Abstract
Whether in the West or the East, the connection between the ear and the rest of the body has been explored for a long time. Especially in the past century or more, the relevant theoretical and applied research on the ear has greatly promoted the development of ear therapy, and finally the concept of transcutaneous auricular vagus nerve stimulation (taVNS) has been proposed. The purpose of taVNS is to treat a disease non-invasively by applying electrical current to the cutaneous receptive field formed by the auricular branch of the vagus nerve in the outer ear. In the past two decades, taVNS has been a topic of basic, clinical, and transformation research. It has been applied as an alternative to drug treatment for a variety of diseases. Based on the rapid understanding of the application of taVNS to human health and disease, some limitations in the development of this field have also been gradually exposed. Here, we comprehensively review the origin and research status of the field.
Keywords: Transcutaneous auricular vagus nerve stimulation, Concept, Application, Development
Introduction
In recent years, the development of neuroscience has given birth to a new treatment strategy, namely neuromodulation therapy, which refers to a wide range of interventional techniques that attempt to change the nervous system and achieve therapeutic effects [1]. Current approaches to neuromodulation may be direct or indirect, and interventions can also be invasive or non-invasive. For example, stimulation of the vagus nerve (VN) mainly includes invasive VN stimulation (iVNS) and transcutaneous VN stimulation (tVNS). In view of the non-invasive nature of the latter, transcutaneous auricular VN stimulation (taVNS), the main tVNS therapy, has received special interest in basic, clinical, and translational studies, due to its having benefits comparable to iVNS, ease of operation, greater accessibility, and a reduced side-effect profile [2, 3]. Techniques for taVNS depend on the clinical context, precisely targeted areas, and parameters, as well as the desired effect. With the current rapid growth in taVNS technology and application, it is time to review the genesis and state-of-the-art in this area.
The Evolution of taVNS
Although it is generally known that the ear is the auditory organ, there is a long history of exploring its connection with the rest of the body and treating diseases by stimulating the external ear, both in the Eastern and Western medicine. The auricle’s importance was noted in Huang Di Nei Jing (Internal Classic of the Yellow Emperor), a classic in traditional Chinese medicine compiled around 500 BC. In this book, the ear is closely associated with the meridians [4, 5]. Hippocrates used the method of pricking the dorsal auricular vein to treat impotence in the 4th century BC. [6]. The Egyptologist Alexandre Varille (1909–1951) recorded that ancient Egyptian women would insert needles or cause heat burns to the outer ear if they did not want to have any more children [7]. In the subsequent two thousand years, with the exchange between Eastern and Western civilizations, more and more diseases were treated by traumatic stimulation of the outer ear, such as cauterization, cutting, and pricking. However, there was no systematic theoretical explanation for this treatment until 1832, when Friedrich Arnold first described the Arnold reflex. Since then, researchers have reported various reflexes and phenomena of a connection between the external ear and the viscera [4], and gradually opened up discussion of the connection between the external ear and the VN.
Anatomical research shows that the only branch of the VN that reaches the body surface is the auricular branch of the VN (ABVN) [8, 9]. The ABVN is mainly distributed in the concha (cymba conchae and cavum conchae), and the cymba conchae is supplied exclusively by the ABVN [10]. The afferent fibers of the ABVN enter the vagal trunk through the jugular ganglion and project to the nucleus of the solitary tract (NTS) [9], where the central integration of autonomic neurons occurs. It collects afferent information and activates the caudal ventrolateral medulla and dorsal motor nucleus to regulate central autonomic activity [11]. It is precisely because of this direct anatomical pathway that the conchae have the possibility of regulating bodily functions [12]. And because of the importance of electricity in the therapeutic armamentarium of clinicians in the past 2000 years [13], the use of electrical stimulation has become the best way to carry out non-invasive or minimally-invasive stimulation of the outer ear [14]. In 2000, inspired by VNS, auricular acupuncture to treat epilepsy, and the distribution of the ABVN in the concha, Enrique Ventureyra formally proposed the concept of transcutaneous VNS for the first time [15]. This concept opened a new chapter in the field of neuromodulation therapy, although there was no reliable standard and abbreviated nomenclature for the method at that time.
The Nomenclature of taVNS
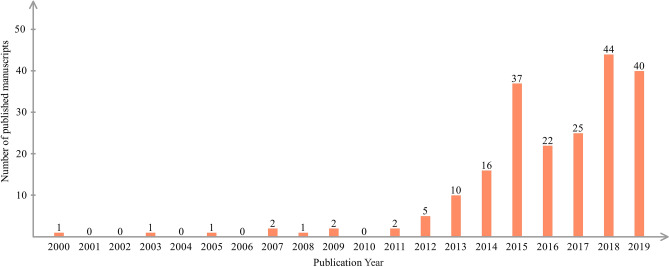
The term of transcutaneous VN stimulation was coined 20 years ago, and research on this method has gradually increased (Fig. 1). Interestingly, we have found that this nomenclature was not immutable in the literature after 2000. Although the different nomenclatures to some extent reflect the evolution of transcutaneous VN stimulation since it was proposed, an in-depth discussion of the inconsistencies in future will not only help to improve the terminology, but also have a positive influence on reaching a consensus in this field. To investigate the cause of the inconsistency in the nomenclature, we carried out a systematic search of, using the bibliographic search engine PubMed for articles published before December 2019.
