Abstract
HPVs representing the most common sexually transmitted disease are a group of carcinogenic viruses with different oncogenic potential. The immune system and the vaginal microbiome represent the modifiable and important risk factors in HPV-induced carcinogenesis. HPV infection significantly increases vaginal microbiome diversity, leading to gradual increases in the abundance of anaerobic bacteria and consequently the severity of cervical dysplasia. Delineation of the exact composition of the vaginal microbiome and immune environment before HPV acquisition, during persistent/progressive infections and after clearance, provides insights into the complex mechanisms of cervical carcinogenesis. It gives hints regarding the prediction of malignant potential. Relative high HPV prevalence in the general population is a challenge for modern and personalized diagnostics and therapeutic guidelines. Identifying the dominant microbial biomarkers of high-grade and low-grade dysplasia could help us to triage the patients with marked chances of lesion regression or progression. Any unnecessary surgical treatment of cervical dysplasia could negatively affect obstetrical outcomes and sexual life. Therefore, understanding the effect and role of microbiome-based therapies is a breaking point in the conservative management of HPV-associated precanceroses. The detailed evaluation of HPV capabilities to evade immune mechanisms from various biofluids (vaginal swabs, cervicovaginal lavage/secretions, or blood) could promote the identification of new immunological targets for novel individualized diagnostics and therapy. Qualitative and quantitative assessment of local immune and microbial environment and associated risk factors constitutes the critical background for preventive, predictive, and personalized medicine that is essential for improving state-of-the-art medical care in patients with cervical precanceroses and cervical cancer. The review article focuses on the influence and potential diagnostic and therapeutic applications of the local innate immune system and the microbial markers in HPV-related cancers in the context of 3P medicine.
Keywords: Predictive preventive and personalized medicine (PPPM/3PM), Gynecology, Obstetrics, Cancer, HPV, Cervical carcinogenesis, Malignant transformation, Cervical cancer, Vaginal microbiome composition, Lactobacillus, Molecular mechanisms, Biomarkers, Innate immunity, Patient stratification, Targeted therapy, Individualized patient profiling, Individual outcomes
Introduction
Women’s health is a cardinal priority of predictive, preventive, and personalized (3P) medicine. 3P medicine, an innovative approach representing individualized treatment strategies and precision medicine, is a cornerstone of the battle against gynecologic cancers [1, 2]
Cervical cancer (CC) is the fourth most common cancer in women, with an estimated age-standardized incidence of 13.1 per 100,000 women and an age-specific mortality rate of 6.9 per 100,000 women globally [3]. Further, 570,000 new CC cases occurred in 2018, and more than 311,000 deaths result from CC every year; incidence and death rates are higher in low- and middle-income countries that lack organized screening and vaccination programs [4, 5]. Moreover, almost 95% of CC biopsies contain high-risk human papillomavirus (HPV) infections [6].
HPV infection is one of the causes of preinvasive and invasive cervical disease [6]. More than 200 HPV genotypes belong to the Papillomaviridae family of DNA viruses, and approximately 30 HPV genotypes infect the anogenital tract. Based on their oncogenic potential, HPV types are classified as high-risk (HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, and HPV-59) and probably high-risk (HPV-26, HPV-53, HPV-66, HPV-67, HPV-68, HPV-70, HPV-73, and HPV-82) with an increased affinity for mucosa. Mucosal HPV genotypes are associated with cervical, penile, vaginal, vulvar, anal, and oropharyngeal pre-cancers and cancers [7, 8].
HPV is the most common sexually transmitted disease (STD) and affects 80% of women during their lifetime [9]. The interaction between the HPV virus and host organism is highly complex and does not represent a one-way process. Fortunately, most of the HPV infections are spontaneously cleared (79% of infections in 24 months) and do not lead to dysplastic changes on the cervical epithelium [10]. In some cases, persistent HPV infection may progress to low- and high-grade lesions [11]. Besides, 90% of screening results in US population are represented by negative cytological finding and HPV negativity. The rest is mainly characterized by mild cytological changes, including atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) either associated with hrHPV (high-risk HPV) positivity or negativity. High-grade squamous intraepithelial lesions (HSILs) form only a small fraction of cytological results. The current management of mild cytological abnormalities consists of a conservative approach in non-suspicious colposcopic findings [12, 13]. This is particularly important in nulliparous women, where the HPV virus incidence is the highest, and any surgical treatment could have adverse effects on future pregnancies [14].
The reasons for HPV infection persistence or progression remain largely unclarified. Biologically based non-modifiable and behaviorally based modifiable risk factors likely play a significant role in preventing viral infection progression and predicting its course. Indeed, in the view of a severe socioeconomic burden on society, overall cancer management requires a shift from a reactive to 3P medicine to implement cost-effective and individualized healthcare that benefits the whole society [15–18]. The vaginal microbiome and innate immune system are highly associated with the pathogenesis of HPV-induced CC [6]; therefore, the individualized patient profiles and targeted preventive, early predictive, or therapeutic strategies as basic pillars of 3P medicine should be implemented in CC management to obtain improved outcomes concerning the individual and society as a whole. In this regard, improved CC management requires identifying novel liquid biopsy biomarkers obtained from specific body fluids. In conclusion, elucidating the malignant potential of particular HPV infections and associated cervical lesions based on the local microenvironment is crucial to the preventive and personalized approach of state-of-the-art medicine.
Non-modifiable and modifiable risk factors of cervical cancer
The best known non-modifiable risk factors for CC development are HPV infections, patient age, ethnic factors, host genetic factors, and the family history of CC [19]. These factors act throughout women’s lives, and it is difficult to isolate their effects on the transformation of primary infections into persistent infections and that of persistent infections into pre-cancer lesions. Moreover, HPV type, viral factors, viral load, and co-infections with multiple HPV genotypes and/or other sexually transmitted diseases (STDs) are major factors in the persistence of HPV and the development of CC [20].
Age, an intrinsic host factor, is associated with the risk of acquiring HPV infection. HPV is most prevalent among adolescents and young adults between 15 and 25 years of age; it is supposed that 75% of young individuals acquire HPV in this age range [21–23]. On the other hand, the risk of HPV infection in puberty or younger age is increased by a lack of immune responses and squamous metaplasia during endocervical reconfiguration to the ectocervix in response to an acidic environment. Therefore, during the metaplastic transformation of the cervical epithelium, basal cells are more susceptible to HPV infection; this may result in cell proliferation and the development of cervical dysplasia or squamous cell cancer [24, 25]. Moreover, the prevalence of HPV increases in post-menopausal women over 50 years of age. This may be a consequence of weakening immunity and reactivation of latent infections, with cumulative risks associated with the number of births and sexual partners over a lifetime [26].
Modifiable risk factors for cervical carcinogenesis mirror the sexual behavior, lifestyle, and socioeconomic status. Moreover, cultural and geographic variations influence the sexual behavior of women and their male partners. Risk factors include certain aspects of a woman’s sexual history: the age of first sexual intercourse, the number of partners and their characteristics, the age at first birth, parity [27], and the intake of oral contraceptives [28]. Numerous sexual partners and/or recent new or older sexual partners are associated with increased HPV risk. On the other hand, marital status and hormonal contraceptive or condom use are associated with decreased risks of HPV infection. Condoms, moreover, have some protective effects against the transmission of HPV and other STDs, including HIV [29]. It remains unclear how parity influences CC risk. HPV-positive women with 7 or more reported full-term pregnancies have a fourfold increased risk of CC compared to HPV-positive nulliparous women [27]. Hormonal factors related to pregnancy and cervical trauma associated with delivery may increase the risk of cervical carcinogenesis [30]. The relationship between long-term oral contraceptive (OC) use and increased CC risk remains controversial. Studies reported an elevated risk of cervical adenocarcinoma due to an abundance of estrogen without progesterone caused by OC pills; endometrial cells respond to this hormonal imbalance through endometrial hyperplasia [31]. On the other hand, women using OC are likely under medical supervision and may participate in screening examinations; therefore, they are at lower risk [32].
An unhealthy lifestyle, negative life events, a lack of social support, smoking, alcohol consumption, and illegal drug abuse are known risk factors for CC, especially in less-educated women. Cigarette smokers are at an increased risk for SIL and CC compared to HPV-positive non-smokers [33]. Tobacco smoke contains carcinogens that could cause immunosuppression and have an instantaneous effect on the transformation of cervical tissue. Chemicals from tobacco smoke were found in cervical mucus; this could allow HPV infections to persist and progress to cancer by integrating viral DNA into the host genome [34]. Substance abuse (e.g., alcohol consumption [35] and illegal drugs [36]) may reduce immune function and thus affect the cervical squamous epithelial microenvironment and support persistent HPV infection.
A low socioeconomic status may lead to an increased risk for health problems. Women living in low-resource countries usually have limited incomes and restricted access to healthcare. These women are often poorly informed about CC risks and suffer from nutritional deficiencies [37]. Consumption of fruits and vegetables containing antioxidant nutrients such as vitamins C and E, carotenoids, and lycopene may prevent DNA damage and protect cells from reactive oxygen species released due to tobacco-induced cervical inflammation [38]. Accordingly, fresh vegetable consumption reduced the risk of HPV persistence by more than 50% [39]. A healthy lifestyle with moderate sexual behavior may reduce the risk of long-term HPV infection and enhance the immune response.
Our review article focuses on two modifiable and important risk factors for HPV-induced carcinogenic processes: the immune system and the vaginal microbiome (VM). These factors could be used as markers and tools in modern personalized diagnostic and therapeutic approaches.
Vaginal microbiome concerning HPV infection and cervical dysplasia
The vaginal microbiome: its composition and interactions
The female genital tract is protected against infections by a complex system composed of the mucosal epithelial barrier, the immune system, and a healthy VM producing lactic acid, hydrogen peroxide, halides, and antimicrobial peptides. Moreover, the VM modulates local inflammatory immune responses, including cytokine secretion [40]. Maintaining or improving the VM represents a new and effective strategy in treating HPV infections and associated precancerous lesions [41].
Estrogen levels have a significant effect on the vaginal microbiome composition. Estrogen influences the amount and viscosity of vaginal secretions, the glycogen content, and the vaginal oxygen and carbon dioxide levels [42]. Regular vaginal lubrication, acidic vaginal pH, and healthy are important and effective defense mechanisms against alien microbial contamination. Vaginal dryness as a part of sicca syndrome represents a low Lactobacillus state with a strong predisposition to frequent vaginal infections and even lichen sclerosus of the vulva [43]. Low-estrogen state in prepubertal age and postmenopausal women is associated with low Lacrobacillus levels and consists of a mixture of anaerobic bacteria [44]. On the contrary, the vaginal microbiome of pregnant women is more stable and typically dominated by L. crispatus or L. iners [45]. Two longitudinal studies showed that the changes in the vaginal microbiome composition are affected by the phase of the menstrual cycle and by sexual activity [46, 47].
Lactobacillus species can colonize both the urinary tract and the rectum. The female reproductive tract microbiome interacts with the gut (vagina–gut axis) and the urinary tract (vagina–bladder axis) and other sites like the oral cavity through direct or estrogen-mediated mechanisms. The rectum is a key lactobacilli reservoir that maintains a healthy vaginal microbiome and local immune system with the subsequent lower incidence of infections [48].
Enteric bacteria can deconjugate estrogens and promote their reabsorption to the circulatory system [49] This leads to the increased glycogen and mucus production and thickening of the epithelium of the lower genital tract. Thus, a reduction in estrogen-metabolizing bacteria could influence the Lactobacillus dominance in vaginal flora [50].
Complex interactions between the microbiome and host that increase the risk of gynecological cancer can be influenced by behavioral, socioeconomic, genetic, environmental, and host factors, including early life factors such as gestation, birth route, and infancy. Table 1 shows a summary of these factors.
Table 1.
