Abstract
The present study was designed to evaluate the anticancer effects of withaferin A against the human endometrial cancer via modulation of transforming growth factor-β (TGF-β) signalling. The results of the present study revealed that withaferin A exerts a dose and time-dependent antiproliferative effects against the human KLE endometrial cancer cells with comparatively lower toxicity against the THESCs normal cells. The IC50 of withaferin A against the KLE endometrial cancer cells was found to 10 μM. The results showed that withaferin A induced apoptosis and G2/M cell cycle arrest of the KLE cells which was associated with alteration of the apoptosis and cell cycle related proteins. In addition, the transwell assays showed that the migration and invasion of the KLE cells were inhibited by 53 and 40%, respectively. Finally, the effects of withaferin A were also examined on the TGF-β signalling pathway. The results showed that withaferin A blocked TGF-β-dependent Smad2 phosphorylation and expression of other TGF-β-related proteins in KLE cells. Summing up, the results suggest that withaferin A inhibits the proliferation of the human endometrial carcinoma via TGF-β signalling.
Keywords: Endometrial cancer, Withanolides, Withaferin A, Apoptosis, TGF-β signalling
Introduction
Endometrial carcinoma comprises of an assembly of distinctive histological subtypes which collectively constitute the most frequent gynaecological disorder across the globe (Green et al. 2020). Alone in United States, about 12,590 deaths and 65,620 new cases of endometrial carcinoma were reported in 2020 (Siegel et al. 2020). Prognosis of endometrial carcinoma is highly based on the clinical stage and histological grade (Torre et al. 2015). Even though, patients with early-stage disease (67%) show 5-year overall survival of above 81%, patients with later stages IVA and IVB endometrial carcinoma show an extremely poor prognosis of 17 and 15%, respectively (Constantine et al. 2019). Mortality associated with endometrial carcinoma is increasing at an alarming pace. From 2005 to 2014, an approximately 1.4% increase in death rate associated with this malignancy was recorded (Siegel et al. 2020; Torre et al. 2015). The key risk factors contributing to the carcinogenesis of endometrial carcinoma includes tamoxifen exposure, obesity, nulliparity, late menopause and early menarche (Raglan et al. 2019; Constantine et al. 2019; Morice et al. 2016). Obesity is one of the leading and modifiable risk factors in endometrial carcinoma accounted for about 50% of cases in United States and Europe. Obesity in endometrial carcinoma patients increases the death risk by almost 6.25 times (Calle et al. 2003). The treatment methodologies for patients with initial-stage endometrial carcinoma include radiation therapy and/or chemotherapy. Patients suffering from recurrent and advanced stage endometrial carcinoma are treated with targeted therapy, cytotoxic therapy or hormonal therapy (Morice et al. 2016). Because of extremely poor prognosis for recurrent and advanced stage disease, the hunt for novel chemopreventives is the need of the hour. Owing to immense structural diversity of natural products, they constitute a remarkable source of drugs (Ji et al. 2009; Majolo et al. 2019). Several natural products have been reported to exhibit strong anticancer activities and some of them have even been already reached clinical trials (Newman 2018). Withanolides belong to naturally occurring steroid lactones class of compounds mostly found in Withania genus (Dhami et al. 2017). Withanolides exhibit remarkable biological and medicinal properties, including anti-inflammatory, antistress, hepatoprotective, antioxidant, immunomodulatory, adaptogenic, antibacterial, insect antifeedant, antiarthritic and anticancer (Singh et al. 2010). The molecule withaferin A, belongs to medicinally active members of withanolides, has been reported to possess strong biological applications, including antiangiogenesis, immunomodulatory, antioxidant, anti-inflammatory, antistress and anticancer activities (vel Szic et al. 2014). Moreover, withaferin A is turning out to be a leading anticancer agent against pancreatic, prostate, uterine and breast cancers (Lee and Choi 2016). The anticancer potential of withaferin A and need for therapeutic agents for endometrial carcinoma prompted us to evaluate the anticancer effects of withaferin A against KLE endometrial carcinoma cells. In addition, attempts were made to unveil the underlying mechanisms associated with withaferin A triggered anticancer effects.