Fig. 1.
Numbers of publications on taVNS from 2000 to 2019.
By sorting the 209 articles retrieved, we extracted 9 common important terms that are the same or similar to tVNS in the description of stimulus methods (Table 1). They are auricular VNS (aVNS or AVNS), auricular transcutaneous vagus stimulation (atVNS), low-level tragus nerve stimulation (LL-TNS), low-level tragus electrical stimulation (LLTS), percutaneous auricular VN stimulation (PVNS), respiratory-gated auricular vagal afferent nerve stimulation (RAVANS), transcutaneous auricular VN stimulation (taVNS or ta-VNS), transcutaneous tragus nerve stimulation (TNS), and transcutaneous VN stimulation (tVNS or TVNS). Among the above terms, taVNS and tVNS account for the majority.
Table 1.
The taVNS nomenclatures in publications
| Full nomenclature | Abbreviated nomenclature |
|---|---|
| Auricular vagus nerve stimulation | aVNS/AVNS |
| Auricular transcutaneous vagus stimulation | atVNS |
| Low-level tragus nerve stimulation | LL-TNS |
| Low-level tragus electrical stimulation | LLTS |
| Percutaneous auricular vagus nerve stimulation | PVNS |
| Respiratory-gated auricular vagal afferent nerve stimulation | RAVANS |
| Transcutaneous auricular vagus nerve stimulation | taVNS/ta-VNS |
| Transcutaneous tragus nerve stimulation | TNS |
| Transcutaneous vagal nerve stimulation | tVNS/TVNS |
We also noted the emergence of transcutaneous cervical VN stimulation in recent years [16–18]. The term of transcutaneous VN stimulation is a bit out of date, due to the fact that transcutaneous cervical VN stimulation is not only abbreviated to tcVNS [19–21], but also often described or abbreviated as tVNS [22–24]. This leads to a confusion between two different forms of transcutaneous VN stimulation. For the sake of a more accurate description of ABVN stimuli, the stimulation target is the key to distinguish the two treatments, and taVNS seem to be more reasonable and are recommended.
TaVNS techniques
In principle, taVNS is similar to conventional transcutaneous electrical nerve stimulation (TENS). They have in common that the stimulation targets and parameters are the main components of these two techniques. The difference is that the stimulus target of taVNS appears to be clearer than that of TENS.
Stimulation Target
In the debate on the best stimulation target of taVNS, the current research is based primarily on considerations of safety and effectiveness. For example, in the choice of unilateral stimulation, the counts of Aβ fibers in the left and right ABVN are similar [25]. Since it is traditionally believed that the VN efferent fibers leading to the heart are usually located on the right side [9, 26], most studies believe that it is safe to perform taVNS only in the left ear [27]. However, it has also been pointed out that right-sided stimulation does not increase the risk of adverse events [27]. Based on increased sensory input to the brainstem, activation of both the left and right ABVN potentially enhances the stimulation effect [12]. Although systematic reviews have shown that tVNS is safe and well tolerated [28], we should recognize that the studies in this field are still insufficient. A great deal of supplementary evidence is required, including controversial issues of unilateral or bilateral and left or right stimulation.
Anatomically, the ABVN is composed of myelinated Aβ fibers, myelinated Aδ fibers, and unmyelinated C fibers [29]. Nerve fibers in the auricle, including the ABVN, are roughly located in the 1 mm–1.5 mm gap between the auricular cartilage and the skin [30]. In addition to the fact that the cymba concha is 100% dominated by the ABVN, other auricle areas are also dominated by the ABVN to varying degrees. The antihelix, cavum conchae, tragus, crus of helix, and crura of anthelix are 73%, 45%, 45%, 20%, and 9% dominated by the ABVN, respectively [8]. When these different areas of the ABVN distribution are stimulated, there are varying degrees of activation along the VN pathway. It is worth emphasizing that this stimulation should be performed on the premise of ensuring accurate stimulation. Based on this, studies have shown that tVNS can activate the VN pathway properly and lead to the strongest activation, so the cymba concha may be the best position for tVNS in the auricle [31]. However, in terms of the convenience of stimulating the ABVN through electrodes, it is easier to apply current to the anterior wall of the external ear canal by clipping onto the tragus rather than inserting and holing an electrode against the conchae [32]. In addition, in practical application, a large surface electrode or poor contact often produces a diffuse electrical field, which may stimulate the non-VN in the ear, thus affecting activation of the VN pathway. More consideration needs to be given to ensure accurate stimulation of the ABVN.