Factors influencing the vaginal microbiome composition [48]
| Genetics/host | Environmental | Socioeconomic | Behavioral | STI status |
|---|---|---|---|---|
| Aging | Geography | Education | Sexual behavior | Bacterial infections |
| Genomics | Early life factors | Income | Contraception | Viral infections |
| Epigenetics | Toxins and carcinogens | Race, ethnicity | Hygiene practices | Fungal infections |
| Pregnancy | Antibiotics, prebiotics, xenobiotics | Access to healthcare | Smoking | Parasitic infections |
| Hormonal status | Stress | Social policy | Alcohol consumption | |
| Comorbidities | HPV vaccine | Diet/nutrition | ||
| Altered immunity | Obesity | |||
| Obesity | Physical activity |
Many recent studies have assessed the relationship between the VM’s composition and HPV infections leading to carcinogenesis. Ravel et al. [51] characterized the VM and vaginal pH of 396 asymptomatic, sexually active women, revealing five major community state types (CSTs) of VM. CST I, which occurred in 26.2% of the women, was dominated by L. crispatus, whereas CST II (6.3%), CST III (34.1%), and CST V (5.3%) were dominated by L. gasseri, L. iners, and L. jensenii, respectively. The remaining CST IV found in 27% of the women was heterogeneous and characterized by higher proportions of strictly anaerobic bacteria, including Gardnerella, Prevotella, Megasphaera, and Sneathia species [51]. CST I had the lowest median pH (4.0 ± 0.3), indicating that other CSTs might produce less lactic acid than group I or have different buffering capabilities [3].
Two faces of Lactobacillus species
Lactobacillus spp. prevent the adherence of pathogenic bacteria to the epithelial tissue and protect the vaginal epithelium through a series of barrier (self-aggregation, adherence) and interference (receptor-binding interference, coaggregation with potential pathogens) mechanisms [52]. Lactobacillus produces organic acids by decomposing glycogen to maintain the vaginal acidic environment [53], which can inhibit the invasion of pathogenic bacteria. H2O2-producing lactobacilli stimulate epithelial cell secretion of antimicrobial substances and increase the antibacterial activity of preexisting protective factors (muramidase and lactoferrin) [54]. Lactobacilli secrete various metabolites and surfactants, such as exopolysaccharides, phosphorylated polysaccharides, and peptidoglycans, which can inhibit harmful microorganisms and carcinogenesis [55–57]. Moreover, Lactobacillus spp. also activate the cellular and humoral components of the immune system [58].
Comparatively, L. crispatus is more effective in preventing bacterial dysbiosis than L. iners, lacking a protective role in vaginal health [59]. L. iners has no antibacterial or antiviral activity, as it can only synthesize L-lactic acid and cannot produce H2O2 [60]. On the other hand, L. iners produces inerolysin, a cytotoxin similar to that produced by Gardnerella vaginalis. Inerolysin forms pores in the vaginal epithelium, increasing the risk of multiple infections [61].
Lactobacillus spp. are associated with the decreased detection of high-risk HPV subtypes (OR 0.64), cervical dysplastic lesions (OR 0.53), and invasive cancers (OR 0.12). L. crispatus (CST I) itself has even better properties for the incidence of high-risk HPV (hrHPV) infections (OR 0.49) and neoplastic changes (OR 0.50) [62]. What more, a Lactobacillus gasseri (CST II)-dominant microbiome causes the rapid clearance of HPV infections (adjusted transition rate ratio (aTRR) 4.43) [63, 64]. Moreover, a specific group of less abundant lactobacilli including L. agilis and L. sanfranciscensis are significantly reduced in HPV-positive women and could play an important role in cervical carcinogenesis [65]. Interestingly, Mitra et al. observed that the overexpression of H2O2-producing L. jensenii and L. coleohominis prevents the progression of low-grade cervical dysplasia [66].
On the other hand, a L. iners-dominated microbiome is commonly associated with HPV positivity. In the vagina, the most common transition observed is from CST III to CST IV; this suggests that L. iners is less able to inhibit colonization by anaerobic bacteria than other Lactobacillus spp. [67]. It was even shown that L. iners became more abundant immediately before the onset of HPV-16 infections [68]. Lactobacillus-depleted CST IV and L. iners are responsible for HPV persistence and progression to preinvasive and invasive lesions [64]. The combination of Gardnerella vaginalis and L. iners or other unclassified lactobacilli increases the risk of high-grade cervical lesions [69, 70]. This CST type leads to a sixfold increase in the risk of cervical dysplasia [71]. Another study compared L. types and showed higher risks of prevalent hrHPV with L. iners than L. crispatus (OR – 1.31). L. iners is associated with almost double the overall risk for LSIL and cancerous lesions (OR 1.95) compared to L. crispatus-dominated CST [70].
Prognostic role of vaginal microbiome composition
Bacterial vaginosis (BV) has a high prevalence (around 9% in the UK [72] and up to 29% in the USA [73]). In women with BV, the native vaginal flora is replaced with invasive pathogens, including Gardnerella vaginalis and Prevotella and Mobiluncus species [74]. The replacement of lactobacilli with G. vaginalis promotes a basic pH that promotes BV. G. vaginalis produces a biofilm that provides a matrix on which other pathogenic bacteria can adhere; this biofilm also reduces the efficacy of antibiotic therapy [67]. CST IV in the cervicovaginal niche is associated with a higher risk of developing persistent HPV infections and, consequently, cervical lesions [75].
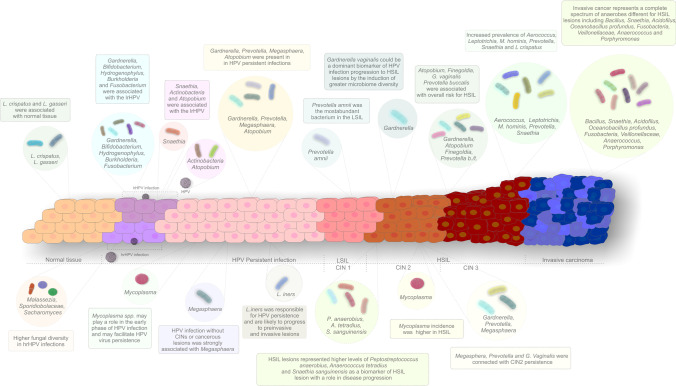
VM composition differs between HPV-positive and HPV-negative patients. Moreover, persistent high-risk HPV infections have a higher prevalence of bacterial vaginosis than HPV clearance [64]. Shannon et al. showed that HPV-positive patients are more likely to have cervico-VMs consistent with CST IV than HPV-negative women (58.8% vs. 29.4%) [76]. The predominance of some pathogens during the early phases of HPV infection may contribute to the subsequent development of cervical dysplasia [77]. A CST IV subgroup, characterized by a dominant presence of Gardnerella, Prevotella, Megasphaera, and Atopobium species, was present in 43% of women with persistent HPV infections but only in 7.4% of women with HPV clearance. G. vaginalis is a substantial risk factor for cervical disease development (OR 10.19) [59]. Its dominance may result from a shift from antimicrobial to antiviral immune responses, with a loss of bacterial control caused by HPV itself [68]. Subsequent production of bacterial sialidase by G. vaginalis leads to creating a biofilm that entraps anaerobic bacteria such as Prevotella and Atopobium, leading to their overgrowth and HPV persistence [78]. G. vaginalis could be a dominant biomarker of HPV infection progression to HSIL lesions through the induction of greater microbiome diversity [79] with an immunosuppressive effect [80]. This vicious cycle leads to overall changes in mucosal metabolism and immune responses [81, 82]. A proinflammatory environment then facilitates the integration of viral DNA; this is a crucial point in cervical carcinogenesis, viral persistence, and disease progression [66].
HPV infection increases VM diversity and richness, leading to gradual increases in the CST IV proportion and consequent increases in the severity of cervical dysplasia [83]. The mycobiome similarly correlates with HPV infection and CIN severity, with higher fungal diversity in hrHPV infections (Malassezia) and ASCUS cytology results (Sporidiobolaceae, Saccharomyces) [84]. Chen et al. distinguished the specific microbes and the vaginal bacterial structure related to the progression of CINs. For example, HPV infection without CINs or cancerous lesions was strongly associated with Megasphaera, while the most abundant bacterium in the low-grade squamous intraepithelial lesion group was Prevotella amnii [83]. HPV-positive patients with high-risk microbial patterns have an OR of 34.1 for LSIL development, compared to HPV-negative women with low-risk microbial scores [64].
Snaethia is a further abundant species connected with HPV infection, as it induces strong inflammation in the vaginal microenvironment [85]. Some studies indicate its potential as a marker of low-risk HPV (lrHPV) genital infection [86]. LrHPV infection is also associated with the abundance of Actinobacteria and Atopobium, which disrupt epithelial barriers [85]. Other common bacterial species in the lrHPV group include Gardnerella, Bifidobacterium, Hydrogenophylus, Burkholderia, and Fusobacterium — all of which have oncogenic potential [66, 87, 88].
The rate of CST IV incidence increased twofold in LSIL, threefold in HSIL, and fourfold in CC groups [66]. HSIL lesions are associated with higher levels of Peptostreptococcus anaerobius, Anaerococcus tetradius, and Snaethia sanguinensis; these species may serve as biomarkers for HSIL lesions [64, 66, 89]. Mycoplasma incidence is also higher in HSIL lesions than LSIL, but it is not detected in cancer. Mycoplasma spp. may play a role in the early phases of HPV infection and may facilitate HPV persistence [77].
So et al. estimated the overall risks for HSIL and CC development associated with particular bacteria, reporting the highest values for Atopobium (OR 4.33), Finegoldia (6.00), Prevotella timonensis (6.00), G. vaginalis (7.33), and Prevotella buccalis (11.00) [59, 65]. Prevotella spp. could be predictive of CIN2 + lesion development in cases of persistent hrHPV infection [65]. CIN2 is a heterogenous disease with marked chances of regression. CST IV species, including Megasphaera, Prevotella, and G. vaginalis, are connected with CIN2 persistence with an OR of 3.85 for 12-month persistence and an OR of 4.25 for 24-month persistence [90]. CIN3 lesions are associated with significantly different VM compositions from CIN2 (Lactobacillus spp., A. vaginae, G. vaginalis, and U. parvum) with the prevalence of Aerococcus, Leptotrichia, M. hominis, Prevotella, Snaethia, and L. crispatus dropping from 70 to 47% [91, 92]. From these studies, it is clear that substantial reductions in lactobacilli are seen in the third grade of dysplastic changes of the cervical epithelium. Invasive cancer is associated with a complete spectrum of anaerobes, including Bacillus, Snaethia, Acidovirus, Oceanobacillus profundus, Fusobacteria, Veillonellaceae, Anaerococcus, and Porphyromonas [83]. An overview of bacterial compositions in cases of HPV infection, different degrees of dysplasia, and invasive cancers is provided in Fig. 1.
Fig. 1.
Changes of the vaginal microbiome composition in the process of cervical carcinogenesis. Abbreviations: HPV, human papillomavirus; hrHPV, high-risk HPV; lrHPV, low-risk HPV; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia; L. crispatus, Lactobacillus crispatus; L. gasseri, Lactobacillus gasseri; G. vaginalis, Gardnerella vaginalis; Prevotella b., Prevotella buccalis; Prevotella t., Prevotella timonensis; M. hominis, Mycoplasma hominis; L. iners, Lactobacillus iners; P. anaerobius, Peptostreptococcus anaerobius; A. tetradius, Anaerococcus tetradius; S. sanguinensis, Snaethia sanguinensis
Personalized diagnostics and treatment of HPV-associated cervical disease based on vaginal microbiome composition
Currently, the complete lack of effective medical therapies for HPV infection poses a significant challenge. Conization treatment remains the gold standard in cases of histologically proven high-grade cervical lesions with possible future adverse effects [64]. Immunohistochemical markers, including p16 and Ki-67, increase the specificity of cytological or histological examinations. Nevertheless, no therapies based on diagnostic markers are included in international guidelines. Therefore, research foci should be diverted away from the ocean of molecular markers with no clinical applicability and toward the promising interactions within and between bodily cells and their prokaryotic symbionts.