Materials and methods
Cell lines, culture and conditions
The cancerous KLE endometrial cells and normal THESCs endometrial cells were procured from American Type Culture Collection (ATCC, Manassas, VA, United States. Withaferin A (> 98% purity by HPLC) was purchased from Sigma Aldrich (St. Louis, MO, United States). All the cell lines were cultured in RMPI-1640 medium containing high glucose (4.5 g/L), 2 mM l-glutamine, Penicillin (50 IU/mL), Streptomycin (50 mg/mL) and 10% fetal bovine serum. All the reagents and medium were purchased from Sigma Aldrich (St. Louis, MO, United States) or else otherwise mentioned. Both the cell lines were maintained under humid conditions at 37 °C in a 5% CO2 and 95% air.
Cell viability assay
The viability of KLE and THESCs cells treated with withaferin A was examined by 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide (MTT) assay. Concisely, each cell line with a density of 2 × 105 cells/well in 96-well plate were exposed to different withaferin A concentrations viz 0, 2.5, 5, 10, 20, 40, 80 and 180 µM for 48 h at 37 °C. Post-treatment, 0.5% MTT reagent (Sigma) was supplied to each well and incubation was performed at 37 °C for additional 4 h. Thereafter, dimethyl sulphoxide (DMSO) was added to each well followed by processing of the cell samples for absorbance measurements to determine cell proliferation at 570 nm.
Cell proliferation assay
The proliferation of the KLE cells was determined by EdU (5-ethynyl-2'-deoxyuridine) assay. Briefly, the KLE cells (2 × 105 cells/well) were treated with 10 µM concentration of withaferin A within 96-well plates maintaining EdU medium for 48 h. EdU assay was performed using EdU assay kit (Ribobio). DAPI staining was used to observe cell nuclei and EdU-positive cells were examined under a fluorescence microscope.
Annexin-V/PI assay
Cancer cell apoptosis was studied through Annexin-V/PI staining assay after withaferin A treatment. In brief, KLE cells were placed in 12-well plates (105 cells/well) and treated with 0 and 10 µM concentrations of with withaferin A for 48 h. Cells were then harvested through centrifugation followed by PBS washing. Post-washing, cells were fixed in ethanol (70%) and then staining was performed using Annexin V-FITC and propidium iodide (PI). Staining was performed by following manufacturers protocol of eBioscience™ Annexin V Apoptosis Detection Kit FITC (Thermo Fisher Scientific). Finally, apoptosis analysis was performed via flow cytometry.
Immunofluorescence analysis
Withaferin A treated (0 and 10 µM) KLE cells were fixed for 15 min in paraformaldehyde (4%) followed by incubation of 30 min with Triton X-100 (Beyotime, China). Subsequently, cells were subjected to incubation overnight at 4 °C with anti-cytochrome C antibody (1:100 dilutions, proteintech, China). Afterwards, cells were washed using PBS followed by incubation with Cy3 goat anti-rabbit IgG (1:200 dilutions, Beyotime, China). Subsequently, the cells were stained with DAPI (Biosharp, China) and finally examined under a fluorescent microscope.
Flow cytometry
Flow cytometry was used to determine the effects of withaferin A on distribution of the KLE cells in different phases of cell cycle. Concisely, KLE cells were placed in 12-well plates (105 cells/well) and treated with 0 and 10 µM concentrations of with withaferin A for 48 h. Thereafter, the cells were fixed and subsequently incubated with RNase (100 µL of concentration 100 µg/mL) and 400 µL of PI dye at 4 °C. Finally, filtration was carried out with 300 mesh (70 µm) cell strainer and ultimately subjected to flow cytometry for cell cycle analysis.
Transwell assay
Cell migration and invasion ability of KLE cells post-withaferin A exposure was measured by using transwell assay. KLE cells were exposed to 0 and 10 µM concentrations of withaferin A for 24 h followed by loading of 2 × 103 cells to upper transwell chamber maintaining serum-free RMPI-1640 medium. RMPI-1640 medium maintaining 10% FBS was supplied to lower chamber. Afterwards, chambers were incubated at 37 °C for 24 h followed by clearing of cells on upper surface of the membrane with a cotton swab. The migrated KLE cells underside of the membrane were subjected to staining for 10 min using crystal violet (0.1%). The cells, which migrated were finally counted and pictured at 200 × magnification field of a light microscope. Consistent procedure was followed for invasion evaluation except transwell chambers were coated with Matrigel.