Stimulus Parameters
Due to the inevitable influence of stimulation devices and stimulation target, the stimulus parameters of taVNS have a large range of variation. This variation results in many combinations among pulse width, frequency, and stimulus intensity (amplitude; mA or V). Of course, whether the combination of these parameters can be effective is also influenced by different diseases. In particular, the current literature lacks a clear consensus on whether the brain regions activated by taVNS depend on specific parameters [33]. According to the current clinical research, the common frequency is 25 Hz or 20 Hz (range, 0.5 Hz–120 Hz) [28]. Although few animal experiments have focused on stimulus frequency, a recent study has shown that 20 Hz intervention has a better antidepressant-like effect on rat models of depression [34]. In reports on pulse width, it is usually 1 ms or 0.25 ms (range 0.02–1) [28]; it has also been suggested that a pulse width of 500 µs is the most biologically active [35]. Another important parameter is intensity, which is not usually specified, as it is adjusted or set by the subjects according to their own tolerance [2, 28]. However, most studies do not disclose details of the stimulus intensity.
Although in every year many studies supplement the parameter data of taVNS, we still do not know which combination of parameters is more important [27]. In addition, due to the diversity of data sources from current parameter studies, and the small sample size included in many studies, the guidance of these data for the clinical application of taVNS is still limited.
Clinical Application of taVNS
Brain Diseases
Epilepsy
Since the concept of taVNS was formally put forward [15], much attention has been paid to the treatment of epilepsy by taVNS. Usually, patients show epileptic discharges during specific ictal periods on the electroencephalogram. In two early studies with small sample sizes (10 and 14 cases), taVNS treatment lasting 6–9 months effectively reduced the frequency of seizure and was well tolerated [36, 37]. Since then, 4 studies with relatively large sample sizes once again showed the effectiveness of taVNS in the treatment of epilepsy [38–41]. A randomized clinical trial conducted on 47 patients suffering from epilepsy showed that after 24 weeks of treatment, 16% of patients were seizure-free, and 38% had reduced seizure frequency [38]. Another controlled clinical trial in 30 epilepsy patients showed that after 12 months of treatment, ~40% had a reduced seizure frequency [39]. A placebo-controlled clinical trial in 27 patients confirmed that taVNS decreased the frequency of seizures after 20 weeks of treatment [40]. A recent, randomized, double-blind controlled trial in 39 patients corroborated that after 20 weeks of treatment, the seizure frequency was significantly reduced [41]. Based on the above studies, taVNS is effective in the treatment of epilepsy, and a recent meta-analysis also supports this view and concludes that taVNS may be an appropriate alternative therapy for epileptic patients who do not want a surgical procedure [42]. Nevertheless, larger-scale and higher-quality clinical trials are still necessary to provide evidence for (or against) the effectiveness of tVNS in the treatment of epilepsy.
Depression
Regarding to the treatment of depression, there have been many studies on iVNS. And a beneficial effect for iVNS in this depressed population has been found through meta-analysis [2]. Previous studies had shown that both taVNS and iVNS stimulate the same neural pathway [43], a placebo-controlled pilot study established the antidepressant effect of taVNS for the first time [44]. Although some small clinical trials had verified these results [45, 46], they still needed to be repeated in a larger patients sample. Since then, a study conducted by Rong et al. [47] has provided more convincing evidence for the antidepressant effect of taVNS, and the study using functional magnetic resonance imaging (fMRI) suggested that this antidepressant effect is mediated by the default network of the nucleus tractus solitarius-limbic lobe [48]. In addition, no side-effects were found in this study. It is worth mentioning that a recent meta-analysis preliminarily demonstrated that taVNS therapy effectively ameliorates the symptoms of depression [49]. These studies provide evidence for the application of taVNS in depression, but we should also be aware of the complexity of the clinical manifestations of depression. That is, it can be comorbid with many other diseases, including chronic pain, cardiovascular disease, inflammatory bowel disease, irritable bowel syndrome, and autism [50]. Therefore, it is particularly important to guide the application of taVNS in depression with the concept of “same treatment for different diseases” in the future.
Others
In addition to the above two diseases, taVNS also shows great potential in the treatment of other brain diseases such as disorders of consciousness (DOCs). Evidence-based guidelines on the treatment of DOCs are rare, and the neuroregulation technique is considered as a potential treatment [51]. Recently, it has been reported that taVNS can promote the recovery of consciousness in patients with severe brain injury, and it is a safe and effective method [52, 53]. In addition, one clinical case study analyzed by fMRI has also shown that the effectiveness of taVNS therapy in insomnia is related to modulation of the default mode network [54].
Cardiovascular Diseases
The autonomic nervous system maintains cardiovascular variables in the steady state. In normal aging or pathological conditions such as heart failure, sympathetic activity is dominant and may adversely affect cardiovascular function. Currently, the main purpose of pharmacological interventions is to inhibit the over-excitation of the sympathetic system, while vagal modulation has been largely ignored [55]. Previous studies have found that VNS influences autonomic activity. VN stimulation can affect the cardiovascular system [56, 57]. Many studies have shown that increased vagal activity reduces cardiovascular risk factors in both animal models and patients [55], VNS can reduce or terminate ventricular arrhythmias caused by myocardial ischemia and increase the ventricular fibrillation threshold [58–62]. Yu et al. [63] found that chronic low-intensity stimulation of the auricular branch of the VN can reduce the induction of ventricular arrhythmia, left stellate ganglion activity, and sympathetic nerve remodeling in dogs after myocardial infarction. Clancy et al. [11] confirmed that taVNS increases the ratio of low to high frequency, improves heart rate variability, and decreases skin sympathetic activity. Their study suggested that taVNS can regulate the cardiac autonomic nervous system and improve the sympathetic or parasympathetic balance in healthy people.