Delineation of the exact composition and immune environment of the VM before HPV acquisition, during persistent infection, and after clearance provides insight into the complex mechanisms of cervical carcinogenesis [68]. A detailed review by Bubnov et al. highlighted the significant role of probiotic-based regimens in treating immune and atopic states, metabolic and inflammatory diseases, and cancers [93].
There is substantial evidence that the gut microbiome is linked with the normal functioning of the brain, heart, skin, respiratory, and urogenital system, leading to new approaches to maintaining health, disease prevention, and treatment of multiple chronic diseases. Consequently, the use of probiotics and prebiotics could have an enormous potential for patient care [94].
Modulating the gut microbiome (sometimes referred to as “bugs as drugs”) includes bacterial therapeutics, probiotics, prebiotics, antibiotics, and microbiota transplantation [48]. Vaginal and fecal microbiota transplantation (VMT, FMT) is a novel, provocative treatment option under investigation for women with BV or vaginal disorders [95].
Prebiotics and probiotics recreating a Lactobacillus-dominant environment in the VM are potential low-cost treatment strategies with minimal side effects [96]. Moreover, probiotics are safe and have proven antiviral activity [97]. Probiotics including L. paracasei and L. rhamnosus acidify the vaginal microenvironment, prevent bacterial adhesion, and act synergistically with the host immune system [98]. This simple approach with oral or vaginal regimens could promote HPV clearance or even reverse carcinogenesis and reduce subsequent morbidity [96, 99]. HPV clearance was markedly increased in patients using probiotics compared to controls (25% vs. 7,7%) [100]. The same effects were seen in cytological abnormalities, with a twofold increase in clearance and normalization in the treated patients [101].
Orally administered lactobacilli can verifiably reach the vagina, restore its microbiota in the presence of microbial imbalance, and eradicate or reduce the incidence of urogenital infections [102, 103]. Orally consumed probiotics ascend to the vaginal tract after they are excreted from the rectum. On the other hand, vaginal administration allows for the direct replacement of probiotics for unhealthy vaginal flora [104]. Daily intake of probiotics containing L. casei improved HPV clearance in LSIL lesions [100]. Bifidobacteria may further enhance antitumor immunity and the efficacy of immunotherapy [105]. Clarification of the exact interactions between specific bacterial types, the immune system, and HPV will support the development of probiotics with high efficacy.
Prebiotics also significantly reduce HPV positivity and low-grade cervical lesion occurrence. Selected prebiotics improve the ectopic structure of the cervical mucosa and enhance the maturation of the metaplastic epithelium, leading to negative colposcopic findings. What is more, prebiotics form a mucoadhesive film that protects the cervical surface from pathogenic microbial agents [41].
Remarkably, research on the penile microbiome has exciting implications. Given the sexually transmitted nature of the pathogenic agents in question, it is wise to consider the male microbiome. Penile anaerobic bacteria such as Prevotella may increase the risk of genital infections such as bacterial vaginosis. On the other hand, penile Corynebacteria and Staphylococcus are associated with healthy cervicovaginal microbiota [106]. In summary, the penis can be a reservoir of BV-associated bacteria, and complex interactions occur in both the male and female genital biomes [107, 108]. Based on the hypotheses above, topical microbicides can fight against HPV infection in the male population [106].
New markers are essential for monitoring and identifying microbial compositions indicative of HPV infection and cervical dysplasia. State-of-the-art molecular methods for VM assessment could support advanced and individualized management strategies (in conjunction with HPV vaccination) to reduce CC incidence [109]. Rapid bedside tests (microchip arrays and metabolomic technologies) focused on identifying patients at highest risk have potential in triage and the selection of patients for intense observation and treatment [64]. For instance, Mitra et al. presented selected vaginal biomarkers for CIN in Caucasian, Black, and Asian women, including Snaethia sanguinensis, Anaerococcus tetradius, and Peptostreptococcus anaerobius [66]. Similar microbial markers could be applicable in tailored surveillance and prognostic evaluation of women with cervical lesions [90].
Innate immune system and HPV infection
The innate immune system plays an essential role during the early stage of HPV infection. Its mechanisms are associated with developing a proinflammatory microenvironment, leading to the recruitment of immune cells and subsequent eradication of infected cells [110, 111]. Importantly, innate immunity is also crucial for the activation of adaptive immunity [112]. Components of the immune system contribute to viral clearance and tumorigenesis due to HPV infection [113]. Innate immunity includes various components, such as physical and anatomical barriers, effector cells and effector molecules (i.e., antimicrobial peptides), and receptors. [114]. The potential of HPV to trigger carcinogenesis in cervical epithelia is associated with the weakened immune system of an infected woman. Indeed, cervical intraepithelial neoplasia (CIN) develops in 15–30% of hrHPV-infected women within 2 years; approximately 10–20% of severe CIN cases develop into invasive CC. Nevertheless, current research into the applicability of vaginal suppositories of the metabolite 3,3′-diindolylmethane for CIN treatment offers potential agents for the personalized prevention of CC [115].
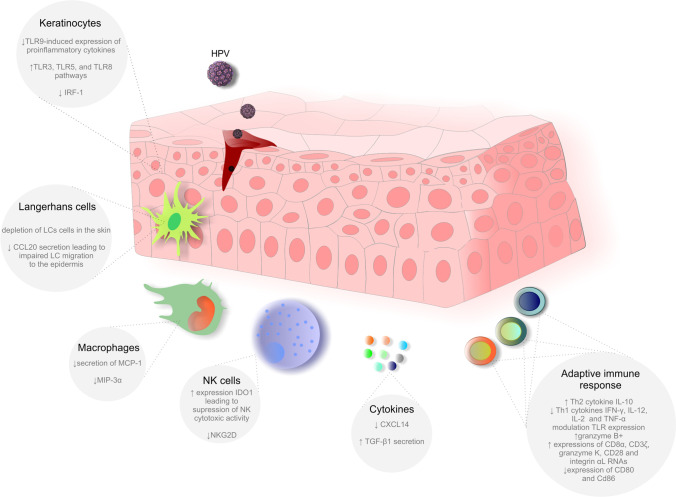
The primary targets of hrHPV in squamous epithelia are undifferentiated keratinocytes [116]. These cells act as non-professional immune cells by serving as physical barriers and expressing pattern recognition receptors (PRRs). PRRs are involved in recognizing pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP). PRRs include Toll-like receptors (TLRs), retinoic acid-inducible gene (RIG) I-like receptors (RLRs), and nucleotide-binding oligomerization domain-like receptors (NLRs) [117]. Keratinocytes express surface and endosomal TLRs; endosomal TLRs are essential for the recognition of viral nucleic acids. TLRs may recognize double-stranded RNA (TLR3), single-stranded RNA (TLR7 and TLR8), and double-stranded CpG-rich DNA (TLR9) [113, 116, 118]. Notably, HPV infection can modulate cytokine secretion and lead to immune evasion. For instance, the HPV18 oncoproteins E6 and E7 suppressed TLR9-induced expression of proinflammatory cytokines [119]. Interestingly, in contrast to TLR9, the TLR3, TLR5, and TLR8 pathways were activated in hrHPV-infected cells [120]. Furthermore, the upregulation of TLR8 correlated with the higher expression of Bcl-2 and VEGF in HeLa cells and CC tissue [120]. Moreover, an HPV oncoprotein (HPV18-E7) inhibited the expression of interferon regulatory factor 1 (IRF-1) [110, 121]. Accordingly, keratinocytes containing episomal copies of hrHPV exhibit deregulated genes associated with antigen presentation, inflammasome action, and proinflammatory and chemotactic cytokines [122].
Dendritic cells (DC) are leukocytes responsible for initiating antigen-specific immune responses through antigen presentation [123]. Due to the localization of HPV infections, a DC subtype known as Langerhans cells (LCs) plays a critical role in initiating and regulating the antiviral immune response [124]. Upon recognition of HPV, LCs undergo maturation, resulting in the upregulation of MHC expression, increased secretion of chemokines and cytokines, and migration to the lymph nodes to activate naive T cells [125]. LCs form tight junctions with adjacent keratinocytes; this close contact mediated by E-cadherin is crucial for retaining LCs in the skin [126]. However, HPV-16 infection reduced E-cadherin expression in infected keratinocytes, resulting in the depletion of LCs in the skin [127]. Notably, chemokine ligand 20 (CCL20) is expressed in various cells and is essential for the migration of immature LCs to the epidermis. The inhibition of CCL20 secretion by keratinocytes by HPV infection impaired LC migration to the epidermis. In-depth analyses revealed that oncoprotein E7 affected molecular cascades, preventing the binding of the transcription factor C/EBP to a CCL20 promoter and inhibiting NF-kB signaling [128]. In addition, recent evidence suggests that HPV-E6-expressing cells inhibit the differentiation of monocytes into LCs [129].
Like DCs, macrophages are professional antigen-presenting cells (APCs) with a critical role in connecting the innate and adaptive immune responses [130, 131]. Macrophages contribute to HPV clearance by eradicating infected host cells. Furthermore, these cells secrete IL-17 and TNF-α to initiate and promote the infiltration of immune cells [132]. HPV utilizes several strategies to modulate macrophage activity. The E6 oncoprotein expressed by infected keratinocytes inhibited the release of monocyte chemoattractant protein-1 (MCP-1) and thus modulated macrophage chemotaxis [133]. In addition, the E6/E7 oncoprotein reduces the secretion of macrophage inflammatory protein 3α (MIP-3α) by infected keratinocytes, resulting in the modulation of macrophage chemotaxis [134].
Viral infection causes the secretion of IFNs and cytokines by DCs and macrophages, leading to the activation of natural killer (NK) cells, which are another important barrier against HPV-infected cells [128]. HPV infection regulates the activity of NK cells in various manners, as demonstrated in several experimental studies. HPV-infected cells expressing HPV-16 E7 expressed indoleamine 2,3-dioxygenase 1 (IDO1), which has an immunosuppressive role and contributes to the impairment of NK cytotoxic activity [135]. Moreover, the natural killer group 2D (NKG2D) receptor is crucial for NK cell activation [136]. The downregulation of NKG2D in NK cells was associated with suppressing cytotoxic activity after contact with SiHa and HeLa HPV-positive cancer cells expressing NKG2D ligands [137].
Finally, HPV infection affects several other components of the innate immune response. The chemokine CXCL14 acts as an immune and inflammatory modulator regulating immune cell migration [138]. Low expression of CXCL14 was documented after HPV infection due to the regulatory impact of E7 oncoprotein-induced hypermethylation of the CXCL14 promoter region [139]. Additionally, low expression of CXCL14 impaired the differentiation of CD14 + DC precursors into LCs [140] and blocked the maturation of macrophages [141]. Similarly, E6/E7 expression correlated with TGF-β1 secretion. TGF-β reduces the immune response via various mechanisms such as suppressing cytokine production or the inhibition of T cell proliferation [142]. Suppression of the immune response via E6-/E7-mediated upregulation of TGF-β1 represents another way to escape from immune surveillance [143].
Understanding the role of HPV in the modulation of the innate immune response is crucial to develop effective therapies against HPV-related cancer. Only more in-depth investigations focused on the relationship between viral pathogens and components of innate immunity can bring novel therapeutic approaches in the context of 3P medicine.
Adaptive immune response to HPV infection
Although the innate immune system is a crucial defense mechanism, it can only recognize a limited number of PAMPs. Effective evasion of innate immune recognition is a hallmark of HPV infections. hrHPV infection modulates the adaptive or acquired immune system to create a suitable microenvironment for persistent infection and lesion progression [144]. Adaptive immunity has evolved for more accurate and broader recognition of both self- and nonself-antigens [145, 146]. The leading players in the adaptive immune response are antibodies and T cells. Antibodies specific for viral surface antigens can block the binding and fusion of viruses with host cells [147]. Moreover, T cell-mediated immune responses play a pivotal role in adaptive immunity. Two viral oncoproteins, E6 and E7, cause the development of cancers induced by HPV infection. Immune responses specific for either or both of these oncoproteins are essential for therapeutic interventions [148]. Therefore, vaccination against HPV can support the prevention of HPV and associated cancers through antibody-driven immunological memory [146]. Intra-muscular delivery of HPV virus-like particle vaccines with high antigen doses enables rapid and direct access to the lymph nodes and the spleen to initiate the adaptive immune response. Two available vaccines against HPV infection, Cervarix® and Gardasil9®, rely on the interaction of antibodies with HPV L1 capsid protein epitopes [148]. However, without vaccination, acquired immunity through natural HPV infection provides only modest protection against subsequent cervical HPV infections in women [149].