Western blotting
Total proteins from withaferin A treated (0 and 10 µM) KLE cells was isolated using RIPA lysis buffer. Proteins were thereafter separated electrophoretically on SDS-PAGE. Afterwards, separated proteins were blotted to PVDF membranes followed by specific primary antibodies treatment overnight at 4 °C. Following primary antibody treatment, the membranes were exposed to a secondary antibody. Finally, visualization of protein bands was performed with the help of enhanced chemiluminescence substrate on X-ray films.
Statistical analysis
Experiments were performed in triplicate and expressed as mean ± standard deviation (SD). Statistical analysis was carried out by performing different statistical tests on SPSS 15.0 software. The measure of statistically significant difference was taken at p < 0.05.
Results
Withaferin A inhibited KLE cell proliferation
To assess the viability and proliferation of normal THESCs and cancerous KLE cells (Fig. 1A)t, we used MTT assay and EdU assay. The results from MTT assay showed that withaferin A exhibits significant (p < 0.05) anti-proliferative effects against KLE cells. The viability of THESCs cells post-48 h prolonged withaferin A treatment (0–160 µM) was observed to be decreased from 100% to almost 25% with an IC50 value of 76 µM (Fig. 1B). In case of endometrial cancer KLE cells, withaferin A showed caused significant inhibition of KLE cell proliferation with an IC50 value of 10 µM (Fig. 1C). Furthermore, withaferin A exerted time-dependent anticancer activity against the KLE cells (Fig. 1D). The results of EdU assay revealed that withaferin A caused remarkable inhibition of KLE cell proliferation (Fig. 1E). Collectively, withaferin A induced potent antiproliferative effects against the human endometrial cancer cells.
Fig. 1.
Withaferin A inhibits the viability and proliferation of endometrial carcinoma cells. A Chemical structure of withaferin A molecule. B Proliferation of THESCs cells post-withaferin A treatment at indicated concentrations. C Proliferation of KLE cells post-withaferin A treatment at indicated concentrations. D Proliferation of the KLE cells at IC50 concentration and at different time periods as indicated. E Proliferation of KLE cells post-withaferin A treatment as depicted by EdU assay. Three replicates were of experiment were performed and final data were presented as mean ± standard deviation. (*p < 0.05)
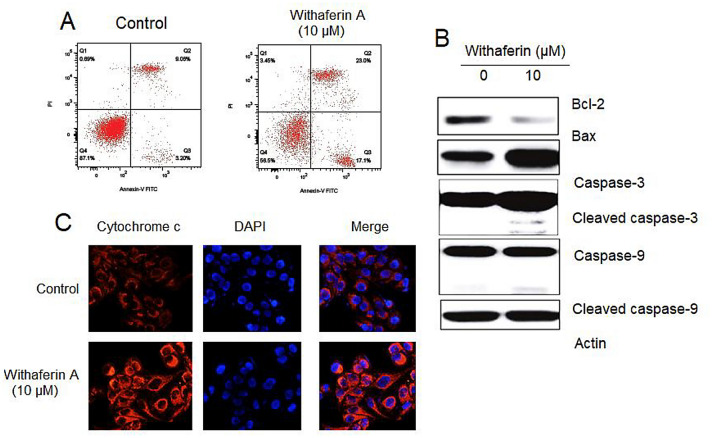
Withaferin A promoted mitochondria-mediated-apoptosis in KLE cells
To detect the apoptosis in KLE cells after withaferin A treatment, annexin-V/PI staining was performed. The results showed that post-withaferin A exposure the percentage of early-stage and late-stage apoptotic cells increased significantly. In comparison to 12.25% of apoptotic cell percentage in control, withaferin A (10 μL) treated cells showed 40.1% of apoptotic cells (Fig. 2A). The results of western blotting indicated that the expression of Bax increased and that of Bcl-2 decreased. In addition, withaferin A treatment increased the cleavage of caspase-3 and caspase-9 (Fig. 2B). Caspase-3 and -9 are the key apoptosis allied proteins that modulate discharge of cytochrome-C into cytoplasm. Therefore, we further performed immunofluorescence analysis to monitor discharge of cytochrome-C. The results showed increased expression of cytochrome-C in withaferin A treated KLE cells in comparison to that of control untreated cells (Fig. 2C). These results indicated that withaferin A promoted apoptosis of KLE cells.
