ge J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Bioprocess. 2017;4:24. doi: 10.1186/s40643-017-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Li N., Zhong X., Jiang Y. Metal-organic frameworks: a potential platform for enzyme immobilization and related applications. Front. Bioeng. Biotechnol. 2020;8:695. doi: 10.3389/fbioe.2020.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-C., Long J.R., Yaghi O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012;112:673–674. doi: 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.