In cases of immunological danger, TLRs link innate and adaptive immunity through their actions on T cells. CD4 + /CD8 + T cells, regulatory T cells, T helper 1 (Th1) and Th2 CD4 + cells, and antibody-producing B cells are adaptive immune cells [150]. TLRs activate downstream signaling pathways, which involve DCs, macrophages, NK cells, NF-κB, mitogen-activated protein kinases (MAPKs), proinflammatory cytokines, and ultimately the induction of adaptive immunity [151, 152]. After the activation of TLRs and consequent signal transduction, DCs undergo maturation characterized by the upregulation of cell surface major histocompatibility complex (MHC) molecules and pathogen-derived peptide co-receptors and fragments (CD40, CD80, and CD86); this finally leads to the activation of T cells and the induction of antigen-specific immune responses [153]. Moreover, reduced CD80 and CD86 expression in DCs in hrHPV + patients positively correlates with CIN grades [154]. Besides, higher levels of stromal DCs are commonly associated with increased HPV infection regression through the activation of the programmed death 1 (PD-1)/PD-1 ligand (PD-L1) pathways [155]. Furthermore, in cervical exudates of hrHPV + patients, increased Th2 cytokine (IL-10) and reduced Th1 cytokine (IFN-γ, IL-12, IL-2, and tumor necrosis factor-α) levels are detected [156, 157]. Moreover, several studies indicate that HPV modulates TLR expression and interferes with TLR signaling pathways; this could point to therapeutic strategies based on TLR agonists to revive host immune responses inhibited by persistent HPV infection [158]. Furthermore, cervical biopsies from women with low-grade and high-grade CIN reveal that cytotoxic T cells are predominant in the intraepithelial region; however, CD4 + and FOXP3 + T cells are present in the stromal compartment. Moreover, in the regression state of low-grade CIN, the levels of the cytotoxic granzyme B + significantly increase. Besides, the correlations between granzyme B + levels and CD8 + T cell populations indicate that the early infiltration of highly active CD8 + T cells has preventive effects against the progression of CIN to invasive cancer [159]. Similarly, the upregulation of CD8α, CD3ζ, granzyme K, CD28, and integrin αL RNAs has been observed in HPV-positive lesions but not in HPV-unrelated tumors. Besides, in HPV-positive tumors, the stroma is strongly infiltrated by CD8α‐ and CD3ζ‐positive T cells, suggesting that an enhanced cytotoxic T cell-mediated antitumor immune response can ameliorate the prognosis of patients with HPV-positive tumors, including HPV-related oropharyngeal tumors [160]. Interestingly, nano-pulse stimulation, a non-thermal pulsed electric field modality, can stimulate an adaptive immune response through the generation of CD8 + T cells that can recognize tumor antigens in an HPV-16-transformed C3.43 murine tumor model [161].
Despite the high efficacy of HPV-like particle vaccines, they remain uncorrelated with protection against infection or disease, and the role of B cell memory remains unclarified. Therefore, these parameters should be evaluated, for example, through the long-term follow-up of vaccinated individuals. However, adaptive immunity obtained by the vaccine is stronger than the acquired immunity by natural HPV infection. A better understanding of the molecular genetic profiles of variations in common immune response genes can improve the HPV vaccine in the prevention of CIN and CC. Figure 2 summarizes the impact of HPV infection on critical components involved in innate and adaptive immune responses.
Fig. 2.
Role of adaptive and innate immunity in HPV infection. Abbreviations: TLR 3, toll-like receptor 3; TLR 5, toll-like receptor 5; TLR 8, toll-like receptor 8; TLR 9, toll-like receptor 9; IRF-1, interferon regulatory factor 1; LC, Langerhans cells; CCL20, chemokine (C–C motif) ligand 20; MCP-1, monocyte chemoattractant protein-1; MIP-3α, macrophage inflammatory protein 3 alpha; IDO1, indoleamine 2,3-dioxygenase 1; NKG2D, natural killer group 2D; CXCL14, chemokine (C-X-C motif) ligand 14; TGF-β1, transforming growth factor beta 1; NK cells, natural killer cells; Th2, T helper type 2; Th1, T helper type 1; IFN-γ, interferon gamma; HPV, human papillomavirus; IL-12, interleukin 12; IL-2, interleukin 2; TNF-α, tumor necrosis factor alpha; CD8α, cluster of differentiation 8 alpha; CD3ζ, cluster of differentiation 3 zeta; CD28, cluster of differentiation 28; CD80, cluster of differentiation 80; CD86, cluster of differentiation 86
Novel immunotherapeutic strategies in HPV-induced cervical carcinogenesis falling into the concept of 3P medicine
The early oncoproteins E6 and E7 from HPV genotypes in cervical lesions are crucial targets of progressive immunotherapeutic strategies. Several HPV therapeutic vaccines were introduced to ameliorate the function of DCs and T lymphocytes [162]. However, established HPV infections with associated neoplastic changes require therapeutic vaccines in combination with cellular immune inducers, such as immunomodulators that enhance HPV-specific cellular responses. These immunomodulators include TLR adjuvants that activate innate immunity, substances that directly enhance adaptive immunity (co-stimulatory molecules and cytokines), and adjuvants that eliminate cancer-induced immunosuppressive processes [162].
Postoperative immunotherapy with inosine pranobex in HPV-positive women receiving cervical conization of established high-grade squamous intraepithelial lesions resulted in significant increases in viral clearance and reductions in disease relapse [163]. Another study demonstrated the clinical importance of combinatory treatments that can (1) induce tumor-associated T cell responses, (2) elevate immune responses in the tumor microenvironment (TME), and (3) reduce immunosuppressive mechanisms in the TME [164]. Combinations of the mentioned clinical strategies may increase the efficacy of therapeutic vaccines in combating HPV-induced cervical neoplasia [165].
Recent data revealed that HPV-transformed cells actively promote chronic stromal inflammation and cooperate with cells within the local microenvironment to support carcinogenesis. This novel insight provides several implications for clinical practice [166]: (a) novel diagnostic approaches such as immunoscores can distinguish non-progressing and progressing precursor lesions; (b) new biomarkers can predict responses to therapy (e.g., IRF1 as a parameter of chemo- or radiochemotherapy); and (c) new immunotherapeutic tools can target specific mechanisms and signaling pathways in HPV-induced carcinogenesis; these tools may include dsRNA-based immunotherapies for individuals with elevated intratumoral RIPK3 expression, IRF3-activating molecules such as dsRNA to eliminate oncogenic β-HPV infection, blockers of the IL-6/JAK/STAT3-pathway for patients with CC after chemo- or radiochemotherapy, and therapies based on IgA antibodies that engage myeloid cells in the killing of tumor cells [166].
Recently, immunotherapy using checkpoint inhibitors was implemented into more targeted CC treatment approaches and showed promise. These novel drugs can reduce the disease burden associated with HPV infection and improve the quality of life of patients [162]. In this regard, the anti-PD1 antibody pembrolizumab was approved by the FDA to treat metastatic or recurrent CC with PD-L1 expression that progresses after one or more lines of chemotherapy. Cemiplimab, another anti-PD1 antibody, demonstrated similar clinical benefits both alone and in combination with radiotherapy. It is currently under evaluation in a phase III trial [167]. Other novel checkpoint inhibitors such as atezolizumab, durvalumab, nivolumab, and camrelizumab are in different stages of clinical evaluations. An additional targeted approach in managing CC involves PARP inhibitors (olaparib and rucaparib, which are both in phase II trials) based on earlier promising data [167].
The molecular etiology of HPV-induced CC provides novel biomarkers that may play an important role in predicting early recurrence and supporting personalized, alternative treatment modalities. Moreover, a wide spectrum of biomarkers may help to tailor immunotherapy and improve responses to these novel approaches.
HLA-related genetics and HPV clearance/persistence: an assessment of individualized risk
The human MHC or human leukocyte complex (HLA) is a cluster of highly polymorphic genes localized on chromosome 6 [168]. HLA genes encode proteins with roles in antigen presentation [169]. There are two classes of HLA (I and II) with essential roles in presenting HPV antigen peptides to T cells. Specific polymorphic variants of the HLA alleles are determinants of HPV persistence and progression. Class I and II HLA polymorphisms were associated with persistent HPV infection and the development of CC (i.e., HLA-A*02, HLA-A*0201, HLA-A*3101, HLA-DQB1*05 HLA-DRB1*04, HLA-DQB1*0602, HLA-DQB1*0403, HLA-A*3303, and HLA-B*3901); other alleles were associated with the reduction of CC risk (i.e., HLA-B*15, HLA-DRB1*13, HLA-DRB1*1310, and HLA-B*1501, HLA-DQB1*0402) [170–177]. Roy-Ospina et al. provided unique insight into the relationship between variations in the HLA-DRB1/DQB1 genes and HPV clearance and redetection. Their study identified alleles and haplotypes and epitope (L1 resp. L2) binding to MHCII molecules from HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, and HPV-58 [178]. Similarly, Bhaskaran et al. analyzed the association between the HLA-A, HLA-B, HLA-DRB, and HLA-DQB genes and HPV-16 persistence and HPV-16-associated CC [179]. Besides, differences in HLA expression correlate with HPV infection. Higher HLA-E expression is associated with better overall survival of patients with CC. Elevated HLA-E expression may be a strategy of host cells against HPV infection due to HLA-E interaction with CD94/NKG2 receptors localized on NK cells; this interaction leads to eradicating infected or cancer cells [180]. Moreover, HLA-G was upregulated in HPV-infected cervical epithelium and CC tissue. In-depth analyses indicated that augmentation of HLA-G favors HPV persistence and neoplastic transformation [181]. As mentioned above, the HLA genes are highly polymorphic. HLA polymorphisms associated with HPV persistence and CC progression and those related to host defense represent promising biomarkers in the context of 3P medical approaches focused on the prevention of HPV-associated CC.
Immune system signatures and cervicovaginal microbiome composition in preventive, predictive, and personalized medicine associated with cervical carcinogenesis and HPV clearance
Due to the role of persistent HPV in CC, an understanding of the mechanisms resulting in HPV clearance is necessary for cancer prevention. The microbiome plays an essential role in human health and immunity [97, 182, 182]; however, the utilization of the microbiome for 3P medicine should be assessed in terms of evidence-based knowledge [93]. Microbiome investigations revealed that G. vaginalis is involved in shaping immune responses, possibly through a shift from antimicrobial to antiviral responses. These bacteria also perform a certain level of inflammatory surveillance to maintain the HPV-negative state. These results suggest that probiotics or proinflammatory agents could be helpful in treating persistent HPV [68]. Moreover, Qingqing et al. reported that cervicovaginal microbiota dysbiosis in cervical secretions to be closely associated with persistent HPV infection identified dysbiosis biomarkers (supported by blood markers) provided new concepts for CC prevention [183–185]. Table 2 provides a detailed overview of the various liquid biopsy markers potentially utilizable in cervical pathogenesis in HPV-infected women.
Table 2.