Although taVNS shows some advantages in the treatment of heart failure, arrhythmias, and other angiocardiopathy, due to the problem of insufficient sample sizes in clinical studies, there are some errors in the results. In addition, changes in hormone levels in the sexes and ages also have an impact on cardiac autonomic nervous regulation. In the future, consideration should be given to adding a study of subjects with different clinical characteristics.
Digestive system Diseases
As noted above, ABVN projects to the NTS, which receives sensory afferents from the viscera and can transmit the integrated information to the viscera. The VN dominates the movements and secretions of the gastrointestinal tract. Therefore, it is possible to regulate the gastrointestinal tract by stimulating the ABVN. Bonaz et al. [64] have shown that the intestinal nervous system can be regulated by VNS or taVNS, so gastric function can be affected. Lu et al. [65] showed that VNS in rats accelerates gastric emptying, leads to greater relaxation or dilation of the pyloric sphincter, and increases the amplitude of anal contraction and peristaltic speed. Frøkjaer et al. [66] stimulated healthy subjects with taVNS, and found that taVNS could regulate the frequency of gastrointestinal contraction, while the visceral pain threshold was not affected, indicating that taVNS may relieve pain. A preliminary study in a mouse model of postoperative ileus (POI) showed that tVNS of the auricular branch reduces intestinal inflammation and relieves POI [67]. It has been reported that the effect of taVNS on colon cancer induced by 1-dimethylhydrazine (DMH) is the same as that of the cholinergic anti-inflammatory pathway (CAP). TaVNS can restore the autonomous functional morphology of cells, reduce oxidative damage, and resist colon cancer induced by DMH [68].
Research on the Mechanism of taVNS
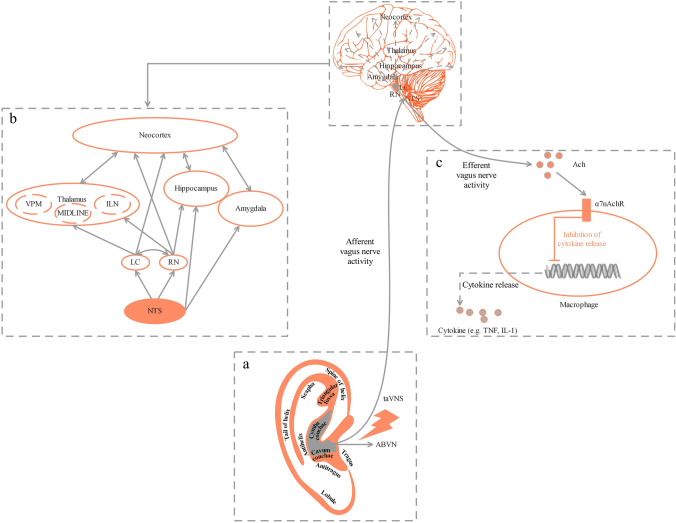
The VN is the 10th cranial nerve and is mainly sensory [69]. Its afferent fibers transmit visceral, somatosensory and taste information from receptors in the peripheral organs to the central nervous system (CNS) [70]. A branch of the VN, ABVN forms a cutaneous receptive field in the pinna of the ear [14] (Fig. 2A). Stimulation of the ABVN is directly connected to the NTS, which is the end point of the afferent fibers of the VN and is recognized as a relay station for visceral sensation, plays a relay role in receiving signals from the ear, and adjusts the function of the body [71]. The NTS can project directly or indirectly from bottom to top to many nuclei such as the parabrachial nucleus, dorsal raphe nucleus, locus ceruleus, hypothalamus, thalamus, amygdala, and hippocampus [72–74] (Fig. 2B). In addition, efferent fibers in the VN can control peripheral organs including the cardiovascular and digestive systems. Therefore, as a way to stimulate the ABVN, taVNS can be used in the treatment of the diseases mentioned above.
Fig. 2.
Potential mechanism of taVNS. A The external ear and its hypothesized cutaneous innervation by the ABVN. B Modulation of brain networks mainly depends on the extensive fibrous connections formed by ABVN projections to multiple brain regions through the NTS to regulate neuroendocrine, emotional, and cognitive functions. C The balance of the production of cytokines depends on the wiring of CAP [87, 88]. CNS, central nervous system; CAP, cholinergic anti-inflammatory pathway; VPM, ventralis posteromedialis nucleus; MIDLINE, midline thalamic nuclei; ILN, intralaminar nuclei; LC, locus coeruleus; RN, raphe nuclei; NTS, nucleus of the solitary tract; ABVN, auricular branch of the vagus nerve; taVNS, transcutaneous auricular vagus nerve stimulation; ACh, acetylcholine; α7nAChR, α7 nicotinic acetylcholine receptor; TNF, tumor necrosis factor; IL-1, interleukin-1.