Fig. 2.
Withaferin A induces apoptosis in KLE cells. A Apoptosis analysis of withaferin A treated KLE cells through Annexin V/PI staining. B Western blots representing expressions of apoptosis associated proteins. Bax, Bcl-2, cleaved caspase-3, caspase-3 and cleaved caspase-9 and caspase-9. C Immunofluorescence analysis indicating expressions of cytochrome-C in withaferin A treated KLE cells. Experiments were repeated thrice
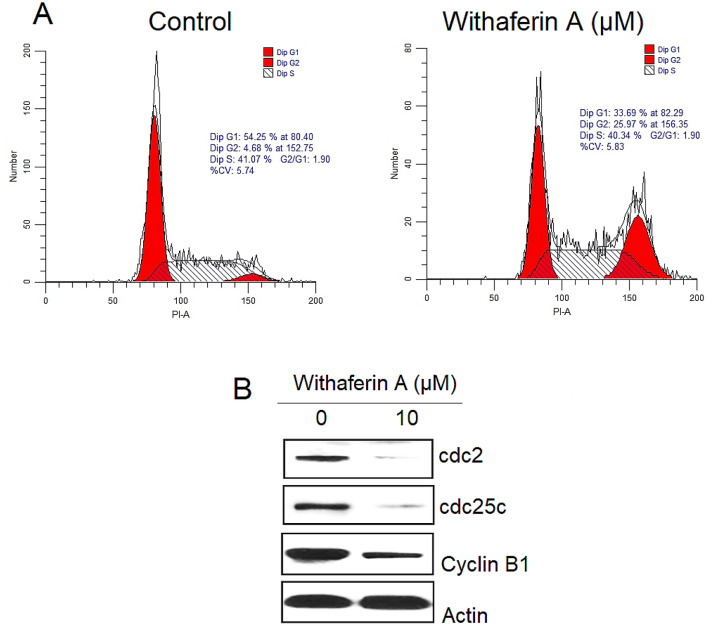
Withaferin A targeted cell cycle in KLE cells
To monitor the distribution of KLE cells in different cell cycle phases treatment with withaferin A at 0 and 10, flow cytometry was performed. The results that withaferin A triggered the accumulation of the KLE cells in the G2/M-phase of the cell cycle. The G2/M-phase cell percentage in control group was 4.68% which enhanced to 25.97% in withaferin A treated group (Fig. 3A). These results indicate that withaferin A induces G2/M phase arrest of the KLE cells. Further, we examined the expression of cell cycle allied proteins in KLE cells post-withaferin A treatment. The results showed decrease in the expression of cdc2, cdc25c and cyclin-B1 in KLE cells.
Fig. 3.
Withaferin A induces G2/M cell cycle arrest. A Flow cytometric analysis for illustration of different cell cycle phases. The figure represented augmented number G2/M-phase cells in treated group as compared to controls. B Western blots showing the expression of cell cycle associated proteins in withaferin A treated KLE cells. The experiments were performed in triplicate
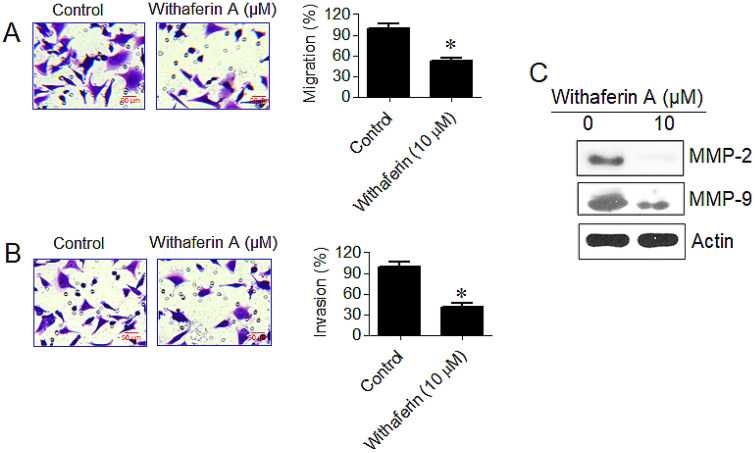
Withaferin A repressed migration and invasion of KLE cells
The transwell assay was performed to detect the effects of withaferin A on migration and invasion of KLE cells. The results indicated withaferin A strongly repressed the migration and invasion potency of KLE cells. Withaferin A repressed the migration percentage from 100% (control) to 53% (treated group) (Fig. 4A). Similarly, withaferin A treatment (0 and 10 µM) to KLE cells resulted in decrease of invasive cell percentage from 100% to almost 40% (Fig. 4B). Moreover, western blotting showed decrease in the expression of MMP-2 and MMP-9 in KLE cells post-withaferin A treatment (Fig. 4C).