Immune system and cervicovaginal microbiome signatures utilizable in cervical carcinogenesis and HPV infection
| Aim of study | Study details | Results | Reference |
|---|---|---|---|
| Clarification of the relationship between locally secreted cancer biomarkers and features of the cervicovaginal microenvironment to understand the interplay between host, virus, and vaginal microbiota | Ctrl HPV − (n = 18), Ctrl HPV + (n = 11), LSIL (n = 12), HSIL (n = 27), and ICC (n = 10) evaluated in cervicovaginal lavage | Cancer biomarkers elevated in ICC vs Ctrl HPV − : proinflammatory cytokines (TNF-α), apoptosis-related proteins (sFas, sFasL, TRAIL), hormones (leptin, prolactin), growth and angiogenic factors (HGF, SCF, VEGF), other multi-functional proteins (OPN, CYFRA 21–1, AFP) | [186] |
| Biomarkers to distinguish ICC from healthy Ctrl HPV − : TNF-α, CYFRA 21–1, MIF, prolactin, SCF measured in the CVL | |||
| Expression of cancer biomarkers related to genital inflammation and VM composition | |||
| 19 out of 23 cancer biomarkers exhibited significant positive correlations with inflammatory scores. Nine cancer biomarkers (MIF, TNFα, sFasL, TRAIL, FGF2, SCF, prolactin, and OPN) correlated negatively with Lactobacillus abundance and positively with vaginal pH. HE4 (an antimicrobial peptide) correlated positively with Lactobacillus abundance and negatively with vaginal pH | |||
| Characterization of the relationship between HPV, vaginal pH, vaginal microbiota, genital immune mediators, and the severity of cervical neoplasms | Ctrl HPV − (n = 20), Ctrl HPV + (n = 31), LGD (n = 12), HGD (n = 27), and ICC (n = 10) evaluated in vaginal swabs and cervicovaginal lavage | Increased vaginal pH at various stages of cervical carcinogenesis and abnormal pH highly associated with cancer | [187] |
| Enriched Sneathia spp.; underrepresented Lactobacillus spp. in ICC, LGD, HGD, Ctrl HPV + | |||
|
BV-associated Atopobium and Parvimonas enriched in LGD and HGD BV-associated Gardnerella, Prevotella, Megasphaera, and Shuttleworthia enriched only in HGD | |||
| Sneathia and Atopobium enriched and underrepresented Lactobacillus spp. in abnormal pH or patients of Hispanic ethnicity | |||
| Increased genital immune mediators and genital inflammatory scores in invasive carcinoma only | |||
| Negative correlations of PD-L1 and LAG-3 and positive correlation of TLR2 with health-associated Lactobacillus dominance | |||
| CD40, CD28, and TLR2 positively correlated with genital inflammation | |||
| Evaluation of the association between the microbiome and inflammatory milieu during HPV-16 pre-acquisition, persistence, and clearance | Women that acquired HPV-16, persistence at least 8 months and cleared (n = 14) and WNHPV (n = 8) evaluated in cervical wash repository samples |
HPV-16: L. Iners, abundance increased immediately before infection but decreased thereafter. G. vaginalis, abundance increased immediately before clearance, significantly increased after clearance, and returned to baseline months later WNHPV: L. iners, more abundant than in samples from the immediate post-clearance visits of HPV-infected subjects G. vaginalis, less abundant than in immediate post-clearance samples but similar to pre- and post-infection levels |
[68] |
| Cytokines (IL-4, IL-5, IL-10, IL-12, and IL-13, IFNγ, IFN-α2, MIP-1α, TNF-α) elevated at the immediate post-clearance visit compared to the pre-acquisition and second post-clearance visits | |||
|
G. vaginalis was associated with elevated cytokines in the post-clearance visit (IL-4, IL-5, IL-10, IL-12, IL-13, TNF-α, IFN-γ, MIP-1α) A G. vaginalis increase preceded final clearance and peaked at the time of the observed cytokine peak | |||
| Correlation of cervicovaginal microbiota dysbiosis and HPV persistence | Persistent and transient HPV infections and healthy women (cervical secretions, blood) | HPV-persistent infection: higher abundance of Proteobacteria, Actinobacteria, Bacteroidetes, and Fusobacteria | [183] |
| Correlation of Prevotella, Sphingomonas, and Anaerococcus with persistent HPV infection. Correlation of Lactobacillus iners with transient HPV infection | |||
| Altered immune microenvironment with cervicovaginal microbiota dysbiosis: IL-6 and TNF-α were upregulated in cervical secretions from persistent HPV infection compared with transient infections and healthy women | |||
| Peripheral blood: regulatory T cells and myeloid-derived suppressor cells in patients with persistent HPV infection were significantly increased |
Abbreviations: AFP, α-fetoprotein; BV, bacterial vaginosis; CD27, cluster of differentiation 27; CD28, cluster of differentiation 28; CD40, cluster of differentiation 40; Ctrl HPV−, HPV-negative controls; Ctrl HPV+, HPV-positive controls; CYFRA 21-1, cytokeratin fragment 21-1; HGD, high-grade dysplasia; HGF, hepatocyte growth factor; HSIL, high-grade intraepithelial lesions; ICC, invasive cervical carcinoma; IFN-γ, interferon gamma; IL, interleukin; LGD, low-grade dysplasia; LSIL, low-grade intraepithelial lesions; MIF, macrophage migration inhibitory factor; MIP-1α, macrophage inflammatory protein-1α; OPN, osteopontin; SCF, stem cell factor; sFas, soluble Fas receptor; sFasL, soluble Fas ligand; TIM-3, T-cell immunoglobulin and mucin domain-containing 3; TLR2, toll-like receptor 2; PD-L1, programmed cell death ligand 1; TNF-α, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor; WNHPV, women with no history of HPV
Indeed, HPV utilizes various mechanisms to evade immune responses and progress from infection to chronic dysplasia and cancer [121]. The presence of invariant natural killer T (iNKT) cells in cervical tissue during the progression from HPV infection to CIN suggests the critical role of iNKT cells in immunosuppression; preventing their accumulation could inhibit CIN development [188]. Moreover, Gutiérrez-Hoya et al. recently demonstrated that CC cells express markers related to the activation and inhibition of the immune system, as well as receptors of natural killer (NK) cells, suggesting that these molecules could potentially facilitate mimicry of the immune cells and evasion of immune responses [189]. Nevertheless, the means through which HPV evades immune mechanisms could promote the identification of new immunological targets applicable in HPV-related cancer therapy; including the use of cytokines to create tumor milieu favoring the destruction of transformed cells, DC vaccines that activate Th1 and cytotoxic T lymphocyte responses, and the activation of NK cells through autologous or allogenic transplants to induce tumor cell lysis [190].
Conclusions and expert recommendations
Viral infections are responsible for approximately 15% of cancer cases worldwide [191]. These oncogenic viruses use different strategies that lead to carcinogenesis, including direct effects on immune responses with subsequent chronic inflammation. A general model of virus–bacteria–host interaction involves altered host gene expression with the consequent promotion of tumorigenesis [192]. On the other hand, a microbiome with multiple bacterial, viral, and fungal species can protect the host from viral infections. This defense system greatly depends on the exact microbiome composition and its complex interactions.
Predictive diagnostics
The disrupted balance of the VM and the innate immune system is one of the most critical risk factors in HPV infection. It could represent an exciting and valuable group of biomarkers for stratifying dysplastic cervical lesions and predicting LSIL and HSIL lesions regression, persistence, or progression to invasive disease. Novel diagnostic approaches such as immunoscores can help to distinguish non-progressing and progressing precursor lesions. Moreover, complex evaluation of the local vaginal microenvironment might potentially assess the malignant potential in elusive histological subgroup of cervical intraepithelial neoplasia grade 2 (CIN2), which has a marked chance regress. A similar approach is also demanding in persisting LSIL lesions, where the guidelines are still incoherent.
Targeted prevention
Prevention is a cornerstone in HPV-associated diseases. There are multiple behavioral, environmental, and host factors influencing the vaginal microbiome and local innate immune system that could be successfully and easily modified. Smoking, a diet rich in fat, with a high glycemic load and nutritional density, and obesity are linked to a higher incidence of bacterial vaginosis [193]. Even hygiene practices, including the frequent douching and use of vaginal lubricants, could alter the local vaginal flora [194]. Gender-specific health concepts raise questions regarding the functional interactions between genital microbiota and the host and the associations between semen and the VM [195]. Multiple studies showed that vaginal microbiome members could be detected in male penile skin, urethral, urine, and semen specimens [196, 197], suggesting that sexual partners share and exchange microorganisms colonizing the urogenital tracts. This fact highlights the need for universal and gender-neutral HPV prevention strategies focused on modifiable risk factors.
Personalization of medical services
Focusing the research on the exact Lactobacillus composition could explain how to prevent the persistent HPV infections and subsequent disease progression [198]. Footprints of these unique ecosystems tightly connected to the immune system could provide valuable insights for the diagnosis and treatment in the context of 3P medicine. This multidisciplinary character of 3P medicine is essential to overcoming barriers between the clinical sphere and the research area [199].
The accumulating evidence connecting HPV-associated gynecological cancers and dysbiosis identifies microbiota as a valuable target for cancer prevention and therapy. In the future, vaginal probiotics, prebiotics, novel antimicrobials, biofilm disruptors, and microbiome transplantation have the potential to be used alone or in combination to modulate the vaginal microbiome by restoring a healthy local microenvironment for the prevention of cervical cancer and/or reduction of vaginal toxic effects associated with cancer therapies [48].
Biomarkers of cervical carcinogenesis obtained from various biofluids (vaginal swabs, cervicovaginal lavage/secretions, or blood) constitute important evidence utilizable in personalized, preventive, and predictive medicine, which is essential for the improvement of medical care due to the need for an individualized approach and reduced economic burden of traditional healthcare in the context of medicine of the twenty-first century. The integration of the unique composition of the VM and the innate immune system allows us to create a complex ecosystem map for targeted prevention and the development of personalized treatment regimens. This could reduce drug toxicity, adverse side effects, and morbidity [200]. What more, high-throughput and cost-effective multi-omics technologies play a clinically relevant role in cancer management and research, especially for identifying biomarkers necessary for the successful implementation of 3P medicine [201, 202].
In conclusion, the concepts of an early predictive diagnosis, targeted prevention, and individualized approach to the patient associated with massive progress in the field of biomedicine bring significant advantages compared to reactive medical approaches. The analysis of disbalance between VM and immune system in HPV-induced CC based on characterization of novel biomarkers demonstrates the innovative approach in the work-frame of the 3P medicine, and only more in-depth research could bring efficient therapeutical strategies for patients with CC.
Author contributions
E.K. was responsible for the paper concepts, draft, and PPPM-related contents. The manuscript was drafted by E.K., A.L., M.S., L.K., V.H., T.R, E.K., and T.P.
D.B., K.Z., P.K., and K.B. critically revised the manuscript. The tables were created by A.L., M.S., and L.K. Figures were prepared by M.S. and E.K.
P.K., D.B., and K.B. provided a skilled assistance and supervised the overall preparation of the manuscript. K.Z. and D.B. were responsible for English corrections.