As a relatively new field, research on taVNS is currently focused on evaluating its clinical efficacy and safety in treating diseases, as well as exploring some translational studies. The research on its mechanism is so limited that little is known about the mechanism of mediating the taVNS effect. After reviewing the progress of taVNS research in the past 20 years, we broadly classify the research on the mechanism of taVNS into 3 types.
Type 1
Explaining the effect of taVNS activation of the vagus pathway based on the anatomical and physiological characteristics of the VN.
He et al. [75] first reported the afferent projections from the ABVN to the NTS in the rat. The relations among the ABVN, the autonomic nervous system, and the CNS is referred to as the “auriculo-vagal afferent pathway” (AVAP), which strongly supports the concept that taVNS has the potential for suppressing epileptiform activity via the AVAP in rats. Deuchars et al. [76] found that non-invasive stimulation of the tragus of the ear increases parasympathetic activity and reduces sympathetic activity. The systemic effects of taVNS are likely to be mostly of sympatho-inhibitory origin [12]. A recent study showed that sensory afferents from the rat tragus project heavily to the dorsal horn of the upper cervical spinal cord, and in the brainstem to a lesser extent in the NTS. This challenges the notion that tragal stimulation is mediated by the ABVN and suggests that alternative mechanisms may be involved [77].
Type 2
Analyzing the effect of taVNS on the CNS by electrophysiology or neuroimaging, or to provide an explanation for the effectiveness of taVNS in the treatment of brain diseases.
In earlier studies, electrophysiological methods usually assessed VN function in the brainstem in healthy participants, and several studies have also shown that taVNS can evoke far-field brainstem potentials [78–80]. In recent years, the neuroimaging method represented by fMRI has been increasingly used in the study of taVNS. fMRI shows specific regulation of various brain structures after taVNS, including the brainstem, NTS, and spinal trigeminal nucleus [81–84]. In addition, in studies of taVNS in the treatment of some brain diseases, fMRI also showed that taVNS can regulate the resting-state functional connectivity of the brain [48, 53, 54].
Type 3
Exploring the intervention effect of taVNS based on the hypothesis of CAP in animal models of some diseases (Fig. 2C).
Advances in biomedical research often rely on the use of animal models as the experimental basis to verify experimental and clinical hypotheses. This is also the approach to further study the mechanism of taVNS. However, at present, there are few studies on taVNS based on animal models, and most of them rely on the CAP hypothesis. For instance, Zhao et al. [85] reported that the effect of taVNS is similar to that of VNS, and taVNS can inhibit the inflammatory response in the endotoxemic rat induced by LPS through α7 nicotinic acetylcholine receptor (α7nAChR)-mediated CAP. Jiang et al. [86] revealed that taVNS can regulate the innate immune response and activate the α7nAChR to inhibit the evolution of inflammation and play a neuroprotective role, which has a positive effect on the 6-OHDA model of Parkinson's disease. Rawat et al. [68] confirmed the efficacy of taVNS on colon cancer induced by 1, 2-DMH, and concluded that this effect is linked to the regulation of CAP by taVNS.
In general, the current research ideas and methods on the mechanism of taVNS have great limitations, which may be attributed to the research interest and knowledge background of practitioners in this field. There are still many disputes about the mechanism of taVNS, such as the projections of ABVN in the CNS, and whether there is a cholinergic anti-inflammatory mechanism similar to CAP in the CNS (Fig. 2), which deserve more attention in future.
Conclusion
Compared to iVNS, taVNS is a more promising neuroregulatory therapy. Based on the anatomical and physiological characteristics of the ABVN, the effect of taVNS on the human body is systematic and extensive. A variety of potential therapeutic applications in clinical practice has been shown. However, since taVNS transmission is not a processing function, its actual stimulation path is very indirect with respect to the peripheral organs [2]. This characteristic of taVNS determines its different roles in different diseases or different stages of diseases.
Based on the review, we believe that the current development of taVNS is restricted by the following factors: (1) Inconsistencies and irregularities in the nomenclature, often leading to different understandings of taVNS. This is not conducive to the promotion of this therapy. (2) The way to choose the appropriate stimulation method is still an urgent problem to be solved, because different stimulation targets and parameters often produce different therapeutic effects. (3) Different subjects and different diseases or stages have their unique clinical characteristics. Clinicians should deal with them individually and personalize treatment for different functional conditions. (4) The way to choose the appropriate evaluation method is also of great importance. Reasonable evaluation methods and appropriate evaluation times directly affect the judgment of its curative effect by clinical researchers. (5) Rigorous trial design is also a key factor. The design includes the principle of random control, strict screening of subjects, and the blind method. (6) The ambiguity of mechanism research limits the development of taVNS.