Fig. 4.
Withaferin A inhibits migration and invasion of KLE cells. A Transwell migration assay. Results showing repressed migration in withaferin A treated KLE cells in comparison to controls. B Transwell invasion assay. Results showing repressed invasion in withaferin A treated KLE cells in comparison to controls. C Western blots representing expressions of cell cycle associated proteins. Results revealed decreased expressions of MMP-2 and MMP-9 in KLE cells post-withaferin A treatment. Three replicates were given to all experimental procedure and final data were presented as mean ± standard deviation. (*p < 0.05)
Withaferin A inhibited TGF-β signalling in KLE cells
To evaluate the effects of withaferin A on TGF-β signalling in KLE cells, western blotting assay was performed. The results showed that the expression of TGF-β1, TGF-β2, p-Smad2 and p-Smad3 were decreased considerably. Nonetheless, Smad2 and Smad3 remained all-over constant (Fig. 5). This indicated that the antiproliferative and proapoptotic effects of withaferin A are mediated by downregulation of TGF-β signalling in KLE cells.
Fig. 5.
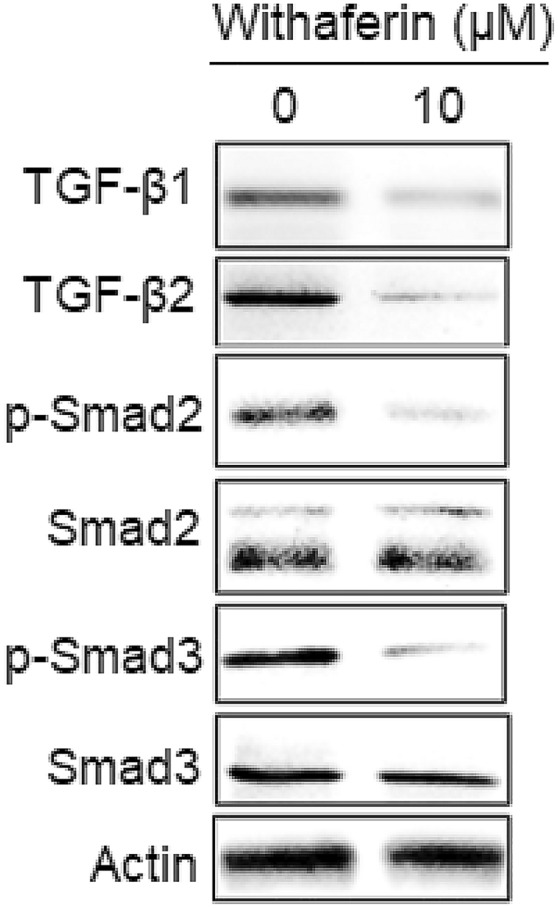
Withaferin A blocks the TGF-β1 pathway. Western blots displaying the activity of TGF-β signalling in withaferin A treated KLE cells. The activity of TGF-β1, TGF-β2, p-Smad2 and p-Smad3 were downregulated and Smad2 and Smad3 remained almost constant. The experiments were performed in triplicate
Discussion
Unfortunately, the endometrial carcinoma incidences and mortality pose a huge threat to female health, globally. Although the recent advancements have led to the development of efficient treatment methodologies and biomarkers for early detection of disease, the overall survival rates for later-stage endometrial carcinoma still remain far from descent. Natural products are ranked as essential modulatory molecules in eukaryotic organisms because they mimic several biological components found in these organisms (Al-Awadhi and Luesch 2020). They have been reported to exert remarkable influence on several physiological processes, developmental processes and health in human beings. Natural products target key survival regulatory proteins in cancer cells to inhibit their proliferation via different molecular mechanisms, such as apoptosis and autophagy (Wang and Feng 2015).