All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding was enabled and organized by Projekt DEAL. This work was supported by the Grant Agency of the Ministry of Education of the Slovak Republic under contract no. 1/0124/17, the Slovak Research and Development Agency under contract no. APVV-16–0021, the Ministry of Health grant no. 2018/20-UKMT-16, and also by the project molecular diagnosis of cervical cancer, ITMS: 26220220113 supported by the Operational Programme Research and Innovation funded by the ERDF. D.B. was supported by a National Priorities Research Program grant (NPRP 11S-1214–170101) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine white paper of the European Association for Predictive. Prev Personal Med EPMA J. 2012;2012(3):14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golubnitschaja O, Yeghiazaryan K, Costigliola V, Trog D, Braun M, Debald M, et al. Risk assessment, disease prevention and personalised treatments in breast cancer: is clinically qualified integrative approach in the horizon? EPMA J. 2013;4:6. doi: 10.1186/1878-5085-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health Elsevier. 2020;8:e191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health Elsevier. 2021;9:e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. Papillomaviruses in the causation of human cancers — a brief historical account. Virology. 2009;384:260–5. 10.1016/j.virol.2008.11.046. [DOI] [PubMed]
- 7.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol. 2014;234:441–451. doi: 10.1002/path.4405. [DOI] [PubMed] [Google Scholar]
- 9.Bosch FX, de Sanjosé S. Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;3–13. 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed]
- 10.Jaisamrarn U, Castellsagué X, Garland SM, Naud P, Palmroth J, Del Rosario-Raymundo MR, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS ONE. 2013;8:e79260. doi: 10.1371/journal.pone.0079260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Demarco M, Lorey TS, Fetterman B, Cheung LC, Guido RS, Wentzensen N, et al. Risks of CIN 2+, CIN 3+, and cancer by cytology and human papillomavirus status: the foundation of risk-based cervical screening guidelines. J Low Genit Tract Dis. 2017;21:261–267. doi: 10.1097/LGT.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE, et al. A study of partial human papillomavirus genotyping in support of the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24:144–147. doi: 10.1097/LGT.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasieni P, Castanon A, Landy R, Kyrgiou M, Kitchener H, Quigley M, et al. Risk of preterm birth following surgical treatment for cervical disease: executive summary of a recent symposium. BJOG. 2016;123:1426–1429. doi: 10.1111/1471-0528.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liskova Alena, Samec Marek, Koklesova Lenka, Kudela Erik, Kubatka Peter, Golubnitschaja Olga. Mitochondriopathies as a clue to systemic disorders: “vicious circle” of mitochondrial injury, analytical tools and mitigating measures in context of predictive, preventive, and personalized (3P) medicine. 2021; 18;22(4):2007 10.3390/ijms22042007. [DOI] [PMC free article] [PubMed]
- 16.Crigna AT, Samec M, Koklesova L, Liskova A, Giordano FA, Kubatka P, et al. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020;1–25. 10.1007/s13167-020-00226-x. [DOI] [PMC free article] [PubMed]
- 17.Liskova A, Samec M, Koklesova L, Giordano FA, Kubatka P, Golubnitschaja O. Liquid biopsy is instrumental for 3PM dimensional solutions in cancer management. J Clin Med Multidiscip Digital Publishing Institute. 2020;9:2749. doi: 10.3390/jcm9092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerner C, Costigliola V, Golubnitschaja O. Multiomic patterns in body fluids: technological challenge with a great potential to implement the advances paradigm of 3P medicine. Mass Spectrom Rev. 2019 doi: 10.1002/mas.21612. [DOI] [PubMed] [Google Scholar]
- 19.Zoodsma M, Sijmons RH, de Vries EG, van der Zee AG. Familial cervical cancer: case reports, review and clinical implications. Hereditary Cancer Clin Pract. 2004;2:99. doi: 10.1186/1897-4287-2-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyervides-Muñoz MA, Pérez-Maya AA, Sánchez-Domínguez CN, Berlanga-Garza A, Antonio-Macedo M, Valdéz-Chapa LD, et al. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses [Internet]. 2020 [cited 2021 Feb 21];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7232502/. 10.3390/v12040380. [DOI] [PMC free article] [PubMed]
- 21.Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–2684. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 22.Wendland EM, Villa LL, Unger ER, Domingues CM, Benzaken AS. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: the POP-Brazil study. Scientific Reports. Nature Publishing Group. 2020;10:4920. doi: 10.1038/s41598-020-61582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Río-Ospina LD, León SCSD, Camargo M, Sánchez R, Mancilla CL, Patarroyo ME, et al. The prevalence of high-risk HPV types and factors determining infection in female Colombian adolescents. PLOS ONE. Public Library of Science. 2016;11:e0166502. doi: 10.1371/journal.pone.0166502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia–cancer sequence. Nat Rev Cancer. 2017;17:594–604. doi: 10.1038/nrc.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedin R, Gasparin VA, Pitilin ÉDB. Fatores associados às alterações cérvico-uterinas de mulheres atendidas em um município polo do oeste catarinense Factors associated to uterine-cervix changes in women assisted in a pole town in western Santa Catarina. R pesq cuid fundam online. 2017;9:167. doi: 10.9789/2175-5361.2017.v9i1.167-174. [DOI] [Google Scholar]
- 26.Kang L-N, Castle PE, Zhao F-H, Jeronimo J, Chen F, Bansil P, et al. A prospective study of age trends of high-risk human papillomavirus infection in rural China. BMC Infect Dis. 2014;14:96. doi: 10.1186/1471-2334-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 28.Moreno V, Bosch FX, Muñoz N, Meijer CJLM, Shah KV, Walboomers JMM, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 29.Orlando G, Tanzi A, Rizzardini G. Modifiable and non-modifiable factors related to HPV infection and cervical abnormalities in women at high risk: a cross-sectional analysis from the VALHIDATE study. 2016;21. Available from: https://air.unimi.it/handle/2434/424485#.YI1A3bUzaUk. Accessed 01 July 2016.
- 30.Roura E, Travier N, Waterboer T, de Sanjosé S, Bosch FX, Pawlita M, et al. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: results from the EPIC cohort. PLoS One [Internet]. 2016 [cited 2021 Mar 3];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4726518/. 10.1371/journal.pone.0147029. [DOI] [PMC free article] [PubMed]
- 31.Asthana S, Busa V, Labani S. Oral contraceptives use and risk of cervical cancer—a systematic review & meta-analysis. Eur J Obstet & Gynecol Reprod Biol. 2020;247:163–175. doi: 10.1016/j.ejogrb.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Mignot S, Ringa V, Vigoureux S, Zins M, Panjo H, Saulnier P-J, et al. Pap tests for cervical cancer screening test and contraception: analysis of data from the CONSTANCES cohort study. BMC Cancer. 2019;19:317. doi: 10.1186/s12885-019-5477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres-Poveda K, Ruiz-Fraga I, Madrid-Marina V, Chavez M, Richardson V. High risk HPV infection prevalence and associated cofactors: a population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC Cancer. 2019;19:1205. doi: 10.1186/s12885-019-6388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonseca-Moutinho JA. Smoking and cervical cancer. ISRN Obstet Gynecol [Internet]. 2011 [cited 2021 Mar 3];2011. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3140050/. 10.5402/2011/847684. [DOI] [PMC free article] [PubMed]
- 35.Oh HY, Kim MK, Seo S, Lee DO, Chung YK, Lim MC, et al. Alcohol consumption and persistent infection of high-risk human papillomavirus. Epidemiol Infect. 2015;143:1442–1450. doi: 10.1017/S0950268814002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minkoff H, Zhong Y, Strickler HD, Watts DH, Palefsky JM, Levine AM, et al. The relationship between cocaine use and human papillomavirus infections in HIV-seropositive and HIV-seronegative women. Infect Dis Obstet Gynecol. 2008;2008:587082 10.1155/2008/587082 [DOI] [PMC free article] [PubMed]
- 37.Shah SC, Kayamba V, Peek RM, Heimburger D. Cancer control in low- and middle-income countries: is it time to consider screening? JGO. Wolters Kluwer; 2019;1–8. 10.1200/JGO.18.00200. [DOI] [PMC free article] [PubMed]
- 38.Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol [Internet]. 2018 [cited 2021 Mar 3];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5966868/. 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed]
- 39.Koshiyama M. The effects of the dietary and nutrient intake on gynecologic cancers. Healthcare (Basel) [Internet]. 2019 [cited 2021 Mar 3];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6787610/. 10.3390/healthcare7030088. [DOI] [PMC free article] [PubMed]
- 40.Castanheira CP, Sallas ML, Nunes RAL, Lorenzi NPC, Termini L. Microbiome and cervical cancer. Pathobiology. 2020;1–11. 10.1159/000511477. [DOI] [PubMed]
- 41.Lavitola G, Della Corte L, De Rosa N, Nappi C, Bifulco G. Effects on vaginal microbiota restoration and cervical epithelialization in positive HPV patients undergoing vaginal treatment with carboxy-methyl-beta-glucan. Biomed Res Int. 2020;2020:1–8. doi: 10.1155/2020/5476389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018;34:451–455. doi: 10.1080/09513590.2017.1407753. [DOI] [PubMed] [Google Scholar]
- 43.Goncharenko V, Bubnov R, Polivka J, Zubor P, Biringer K, Bielik T, et al. Vaginal dryness: individualised patient profiles, risks and mitigating measures. EPMA J. 2019;10:73–79. doi: 10.1007/s13167-019-00164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. doi: 10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Wijgert JHHM, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? Fredricks DN, editor. PLoS ONE. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52–132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17:232–250. doi: 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boris S, Suárez JE, Vázquez F, Barbés C. Adherence of human vaginal lctobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun. 1998;66:1985–1989. doi: 10.1128/IAI.66.5.1985-1989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medina-Colorado AA, Vincent KL, Miller AL, Maxwell CA, Dawson LN, Olive T, et al. Vaginal ecosystem modeling of growth patterns of anaerobic bacteria in microaerophilic conditions. Anaerobe. 2017;45:10–18. doi: 10.1016/j.anaerobe.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.V. Sgibnev A, A. Kremleva E. Vaginal protection by H2O2-producing lactobacilli. Jundishapur J Microbiol [Internet]. 2015 [cited 2021 Mar 7];8. Available from: https://sites.kowsarpub.com/jjm/articles/56511.html. 10.5812/jjm.22913. [DOI] [PMC free article] [PubMed]
- 55.Zadravec P, Štrukelj B, Berlec A. Improvement of LysM-mediated surface display of designed ankyrin repeat proteins (DARPins) in recombinant and nonrecombinant strains of Lactococcus lactis and Lactobacillus species. Björkroth J, editor. Appl Environ Microbiol. 2015;81:2098–106. doi: 10.1128/AEM.03694-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Homburg C, Bommer M, Wuttge S, Hobe C, Beck S, Dobbek H, et al. Inducer exclusion in Firmicutes: insights into the regulation of a carbohydrate ATP binding cassette transporter from Lactobacillus casei BL23 by the signal transducing protein P-Ser46-HPr. Mol Microbiol. 2017;105:25–45. doi: 10.1111/mmi.13680. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front Microbiol [Internet]. 2017 [cited 2021 Mar 7];08. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00564/full10.3389/fmicb.2017.00564. [DOI] [PMC free article] [PubMed]
- 58.Yang X, Da M, Zhang W, Qi Q, Zhang C, Han S. Role of Lactobacillus in cervical cancer. CMAR. 2018;10:1219–1229. doi: 10.2147/CMAR.S165228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.So KA, Yang EJ, Kim NR, Hong SR, Lee J-H, Hwang C-S, et al. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. Consolaro MEL, editor. PLoS ONE. 2020;15:e0238705. 10.1371/journal.pone.0238705. [DOI] [PMC free article] [PubMed]
- 60.Amabebe E, Anumba DOC. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. Landay A, editor. PLoS ONE. 2013;8:e80074. 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed]
- 62.Wang H, Ma Y, Li R, Chen X, Wan L, Zhao W. Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J Infect Dis. 2019;220:1243–1254. doi: 10.1093/infdis/jiz325. [DOI] [PubMed] [Google Scholar]
- 63.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyrgiou M, Mitra A, Moscicki A-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168–182. doi: 10.1016/j.trsl.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao X-P, Sun T-T, Wang S, Fan Q-B, Shi H-H, Zhu L, et al. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int J Gynecol Cancer. 2019;29:28–34. doi: 10.1136/ijgc-2018-000032. [DOI] [PubMed] [Google Scholar]