Therefore, in the future, scientists need to further standardize the stimulation methods of taVNS, combine electrophysiological and imaging evaluation methods to reduce the bias of the subjective scale, stick to follow-up observations, and develop a more optimal scheme for different diseases or functional conditions. In addition, more studies should be done on the mechanism of taVNS at the molecular, cellular, and neural circuit levels to provide more evidence for clinical application.
Acknowledgements
This review was supported by the National Key R&D Program of China (2018YFC1705800), the National Natural Science Foundation of China (81803872, 81674072), the Joint Sino-German Research Project (GZ1236), and the Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ201813006).
Conflict of interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rossi S, Santarnecchi E, Valenza G, Ulivelli M. The heart side of brain neuromodulation. Philos Trans A Math Phys Eng Sci. 2016;374:20150187. doi: 10.1098/rsta.2015.0187. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson WC, Kempf MC, Moneyham L, Vance DE. The potential role of vagus-nerve stimulation in the treatment of HIV-associated depression: a review of literature. Neuropsychiatr Dis Treat. 2017;13:1677–1689. doi: 10.2147/NDT.S136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22:1260–1268. doi: 10.1111/ene.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W, Wang X, Shi H, Shang H, Li L, Jing X, et al. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med. 2012;2012:786839. doi: 10.1155/2012/786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sator-Katzenschlager SM, Michalek-Sauberer A. P-Stim Auricular electroacupuncture stimulation device for pain relief. Expert Rev Med Devices. 2007;4:23–32. doi: 10.1586/17434440.4.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Nogier P. From Acuriculotherapy to Auriculomedicine. Sainte-Ruffine, France: Maisonneuve; 1981. [Google Scholar]
- 7.Gori L, Firenzuoli F. Ear acupuncture in european traditional medicine. Evid Based Complement Alternat Med. 2007;4:13–16. doi: 10.1093/ecam/nem106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Yu L, Ouyang F, Liu Q, Wang Z, Wang S, et al. The right side or left side of noninvasive transcutaneous vagus nerve stimulation: Based on conventional wisdom or scientific evidence? Int J Cardiol. 2015;187:44–45. doi: 10.1016/j.ijcard.2015.03.351. [DOI] [PubMed] [Google Scholar]
- 10.Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014;1:64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7(6):871–877. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation I - A physiological perspective. Front Neurosci. 2019;13:854. doi: 10.3389/fnins.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis PM, Thomson RH, Rosenfeld JV, Fitzgerald PB. Brain neuromodulation techniques: A review. Neuroscientist. 2016;22(4):406–421. doi: 10.1177/1073858416646707. [DOI] [PubMed] [Google Scholar]
- 14.Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation II - An engineering perspective. Front Neurosci. 2019;13:772. doi: 10.3389/fnins.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16:101–102. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- 16.Ay I, Nasser R, Simon B, Ay H. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 2016;9:166–173. doi: 10.1016/j.brs.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Qiao P, Li Q, Wang Y, Zhang L, Yan LJ, et al. Vagus nerve stimulation as a promising adjunctive treatment for ischemic stroke. Neurochem Int. 2019;131:104539. doi: 10.1016/j.neuint.2019.104539. [DOI] [PubMed] [Google Scholar]
- 18.Lendvai IS, Maier A, Scheele D, Hurlemann R, Kinfe TM. Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: a review. J Pain Res. 2018;11:1613–1625. doi: 10.2147/JPR.S129202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broncel A, Bocian R, Kłos-Wojtczak P, Kulbat-Warycha K, Konopacki J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull. 2020;155:37–47. doi: 10.1016/j.brainresbull.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Bucksot JE, Morales Castelan K, Skipton SK, Hays SA. Parametric characterization of the rat Hering-Breuer reflex evoked with implanted and non-invasive vagus nerve stimulation. Exp Neurol. 2020;327:113220. doi: 10.1016/j.expneurol.2020.113220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MMH, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020;13:47–59. doi: 10.1016/j.brs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimmele F, Jürgens TP. Neuromodulation in primary headaches: current evidence and integration into clinical practice. Curr Opin Neurol. 2020;33:329–337. doi: 10.1097/WCO.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 23.Magis D, Gérard P, Schoenen J. Transcutaneous Vagus Nerve Stimulation (tVNS) for headache prophylaxis: initial experience. J Headache Pain. 2013;14:198. [Google Scholar]
- 24.Muthulingam JA, Olesen SS, Hansen TM, Brock C, Drewes AM, Frøkjær JB. Study protocol for a randomised double-blinded, sham-controlled, prospective, cross-over clinical trial of vagal neuromodulation for pain treatment in patients with chronic pancreatitis. BMJ Open. 2019;9:e029546. doi: 10.1136/bmjopen-2019-029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safi S, Ellrich J, Neuhuber W. Myelinated axons in the auricular branch of the human vagus nerve. Anat Rec (Hoboken) 2016;299:1184–1191. doi: 10.1002/ar.23391. [DOI] [PubMed] [Google Scholar]