Withaferin A is a medicinally high rated natural product shown with strong antiproliferative effects against several human cancer cells, including colon, gastric, prostrate and pancreatic cancer (Lee and Choi 2016). Herein, the antiproliferative effects of withaferin A were evaluated against KLE cells. Withaferin A demonstrated strong proliferation inhibitory effects against KLE cells (IC50 value of 10 µM) in a concentration- and time-reliant mode. Cytotoxicity of withaferin A was comparatively lower against normal THESCs endometrial cells (IC50 value of 76 µM) when compared to cancerous KLE cells.
On establishment of antiproliferative effects of withaferin A against KLE cells, further studies were carried out to determine the underlying molecular mechanism. Programmed cell death (apoptosis) is a highly organized process in which the intracellular content is collected in membrane packets for destruction by immune cells (Elmore 2007). Apoptosis maintains normal balance in multicellular organisms by eliminating malfunctioning, damaged, virus-infected or cancerous cells during development. Herein, withaferin A demonstrated pro-apoptotic activity in KLE cells which was supported by upregulation of pro-apoptotic proteins Bax, downregulation of Bcl-2 and triggered cleavage of caspase-3 and 9. Moreover, immunofluorescence analysis revealed discharge of cytochrome-c into cytoplasm post-withaferin A exposure of KLE cells. This indicated that withaferin A induces intrinsic apoptosis in KLE cells. Our results were in consistency with the previous studies where withaferin A was shown to regulate apoptosis in human melanoma and leukemia cancer cells by targeting the key modulatory processes and proteins including activation of caspase-3 and -9, DNA cleavage, mitochondrial membrane potential loss, cell cycle arrest, upregulation of Bax and downregulation of Bcl-2 (Mayola et al. 2011; Mandal et al. 2008).
Uncontrolled and rapid cell division is one of the key features of cancer cells (Marte 2004). Cell cycle proceeds through several check points during its course. These check points serve as main targets for chemopreventives to supress rapid cell division of cancer cells. Herein, withaferin A showed significant inhibition of cell division at G2/M-phase of KLE cell cycle. Further, western blotting showed that withaferin A reduced the expression levels of cell cycle allied proteins including cyclin-B1, cdc25c and cdc2. Withaferin A has been already been reported to exert cell cycle inhibitory effects against different cancer cells. Withaferin A has been shown to inhibit progression of cell cycle in breast and prostate cancer cells at G2/M-phase via downregulation of cdc25b, cdc25c and cdc2 (Stan et al. 2008; Roy et al. 2013).
Cell migration and invasion play critical role in cancer metastasis. Withaferin A has been previously reported of anti-migration and anti-invasion effects against oral carcinoma cells (Yu et al. 2020). Herein, withaferin A showed significant inhibition of migration as well as invasion of KLE cells. Moreover, post-withaferin A exposure the expressions of MMP-2 and MMP-9 reduced remarkably in KLE cells indicating inhibition of migration and invasion.
TGF-β signalling is an important pathway that plays a crucial role in fibrosis, cell growth, differentiation and apoptosis (Morris et al. 2015). Cancer cells mostly show elevated TGF-β signalling. TGF-β signalling is modulated by non-Smad and Smad pathways regulated by TGF-β ligands, type-1 and type-2 receptors and non-Smad or Smad proteins (Liu et al. 2018). Mammals have three TGF-β types including TGF-β1, TGF-β2 and TGF-β3. Herein, withaferin A induced inhibition of TGF-β signalling through Smad pathway. The expressions of TGF-β1, TGF-β2, p-Smad2 and p-Smad3 were downregulated and Smad2 and Smad3 remained almost constant in KLE cells post-withaferin A exposure. Our results were similar to that of Bale et al., wherein withaferin A induced inhibition of TGF-β/Smad signalling in scleroderma (Bale et al. 2018).