- 66.Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210:338–343. doi: 10.1093/infdis/jiu089. [DOI] [PubMed] [Google Scholar]
- 68.Moscicki A-B, Shi B, Huang H, Barnard E, Li H. Cervical-vaginal microbiome and associated cytokine profiles in a prospective study of HPV 16 acquisition, persistence, and clearance. Front Cell Infect Microbiol. 2020;10:569022. doi: 10.3389/fcimb.2020.569022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piyathilake CJ, Ollberding NJ, Kumar R, Macaluso M, Alvarez RD, Morrow CD. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev Res (Phila) 2016;9:357–366. doi: 10.1158/1940-6207.CAPR-15-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta‐analysis. BJOG. Int J Obstet Gy. 2020;127:171–80. doi: 10.1111/1471-0528.15854. [DOI] [PubMed] [Google Scholar]
- 71.Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21(674):e1–9. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Lamont RF, Morgan DJ, Wilden SD, Taylor-Robinson D. Prevalence of bacterial vaginosis in women attending one of three general practices for routine cervical cytology. Int J STD AIDS. 2000;11:495–498. doi: 10.1258/0956462001916371. [DOI] [PubMed] [Google Scholar]
- 73.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. 10.15585/mmwr%20.rr6404a1. [PMC free article] [PubMed]
- 75.Curty G, de Carvalho PS, Soares MA. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int J Mol Sci [Internet]. 2019 [cited 2021 Jan 27];21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6981542/. 10.3390/ijms21010222. [DOI] [PMC free article] [PubMed]
- 76.Shannon B, Yi TJ, Perusini S, Gajer P, Ma B, Humphrys MS, et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017;10:1310–1319. doi: 10.1038/mi.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei Z-T, Chen H-L, Wang C-F, Yang G-L, Han S-M, Zhang S-L. Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front Public Health. 2021;8:587298. doi: 10.3389/fpubh.2020.587298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Machado A, Cerca N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis. 2015;212:1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 79.Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. Silvestri G, editor. PLoS Pathog. 2020;16:e1008376. doi: 10.1371/journal.ppat.1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(Suppl 1):S29–35. doi: 10.1093/infdis/jiw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng LT, Xue Y, Yue T, Yang L, Gao L, An RF. Relationship of HPV infection and BV, VVC, TV: a clinical study based on 1 261 cases of gynecologic outpatients. Zhonghua Fu Chan Ke Za Zhi. 2016;51:730–733. doi: 10.3760/cma.j.issn.0529-567X.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Zhou D, Cui Y, Wu FL, Deng WH. The change of vaginal lactobacillus in patients with high-risk human papillomavirus infection. Zhonghua Yi Xue Za Zhi. 2016;96:2006–2008. doi: 10.3760/cma.j.issn.0376-2491.2016.25.010. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20:629. doi: 10.1186/s12879-020-05324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a hispanic population. Front Microbiol. 2018;9:2533. doi: 10.3389/fmicb.2018.02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis. 2014;209:1989–1999. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 86.Larsen AK, Nymo IH, Briquemont B, Sørensen KK, Godfroid J. Entrance and survival of Brucella pinnipedialis hooded seal strain in human macrophages and epithelial cells. PLoS ONE. 2013;8:e84861. doi: 10.1371/journal.pone.0084861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y, Wang L, Pei F, Ji M, Zhang F, Sun Y, et al. Patients with LR-HPV infection have a distinct vaginal microbiota in comparison with healthy controls. Front Cell Infect Microbiol. 2019;9:294. doi: 10.3389/fcimb.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE. 2016;11:e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JE, Lee S, Lee H, Song Y-M, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE. 2013;8:e63514. doi: 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun. 2020;11:1999. doi: 10.1038/s41467-020-15856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caselli E, D’Accolti M, Santi E, Soffritti I, Conzadori S, Mazzacane S, et al. Vaginal microbiota and cytokine microenvironment in HPV clearance/persistence in women surgically treated for cervical intraepithelial neoplasia: an observational prospective study. Front Cell Infect Microbiol. 2020;10:540900. doi: 10.3389/fcimb.2020.540900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE. 2012;7:e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benef Microbes. 2017;8:521–533. doi: 10.3920/BM2016.0222. [DOI] [PubMed] [Google Scholar]
- 95.Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25:1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 96.Vujic G, Jajac Knez A, Despot Stefanovic V, Kuzmic VV. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2013;168:75–79. doi: 10.1016/j.ejogrb.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 97.Abdelhamid AG, El-Masry SS, El-Dougdoug NK. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10:337–350. doi: 10.1007/s13167-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Yu T, Yan H, Li D, Yu T, Yuan T, et al. Vaginal microbiota and HPV infection: novel mechanistic insights and therapeutic strategies. IDR. 2020;13:1213–1220. doi: 10.2147/IDR.S210615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 100.Verhoeven V, Renard N, Makar A, Van Royen P, Bogers J-P, Lardon F, et al. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur J Cancer Prev. 2013;22:46–51. doi: 10.1097/CEJ.0b013e328355ed23. [DOI] [PubMed] [Google Scholar]
- 101.Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis. 2018;18:13. doi: 10.1186/s12879-017-2938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32:37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 103.Morelli L, Zonenenschain D, Del Piano M, Cognein P. Utilization of the intestinal tract as a delivery system for urogenital probiotics. J Clin Gastroenterol. 2004;38:S107–110. doi: 10.1097/01.mcg.0000128938.32835.98. [DOI] [PubMed] [Google Scholar]
- 104.Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18:79–86. 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed]
- 105.Liwen Z, Yu W, Liang M, Kaihong X, Baojin C. A low abundance of Bifidobacterium but not Lactobacillius in the feces of Chinese children with wheezing diseases. Medicine (Baltimore) 2018;97:e12745. doi: 10.1097/MD.0000000000012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Onywera H, Williamson A-L, Ponomarenko J, Meiring TL. The penile microbiota in uncircumcised and circumcised men: relationships with HIV and human papillomavirus infections and cervicovaginal microbiota. Front Med. 2020;7:383. doi: 10.3389/fmed.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, et al. The effects of circumcision on the penis microbiome. PLoS ONE. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zozaya M, Ferris MJ, Siren JD, Lillis R, Myers L, Nsuami MJ, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi: 10.1186/s40168-016-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(9–18):e8. doi: 10.1016/j.ajog.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5:2624–2642. doi: 10.3390/v5112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samec M, Liskova A, Koklesova L, Samuel SM, Murin R, Zubor P, et al. The role of plant-derived natural substances as immunomodulatory agents in carcinogenesis. J Cancer Res Clin Oncol. 2020;146:3137–3154. doi: 10.1007/s00432-020-03424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nunes RAL, Morale MG, Silva GÁF, Villa LL, Termini L. Innate immunity and HPV: friends or foes. Clinics (Sao Paulo) [Internet]. 2018 [cited 2021 Jan 29];73. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6157093/. 10.6061/clinics/2018/e549s. [DOI] [PMC free article] [PubMed]
- 114.Aristizábal B, González Á. Innate immune system [Internet]. Autoimmunity: from bench to bedside [Internet]. El Rosario University Press; 2013 [cited 2021 Jan 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459455/.
- 115.Ashrafian L, Sukhikh G, Kiselev V, Paltsev M, Drukh V, Kuznetsov I, et al. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention. EPMA J. 2015;6:25. doi: 10.1186/s13167-015-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karim R, Tummers B, Meyers C, Biryukov JL, Alam S, Backendorf C, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLOS Pathogens. Public Library of Science; 2013;9 e1003384 10.1371/journal.ppat.1003384 [DOI] [PMC free article] [PubMed]
- 117.Garcia M, Alout H, Diop F, Damour A, Bengue M, Weill M, et al. Innate immune response of primary human keratinocytes to West Nile virus infection and its modulation by mosquito saliva. Front Cell Infect Microbiol [Internet]. 2018 [cited 2021 Jan 29];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224356/. 10.3389/fcimb.2018.00387. [DOI] [PMC free article] [PubMed]
- 118.Hasan U. Human papillomavirus (HPV) deregulation of Toll-like receptor 9. Oncoimmunology [Internet]. Taylor & Francis; 2014 [cited 2021 Jan 29];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3935924/. 10.4161/onci.27257. [DOI] [PMC free article] [PubMed]
- 119.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Yang H, Barnie PA, Yang P, Su Z, Chen J, et al. The expression of Toll-like receptor 8 and its relationship with VEGF and Bcl-2 in cervical cancer. Int J Med Sci. 2014;11:608–613. doi: 10.7150/ijms.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: an update. Int J Cancer. 2018;142:224–229. doi: 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 122.Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GLB, et al. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol [Internet]. 2019 [cited 2021 Jan 29];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6348254/. 10.3389/fimmu.2018.03176. [DOI] [PMC free article] [PubMed]
- 124.Da Silva DM, Movius CA, Raff AB, Brand HE, Skeate JG, Wong MK, et al. Suppression of Langerhans cell activation is conserved amongst human papillomavirus α and β genotypes, but not a μ genotype. Virology. 2014;0:279–286. doi: 10.1016/j.virol.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Muñoz-Molina R, Balderas-Carrillo O, de la Luz D-S, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans’ cells in the human female genital tract. Immunology. 2006;117:220–228. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su Q, Igyártó B. Keratinocytes affect biology of Langerhans cells through mRNA transfer. The Journal of Immunology. American Association of Immunologists. 2018;200:109.12–109.12. doi: 10.1016/j.jid.2019.05.006. [DOI] [Google Scholar]
- 127.Jackson R, Eade S, Zehbe I. An epithelial organoid model with Langerhans cells for assessing virus-host interactions. Philosophical Transactions of the Royal Society B: Biological Sciences. Royal Society. 2019;374:20180288. doi: 10.1098/rstb.2018.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wakabayashi R, Nakahama Y, Nguyen V, Espinoza JL. The host-microbe interplay in human papillomavirus-induced carcinogenesis. Microorganisms [Internet]. 2019 [cited 2021 Jan 30];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6680694/. 10.3390/microorganisms7070199. [DOI] [PMC free article] [PubMed]
- 129.Iijima N, Goodwin EC, Dimaio D, Iwasaki A. High-risk human papillomavirus E6 inhibits monocyte differentiation to Langerhans cells. Virology. 2013;444:257–262. doi: 10.1016/j.virol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol [Internet]. Frontiers; 2019 [cited 2021 Jan 30];9. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2019.00682/full. 10.3389/fonc.2019.00682. [DOI] [PMC free article] [PubMed]
- 131.Eiz-Vesper B, Schmetzer HM. Antigen-presenting cells: potential of proven und new players in immune therapies. Transfus Med Hemother. 2020;47:429–431. doi: 10.1159/000512729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tran LS, Mittal D, Mattarollo SR, Frazer IH. Interleukin-17A promotes arginase-1 production and 2,4-dinitrochlorobenzene-induced acute hyperinflammation in human papillomavirus E7 oncoprotein-expressing skin. J Innate Immun. 2015;7:392–404. doi: 10.1159/000374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hacke K, Rincon-Orozco B, Buchwalter G, Siehler SY, Wasylyk B, Wiesmüller L, et al. Regulation of MCP-1 chemokine transcription by p53. Mol Cancer. 2010;9:82. doi: 10.1186/1476-4598-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J Virol. 2005;79:14852–14862. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mittal D, Kassianos AJ, Tran LS, Bergot A-S, Gosmann C, Hofmann J, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. 2013;133:2686–2694. doi: 10.1038/jid.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer [Internet]. 2019 [cited 2021 Jan 30];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6391774/. 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed]