- 26.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 27.Badran BW, Yu AB, Adair D, Mappin G, DeVries WH, Jenkins DD, et al. Laboratory administration of transcutaneous auricular vagus nerve stimulation (taVNS): Technique, targeting, and considerations. J Vis Exp 2019, 143:10.3791/58984. [DOI] [PMC free article] [PubMed]
- 28.Redgrave J, Day D, Leung H, Laud PJ, Ali A, Lindert R, et al. Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans: a systematic review. Brain Stimul. 2018;11:1225–1238. doi: 10.1016/j.brs.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): A transganglionic HRP study in the cat. Brain Res. 1984;292:199–205. doi: 10.1016/0006-8993(84)90756-x. [DOI] [PubMed] [Google Scholar]
- 30.Bermejo P, López M, Larraya I, Chamorro J, Cobo JL, Ordóñez S, et al. Innervation of the human cavum conchae and auditory canal: anatomical basis for transcutaneous auricular nerve stimulation. Biomed Res Int. 2017;2017:7830919. doi: 10.1155/2017/7830919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- 32.Badran BW, Brown JC, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, et al. Tragus or cymba conchae? Investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS) Brain Stimul. 2018;11:947–948. doi: 10.1016/j.brs.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. 2020;236:588–611. doi: 10.1111/joa.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Wang Y, Gao G, Guo X, Zhang Y, Zhang Z, et al. Transcutaneous auricular vagus nerve stimulation at 20 Hz improves depression-like behaviors and down-regulates the hyperactivity of HPA axis in chronic unpredictable mild stress model rats. Front Neurosci. 2020;14:680. doi: 10.3389/fnins.2020.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11:492–500. doi: 10.1016/j.brs.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia. 2012;53:e115–118. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 37.He W, Jing X, Wang X, Rong P, Li L, Shi H, et al. Transcutaneous auricular vagus nerve stimulation as a complementary therapy for pediatric epilepsy: a pilot trial. Epilepsy Behav. 2013;28:343–346. doi: 10.1016/j.yebeh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Rong P, Liu A, Zhang J, Wang Y, He W, Yang A, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: A randomized controlled trial. Clin Sci (Lond) 2014. [DOI] [PubMed]
- 39.Aihua L, Lu S, Liping L, Xiuru W, Hua L, Yuping W. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 2014;39:105–110. doi: 10.1016/j.yebeh.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: A randomized, double-blind clinical trial (cMPsE02) Brain Stimul. 2016;9:356–363. doi: 10.1016/j.brs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Hamer HM, Bauer S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS) Epilepsy Res. 2019;153:83–84. doi: 10.1016/j.eplepsyres.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Wu K, Wang Z, Zhang Y, Yao J, Zhang Z. Transcutaneous vagus nerve stimulation for the treatment of drug-resistant epilepsy: a meta-analysis and systematic review. ANZ J Surg. 2020;90:467–471. doi: 10.1111/ans.15681. [DOI] [PubMed] [Google Scholar]
- 43.Assenza G, Campana C, Colicchio G, Tombini M, Assenza F, Di Pino G, et al. Transcutaneous and invasive vagal nerve stimulations engage the same neural pathways: in-vivo human evidence. Brain Stimul. 2017;10:853–854. doi: 10.1016/j.brs.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna) 2013;120:821–827. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- 45.Trevizol AP, Taiar I, Barros MD, Liquidatto B, Cordeiro Q, Shiozawa P. Transcutaneous vagus nerve stimulation (tVNS) protocol for the treatment of major depressive disorder: a case study assessing the auricular branch of the vagus nerve. Epilepsy Behav. 2015;53:166–167. doi: 10.1016/j.yebeh.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Trevizol AP, Shiozawa P, Taiar I, Soares A, Gomes JS, Barros MD, et al. Transcutaneous vagus nerve stimulation (taVNS) for major depressive disorder: an open label proof of concept trial. Brain Stimul. 2016;9:453–454. doi: 10.1016/j.brs.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79:266–273. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Liu P, Fu H, Chen W, Cui S, Lu L, et al. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e13845. doi: 10.1097/MD.0000000000013845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong J, Fang J, Park J, Li S, Rong P. Treating depression with transcutaneous auricular vagus nerve stimulation: state of the art and future perspectives. Front Psychiatry. 2018;9:20. doi: 10.3389/fpsyt.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang T. Recent progress in basic and clinical research on disorders of consciousness. Neurosci Bull. 2018;34:589–591. doi: 10.1007/s12264-018-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noé E, Ferri J, Colomer C, Moliner B, O'Valle M, Ugart P, et al. Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimul. 2020;13:427–429. doi: 10.1016/j.brs.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Yu YT, Yang Y, Wang LB, Fang JL, Chen YY, He JH, et al. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: The first case report. Brain Stimul. 2017;10:328–330. doi: 10.1016/j.brs.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhao B, Li L, Jiao Y, Luo M, Xu K, Hong Y, et al. Transcutaneous auricular vagus nerve stimulation in treating post-stroke insomnia monitored by resting-state fMRI: The first case report. Brain Stimul. 2019;12:824–826. doi: 10.1016/j.brs.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Zhao M, Yu X, Zang W. Pharmacological modulation of vagal nerve activity in cardiovascular diseases. Neurosci Bull. 2019;35:156–166. doi: 10.1007/s12264-018-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 57.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. European Heart Journal. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 58.Scherlag BJ, Helfant RH, Haft JI, Damato AN. Electrophysiology underlying ventricular arrhythmias due to coronary ligation. Am J Physiol. 1970;219:1665–1671. doi: 10.1152/ajplegacy.1970.219.6.1665. [DOI] [PubMed] [Google Scholar]