In conclusion, we demonstrated that withaferin A induced time and dose-dependent anticancer effects against human endometrial cancer cells. We further established the mechanism by which anticancer effects of withaferin A are regulated. The results revealed Withaferin A stimulated apoptosis, blocked cell cycle, inhibited migration and invasion and targeted TGF-β signalling in KLE cells.
Acknowledgements
We acknowledge the central instrumentation section of Ningbo First Hospital, Ningbo, Zhejiang, 315010, China for providing the instrumentation facility.
Author contribution
Conceptualization: KX and JQ; methodology: KX, HS and YD; formal analysis and investigation: KX, HS and YD; writing—original draft preparation: YX, HS and YD; writing—review and editing critically for important intellectual content: JQ; supervision: JQ.
Funding
Not applicable.
Declarations
Conflict of interest
All the authors declare that he has no conflict of interest.
References
- Al-Awadhi FH, Luesch H. Targeting eukaryotic proteases for natural products-based drug development. Nat Prod Rep. 2020;37:827–860. doi: 10.1039/C9NP00060G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale S, Pulivendala G, Godugu C. Withaferin A attenuates bleomycin-induced scleroderma by targeting FoxO3a and NF-κβ signaling: connecting fibrosis and inflammation. BioFactors. 2018;44:507–517. doi: 10.1002/biof.1446. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women's Health Initiative: an assessment of risk factors. J Women's Health. 2019;28:237–243. doi: 10.1089/jwh.2018.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami J, Chang E, Gambhir SS. Withaferin A and its potential role in glioblastoma (GBM) J Neurooncol. 2017;131:201–211. doi: 10.1007/s11060-016-2303-x. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AK, Feinberg J, Makker V. A review of immune checkpoint blockade therapy in endometrial cancer. Am Soc Clin Oncol Educ Book. 2020;40:238–244. doi: 10.1200/EDBK_280503. [DOI] [PubMed] [Google Scholar]
- Ji HF, Li XJ, Zhang HY. Natural products and drug discovery: can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10:194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Choi BY. Withaferin-A—a natural anticancer agent with pleitropic mechanisms of action. Int J Mol Sci. 2016;17:290. doi: 10.3390/ijms17030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chen S, Zeng J. TGF-β signaling: a complex role in tumorigenesis. Mol Med Rep. 2018;17(1):699–704. doi: 10.3892/mmr.2017.7970. [DOI] [PubMed] [Google Scholar]
- Majolo F, Delwing LK, Marmitt DJ, Bustamante-Filho IC, Goettert MI. Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochem Lett. 2019;31:196–207. doi: 10.1016/j.phytol.2019.04.003. [DOI] [Google Scholar]
- Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13:1450–1464. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- Marte B. Cell division and cancer. Nature. 2004;432:293. doi: 10.1038/432293a. [DOI] [Google Scholar]
- Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- Morris SM, Carter KT, Baek JY, Koszarek A, Yeh MM, Knoblaugh SE, Grady WM. TGF-β signaling alters the pattern of liver tumorigenesis induced by Pten inactivation. Oncogene. 2015;34:3273–3282. doi: 10.1038/onc.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ. From natural products to drugs. Phys Sci Rev. 2018;4:20180111. [Google Scholar]
- Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- Roy RV, Suman S, Das TP, Luevano JE, Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76(10):1909–1915. doi: 10.1021/np400441f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1:56–63. [Google Scholar]
- Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60:51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- vel Szic KS, de Beeck KO, Ratman D, Wouters A, Beck IM, Declerck K, Heyninck K, Fransen E, Bracke M, De Bosscher K, Lardon F. Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS ONE. 2014;9:e87850. doi: 10.1371/journal.pone.0087850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Feng Y. Elaborating the role of natural products-induced autophagy in cancer treatment: achievements and artifacts in the state of the art. Biomed Res Int. 2015;2015:934207. doi: 10.1155/2015/934207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TJ, Tang JY, Ou-Yang F, Wang YY, Yuan SS, Tseng K, Lin LC, Chang HW. Low concentration of Withaferin A inhibits oxidative stress-mediated migration and invasion in oral cancer cells. Biomolecules. 2020;10:777. doi: 10.3390/biom10050777. [DOI] [PMC free article] [PubMed] [Google Scholar]