- 137.Jimenez-Perez MI, Jave-Suarez LF, Ortiz-Lazareno PC, Bravo-Cuellar A, Gonzalez-Ramella O, Aguilar-Lemarroy A, et al. Cervical cancer cell lines expressing NKG2D-ligands are able to down-modulate the NKG2D receptor on NKL cells with functional implications. BMC Immunol. 2012;13:7. doi: 10.1186/1471-2172-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm (Lond) [Internet]. 2016 [cited 2021 Jan 30];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4700668/. 10.1186/s12950-015-0109-9. [DOI] [PMC free article] [PubMed]
- 139.Cicchini L, Westrich JA, Xu T, Vermeer DW, Berger JN, Clambey ET, et al. Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio. 2016;7. 10.1128/mBio.00270-16. [DOI] [PMC free article] [PubMed]
- 140.Schaerli P, Willimann K, Ebert LM, Walz A, Moser B. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity Elsevier. 2005;23:331–342. doi: 10.1016/j.immuni.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 141.Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney–expressed chemokine (Brak) in macrophage development. J Exp Med. 2001;194:855–862. doi: 10.1084/jem.194.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshimura A, Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 143.Iancu IV, Botezatu A, Goia-Ruşanu CD, Stănescu A, Huică I, Nistor E, et al. TGF-beta signalling pathway factors in HPV-induced cervical lesions. Roum Arch Microbiol Immunol. 2010;69:113–8. ISSN 1222–3891. [PubMed]
- 144.Stanley MA, Sterling JC. Host responses to infection with human papillomavirus. Curr Probl Dermatol. 2014;45:58–74. doi: 10.1159/000355964. [DOI] [PubMed] [Google Scholar]
- 145.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 146.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ambagala APN, Solheim JC, Srikumaran S. Viral interference with MHC class I antigen presentation pathway: the battle continues. Vet Immunol Immunopathol. 2005;107:1–15. doi: 10.1016/j.vetimm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 148.Clark KT, Trimble CL. Current status of therapeutic HPV vaccines. Gynecol Oncol. 2020;156:503–510. doi: 10.1016/j.ygyno.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 149.Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis. 2016;213:1444–1454. doi: 10.1093/infdis/jiv753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer (Review) Oncol Lett Spandidos Publications. 2015;10:600–606. doi: 10.3892/ol.2015.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. Nature Publishing Group. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 153.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang W, Song Y, Lu Y-L, Sun J-Z, Wang H-W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139:513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sheu BC, Chang WC, Lin HH, Chow SN, Huang SC. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J Obstet Gynaecol Res. 2007;33:103–113. doi: 10.1111/j.1447-0756.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 156.Scott ME, Shvetsov YB, Thompson PJ, Hernandez BY, Zhu X, Wilkens LR, et al. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study. Int J Cancer. 2013;133:1187–1196. doi: 10.1002/ijc.28119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peghini BC, Abdalla DR, Barcelos ACM, Teodoro L das GVL, Murta EFC, Michelin MA. Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Hum Immunol. 2012;73:920–6. doi: 10.1016/j.humimm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 158.Zhou Q, Zhu K, Cheng H. Toll-like receptors in human papillomavirus infection. Arch Immunol Ther Exp (Warsz) 2013;61:203–215. doi: 10.1007/s00005-013-0220-7. [DOI] [PubMed] [Google Scholar]
- 159.Woo YL, Sterling J, Damay I, Coleman N, Crawford R, van der Burg SH, et al. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG. 2008;115:1616–21; discussion 1621–1622. 10.1111/j.1471-0528.2008.01936.x. [DOI] [PubMed]
- 160.Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013;132:E26–36. doi: 10.1002/ijc.27776. [DOI] [PubMed] [Google Scholar]
- 161.Skeate JG, Da Silva DM, Chavez-Juan E, Anand S, Nuccitelli R, Kast WM. Nano-pulse stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE. 2018;13:e0191311. doi: 10.1371/journal.pone.0191311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee S-J, Yang A, Wu T-C, Hung C-F. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol [Internet]. 2016 [cited 2021 Feb 13];27. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4944018/. 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed]
- 163.Kovachev SM. Immunotherapy in patients with local HPV infection and high-grade squamous intraepithelial lesion following uterine cervical conization. Immunopharmacol Immunotoxicol. 2020;42:314–318. doi: 10.1080/08923973.2020.1765374. [DOI] [PubMed] [Google Scholar]
- 164.Rumfield CS, Pellom ST, Ii YMM, Schlom J, Jochems C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J Immunother Cancer. BMJ Specialist Journals. 2020;8:e000612. doi: 10.1136/jitc-2020-000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Van de Wall S, Nijman HW, Daemen T. HPV-specific immunotherapy: key role for immunomodulators. Anticancer Agents Med Chem. 2014;14:265–279. doi: 10.2174/187152061402140128163306. [DOI] [PubMed] [Google Scholar]
- 166.Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9. 10.3390/v9090254. [DOI] [PMC free article] [PubMed]
- 167.Cohen AC, Roane BM, Leath CA. Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy. Drugs. 2020;80:217–227. doi: 10.1007/s40265-019-01249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tumer G, Simpson B, Roberts TK. Genetics, human major histocompatibility complex (MHC). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2021 Feb 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK538218/. [PubMed]
- 169.Mak TW, Saunders ME, Jett BD, editors. Chapter 6 - The major histocompatibility complex. Primer to the Immune Response (Second Edition) [Internet]. Boston: Academic Cell; 2014 [cited 2021 Feb 12]. p. 143–59.Available from: https://www.sciencedirect.com/science/article/pii/B9780123852458000066. ISBN 978–0–12–385245–8.
- 170.Paaso A, Jaakola A, Syrjänen S, Louvanto K. From HPV infection to lesion progression: the role of HLA alleles and host immunity. Acta Cytol. 2019;63:148–158. doi: 10.1159/000494985. [DOI] [PubMed] [Google Scholar]
- 171.Zheng Z, He Q, An L, Li D, Wang N, Wang L, et al. HLA Class II alleles and association with HPV Infection prevalence in high-risk HPV-positive Han women in southern China. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 172.Gokhale P, Mania-Pramanik J, Sonawani A, Idicula-Thomas S, Kerkar S, Tongaonkar H, et al. Cervical cancer in Indian women reveals contrasting association among common sub-family of HLA class I alleles. Immunogenetics. 2014;66:683–691. doi: 10.1007/s00251-014-0805-2. [DOI] [PubMed] [Google Scholar]
- 173.Wang SS, Hildesheim A, Gao X, Schiffman M, Herrero R, Bratti MC, et al. Comprehensive analysis of human leukocyte antigen class I alleles and cervical neoplasia in 3 epidemiologic studies. J Infect Dis. 2002;186:598–605. doi: 10.1086/342295. [DOI] [PubMed] [Google Scholar]
- 174.Leo PJ, Madeleine MM, Wang S, Schwartz SM, Newell F, Pettersson-Kymmer U, et al. Defining the genetic susceptibility to cervical neoplasia—a genome-wide association study. PLOS Genetics. Public Library of Science. 2017;13:e1006866. doi: 10.1371/journal.pgen.1006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ivansson EL, Magnusson JJ, Magnusson PKE, Erlich HA, Gyllensten UB. MHC loci affecting cervical cancer risk: distinguishing the effects of HLA-DQB1 and non-HLA genes TNF, LTA, TAP1 and TAP2. Genes Immun. 2008;9:613–623. doi: 10.1038/gene.2008.58. [DOI] [PubMed] [Google Scholar]
- 176.Chan PKS, Cheung JLK, Cheung T-H, Lin C, Tam AOY, Chan DPC, et al. HLA-B alleles, high-risk HPV infection and risk for cervical neoplasia in southern Chinese women. Int J Cancer. 2006;118:1430–1435. doi: 10.1002/ijc.21528. [DOI] [PubMed] [Google Scholar]
- 177.Hu JM, Sun Q, Li L, Liu CX, Chen YZ, Zou H, et al. Human leukocyte antigen-DRB1*1501 and DQB1*0602 alleles are cervical cancer protective factors among Uighur and Han people in Xinjiang, China. Int J Clin Exp Pathol. 2014;7:6165–71. [PMC free article] [PubMed]
- 178.Del Río-Ospina L, Camargo M, Soto-De León SC, Sánchez R, Moreno-Pérez DA, Patarroyo ME, et al. Identifying the HLA DRB1-DQB1 molecules and predicting epitopes associated with high-risk HPV infection clearance and redetection. Sci Rep [Internet]. 2020 [cited 2021 Feb 12];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7190668/. 10.1038/s41598-020-64268-x. [DOI] [PMC free article] [PubMed]
- 179.Bhaskaran M, Murali SV, Rajaram B, Krishnasamy S, Devasena CS, Pathak A, et al. Association of HLA-A, -B, DRB, and DQB alleles with persistent HPV-16 infection in women from Tamil Nadu. India Viral Immunol. 2019;32:430–441. doi: 10.1089/vim.2019.0094. [DOI] [PubMed] [Google Scholar]
- 180.Franciosi JR, Gelmini GF, Roxo VS, Carvalho NSde, Bicalho MdaG. Is there a role played by HLA-E, if any, in HPV immune evasion? Scand J Immunol. 2020;91:e12850. doi: 10.1111/sji.12850. [DOI] [PubMed] [Google Scholar]
- 181.Aggarwal R, Sharma M, Mangat N, Suri V, Bhatia T, Kumar P, et al. Understanding HLA-G driven journey from HPV infection to cancer cervix: adding missing pieces to the jigsaw puzzle. J Reprod Immunol. 2020;142:103205. doi: 10.1016/j.jri.2020.103205. [DOI] [PubMed] [Google Scholar]
- 182.Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J. 2018;9:205–223. doi: 10.1007/s13167-018-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qingqing B, Jie Z, Songben Q, Juan C, Lei Z, Mu X. Cervicovaginal microbiota dysbiosis correlates with HPV persistent infection. Microb Pathog. 2020;104617. 10.1016/j.micpath.2020.104617. [DOI] [PubMed]
- 184.Lazarenko LM, Nikitina OE, Nikitin EV, Demchenko OM, Kovtonyuk GV, Ganova LO, et al. Development of biomarker panel to predict, prevent and create treatments tailored to the persons with human papillomavirus-induced cervical precancerous lesions. EPMA J. 2014;5:1. doi: 10.1186/1878-5085-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10:365–381. doi: 10.1007/s13167-019-00194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Łaniewski P, Cui H, Roe DJ, Barnes D, Goulder A, Monk BJ, et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci Rep [Internet]. 2019 [cited 2021 Jan 27];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6517407/. 10.1038/s41598-019-43849-5. [DOI] [PMC free article] [PubMed]
- 187.Łaniewski P, Barnes D, Goulder A, Cui H, Roe DJ, Chase DM, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep [Internet]. 2018 [cited 2021 Jan 27];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5954126/. 10.1038/s41598-018-25879-7. [DOI] [PMC free article] [PubMed]
- 188.Hu T, Yang P, Zhu H, Chen X, Xie X, Yang M, et al. Accumulation of invariant NKT cells with increased IFN-γ production in persistent high-risk HPV-infected high-grade cervical intraepithelial neoplasia. Diagn Pathol. 2015;10:20. doi: 10.1186/s13000-015-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, et al. Cervical cancer cells express markers associated with immunosurveillance. J Immunol Res. 2019;2019:1242979. doi: 10.1155/2019/1242979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barros MR, de Melo CML, Barros MLCMGR, de Cássia Pereira de Lima R, de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV-related cancers. Journal of Experimental & Clinical Cancer Research. 2018;37:137. 10.1186/s13046-018-0802-7. [DOI] [PMC free article] [PubMed]
- 191.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 192.Vyshenska D, Lam KC, Shulzhenko N, Morgun A. Interplay between viruses and bacterial microbiota in cancer development. Semin Immunol. 2017;32:14–24. doi: 10.1016/j.smim.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. 2017;129:643–654. doi: 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilkinson EM, Łaniewski P, Herbst-Kralovetz MM, Brotman RM. Personal and clinical vaginal lubricants: impact on local vaginal microenvironment and implications for epithelial cell host response and barrier function. J Infect Dis. 2019;220:2009–2018. doi: 10.1093/infdis/jiz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mändar R, Punab M, Borovkova N, Lapp E, Kiiker R, Korrovits P, et al. Complementary seminovaginal microbiome in couples. Res Microbiol. 2015;166:440–447. doi: 10.1016/j.resmic.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 196.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS ONE. 2010;5:e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS ONE. 2012;7:e36298. doi: 10.1371/journal.pone.0036298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Golubnitschaja O, Kinkorova J, Costigliola V. Predictive, preventive and personalised medicine as the hardcore of ‘Horizon 2020’: EPMA position paper. EPMA J. 2014;5:6. doi: 10.1186/1878-5085-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu R, Wang X, Zhan X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013;4:2. doi: 10.1186/1878-5085-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9:77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu JC, Khodadadi H, Baban B. Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019;10:43–50. doi: 10.1007/s13167-019-00163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.