- 59.Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation 1973, 47: 291–298. [DOI] [PubMed]
- 60.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 61.Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, Goldstein RE, et al. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974;49:943–947. doi: 10.1161/01.cir.49.5.943. [DOI] [PubMed] [Google Scholar]
- 62.James RG, Arnold JM, Allen JD, Pantridge JF, Shanks RG. The effects of heart rate, myocardial ischemia and vagal stimulation on the threshold for ventricular fibrillation. Circulation. 1977;55:311–317. doi: 10.1161/01.cir.55.2.311. [DOI] [PubMed] [Google Scholar]
- 63.Yu L, Wang S, Zhou X, Wang Z, Huang B, Liao K, et al. Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. JACC Clin Electrophysiol. 2016;2:330–339. doi: 10.1016/j.jacep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. The Journal of Physiology. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu KH, Cao J, Oleson S, Ward MP, Phillips RJ, Powley TL, et al. Vagus nerve stimulation promotes gastric emptying by increasing pyloric opening measured with magnetic resonance imaging. Neurogastroenterol Motil. 2018;30:e13380. doi: 10.1111/nmo.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frøkjaer JB, Bergmann S, Brock C, Madzak A, Farmer AD, Ellrich J, et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol Motil. 2016;28:592–598. doi: 10.1111/nmo.12760. [DOI] [PubMed] [Google Scholar]
- 67.A Zillekens, GS Hong, B Schneiker, A Schmidt, JC Kalff, S Wehner. Untersuchung des Effektes einer transkutanen Vagus nerve stimulation auf den postoperativen Ileus im Maus model. Z Gastroenterol 2014, 52 - FV39.
- 68.Rawat JK, Roy S, Singh M, Guatam S, Yadav RK, Ansari MN, et al. Transcutaneous vagus nerve stimulation regulates the cholinergic anti-inflammatory pathway to counteract 1, 2-dimethylhydrazine induced colon carcinogenesis in albino wistar rats. Front Pharmacol. 2019;10:353. doi: 10.3389/fphar.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 71.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 72.Brandt C, Volk HA, Loscher W. Striking differences in individual anticonvulsant response to phenobarbital in rats with spontaneous seizures after status epilepticus. Epilepsia. 2004;45:1488–1497. doi: 10.1111/j.0013-9580.2004.16904.x. [DOI] [PubMed] [Google Scholar]
- 73.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 74.Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience. 2005;134:657–669. doi: 10.1016/j.neuroscience.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 75.He W, Jing XH, Zhu B, Zhu XL, Li L, Bai WZ, et al. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci. 2013;14:85. doi: 10.1186/1471-2202-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deuchars SA, Lall VK, Clancy J, Mahadi M, Murray A, Peers L, et al. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol. 2018;103:326–331. doi: 10.1113/EP086433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahadi KM, Lall VK, Deuchars SA, Deuchars J. Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways. Brain Stimul. 2019;12:1151–1158. doi: 10.1016/j.brs.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Fallgatter AJ, Ehlis AC, Ringel TM, Herrmann MJ. Age effect on far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Int J Psychophysiol. 2005;56:37–43. doi: 10.1016/j.ijpsycho.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2003;110:1437–1443. doi: 10.1007/s00702-003-0087-6. [DOI] [PubMed] [Google Scholar]
- 80.Polak T, Markulin F, Ehlis AC, Langer JB, Ringel TM, Fallgatter AJ. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm (Vienna) 2009;116:1237–1242. doi: 10.1007/s00702-009-0282-1. [DOI] [PubMed] [Google Scholar]
- 81.Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37:443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 82.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 83.Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal-a pilot study. Brain Stimul. 2013;6:798–804. doi: 10.1016/j.brs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Dietrich S, Smith J, Scherzinger C, Hofmann-Preiss K, Freitag T, Eisenkolb A, et al. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl) 2008;53:104–111. doi: 10.1515/BMT.2008.022. [DOI] [PubMed] [Google Scholar]
- 85.Mercante B, Ginatempo F, Manca A, Melis F, Enrico P, Deriu F. Anatomo-physiologic basis for auricular stimulation. Med Acupunct. 2018;30:141–150. doi: 10.1089/acu.2017.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang Y, Cao Z, Ma H, Wang G, Wang X, Wang Z, et al. Auricular vagus nerve stimulation exerts antiinflammatory effects and immune regulatory function in a 6-OHDA model of Parkinson's disease. Neurochem Res. 2018;43:2155. doi: 10.1007/s11064-018-2639-z. [DOI] [PubMed] [Google Scholar]
- 87.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 88.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Vest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]